Archived - Health Product InfoWatch – November 2019

Download the alternative format
(PDF format, 715 KB, 8 pages)
Health Products and Food Branch
Marketed Health Products Directorate
Health Product InfoWatch Editorial Team
ISSN: 2368-8025
Cat.: H167-1E-PDF
Pub.: 190000
Contents
- Health products mentioned in this issue
- Monthly recap
- New information
- Health Canada News: Preparing for Mandatory Reporting
- Scope
- Helpful links
- Suggestions?
- Copyright
Health products mentioned in this issue
Pharmaceuticals and Biologics
- Balversa (erdafitinib)
- Bavencio (avelumab)
- EpiPen and EpiPen Jr (epinephrine)
- Lemtrada (alemtuzumab)
- Ranitidine
- Xarelto (rivaroxaban)
Medical Devices
Natural Health Products
Other
Monthly recap of health product safety information
The following is a list of health product advisories, type I recalls as well as summaries of completed safety reviews published in October 2019 by Health Canada.
Avalon Fetal Monitor
This safety review evaluated the risk of inaccurate fetal heart rate (FHR) monitoring associated with the Avalon Fetal Monitor. Health Canada's review of the available information found that inaccurate FHR monitoring is occurring, however, there was insufficient evidence to establish a link between the use of the Avalon Fetal Monitor and the reported incidents. There was no evidence of device malfunction in these incidents. While the Avalon Fetal Monitor Instructions for Use (IFU) contains detailed information regarding the correct use of the device, key instructions are not prominently highlighted. As a result, users may not be aware of certain key steps during the use of the device, which could contribute to inaccurate FHR monitoring. Health Canada has worked with the manufacturer to update the IFU to ensure that important instructions are appropriately highlighted. Health Canada has also communicated this information to healthcare professionals.
Health Professional Risk Communication - Avalon Fetal Monitor
Summary Safety Review - Avalon Fetal Monitor
EpiPen and EpiPen Jr (epinephrine)
In a very small number of cases, some EpiPen (0.3 mg) and EpiPen Jr (0.15 mg) auto-injector devices may not slide out of their carrier tube easily, or at all. The issue is with the carrier tube and not with the device itself or the drug that it delivers. The company has identified the root cause of this issue and has put corrective measures in place. Products are not being recalled at this time because the risk can be easily addressed by consumers and pharmacists checking devices, before an emergency situation arises, to make sure they slide easily out of their carrier tube.
Advisory - EpiPen and EpiPen Jr (epinephrine)
Foreign health products
These foreign health products have been found by regulators in the United States, Singapore, Australia and Hong Kong to contain undeclared drug ingredients, which may pose serious health risks. These products are not authorized for sale in Canada and have not been found in the Canadian marketplace, but it is possible that they may have been brought into the country by travellers or purchased over the Internet.
Lemtrada (alemtuzumab)
Life-threatening and fatal cases have been reported in patients with relapsing remitting multiple sclerosis (MS) receiving Lemtrada (alemtuzumab). This includes reports of autoimmune hepatitis and haemophagocytic lymphohistiocytosis, as well as temporally associated serious cardiovascular reactions. The Canadian product monograph has been updated to include a revised indication for use and safety information.
Health Professional Risk Communication - Lemtrada (alemtuzumab)
Passion X and Passion Fem
All lots of "Passion X" and "Passion Fem" were recalled from retail because they contain undeclared sildenafil. The products are marketed to help support emotional aspects of sexual health. In addition, Health Canada suspended all Pharm Canada Inc.'s sexual health product licences, which means no person or company is permitted to sell these products in Canada.
Advisory - Passion X and Passion Fem
Ranitidine
Health Canada is assessing the issue of an impurity, N-nitrosodimethylamine (NDMA), detected in some ranitidine drugs. Current evidence suggests that NDMA may be present in ranitidine, regardless of the manufacturer. At Health Canada's request, companies marketing ranitidine products in Canada have stopped any further distribution until evidence is provided to demonstrate that they do not contain NDMA above acceptable levels. A table with detailed information on the recalled lots is provided in the information update.
