Pest Management Regulatory Agency Annual Report 2022–2023

Download the alternative format
(PDF format, 2.24 Mb, 55 pages)
- Organization: Health Canada
- Date published: 2024-09-20
Health Canada
20 September 2024
Table of Contents
- Message from the Assistant Deputy Minister
- Message from the Executive Director
- 2022–2023 pesticide regulation numbers at a glance
- What are pesticides?
- About the Pest Management Regulatory Agency
- Scientific assessment of pesticides
- Pesticide registrations and decisions
- Transforming our regulatory approach toward a continuous oversight model for registered pesticides
- Advancing science in pesticide risk assessment
- Health Canada's compliance and enforcement activities on pesticides
- Legislative and regulatory modernization
- Improving transparency in pesticide regulation
- External communications and outreach
- External advisory bodies
- International cooperation
- Financial profile
- Appendices
Message from the Assistant Deputy Minister
Health Canada's Pest Management Regulatory Agency (PMRA) upholds the highest standards of health and environmental protection in the regulation of pesticides. In 2022, we continued to focus on delivering the federal commitments made in August 2021 to strengthen the pesticide review process in Canada and improve its transparency. I am pleased to present this year's report as yet another mechanism to inform Canadians on the progress made to date on the PMRA's Transformation Agenda.
In its second year of transformation, the Agency has already achieved success on many fronts and will begin its transition into everyday operations in 2023–2024. We have advanced several key aspects of modernizing our business processes, collected and disclosed real-world data to inform the development of a national pesticide monitoring program, and sought independent scientific advice from the Science Advisory Committee on Pest Control Products (SAC-PCP) to better inform our decisions. We continue to integrate improved transparency and communication products throughout Agency activities, including in our decisions, web presence, and access to information and data.
Canada committed to the Kunming-Montreal Global Biodiversity Framework (GBF) in December 2022. The Framework will guide global actions through 2030 to support the conservation and sustainable use of nature. A component of the GBF's Target 7, which the PMRA co-leads, aims to reduce the overall risk from pesticides by at least half by 2030, including through integrated pest management, based on science, while taking food security and livelihoods into account. I am personally very excited to see Canada's global leadership in pesticide management come to the forefront through this ambitious commitment.
There is still much work to be done, and the Agency is motivated more than ever to continue achieving visible results, as demonstrated by this year's report. I would like to thank all of our stakeholders and partners who have helped to shape our path forward. I personally would like to ask you all to stay the course with us; we will have more opportunities for engagement and consultations throughout the next year as we continue to progress on formalizing our policies and regulations. I would also like to acknowledge the dedication exemplified by the PMRA's employees as they balance the innovation and coordination of Transformation activities while upholding core regulatory duties. Frederic and I take great pride in their commitment to protecting the health and safety of Canadians and the environment.
I personally commit to continuing engagement with all stakeholders to continue to inform and advance our efforts. We will pursue our transformation with steadfast dedication to evidence-based decision-making and upholding Canada's reputation as a global leader in pesticide management.
Manon Bombardier, Ph.D., MBA
Assistant Deputy Minister, Transformation
Pest Management Regulatory Agency
Message from the Executive Director
I am pleased to present to you Health Canada's Pest Management Regulatory Agency (PMRA) Annual Report for 2022–2023.
As Manon Bombardier outlined in her message, this report describes the many ways in which the PMRA is transforming its approach to pesticide regulation, while the important work of assessing and managing pesticide risks continues.
This core purpose represents a substantial amount of work, some of which is unpredictable as submission numbers and types vary each year, while others, such as re-evaluations, require growing effort.
This past year, the PMRA prioritised and completed the re-evaluation of legacy pesticides registered before 1995, while making significant progress on cyclical re-evaluations and special reviews. This was achieved while also piloting new approaches that will enable a sharper focus of the PMRA's resources on pesticides presenting a higher level of potential risk.
Work is continuing to pick up on international cooperation as we continue to liaise with our partners on key issues at the Organization for Economic Cooperation and Development (OECD), Canada-United States-Mexico Agreement (CUSMA), Stockholm and Rotterdam Conventions, and bilaterally with various jurisdictions. At these fora, the PMRA participated in discussions and negotiations around topics such as food safety, maximum residue limits, biotechnology and agreements on the management of toxic chemicals.
This year, our workforce transitioned from almost entirely remote, to a hybrid model that combines in-person and virtual work. This allowed staff to combine the productivity-enhancing benefits of remote work, with the opportunities for creative cooperation of in-person work.
The pace of change in the factors that drive our work is unprecedented right now. Evolving science, agricultural technologies, climate change, globalization and pest pressures require a workforce that is aware, agile and responsive.
The accomplishments described in this report show that one of our key strengths of our workforce is our ability to view change as an opportunity to transform, while maintaining unwavering focus on the protection of human health and the environment from the potential risks of pesticides.
Frédéric Bissonnette
Executive Director
Pest Management Regulatory Agency
2022–2023 pesticide regulation numbers at a glance
- New active ingredients: 14
- New generic products (active ingredient and end-use): 100
- New minor uses: 64
- Emergency registrations: 17
- New active Joint Reviews: 1
- Final re-evaluation decisions: 26
- Proposed re-evaluation decisions: 16
- Final special review decisions: 1
- Proposed special review decisions: 3
- Pesticide incident reports received: 1566
- Scientific studies received through incident reporting: 88
- Compliance verifications: 879
- Enforcement actions taken: 1885
- Compliance promotion activities: 89
What are pesticides?
When most people hear the word "pesticide", they tend to think of chemicals used to control agricultural pests. In Canada, the definition of a pesticide, also referred to as a "pest control product", is broad:
- any product, device, organism, substance or thing that is manufactured, represented, sold or used as a means for directly or indirectly controlling, preventing, destroying, mitigating, attracting or repelling any pest
While most of these products are chemical in nature, they can also include:
- devices like traps or attractants
- plant-based substances like essential oils or garlic juice
- viruses and strains of bacteria
The pests these products are intended to control are often weeds, insects and fungi that can threaten crops, but these can also be:
- invasive species of plants and animals
- disease-carrying insects such as mosquitoes, ticks, and fleas
- rodents such as mice and rats
- insects and diseases that can threaten our forests
- toxic weeds like poison ivy and giant hogweed
About the Pest Management Regulatory Agency
Under Canadian law (the Pest Control Products Act), any pesticide intended for use in Canada must be assessed and registered or otherwise authorized by Health Canada's Pest Management Regulatory Agency (PMRA). PMRA scientists thoroughly assess a product's potential health and environmental risks, as well as its value as a pest control product, before it can be registered for use. Under the Act, a pest control product can only be approved for use if the product has value and there is reasonable certainty that no harm to human health or the environment will result from the use of the product, taking into account its conditions of registration.
The Pest Control Products Act requires that regulatory decisions about pesticides be based on a scientific evaluation of health and environmental risks, and value. This requirement ensures pesticide regulation in Canada is based on evidence. This prevents the registration of unsafe pesticides, and conversely, allows the registration of pesticides whose risks have been scientifically determined to be acceptable under approved conditions of use.
Once a pesticide is registered it is subject to "post-market" (in other words, after registration) oversight and risk management that considers the body of scientific knowledge about the product, as well as new data that becomes available as the product is used.
This work is carried out by a highly skilled workforce, the majority of whom are scientists, with expertise in areas such as:
- toxicology
- plant science
- entomology
- chemistry
- epidemiology
- water monitoring
- environmental exposure modelling
The PMRA workforce also includes experts in areas such as regulatory and policy development, stakeholder engagement, international collaboration, science communications, data scientists and information management.
Scientific assessment of pesticides
The PMRA's mandate is to prevent unacceptable risks to Canadians and the environment from the use of pesticides. The PMRA applies current, evidence-based scientific approaches to assess whether the health and environmental risks of pesticides proposed for registration are acceptable, and if the products have value.
These science-based risk assessments include the following:
- an examination of all sources and routes of potential exposure (for example, mouth, skin, breathing) to a given pesticide, including exposure through diet, from drinking water and from contact with treated areas like lawns and gardens
- an estimation of the amount of pesticides that people, including children, may come in contact with, both during and after a pesticide application
- a human health risk assessment, including a sex- and gender-based analysis (SGBA+) with a particular focus on risk factors in various sub-populations, including pregnant people, infants, children, women, and seniors; this considers the potential for a pesticide to cause health effects such as cancer, birth defects and hormone effects, and allows registration only for those pesticides with exposures well below levels that may cause effects
- a determination of what happens to a pesticide once it enters the environment, including its behaviour in soil, water and air, the potential for its uptake by plants or animals, the potential for bioaccumulation in organisms, as well as likely exposure levels for non-target organisms
- an examination of the toxicity of a pesticide to non-target plants and animals (for example, birds, mammals, beneficial insects, aquatic organisms), both on land and in bodies of water
- an environmental risk assessment which integrates the environmental exposure and ecotoxicology information to estimate the potential for adverse effects on non-target species
- a value assessment that considers the contribution of the product to pest management, as well as its health, safety and environmental benefits, and social and economic impact
If the health or environmental risks of a pest control product, or its value, are found to be unacceptable, it will not be registered for use. When a pesticide is registered for use, in addition to post-market consideration and re-evaluation of the scientific information described above, the following information contributes to the monitoring and management of the risks of registered pesticides:
- additional environmental data, such as levels of pesticides detected in water across Canada or the United States
- Canadian Food Inspection Agency's (CFIA) monitoring data for pesticide residues in both domestic and imported food commodities as part of its National Chemical Residue Monitoring Program
- pesticide sales data
- reports of incidents from Canada or other jurisdictions where the pesticide is already registered
- any other evidence-based information that contributes to the ongoing assessment and monitoring of the health and environmental risks and the value of the pest control product (for example, pesticide use information, literature reviews, biomonitoring data from other programs, compliance- and enforcement-related activities)
Using pesticides safely (read the label)
The product label is a critical component of managing the risks of using a pesticide and is a key outcome of the scientific assessment process. When a pesticide is registered, the product label is a legal document that must be followed, and failure to do so is a contravention of the Pest Control Products Act, subject to compliance and enforcement action. The label is very specific about the conditions under which the product can be used. This can include:
- who is allowed to use it (some products require certification and/or training)
- where it can be applied, for example on specific crops, in or on structures, on people (for example, bug spray) or specific animals
- the pests it can be used to treat
- the environmental conditions under which it can be used (indoor/outdoor, time of year, weather conditions, distance from water and sensitive habitats, etc.)
- how much can be used in a specified area at once and how often it can be re-applied
- the equipment that may or must be used to mix and apply a pesticide
- methods of application
- protective equipment that must be worn during mixing and application
- the required wait time before re-entering a treated area
- other precautions, such as consideration of the presence of pollinators or species at risk
Health Canada's Regulatory Operations and Enforcement Branch is responsible for promoting, monitoring, and enforcing compliance with the Pest Control Products Act across Canada.
Pesticide registrations and decisions
The general term "pesticide" or "pest control product" can refer to the "active ingredient" or "active" (the substance with the pesticidal effect, for example DEET), or it could refer to an "end-use product" that contains the active ingredient along with other formulants and additives (for example various brands of insect repellent). Both active ingredients and end-use products must be evaluated by the PMRA before they may be registered for use.
A new active ingredient could be a completely novel pesticide, or it could be a pesticide that is already registered elsewhere with a history of use. Active ingredients can be:
- synthetic chemicals, often referred to as "conventional chemical pesticides"
- "biopesticides", which are derived from natural sources such as bacteria, fungi, viruses, plants, animals, and minerals
- "antimicrobials", which are products used for the control of microorganisms (bacteria, fungi, algae, protozoa, or viruses) and fouling organisms (those that attach themselves to wet surfaces such as zebra mussels or barnacles) on or in inanimate objects, industrial processes, water or air
New end-use products can be products that contain already registered active ingredients.
Registrants must submit an application to register a new product, or to make a change to an existing product registration. Appendix Table 1 describes the various product submission categories, as well as the service standards for processing these applications.
