Archived - Health Product InfoWatch – January 2019

Download the alternative format
(PDF format, 558 KB, 10 pages)
Health Products and Food Branch
Marketed Health Products Directorate
Health Product InfoWatch Editorial Team
ISSN: 2368-8025
Cat.: H167-1E-PDF
Pub.: 180663
Organization: Health Canada
Published: 2019-01-31
Contents
- Health products mentioned in this issue
- Announcement – Trelegy Ellipta
- Opioid Updates
- Monthly recap
- New information
- Review article: Prolonged use of hydrochlorothiazide and the risk of non-melanoma skin cancer
- Vaccine safety biannual summary: Report for January 1, 2018 to June 30, 2018
- Product monograph update: Biaxin BID, Biaxin XL, and Biaxin (clarithromycin)
- Product monograph update: Lamictal (lamotrigine)
- Product monograph update: Revlimid (lenalidomide)
- Scope
- Helpful links
- Suggestions?
- Copyright
Health products mentioned in this issue
Pharmaceuticals and Biologics
- Acetaminophen oral drops
- Biaxin (clarithromycin)
- Equate brand Lens Care System and Multi-Purpose Solution
- Hydrochlorothiazide
- Lamictal (lamotrigine)
- Revlimid (lenalidomide)
- Sartan drugs
- Trelegy Ellipta
- Vascular endothelial growth factor receptor tyrosine kinase inhibitors
- Xarelto (rivaroxaban)
Natural Health Products
Other
Announcement
Trelegy Ellipta
Health Canada has recently been made aware that healthcare professionals may have received information that is inconsistent with the Canadian product monograph for Trelegy Ellipta. Health Canada wishes to remind healthcare professionals that:
- Trelegy Ellipta is an inhaler that contains the following medicinal ingredients:Footnote 1
- Fluticasone furoate, an inhaled corticosteroid (ICS)
- Umeclidinium, a long acting muscarinic antagonist (LAMA)
- Vilanterol, a long acting beta2-adrenergic agonist (LABA)
- Trelegy Ellipta is indicated for the long-term, once daily, maintenance treatment of chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema in patients who are not adequately treated by a combination of an ICS and a LABA.Footnote 1
- Trelegy Ellipta is not indicated for the relief of acute bronchospasm or for the treatment of asthma.Footnote 1
Healthcare professionals are encouraged to contact Health Canada at MHPD_DPSC-Advertising_Reg_Publicite@hc-sc.gc.ca if any health product information suspected to be false or misleading is encountered.
Reference
- Footnote 1
-
Trelegy Ellipta (fluticasone furoate, umeclidinium, and vilanterol) [product monograph]. Mississauga (ON): GlaxoSmithKline Inc.; 2018.
Opioid Updates
Now online - A summary of what Health Canada heard in response to the Notice of Intent to restrict the marketing and advertising of opioids
On June 19, 2018, the Minister of Health published a Notice of Intent to restrict the marketing and advertising of opioids. As part of Health Canada's commitment to transparency, Health Canada recently published a What We Heard report summarizing the comments received. The summary draws on more than 40 submissions from healthcare professionals and associations, patients, academics, and pharmaceutical industry representatives on further restrictions to opioid marketing and advertising.
The feedback received is informing next steps for federal action. Health Canada will continue to share updates with Canadians on the Restricting the marketing and advertising of opioids Web page.
Monthly recap of health product safety information
The following is a list of health product advisories, type I recalls as well as summaries of completed safety reviews published in December 2018 by Health Canada.
Acetaminophen oral drops
Laboratoire Riva Inc. and Laboratoires Trianon Inc. recalled 5 over-the-counter strawberry-flavoured acetaminophen oral drops for infants. The products are labelled as Biomedic, Option+, Personnelle, Selection, or Laboratoires Trianon Inc. They are being recalled because the child-resistant safety cap may be defective. This recall is in addition to previous recalls of children's acetaminophen syrups for the same issue.
