Canadian biosafety guideline: Biosafety program management

Download in PDF format
(PDF format, 6.23 MB, 63 pages)
Organization: Public Health Agency of Canada
Cat.: HP45-33/2023E-PDF
ISBN: 978-0-660-47918-7
Pub.: 220805
Published: 2023-03-31
Table of contents
- Preface
- Abbreviations and acronyms
- 1. Introduction
- 2. Plan a biosafety program
- 3. Implement the biosafety program
- 4. Ongoing improvement of the biosafety program
- 5. Glossary
- 6. References
Preface
In Canada, the handling or storing of Risk Group 2 (RG2), RG3, and RG4 human pathogens or toxins is regulated by the Public Health Agency of Canada (PHAC) under the Human Pathogens and Toxins Act (HPTA) and the Human Pathogens and Toxins Regulations (HPTR). Under the Health of Animals Act (HAA) and the Health of Animals Regulations (HAR), the PHAC and the Canadian Food Inspection Agency (CFIA) regulate the importation of animal pathogens or part of one (e.g., toxin), animals naturally or experimentally exposed to an animal pathogen or part of one (e.g., toxin), and animal products or by-products (e.g., tissue, serum), or other organisms carrying an animal pathogen or part of one (e.g., toxin).
The following figure depicts the document hierarchy used by the PHAC and the CFIA to oversee biosafety and biosecurity operations. Each tier of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards. Acts and regulations are found at the top of the pyramid, as they are the documents that convey the PHAC's and the CFIA's legal authorities. Guidance material and technical pieces are found at the bottom of the pyramid, as they are only intended to summarize recommendations and scientific information.
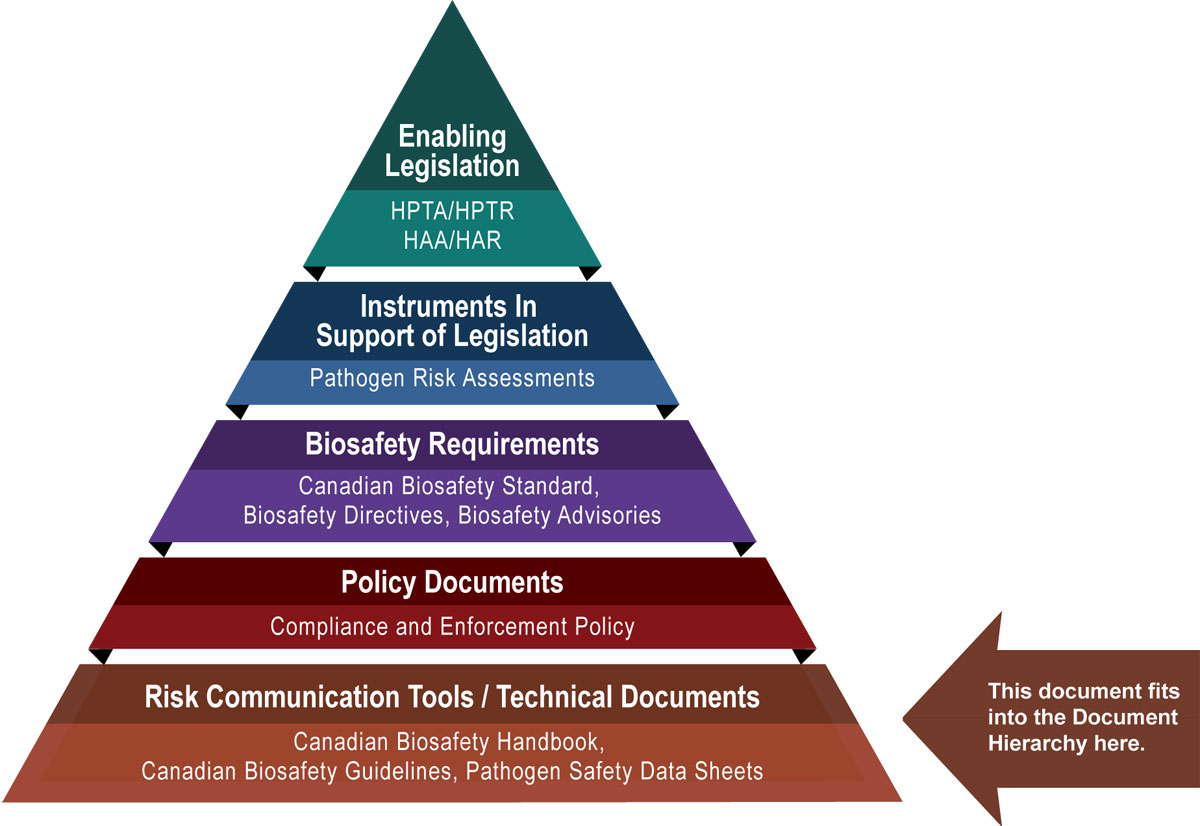
Figure 1 - Text description
Figure in the form of a pyramid depicting the document hierarchy used by the PHAC to oversee biosafety and biosecurity operations. Each of the five tiers of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards.