Information Update - Ranitidine
Unauthorized health products
Health Canada advised Canadians about various unauthorized health products being sold at retail locations across Canada or online that may pose serious health risks.
Multiple unauthorized health products
Sexual enhancement, weight loss and sleep aid products from Vikings Nutrition
Soft-shelled hyperbaric chambers
Yunnan Baiyao Toothpaste by LinkGlobal Food Inc.
Xarelto (rivaroxaban)
This safety review evaluated the risk of liver injury associated with Xarelto (rivaroxaban). Following completion of a review in 2015, Health Canada had published an article in the August 2015 issue of the Health Product InfoWatch to summarize the Department's understanding of the risk of liver injury associated with Xarelto use. In 2019, Health Canada reviewed all new available evidence regarding the potential risk of liver injury with Xarelto. Health Canada's review of the available information did not establish a definitive link between the use of Xarelto and the risk of liver injury. Health Canada will continue to monitor safety information involving Xarelto, as it does for all health products on the Canadian market.
Summary Safety Review - Xarelto (rivaroxaban)
New health product safety information
The following topics have been selected to raise awareness and, in some cases, to stimulate reporting of similar adverse reactions.
Vaccine safety biannual summary
Health Canada and the Public Health Agency of Canada (PHAC) share the responsibility for monitoring the safety of vaccines in Canada.
Market authorization holders are required to report serious adverse events following immunization to the Canada Vigilance Program in Health Canada. The Canada Vigilance Program also receives voluntary reports from healthcare professionals and consumers.
Provincial and territorial public health authorities report adverse events following immunization (AEFIs) from publicly-funded vaccine programs to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) in PHAC to monitor the safety of immunization programs.
Report for January 1, 2019 to June 30, 2019
Key Messages:
- No new safety signals (potential safety issues) were identified during this period.
- From January 1, 2019 to June 30, 2019, the Canada Vigilance Program received 594 reportsFootnote 1 of adverse events following immunization for which vaccines were a suspected cause.
This biannual vaccine safety summary includes adverse events following immunization reports received by the Canada Vigilance Program between January 1, 2019 and June 30, 2019. To access reports published by the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS), please visit the CAEFISS Web site.
- From January 1, 2019 to June 30, 2019, the Canada Vigilance Program received 594 reportsFootnote 1 of adverse events following immunization for which vaccines were a suspected cause.
- The largest proportion of the reports was from spontaneous reporting (Figure 1). The source of the reports is presented in Figure 2 (Note: 3 reports with unknown reporter type were not included in the graph).
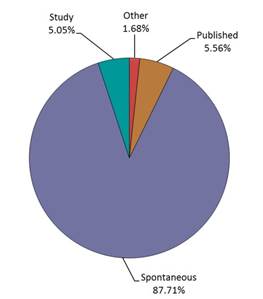
Figure 1: Total number of reports received by report type
| Report Type | Percentage (%) |
|---|---|
| Other | 1.68 |
| Published | 5.56 |
| Spontaneous | 87.71 |
| Study | 5.05 |
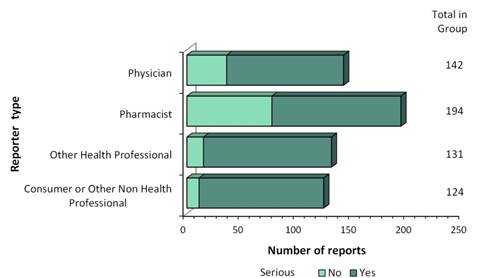
Figure 2: Total number of reports received by type of originating reporter
| Originating reporter | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Consumer Or Other Non Health Professional | 11 | 113 |
| Other Health Professional | 15 | 116 |
| Pharmacist | 77 | 117 |
| Physician | 36 | 106 |
- Most of the reports involved females (Figure 3), and the most common age group was adults from 19 to 64 years of age (Figure 4).