- Advisory – Acetaminophen oral drops
- Drug recall – Acetaminophen 80 mg / mL, Laboratoire Riva Inc. (2018-12-06)
- Drug recall – Acetaminophen 80 mg / mL, Laboratoire Trianon Inc. (2018-12-06)
Equate brand Lens Care System and Multi-Purpose Solution
One lot (Lot 150261) of these two products was recalled because of a labelling error. While the outer carton of Equate brand Lens Care System is correctly labelled, the bottle within the carton is mislabelled as Equate brand Multi-Purpose Solution. Because of the labelling error, the company is recalling both products labelled with Lot 150261. Bottles labelled as Equate Multi-Purpose Solution should contain a 0.0001% w/v polyhexanide based disinfecting solution for rinsing. The mislabelled bottles contain the Equate Lens Care System, which is a 3.3% hydrogen peroxide cleaning and disinfecting solution and should not be used for rinsing.
Foreign health products
Quizz capsules are a foreign health product that has been found to contain undeclared lead and mercury. This product is not authorized for sale in Canada and has not been found in the Canadian marketplace, but it is possible that it may have been brought into the country by travellers or purchased over the Internet. Health Canada is warning consumers not to use this unauthorized product after it was associated with a Canadian case of lead poisoning. The product is promoted for the treatment of diabetes.
Health products manufactured by Professional Botanicals Inc.
All products manufactured by Professional Botanicals Inc. may pose serious health risks. The company is a contract manufacturer that also operates under the name Healthy Botanicals Inc. Health Canada's assessment indicated that the company was manufacturing products under unsanitary conditions at a site that was not licensed by Health Canada at the time. Health Canada has seized all of the products, prescription drug ingredients and manufacturing equipment found at the site, and suspended the 11 natural health product licences held by the company.
Sartan drugs
Health Canada has released the results of its testing of sartan drugs in Canada. Health Canada tested 48 samples of certain sartan drugs (valsartan, candesartan, irbesartan, losartan, and olmesartan), representing 43 different products, and did not identify any new safety concerns. Of the 48 samples, 6 valsartan samples representing 4 products were found to contain levels of impurities that were, on average, higher than what is considered to be reasonably safe. All 4 of the products have already been recalled from the Canadian market.
Talc
Health Canada and Environment and Climate Change Canada's draft screening assessment of talc proposes that breathing in loose talc powder may cause lung effects, such as coughing, trouble breathing, decreased lung function and fibrosis and that exposure to the perineal area from the use of certain products containing talc is a possible cause of ovarian cancer. Healthcare professionals are advised to remind patients to avoid inhaling loose talc powders, avoid female genital exposure to products containing talc, keep baby powder away from a child's face to avoid inhalation, check product labels for talc and choose talc-free alternatives if concerned.
Unauthorized health products
Health Canada advised Canadians about various unauthorized health products being sold at retail locations across Canada or online that may pose serious health risks.
Vascular endothelial growth factor receptor tyrosine kinase inhibitors
This safety review evaluated the risk of artery dissections and artery aneurysms associated with vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR TKIs). Health Canada's review concluded that there may be a link between the use of VEGFR TKIs and artery dissections/artery aneurysms. Health Canada is working with the manufacturers to update the Canadian product monographs of all VEGFR TKIs to inform about this risk.
Xarelto (rivaroxaban)
Increased all-cause mortality, thromboembolic and bleeding events have been reported in a phase III clinical study among Xarelto-treated patients who had transcatheter aortic valve replacement (TAVR). Based on these preliminary results, the clinical study has been terminated and further analysis of the study results is ongoing. Healthcare professionals are reminded that Xarelto is not authorized for thromboprophylaxis in patients with prosthetic heart valves, including patients who have undergone TAVR, and should not be used in such patients. Xarelto treatment should be stopped in patients who undergo TAVR and switched to standard of care.
New health product safety information
The following topics have been selected to raise awareness and, in some cases, to stimulate reporting of similar adverse reactions.
Review article: Prolonged use of hydrochlorothiazide and the risk of non-melanoma skin cancer
Hydrochlorothiazide (HCTZ) is a diuretic commonly used in Canada to treat hypertension and edema.