At the top sits the Enabling Legislation, that is, the HPTA, HPTR, HAA, and HAR, that convey the PHAC's legal authorities. Below the acts and regulations sit Instruments in Support of Legislation, which are the Pathogen Risk Assessments. The next tier down are the Biosafety Requirements, which include the Canadian Biosafety Standard, Biosafety Directives, and Biosafety Advisories. In the second lowest tier are the Policy Documents, which include the Compliance and Enforcement Policy. Guidance material and technical pieces found at the bottom of the pyramid, under the Risk Communication Tools and Technical Documents heading, are intended to summarize recommendations and scientific information only. These include the Canadian Biosafety Handbook, Canadian Biosafety Guidelines, and Pathogen Safety Data Sheets.
The Biosafety Program Management guideline was developed by the PHAC and the CFIA as part of a series of electronic publications that expand upon the biosafety and biosecurity concepts discussed in the current edition of the Canadian Biosafety Handbook (CBH), the companion document to the Canadian Biosafety Standard (CBS). This guideline provides information and considerations that can assist in the development and implementation of the policies and procedures that organizations need in order to safeguard personnel, the community, and the environment from the pathogens and toxins handled and stored in their facilities. Such biosafety policies and procedures include those needed to meet the requirements of the HPTA, HPTR, HAA, and HAR, as well as the applicable requirements specified in the CBS.Footnote 1Footnote 2 By taking a comprehensive approach, biosafety policies and procedures can be expanded to address other biological risks within an organization, where appropriate, such as for work with aquatic animal pathogens, plant pests, arthropods, and new organisms.Footnote 3Footnote 4Footnote 5
Abbreviations and acronyms
- BSC
- Biological safety cabinet
- BSO
- Biological safety officer
- CBH
- Canadian Biosafety Handbook
- CBS
- Canadian Biosafety Standard
- CFIA
- Canadian Food Inspection Agency
- CL
- Containment level (i.e., CL1, CL2, CL3, CL4)
- HAA
- Health of Animals Act
- HAR
- Health of Animals Regulations
- HPTA
- Human Pathogens and Toxins Act
- HPTR
- Human Pathogens and Toxins Regulations
- IBC
- Institutional biosafety committee
- LRA
- Local risk assessment
- PDCA
- Plan-do-check-act
- PHAC
- Public Health Agency of Canada
- PPE
- Personal protective equipment
- RG
- Risk group (i.e., RG1, RG2, RG3, RG4)
- SOP
- Standard operating procedure
- SSBA
- Security sensitive biological agent
1. Introduction
The words in bold type are defined in the Glossary.
A biosafety program incorporates a broad range of actions with the intent of identifying and quantifying biosafety and biosecurity risks associated with the regulated materials handled or stored within a facility, and implementing appropriate mitigation measures to reduce these risks. The biosafety program can also address other biological material, such as Risk Group 1 (RG1) microorganisms, cell lines, animal products and by-products, and human clinical samples, which may or may not be regulated. This organization-wide system serves to create and maintain a biosafety framework that is integrated into the overall governance structure. The biosafety program can be a standalone system or it can be combined with other programs (e.g., occupational health and safety, security) to produce a comprehensive program for biosafety, workplace health, safety, and security.