New pesticide registrations in 2022–2023:
- 14 new active ingredients
- 7 biopesticides
- 7 conventional chemical pesticides
- 18 new related end-use products containing these actives
- 278 total new end-use products
Some examples of end-use products registered in 2022–2023 include:
- a new insecticide that is applied indoors as a crack-and-crevice and spot treatment, including for furniture and mattresses, to control bed bugs and bed bug eggs
- a new active ingredient for control of invasive quagga and zebra mussels in water reservoirs and other water bodies, and in agricultural irrigation pipelines and closed systems, including fire suppression systems in hydroelectric plants
- a new ozone-generating device for use as a sanitizer and disinfectant on hard, non-porous surfaces in commercial and industrial areas
- a fungus isolated from soil, used to control pests on a wide variety of greenhouse-grown ornamental and food crop plants, including plants grown for transplanting, and cannabis grown in greenhouses and enclosed structures
- a combination of two new microbial active ingredients for fungal disease management on various field and greenhouse crops, including strawberries, greenhouse ornamentals and cannabis produced commercially indoors
Please see Appendix Table 2 for a full list of new active ingredients registered.
Generic registrations
When a new pesticide is developed, the innovator invests substantial funds into the studies required to show that the product has value (for example, works as intended) and poses no unacceptable health and environmental risks. The data supporting an innovation to Canada (in other words, a new active ingredient) receives exclusive use protection for a period of time, to prevent it from being used for the benefit of a competitor without the innovator's approval. Data subsequently used to amend or maintain a registration or register a new product are given "compensable protection", meaning applicants who wish to rely on the data are required to pay compensation to the applicable registrant. These data protection provisions are described in section 17.01 of the Pest Control Product Regulations.
This practice allows the innovator the opportunity to recover their investment, but also encourages further innovation by allowing competition on the market after a period of time. Allowing timely introduction of equivalent products by generic manufacturers following the exclusive period can enhance market competition to the benefit of users, including growers. These regulations are important to innovators, generic companies and to growers.
New generic registrations in 2022–2023:
- 269 applications
- 100 generic registrations
- 34 were active ingredients
- 66 were end-use products
- 95% were agricultural
- 73% herbicides
- 26% fungicides
- 1% insecticides
Joint reviews
Joint reviews are pesticide assessments conducted in cooperation with other jurisdictions. Joint reviews increase the efficiency of the registration process and facilitate collaboration between Canada and other countries such as the United States. Registrants must apply to register their product in each participating jurisdiction at the same time for a joint review to be conducted.
In 2022–2023, one of the 14 new active ingredients registered was a joint review (pyraziflumid). The PMRA is continuing to pilot new joint review approaches with the United States Environmental Protection Agency to increase efficiencies of the review process. The pilot approaches are being shared with international partners with the aim of increasing international interest in joint reviews, potentially leading to more joint reviews in the future.
Minor uses
The term "minor use" describes a potential use for a pest control product whose anticipated volume of sales is not sufficient to persuade a manufacturer to register and sell the product in Canada. The definition emphasizes that it is the projected sales of the pest control product that is minor and not necessarily the size of the crop. A minor use may be registered on a major crop because the use may be needed only occasionally or is limited to a small percentage of the total area of the crop.
To help resolve these pesticide access issues for Canadian growers, the PMRA works with Agriculture and Agri-Food Canada's Pest Management Centre to support growers and grower associations in identifying priorities for new minor use registrations in Canada. The PMRA also works directly with the provinces to assist in addressing regional minor use needs.
Minor use decisions in 2022–2023:
- 64 regulatory decisions were made in support of minor uses
- 39 addressed provincial/needs
- 25 addressed grower priorities identified at the national Minor Use Pesticide Priority Setting Workshop
Of the 64 decisions, 4 were joint reviews with the United States Environmental Protection Agency
Research applications
Researchers looking to generate test data on human health and environmental effects or value using unregistered pest control products or registered products outside of the labelled use pattern are subject to the Pest Control Product Regulations for research. There are three main categories of research described in the Pest Control Product Regulations:
- Exemption – The research is small scale and meets a defined set of criteria. The researcher does not need to make an application to the PMRA and can proceed with the research.
- Research notification – The research is small to medium scale and meets a set or criteria. The researcher must notify the PMRA by submitting an application for Notification
- Research authorization – The research is larger scale or does not meet exemption or notification criteria. The researcher must make an application to the PMRA for Authorization.
Research can be authorized for up to three years.
The number of applications for research that the PMRA receives can vary from year to year. Applications may be received in one year but completed in the next, depending on the time allotted for review of the application under the PMRA's Management of Submission Policy (MOSP).
Research application decisions in 2022–2023:
- 75 research authorizations
- 70 research notifications
Research authorizations for pesticide application by drone
Drones used for the purpose of applying pesticides represent a new method of aerial application for the PMRA. Products registered for application by drone will have "Remotely Piloted Aircraft Systems" and/or "RPAS" on the label.
To generate data that would support future registrations and label amendments for this method of aerial application, authorization is required for any research conducted in Canada using drones.
Considering the increasing interest within the agricultural community for this method of application, the PMRA granted six research authorizations in 2022–2023 where the method of application was using a remotely piloted aircraft system.
In the coming years, it is anticipated that there will be an increasing number of research authorization applications requesting drone application as the industry generates the required data that allows the PMRA to characterize the risks and value associated with this use.
Emergency registrations
A pest control product can be registered for up to one year for the emergency control of serious pest infestations, for example, to control or eliminate an invasive species and protect native biodiversity. The human health and environmental risks of the product must be acceptable, and the product must have value.
The number of emergency registration submissions that the PMRA receives can vary from year to year, depending on pest outbreaks, environmental conditions, and the availability of alternative products and control methods. If a submission is received late in the year, or if it requires significant risk assessment, the registration decision may occur in the following year.
- In 2022–2023, the PMRA granted 17 emergency registrations based on requests from provinces, and one from Natural Resources Canada's Canadian Forest Service.
Emergency registrations for control of invasive species: hemlock woolly adelgid
The hemlock woolly adelgid is an invasive insect that causes a rapid and severe decline in the health of affected hemlock trees, eventually resulting in their death. First reported in Virginia over 70 years ago, it has since spread throughout the eastern United States wherever hemlocks grow. Over the past few years, this invasive insect has been seen with increased frequency in Canada, including several occurrences in southern Ontario and, most recently, a widespread infestation in Nova Scotia that is posing a severe threat to remaining eastern hemlock stands in that province.
An emergency registration, requested by the Canadian Forest Service, was granted in 2022 for a product that is specifically suitable for the types of rapid and widespread applications that are critical for an efficacious response to large scale forest infestations. Without effective control of this invasive insect, the dense canopy and shading provided by hemlock tree stands would be lost, resulting in detrimental effects on the entire surrounding ecosystem, including destruction of habitat and shelter for numerous animals that depend on these hemlock populations.
Maximum residue limits
A maximum residue limit (MRL) is the highest amount of pesticide residue that may remain on food when a pesticide is used according to label directions. These are set at levels well below the amount that could pose a health concern and are established for each combination of pesticide and treated food crop.
The PMRA sets science-based MRLs to ensure the food Canadians eat is safe. Typically, an MRL applies to the identified raw agricultural food commodity as well as to any processed food product derived from these raw commodities. If it is determined that an unacceptable risk exists based upon how the pesticide is intended to be used, the pesticide will not be permitted for sale or use in Canada and MRLs will not be set. As of December 2022, Canada had approximately 25 750 pesticide MRLs set (Figure 1), with 912 MRLs set between January and December 2022. As a result of the pause on all MRL increase decisions announced in August 2021, there were no proposed MRL increases over the reporting period.
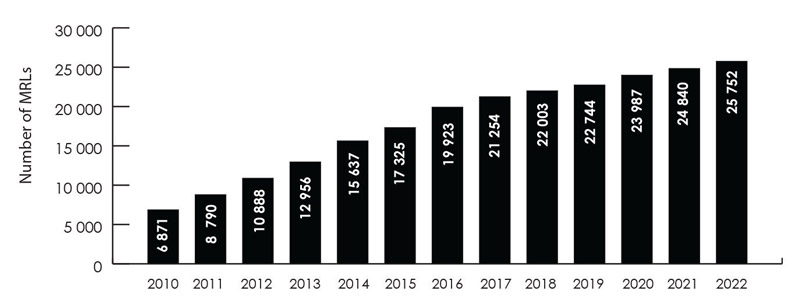
Figure 1 - Text equivalent
Number of Canadian MRLs over time, including new MRLs
- 2010 = 6871
- 2011 = 8790
- 2012 = 10 888
- 2013 = 12 956
- 2014 = 15 637
- 2015 = 17 325
- 2016 = 19 923
- 2017 = 21 254
- 2018 = 22 003
- 2019 = 22 744
- 2020 = 23 987
- 2021 = 24 840
- 2022 = 25 752
The CFIA is responsible for monitoring MRL compliance in foods in the Canadian marketplace. In their most recent report from 2019–2020 the compliance rate for imported and domestic fresh fruit and vegetable samples was 94.3% and 99.2%, respectively, which is consistent with observed compliance rates in past years and continues to indicate that the vast majority of food on the market meets Canadian pesticide standards.
Differences in MRLs between countries can lead to trade barriers. If an importing country's MRL for a given commodity is set lower than Canada's, this can lead to the importing country refusing entry to the Canadian commodity, even though the difference does not reflect a health risk.
International differences in MRLs can occur because of differences in both use patterns and data available to regulators at the time of MRL establishment, as well as other factors. Aligning MRLs globally has become increasingly important to reduce barriers to the movement of treated agricultural food commodities around the world. Domestic and international collaboration is critical in resolving these issues. This is especially important to help ensure that Canadian crops can be exported to international markets and that Canadians have access to foods they want and need at home, which is central to supporting food security.
The PMRA plays an active role in the World Health Organization (WHO)/Food and Agriculture Organization (FAO) Codex Committee on Pesticide Residues, which is responsible for setting international food standards. The Government of Canada also participates in the World Trade Organization (WTO) Agreement on the Application of Sanitary and Phytosanitary Measures (SPS Agreement), which sets out basic rules for food safety and animal and plant health standards. Member countries are encouraged to use international standards, although they can set their own standards based on science and an appropriate assessment of risks, which may differ across jurisdictions due to different use patterns.
The PMRA continues to work with its international partners under the Canada-United States-Mexico Agreement (CUSMA), the Organisation for Economic Co-operation and Development (OECD) and Codex Alimentarius Commission, on science policies relevant to establishing MRLs.
The PMRA is also continuing work on an import MRL pilot project initiated in 2020 to explore the feasibility of specifying import MRLs using only foreign country reviews, if available, which is based on a similar pilot project conducted by the United States Environmental Protection Agency's Office of Pesticide Programs. Preference is given to reviews prepared by the Joint Food and Agriculture Organization/World Health Organization Meeting on Pesticide Residues, in which Canada actively participates, along with the European Food Safety Authority, the United States, and other OECD countries such as Australia and New Zealand.
The absence of an MRL for a particular pesticide-crop combination in an export market (sometimes called a "missing MRL"), or MRL differences can also be a challenge for agricultural exporters. The PMRA continues to support Agriculture and Agri-Food Canada in efforts to address this challenge by providing policy and technical expertise to promote Canada's interests in international standard-setting for pesticide MRLs on agricultural commodities.
Improving transparency on MRLs
In August 2021, the Government of Canada announced a pause on all proposed increases to MRLs. The pause on MRL increases was put in place in response to public concerns regarding a proposed increase to the MRLs for glyphosate in May 2021 for certain imported commodities, which would have brought Canada into international alignment with the Codex Alimentarius Commission.
This pause allowed Health Canada to work with partners and engage with stakeholders to better understand the expectations of people in Canada about the pesticide regulatory review process, including the setting of MRLs. During the reporting period, several measures were taken in preparation for the lifting of the pause in spring of 2023, including:
- Updated the PMRA's webpages to help users find information related to consultations and decisions, including on proposed MRLs
- Developed plain language communication products to improve understanding of MRL applications and proposed decisions, and
- Conducted public opinion research and user testing on new science communication products on MRLs, including an infographic and a video.
An increase to an MRL does not change whether the pesticide meets health and environmental protection requirements. An MRL will only be increased if Health Canada scientists determine through the risk assessment process that it is safe to do so.
Lifting the pause and resuming public consultations for proposed MRL increases is important to allow people in Canada to maintain reliable access to affordable and nutritious food, provide predictability for farmers to access the required tools to fight against new pests, and facilitate trade, which is central to supporting food security.