Recent pharmacoepidemiologic studies have suggested a cumulative dose-dependent increased risk of non-melanoma skin cancer (NMSC) with the use of HCTZ.Footnote 2,Footnote 3
NMSC is the most commonly diagnosed cancer in Canada with basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) being the two most common types.Footnote 4 One in 8 Canadians will develop BCC in their lifetime, and 1 in 20 will develop SCC.Footnote 5 NMSCs are generally curable if treated early and fatal cases are uncommon.Footnote 6 Ultraviolet exposure, photosensitizing medications, and immunosuppression are important risk factors for NMSCs; individuals with light coloured skin are at particularly high risk.Footnote 4
HCTZ is known to increase the sensitivity of the skin to sunlight and ultraviolet radiation.Footnote 7,Footnote 8
Health Canada has completed a review of the relevant studies using systematic review and meta-analytic methods to investigate the strength of the association between HCTZ and NMSC. Certainty of evidence was also assessed using standard methods (the GRADE approachFootnote 9).
Pooling of the study level dataFootnote 10 showed that continued use of HCTZ (alone or as combination therapy) for several years could lead to:
- 122 more cases (95% confidence interval [CI], 112-133) of SCC per 1000 patients treated with HCTZ compared with those not treated with HCTZ (meta-analysis of 3 observational studies, 2 published studies and one unpublished;Footnote 2,Footnote 11,Footnote 12 very low certainty evidence)
- 31 more cases (95% CI, 24-37) of BCC per 1000 patients treated with HCTZ compared with those not treated with HCTZ (meta-analysis of 2 observational studies, one published study and one unpublished;Footnote 2,Footnote 12 very low certainty evidence)
However, Health Canada noted important methodological limitations (e.g., confounding due to lack of data on sun exposure and imbalance in the duration of hypertension, etc.) in the reviewed studies, which resulted in very low certainty for the above estimates of harm. According to the GRADE approach, very low certainty means that the true effect is likely to be substantially different. The aforementioned findings, however, reflect the current best evidence.
Based on this review, Health Canada concluded that NMSC is a potential risk of prolonged use of HCTZ. Due to the seriousness of this potential risk and the wide use of this drug in Canada, Health Canada is taking a precautionary approach and is currently working with manufacturers to update the Canadian product safety information to inform the public and healthcare professionals about this new potential risk and the preventive measures to consider when taking HCTZ.
Key messages for healthcare professionals:
- Health Canada's review of the relevant evidence concluded that there is a potential risk of developing NMSC with prolonged use of HCTZ. However, uncertainty remains due to limitations noted in all the reviewed studies. The photosensitizing action of HCTZ may be a mechanism for this effect.
- Patients taking HCTZ-containing products should be informed of the potential risk of NMSC and advised to regularly check their skin for new lesions as well as changes to existing lesions and report any suspicious skin lesions.
- Advise patients to practice routine sun-safety by limiting sun exposure time and protecting themselves from the sun (e.g., SPF 30 or higher sunscreen and cover up with clothing and a hat). Use of tanning equipment should be avoided.
- Alternatives to HCTZ may be considered for patients who are at a particularly high risk for NMSC (e.g., light coloured skin, known personal or family history of skin cancer, ongoing immunosuppressive therapy).
Healthcare professionals are encouraged to report to Health Canada any case of BCC or SCC suspected to be associated with HCTZ therapy. Information to be provided in the reports includes dosage, dates of treatment initiation and discontinuation, concomitant medications, comorbidities, life style reflecting sun exposure or indoor tanning, and date of onset of the adverse reaction. This information will support ongoing monitoring of this potential safety issue.
Article citation: Health Canada. Prolonged use of hydrochlorothiazide and the risk of non-melanoma skin cancer. Health Product InfoWatch January 2019.
References
- Footnote 2
-
Pedersen SA, Gaist D, Schmidt SAJ, et al. Hydrochlorothiazide use and risk of nonmelanoma skin cancer: A nationwide case-control study from Denmark. J Am Acad Dermatol 2018;78(4):673-81. [PubMed]
- Footnote 3
-
Pottegård A, Hallas J, Olesen M et al. Hydrochlorothiazide use is strongly associated with risk of lip cancer. J Intern Med 2017;282(4):322-31. [PubMed]
- Footnote 4
-
Guenther LC, Barber K, Searles GE, et al. Non-melanoma skin cancer in Canada Chapter 1: Introduction to the Guidelines. J Cutan Med Surg 2015;19(3):205-15. [PubMed]
- Footnote 5
-
Non melanoma skin cancer. Ottawa (ON): Public Health Agency of Canada; 2014 May 28. (accessed 2019 January 10).
