Health product highlights 2021: Lower-risk health products
On this page:
- Message from the Directors General
- Lower-risk health product approved
- Post-market vigilance
- Building partnerships
- Enhancing our regulatory approach
Message from the Directors General
One of Health Canada’s roles is to regulate lower-risk health products – including non-prescription (“over-the-counter”) drugs and natural health products so that Canadians can have confidence that the products they use are safe, effective and of high quality. These include important products that Canadians use every day, such as painkillers, heartburn drugs, vitamins, minerals, probiotics, homeopathic and traditional products. Demand for them has increased in recent years, and accelerated since the pandemic began, as more Canadians are relying on lower-risk health products to support their own health and that of their families.
Our focus for 2021 was to continue our pandemic response as well as to advance regulations that modernize our approach to lower-risk health products and biocides.
This year, we continued to respond to increased demand for disinfectants and hand sanitizers due to the COVID-19 pandemic. Early in the pandemic, Health Canada responded quickly to introduce interim measures, new guidance and streamlined applications to facilitate the availability of these products on the Canadian market. Since March 2020, we have approved over 4,000 hand sanitizers alone, and temporarily authorized over 2,000 sites to manufacture, package, label and/or import them. Throughout the pandemic, we have collaborated closely with stakeholders and other regulators to expedite access to urgently needed products.
In 2021, the Commissioner of the Environment and Sustainable Development tabled his spring report and presented the results of an Audit on Health Canada’s Natural Health Products Program. The Audit focused on natural health products available for sale in Canada to ensure that they are safe, effective and accurately represented to consumers. Overall, it identified both strengths and areas for improvement within the program. Health Canada has accepted all of the Commissioner’s recommendations and has begun taking steps to strengthen the program, including the advancement of the Self-Care Framework – a multi-year initiative to update our regulatory approach for self-care products, including natural health products.

Director General, Natural and Non-Prescription Health Products

Director General,
Marketed Health
Products
Lower-risk health products approved
Non-prescription drugs are health products that can be bought without a doctor's prescription. Health Canada regulates non-prescription drugs to make sure they are safe to use and reduce health risks to Canadians.
In 2021, we reviewed 516 non-prescription drug submissions, including new products and changes to existing products such as antiseptics, pain relievers, cold and cough medicines and sunscreens. Of the total reviewed, 495 submissions were approved.
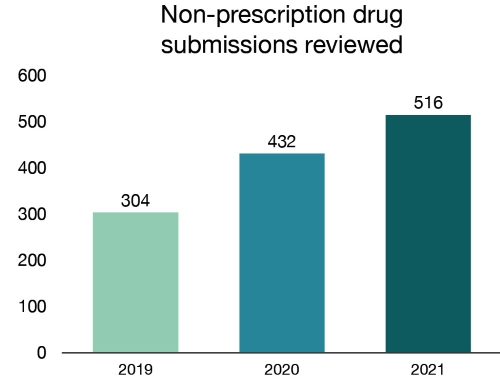
Figure 7 - Text description
| Year | Non-prescription drug submissions reviewed |
|---|---|
| 2019 | 304 |
| 2020 | 432 |
| 2021 | 516 |
We also reviewed 559 surface disinfectant submissions, with 400 of these approved for direct and indirect claims against SARS-CoV-2 (the virus which causes COVID-19). You can find more information on the List of disinfectants with evidence for use against COVID-19. More information on all other approved disinfectants and other drug products can be found on Health Canada’s Drug Product Database.
We reviewed 11,697 natural health product applications, including sanitizers, probiotics, herbal remedies, vitamins and minerals. This resulted in 8,165 new products being licensed. Of these new natural health products, 176 were alcohol-based hand sanitizers, which may be used as part of hand hygiene practices to help reduce the spread of micro-organisms. More information can be found within the Licensed Natural Health Products Database.
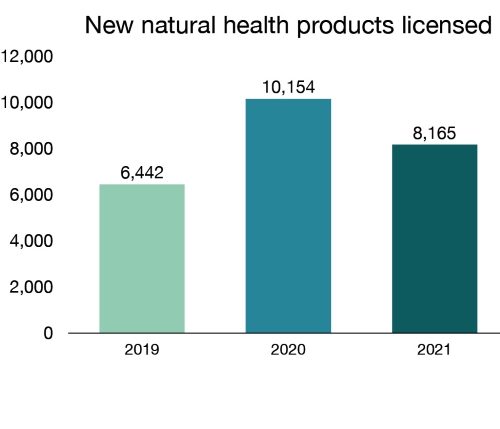
Figure 8 - Text description
| Year | New natural health products licensed |
|---|---|
| 2019 | 6,442 |
| 2020 | 10,154 |
| 2021 | 8,165 |
Post-market vigilance
After we approve a non-prescription drug or natural health product for sale in Canada, Health Canada continues to monitor and evaluate reports of suspected adverse reactions. We evaluate potential safety and efficacy issues, and take action when there are identified problems.
