Health product highlights 2021: Drugs for human use
On this page
- Message from the Directors General
- Clinical trials
- New drugs for human use approved
- Special Access Program
- Post-market vigilance
- Transparency of decision making
- Building partnerships
- Addressing antimicrobial resistance
- Enhancing our regulatory approach
Message from the Directors General
One of Health Canada’s roles is to regulate drugs that can help Canadians maintain and improve their health. This section will discuss drugs, including prescription medicines, vaccines and blood and plasma for human use. Health Canada evaluates drugs before they reach the Canadian market and continues to monitor real world evidence after they are on the market. We are involved throughout the lifecycle of drugs for human use, from clinical trials to once a drug is in use in Canada.
The COVID-19 pandemic created an urgent need for access to safe, effective and high quality vaccines and treatments. At the end of 2021, 4 COVID-19 vaccines were approved for use in Canada. Our vaccine work also included dedicated reviews to ensure the safety and efficacy of booster shots and a pediatric formulation for children aged 5-12 years. In 2021, we also approved 2 new COVID-19 treatments, bringing us to 4 approved COVID-19 treatments in total and with the review of other treatment submissions received continuing into 2022. We expedited the review of these products while maintaining our rigorous scientific standards by using agile regulatory tools, such as rolling submissions, along with staff surge capacity and increased resources within Health Canada.
While responding to the pandemic, we continued to review products not related to COVID-19 to ensure their safety, efficacy and quality. We continued to strengthen our focus on diversity and vulnerable populations, such as children. For example, we worked to enhance access to treatments for rare diseases, approving 10 new drugs for rare diseases in 2021. We advanced implementation of the Pediatric Drug Action Plan to further increase access to drugs for pediatric populations in Canada. Health Canada also supported access to treatments not available in Canada through our Special Access Program.
Our post-market risk management planning starts at the pre-market stage. We review risk management plans which help ensure that appropriate risk mitigation measures for known and potential safety risks and uncertainties are in place. These risk management plans are updated as new safety information became available. We also enhanced our monitoring and assessment activities of emerging safety issues, including for products used for COVID-19, while collaborating and sharing information with our partners in Canada and around the world.
In 2021, we continued to see the benefit of mandatory reporting of serious adverse drug reactions by hospitals, which was implemented in 2019. Since these regulations have been in force, the number of serious adverse drug reaction reports submitted by hospitals has increased significantly – with 4,500 reports in 2021 alone. This reporting strengthens our post-market knowledge base to reduce uncertainty associated with the real-world benefits and risks of therapeutic products.

Director General,
Therapeutic
Products

Director General,
Biologic and
Radiopharmaceutical Drugs

Director General,
Marketed Health
Products
Clinical trials
Clinical trials are conducted by sponsors (manufacturers or researchers) to gather information on a drug’s safety and efficacy in humans. Sponsors of clinical trials submit their applications to conduct a clinical trial with a drug in Canada. Health Canada reviews these applications to ensure that trials are well designed and that participants are not exposed to undue risk.
In 2021, we authorized the use of investigational drugs in 1,158 new clinical trial applications for drugs. Of these, 32 were for COVID-related products (13 vaccines and 19 drugs).
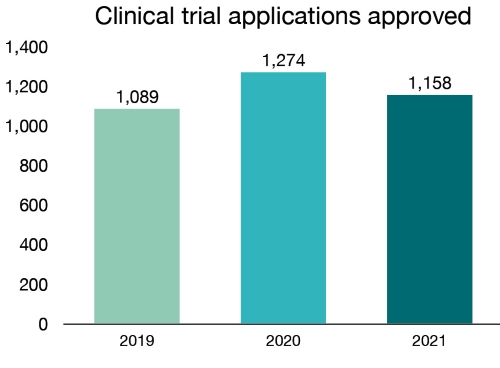
Figure 1 - Text description
| Year | Number of clinical trials approved |
|---|---|
| 2019 | 1,089 |
| 2020 | 1,274 |
| 2021 | 1,158 |
The trials varied significantly in type, size and intent. For example, we authorized the use of investigational products in a phase II/III trial designed to rapidly evaluate several different novel products with the potential to be effective therapies for non-hospitalized COVID-19 patients.