- Footnote 6
-
Fahradyan A, Howell AC, Wolfswinkel EM, et al. Updates on the management of non-melanoma skin cancer (NMSC). Healthcare (Basel) 2017;5(4):82. doi:10.3390/healthcare5040082 [PubMed]
- Footnote 7
-
Addo HA, Ferguson J, Frain-Bell W. Thiazide-induced photosensitivity: a study of 33 subjects. Br J Dermatol 1987;116(6):749-60. [PubMed]
- Footnote 8
-
Robinson HN, Morison WL, Hood AF. Thiazide diuretic therapy and chronic photosensitivity. Arch Dermatol 1985;121(4):522-4. [PubMed]
- Footnote 9
-
Guyatt G, Oxman AD, Akl EA et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64(4):383-94. [PubMed]
- Footnote 10
-
Health Canada has the data on file.
- Footnote 11
-
Friedman GD, Asgari MM, Warton EM, et al. Antihypertensive drugs and lip cancer in non-Hispanic whites. Arch Intern Med 2012;172(16):1246-51. [PubMed]
- Footnote 12
-
Health Canada has the data on file.
Vaccine safety biannual summary
Health Canada and the Public Health Agency of Canada (PHAC) share the responsibility of monitoring of the safety of vaccines in Canada.
Market authorization holders are required to report serious adverse events following immunization to the Canada Vigilance Program in Health Canada. The Canada Vigilance Program also receives voluntary reports from healthcare professionals and consumers.
Provincial and territorial public health authorities report adverse events following immunization (AEFIs) from publicly-funded vaccine programs to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) in PHAC to monitor the safety of immunization programs.
Report for January 1, 2018 to June 30, 2018
Key messages:
- No new safety signals (potential safety issues) were identified during this period.
- From January 1, 2018 to June 30, 2018, the Canada Vigilance Program received 331 reportsEndnote a of adverse events following immunization for which vaccines were a suspected cause.
This biannual vaccine safety summary includes reports of adverse events following immunization received by the Canada Vigilance Program between January 1, 2018 and June 30, 2018. To access reports published by the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS), please visit the CAEFISS Web site.
- From January 1, 2018 to June 30, 2018, the Canada Vigilance Program received 331 reportsEndnote a of adverse events following immunization for which vaccines were a suspected cause.
Figure 1. Total number of reports received by type of originating reporterEndnote b
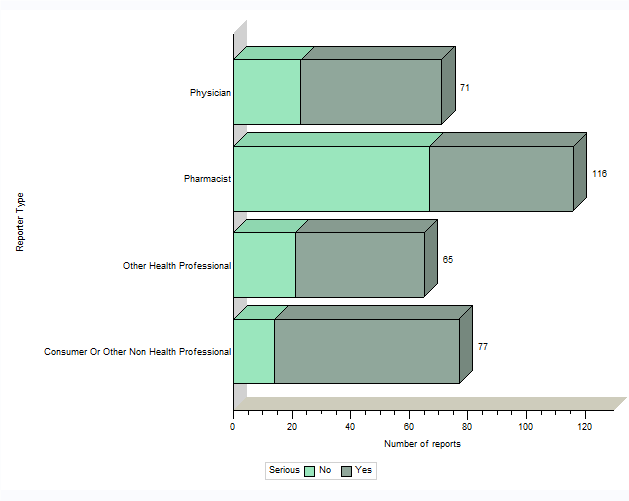
Figure 1: Total number of reports received by type of originating reporter - Text Description
The figure shows the total number of reports received from January 1st, 2018 to June 30, 2018, by type of originating reporter and severity.
| Originating reporter | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Physicians | 23 | 48 |
| Pharmacists | 67 | 49 |
| Other health professional | 21 | 44 |
| Consumer or other non health professional | 14 | 63 |
Figure 2. Total number of reports received by age group
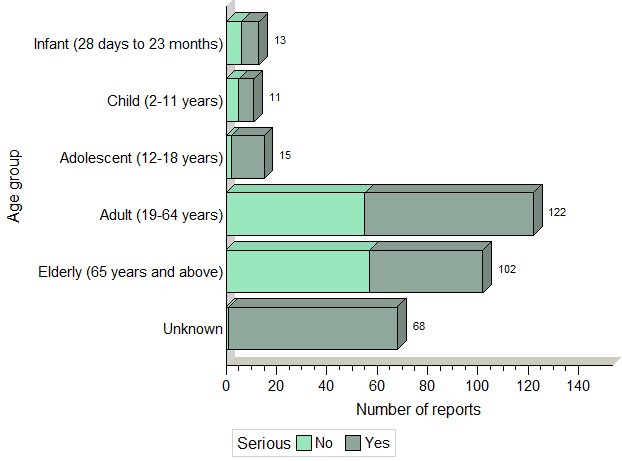
Figure 2: Total number of reports received by age group - Text Description
The figure shows the total number of reports received from January 1st, 2018 to June 30, 2018, by age group and severity.