Microorganisms are classified into one of four risk groups (i.e., RG1 to RG4) based on the outcome of a pathogen risk assessment, which evaluates the organism's inherent characteristics that contribute to the risk it poses to an individual human or animal, and to public health and the animal population. Containment levels describe the minimum physical containment and operational practices that a containment zone (i.e., an identified physical area that meets the requirements for a specified containment level) requires for the safe handling of regulated materials. There are four containment levels ranging from a basic laboratory for work with biological material (i.e., Containment Level 1 [CL1]) to the highly sophisticated facilities for work with the highest risk pathogens (i.e., CL4). In general, the risk group of a pathogen is the same as the containment level at which it must be handled (i.e., RG2 pathogens are typically handled at CL2); where these do not align, the Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA) may develop Biosafety Directives to clarify containment requirements.Footnote 6 In addition, requirements can vary according to the type of work area, including laboratory work area, large scale production area, animal work area, and areas where prions or security sensitive biological agents (SSBAs) are handled.
The level of complexity and detail of the biosafety program varies according to the nature and size of the organization, the types of pathogens handled, and the activities performed. For example, a small company where only one low-risk pathogen is handled in a single-room CL2 laboratory work area will have a less complex biosafety program than a large company or university with multiple campuses where research, diagnostics, and in vivo work involving diverse low to high-risk pathogens (i.e., RG2, RG3, RG4) are performed. Regardless of the size and complexity of the organization, all elements of the biosafety program apply, but the manner in which these elements are implemented may vary between organizations. An effective biosafety program promotes and reinforces safe work practices, improves overall biosafety, increases compliance with applicable regulatory requirements, and supports a culture of biosafety throughout the organization.
Proactive management is critical for a successful biosafety program. A standardized program management process helps organizations to effectively identify, assess, control, and monitor the risks related to the handling and storing of pathogens and toxins. This reduces the potential for biosafety and biosecurity incidents and establishes an appropriate organizational response to incidents. Within the organization, effective biosafety program management can improve compliance, safety performance, and efficiency. Outside the organization, biosafety program management is critical to protecting the safety of the community from the pathogens and toxins handled and stored on-site.
Effective management of the biosafety program can be accomplished by following the plan-do-check-act (PDCA) management cycle.Footnote 7Footnote 8Footnote 9 Figure 1-1 shows how the PDCA cycle can be applied to establish and maintain a biosafety program: Plan – biosafety and biosecurity hazards and risks are identified and assessed; Do – mitigation measures are implemented and documented; Check – planned and responsive monitoring of the program and its elements are performed; Act – processes are adapted and modified to continually improve the program.