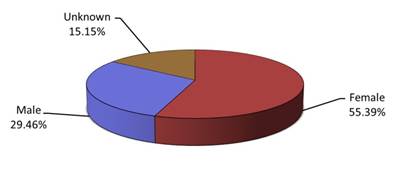
Figure 3: Total number of reports received by gender
| Sex | Percentage (%) |
|---|---|
| Female | 55.39 |
| Male | 29.46 |
| Unknown | 15.15 |
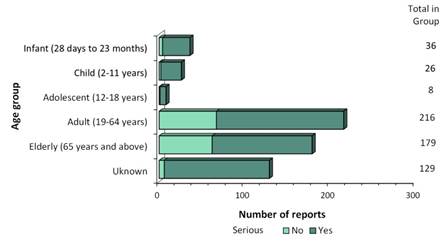
Figure 4: Total number of reports received by age group
| Age | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Infant (28 days to 23 months) | 4 | 32 |
| Child (2-11 years) | 2 | 24 |
| Adolescent (12-18 years) | 1 | 7 |
| Adult (19-64 years) | 67 | 149 |
| Elderly (65 years and above) | 62 | 117 |
| Unknown | 6 | 123 |
- There were 452 (76%) serious reports. Most of these involved patients with underlying medical conditions and the serious adverse events were unlikely related to the vaccination.
- The highest number of reports (serious and non-serious) involved herpes zoster vaccines (306 reports; 52%); followed by pneumococcal vaccines (133 reports; 22%) and influenza vaccines (55 reports; 9%).
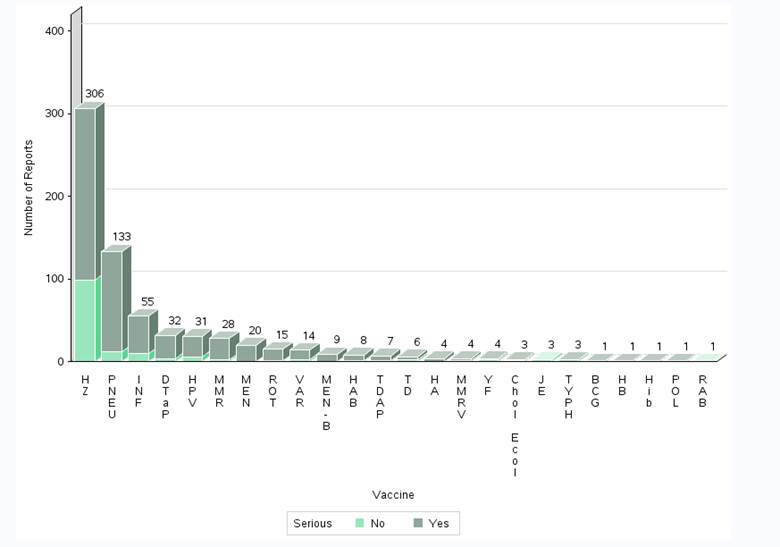
Figure 5: Total number of reports received by vaccine (some reports include multiple vaccines)
| Vaccine Type | Number of non-serious reports | Number of serious reports | |
|---|---|---|---|
| Bacille Calmette-Guérin | BCG | 0 | 1 |
| Cholera and Enterotoxigenic Escherichia Coli Travellers' Diarrhea | Chol Ecol | 1 | 2 |
| Diphtheria, tetanus, acellular pertussis | DTaP | 4 | 28 |
| Hepatitis A | HA | 0 | 4 |
| Hepatitis A, B | HAB | 1 | 7 |
| Hepatitis B | HB | 0 | 1 |
| Haemophilus influenzae type b vaccines | Hib | 0 | 1 |
| Human papillomavirus | HPV | 6 | 25 |
| Herpes zoster | HZ | 99 | 207 |
| Influenza | INF | 10 | 45 |
| Japanese encephalitis | JE | 3 | 0 |
| Meningococcal | MEN | 0 | 20 |
| Meningococcal B | MEN-B | 0 | 9 |
| Measles, mumps, rubella | MMR | 2 | 26 |
| Measles, mumps, rubella, varicella | MMRV | 1 | 3 |
| Pneumococcal | PNEU | 12 | 121 |
| Poliomyelitis | POL | 0 | 1 |
| Rabies | RAB | 1 | 0 |
| Rotavirus | ROT | 1 | 14 |
| Tetanus, diphtheria (reduced) | TD | 2 | 4 |
| Tetanus, diphtheria (reduced), acellular pertussis | TDAP | 1 | 6 |
| Typhoid | TYPH | 2 | 1 |
| Varicella | VAR | 3 | 11 |
| Yellow Fever | YF | 2 | 2 |
- The majority of reports for herpes zoster vaccines (306 reports) were for Shingrix (292 reports). Of these 292 reports for Shingrix, 57 were described as social/digital media cases retrieved by the marketing authorization holder (MAH). None of the social/digital media reports were assessable for the relatedness to the vaccine because of missing information.