We authorized the MOSAIC Study, led by the Canadian Immunization Research Network, which is examining the safety and immune response of mixing and matching approved COVID-19 vaccines using various time intervals in adults.
Further, in March 2021, Medicago’s Recombinant Coronavirus-Like Particle COVID-19 Vaccine entered phase III clinical trials. Medicago’s COVID-19 vaccine was the first Canadian manufactured vaccine in a phase III trial. It is also unique in that it incorporates a plant-based protein.
In November 2021, Health Canada authorized the use of the investigational drug ION582 in an early stage, open-label clinical trial. This trial was designed to evaluate the safety and tolerability of ascending doses of this drug in patients with Angelman Syndrome. Angelman Syndrome is an incurable genetic disorder resulting in delayed development, problems with speech and balance, intellectual disability and seizures. Current treatment focuses on managing medical, sleep and developmental issues.
In 2021, we dedicated significant effort to continuing to provide enhanced regulatory advice for clinical trials and drug reviews, including guidance on the management of clinical trials during the pandemic and the publication of a dedicated list of authorized clinical trials for COVID-19-related drugs and vaccines. We also continued to engage the Canadian Institutes of Health Research and the Canadian Association of Research Ethics Boards, along with provincial stakeholders, to share information on clinical trials and COVID-19.
New drugs for human use approved
When a company decides that it would like to market a drug in Canada, it files a submission with Health Canada. A new drug submission contains detailed scientific information about the drug’s safety, quality and efficacy. Our scientists and medical officers perform a thorough review of the information submitted, and may also consult with advisory committees or external consultants. Reviewers evaluate the safety, efficacy and quality data to assess the benefits and potential risks of the drug, and review the information that will be provided to health care practitioners and consumers about the drug.
In 2021, we approved 78 new drugs, providing patients with more options for the treatment, prevention and diagnosis of various health conditions. Of these:
- 37 were drugs for rare diseases
- 8 were new drugs with pediatric indications
Forty-three of the new drugs approved in 2021 contained medicinal ingredients that had never been approved for sale in Canada, or what we call “new active substances.” Of these, 37% were approved through an expedited pathway, including those that target specific health care needs.
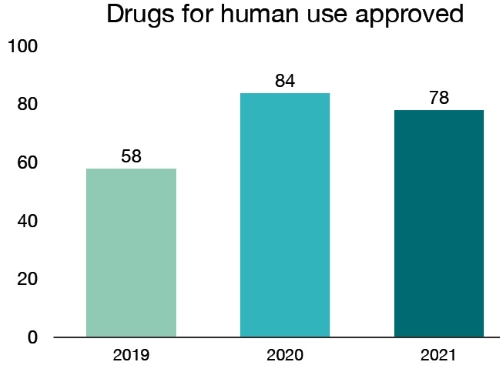
Figure 2 - Text description
| Year | Drugs for human use approved |
|---|---|
| 2019 | 58 |
| 2020 | 84 |
| 2021 | 78 |
One such example is Trikafta, a breakthrough therapy authorized by Health Canada in June 2021 following a priority review. Trikafta is indicated for all cystic fibrosis patients aged 12 years and older with the most common gene mutation, providing access to an additional effective therapy for cystic fibrosis patients, including adolescents.
Another example is Rukobia, an HIV drug which represents a new hope for patients facing multi-drug resistant HIV—a rare subset of the overall HIV population—who may otherwise have no treatment options. Given the importance of this new treatment for HIV patients, the drug submission was granted priority review status and approved in October 2021.
In 2021, we also approved 147 new generic drugs. A generic drug contains the same medicinal ingredients as the brand name drug, and is considered bioequivalent to the brand name drug. These products bring greater choice and affordable options for Canadians. We also approved 11 new biosimilars, which are biologic drugs that are highly similar to a biologic drug already authorized for sale.