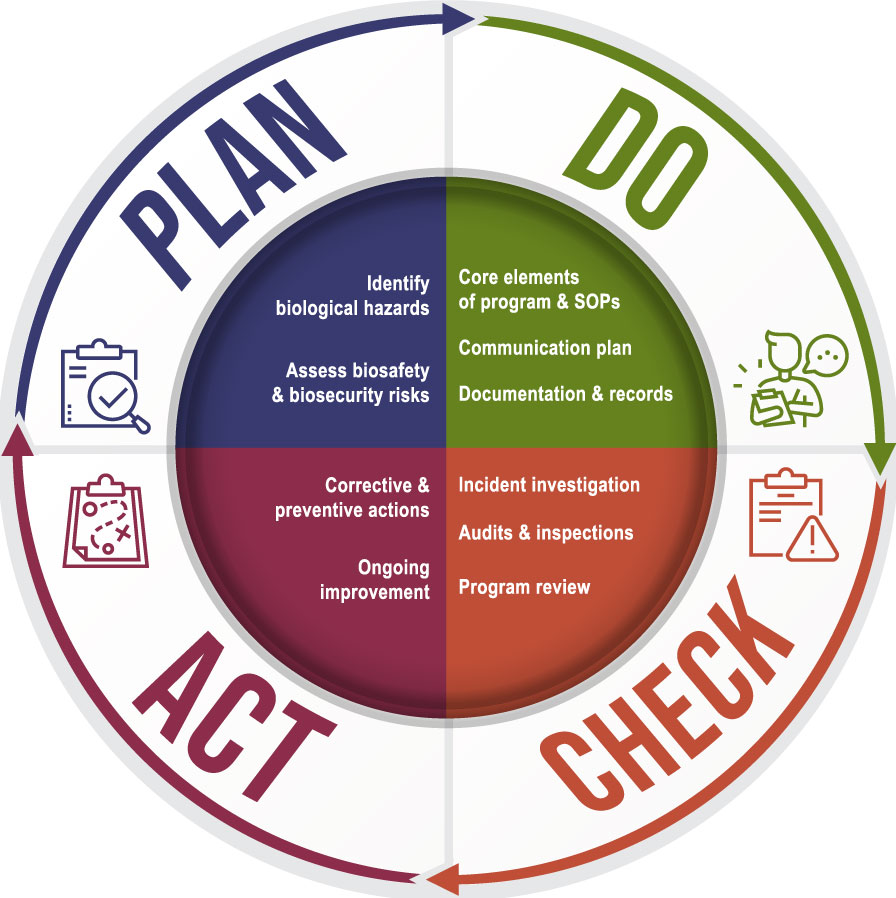
Figure 1-1 - Text description
Figure in the form of a cycle with four quadrants. The first quadrant corresponds to the Plan portion of the cycle and lists identify biological hazards and assess biosafety and biosecurity risks as components. The second quadrant represents the Do portion of the cycle and includes core elements of the program and SOPs, communication plan and documentation and records. The third quadrant is indicated as the Check portion of the cycle and includes incident investigation, audits and inspections and program review as the components of this section. The last quadrant is the Act portion of the cycle and includes corrective & preventative actions and ongoing improvement as components.
1.1 Scope
The Biosafety Program Management guideline provides comprehensive guidance on how to develop, implement, and manage a biosafety program in facilities where human and terrestrial animal pathogens and toxins are handled and stored. The guideline outlines the steps essential to the development and execution of a comprehensive biosafety program, which includes planning, implementing, reviewing, and improving the program. This guideline is intended to be used in conjunction with the CBS. Footnote 10
The information provided in this document is intended as guidance only and is not meant to be interpreted as requirements. Regulated parties may choose alternative approaches to meet the requirements specified in the CBS.
1.2 How to use the Biosafety Program Management Guideline
A detailed list of all abbreviations and acronyms used throughout this guideline is located at the beginning of this document. Each word or term is spelled out upon first use in the guideline, with the abbreviation immediately following in brackets. After its initial definition, the abbreviation is used exclusively throughout the remainder of the document. A comprehensive glossary of definitions for technical terms is located in section 5 of this document. Terms defined in the glossary appear in bold type upon first use in the guideline. Where the guidance relates to a specific requirement in the CBS, the requirement matrix or matrices are referenced (e.g., CBS Matrix 4.1). A full list of the references as well as other resources is provided in the References section.
2. Plan a biosafety program
Planning a biosafety program starts with determining an organization's needs (based on its program intent and risk assessments), identifying measures to mitigate risks, and determining the human and financial resources required to implement the identified mitigation measures.
While the focus of this document is on biosafety and the protection of public health, the animal population, and the environment, many of the elements, including the program itself, may be integrated into an organization's existing health, safety, and security programs for a more efficient use of resources. Since organizations may be subject to numerous requirements (e.g., regulatory, national and international standards, agreements), combining common elements and internal processes may streamline efforts and reduce the resources needed. For example, a single internal inspection can assess compliance with biological, chemical, and radioactive material requirements. Similarly, internal reporting requirements can be integrated into a single system for any incident, including those involving biological material, chemicals, radiation, information technology, health and safety, and security.