In 2021, Health Canada launched a proactive surveillance program, enhanced with an augmented artificial intelligence tool, to detect and take action against false and misleading advertising for COVID-19 health products. Building on this success, we expanded the scope of the initiative to detect non-compliant cancer-related claims in advertising of natural health products. Since its launch, over 3,000 cases of potentially misleading advertising incidents have been assessed, with subsequent regulatory action taken to protect the health and safety of Canadians.
We have also increased quality requirements for natural health product site licensing by transitioning temporary COVID-19 site licenses to regular site licenses in full compliance with the natural health product quality standard. In addition, a natural health product inspection pilot program was launched in March 2021 that will inform the development of a permanent inspection program.
Building partnerships
A key part of our core business is to engage both internationally and domestically to help provide Canadians with timely access to health products, including non-prescription drugs and natural health products. Our valued relationships with stakeholders are critical to advancing this work.
In an effort to bring greater consistency and alignment in the regulatory approach to non-prescription drugs and natural health products, Health Canada continued regular engagement with our key international regulatory counterparts to share information and advance mutual priorities.
We continued to participate in quarterly meetings with the United States Food and Drug Administration, with discussions including areas of common interest such as health products containing cannabis or cannabis-derived compounds. We also met regularly with the United States Environmental Protection Agency to discuss trends in the disinfectant industry.
As part of the COVID-19 response, we collaborated with the United Kingdom Medicines and Healthcare products Regulatory Agency from May to July 2021 to support the development of interim measures to expedite the review and licensing of sanitizers.
We are also part of the World Health Organization’s International Regulatory Cooperation for Herbal Medicines steering committee, where we work with other regulators to enhance our knowledge and evidence base when developing policies and approaches related to the regulation of herbal medicines.
Domestically, we engaged regularly with stakeholders, including industry associations, medical professionals, patient and consumer groups and academics, on key program priorities. Input from stakeholders has been integral to advancing regulatory priorities and meeting operational targets, as well as understanding the important issues that stakeholders face to make sure the program is responsive and agile. We continued to meet quarterly with industry associations to provide updates on natural health product workload and progress in eliminating remaining submission backlogs resulting from efforts to increase the supply of hand sanitizers for Canadians at the onset of the pandemic.
Enhancing our regulatory approach
Improved labelling for natural health products
A significant milestone in advancing the Self-Care Framework and responding to the Audit of the Natural Health Products Program by the Commissioner of the Environment and Sustainable Development was achieved in June 2021 with the publication of proposed regulations to improve natural health product labelling for formal consultation in Canada Gazette, Part I. The proposed regulatory changes are intended to make natural health product labels clearer, more legible and easier to understand so that consumers can make informed purchasing decisions and use these products safely. The regulations and guidance were available for comment for 90 days, and Health Canada held five technical sessions to provide more information to stakeholders and gather feedback.
Advancing a tailored framework to regulate biocides
In an effort to further expedite access to surface sanitizers and disinfectants, we advanced policy development on a regulatory framework for biocides. Currently, biocides are regulated under several regulatory frameworks with rules that are not in line with the lower-risk nature of these products. Our goal is to create a standalone set of regulations for biocides to reduce the regulatory burden for industry and increase access for Canadians. This framework would enable Health Canada to leverage regulatory decisions by other international regulators for biocides to expedite products to the Canadian market, facilitate entry of innovative products, promote trade and eliminate duplicative reviews. These efforts will ensure appropriate oversight while expediting access to these important products.
Preliminary consultations with key stakeholders took place in spring 2021. Health Canada will continue to leverage the insight and feedback of stakeholders as this initiative moves forward.
Focus on … Audit of Natural Health Products Program
In April 2021, the Commissioner of the Environment and Sustainable Development tabled his spring report and presented the results of the Audit on Health Canada’s Natural Health Products Program, identifying both strengths and areas for improvement. The Audit found that Health Canada licensed natural health products appropriately, based on evidence of safety and efficacy, and found that when an issue was brought to Health Canada’s attention, immediate action was taken. However, the Audit presented several recommendations on how to further strengthen the program. Health Canada has accepted all of the Commissioner’s recommendations and is actively working to increase oversight of quality, advertising and labelling, enhance compliance and enforcement efforts as well as ensure the Department has the tools to protect the health and safety of Canadians when a serious health risk arises.