Reinforcing trust in the PMRA's regulatory decisions by improving transparency, including plain language communication on how MRLs are established and their safety for people in Canada, and increasing the use of independent data and advice are some of the key objectives of PMRA's transformation.
Transforming our regulatory approach toward a continuous oversight model for registered pesticides
Under the Pest Control Products Act, registered pesticides in Canada are subject to re-evaluations, which are initiated on a 15-year cycle based on the most recent major decision affecting the registration, including its initial registration.
In 2022–2023, the PMRA advanced the development of a continuous oversight framework that will integrate key new approaches and processes, building on existing activities, to create a modern regulatory model that improves oversight throughout the pesticide regulatory lifecycle to ensure that risks from pesticides to the people who live in Canada, and the environment, continue to be acceptable.
To inform development of the new Continuous Oversight framework, the PMRA conducted four pilot studies involving pesticides with upcoming re-evaluations. The results of these pilots will inform the draft policy on Continuous Oversight that will be made available for public consultation in 2023–2024.
As a part of this new proposed framework, the PMRA has also advanced the development of a Proportional Effort policy that will be applied across the product life-cycle (in other words, in pre- and post-market reviews), to allocate review effort taking into consideration the properties and risks of the pesticide, and enable a sharper focus of the PMRA's resources on products presenting a higher level of potential risk.
The development of the Proportional Effort approach was informed by targeted consultations sessions with various stakeholder groups throughout 2022–2023 and a draft policy is slated to be released for public consultation in 2023–2024.
The development of the proposed Continuous Oversight and Proportional Effort frameworks has been supported by various activities undertaken during the reporting period, including:
- Ongoing consultation with experts, partners and stakeholders on the development and planned implementation of the new policies
- Developed new digital tools to capture and efficiently manage information collected throughout the lifecycle
- Developed an approach to expand the monitoring and consideration of information in the published scientific and grey literature (scientific databases such as government science journals, monographs and programs that routinely update and release science information, pesticide monitoring, biomonitoring and draft/preliminary risk assessments on pesticides), and sought advice from the Science Advisory Committee on Pest Control Products to help inform this approach
- Continued implementation of a streamlined approach for re-evaluations of lower priority actives. In addition, as required under the Pest Control Products Act, final decisions of lower priority actives are informed by public consultation.
Re-evaluation and special review programs
Under the re-evaluation program, new methodologies, data, and scientific approaches are incorporated into the assessments to ensure that registered pesticides continue to meet modern standards for health and environmental protection and have value.
Special reviews are another mechanism used under the Pest Control Products Act to determine the continued acceptability of registered pesticides. Unlike a re-evaluation, the intent of a special review is to address the specifically identified aspect(s) of concern, and is triggered when:
- there are reasonable grounds to believe that the health or environmental risks of the product are, or its value is, unacceptable; or
- an OECD member country prohibits all uses of an active ingredient for health or environmental reasons.
As per the Pest Control Products Act, an identified aspect(s) of concern that would otherwise prompt a new special review can also be addressed through an ongoing re-evaluation or special review, reducing the need to duplicate work that is already being done. As with the re-evaluation program, a science-based approach is taken for special review decisions.
Re-evaluation work planning
As part of its commitment to improve transparency, the PMRA published its five-year Pest Management Regulatory Agency Re-evaluation and Special Review Work Plan 2022–2027 (Re-evaluation Note REV2022-01). This work plan includes the target timelines to publish proposed and final decisions for ongoing re-evaluations and special reviews, as well as the list of anticipated re-evaluation initiations in the next five years.
As of 31 March 2023, PMRA completed the re-evaluations of all legacy pesticides (registered prior to 1995). As of 31 March 2023, 161 re-evaluations and special reviews were underway with a requirement to initiate 34 new re-evaluations later in fiscal year 2023–2024. As the PMRA focused its resources on completing older complex pesticide re-evaluations and special reviews, progress on the review of cyclical re-evaluations was impacted.
In 2022–2023, the PMRA completed 36 final post-market decisions (re-evaluations and special reviews) and 27 proposed decisions impacting over 1000 end use products. These decisions resulted in various risk management requirements to further reduce potential risk to human health (for example, additional personal protective equipment and/or engineering controls, changes to restricted entry intervals, reduction of application rates) and the environment (for example, updated spray buffer zones, additional precautionary/hazard statements, statements to reduce pesticide runoff).
Over the past five years, the PMRA has completed an average of 26 final decisions per year for re-evaluations and special reviews. Though this is an improvement over previous years, workload continues to increase as new re-evaluations and special reviews are initiated. Based on the projected number of re-evaluation initiations for the next five years, along with the average number of final decisions made per year, work on hand will continue to grow. Implementation of continuous oversight and proportional effort approaches will further improve the efficiency of reviews of registered pesticides.
Sales information reporting
In 2022–2023, the PMRA published the annual sales report for the 2020 calendar year.
Sales of pest control products in Canada increased from 120.1 million kg of active ingredients (kg a.i.) in 2016 to 126.4 million kg a.i. in 2020 (Figure 2).
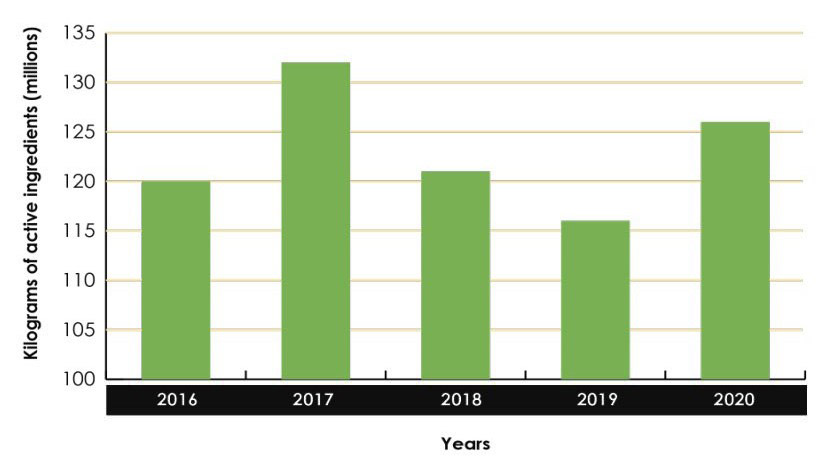
Figure 2 - Text equivalent
Quantity of pesticides sold in Canada (2016–2020)
- 2016 = 120 100 000 kilograms of active ingredients
- 2017 = 132 100 000 kilograms of active ingredients
- 2018 = 121 300 000 kilograms of active ingredients
- 2019 = 116 000 000 kilograms of active ingredients
- 2020 = 126 000 000 kilograms of active ingredients
In 2020, 72.4% of pesticide sales in Canada were agricultural sector products (Figure 3), whereas 23.3% were non-agricultural sector products, and 4.3% were domestic sector products.
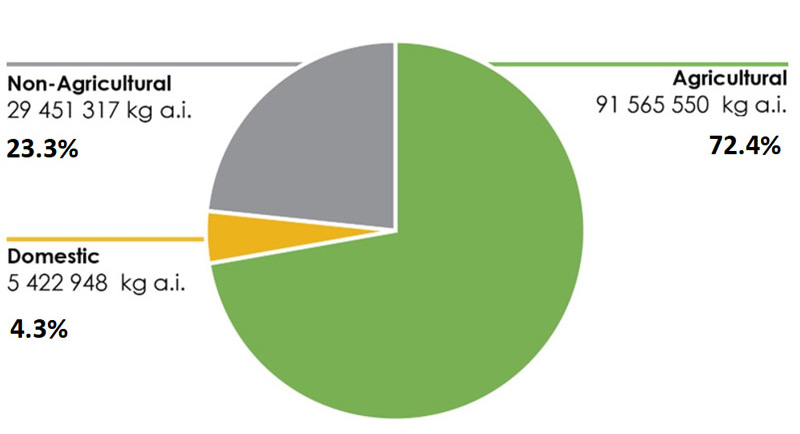
Figure 3 - Text equivalent
Quantity of pesticides sold in Canada in 2020 by sector
- Non-agricultural = 23.3%, 29 451 317 kilograms of active ingredients
- Domestic = 4.3%, 5 422 948 kilograms of active ingredients
- Agricultural = 72.4%, 91 565 550 kilograms of active ingredients
Glyphosate remained the top active ingredient sold in Canada in 2020 (Table 1). Seven of the top 10 active ingredients sold in 2020 had been among the top 10 selling active ingredients since 2016. These top 10 active ingredients accounted for 71.9% of all pesticides sold in Canada in 2020.
| Active ingredient | Product type |
|---|---|
| Glyphosate | Herbicide |
| Available chlorine, present as sodium hypochlorite | Antimicrobial |
| Borates | Insecticide/Fungicide/Antimicrobial |
| Creosote | Antimicrobial |
| Glufosinate ammonium | Herbicide |
| Surfactant blend | Other |
| 2,4-D | Herbicide |
| Hexahydro-1,3,5-tris(2-hydroxyethyl)-s-triazine | Antimicrobial |
| MCPA | Herbicide |
| Corn gluten meal | Herbicide |
Incident reporting
The PMRA has been collecting pesticide incident reports since 2007. A pesticide incident is any unintended effect to humans, animals (includes pets, livestock, or wildlife) or plants that may have resulted from exposure to a pesticide. This includes pesticide packaging failure incidents that may result in human exposure or injury, or effects seen in scientific studies, such as a new hazard or increased risk.
Pesticide registrants are required by law to report all incidents related to their products to the PMRA. Canadians may also report pesticide incidents either to product manufacturers or directly to the PMRA using the Voluntary Incident Reporting Form available in Public Engagement Portal.
The PMRA uses incident reports to identify hazards and characterize potential risks to humans or the environment from the use of pesticides. Priority for in-depth reviews is given to incident reports that are serious in nature, such as death or life-threatening effects, that involve multiple people or animals, or that indicate a recurring problem with a pesticide product use.
In addition, when the PMRA reviews new active ingredients or conducts re-evaluations of older pesticides, a complete analysis of all incident reports involving that pesticide is integrated into the risk assessment.
Another component of the incident reporting program includes scientific study incidents. Registrants must provide a scientific study they have sponsored if it indicates either a new health or environmental hazard, increased health or environmental risk or the presence of a component or derivative that has not been previously detected. In addition to completed studies, this requirement also includes studies that are ongoing, or discontinued before completion, if they indicate an adverse effect.
Scientific study incidents are evaluated by the PMRA as they are received. The purpose of this evaluation is to determine if the study changes the existing risk profile of a pesticide and if such a change may affect the acceptability of the pest control product.
In 2022–2023, a total of 1566 pesticide incident reports were submitted to the PMRA. Details of these reports can be found by visiting Canada.ca/pesticides and selecting the link for the Pesticide Product Information Database. An overview of the incidents is provided below:
- There were 953 reported incidents that occurred in Canada and 525 reported incidents, relevant to Canadian products, that occurred in the United States. The remaining 88 reports were scientific studies.
- Overall, 207 distinct pesticide products were reported in Canadian incidents. The majority of these products are marketed as domestic class and are registered for use on companion animals or at residential sites.
- In general, the majority of products in reported incidents involved pets (mainly cats and dogs - 1019 reports). Minor adverse effects, such as itchy skin, were frequently reported in animals following the use of companion animal products. There were 163 incident reports that involved people, with reported exposures due to applying a pesticide product to a residential site or contacting an area treated with a pesticide. The severity of effects reported in people were frequently minor, of short duration, and resolved quickly without medical treatment.
- Registrants reported 99% of incidents over this period via the mandatory pesticide incident reporting This includes incidents reported to registrants by the public, including users. The Canadian public reported 1% of all incidents directly to PMRA through the voluntary incident reporting process.
During the 2022–2023 period, the PMRA put in place several risk reduction measures in response to the incident report data received in the period up to 2022. For example, the review of human and domestic animal incidents resulted in label changes for pyrethrins/piperonyl butoxide products used in residential sites and enclosed areas, and for pet shampoo or spray products registered for the control of fleas and ticks. These included:
- Adding label statements to inform the consumer of the potential side effects that may occur in people and pets following the using of pyrethrin products co-formulated with piperonyl butoxide or other synthetic pyrethroids.
- Improving the "Direction of use" statements and instructions for all domestic class pyrethrins/piperonyl butoxide products (for example, space sprays, surface sprays, companion animal sprays and shampoos) to allow the user to better understand how to apply the product correctly.
