Health product highlights 2021: Medical devices
On this page:
- Message from the Directors General
- Investigational testing (clinical trials)
- New medical devices approved
- Special Access Program
- Post-market vigilance
- Transparency of decision making
- Building partnerships
- Enhancing our regulatory approach
Message from the Directors General
One of Health Canada's roles is to regulate medical devices that can help Canadians maintain and improve their health. Medical devices are used in the treatment, diagnosis or prevention of diseases or physical conditions. In Canada, we categorize medical devices into four groups based on the level of risk associated with their use. These groups are called “Classes” and they range from I to IV. Class I devices are considered low-risk devices – for example, a wheelchair. Class IV devices present the greatest potential risk – for example, a defibrillator.
In 2021, Health Canada continued to prioritize our response to the COVID-19 pandemic, while also advancing key non-COVID-related priorities. In particular, we made efforts to increase the number of self-tests for COVID-19 available on the Canadian market. The rate at which the Omicron variant spread across the world reinforced the importance of these devices, which help to slow transmission by allowing individuals to quickly identify if they are infected. Over 115 COVID-19 testing devices are now available in Canada, and there are more in the pipeline.
We also actively scanned for emerging COVID-19 products, enabling us to seek out and encourage submissions for important and innovative technologies that otherwise may not have been filed in Canada.
Building on the progress made through the Medical Devices Action Plan (MDAP), Health Canada is advancing regulatory changes as part of the Agile Licensing initiative. A Notice of Intent was published in 2021 outlining our plans to amend the Food and Drug Regulations and the Medical Devices Regulations to support enhanced regulatory agility. The expanded ability to use terms and conditions on medical device licenses will better support the effective oversight of devices across their lifecycle.
As we move forward responding to COVID-19 and advancing our key priorities, we will continue to put the health and safety of Canadians first, while supporting innovation and improved regulatory solutions.

Director General,
Medical Devices

Director General,
Marketed Health Products
Investigational testing (clinical trials)
Clinical trials are conducted by sponsors (manufacturers or importers) to gather information on a medical device’s safety and efficacy in humans. Sponsors of investigational tests submit their applications to conduct investigational testing (clinical trials) with a medical device in Canada. Health Canada reviews these applications before the testing is conducted in Canada. New trials mean Canadians may have access to more innovative product choices. In 2021, 140 new investigational testing applications for medical devices were approved.
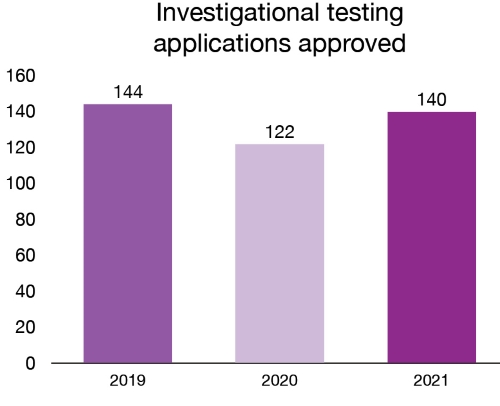
Figure 4 - Text description
| Year | Investigational testing applications approved |
|---|---|
| 2019 | 144 |
| 2020 | 122 |
| 2021 | 140 |
We enabled the testing of new COVID-19-related medical devices, including COVID-19 diagnostic test kits, physiological monitoring devices and respiratory treatment-related devices.
We also authorized investigational testing for a number of novel devices used for certain cardiovascular surgical procedures, as well as for a point-of-care magnetic resonance imaging device to be used for imaging in remote regions. This device allows health professionals to carry out imaging where patients are located, instead of requiring them to travel to larger centres.
New medical devices approved
As part of Health Canada’s mission to help Canadians maintain and improve their health, we evaluate medical devices before and after they reach the Canadian market. Health Canada is involved throughout the lifecycle of a medical device, from investigational testing to after the device is being sold in Canada.
In 2021, we licensed 272 new Class III and 45 new Class IV medical devices. These new devices provide patients and health care professionals with new and innovative options for the treatment, prevention and diagnosis of various health conditions. For example, in 2021, we licensed the first medical implant made in Canada with a 3D printer. The technology used makes it possible to produce custom jaw prosthesis, adapted to the anatomy of each patient. For a list and description of the new Class IV medical devices approved in 2021, please see the the annex.