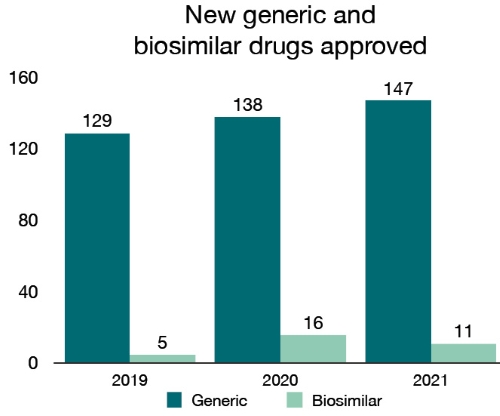
Figure 3 - Text description
| Year | New generic drugs approved | New biosimilars approved |
|---|---|---|
| 2019 | 129 | 5 |
| 2020 | 138 | 16 |
| 2021 | 147 | 11 |
In 2021, we also continued to prioritize the review and approvals of biologic drug lots for release, maintaining Canadians’ access to their biological drugs or enabling equivalent alternative treatments. The lot release program provides Health Canada with a real-time system to monitor product quality, through review and testing, of many of the biological drugs that we regulate. The program covers both pre- and post-market stages for biologic drugs, with each subject to a lot release program based on the degree of risk linked to the product, in order to ensure proper oversight.
Focus on … Blood and plasma for human use
Health Canada is responsible for the regulatory oversight of the blood system in Canada to help ensure the safety of blood and blood components for Canadians. Under the Blood Regulations, those Canadian establishments that collect blood, blood components and plasma are required to make submissions for any proposed changes to Health Canada. These submissions must contain scientific data from studies that support the safety of any proposed changes. Health Canada considers the risks and benefits of any proposed changes to blood operations before granting approval. In 2021, we also approved 23 submissions of blood and plasma products for human use.
We have continued to advance work on pathogen reduction technologies for platelets. For example, we approved the Canadian Blood Services’ submission pertaining to the use of the Intercept pathogen inactivation technology for platelets with a 5-day shelf life. This technology is used to inactivate a broad range of pathogens in order to reduce the risk of transfusion-transmitted infections, and serves as a replacement for sterility testing.
Special Access Program
Through our Special Access Program, we enable access to a wide range of drugs not available for sale in Canada for serious diseases such as cystic fibrosis, rare forms of epilepsy, complicated infections, cancers, ALS, cardiac diseases, hemophilia and other blood disorders. In 2021, we authorized 12,456 requests for special access to drugs and added 140 new drugs to the program. These drugs are either in development or have been approved in other jurisdictions.
In 2020, we finalized changes to the Food and Drug Regulations to modernize the Special Access Program for human drugs. These changes improved the processes used by health care providers and reduced the administrative burden for requests to access drugs that are not yet authorized for sale in Canada.
We also continue to actively reduce the need for the Special Access Program by working with regulatory partners to approve needed drugs, including through information sharing and parallel reviews as well as by encouraging industry to submit drug applications in Canada. For example, following their Canadian market authorization in 2021, 35 drugs released through the program will no longer require special access. These included Ranexa (ranolazine) to treat heart-related chest pains, Evrysdi (risdiplam) to treat spinal muscular atrophy and Effient (prasugrel) to prevent the formation of blood clots. Health professionals previously requested these drugs on average more than 50 times per year through the Special Access Program, but they can now be prescribed directly.
Post-market vigilance
After we approve a drug for sale in Canada, we continue to monitor and evaluate reports of suspected adverse reactions. Adverse reactions are undesirable effects that may be associated with a drug.
We evaluate potential safety and efficacy issues, and take action when there are identified problems. As part of the Canada Vigilance Program, Health Canada collects safety information about a product from a variety of sources, including suspected adverse reactions reported after products are approved for sale. Health Canada evaluates the data from several sources to detect new safety signals, which we then investigate more closely. A safety signal can be defined as information on a new or known adverse event that may be associated with a drug. These investigations are called signal assessments and they may result in recommendations for actions to be taken by the company, by Health Canada, or both. These regulatory actions can include informing the public and health care professionals of new safety information or recommending labelling changes. In the most serious situations, we may remove a drug from the market.
