Guidance to market authorization holders on issuing health product risk communications: Roles, responsibilities and process
On this page
- Roles and responsibilities
- Correspondence and submissions
- Process to develop and disseminate health product risk communications
Roles and responsibilities
In Canada, many different organizations and individuals play a role in communicating risks associated with health products.
Shared responsibility
Organizations and individuals involved in communicating risk associated with health products include:
- the MAH, which is responsible for monitoring the continued safe use of its products and communicating new information on the safety of a health product in an effective and timely manner
- Health Canada, which is responsible for authorizing health products for sale in Canada, coordinating and monitoring the continued safety of health products on the market and communicating relevant information
- provincial licensing authorities, which set the standards of practice for health professionals and function as a trusted source of safety information for its members
- health care professionals (for example, physicians, pharmacists, nurses, naturopaths), who prescribe, dispense and provide information about treatments to their patients, as well as monitor and report adverse reactions
- patients, who, with guidance from their health professionals and other resources, make informed decisions about their treatment while monitoring and reporting any adverse reactions they may experience
This document focuses on the role of MAHs and Health Canada, who are jointly responsible for developing and disseminating HPRCs.
Market authorization holder (MAH)
An MAH has a responsibility to inform Health Canada if it becomes aware of the need to issue an HPRC, as a result of new or emerging risk information either in Canada or internationally. Prior to the HPRC development process, the MAH should engage in discussions with the appropriate lead directorate within Health Canada to confirm the need for an HPRC. These discussions help to ensure that both the MAH and Health Canada have:
- a clear understanding of the risk and the risk mitigation strategy
- an agreement that the HPRC is the ideal risk communication tool to convey information about the risk
When Health Canada and the MAH agree upon the need for an HPRC, the MAH is expected to take a leading role in the development and dissemination of the HPRC.
Key MAH activities include:
- preparing the first draft of the HPRC
- developing a dissemination strategy in collaboration with Health Canada
- collaborating with Health Canada on the review and approval of the HPRC within agreed timelines
Additional details about these activities are found in the process to develop and disseminate health product risk communications.
Health Canada
Health Canada is responsible for ensuring that HPRCs are developed in accordance with the guidelines and procedures set out in this document. This is to ensure that HPRCs are developed consistently and accurately, and are disseminated to appropriate audiences in a timely manner.
Key Health Canada activities include:
- coordinating the overall HPRC development process
- reviewing, editing and approving HPRCs and dissemination strategies
- supporting dissemination efforts
Additional details about these activities are found in the process to develop and disseminate health product risk communications.
Health Canada may also independently identify a risk it determines warrants an HPRC. In this case, Health Canada will request that an MAH undertake the required activities to issue an HPRC. If the MAH receives such a request from Health Canada and doesn't believe that an HPRC is necessary, the MAH should provide a rationale for this decision to Health Canada in writing.
Health Canada and the MAH will take action to resolve an impasse on a case-by-case basis, taking into consideration the significance and urgency of the issue. Health Canada and the MAH will seek to resolve the impasse on the premise that actions are transparent, fair and appropriate to the risk. Ultimately, the responsibility and authority for resolving impasses rests with Health Canada and, if required, Health Canada will independently develop and disseminate the HPRC.
Issuance by more than 1 MAH
In the simplest scenario, the risk to be communicated will be linked to a single health product marketed by a single MAH. However, there may be situations in which more than 1 product and MAH is implicated, such as when:
- the risk is linked to an ingredient or component found in several types of products
- the risk associated with 1 product is due to an interaction with another type of product
- there is a risk associated with 1 or more groups of products
- a product has both an innovator and generic companies that market the product in Canada
One of the primary objectives of the HPRC tool is to ensure clear and effective messaging. The issuance of multiple HPRCs from different MAHs on the same issue may lead to confusion or message fatigue among health care professionals.
Where possible, MAHs should work together to issue a single, joint HPRC. MAHs should share any costs associated with developing and disseminating the HPRC. When MAHs can't agree to issue a single communication, Health Canada may develop and disseminate the HPRC.
Correspondence and submissions
MAHs must work closely with Health Canada throughout the HPRC development process. This involves the submission of key documents and routine correspondence.
Correspondence
HPRCs are coordinated through a centralized process at Health Canada. The Marketed Health Product Directorate's Risk Communication Section (RCS) administers this process. Once Health Canada's lead directorate and the MAH have decided to issue an HPRC, MAHs should address all HPRC-related correspondence to the risk communications project manager. The risk communications project manager can be reached by email at riskcommunications-communicationdesrisques@hc-sc.gc.ca.