| Age | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Infant (28 days to 23 months) | 6 | 7 |
| Child (2-11 years) | 5 | 6 |
| Adolescent (12-18 years) | 2 | 13 |
| Adult (19-64 years) | 55 | 67 |
| Elderly (≥ 65 years) | 57 | 45 |
| Unknown | 1 | 67 |
- There were 205 (62%) serious reports, all of which were individually assessed. Most of these involved patients with underlying medical conditions and were unlikely related to the vaccination.
- The highest number of reports (serious and non-serious) involved herpes zoster vaccines (50%), followed by pneumococcal vaccines (15%) and influenza vaccines (9%).
- The majority of reports for herpes zoster vaccines (149/176 reports) were for Shingrix. Of the 149 reports for Shingrix, 36 reports were from social media. The information provided from these reports was not sufficient to adequately assess the causal association with the vaccine.
- There were 4 reports with an outcome of death. All were female; 1 report was for influenza vaccine and another was a social media report for Shingrix. In both cases, the patient died of unknown causes and the information provided was not sufficient to adequately assess the causal association with the vaccine. The other 2 fatal reports were for Gardasil; 1 was previously assessed in 2015. A copy of the Summary Safety Review is available on the Health Canada Web site. The other case lacked sufficient information to adequately assess the causal association with the vaccine.
- The most frequently reported adverse events (serious and non-serious) included herpes zoster, vaccination failure, pyrexia, fatigue and pain. These adverse events were mostly reported for Shingrix.
- The adverse events of vaccination failure and herpes zoster were primarily from social media extracted by the company and the information provided was not sufficient to adequately assess the causal association with the vaccine.
- No new safety signals (potential safety issues) were identified during this period.
- Health Canada, in collaboration with PHAC, will continue to closely monitor the safety of vaccines authorized in Canada and will take appropriate action if any new health risks are identified.
For additional information, contact the Marketed Health Products Directorate.
Note that because of updated information received by the Canada Vigilance Program, there may be differences in the number of reports and adverse events retrieved at different dates.
Product Monograph Updates
The following safety labelling updates, which were recently made to the Canadian product monograph, have been selected for your awareness. A complete list of safety labelling updates is available on Health Canada's Product Monograph Brand Safety Updates page. Canadian product monographs can be accessed through Health Canada's Drug Product Database.
Biaxin BID, Biaxin XL, and Biaxin (clarithromycin)
The use of Biaxin BID, Biaxin XL, or Biaxin is now contraindicated in concomitant therapy with domperidone. This information and updates on the risk of adverse cardiovascular outcomes with macrolides have been included in the Contraindications, Warnings and Precautions, Drug Interactions, and Patient Medication Information sections of the Canadian product monograph for Biaxin BID, Biaxin XL, and Biaxin.
Key messages for healthcare professionals:Footnote 13
- Concomitant administration of clarithromycin with domperidone is contraindicated.
- Epidemiological studies investigating the risk of adverse cardiovascular outcomes with macrolides have shown variable results. Studies have identified risks of arrhythmia, myocardial infarction and cardiovascular mortality associated with macrolides including clarithromycin. Consideration of these findings should be balanced with treatment benefits when prescribing clarithromycin.
Reference
- Footnote 13
-
Biaxin BID, Biaxin XL, and Biaxin (clarithromycin) [product monograph]. Etobicoke (ON): BGP Pharma ULC; 2018.
Lamictal (lamotrigine)
The risk of Brugada-type ECG has been included in the Warnings and Precautions and Consumer Information sections of the Canadian product monograph for Lamictal.