Health Canada is continuously looking for ways to strengthen the post-market knowledge base to reduce the uncertainty associated with the real-world benefits and harms of therapeutic products. In 2021, Health Canada received over 1.1 million reports of suspected adverse reactions to drugs for human use, including those submitted by Canadian hospitals as part of mandatory reporting obligations that came into force in December 2019. These reports help Health Canada further investigate potential risks to Canadians’ health and safety.
In 2021, we investigated 12 safety signals and undertook 9 regulatory actions resulting from the assessments for COVID-19 vaccines and treatments. We also completed 14 safety signal assessments and undertook 10 regulatory actions stemming from these assessments for non-COVID-19 pharmaceutical and biologic drugs. In 2021, we also completed 4 medication incident analyses and undertook 3 regulatory actions stemming from these analyses.
Health Canada also uses Risk Management Plans to support ongoing evaluation of information that could have an impact on a drug’s benefit-risk profile. The decision to approve a drug is based on its benefit-risk profile, based on the information available at the time of approval. The knowledge related to the safety profile of the drug can change over time through expanded use in terms of patient characteristics and the number of patients using the drug. Risk Management Plans describe activities to be undertaken following marketing authorization to identify, characterize, prevent or minimize risks related to drugs once they are in use. In 2021, we reviewed 413 Risk Management Plans for drugs, 35 of which were for COVID-19 treatments and vaccines.
In 2021, we also issued an increased number of Health Product Risk Communications, with a continued focus on issues related to the availability of and safety risks related to COVID-19 vaccines and treatments. Health Product Risk Communications are designed to clearly and effectively communicate new and clinically significant information to healthcare professionals about new health-related risks associated with marketed health products. Improved collaboration with stakeholders also contributed to the successful issuance of 33 Health Product Risk Communications, 21 of which were related COVID-19 vaccines and treatments, between January 1, 2021 and December 31, 2021.
In addition, Health Canada continued to identify a large number of advertising incidents, including through active monitoring of COVID-19-related advertisements. From January to December 2021, we took action on more than 1,100 false and misleading advertisements, 530 of which were related to COVID-19.
Transparency of decision making
In 2021, we continued to advance our openness and transparency efforts by expanding the amount of regulatory health and safety information that was made available to Canadians. We published 147 regulatory decision summaries and 48 summary basis of decision documents, which explain Health Canada’s decisions for certain drugs seeking market authorization.
We continue to publish regulatory and product information on the Health Canada website and the COVID-19 vaccines and treatments portal to respond to the high demand for credible scientific data. Health Canada also continues to work with the Public Health Agency of Canada to provide weekly updates on reported side effects following COVID-19 vaccination in Canada.
Building partnerships
Health Canada has increased its efforts to collaborate with regulators in other countries in recent years. We have worked to enhance regulatory alignment, scientific cooperation and undertake more collaborative reviews, all with the goal of enhancing our approval processes and bringing needed drugs to Canadians more quickly.
Our international cooperation related to drugs continued in 2021, both on COVID-19-related files and in our core activities. We completed 17 drug reviews in collaboration with other regulators.
For example, as a founding member of the Access Consortium, we have continued to work with Australia, Singapore, Switzerland, and as of last year the United Kingdom, to get products to market faster. Through Access we have completed reviews of numerous new drugs by using a work-sharing model that represents a collective population base of 150 million. This included, for example, Kesimpta – a treatment for relapsing multiple sclerosis that was authorized as part of Access with work sharing alongside Australia and Switzerland. We were also able to leverage the Access partnership on COVID-19 vaccine and treatment safety monitoring and surveillance.
We also collaborated with the United States Food and Drug Administration and other partners as part of Project Orbis, to provide patients with enhanced and earlier access to promising cancer treatments. Through Orbis, cancer drugs were approved on average 109 days earlier than the regular 300 day service standard.
Health Canada participated in the European Medicines Agency’s OPEN initiative, along with the European Union, Australia, Japan, Switzerland and the World Health Organization. The initiative helped to increase international collaboration in the evaluation of COVID-19 vaccines and treatments as well as ongoing vaccine safety monitoring. Collaboration with the European Medicines Agency also continued with the publication of clinical information about drugs.