MAHs will also need to designate an individual (for example, director of regulatory affairs or regulatory affairs manager) to serve as the company contact for all HPRC-related activities. Designated individuals are welcome to correspond with RCS in either official language.
Meetings between an MAH and Health Canada aren't typically required during the HPRC development process. A meeting may be scheduled in cases where complex issues arise.
Submissions
RCS will request key documents from the MAH at progressive stages of the HPRC development and dissemination process. In general, these documents include:
- the first draft of the HPRC and an accompanying dissemination strategy
- subsequent draft HPRCs generated as a result of the editorial process
- a translated version of the Health Canada-approved HPRC
- MAH signed and dated copies of the final HPRC (in both official languages)
- notification of dissemination
Additional details about these documents are found in the process to develop and disseminate health product risk communications and the submission checklist for MAHs.
MAHs should submit all documents to the risk communications project manager via email. Documents that may require further editing, such as draft HPRCs, should be submitted in an editable file format (for example, Microsoft Word).
MAHs should submit draft and final HPRCs to Health Canada in Electronic Common Technical Document (eCTD) format.
Process to develop and disseminate health product risk communications
The development and dissemination of an HPRC follows a standardized process with 3 major phases:
- request and submission
- review and approval (including translation and verification of translation)
- dissemination, notification and evaluation
HPRCs may be subject to different timelines depending on the level of urgency associated with the risk. Despite varied timelines, the phases of HPRC development and their associated activities will generally remain consistent. Health Canada may consider deviations from the standardized process on a case-by-case basis.
The MAH and lead directorate should determine a timeline for a particular HPRC prior to initiating the HPRC process.
Process overview
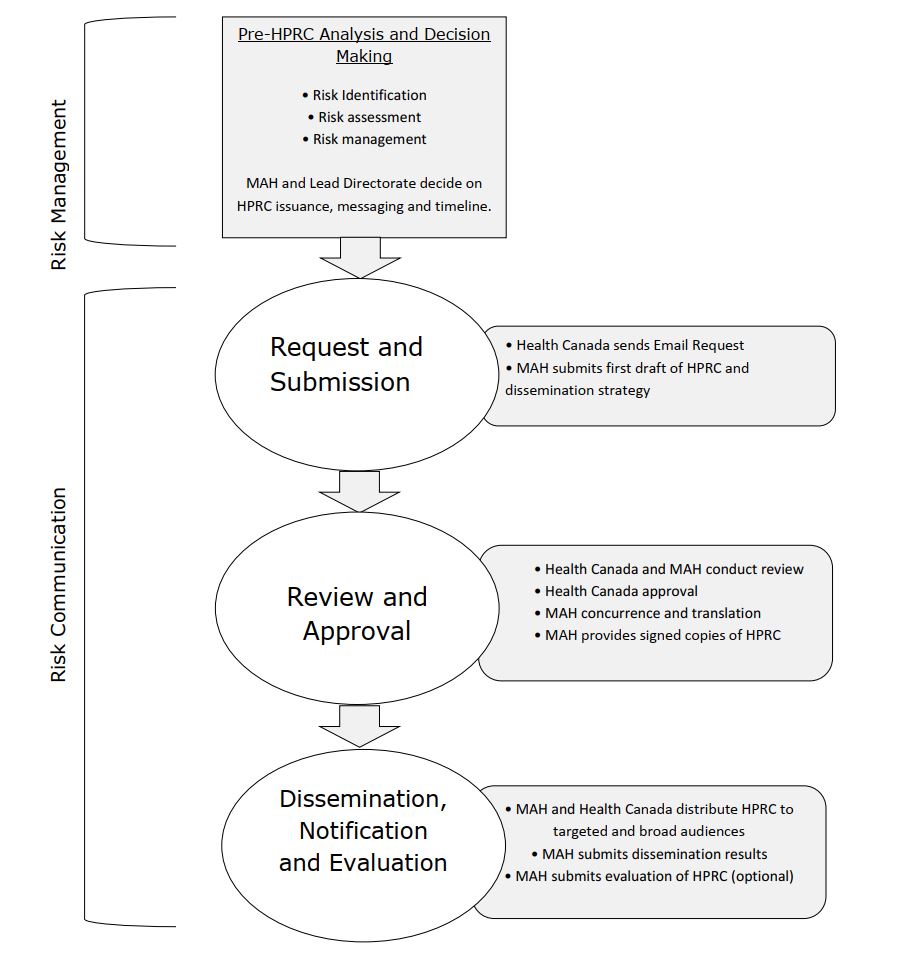
Figure 1 - Text description
This diagram illustrates the HPRC development and dissemination process.