- There were 7 reports with an outcome of death. Two were male, 2 were female and 3 were of unknown gender. Age was not provided in 2 of the reports. In the remaining reports, 3 were elderly, 1 was an adult, and 1 was an infant. The reported vaccines were: tetanus, diphtheria [Td] vaccines (2), diphtheria, tetanus, acellular pertussis [DTaP] vaccine (1), human papillomavirus [HPV] vaccine (1), influenza vaccine (1), measles, mumps, rubella, varicella [MMRV] vaccine (1) and pneumococcal vaccine (1). The information provided from these reports was not sufficient to adequately assess the causal association with the vaccine.
- The most frequently reported adverse events (serious and non-serious) included vaccination failure, pyrexia (fever), herpes zoster, headache and pain. These were mostly reported for Shingrix. The adverse events of vaccination failure and herpes zoster were from social/digital media cases submitted by the MAH. None of the social/digital media reports were assessable for the relatedness to the vaccine because of 2 or more of the following missing information: doses received (first or second dose or both), time to onset, pre-existing and concurrent conditions prior to vaccination, medical history, concomitant treatments, corrective treatment, and medical confirmation/test/results of the reported adverse events.
- No new safety signals (potential safety issues) were identified during this period.
- The benefits of vaccines authorized in Canada continue to outweigh the risks.
- Health Canada, in collaboration with the Public Health Agency of Canada (PHAC), will continue to closely monitor the safety of vaccines authorized in Canada.
For additional information, contact the Marketed Health Products Directorate.
Note that because of updated information received by the Canada Vigilance Program, there may be differences in the number of reports and adverse events retrieved at different dates.
Notice of market authorization with conditions
A Notice of Compliance with Conditions (NOC/c) is a form of market authorization with conditions granted to a product on the basis of promising evidence of clinical effectiveness following review of the submission by Health Canada. Communicating a NOC/c is intended to raise awareness on the details of the drug and the nature of authorization granted.
Healthcare professionals are encouraged to report to Health Canada any adverse reactions suspected of being associated with marketed health products, including drugs authorized under the NOC/c policy.
The content of these notices reflects current information at the time of publication. Conditions associated with the NOC/c will remain until they have been fulfilled and authorized by Health Canada, in accordance with the NOC/c Policy. For the most up-to-date information, consult Health Canada's NOC database.
Balversa (erdafitinib): Authorization with conditions
Health Canada has issued a Notice of Compliance, under the Notice of Compliance with Conditions policy for Balversa (erdafitinib), 3 mg, 4 mg, and 5 mg oral tablets. Balversa is indicated for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma:
- whose tumors have susceptible fibroblast growth factor receptor (FGFR)2 or FGFR3 genetic alterations; and
- who have disease progression during or following at least one line of prior chemotherapy, including within 12 months of neoadjuvant or adjuvant chemotherapy.
Patients should be advised about the conditional market authorization for this indication.
For the complete prescribing information and information available for the patients/caregivers, please consult the Balversa Canadian product monograph. The product monograph can be accessed through Health Canada's Drug Product Database, the Janssen Inc. Web site or by contacting Janssen Inc. at 1-800-567-3331 or 1-800-387-8781. Contact the company for a copy of any references, attachments or enclosures.
Bavencio (avelumab): Authorization with conditions
Health Canada has issued a Notice of Compliance, under the Notice of Compliance with Conditions policy, for an expansion of the indication for BavencioFootnote * (avelumab), solution for intravenous infusion, 20 mg/mL single-use vial. The expanded indication for Bavencio is for the treatment of adult patients with metastatic Merkel cell carcinoma (MCC). Patients should be advised about the conditional market authorization for this indication.
