Health product highlights 2021: Drugs for veterinary use
On this page:
- Message from the Director General
- Experimental studies
- Emergency Drug Release program
- Post-market vigilance
- Addressing antimicrobial resistance related to the use of antimicrobials in animals
- Building partnerships
- Enhancing our regulatory approach
Message from the Director General
One of Health Canada’s roles is to regulate drugs for veterinary use, which play an important role in protecting human and animal health. We evaluate and monitor the safety, efficacy and quality of veterinary drugs. In doing so, we work to protect animals and Canada’s food supply.
This year we continued to advance work on several key priorities, including the implementation of a pilot project for veterinary health products that can be mixed into livestock feed. A partnership with the Canadian Food Inspection Agency, this initiative offers additional tools for the maintenance of animal health and wellness and may help to reduce the need for the routine use of antimicrobials.
We also continued our tracking and analysis of veterinary antimicrobial sales data to better support surveillance efforts. This report marks the third year of sales reporting, with its key findings painting a comprehensive picture of antimicrobials available for veterinary use broken down by species, and province or territory. These annual reports play a key role in our antimicrobial resistance surveillance program and stewardship efforts.

Director General,
Veterinary Drugs
Experimental studies
We review applications to allow companies and researchers to conduct studies on drugs for veterinary use in Canada. New veterinary drug trials (called investigational new drugs and experimental studies) support access to new products in the future. In 2021, we authorized 125 experimental studies that support clinical trials or research activities.
New drugs for veterinary use approved
When a company decides that it would like to market a veterinary drug in Canada, it files a submission with Health Canada that contains detailed scientific information about the drug’s safety, efficacy and quality. These submissions are reviewed by our scientists to assess the potential benefits and risks to human and animal health. They also help to ensure veterinary drug labels have clear directions for use and warning statements.
In 2021, we approved 6 new drugs for companion or food-producing animals. This enabled greater stakeholder access to innovative new products and therapies to help maintain and improve the health of animals. We also approved 13 new generic drugs, which provide additional options for cost-effective prevention and treatment.
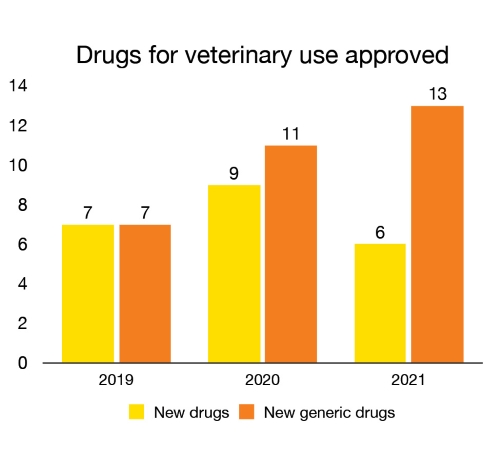
Figure 9 - Text description
| Year | New drugs for veterinary use approved | New generic drugs for veterinary use approved |
|---|---|---|
| 2019 | 7 | 7 |
| 2020 | 9 | 11 |
| 2021 | 6 | 13 |
We approved the first two monoclonal antibody products authorized as veterinary drugs. The two products, Librela and Solensia (Zoetis), are indicated for the treatment of osteoarthritis pain in dogs and cats respectively. Methods to reduce pain other than non-steroidal anti-inflammatories (NSAIDs) are of interest to owners of aging pets, which may be sensitive to the effects of NSAIDs on the kidney, liver and gastrointestinal system.
To allow sponsors to send in their veterinary drug submissions easily and securely, all submissions became electronic either through the Regulatory Enrolment Process or through new tools such as the Secure File Transfer Protocol. In addition, the Drug Product Database, which contains product-specific information on veterinary, human and disinfectant products approved for use in Canada, was updated to permit product searches by species. This is particularly useful for veterinary health care practitioners and producers interested in finding out which drugs are available to treat particular animals.
Emergency Drug Release program
Through our Emergency Drug Release (EDR) program, veterinarians are able to request authorization for drugs for veterinary use that are not available in Canada, for emergency situations. Veterinarians may request access to veterinary drugs to treat patients (an animal or group of animals) with serious or life-threatening conditions. Access to these drugs is only considered when conventional therapies have failed, are unsuitable or are unavailable. In 2021, we authorized 333 requests under the Emergency Drug Release program.
Health Canada continued to implement the changes made in October 2020 to the Food and Drug Regulations, which minimized the burden associated with providing access to unauthorized veterinary drugs. This included working to streamline the process for veterinarians. In addition, we worked with manufacturers to permit the early importation and placement in Canadian facilities. This process, also known as “pre-positioning,” facilitates the immediate distribution of a drug once authorized, making it available as early as possible.