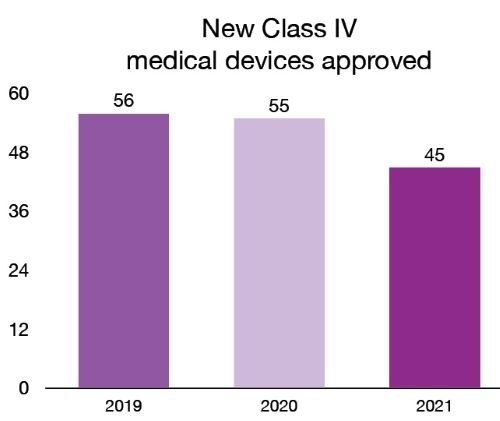
Figure 5 - Text description
| Year | New class IV medical devices approved |
|---|---|
| 2019 | 56 |
| 2020 | 55 |
| 2021 | 45 |
In 2021, we prioritized the review of testing device applications in support of public health needs. We approved 55 COVID-19-related diagnostic test devices. This included tests for use in asymptomatic populations, the first rapid self-tests for home use, and multiplex tests that can be used to simultaneously diagnose other respiratory viruses as well as COVID-19.
In addition, we authorized the sale of 10 ventilators, and expanded the intended use of some continuous glucose monitors to include pregnant women, following a Health Canada review and recommendation. Continuous glucose monitors offer the ability for healthcare providers to remotely view patients’ glucose control data and conduct virtual clinic visits, which has helped reduce the need for in-person visits for pregnant women who were identified as having a higher risk of serious COVID-19 outcomes.
Special Access Program
Through the Special Access Program, we grant healthcare professionals access to custom-made devices and unlicensed medical devices for emergency use or when conventional therapies have failed, are unavailable or are unsuitable to treat a patient. In 2021, we authorized 3,121 requests for medical devices under this program.
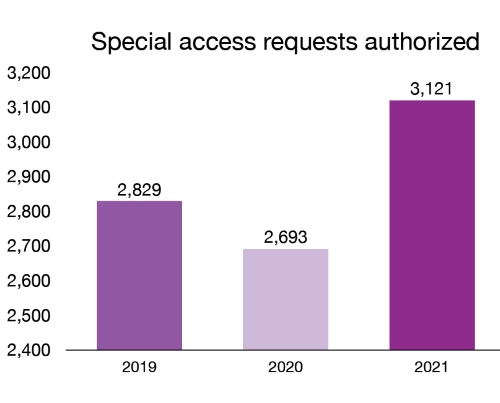
Figure 6 - Text description
| Year | Special access requests authorized |
|---|---|
| 2019 | 2,829 |
| 2020 | 2,693 |
| 2021 | 3,121 |
Post-market vigilance
After a medical device is approved and available for sale in Canada, we continue to monitor its use in the broader Canadian population. We evaluate potential safety and efficacy issues and take action when there are identified problems. Actions can include informing the public and health care professionals of safety information, requesting labelling changes or affirming our current understanding recommending compliance actions. For example, we implemented comprehensive labelling changes for breast implants, similar to those required by the United States Food and Drug Administration.
In 2021, we received 39,601 reports of suspected medical device incidents and undertook 5 regulatory actions related to medical devices. This follows the coming into force of regulations requiring hospitals to report serious adverse drug reactions and medical device incidents in December 2019. Since the regulations came into force, we have seen an increase in the number of medical device incident reports received – almost 1,200 in 2021 alone. This helps Health Canada take action against products that may pose a risk to Canadians’ health and safety.
In 2021, we continued to monitor the safety and efficacy of health products related to COVID-19, and took action as needed to protect Canadians where there were issues with safety or efficacy of a device. This included taking proactive steps to identify incidents related to medical devices used for COVID-19 and working with government partners to monitor retailers and advertisements making false, misleading and illegal claims related to COVID-19. We worked closely with domestic and international partners and published risk communications about potential safety and efficacy concerns (for example, a Notice on limitations and best practices to ensure accurate readings when using non-contact infrared thermometers).
New medical device post-market surveillance regulations came into effect in 2021, which further support the lifecycle approach to the regulation of medical devices by strengthening post-market authorities. We published guidance documents to inform manufacturers how to comply with the new regulations related to foreign risk notification, issue-related analyses and summary reports for licensed medical devices. Together these will help to reduce risks associated with medical devices, and improve their safety, efficacy and quality.
Transparency of decision making
In 2021, we continued to advance our openness and transparency efforts by expanding the amount of health and regulatory safety information that is made available to Canadians.
Through our Clinical Information Portal, we published 2,100 pages of clinical information on 13 medical devices. Companies provide this information when they seek approval to sell a medical device in Canada.
We also published summaries of our safety reviews, which describe Health Canada’s decisions related to potential safety issues, for dental amalgam and contact lenses in 2021. These summaries complement other safety-related information to help Canadians make informed decisions.
The Canada Vigilance Program collects suspected adverse drug reactions and medical device incidents. For medical devices, incident and recalls data are made publically available through the Medical Devices Incidents Database, and are updated on a quarterly basis.