- Updating existing product labels to current standards, as outlined in the 2020 PMRA Guidance Document, Structural Pest Control Products Label Updates or the 2019 PMRA Guidance Document, Label Improvements for Spot-on Pesticides Used on Companion Animals, when pertinent.
Incident reports are an essential element of post-market monitoring. Monitoring incident reports for unanticipated effects or changes in a pesticide's risk profile is an ongoing process that may include re-assessing previous conclusions, as necessary. In cases where actions to reduce pesticide exposure have been adopted, the PMRA monitors the incident report data to determine if the actions were effective in managing the identified risk.
To report a pesticide incident, visit Canada.ca/pesticides and select the link for Report a Pesticide Incident.
Advancing science in pesticide risk assessment
Water monitoring
In 2022–2023, the PMRA continued work on the development of a framework for a National-Scale Water Monitoring Program for Pesticides (NWMPP) that will support consistency in measurement and reporting, and the implementation of a long-term water monitoring program by the PMRA.
Water monitoring data allows for the identification of areas where risks to human health and aquatic organisms may be present and further investigation is needed to inform regulatory action.
In 2022–2023, the PMRA continued to engage with the Water Monitoring Technical Working Group, including federal/provincial/territorial partners, manufacturers, academia, grower groups, and non-government organizations, to inform the development of the NWMPP framework.
The PMRA implemented year one of a two-year water monitoring pilot program for pesticides in partnership with Environment and Climate Change Canada (ECCC) and Agriculture and Agri-Food Canada (AAFC), conducting water sampling at 89 sites across Canada.
Health Canada's Regulatory Operations and Enforcement Branch's Pesticide Laboratory contributed the analysis of nearly 1300 water samples for 185 currently registered pesticides and two transformation products in water. The University of Guelph also analyzed 200 of these samples for an additional five currently registered pesticides and six transformation products.
The PMRA collaborated with ECCC to make this water monitoring data accessible to the public through the Government of Canada Open Data portal. The PMRA also published new water monitoring web pages, including an overview of the PMRA's water monitoring activities, a summary of the water monitoring pilot program, an explanation of, and public access to, Aquatic Life Reference Values and Human Health Reference Values used in the risk assessment of pesticides, and a glossary of relevant terms. The PMRA also initiated work with the Public Health Agency of Canada's Infobase to develop a public-facing dashboard that will provide interactive data summaries and comparisons of pesticide concentrations with Aquatic Life Reference Values and Human Health Reference Values.
Planning for water sampling in year two of the pilot program in 2023–2024 was initiated with AAFC and ECCC as well as other partners, such as provinces, Indigenous communities, conservation authorities, and watershed alliances.
Pesticide use information program
In 2022–2023, the PMRA continued to develop a systematic approach to identify, access, and manage information about pesticide use following registration. This framework will enhance the PMRA's ability to make robust and timely pesticide risk management decisions.
In 2022–2023, the PMRA consulted extensively with government partners, user groups, industry, non-government organizations and academia in various Technical Working Groups to develop strategies for gathering pesticide use information.
The program development focus in 2022–2023 was on the agricultural crop sectors. Pilot opportunities for the agricultural crop sector are being considered for initiation in 2023–2024 in collaboration with the targeted sectors.
In December 2022, consultations began with non-crop sectors, focusing on the livestock, forestry and structural uses. Technical working groups were established, with initial meetings held in February and March 2023.
This stakeholder input will be used to inform the development of the Pesticide Use Information framework to be subject to public consultation in 2024.
Reducing animal testing
The PMRA continues to be an active participant in various international activities aimed at reducing animal testing while ensuring the protection of human health. Recent collaborations on developing alternative and new approach methods, or NAMs, to animal testing, include participation in a workshop led by the Center for Alternatives to Animal Testing (CAAT) that focused on the role of the 90-day dog study in global regulatory decisions on agrochemicals and potential strategies for reducing its use.
This collaboration resulted in a report on the challenges and opportunities for overcoming use of testing in dogs for agrochemical evaluation and registration, which emphasized that further collaboration and efforts are required to develop guidance on when the study would not be useful in informing human safety and risk assessment. The PMRA also contributed to discussions and analysis related to dermal absorption data that led to the validation of the use of in vitro data for the estimation of dermal absorption.
The PMRA's ongoing involvement with in vitro initiatives, led by the OECD and CAAT, include examining alternative in vitro tests and defining approaches for eye and skin irritation, dermal sensitization, immunotoxicity, and developmental neurotoxicity. Recent advancements in this area include the release of defined approaches for skin sensitization and for serious eye damage and eye irritation by the OECD.
Finally, the PMRA is a member of the Health and Environmental Sciences Institute (HESI) Transforming the Evaluation of Agrochemicals (TEA) Committee, which is developing a fit-for-purpose framework for the safety evaluation (health and environment) for agrochemicals that includes a focus on exploring possibilities for reducing animal testing.
Gene-edited organisms for pest control
Advances in gene editing tools and technologies have made the process of changing an organism's genome more efficient, opening up a range of potential applications. One such application is in pest control. By editing genomes of organisms, and introducing them to wild populations, it is possible to control insect-borne disease and invasive species, or reverse insecticide resistance in pests. However, the full implications of using these methods remains uncertain.
In February 2022, the PMRA partnered with Council of Canadian Academies (CCA) to sponsor an initiative to examine the scientific, bioethical, and regulatory challenges associated with the use of gene-edited organisms and technologies for pest control. The independent CCA assessment is currently ongoing and outcomes will be described in a future report.
Remote piloted aircraft systems (drones) for pesticide application
The PMRA continues to receive inquiries related to the application of pesticides by Remote Piloted Aircraft Systems (RPAS, or drones). In September 2022, the first pest control product amendments to allow the use of drones for pesticide application were approved in Canada. The restricted class biological larvicide products approved for this application method are of low concern to human health and the environment, so the need for drone-specific data was not required in this case.
A variety of factors need to be considered before a pesticide can be approved for application using drones. If a pesticide is known to be of very low concern to human health and the environment, for example a bacteria-based larvicide, data related to the risks of using a drone application method may not be required. For pesticides whose health and/or environmental risks need to be managed, data that assesses the risks of using drone application is required. As of the end of the reporting period, no data has yet been submitted that will allow the PMRA to characterize and evaluate risks associated with drone application of these kinds of pesticides. In 2022–2023, the PMRA continued to issue Research Authorizations in support of data generation for drone regulatory applications.
The PMRA is involved at the national and international level in identifying and addressing data needs to support the application of pesticides by drones. Areas of discussion include off-site drift, worker exposure, impact on crop residues and product efficacy. To determine whether the levels of pesticide residues are similar in agricultural crops when a pesticide is applied by either drone application or by conventional application equipment, the PMRA is providing regulatory support for a crop residue comparison study managed by Agriculture and Agri-Food Canada (AAFC), and planned for the 2023 growing season.
To continue to advance the science on drone application of pesticides, in 2022–2023, the PMRA worked closely with three domestic and international working groups to coordinate information sharing on the health and environmental safety of pesticide application by drone, in support of regulatory risk assessment:
- The OECD Working Group on Pesticides Drone/Unmanned Aerial Spray Systems Subgroup – The PMRA committed to co-lead the production of an empirical spray drift curve and a mechanistic model for estimating spray drift. The PMRA provided updates on Canada's regulatory position on drone pesticide applications and the proposed AAFC residue trials (see above and below).
- The North American Remotely Piloted Aerial Application Systems (RPAAS) working group – The PMRA provided an update on Canada's regulatory position on drone pesticide applications at the October 2022 North American RPAAS Workshop in Penticton, British Columbia, and was engaged with the working group on developments in drone research.
- Agriculture and Agri-Food Canada's (AAFC) residue trial working group – The PMRA was involved in an advisory role for the AAFC residue trials starting in 2023, as described above.
The PMRA helped organize, and participated in, the August 2022 Fall Meeting of the American Chemical Society's (ACS) "Unmanned Aerial Systems (UAS) (in other words, Drones): Pesticide Spraying and Other Agricultural Applications" symposium. The symposium focused on technology development and applications of drones in agriculture, public health, and industrial vegetative management. Regulatory and policy development, and best management practices for UAS uses in these areas were also highlighted. The PMRA committed to helping organize the 2023 ACS Fall Meeting symposium on drones.
The PMRA continues to move forward, in conjunction with international regulatory partners, on identifying regulatory data needs for this rapidly emerging spray technology.
Health Canada's compliance and enforcement activities on pesticides
Health Canada's Pesticide Compliance Program (PCP), managed by the Regulatory Operations and Enforcement Branch, is responsible for promoting, verifying and enforcing compliance with the Pest Control Products Act and its Regulations.
The PCP conducts a variety of compliance promotion and verification activities targeted to those regulated by the Pest Control Products Act including pesticide registrants, importers, retailers, and users.
Compliance promotion activities aim to foster compliance by providing important information to regulated parties pertaining to the Pest Control Products Act and its Regulations.
Compliance verification activities include conducting inspections and collecting samples to assess compliance. They may be planned or conducted in response to complaints or importation referrals from the Canada Border Services Agency (CBSA).
When required, enforcement action is taken against regulated parties to address identified non-compliance with the Pest Control Products Act and its Regulations. Any contravention of the Pest Control Products Act and its Regulations is considered a non-compliance. The PCP uses a range of enforcement tools including warning letters, compliance orders, and seizure. The PCP also partners with the Canada Border Services Agency to refuse entry of unauthorized pesticides into Canada.
The PCP also issues notices of violation under the Agriculture and Agri-Food Administrative Monetary Penalties Act with warning or monetary penalty (the amount for businesses varies from $1300 to $10 000 per violation depending on the severity), and where appropriate, can also make recommendations to the Public Prosecution Service of Canada for prosecution. The choice of enforcement actions reflects the severity of the risks posed by the identified contraventions and multiple enforcement actions may be taken against a single regulated party if necessary.
The delivery of compliance activities is prioritized based on criteria including, but not limited to, potential risks to human health and the environment, compliance history, considerations such as observations from the field, information from the PMRA and provincial and territorial regulators, complaints received, and data analysis. Regulatory changes including the decisions made by PMRA through the re-evaluation and special review processes are also used to identify priority areas for compliance verification as these processes can result in the cancellation of products or significant label changes that include new risk mitigation measures.
The PCP works collaboratively with international, federal, provincial and territorial pesticide regulators to share best practices and information on existing and emerging issues, as well as to optimize compliance promotion, verification or enforcement actions.
Compliance and enforcement activities in 2022–2023
The PCP publishes an annual report describing compliance and enforcement activities in greater detail, and these reports are listed on Health Canada's PMRA Corporate plans and reports page. The following is a summary of those activities.
In 2022–2023, the PCP conducted a number of activities to promote, verify, and enforce the Pest Control Products Act and its Regulations. Regulated parties selected for compliance verifications continued to include some previously found to be non-compliant with the Pest Control Products Act and its Regulations.
A total of 879 compliance verifications were conducted, which included both planned and reactive activities (such as complaints) and 1554 admissibility recommendations on importation were issued to the CBSA.
The PCP issued a total of 1885 enforcement actions addressing single or multiple violations to non-compliant regulated parties, including:
- 1855 warning letters, which includes 1473 issued to importers of unauthorized pesticides referred by the CBSA to the PCP
- 19 compliance orders
- 4 seizures or detentions
- 7 administrative monetary penalties under the Agriculture and Agri-Food Administrative Monetary Penalties Act, for a total value of $71 000 in penalties
In addition, the PCP conducted 89 compliance promotion activities directed to associations, during industry trade shows, or through targeted dissemination of compliance promotion materials.
Legislative and regulatory modernization
The Pest Control Products Act governs how pesticides are regulated based on scientific risk assessment and risk management, before and after they are registered for use. Regulations made under the Pest Control Products Act further establish certain requirements to enable behaviours or outcomes, with a view to achieving the Act's objectives.
Regulations under the Pest Control Products Act include:
- List of Pest Control Product Formulants and Contaminants of Health or Environmental Concern (SI/2005-114)
- Pest Control Products Fees and Charges Regulations (SOR/2017-9)
- Pest Control Products Incident Reporting Regulations (SOR/2006-260)
- Pest Control Products Regulations (SOR/2006-124)
- Pest Control Products Sales Information Reporting Regulations (SOR/2006-261)
- Review Panel Regulations (SOR/2008-22)
Targeted review of the Pest Control Products Act
As part of the Transformation Agenda, in Spring 2022 the PMRA launched a targeted review of the Pest Control Products Act to ensure the pesticide approval process meets the expectations of Canadians, namely to further strengthen human health and environmental (including wildlife) protection and improve transparency.