Post-market vigilance
After we approve a drug for sale in Canada, we continue to monitor and evaluate reports of suspected adverse veterinary drug reactions to improve information and access to animal owners, veterinary health professionals and drug manufacturers. The Adverse Event Reporting form for veterinary drugs was updated to improve its usability with clearer and more comprehensive information provided about adverse event reporting. A new PDF-fillable and more accessible Adverse Event Reporting form was created and made available on July 15, 2021, making it easier to report any adverse events.
Addressing antimicrobial resistance related to use of antimicrobials in animals
Antimicrobial resistance is a growing public health threat in Canada and worldwide. The overuse and misuse of antimicrobial drugs allow illness-causing germs like bacteria and fungi to evolve and become resistant to antimicrobials.
Antimicrobial use in animals can contribute to the development and spread of resistant bacteria in humans. A “One Health” approach acknowledges the interconnection between the health of humans, animals and their shared environment, and the need for collaborative efforts across sectors to improve health for all. In 2021, Health Canada continued to focus on our veterinary drug antimicrobial resistance initiatives, to reduce the routine use of antimicrobials and promote their responsible use when they are needed.
For example, in collaboration with the Public Health Agency of Canada we published the 2019 Veterinary Antimicrobial Sales Highlights Report in August 2021. This report marks the second year of sales reporting, which supports our antimicrobial resistance surveillance program and stewardship. This annual report provides a comprehensive picture of antimicrobials available in 2019 for veterinary use broken down by species and by province/territory.
By keeping animals healthy, we can also reduce the need to use drugs, including antimicrobials. This past year we implemented a pilot project for veterinary health products that can be mixed into livestock feed, in partnership with the Canadian Food Inspection Agency. In 2021, 12 products were notified through the veterinary health product web application that were allowed to be mixed into livestock feed as an interim pilot. This initiative provides access to additional tools for maintaining the health and wellness of animals.
We also made significant online updates to List C, the list of permitted substances used to make veterinary health products. In 2021, 97 new substances were added and 106 modifications were made to current listings, including substances used for a number of species such as fin fish and dairy cattle. As of December 2021, List C included a total of 781 active substances, with almost 300 for food producing animals. Expanding the number of substances creates more opportunities for industry to bring a wider range of products to market in support of animal health. In 2021, a total of 496 veterinary health products were notified through the veterinary health products notification program.
Building partnerships
In 2021, Health Canada has continued to work closely with domestic stakeholders and regulators around the world on issues related to drugs for veterinary use, supporting expanded access to treatment options for animals in Canada while reducing regulatory burden for industry.
We continued our simultaneous reviews of veterinary drugs in partnership with the United States Food and Drug Administration’s Center for Veterinary Medicine, along with collaborating on joint reviews with Australia and New Zealand.
In January 2021, Canada and the United Kingdom also published new guidance on the veterinary drug simultaneous review processes for regulatory cooperation. This collaboration creates opportunities for manufacturers to access two major markets simultaneously, helping to expand treatment options for animals and support food producers stay competitive globally.
Focus on … Simultaneous review with the United Kingdom
Building on other veterinary drug international collaborative successes, Health Canada advanced the process for simultaneous reviews with the United Kingdom. In 2021, Health Canada began its first simultaneous review with the United Kingdom. The Guidance on Veterinary Drug Simultaneous Reviews with the United Kingdom was also published and outlines the simultaneous review process for veterinary drugs.
Enhancing our regulatory approach
Regulatory Innovation
Agile licensing for drugs
In 2021, a Notice of Intent was published in the Canada Gazette informing stakeholders of plans to amend the Food and Drug Regulations. These planned amendments would give the Minister of Health the ability to impose terms and conditions on drug authorizations that will better support the oversight of products at the time of approval and afterwards. The proposed amendments would also create an optional application pathway for rolling submissions to facilitate timely access to veterinary drugs that address significant new and emerging infectious diseases, and for the treatment, prevention or diagnosis of serious or severely debilitating diseases or conditions.
Focus on … Health Canada’s role in food safety
Human safety assessment of veterinary drugs used in food producing animals helps to ensure that food derived from treated animals is safe for human consumption. Product sponsors are required to provide to Health Canada scientific data or relevant information necessary to demonstrate that residues of the veterinary drug in edible tissues of treated animals are safe for human consumption. Health Canada establishes food safety standards in the form of maximum residue limits, which are levels of residue that could safely remain in the tissue or food product derived from a food-producing animal that has been treated with a veterinary drug. Health Canada consulted on establishing maximum residue limits for 6 new drugs in 2021. These will be added to the List of Maximum Residue Limits for veterinary drugs in foods in early 2022.