We continued to be an active member of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), and acted as both vice-chair of the ICH Assembly and as chair of the ICH Financial Committee. We continued our work with regulators and industry globally to develop internationally harmonized technical guidelines to ensure that safe, effective and high-quality medicines are developed, registered and maintained in the most resource efficient manner while meeting high standards.
We also continued to participate actively in the International Pharmaceutical Regulators Programme (IPRP). The purpose of IPRP is to create a forum for regulatory members and observers to exchange information on issues of mutual interest, enable cooperation and promote convergence of regulatory approaches for pharmaceutical medicinal products for human use. Through its Management Committee and various working groups, IPRP facilitates discussions on global regulatory issues and on emerging technologies.
Health Canada is also an executive committee member of the International Coalition of Medicines Regulatory Authorities (ICMRA), and plays an integral role in setting its strategic direction. ICMRA includes 37 participating regulatory organizations from jurisdictions around the world. In response to the COVID-19 pandemic, ICMRA expanded its scope of work to provide a global strategy for aligning the approaches of regulators to COVID-19 treatments and vaccines. Health Canada led and contributed directly to this work.
Along with ICMRA members, Health Canada has collaborated with our international partners to publish statements on COVID-19 diagnostics, therapeutics, clinical trials, and vaccine confidence. These statements provide important COVID-19 information to a wide variety of audiences.
Domestically, we have similarly enhanced our collaboration with health system partners. For example, through the Regulatory Review of Drugs and Devices initiative, we worked with Health Technology Assessment organizations to help reduce the time between our approvals and their reimbursement recommendations – helping speed patient access to drugs. In 2021, we completed 20 aligned reviews alongside the Canadian Agency for Drugs and Technologies in Health (CADTH) and the Institut national d'excellence en santé et en services sociaux (INESSS). This process reduced the overall time needed for Health Canada, CADTH and INESSS to complete their reviews and recommendations, supporting faster access to medicines for Canadians.
Focus on … Nitrosamines
Nitrosamines are compounds that can form in certain drugs during the manufacturing process. In recent years, they have been found in an increasing number of drugs. Some nitrosamines may increase the risk of cancer if people are exposed to them over long periods of time.
In response to this important challenge, Health Canada has taken a leadership role internationally by chairing the Nitrosamines International Strategic Group and the Nitrosamines International Technical Working Group, to coordinate the activities of the different regulators and facilitate the rapid exchange of information with respect to the management of nitrosamine impurities in pharmaceutical products. These groups are forums for regulators, including from the European Union, United States, Japan, Australia, Singapore and Switzerland, to share intelligence on new risk signals, converge on acceptable limits for specific nitrosamines, develop technical and regulatory positions and address new and emerging concerns.
Health Canada will continue to work with industry and partners to better understand the causes of this contamination, and to determine how to prevent these impurities in the future. Most importantly, we will continue to communicate with Canadians regarding affected drugs so they can make informed decisions about their health.
Addressing antimicrobial resistance
Antimicrobials, such as antibiotics and antifungals, are essential to modern health care. However, the widespread use of these products has resulted in increasing levels of antimicrobial resistance. Commonly used antimicrobials become less effective as the pathogens they target (bacteria, viruses, fungi and parasites) become resistant to them.
Antimicrobial resistance is a global challenge and a growing threat to public health, the health care system and health security. When there are fewer effective antimicrobials available, it will be harder to protect Canadians from common infectious diseases. In 2021, we continued to take important steps to encourage the development of new and innovative therapeutic products to help combat antimicrobial resistance.
For example, in March 2021, we published the first update to Health Canada’s Pathogens of Interest List following the completion of a public consultation. The List contains bacterial and fungal pathogens that may cause serious, life-threatening infections in the Canadian population, and for which there are (or exists the potential to be) limited or no treatment options available.
In October 2021, in collaboration with the Public Health Agency of Canada and Canadian Institutes of Health Research, Health Canada hosted a Best Brains Exchange meeting that convened relevant policymakers, subject matter experts and industry stakeholders to discuss challenges with the business model for antimicrobials and potential incentives to improve access and promote innovation in Canada.