Consultations, public information sessions and ad hoc meetings were held with a variety of groups including the general public, pesticide manufacturer associations, agricultural and non-agricultural pesticide users, non-governmental organizations (NGOs), academia, Indigenous organizations, provinces and territories, and foreign jurisdictions including the United States, Australia and the European Union.
A summary of the feedback from these consultations was published on 1 November 2022, in the "What We Heard" report. An analysis of all comments received is informing the path forward to strengthen the pesticide review process and improve transparency.
Regulating ultraviolet radiation-emitting and ozone-generating devices
Ultraviolet radiation-emitting (UV) and ozone-generating devices that make claims to control or kill bacteria and viruses on surfaces, objects, in water, and in the air have been more widely and increasingly available for sale in Canada since the start of the COVID-19 pandemic. Health Canada had not yet received sufficient evidence to demonstrate that all UV and ozone-generating devices can be used safely or work as claimed.
Devices that have not been evaluated against the requirements of the Pest Control Products Act may, therefore, pose a serious health and safety risk. To address this risk, the PMRA amended the Pest Control Products Regulations in May 2022, to provide a regulatory pathway for UV and ozone-generating devices. The amendments continued protections put in place via an Interim Order made by the Minister on 7 June 2021.
Regulatory guidance for sanitizers
Since the beginning of the COVID-19 pandemic, Health Canada's PMRA has faced a significant increase in requests for regulatory guidance from manufacturers, distributors and importers of sanitizers and similar type products (for example, UV radiation-emitting devices, self-sanitizing coatings) who wish to bring their products to market in Canada.
Biocides, including surface sanitizers, have historically been regulated under separate regulatory frameworks in Canada, with surface sanitizers and disinfectants having different associated requirements, despite having similar risks, benefits, uses, and ingredients. While not the lead on this work, in recent years PMRA has supported other Health Canada branches in the development of a single regulatory framework for biocides under the Food and Drugs Act. This
encompasses disinfectants that are currently regulated under the Food and Drug Regulations, as well as surface sanitizers regulated under the Pest Control Products Act that meet the definition of a drug.
The proposed Biocides Regulations were published in the Canada Gazette, Part I in May 2022. The PMRA continues to support this work to develop the final regulations.
Update on the targeted regulatory review of the agri-food and aquaculture sector
The Government of Canada announced in Budget 2018 that it would fund "targeted reviews of regulatory requirements and practices that are bottlenecks to economic growth and innovation."
As part of this initiative, in 2018, the PMRA participated in the targeted regulatory review of the agri-food and aquaculture sector.
In 2022–2023, the PMRA continued work on regulatory modernization initiatives related to implementing the Agri-food and Aquaculture Regulatory Review Roadmap (released in 2019), including those related to the post-market review process, labelling, data compensation, and the authorization of pesticides not requiring registration.
For example, in June 2022, the PMRA published proposed amendments to the Pest Control Products Regulations in the Canada Gazette, Part I pertaining to exclusive rights and compensable data. The proposed amendments will clarify data compensation provisions for re-evaluations and special reviews by specifying a process under which registrants who are data holders and registrants who are relying on the data of data holders could establish compensation payable, by clarifying what data is subject to compensation, and when to initiate negotiations and binding arbitration (if necessary).
The PMRA also continued work on other Roadmap initiatives, including possible statutory amendments to broaden the Minister's powers to make risk-based authorizations and exercise appropriate post-market oversight over authorized products. The proposed amendments to the Pest Control Products Act with respect to a possible new ministerial authorization pathway, initially proposed under Bill S-6 (the Annual Regulatory Modernization Bill), were incorporated into the targeted review of the Pest Control Products Act under the Transformation Agenda.
Pest Control Products Regulations review
In 2022–2023, the PMRA continued its comprehensive review of the Pest Control Products Regulations. The review is aimed at ensuring the Regulations continue to meet program objectives (for example, of health and environmental protection) in an effective and efficient manner, while attempting to minimize regulatory burden (if any) on regulated parties.
In 2022–2023, the review included developing two regulatory proposals pertaining to the following:
- Applications and Imports: In December 2022, Health Canada amended certain application and importation requirements for pest control products in Canada, including clarifying regulatory requirements for treated articles and establishing criteria for authorizing certain treated articles.
- Research: In December 2022, the PMRA published a consultation document that proposed amendments to the Pest Control Products Regulations pertaining to research. The proposed amendments aim to facilitate beneficial pest control product research by modernizing regulations while ensuring that research is conducted under conditions that protect human health and the environment.
Improving transparency in pesticide regulation
The PMRA recognizes that transparency and engagement is critical to strengthening trust in regulatory decisions about pesticides. The PMRA continually works to improve communication with the public, stakeholders and government partners through bilateral and multilateral engagement, and through new and long-standing committees, events, outreach materials, and information and applications on Canada.ca.
Transformation initiatives to improve transparency
One of the key strategic objectives of the Transformation Agenda is to enable more meaningful public participation in the PMRA's regulatory decision-making processes. In 2022–2023, work focused on three primary work themes as described below.
Improving science communications
- plain language content was integrated in the public consultation notice and public summary for the proposed special review decision for atrazine, as a first test case
- baseline user testing on existing web content about risk and value assessments was conducted to inform work in this area
- a new template for consultation notices was developed and applied to key proposed decisions
- the PMRA continues to build a library of foundational science (information about science concepts that form the basis of the PMRA's risk assessment and regulation processes) to increase awareness and trust in how science is conducted at PMRA and how it informs decision-making
Enhancing access to PMRA processes and decisions
- consulted stakeholders on priority needs for access to information/data in the public registry of pesticides
- key PMRA webpages (Public Registry, Pesticides and pest management consultations, Decisions and updates) were updated and redesigned for easier navigation and use
- a notice of intent was published to disclose the names of applicants and registrants for certain pest control product regulatory activities, while an application is under review
Modernizing access to confidential test data
Previously, confidential test data inspections were required to be done in person at the PMRA's Ottawa headquarters in a physical "Reading Room". However, the pandemic severely limited this option. In response, an interim process was piloted starting in July 2021, providing the public with the ability to inspect confidential test data from any location in Canada via an encrypted USB key loaded with robust document protection software.
- Since the introduction of the interim approach, the PMRA received five CTD inspection requests in 2022–2023 of which four met the criteria and received a secure USB key containing the confidential test data for inspection. This new approach is a successful demonstration of the PMRA's commitment to improved transparency as part of the Transformation Agenda, while efforts continue to make this data more accessible.
Stakeholder engagement
In 2022–2023, the PMRA continued to engage stakeholders extensively – both external and within the Government of Canada – focusing on building relationships to establish a foundation for ongoing, effective engagement in the future. This included engagements with partners and various stakeholder organizations in the context of its Transformation Agenda, including the targeted review of the Pest Control Products Act. Summaries of these meetings and engagement activities were publicly shared online that PMRA continues to update on a regular basis. Transformation engagements are being undertaken in coordination with PMRA Core Operations to support an integrated approach and a comprehensive dialogue with key partners and stakeholders.
This work has allowed the PMRA to share information and better understand stakeholder concerns, with the goal of better integrating stakeholder views in the development or modernization of PMRA policies, tools, and processes.
Federal/Provincial/Territorial Committee on Pest Management and Pesticides (FPT-CPMP)
The FPT-CPMP was established to help strengthen FPT relationships in the area of pest management and pesticides. The Committee also provides advice and direction to FPT governments on programs, policies and issues.
The purpose of FPT-CPMP is to:
- strengthen federal/provincial/territorial relationships in the area of pest management and pesticides
- promote information exchange in the area of pest management and pesticides.
- provide advice and direction to federal, provincial and territorial governments on programs, policies and issues for pesticides with the aim of enhancing sustainable pest management practices
- seek harmonization where applicable in programs and policies
In 2022, members of the FPT-CPMP held six virtual meetings to discuss issues of national interest, and met in a hybrid virtual and in-person format in Charlottetown, Prince Edward Island (PEI), for their annual meeting in October 2022.
This was the first in-person meeting since 2019. During the annual meeting, co-chaired by the PMRA and the Government of Prince Edward Island, the FPT-CPMP discussed:
- compliance and enforcement
- a study on Teton® (Endothall) application to control algae in irrigation Canada
- updates on PMRA Transformation initiatives
- updates to the FPT-CPMP Terms of Reference
- updates on the Notice of Objection process
- Agriculture and Agri-Food Canada's remotely piloted aircraft system demonstration
- activities of the Sub-Committee on Pesticide Education, Training and Certification
- activities of the Minor Use Working Group
Public opinion research
In March of 2022, a third round of public opinion research was conducted to assess public knowledge, behaviours and opinions related to:
- pesticides and their uses
- perceived safety and acceptability of specific pesticide uses
- personal use habits
- information-seeking preferences
- general awareness and knowledge about how pesticides are regulated in Canada
- perceptions and confidence in Health Canada's PMRA as the responsible regulatory authority
Results and analysis will be described in the 2023–2024 Annual Report.
The survey was first initiated in 2016 to measure progress on the PMRA's efforts to build public confidence in the PMRA's work, and repeated in 2020.
Key comparative findings from 2020 indicated that despite low levels of knowledge about the pesticides regulatory process itself, Canadians exhibited an increased confidence that Health Canada's PMRA protects human health and the environment, and increased confidence in Health Canada scientists as a trusted source of information on pesticides.
The 2022 round of research was developed to gauge current public opinion compared to previous results, and where possible, to support key transparency initiatives being undertaken as part of the PMRA's Transformation Agenda announced in 2021.
New survey questions were introduced to gauge public awareness and experience with the public consultation process for pesticide decisions. The survey (quantitative) data were weighted to the Canadian population data by region, gender, and age, and included 2SLGBTQQIA+ community representation.
In efforts to improve Health Canada's knowledge of Indigenous perspectives on the topic, the survey included 200 individuals who identify as a member of the Indigenous Peoples of Canada, and an additional online focus group (qualitative) was conducted with Indigenous Canadians.
Focus group discussions included reactions to:
- a new infographic on maximum residue limits (MRLs)
- updated Public Registry webpage to improve accessibility to pesticide information in one place
- establishment of an external advisory committee to provide scientific advice to the PMRA on proposed pesticide decisions
- National Water Monitoring Program
The 2023 public opinion research report will be available to the public along with previous reports that are published on the Library and Archives Canada website.
Online panels were used as sources of sample for these public opinion surveys, resulting in non-probability samples. According to the Standards for the Conduct of Government of Canada Public Opinion Research, the findings of a non-probability sample should not be generalized to the overall Canadian population.
External communications and outreach
Pest management information service
The PMRA's Pest Management Information Service (InfoServ) is a stakeholder outreach service intended to facilitate communications and engagement with all external stakeholders. InfoServ responds to inquiries, comments, reports and complaints via its Public Engagement Portal found online, by email inquiries sent to its generic email inbox, as well as all telephone calls made to its toll-free 1-800 telephone line, on the subject of pesticides and/or the federal pesticide regulatory system.
Origin of InfoServ inquiries in 2022–2023:
- total number: 3460
- pesticide manufacturers (58%)
- general public and consumers (13%)
- provincial and territorial governments (4%)
- federal government (4%)
- growers (3%)
- non-government organizations (NGOs) (2%)
- municipal governments (1%)
Most commonly discussed InfoServ topics in 2022–2023 included:
- registrant/applicant support (58%)
- publications, policy and mandate (18%)
- compliance (10%)
- health and safety (5%)
Correspondence services
The PMRA receives a high volume of correspondence in the form of emails, letters, meeting requests and petitions on the subject of pesticides and/or the federal pesticide regulatory system. The information received through correspondence allows PMRA to monitor and respond to the concerns of the public and stakeholders.