Key messages for healthcare professionals:Footnote 14
- Cases of arrhythmogenic ST-T abnormality and typical Brugada ECG pattern have been reported in patients treated with lamotrigine.
- The use of lamotrigine should be carefully considered in patients with Brugada syndrome.
Reference
- Footnote 14
-
Lamictal (lamotrigine) [product monograph]. Mississauga (ON): GlaxoSmithKline Inc.; 2018.
Revlimid (lenalidomide)
The risk of progressive multifocal leukoencephalopathy has been included in the Warnings and Precautions, Adverse Reactions (Post-Market Adverse Drug Reactions), and Consumer Information sections of the Canadian product monograph for Revlimid.
Key messages for healthcare professionals:Footnote 15
- Cases of progressive multifocal leukoencephalopathy (PML), including fatal cases, have been reported with the use of lenalidomide in combination with immunosuppressive therapy including dexamethasone.
- PML should be considered in the differential diagnosis in patients with new or worsening neurological, cognitive or behavioral signs or symptoms and appropriate diagnostic measures for PML are recommended.
- If PML is suspected, further lenalidomide dosing must be suspended until PML has been excluded.
- If PML is confirmed, lenalidomide must be permanently discontinued.
Reference
- Footnote 15
-
Revlimid (lenalidomide) [product monograph]. Mississauga (ON): Celgene Inc.; 2018.
Health Canada News
Medical Device Action Plan
Health Canada has developed an Action Plan to accelerate its efforts to strengthen the regulation of medical devices in Canada. The plan proposes actions to further improve how medical devices get on the market; strengthen monitoring and follow-up for devices already in use; and provide Canadians with more information about the medical devices they rely on.
New Drug and Medical Device Authorizations
Health Canada informs Canadians of new authorizations of drugs and Class IV medical devices via Twitter and LinkedIn using the hashtag #drugandmeddevice. Health Canada will now also provide quarterly updates listing the drugs and medical devices that have been authorized during the previous three months.
Consultation: Regulation of edible cannabis, extracts and topicals
Health Canada has launched a public consultation on draft regulations addressing additional cannabis products, namely edible cannabis, cannabis extracts and cannabis topicals.
The online consultation will be open until February 20, 2019.
Scope
This monthly publication is intended primarily for healthcare professionals and includes information on pharmaceuticals, biologics, medical devices and natural health products. It provides a summary of key health product safety information published in the previous month by Health Canada, as well as a selection of new health product safety information meant to raise awareness. New information contained in this issue is not comprehensive but rather represents a selection of clinically relevant items warranting enhanced dissemination.
Reporting Adverse Reactions
Canada Vigilance Program
Telephone: 1-866-234-2345
Fax or mail: Form available on MedEffect Canada
For more information on how to report an adverse reaction, visit the Adverse Reaction and Medical Device Problem Reporting page.
Helpful links
- MedEffect™ Canada
- Recalls and Safety Alerts Database
- New Safety Reviews
- Canada Vigilance Adverse Reaction Online Database
- Drug Product Database
- Medical Devices Active Licence Listing
- Licensed Natural Health Products Database
- The Drug and Health Product Register
- Drug Shortages Canada
- Annual trends for adverse reaction case reports and medical device problem incidents
Suggestions?
Your comments are important to us. Let us know what you think by reaching us at HC.infowatch-infovigilance.SC@canada.ca
Health Canada
Marketed Health Products Directorate
Address Locator 1906C
Ottawa ON K1A 0K9
Telephone: 613-954-6522
Fax: 613-952-7738
Copyright
© 2019 Her Majesty the Queen in Right of Canada. This publication may be reproduced without permission provided the source is fully acknowledged. The use of this publication for advertising purposes is prohibited. Health Canada does not assume liability for the accuracy or authenticity of the information submitted in case reports.
Adverse reactions (ARs) to health products are considered to be suspicions, as a definite causal association often cannot be determined. Spontaneous reports of ARs cannot be used to estimate the incidence of ARs because ARs remain underreported and patient exposure is unknown.
Due to time constraints relating to the production of this publication, information published may not reflect the most current information.
Endnotes
- Endnote a
-
Glossary of Fields in the Canada Vigilance Adverse Reaction Online Database
- Endnote b
-
Two with unknown reporter type were not included in the graph.