Before the HPRC process can begin, the MAH and the lead directorate within Health Canada must first identify the risk issue, assess the risk and decide on an appropriate risk mitigation strategy. If the MAH and lead directorate decide that an HPRC is needed as part of this strategy, they should also determine what key messages should be included in the HPRC and when to issue the HPRC. These activities and decisions must be completed or taken prior to initiating the HPRC process.
The first part of the HPRC process is the "request and submission" phase. This phase begins with Health Canada requesting key documents from the MAH, including the first draft of the HPRC and the dissemination strategy, and ends with the submission of these documents.
The second part of the HPRC process is the "review and approval" phase. Health Canada and the MAH review the HPRC and dissemination strategy until both parties agree with the final drafts. The MAH is responsible for translating the HPRC and providing signed copies of the final HPRC to Health Canada.
The third part of the HPRC process is the "dissemination, notification and evaluation" phase. The MAH and Health Canada distribute the HPRC to targeted and broad audiences. The MAH must also provide the department with the results of their dissemination efforts within 14 days of issuing the HPRC. MAHs are also encouraged to evaluate the effectiveness of the HPRC and submit their results to the department.
Phase 1: Request and submission
Phase 1 of the process involves the submission of key documents by the MAH following a request from RCS.
Email request
Once the MAH and the lead directorate have identified and agreed upon the need for an HPRC, the RCS will send the MAH an email request. This correspondence represents Health Canada's formal request to the MAH to begin drafting the HPRC. The email request will also include other important information for the MAH, including a summary of key deliverables and expected timelines for developing and disseminating the HPRC.
Initial submission
The MAH's initial HPRC submission to the RCS should include:
- the first draft of the HPRC
- any graphics (for example, images, figures) to be included in the HPRC
- copies of any medical or scientific literature referenced in the HPRC
- copies of any risk communications issued within Canada or foreign jurisdictions that are related to the issue
- the dissemination strategy
The MAH should submit these documents on the date specified by the RCS in the email request.
Standard format and content of a health product risk communication
MAHs must develop HPRCs using the standardized HPRC template. This template organizes information under specific headings in order to facilitate clear and consistent messaging. A guide for using the standardized HPRC template provides step-by-step instructions.
In addition to the instructions supplied in the guide, HPRCs should also adhere to the following formatting guidelines:
- the HPRC should generally not exceed 3 pages in either official language
- True Type (TT) Verdana (11 pt) font should be used
- text should be left justified and the document should be paginated on the bottom right of the page
- underlining and indenting should not be used
- a "draft" watermark, the date (mm/dd/yyyy) and version number should be included
- the MAH logo should be inserted at the beginning of the HPRC
- for HPRCs intended for hospitals and other health care facilities, this text should be included before the subject line:
"Please distribute to relevant departments [Surgery, Emergency Medicine, Pharmacy, Paediatrics, Anaesthesia, Geriatrics, Internal Medicine, Nursing, Dentistry, Intensive Care and/or other departments as required], and other involved professional staff and post this notice in your institution."
When drafting an HPRC, MAHs should bear in mind the purpose of the risk communication and ensure that messages about the risk are linked to the goals of the HPRC (for example, raise awareness, encourage a change in behaviour). Recognizing that health care professionals are the target audience, HPRCs should aim to provide details about the product(s) affected and the potential clinical impact of the risk, and include action-oriented statements that support the objective of the communication.
Some risk issues may be highly technical or complex in nature. To ensure effective messaging, MAHs should consider soliciting input, as needed when drafting an HPRC, from implicated stakeholders such as specialists and other subject matter experts
Although HPRCs are targeted to health care professionals, the standardized HPRC template includes a section dedicated to the general public. This section should contain easy-to-understand information on the health product and the related risk. It should also contain practical information on what a patient or consumer can do about the risk, in consideration of social, cultural, economic and other important factors that may influence public risk perception.
MAHs shouldn't use the HPRC template for risk communications that haven't been approved by Health Canada as part of the HPRC development process (that is, company-issued risk communications).
Phase 2: Review and approval
Phase 2 of the process involves the review and approval of the HPRC, including translation.
Review
The RCS conducts Health Canada's review of the draft HPRC and dissemination strategy in collaboration with subject matter experts from the lead directorate. The purpose of this review is to ensure:
- accuracy, clarity and consistency of the HPRC content and format
- acceptability of methodology and recipient selection of the dissemination strategy
Following Health Canada's review, RCS will provide the MAH with proposed revisions for review within expected timelines.