Every year Health Canada joins the World Health Organization, international health agencies and other national authorities to support World Antimicrobial Awareness Week. In 2021, we collaborated with the Public Health Agency of Canada to host a One Health webinar, featuring sessions on innovative approaches and products to combat AMR in humans, foodborne AMR as well as strategies to combat AMR in food-producing animals. Presentations were delivered by experts from Health Canada as well as other departments and stakeholder groups.
These efforts, involving collaboration within government, with multi-stakeholder groups and with national and international partners, are important steps as we move forward on this issue.
Enhancing our regulatory approach
In 2021, we worked to ensure that Canadians continued to benefit from access to needed COVID-19 vaccines and treatments by maintaining some of the key flexibilities leveraged under our agile regulatory response to the pandemic. These flexibilities included expanded access to clinical trials for COVID-19 health products, the use of terms and conditions to better manage products across their lifecycle, as well as accepting rolling submissions, which allowed us to review evidence and information about a product as soon as it became available.
In March 2021, transitional provisions to the Food and Drug Regulations which maintained certain of these key flexibilities for COVID-19 vaccines and treatments were published in the Canada Gazette, Part II. Further, in May 2021, the Minister of Health approved a second Interim Order which maintained flexibilities for clinical trials related to COVID-19 drugs and medical devices. The insights learned from these flexibilities have reinforced the importance of being an agile regulator. We will continue to build on these insights as we advance our broader regulatory innovation plans.
Regulatory Innovation
Modernizing clinical trial regulations
Over the last decade, we have observed a shift in the focus of clinical trials. Increasingly, trials aim to find a therapy for a serious, life-threatening or rare condition where an unmet medical need exists. The operational complexity and expense of running a clinical trial has also increased dramatically. As a result of these changes, clinical trial design is evolving quickly. These changes are occurring in tandem with the increased development of personalized health products, gene therapies and products intended for the treatment of rare diseases, each of which poses its own unique challenges regarding the conduct of clinical trials. Appropriate trial design and requirements are therefore critical to support the introduction of novel safe and effective therapies to the Canadian market.
In response, Health Canada has put forward a vision for the modernization of Canada's clinical trial regulatory framework. The new framework will better respond to these changes and help encourage clinical trials in Canada by creating an environment that supports the safe innovation of health products. In May 2021, we published a consultation document outlining our plans to modernize Canada’s clinical trial regulations to improve access to novel therapies, while continuing to ensure patient safety. This was complemented by stakeholder consultations through the spring and summer.
Agile licensing for drugs
In the past, a common set of regulatory rules worked well for most products. However, as the market has evolved and now includes a much larger number of drugs, including more complex and personalized therapies, our regulatory system also needs to evolve. To continue to advance our work in this area, we posted a Notice of Intent in the Canada Gazette informing stakeholders of plans to amend the Food and Drug Regulations. These planned amendments would give the Minister of Health the ability to accept submissions on a rolling basis in certain circumstances, impose terms and conditions on drug authorizations if needed and require Risk Management Plans. These regulatory abilities were leveraged as part of our response to COVID-19 and which we can now build on to better support the oversight of a broader range of products.
Advanced therapeutic products pathway
Some products are so innovative or complex that they need a different regulatory approach – we refer to these as advanced therapeutic products. In 2021, we established an External Reference Group to help guide early thinking around requirements to establish a tailored regulatory pathway for fecal microbiota therapy. The intent of this External Reference Group is to bring relevant experts together to provide evidence-based advice to help tackle regulatory issues and enable safe patient access. We will continue to leverage the insight and feedback of stakeholders as this initiative moves forward.
Focus on … Innovation – Fecal Microbiota Therapy
Fecal Microbiota Therapy (FMT) involves the transfer of bacteria from a healthy donor into a patient’s intestinal tract to establish a healthy microbial community to help fight antimicrobial resistance, such as when treating recurrent C. difficile infections. Health Canada currently oversees FMT using an Interim Policy, and is limited to classical FMT dosage forms (i.e., fresh or frozen) used for the treatment of patients with recurrent C. difficile that are not responsive to conventional therapies. By creating a tailored advanced therapeutic product pathway for FMT, it will allow for appropriate oversight under a pathway that has the force of law, with more enforcement tools and will provide a path to market for researchers and innovators.