Building partnerships
We have continued to collaborate with our international counterparts, as well as with the World Health Organization, to harmonize regulatory and communication strategies and guidance on medical device management. In 2021, we participated in discussions on safety and quality issues observed with several COVID-19 medical devices, including respirators, serological and antibody diagnostic tests, reprocessed and decontaminated ventilators, facemasks and 3D printed testing swabs.
Under the International Medical Device Regulators Forum (IMDRF), Health Canada contributed to the guidance document on terms and definitions for machine learning-enabled medical devices and on the recognition process of conformity assessment bodies conducting regulatory reviews, both published in 2021. We also continue our routine work with the IMDRF Adverse Events Working Group to harmonize patient and device codes for medical device incident reports.
Under the Regulatory Co-operation Council, Health Canada continued to work with the United States Food and Drug Administration to build a Medical Device Single Review Program. This program works to improve patient access to medical devices, support innovation and strengthen the development of standards. Through the initiative, we have finished a first review under a pre-pilot proof of concept of the Affinity NT Oxygenator and will begin a second simultaneous review of a percutaneous transluminal coronary angioplasty catheter in 2022.
As part of our COVID-19 regulatory response, we have worked closely with companies in the Canadian ventilator sector, the Public Health Agency of Canada, Public Services and Procurement Canada, Innovation, Science and Economic Development Canada and the National Research Council, to provide guidance regarding Health Canada’s ventilator requirements and application process. In addition, Health Canada worked with companies by providing guidance about the Health Canada Interim Order authorization process and communicating Health Canada ventilator requirements with respect to the necessary safety and efficacy information to be submitted for an Interim Order authorization.
Enhancing our regulatory approach
In 2021, we worked to ensure that Canadians continued to benefit from access to needed COVID-19 medical devices by maintaining several key flexibilities leveraged under our agile regulatory response to the pandemic.
In March 2021, the Minister of Health approved a second Interim Order which maintained the expedited pathway and regulatory flexibilities for the import and sale of needed COVID-19 medical devices. Further, in May 2021, the Minister of Health approved a second Interim Order which maintained a number of regulatory flexibilities for the conduct of clinical trials related to COVID-19 drugs and medical devices. The insights learned from these interim measures have reinforced the importance of being an agile regulator. We will continue to build on these insights as we advance our broader regulatory innovation plans.
Regulatory Innovation
Modernizing clinical trial regulations
In May 2021, we published a consultation document outlining our plans to modernize Canada’s clinical trial regulations to improve access to novel therapies, while continuing to ensure patient safety. This was complemented by stakeholder consultations through the spring and summer. The new framework will help encourage clinical trials in Canada by creating an environment that supports safe innovation. The proposed regulations would allow independent researchers and medical professionals to conduct clinical trials on medical devices.
Advanced therapeutic products pathway
As discussed above in the Drugs for Human Use section, some products are so innovative or complex that they need a different regulatory approach, and we refer to these as advanced therapeutic products. In 2021, we established an External Reference Group to help guide early thinking around requirements to establish a tailored pathway for adaptive machine-learning enabled medical devices. The intent of this External Reference Group is to bring relevant experts together to provide evidence-based insight and advice on the development of requirements to bring adaptive machine-learning enabled medical devices to the Canadian market while effectively managing the risks, benefits and uncertainties of these products. We will continue to leverage the insight and feedback of stakeholders as this initiative moves forward.
Agile licensing for medical devices
In 2021, we posted a Notice of Intent in the Canada Gazette informing stakeholders of plans to make improvements to the Medical Devices Regulations. These proposed amendments would help to enable more adaptive licensing of medical devices and provide Health Canada with agile regulatory tools to protect the health and safety of Canadians. The planned amendments would broaden the scope of terms and conditions for medical devices, to help manage known risks or uncertainties relating to the benefits or risks of the device. This would build on our experience with the use of terms and conditions as part of our response to COVID-19.
Medical Devices Action Plan
In May of 2021, Health Canada published a progress report on the Medical Devices Action Plan outlining activities undertaken to improve the safety and efficacy of medical devices, including:
- Consulting Health Canada's Scientific Advisory Committees with respect to health products for women, digital health technologies and medical devices used in the cardiovascular system.
- Hosting four webinars to provide guidance on the strengthened final regulations regarding the post-market surveillance of medical devices.
Furthermore, additional regulations related to post-market surveillance came into force in December 2021 and will enable Health Canada to better monitor medical devices through annual or bi-annual summary reports prepared by the manufacturer.
Focus on … Innovation – adaptive machine learning-enabled medical devices (MLMD)
Adaptive machine learning-enabled medical devices are highly sophisticated tools that leverage artificial intelligence to learn and improve over time, particularly for medical imaging. Currently, Health Canada has well-established protocols for oversight of traditional devices with static algorithms. These medical devices with adaptive algorithms have the potential to revolutionize health care, and by creating a tailored advanced therapeutic product pathway to enable their use, this will allow patients to gain access to these complex and unique products.