Interim guidance for producing ethanol for use in alcohol-based hand sanitizers: Quality grades and recommended formulations
On this page
Quality standards
Ethanol used in the production of hand sanitizers should meet quality and purity standards. We have noted any deviations to these standards in this interim guidance.
As outlined in the Quality of Natural Health Products Guide, the current official version of the quality standard (or pharmacopoeia) should be used. Moreover, the quality standards as a whole, including all other pharmacopoeial requirements, should be applied. While some of these standards are free, others require payment for full access:
- USP monograph
- European Pharmacopeia (Ph. Eur.)
- Food Chemical Codex (FCC)
- British Pharmacopoeia (BP)
- Pharmacopée française (Ph.f.) (see the monographs in subfolder "13-Formulaire national")
- Pharmacopoeia Internationalis (Ph.I.)
- Japanese Pharmacopoeia (JP) (see page 896)
- National Formulary (NF)
Grades of ethanol that do not conform to these quality and purity standards are unacceptable for use in the manufacture of alcohol-based hand sanitizers. The exception is technical-grade ethanol (TGE) that Health Canada has reviewed and authorized and are from an accepted supplier or distributor.
Ethanol producers that wish to supply TGE for use in hand sanitizers or hard-surface disinfectants must receive written authorization from Health Canada before proceeding. Please note that TGE approvals are based on an assessment of the ethanol before denaturants are added. For more information on the use of TGE, see the notice to industry on using technical-grade ethanol in hand sanitizers and hard-surface disinfectants.
For the names of accepted ethanol grades and their manufacturers, see the list of approved suppliers for technical grade ethanol. For authorized distributors of these ethanol grades, see the list of approved distributors.
Companies that wish to manufacture hand sanitizers containing TGE supplied by these authorized suppliers and distributors must first notify Health Canada using a notification form. Companies that meet certain conditions, such as special labelling, will receive a No Objection Letter, granting temporary authorization for the use of TGE. These companies and products are on the list of manufacturers of hand sanitizers and hard-surface disinfectants.
TGE may only be used on a temporary basis, until the supply of acceptable grades of ethanol stabilizes.Recommended formulations
All hand sanitizer formulations must meet the safety and efficacy requirements outlined in the monograph on antiseptic skin cleansers (personal domestic use).
Health Canada recommends using the WHO formulation to manufacture ethanol-based hand sanitizer. The WHO's hand-rub formulation suggests a final concentration of 80% v/v ethanol. While Health Canada's monograph stipulates a range of 60% to 80 v/v ethanol, an 80% v/v concentration is recommended for increased efficacy.
Formulation for a 10-litre preparation of 80% ethanol:
- Ethanol 96%: 8,333 ml
- Hydrogen peroxide 3%: 417 ml
- Glycerol 98%: 145 ml
- Water: amount to make up the remainder of the 10 L
Another example of an acceptable formulation is included in the USP guidance (updated on March 25, 2020).
Record-keeping
Records must be maintained on how the hand sanitizer is prepared, including how the final ethanol dilution in the finished product was obtained. The amount of ethanol needed in the formulation should be calculated using the following equation (set out in the USP guidance):
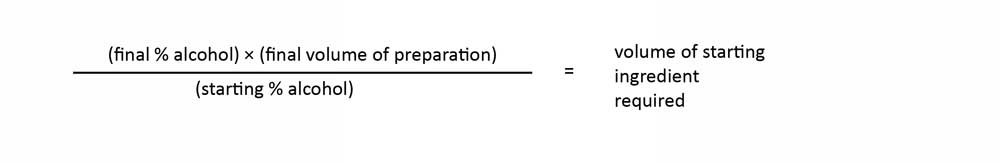
Figure - Text description
The final percentage of alcohol times the final volume of preparation divided by the starting volume of alcohol equals the volume of starting ingredient required.
Licence holders are also responsible for ensuring the quality of their licensed natural health products (NHPs) before marketing these products. This includes setting up and maintaining product specifications, as outlined in section 44 of the Natural Health Products Regulations. They must also declare that each lot of batch of finished product meets those specifications.
All products must be manufactured, packaged, labelled and imported according to the requirements of Part 3 (good manufacturing practices) of the regulations. This includes maintaining records that show compliance for a period of 1 year after the product's expiry date (see sections 53 to 58 of the regulations).
If the product licence holder relies on another party to produce the NHP, the roles and responsibilities for the development, maintenance and storage of these records must be clearly defined.