Origin of correspondence inquiries in 2022–2023:
- general public or consumers (54%)
- members of Parliament (on behalf of constituents) (13%)
- industry/pesticide manufacturers (7%)
- Indigenous partners (6%)
- non-government organizations (6%)
- provincial governments (3%)
Most commonly discussed correspondence topics in 2022–2023 included:
- human health and environmental concerns related to pesticide use (80%, with glyphosate as the most common concern)
- PMRA's Transformation Activities (20%)
External advisory bodies
Science Advisory Committee on Pest Control Products (SAC-PCP)
As part of the 4 August 2021 Government of Canada announcement with respect to strengthening the capacity and transparency of the review process for pesticides, the government committed to the creation of an external science advisory committee.
The SAC-PCP provides Health Canada with independent scientific advice to support evidence-based decision making on pesticide health and environmental risk and value assessments as well as the development of risk management options.
This committee acts in an advisory role to Health Canada while Health Canada's PMRA maintains the responsibility and the sole authority to make regulatory decisions on pesticides taking into consideration the advice provided by the Committee.
Together, the committee members have a wide range of knowledge, expertise, and experience to support the mandate of the advisory body. In addition to the core members of the committee, a roster of scientific experts, known as the Community of Specialized Experts, was established to provide supplementary scientific expertise and advice on specific issues or assessments under consideration as needed.
The membership of the SAC-PCP was officially announced on 27 June 2022. The inaugural Committee meeting was held on 8 July 2022 and additional meetings were held on October 20 and December 5, 2022.
As of 31 March 2023, the Committee had considered three scientific questions, which focused on the following topics:
- the proposed Ministerial Authorizations pathway for low-risk or well-characterized-risk products
- scientific communications of maximum residue limits
- science literature review strategy under the proposed continuous oversight approach
The PMRA received 32 recommendations from the SAC-PCP on the questions regarding the scientific communication of MRLs and continuous oversight. The implementation of these recommendations will be described in more detail in the 2023–2024 Annual Report. At the time of writing this report, of these, the PMRA implemented 21 (66%), partially implemented 8 (25%), and is planning to implement or consider 2 (6%). One recommendation (3%) will not be implemented. PMRA received 10 recommendations for the first science question on the proposed new Ministerial Authorization Pathway; however this proposal is currently paused.
A summary of the SAC-PCP's advice and recommendations, along with the PMRA's response to them, are posted online to Health Canada's SAC-PCP webpage once available. The most up-to date information on Health Canada's SAC-PCP can be found on the Science Advisory Committee on Pest Control Products page on Canada.ca.
Pest Management Advisory Council (PMAC)
The Pest Management Advisory Council is a multi-stakeholder group that fosters communication and dialogue among stakeholders and with the PMRA, and provides advice directly to the Minister of Health on policies and issues relating to the federal pest management regulatory system. The Council's role is advisory. Decision-making remains the responsibility of the PMRA.
The purpose of PMAC is to:
- provide recommendations on broad strategic directions, management, and overall priorities for PMRA
- provide a challenge function to ensure that PMRA programs are consistent with the needs of Canadians within the overall national and global environmental, social, and economic context
- provide advice and a forum for the exchange of views of all key stakeholders on issues affecting the management of pest control products in Canada
- receive and review reports from Council working groups
In October 2022, PMAC met virtually, where the PMRA presented its accomplishments to date with respect to the Transformation Agenda, provided a demonstration of PMRA's draft infographic on MRLs as well as an overview of the Notice of Objection process. A summary of the report is available on our website.
The Council was supportive of the PMRA's planned next steps and recommended that funding to the PMRA be continued so the organization can continue to meet its overall legislative mandate.
International cooperation
Canada's internationally respected regulatory model has allowed Canada to form strong partnerships with other regulators.
The PMRA's work with international partners enhances pesticide risk management beyond our borders through:
- development of collaborative approaches to joint pesticide reviews
- promotion of international regulatory alignment
- addressing barriers to agricultural innovation and trade
- promotion of regulatory capacity building among other national and international pesticide regulatory authorities
International engagement on the Transformation Agenda
Throughout the fiscal year, PMRA officials have met with international counterparts at the United States Environmental Protection Agency (USEPA), the European Food Safety Authority (EFSA), and other regulators such as the Australian Pesticides and Veterinary Medicines Authority (APVMA). Discussions included issues of mutual interest, collaboration and cooperation in communications and policy approaches, as well as the opportunity for technical discussions and information exchange. International partners have been particularly interested in the ongoing transformation of PMRA and common concerns of stakeholders.
Canada-United States-Mexico Agreement
In North America, the PMRA continues to collaborate with its partners under the Canada-United States-Mexico Agreement (CUSMA) as part of the Technical Working Group on Pesticides, to serve as a focal point for addressing pesticide issues arising in the context of liberalized trade among the countries. In 2022–2023, technical meetings were held virtually.
Stockholm Convention
The Stockholm Convention is a multilateral treaty with a focus on global elimination or restrictions on the production and use of persistent organic pollutants (POPs). The PMRA is the responsible federal authority for meeting the obligations of, and for ongoing participation in, the Stockholm Convention as it pertains to pesticides.
The PMRA collaborates with other federal partners by providing scientific experts to work with the Persistent Organic Pollutants Review Committee (POPRC) and the Conference of the Parties (COP) of the Stockholm Convention.
At POPRC, the PMRA actively participates in the review of the scientific basis for identifying substances as POPs and making recommendations on how these substances can be managed globally.
At the COP meetings, the PMRA provides experts to participate in international negotiations working toward the elimination of POPs at the global level.
In September 2022, at the 18th Meeting of the POPRC, the committee continued the review of five potential POPs, including four industrial substances and the pesticide chlorpyrifos. The POPRC deferred a decision on whether chlorpyrifos met the convention's POP criteria until the 19th meeting of the POPRC, scheduled for Fall 2023.
Part two of the 10th Meeting of the Conference of Parties was scheduled for May 2023, and the 11th Meeting of the Conference of the Parties will take place in Spring 2025.
Rotterdam Convention
The Rotterdam Convention is a multilateral treaty that promotes information exchange and prior informed consent (PIC) in the international trade of chemicals, with the aim of protecting human health and the environment.
Substances listed to Annex III of the Convention include pesticides and industrial chemicals that have been banned or severely restricted for health or environmental reasons by two or more Parties, and which the COP has decided to subject to the Convention's PIC procedure. The Convention also calls on exporters to use proper labelling, include directions on safe handling, and inform purchasers of any known restrictions or bans.
The PMRA collaborates with other federal partners by providing scientific experts to work with the Chemical Review Committee (CRC) and the COP of the Rotterdam Convention. For the CRC, the PMRA actively reviews submissions to the Rotterdam Convention against established Convention criteria.
In 2022, the PMRA participated in the 18th Meeting of the CRC (CRC.18). At CRC.18 the Committee finalized draft decision guidance documents recommending the listing of the pesticides iprodione and terbufos in Annex III to the Convention and reviewed 10 additional pesticides against the criteria for listing:
- amitrole
- carbaryl
- carbon tetrachloride
- chlorfenvinphos
- methidathion
- methyl Bromide
- methyl parathion
- paraquat
- mirex
- thiodicarb
Of these, two pesticides, methyl bromide and paraquat, were recommended for listing to Annex III. Draft decision guidance documents for these chemicals will be developed in the intersessional period for consideration at CRC.19 in 2023.
Organisation for Economic Co-operation and Development
The PMRA contributes input (via the Canadian Delegation) to the OECD Joint Meeting of the Chemicals and Biotechnology Committee (CBC), as required. In addition, the PMRA is involved with several other OECD initiatives, including various task forces and expert group projects.
The PMRA routinely participates in meetings of both the OECD Working Party on Pesticides (WPP) as well as the OECD Working Party on Biocides (WPB). Both working groups function as vehicles for global cooperation, information exchange and alignment of approaches with respect to pesticides and biocides assessment. In support of the OECD WPP's objectives, the PMRA leads the work and/or actively participate in each sub-group relevant to pesticide assessments. Some examples of OECD WPP initiatives include:
- active contribution in the discussions to resolve challenges and determine potential updates to microbial pesticide hazard test guidelines
- collaboration to develop New Approach Methods (NAMs) for pesticide assessment, aimed at reducing animal testing
- identification of residues, metabolites and degradation products
- ongoing dialogue related to pollinator protection
- aligning risk assessment of new digital and mechanical technologies for applying pesticides, such as innovative drone technology
New area of collaboration: Global Biodiversity Framework
In December 2022 at COP15 in Montreal, the Parties to the United Nations Convention on Biological Diversity (CBD), including Canada, secured agreement on a global framework to halt and reverse the loss of nature around the world.
The Kunming-Montreal Global Biodiversity Framework is an ambitious global plan for nature, committing the global community to halt and reverse nature loss by 2030, including by conserving 30% of its lands and oceans by 2030, while ensuring that at least 30% of the world's degraded ecosystems are under restoration by 2030.
The Framework addresses all five of the direct drivers of global biodiversity loss and includes four long-term goals (by 2050) and 23 targets to be achieved by 2030. This includes Target 7, which is focused on reducing risks and impacts from all pollution sources; and includes reducing the overall risk of pesticides by at least half by 2030, for example through integrated science-based pest management, while taking into account food security.
ECCC is leading work on a whole-of-government approach to implement the GBF, including the development of Canada's 2030 National Biodiversity Strategy. Heath Canada, including the PMRA, are working with federal partners on Canada's implementation strategy to meet Target 7.
Financial profile
| 2022–2023 funding and revenue (in millions of dollars) | Total |
|---|---|
A-Base |
26.3 |
Revenue - Application fees $6.8 and Annual charge $9.4 |
16.2 |
Canadian Agricultural Partnership |
3.3 |
Chemicals Management Plan |
5.3 |
Transformation - TB submission |
11.3 |
Transformation - Funding Reprofile from 2021–2022 |
8.1 |
Total PMRA fiscal year 2022–2023 |
$70.5 |
- Financial profile includes employee benefit plan (EBP) and is per Annual Reference Level Update (ARLU)/main estimates.
- The revenue amounts reported are revenue actuals collected (in other words, they include the portion of the revenues that are allocated to support employee benefit plans and internal services and are therefore not respendable. The cost recovery regime for pesticides is currently under review.
- The PMRA received $3.3 million through the Canadian Agricultural Partnership initiative to support the registration of minor use products. As a result, newer, more environmentally sustainable, and more modern products have been made available to Canadian producers, which helps sustain Canada's competitive position globally.
- Through Canada's Chemicals Management Plan, the PMRA received $5.3 million to re-evaluate older pesticides, improve risk management approaches through Incident Reporting and Sales Reporting Regulations, and contribute to the development of scientific and regulatory approaches with other jurisdictions on high-priority issues. For more information, please consult the Chemicals Management Plan
- Strengthening the capacity and transparency of the pesticide review process is the new Treasury Board submission funding approved total of $11.3 M for 2022–2023.
- Strengthening the capacity and transparency of the pesticide review process funding for 2021–2022 re-profiled/moved to 2022–2023.