The MAH is expected to review these revisions in a timely manner and either concur or recommend additional changes. All proposed revisions should be incorporated as annotated text within updated versions of the documents.
Given the inherent urgency associated with HPRCs, the review process has been designed to limit the number of iterations generated between Health Canada and the MAH. Generally, 1 round of review per party (before the approval phase) is sufficient. The efficiency of this process depends heavily on the concurrence (of intended messaging, timing and so on) established between the lead directorate and the MAH prior to initiating the HPRC process.
Health Canada approval and MAH concurrence
The RCS will initiate the Health Canada approval process after the review process has been completed. Health Canada may propose additional revisions to the HPRC and dissemination strategy during this stage. The RCS will notify the MAH in writing when Health Canada has approved the HPRC and dissemination strategy. At that time, Health Canada will:
- seek concurrence from the MAH on the approved documents (if changes have been made)
- request that the MAH translate the final HPRC into the other official language
Translation
MAHs are responsible for translating the HPRC in both official languages. The MAH should use the HPRC bilingual template to generate translated versions. The MAH should provide the translated HPRC to the RCS within designated timelines. The RCS will review the translation to ensure consistency between the different language versions.
MAH signed copies
The RCS will request signed and dated copies of the final HPRC in both official languages from the MAH upon completing the translation. MAHs should provide signed documents in PDF format.
Phase 3: Dissemination, notification and evaluation
Phase 3 of the process involves disseminating the HPRC, notifying that the HPRC has been disseminated and evaluating the effectiveness of the HPRC.
Dissemination
MAHs are responsible for ensuring that new and emerging information related to their marketed health product is communicated to health care professionals and the public in an effective and timely manner. This responsibility extends to the dissemination of an HPRC.
At the time of submitting the initial draft HPRC, MAHs should provide a draft dissemination strategy for Health Canada review and approval. An effective strategy should consider:
- the goal of the HPRC
- impacted persons (such as specific health professional groups, health care facilities)
- different distribution channels and their expected reach
An effective strategy should also attempt to leverage the distribution capacity of existing health care professional networks.
At a minimum, a dissemination strategy should include:
- a proposed distribution list of targeted groups (for example, health care professionals, health care professional associations and regulatory bodies, hospitals, other health care facilities)
- the proposed methods of dissemination (for example, email, fax, mail, web-posting, publication in journals and/or newsletters)
In addition to targeted methods of dissemination, Health Canada recommends (as a matter of transparency and continued accessibility) that MAHs post the HPRC on their company website.
Health Canada will supplement the MAH's dissemination efforts using broader distribution mechanisms. These mechanisms include:
- posting the HPRC on the Recalls and Safety Alerts Database
- sending a mass email notification to subscribers of the MedEffect™ e-Notice email notification system
- posting through MedEffect™ Canada RSS Feeds and on social media channels such as LinkedIn and Twitter
Table 1 shows a summary of HPRC dissemination methods typically used by MAHs and Health Canada.
Health Canada will also share the approved HPRC with foreign regulatory authorities. At a minimum, Health Canada will notify countries and organizations with which it maintains formal and regular communication, such as:
- the founding members of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use:
- Japan
- European Union
- United States of America
- the Forum for the Harmonization of Herbal Medicines countries:
- Japan
- China
- Vietnam
- Australia
- Singapore
- Hong Kong
- South Korea
On the pre-selected date of issuance, the MAH should disseminate the HPRC in both official languages to the target audiences identified in the approved dissemination strategy. Health Canada will concurrently disseminate the HPRC through its standard mechanisms.
| Disseminating organization | General approach | Typical dissemination methods |
|---|---|---|
| MAH | targeted dissemination |
|
| Health Canada | broad dissemination |
|
Notification
MAHs must provide Health Canada with a list of targeted groups to whom the HPRC was issued, including the date of issuance and the methods of distribution that were used, within 14 days of issuing the HPRC. This list should also identify the number of recipients successfully reached.
Where Health Canada isn't satisfied with the degree of distribution, we will send a written request to the MAH to correct the situation.
Evaluating the effectiveness of the HPRC
MAHs can contribute to the continuous improvement of risk communications by evaluating the effectiveness of its risk communication efforts. To determine whether an HPRC has produced its intended effect, Health Canada encourages MAHs to conduct routine evaluations of its HPRCs in order to assess the effectiveness of its reach, comprehension and usefulness. For HPRCs that are intended to modify behaviour, these evaluations should also ideally examine the extent to which behaviour was changed as a result of the recommendations in the HPRC.
Page details
- Date modified: