Overview of the licence application process: Cultivation, processing and sale for medical purposes licence
On this page
- 1.0 Who is this for
- 2.0 Diagram of licence application process
- 3.0 Licence application checklists
- 4.0 Contact us
- 5.0 Foreword and disclaimer
1.0 Who is this for
Use the content in these pages to apply for the following Health Canada cannabis licences:
- micro-cultivation, nursery and standard cultivation
- micro-processing and standard processing
- sale for medical purposes
These pages don't include all types of cannabis licences. For other licences, refer to:
- Application requirements for a cannabis analytical testing licence
- Application requirements for a cannabis research licence
- Application for a cannabis drug licence
- Industrial hemp licensing application guide
Important: For micro-cultivation, nursery and micro-processing licences
What you need to submit in the Cannabis Tracking and Licensing System (CTLS) for micro-cultivation, nursery and micro-processing licences is mostly the same as for standard cultivation, standard processing and sale for medical purposes with possession of cannabis licences. However, your site evidence package will be different. The main difference is the physical security requirements you have to meet.
2.0 Diagram of licence application process
Important: You can only start your cannabis activities after you've received your licence from Health Canada. Even after the issuance of your licence, your activities can be restricted.
When starting the application process, Before you start applying for a licence has more information about what you should consider before applying. It may also be helpful to use the applicable checklists that can guide you through this process.
Figure 1 provides an overview of the general licence application process and the things you need to do as an applicant.
When applying only for a sale for medical purposes without possession of cannabis licence, you don't need to:
- send notices to local authorities
- submit a site evidence package
Indigenous affiliated applicants are able to ask for a 2-stage review that lets you start applying without a fully built site.
Self-identify as an Indigenous affiliated applicant
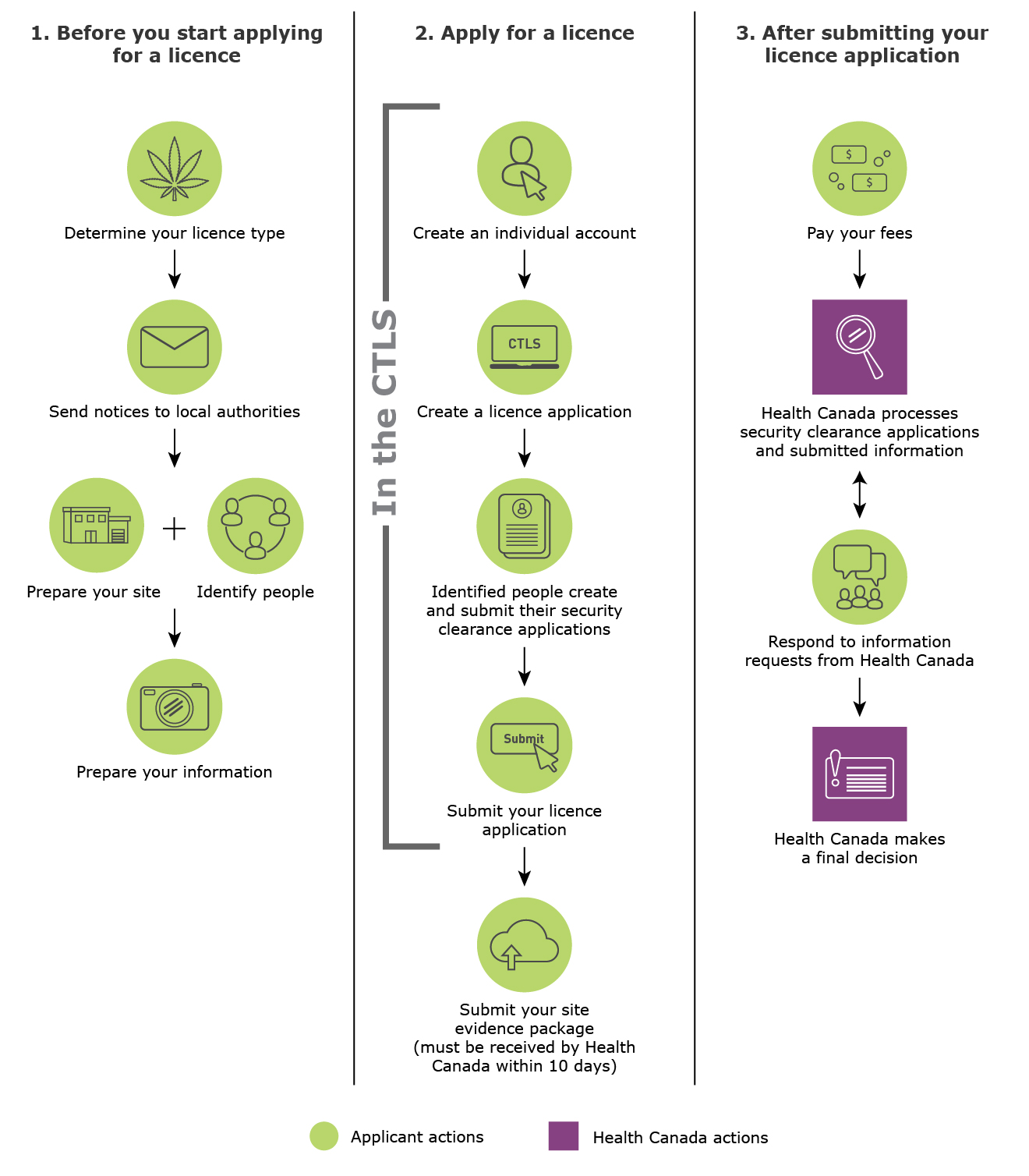
Figure 1 - Text description
- The key steps you need to do before you start applying for a licence
- Determine your licence type
- Send notices to local authorities
- Prepare your site and identify people
- Prepare your information
- The key steps you need to do when you apply for a licence
- Create an individual account in the CTLS
- Create a licence application in the CTLS
- Identified people create and submit their security clearance applications in the CTLS
- Submit your licence application in the CTLS
- Submit your site evidence package within 10 days after submitting your application in the CTLS
- The key steps after you've submitted your licence application
- You need to pay your fees
- Health Canada processes security clearance applications and submitted information
- You need to respond to information requests from Health Canada
- Health Canada makes a decision on your licence application
- The key steps you need to do before you start applying for a licence
- Determine your licence type
- Send notices to local authorities
- Prepare your site and identify people
- Prepare your information
- The key steps you need to do when you apply for a licence
- Create an individual account in the CTLS
- Create a licence application in the CTLS
- Identified people create and submit their security clearance applications in the CTLS
- Submit your licence application in the CTLS
- Submit your site evidence package within 10 days after submitting your application in the CTLS
- The key steps after you've submitted your licence application
- You need to pay your fees
- Health Canada processes security clearance applications and submitted information
- You need to respond to information requests from Health Canada
- Health Canada makes a decision on your licence application
3.0 Licence application checklists
The checklists are tools that can guide you through the licence application process. The checklists give a summary of the key tasks:
- before you start applying for a licence
- when you're applying for a licence
- after you've submitted your licence application
The checklists link to the sections in these web pages where you can find more information. The checklists are provided in the web page format and in a printable PDF format. The printable PDF format includes the suggested naming convention for each document listed in the checklist.
Important: Health Canada recommends using a document naming convention for all the information you have to submit. We can process your application more efficiently when you follow this document naming convention. It generally follows this layout: "MainCategory"_APP-#_"SubCategory"_"YYYY-MM-DD"."PDF".
- MainCategory: Main category of the information type
- APP-#: Your Licence application ID, which is available after you've created a licence application in the CTLS
- SubCategory: Sub-category of the information type
- YYYY-MM-DD: The date (year-month-date) of when your document was created
- PDF: Document file type
- For your corporate profile, if applicable:
- "CompanyName"_"DocumentCategory"_"YYYY-MM-DD"."PDF"
- CompanyName: Name of your corporation, cooperative or partnership
- DocumentCategory: Category of the information type
- YYYY-MM-DD: The date (year-month-date) of when your document was created
- PDF: Document file type
- "CompanyName"_"DocumentCategory"_"YYYY-MM-DD"."PDF"
3.1 Checklist: Applying for cultivation, processing and sale for medical purposes with possession of cannabis licences
Use this checklist if you're applying for:
- micro-cultivation licence
- nursery licence
- micro-processing licence
- standard cultivation licence
- standard processing licence
- sale for medical purposes with possession of cannabis licence
For more information on the types of cannabis licences, refer to Types of cannabis and industrial hemp licences.
View printable version of the checklist (PDF)
Section 1: Before you apply
- Determine which licence types to apply for
- Familiarize yourself with the legislation
- If you intend to self-identify as an Indigenous or Indigenous-affiliated applicant, you may want to contact the Indigenous Navigator Service at navigator-navigateur@hc-sc.gc.ca for additional guidance on the application process
- Know your fees
- Application-screening fee
- Security clearance application fees
- Annual regulatory fee
- Send notices to your local authorities
- Local police force or the Royal Canadian Mounted Police detachment:
- Prepare a written notice, or fill out the Notices to local authorities template
- Send the written notice
- Gather contact information
- Local fire authority
- Prepare a written notice, or fill out the Notices to local authorities template
- Send the written notice
- Gather contact information
- Local government
- Prepare a written notice, or fill out the Notices to local authorities template
- Send the written notice
- Gather contact information
- Local police force or the Royal Canadian Mounted Police detachment:
- Prepare your site
- Identify the following people
- Key site personnel
- People with direct control, if applicable
- Associated individuals, if applicable
- All identified people need to create their own CTLS account and give you their CTLS account ID
Proposed document naming conventions
- Sending notices to your local authorities
- Local police force or the Royal Canadian Mounted Police detachment: "NoticePolice_APP-#_YYYY-MM-DD.PDF"
- Local fire authority: "NoticeFire_APP-#_YYYY-MM-DD.PDF"
- Local government: "NoticeGovernment_APP-#_YYYY-MM-DD.PDF"
Section 2: Information to prepare
Part A: Information to prepare to submit into the CTLS
- For corporations, cooperatives or partnerships
- If you're a corporation: Copy of your certificate of incorporation, amalgamation or amendment
- If you're a cooperative or a partnership: Copy of your business name registration or partnership agreement
- If you want to use another name besides your legal business name, you can request that the other name appear on the licence. For example, Legal corporation name d.b.a. Other name. Some provinces and territories may require that this other name be registered. It is your responsibility to consult the registry of the jurisdiction where you plan to do business on the requirements for registration
- Site details
- Health Canada licences, registrations and authorizations
- Aerial view
- If your site has multiple addresses, document listing all addresses
- Identified people
- Associated individuals, if applicable:
- Associated individuals requiring security clearances
-
For micro-processing and standard processing licences: Quality assurance person (QAP). Also, 1 or more alternate QAPs (AQAP), if you plan to have any
- Qualifications of the proposed QAP or alternate form, which will indicate whether a resume or curriculum vitae (CV), copy of degrees or diplomas, or other supporting documents are required
- Additional information, if there isn't enough space in the Qualifications of the proposed QAP or alternate form
- Associated individuals, if applicable:
- Site ownership
- If other individuals or organizations own the site: Site owner information and consent for each site owner
- Organizational security plan
- Business overview
- Template or primary document
- People requiring a security clearance
- Head of security
- Organizational chart
- Descriptions of roles and responsibilities
- Descriptions of standard operating procedures (SOPs)
- Attestation
- Good production practices
- Good production practices report
- For micro-processing and standard processing licences: Good production practices attestation
- Record keeping
- Record keeping attestation
- For sale for medical purposes licences: Proposed record keeping methods
- Record keeping methods
- Medical document verification
- Example of your registration document
- Client's orders and refusal to fill client's order example
- Process to limit quantities of 150 g of dried cannabis (or its equivalent amount) per order
-
Key investor report (choose 1 of the 2 options):
- a Key investor report
- a Key investor attestation
- For micro-cultivation, nursery and standard cultivation licences: Source of starting material
- If you're not sourcing solely from an authorized source: Declaration under subsection 10(2) of the Regulations
Part B: Information to prepare for the site evidence package
- For all licences
- Site plan
- Floor plans
- Description of physical barrier and site design
- Description of restricted access measures
- Guided video tour
- For micro-cultivation, nursery and micro-processing licences
- Physical security measures attestation for micro-cultivation, nursery and micro-processing licences
- For standard cultivation, standard processing and sale for medical purposes with possession of cannabis licences
- Visual monitoring devices detail
- Intrusion detection devices detail
- Physical security measures attestation for standard cultivation, standard processing and sale for medical purposes with possession licences
- For standard cultivation licences
- Security devices for outdoor areas, if applicable
Proposed document naming conventions
Part A: Information to prepare to submit into the CTLS
- For corporations, cooperatives or partnerships
- Certificate of incorporation: "CompanyName_Certificate-of-Incorporation_YYYY-MM-DD.PDF"
- Certificate of amalgamation: "CompanyName_Certificate-of-Amalgamation_YYYY-MM-DD.PDF"
- Certificate of amendment: "CompanyName_Certificate-of-Amendment_YYYY-MM-DD.PDF"
- Business name registration: "CompanyName_BusinessNameRegistration_YYYY-MM-DD.PDF"
- Partnership agreement: "CompanyName_PartnershipAgreement_YYYY-MM-DD.PDF"
- Site details
- Health Canada licences, registrations and authorizations: "LicencesRegistrationsAuthorizations_APP-#_YYYY-MM-DD.PDF"
- Aerial view: "AerialView_APP-#_YYYY-MM-DD.PDF"
- If your site has multiple addresses, document listing all addresses: "SiteAddresses_APP-#_YYYY-MM-DD.PDF"
- Identified people
- Associated individuals, if applicable: Associated individuals requiring security clearances: "People_APP-#_AssociatedIndividuals_YYYY-MM-DD.PDF"
- For micro-processing and standard processing licences: Quality assurance person (QAP) and 1 or more alternate QAP (AQAP), if applicable
- Qualifications of the proposed QAP or alternate form: "QAP_APP-#_Form_YYYY-MM-DD.PDF" or "AQAP-APP-#_Form_YYYY-MM-DD.PDF"
- Proposed QAP's or AQAP's resume or curriculum vitae (CV): "QAP_APP-#_CVResume_YYYY-MM-DD.PDF" or "AQAP_APP-#_CVResume_YYYY-MM-DD.PDF"
- Proposed QAP's or AQAP's copies of degree or diploma supporting formal education relevant to QAP responsibilities: "QAP_APP-#_Education_YYYY-MM-DD.PDF" or "AQAP_APP-#_Education_YYYY-MM-DD.PDF"
- Supporting documents for QAP or AQAP: "QAP_APP-#_SupportingDocs_YYYY-MM-DD.PDF" or "AQAP_APP-#_SupportingDocs_YYYY-MM-DD.PDF"
- Additional information: "QAP_ APP-#_Qualifications_YYYY-MM-DD.PDF" or "AQAP_ APP-#_Qualifications_YYYY-MM-DD.PDF"
- Site ownership
- If other individuals or organizations own the site: Site owner information and consent for each site owner: "SiteOwnership_APP-# _YYYY-MM-DD.PDF"
- Organizational security plan
- Business overview: "OSP_APP-#_BusinessOverview_YYYY-MM-DD-PDF"
- Template or primary document: "OSP_APP-#_YYYY-MM-DD.PDF"
- People requiring a security clearance: "OSP_APP-#_PeopleSC_YYYY-MM-DD.PDF"
- Head of security: "OSP_APP-#_HeadOfSecurity_YYYY-MM-DD.PDF"
- Organizational chart: "OSP_APP-#_OrganizationalChart_YYYY-MM-DD.PDF"
- Descriptions of roles and responsibilities: "OSP_APP-# _DescriptionsRoles_YYYY-MM-DD.PDF"
- Descriptions of standard operating procedures (SOPs): "OSP_APP-#_SOPs_YYYY-MM-DD.PDF"
- Attestation: "OSP_APP-#_Attestation_YYYY-MM-DD.PDF"
- Good production practices
- Good production practices report: "GPP_APP-#_Report or Section_YYYY-MM-DD.PDF"
- For micro-processing and standard processing licences: Good production practices attestation: "GPP_APP-#_Attestation_YYYY-MM-DD.PDF"
- Record keeping
- Record keeping attestation: "RK_APP-#_Attestation_YYYY-MM-DD.PDF"
- For sale for medical purposes licences: Proposed record keeping methods
- Record keeping methods: "RK _APP-#_SmpMethods_YYYY-MM-DD.PDF"
- Medical document verification: "RK _APP-#_SmpVerification_YYYY-MM-DD.PDF"
- Example of your registration document: "RK _APP-#_SmpRegistration_YYYY-MM-DD.PDF"
- Client's orders and refusal to fill client's order example: "RK_APP-#_SmpOrders_YYYY-MM-DD.PDF"
- Process to limit quantities of 150 g of dried cannabis (or its equivalent amount) per order: "RK _APP-#_Smp150g_YYYY-MM-DD.PDF"
- Key investor report (choose 1 of the 2 options)
- Key investor report: "KeyInvestor_APP-#_Report _YYYY-MM-DD.PDF"
- Key investor attestation: "KeyInvestor_APP-#_Attestation _YYYY-MM-DD.PDF"
- For micro-cultivation, nursery and standard cultivation licences: Source of starting material
- If you're not sourcing solely from an authorized source: Declaration under subsection 10(2) of the Regulations: "Declaration_10-2_APP-# _YYYY-MM-DD.PDF"
Part B: Information to prepare for the site evidence package
- For all licences
- Site plan: "SitePlan_APP-#_YYYY-MM-DD.PDF"
- Floor plans: "FloorPlan_APP-#_YYYY-MM-DD.PDF"
- Description of physical barrier and site design: "BarrierDesign_APP-#_YYYY-MM-DD.PDF"
- Description of restricted access measures: "RestrictedAccess_APP-#_YYYY-MM-DD.PDF"
- Guided video tour: "Video_APP-#_YYYY-MM-DD.MP4"
- For micro-cultivation, nursery and micro-processing licences
- Physical security measures attestation for micro-cultivation, nursery and micro-processing licences: "PSM_Attestation_APP-#_YYYY-MM-DD.PDF"
- For standard cultivation, standard processing and sale for medical purposes with possession of cannabis licences
- Visual monitoring devices detail: "VisualMonitoring_APP-#_YYYY-MM-DD.PDF"
- Intrusion detection devices detail: "IntrusionDetection_APP-#_YYYY-MM-DD.PDF"
- Physical security measures attestation for standard cultivation, standard processing and sale for medical purposes with possession licences: "PSM_Attestation_APP-#_YYYY-MM-DD.PDF"
- For standard cultivation licences
- Security devices for outdoor areas detail, if applicable: "OutdoorSecurity_APP-#_YYYY-MM-DD.PDF"
Section 3: Create a licence application
- Create an account in the CTLS, if you don't have one already
- If you're applying as a part of a corporation, a cooperative, or a partnership, create a corporate profile, if you don't already have one
- Create a new licence application in the CTLS
Section 4: Submit your information
Part A: Submit your security clearance applications in the CTLS
- Identified people to submit their security clearance applications, if applicable, no more than 1 month before you submit your licence application
Part B: Submit your information in the CTLS
- Submit your information into the CTLS
Part C: Submit your site evidence package
- Submit your site evidence package (must be received by Health Canada within 10 business days of the licence submission in the CTLS)
Section 5: After you've submitted your licence application
- Pay fees (licence application-screening fee, security clearance application fees, if applicable)
3.2 Checklist: Applying for a sale for medical purposes without possession of cannabis licence
View printable version of the checklist (PDF)
Section 1: Before you apply
- Determine which licence types to apply for
- Familiarize yourself with the legislation
- If you intend to self-identify as an Indigenous or Indigenous-affiliated applicant, you may want to contact the Indigenous Navigator Service at navigator-navigateur@hc-sc.gc.ca for additional guidance on the application process
- Know your fees
- Application-screening fee
- Security clearance application fees
- Annual regulatory fee
- Prepare your site
- Identify the following people
- Key site personnel
- People with direct control, if applicable
- Associated individuals, if applicable
- All identified people need to create their own CTLS account and give you their CTLS account ID
Section 2: Information to prepare
Information to prepare to submit into the CTLS
- For corporations, cooperatives or partnerships
- If you're a corporation: Copy of your certificate of incorporation, amalgamation or amendment
- If you're a cooperative or a partnership: Copy of your business name registration or partnership agreement
- If you want to use another name besides your legal business name, you can request that the other name appear on the licence. For example, Legal corporation name d.b.a. Other name. Some provinces and territories may require that this other name be registered. It is your responsibility to consult the registry of the jurisdiction where you plan to do business on the requirements for registration
- Site details
- Health Canada licences, registrations and authorizations
- Aerial view
- If your site has multiple addresses, document listing all addresses
- Identified people
- Associated individuals, if applicable: For associated individuals requiring security clearances
- Organizational security plan
- Business overview
- Template or primary document
- People requiring a security clearance
- Head of security
- Organizational chart
- Descriptions of roles and responsibilities
- Descriptions of standard operating procedures (SOPs)
- Attestation
- Site plan
- Floor plan
- Record keeping
- Record keeping attestation
- Proposed record keeping methods
- Record keeping methods
- Medical document verification
- Example of your registration document
- Client's orders and refusal to fill client's order example
- Process to limit quantities of 150 g of dried cannabis (or its equivalent amount) per order
- Key investor report (choose 1 of the 2 options):
- a Key investor report
- a Key investor attestation
Proposed document naming conventions
- For corporations, cooperatives or partnerships
- Certificate of incorporation: "CompanyName_Certificate-of-Incorporation _YYYY-MM-DD.PDF"
- Certificate of amalgamation: "CompanyName_Certificate-of-Amalgamation _YYYY-MM-DD.PDF"
- Certificate of amendment: "CompanyName_Certificate-of-Amendment_YYYY-MM-DD.PDF"
- Business name registration: "CompanyName_BusinessNameRegistration_YYYY-MM-DD.PDF"
- Partnership agreement: "CompanyName_PartnershipAgreement_YYYY-MM-DD.PDF"
- Site details
- Health Canada licences, registrations and authorizations: "LicencesRegistrationsAuthorizations_APP-#_YYYY-MM-DD.PDF"
- Aerial view: "AerialView_APP-#_YYYY-MM-DD.PDF"
- Document listing all addresses: "SiteAddresses_APP-#_YYYY-MM-DD.PDF"
- Identified people
- Associated individuals requiring security clearances: "People_APP-#_AssociatedIndividuals_YYYY-MM-DD.PDF"
- Organizational security plan
- Business overview: "OSP_APP-#_BusinessOverview_YYYY-MM-DD-PDF"
- Template or primary document: "OSP_APP-#_YYYY-MM-DD.PDF"
- People requiring a security clearance: "OSP_APP-#_PeopleSC_YYYY-MM-DD.PDF"
- Head of security: "OSP_APP-#_HeadOfSecurity_YYYY-MM-DD.PDF"
- Organizational chart: "OSP_APP-#_OrganizationalChart_YYYY-MM-DD.PDF"
- Descriptions of roles and responsibilities: "OSP_APP-# _DescriptionsRoles_YYYY-MM-DD.PDF"
- Descriptions of standard operating procedures (SOPs): "OSP_APP-#_SOPs_YYYY-MM-DD.PDF"
- Attestation: "OSP_APP-#_Attestation_YYYY-MM-DD.PDF"
- Site plan: "SitePlan_APP-#_YYYY-MM-DD.PDF"
- Floor plan: "FloorPlan_APP-#_YYYY-MM-DD.PDF"
- Record keeping
- Record keeping attestation: "RK_APP-#_Attestation_YYYY-MM-DD.PDF"
- Proposed record keeping methods
- Record keeping methods: "RK _APP-#_SmpMethods_YYYY-MM-DD.PDF"
- Medical document verification: "RK _APP-#_SmpVerification_YYYY-MM-DD.PDF"
- Example of your registration document: "RK _APP-#_SmpRegistration_YYYY-MM-DD.PDF"
- Client's orders and refusal to fill client's order example: "RK_APP-#_SmpOrders_YYYY-MM-DD.PDF"
- Process to limit quantities of 150 g of dried cannabis (or its equivalent amount) per order: "RK _APP-#_Smp150g_YYYY-MM-DD.PDF"
- Key investor report (choose 1 of the 2 options)
- Key investor report: "KeyInvestor_APP-#_Report _YYYY-MM-DD.PDF"
- Key investor attestation: "KeyInvestor_APP-#_Attestation _YYYY-MM-DD.PDF"
Section 3: Create a licence application
- Create an account in the CTLS, if you don't have one already
- If you're applying as a part of a corporation, a cooperative, or a partnership, create a corporate profile, if you don't already have one
- Create a new licence application in the CTLS
Section 4: Submit your information
Part A: Submit your security clearance applications in the CTLS
- Identified people to submit their security clearance applications, if applicable, no more than 1 month before you submit your licence application
Part B: Submit your information in the CTLS
- Submit your information into the CTLS
Section 5: After you've submitted your licence application
- Pay fees (licence application-screening fee, security clearance application fees, if applicable)
4.0 Contact us
For questions about a specific licence application, email licensing-cannabis-licences@hc-sc.gc.ca. Include your Licence application ID found in the CTLS. Use the subject line "Questions about APP #".
For all other questions, refer to the Cannabis and industrial hemp contact information for licence holders, applicants and industry to find the appropriate email address.
For general questions, email at cannabis@hc-sc.gc.ca or call 1-866-337-7705 (toll free).
5.0 Foreword and disclaimer
5.1 Foreword
The Cannabis Act (the Act) sets out that an applicant must file a licence application in the form and manner set by the Minister of Health, and include all information required. These pages set out:
- the application process, including the requirements of a licence application
- the information that the applicant must submit
Under the Act, Health Canada may require more information to process your licence application. If you don't submit the information requested, the Minister may refuse to consider your application.
5.2 Disclaimer
You need to read these pages along with the Act and the Cannabis Regulations. If there are differences between these pages and the legislation, the legislation is correct. If there are differences between the Cannabis Tracking and Licensing System (CTLS) and these pages, these pages are correct.
Health Canada's CTLS is a Secure Web Portal and a single point of access for licence application submissions. You can access the CTLS directly at Health Canada's Secure Web portal. You should familiarize yourself with the use of this system and should refer to the CTLS getting started guide for more information.