Appendices
| Submission category | Service standard in days |
|---|---|
| Category A New active ingredients or integrated system products, their related end-use products and manufacturing-use products; major new use of registered pest control products; maximum residue limits for an unregistered active ingredient; and user requested minor use registrations (URMUR). |
|
| Conventional Chemicals and import MRLs for an unregistered active ingredient | 665 |
| Reduced risk, other biopesticides, non-conventionals, non-straight chain lepidopteran pheromone (NSCLP) | 555 |
| Microbials, and URMUR for all pesticide types (conventional chemical, reduced risk, microbial, other biopesticides, non-conventionals, NSCLP) | 470 |
| Straight Chain Lepidopteran Pheromone (SCLP), including URMUR | 285 |
| Applications with atypical timelines (joint reviews, tailgaters, renegotiated timelines, synchronized timelines, coordination with Re-evaluation | Variable |
| Category B New pest control products containing registered active ingredients; an amendment to existing pest control products (for example, product chemistry, labelling); Emergency Registration; the addition of import MRLs for previously assessed active ingredients. |
|
| Conventional Chemicals (including emergency use) and new import MRL for previously assessed active ingredient | 425 |
| Reduced risk, other biopesticides, non-conventionals, NSCLP (including emergency use) | 360 |
| Microbials and SCLP (including emergency use) | 240 |
| Streamlined applications (application rate changes, tank mixes, new pests, or changes to level of control) | 158 |
| Applications with atypical timelines (joint reviews, tailgaters, renegotiated timelines, synchronized timelines, coordination with re-evaluation) | Variable |
| Category C Product registrations and amendments with no data requirements. These applications involve minor label or formulation reviews, such as product registration based on registered precedent products. |
|
| New / changes to product labels; addition of approved minor use; similar product | 240 |
| New/changes to Technical Grade Active Ingredient, Integrated System Product, Manufacturing Concentrate or End-use Product chemistry; administrative changes; administrative re-instatement | 180 |
| Applications with atypical timelines (tailgaters, renegotiated/ synchronized timelines, coordination with re-evaluation) | Variable |
| Category D Submissions within particular programs. |
|
| Registration renewal | 287 |
| Registration/ amendment to registration of active ingredient to be used in pest control product manufactured for export only | 46 |
| Master copies | 42 |
| Private labels | 10 |
| Own Use Import Equivalency and PermitsTable 3 Footnote * | 70 (Equivalency) |
| 30 (Permits) | |
| Grower Requested Own Use Equivalency and PermitsTable 3 Footnote * | TBD (Equivalency) |
| 30 (Permits) | |
| DiscontinuationsTable 3 Footnote * | 45 |
| Category E Authorizations and notifications for research in Canada. |
|
| Research authorization for new technical grade active ingredients | 159 |
| Research authorization for new uses of registered active ingredients | 69 |
| Research notification for research carried out in Canada | 30 |
| Category F Notification |
|
| Registration and amendments to registered pest control products via notification | 45 |
| Category L Submissions to register or amend products where the applicant wishes to use or rely upon data provided by another registrant. |
|
| Equivalency and data compensation assessment of end-use product and manufacturing concentrate with partial data package (conventional chemical) | 425 |
| Equivalency and data compensation assessment of active ingredient, end-use product and manufacturing concentrate with no data (all product types) | 365 |
| Equivalency and data compensation assessment of end-use product and manufacturing concentrate with partial data package (reduced risk, other biopesticide, non-conventional, NSCLP) | 360 |
| Equivalency and data compensation assessment of end-use product and manufacturing concentrate with partial data package (microbial and SCLP) | 240 |
| Applications with atypical timelines (tailgaters, renegotiated/ synchronized timelines, coordination with re-evaluation) | Variable |
| Regulatory DecisionTable 3 Footnote * | 45 |
| Requests to extend the exclusive use protection period based upon minor usesTable 3 Footnote * | 240 |
| Category P Pre-submission consultations |
|
| Pre-submission Consultations excluding those for Joint Reviews and Subject to Registration inquiriesTable 3 Footnote * | 80 |
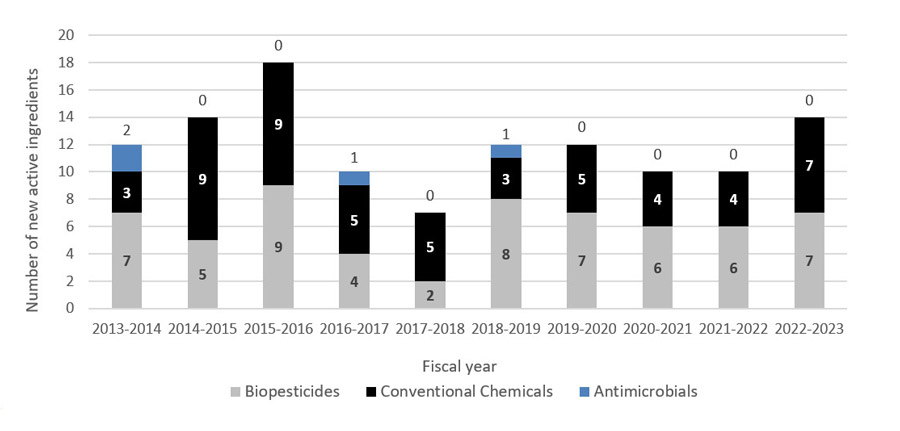
Appendix Figure 1 - Text equivalent
Number of new active ingredients registered by the PMRA from 1 April 2013 to 31 March 2023
- 2013-2014 - Biopesticides 7, Conventional Chemicals 3, Antimicrobials 2
- 2014-2015 - Biopesticides 5, Conventional Chemicals 9, Antimicrobials 0
- 2015-2016 - Biopesticides 9, Conventional Chemicals 9, Antimicrobials 0
- 2016-2017 - Biopesticides 4, Conventional Chemicals 5, Antimicrobials 1
- 2017-2018 - Biopesticides 2, Conventional Chemicals 5, Antimicrobials 0
- 2018-2019 - Biopesticides 8, Conventional Chemicals 3, Antimicrobials 1
- 2019-2020 - Biopesticides 7, Conventional Chemicals 5, Antimicrobials 0
- 2020-2021 - Biopesticides 6, Conventional Chemicals 4, Antimicrobials 0
- 2021-2022 - Biopesticides 6, Conventional Chemicals 4, Antimicrobials 0
- 2022-2023 - Biopesticides 7, Conventional Chemicals 7, Antimicrobials 0
- This figure provides the number of new active ingredients registered over the course of the last 10 fiscal years. It represents active ingredients that have been registered for use in Canada and excludes any new active ingredients for which only a maximum residue limit on imported food was established as well as new actives for which only Importation for Manufacturing and Export Program (IMEPs) exist.
Appendix Figure 2 - Text equivalent
| Year | Category A | Category B | Category C | Category D | Category E | Category F | Category L | Category P |
|---|---|---|---|---|---|---|---|---|
| 2020-2021 | 39% | 83% | 97% | 96% | 83% | 99% | 79% | 83% |
| 2021-2022 | 82% | 92% | 98% | 99% | 97% | 99% | 88% | 74% |
| 2022-2023 | 80% | 91% | 99% | 99% | 91% | 99% | 91% | 45% |
- This figure shows the percentages of submissions by submission category that met the applicable review timelines outlined in the Management of Submissions Policy (MOSP) over the last three fiscal years.
- All categories of pre-market submissions have a performance standard of 90% against the established review timelines for the different submission categories.
- In 2022–2023, the PMRA's pre-market performance results remained relatively stable compared to the previous year. Six of the eight categories of pre-market evaluations exceeded the 90% performance target in 2022–2023 compared to five of the eight in 2021–2022.
- The pre-market evaluation categories that missed the 90% performance target in 2022–2023 were Category A at 80% and Category P at 45%. See Appendix Table 1 for a description of categories, and Appendix Figure 2 for full details on pre-market performance.
- Category A performance was affected by several factors. These factors include elections, an increasingly complex workload and a delay in the publication of consultation and final decision documents to address comments on the proposed registration decisions.
- Another area that experienced challenges in 2022–2023 was Category P. Despite being a free service, the PMRA assigns a service standard to this category and works to meet the 90% performance target. The PMRA failed to meet this target in 2022–2023 due to increased complexity in submissions, in addition to data packages that are often low quality, as well as a more limited number of employees available to manage the high workload.
- As part of its commitment to continuous improvement, the PMRA hosted a Registration Workshop to educate and encourage the regulatory community to submit complete and high-quality application packages. Additionally, the PMRA created a framework for the Expanded Renewal pilot and released the Statement of Product Specification SMART form.
| No. | New active ingredient | End-use product(s) | Product type | Product category | Uses/Sites |
|---|---|---|---|---|---|
1 |
Sheep fat |
Trico® |
Animal Repellent |
Biopesticide |
Apple orchards and grape vineyards (new and established plantations) |
Trico® garden |
Animal Repellent |
Biopesticide |
Outdoor ornamentals (including flowers, trees and shrubs), apple trees and grapevines |
||
2 |
Potassium chloride |
Potash molluscicide |
Molluscicide |
Biopesticide |
Control of zebra and quagga mussels |
3 |
98Sumithrin |
MGK formula 31451 |
Insecticide |
Conventional Chemical |
Control of bed bugs and bed bug eggs |
4 |
Tiafenacil |
Tiafenacil 70WG herbicide |
Herbicide |
Conventional Chemical |
Field corn, fallow, grape, spring wheat, soybeans, wild buckwheat, kochia, common lamb's quarters, redroot pigweed, prickly lettuce, Russian thistle, velvetleaf, waterhemp |
Insight 339SC herbicide |
Herbicide |
Conventional Chemical |
|||
5 |
cis-JASMONE |
Trunemco |
Nematicide |
Biopesticide |
Field corn, popcorn, sweet corn (includes seed production), soybean |
6 |
Trichoderma gamsii Strain ICC 080, Trichoderma asperellum STRAIN ICC 012 |
Foretryx |
Fungicide |
Biopesticide |
Ornamentals; Fruiting Vegetables (crop group 8): Eggplant, groundcherry, pepino, pepper (includes bell pepper, chili pepper, cooking pepper, pimento, sweet pepper), tomatillo, tomato, strawberries, cannabis, squash (summer and winter), lettuce. |
7 |
Beauveria bassiana Strain R444 |
Bassidor |
Insecticide |
Biopesticide |
Cannabis grown in greenhouses and enclosed structures; Ornamentals Grown in greenhouse or in protected environments; Fruiting Vegetables (Crop Group 8-09): African eggplant, bell pepper, bush tomato, cocona, currant tomato, eggplant, garden huckleberry, goji berry, groundcherry, martynia, naranjilla, non-bell pepper, okra, pea eggplant, pepino, roselle, scarlet eggplant, sunberry, tomatillo, tomato, tree tomato, and cultivars, varieties and hybrids of these. Cucurbit Vegetables (Crop Group 9): chayote (fruit), Chinese wax gourd (Chinese preserving melon), citron melon, cucumber, gherkin, gourd (edible, includes Chinese okra, cucuzza, hechima, hyotan), Momordica spp. (includes balsam apple, balsam pear, bitter melon, Chinese cucumber), muskmelon (includes cantaloupe, casaba, crenshaw melon, golden pershaw melon, honey balls, honeydew melon, mango melon, Persian melon, pineapple melon, Santa Claus melon, snake melon, true cantaloupe), pumpkin, summer squash (includes crookneck squash, scallop squash, straightneck squash, vegetable marrow, zucchini), watermelon, winter squash (includes acorn squash, butternut squash, calabaza, hubbard squash, spaghetti squash). Leafy Vegetables (Crop Group 4-13): Abyssinian cabbage, arugula, bitter lettuce, blackjack, broccoli raab, cat's whiskers, cham-chwi, cham-na-mul, chervil (fresh leaves), Chinese amaranth, Chinese broccoli, Chinese cabbage (bok choy), Chinese violet, chipilin, cilantro (fresh leaves), collards, corn salad, cosmos, dandelion, dang-gwi, dillweed (fresh leaves), dock, dol-nam-mul, ebolo, endive, escarole, English primrose, fameflower, feather cockscomb, garden cress, garden purslane, garland chrysanthemum, Good-King-Henry, Hanover salad, head lettuce, huauzontle, jute leaves, kale, Indian aster, leaf lettuce, leafy amaranth, maca, Malabar spinach, mizuna, mustard greens, New Zealand spinach, orach, parsley (fresh leaves), plantain (including buckhorn plantain and common plantain), radicchio (red chicory), radish leaves, rape greens, seakale cabbage, shepherd's purse, spinach, Swiss chard, tanier spinach, tree spinach, turnip greens, upland cress, watercress, wild rocket, winter purslane, and cultivars, varieties and hybrids of these. Brassica Head and Stem Vegetables (Crop Group 5-13): Broccoli, Brussels sprouts, cabbage, Chinese cabbage (napa), cauliflower, and cultivars, varieties and hybrids of these. Berries and Small Fruits (Crop Group 13-07) GROWN FOR TRANSPLANT ONLY: Blackberry, highbush blueberry, lowbush blueberry, black currant, red currant, elderberry, gooseberry, huckleberry, black and red raspberry, amur river grape, aronia berry, bayberry, bearberry, bilberry, buffalo currant, buffaloberry, che, Chilean guava, chokecherry, cranberry, European barberry, grape, highbush cranberry, edible honeysuckle, jostaberry, juneberry, fuzzy kiwifruit, hardy kiwifruit, lingonberry, maypop, mountain pepper berry, mulberry, muntries, native currant, partridgeberry, phalsa, pin cherry, riberry, salal, schisandra berry, sea buckthorn, serviceberry, strawberry, wild raspberry and cultivars, varieties and hybrids of these. Strawberry; Stalk, Stem, and Leaf Petioles (Crop Group 22): agave, aloe vera, asparagus, bamboo shoots, cardoon, celery, Chinese celery, Florence fennel (fresh leaves and stalk), fern (edible fiddlehead, including black lady fern, bracken fern, broad buckler fern, cinnamon fern, lady fern, leather fern, mother fern, ostrich fern, vegetable fern, zenmai fern), fuki, kohlrabi, palm hearts, prickly pear, rhubarb, sea kale, Texas prickly pear, udo, zuiki, and cultivars, varieties and hybrids of these. Herbs and Spices (Crop Group 19): Allspice, angelica, anise (seed), annatto, balm (lemon balm), basil, black caraway, borage, burnet, camomile, caper buds, caraway, cardamom, cassia bark, cassia buds, catnip, celery seed, chervil (dried), Chinese chive, chive, cinnamon, clary, clove buds, cilantro (leaf), coriander (cilantro seed), costmary, culantro (leaf), culantro (seed), cumin, curry (leaf), dill (dillweed), dill (seed), fennel, fenugreek, Florence fennel (seed), grains of paradise, horehound, hyssop, juniper berry, lavender, lemongrass, lovage (leaf), lovage (seed), mace, marigold, marjoram (includes sweet or annual marjoram, wild marjoram or oregano and pot marjoram), mustard (seed), nasturtium, nutmeg, parsley (dried), pennyroyal, pepper, poppy (seed), rosemary, rue, saffron, sage, savory, star anise, sweet bay (bay leaf), tansy, tarragon, thyme, vanilla, wintergreen, woodruff, wormwood. |