For the complete prescribing information and information available for the patients/caregivers, please consult the Bavencio Canadian product monograph. The product monograph can be accessed through Health Canada's Drug Product Database, the EMD Serono Web site or by contacting EMD Serono at 1-888-737-6668. Contact the company for a copy of any references, attachments or enclosures.
Health Canada News: Preparing for Mandatory Reporting
New regulations requiring hospitals to report serious adverse drug reactions (ADRs) and medical device incidents (MDIs) to Health Canada will come into force on December 16, 2019.
Each regulated hospital has been assigned a unique hospital identifier by Health Canada. Institutions that provide their Health Canada Institutional ID will not need to complete the organization address details on the serious ADR and MDI forms. Hospitals can obtain this ID by sending a request to the Canada Vigilance Program, hc.canada.vigilance.sc@canada.ca.
Hospitals will be able to submit reports to Health Canada electronically (e.g., via a secure File Transfer Protocol - sFTP), using online reporting applications, by fax or by mail. The sFTP is an efficient way of securely sending reports to Health Canada. This method supports single or bulk submissions of PDFs, MS Word, MS Excel, and XML formats. For sFTP submissions, hospitals are required to complete a registration process, at which time they will receive additional details on how to use sFTP. To register for sFTP submissions, please send a request to the Canada Vigilance Program, hc.canada.vigilance.sc@canada.ca.
Hospitals may choose to use a third party, such as a regional health authority, other reporting programs or another agent to send reports to Health Canada, on the hospital's behalf. For third party reporting, an agreement between the hospital and the third party would be required in order to authorize the collection of information by the third party on behalf of the hospital and provide reports that meet the legal requirements of the regulations. This agreement would need to be signed by both the hospital and the third party, and be provided to Health Canada.
For more information please see Health Canada's Guidance Document, "Mandatory reporting of serious adverse drug reactions and medical device incidents by hospitals" or Canada.ca/drug-device-reporting.
Scope
This monthly publication is intended primarily for healthcare professionals and includes information on pharmaceuticals, biologics, medical devices and natural health products. It provides a summary of key health product safety information published in the previous month by Health Canada, as well as a selection of new health product safety information meant to raise awareness. New information contained in this issue is not comprehensive but rather represents a selection of clinically relevant items warranting enhanced dissemination.
Reporting Adverse Reactions
Canada Vigilance Program
Telephone: 1-866-234-2345
Fax or mail: Form available on MedEffect Canada
For more information on how to report an adverse reaction, visit the Adverse Reaction and Medical Device Problem Reporting page.
Helpful links
- MedEffect™ Canada
- Recalls and Safety Alerts Database
- New Safety and Effectiveness Reviews
- Canada Vigilance Adverse Reaction Online Database
- Drug Product Database
- Medical Devices Active Licence Listing
- Licensed Natural Health Products Database
- The Drug and Health Product Register
- Drug Shortages Canada
- Annual trends for adverse reaction case reports and medical device problem incidents
- Stop Illegal Marketing of Drugs and Devices
Suggestions?
Your comments are important to us. Let us know what you think by reaching us at HC.infowatch-infovigilance.SC@canada.ca
Health Canada
Marketed Health Products Directorate
Address Locator 1906C
Ottawa ON K1A 0K9
Telephone: 613-954-6522
Fax: 613-952-7738
Copyright
©2019 Her Majesty the Queen in Right of Canada. This publication may be reproduced without permission provided the source is fully acknowledged. The use of this publication for advertising purposes is prohibited. Health Canada does not assume liability for the accuracy or authenticity of the information submitted in case reports.
Adverse reactions (ARs) to health products are considered to be suspicions, as a definite causal association often cannot be determined. Spontaneous reports of ARs cannot be used to estimate the incidence of ARs because ARs remain underreported and patient exposure is unknown.
Due to time constraints relating to the production of this publication, information published may not reflect the most current information.
Footnotes
- Footnote *
-
Bavencio's updated product monograph with this NOC/c indication is dated November 2019.
- Footnote 1
-
Glossary of Fields in the Canada Vigilance Adverse Reaction Online Database