8 |
Attenuated Cucumber green mottle mosaic virus strain ON-BM3; Al-Bio10 |
CUC-Guard |
Non -parasitic plant disease control |
Biopesticide |
Greenhouse cucumber |
9 |
Fenazaquin |
Magister SC miticide/fungicide |
Acaricide, Fungicide, Insecticide |
Conventional Chemical |
Bushberries (Crop Subgroup 13-07B): Aronia berry; highbush blueberry, lowbush blueberry, buffalo currant; Chilean guava; highbush cranberry, black currant, red currant, elderberry, European barberry, gooseberry, edible honeysuckle, huckleberry, jostaberry, Juneberry (Saskatoon berry), lingonberry, native currant, salal, sea buckthorn, cultivars, varieties, and/or hybrids of these. Caneberries (Crop Subgroup 13-07A): Blackberry (including Andean blackberry, arctic blackberry, bingleberry, black satin berry, boysenberry, brombeere, California blackberry, Chesterberry, Cherokee blackberry, Cheyenne blackberry, common blackberry, coryberry, darrowberry, dewberry, Dirksen thornless berry, evergreen blackberry, Himalayaberry, hullberry, lavacaberry, lowberry, Lucretiaberry, mammoth blackberry, marionberry, mora. mures deronce, nectarberry, northern dewberry, olallieberry, Oregon evergreen berry, phenomenalberry, rangeberry, ravenberry, rossberry, Shawnee blackberry, southern dewberry, tayberry, youngberry, zarzamora), loganberry, red and black raspberry, wild raspberry, cultivars, varieties, and/or hybrids of these. Cucurbit Vegetables (Crop Group 9): Chinese waxgourd (Chinese preserving melon), citron melon, cucumber, gherkin, edible gourd, (includes hyotan, cucuzza, hechima, Chinese okra); Momordica spp. (includes balsam apple, balsam pear, bitter melon, Chinese cucumber); muskmelon (includes cantaloupe, casaba, crenshaw melon, golden pershaw melon, honeydew melon, honey balls, mango melon, Persian melon, pineapple melon, Santa Claus melon, snake melon), pumpkin summer squash (includes crookneck squash, scallop squash, straightneck squash, vegetable marrow, zucchini), winter squash, (includes butternut squash, calabaza, hubbard squash, acorn squash, spaghetti squash), watermelon. Fruiting Vegetables (Crop Group 8-09): African eggplant, currant tomato, eggplant, garden huckleberry, goji berry, groundcherry, martynia, okra, pea eggplant, pepino, bell pepper, non-bell pepper, scarlet eggplant, sunberry, tomatillo, tomato, cultivars, varieties, and/or hybrids of these. Low Growing Berries (Crop Subgroup 13-07G): Bearberry, bilberry, lowbush blueberry, cloudberry, cranberry, lingonberry, muntries, partridgeberry, strawberry, cultivars, varieties, and/or hybrids of these. Pome Fruits (Crop Group 11-09): Azarole apple, crabapple, mayhaw apple, medlar, Asian pear, Chinese quince, Japanese quince, tejocote, cultivars, varieties, and/or hybrids of these. Small Fruit Vine Climbing, Except Fuzzy Kiwifruit (Crop Subgroup 13-07F): Amur river grape, gooseberry, grape, hardy kiwifruit, maypop, schisandra berry, cultivars, varieties, and/or hybrids of these. Stone Fruits (Crop Group 12-09): Japanese apricot, black cherry, Nanking cherry, sweet cherry, tart cherry, Chinese jujube, nectarine, peach, American plum, beach plum, Canada plum, cherry plum, Chicksaw plum, Damson plum, Japanese plum, Klamath plum, prune plum, plumcot, sloe, cultivars, varieties, and/or hybrids of these. Ornamental plants: Including fruit and nut tree seedlings (greenhouse), non-bearing fruit and nut trees (field grown, outdoor nursery, shade house), ornamental plants in residences, commercial buildings and shopping malls, ornamental plants in residential areas, public areas, commercial areas, institutional areas, industrial areas, rights of way and other easements, and recreational sites such as campgrounds, golf courses, parks and athletic fields. |
Magus SC miticide |
Acaricide, Fungicide, Insecticide |
Conventional Chemical |
Ornamental plants: Including fruit and nut tree seedlings (greenhouse), non-bearing fruit and nut trees (field grown, outdoor nursery, shade house), ornamental plants in residences, commercial buildings and shopping malls, ornamental plants in residential areas, public areas, commercial areas, institutional areas, industrial areas, rights of way and other easements, and recreational sites such as campgrounds, golf courses, parks and athletic fields. |
||
10 |
Pyrifluquinazon |
Pyrifluquinazon 20sc insecticide |
Insecticide |
Conventional Chemical |
Indoor greenhouse use on lettuce, tomato, eggplant, pepper, cucumber and herbaceous and woody deciduous ornamental plants. |
11 |
Pyraziflumid |
Parade fungicide |
Fungicide |
Conventional Chemical |
Apple |
12 |
Ipflufenoquin |
Kinoprol 20 SC |
Fungicide |
Conventional Chemical |
Pome Fruits (Crop Group 11-09): Azarole apple, crabapple, mayhaw apple, medlar, Asian pear, Chinese quince, Japanese quince, tejocote, cultivars, varieties, and/or hybrids of these. |
13 |
Florylpicoxamid |
GF-3840 fungicide |
Fungicide |
Conventional Chemical |
Wheat (spring, winter, durum), canola, sugar beets, established turf, golf course, turf/sod farms only |
Zetigo PRM fungicide |
Fungicide |
Conventional Chemical |
Lentil and canola |
| Active ingredient | Document number | Summary of Decision or Proposed Decision | Number of impacted end-use products |
|---|---|---|---|
| Re-evaluation Decisions | |||
| Florasulam | RVD2022-03 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 40 |
| Isoxaflutole | RVD2022-04 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 5 |
| Difenoconazole | RVD2022-05 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 27 |
| Straight Chain Lepidopteran Pheromones | RVD2022-06 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 8 |
| Mustard Seed Powder (Brassica hirta) and Sodium Alpha-olefin Sulfonate | RVD2022-07 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 1 |
| Dried Blood | RVD2022-08 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 4 |
| Ancymidol | RVD2022-09 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 3 |
| Kaolin | RVD2022-10 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 1 |
| p-Menthane-3,8-diol | RVD2022-11 | Acceptable for continued registration. No label changes required. | 5 |
| Trinexapac-ethyl | RVD2022-12 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 7 |
| Ziram (preservative in adhesives) | RVD2022-13 | Cancellation of all products due to human health risk concerns. | 1 |
| Dazomet (preservatives in paints, coatings and related uses) | RVD2022-14 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health. Cancellation of other uses due to health risk concerns. | 12 |
| Sodium Omadine (preservatives in paints, coatings and related uses) | RVD2022-15 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health. | 2 |
| Folpet (preservative in paints and vinyl plastics) | RVD2022-16 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health. Cancellation of other uses due to health risk concerns. | 3 |
| Chlorothalonil (preservative in paints) | RVD2022-17 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health. | 7 |
| Methyl Nonyl Ketone | RVD2022-18 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 15 |
| Putrescent Whole Egg Solids | RVD2023-01 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 4 |
| Corn Gluten Meal | RVD2023-02 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 12 |
| Flucarbazone | RVD2023-03 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 13 |
| Zoxamide | RVD2023-04 | Acceptable for continued registration. Mitigation includes new/revised label statements to further protect the environment. | 3 |
| Formic Acid | RVD2023-05 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 5 |
| Octadec-9-enoic Acid, Methyl Ester, and Octadec-9-enoic Acid, Ethyl Ester | RVD2023-08 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 3 |
| Pyrethrins | RVD2023-06 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health risk concerns or value not shown to be acceptable. | 406 |
| Piperonyl Butoxide | RVD2023-07 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health and the environment. Cancellation of other uses due to health risk concerns. | 293 |
| Verbenone | RVD2023-09 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 3 |
| Capsaicin and related Capsaicinoids | RVD2023-10 | Acceptable for continued registration. Label improvements required to meet current labelling standards. | 40 |
| Special Review Decision | |||
| Diodofon | SRD2022-03 | Acceptable for continued registration for certain uses. Mitigation includes new/revised label statements to further protect human health. Cancellation of other uses due to health risk concerns. | 5 |
| Re-evaluation Note | |||
| Pentachlorophenol | REV2022-02 | Discontinuation of all products | 2 |
| Proposed Re-evaluation Decisions | |||
| (Z)-9-tricosene | PRVD2022-07 | Proposed for continued registration. Label improvements required to meet current labelling standards. | 10 |
| Methyl Nonyl Ketone | PRVD2022-08 | Proposed for continued registration. Label improvements required to meet current labelling standards. | 15 |
| Bacillus amyloliquefaciens strain MBI600 and Bacillus subtilis strain QST 713 | PRVD2022-09 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect the environment. | 18 |
| 1,3-bis(hydroxymethyl)-5,5-dimethylhydantoin and hydroxymethyl-5,5-dimethylhydantoin | PRVD2022-10 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 5 |
| Silica Aerogel and Silicon Dioxide | PRVD2022-12 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 69 |
| Putrescent Whole Eggs | PRVD2022-11 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health. | 4 |
| Capsaicin and Related Capsaicinoids | PRVD2022-14 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 40 |
| Bacillus sphaericus Strain 2362 | PRVD2022-13 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health. | 3 |
| Formic Acid | PRVD2022-15 | Proposed for continued registration. Label improvements required to meet current labelling standards. | 5 |
| Dodecylguanidine Hydrochloride | PRVD2022-16 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 2 |
| Quizalofop-p-ethyl | PRVD2022-17 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 14 |
| Strychnine and Sodium Monofluoroacetate | PRVD2022-18 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 4 |
| Verbenone | PRVD2022-19 | Proposed for continued registration. Label improvements required to meet current labelling standards. | 3 |
| Nucleopolyhedrovirus for Douglas-Fir Tussock Moth and Neodiprion abietis Nucleopolyhedrovirus | PRVD2022-20 | Proposed for continued registration. Label improvements required to meet current labelling standards. | 3 |
| Sodium Hypochlorite and Calcium Hypochlorite | PRVD2022-21 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health and the environment. | 83 |
| Abamectin | PRVD2023-01 | Proposed continued registration for certain uses. Mitigation includes new/revised label statements to further protect the environment. Proposed cancellation of other uses due to health risk and environmental concerns. | 18 |
| Proposed Special Review Decisions | |||
| Potassium Dimethyldithiocarbamate | PSRD2022-02 | Proposed for continued registration. Mitigation includes new/revised label statements to further protect human health. | 27 |
| Picoxystrobin | PSRD2022-03 | Proposed for continued registration. | 4 |
| Atrazine | PSRD2023-01 | Proposed continued registration for certain uses. Mitigation includes new/revised label statements to further protect the environment. Proposed cancellation of other uses due to health risk concerns. | 10 |