2.1 Promoting a culture of biosafety
A strong biosafety culture is foundational to the success of a biosafety program and is driven by responsibility and accountability.Footnote 11 A biosafety culture can be strengthened and supported with a demonstrated commitment to biosafety by senior management, and its success necessitates engagement by personnel from all levels of the organization. The importance and the status of biosafety within the organization is conveyed when biosafety is made a core value of the organization, and by the amount of resources dedicated towards establishing, implementing, maintaining, reviewing, and improving the biosafety program. Active and visible participation and advocacy of senior management in biosafety-related activities further demonstrates a commitment to biosafety.
The development of a clear policy and set of biosafety targets, with the early and active involvement of personnel from all levels of the organization, promotes ownership and responsibility for biosafety. Personnel engagement can be maintained with open and consistent communication in a responsive and non-punitive environment.Footnote 11 A strong culture of biosafety can lead to increased compliance with biosafety requirements and integration of biosafety awareness in all activities undertaken in a facility.
2.1.1 Roles and responsibilities
The roles and responsibilities of all individuals involved in the biosafety program need to be clearly delineated, documented, and communicated in order for management to be effective. In defining these roles, potential conflicts of interest can be identified and managed. Additionally, when individuals understand their roles and responsibilities, it helps contextualize how they contribute to the organization's biosafety program as a whole.
2.1.1.1 Senior management
As leaders in their organization, members of senior management are in a unique position to direct the development of the biosafety program and promote a culture of biosafety amongst all personnel. Some of the responsibilities of senior management may include the following:Footnote 7Footnote 12
- create the mechanism(s) (e.g., policy, code, strategy) that highlight(s) their commitment to manage and control biosafety and biosecurity risks within the organization;
- allocate sufficient human and financial resources for biosafety throughout the organization;
- define the roles, responsibilities, and authorities related to the biosafety program and assign these to personnel within the organization, or designate the authority to do so (e.g., to the biological safety officer [BSO]), and have these communicated to all personnel;
- review and approve the Plan for Administrative Oversight for Pathogens and Toxins in a Research Setting when scientific research with pathogens and toxins is conducted;Footnote 12
- provide biosafety program oversight, including participation in the program review process;
- participate in biosafety committees (or designate a senior manager to participate); and
- encourage ongoing communication of biosafety-related issues and concerns between personnel and internal authorities (e.g., senior management, BSO) using a non-punitive approach.
2.1.1.2 Pathogen and Toxin Licence holder and Terrestrial Animal Pathogen importer
Under the Human Pathogens and Toxins Act (HPTA), controlled activities involving human pathogens and toxins generally require a Pathogen and Toxin Licence (hereafter, " licence") issued by the PHAC. While all persons knowingly conducting controlled activities are accountable, the licence holder has specific obligations related to the biosafety program and its management. The licence holder is responsible for, among others, designating a BSO and communicating all licence conditions, including all the applicable requirements specified in the HPTA, the HPTR, and CBS, to everyone conducting activities under that licence. While the licence holder can be a corporate entity, the day-to-day responsibilities of the licence holder will generally fall on senior management or another authorized individual. When identifying an appropriate member from senior management to be the licence holder (or for taking on licence holder responsibilities) for the facility or organization, considerations include selecting an individual who is:
- authorized to make decisions with financial and legal implications;
- authorized to make decisions associated with compliance to the HPTA and HPTR, and will do so based on the organization's internal recommendations;
- supported by the organization's existing administrative oversight arrangements;
- able to influence decisions and behaviour throughout all levels of the organization; and
- aware of the overall organizational risks related to human pathogens and toxins handled and stored within the facility.
In facilities where a terrestrial animal pathogen or toxin has been imported under a terrestrial animal pathogen import permit issued by the CFIA, the person identified on the permit as the applicant is accountable for the imported regulated materials. The applicant may be the person who will be using the biological material (e.g., primary investigator, researcher, technician), senior management, or the organization (i.e., corporate entity). In the case where the applicant is the person who will be using the material, it is important to keep the BSO informed of the imported terrestrial animal pathogens and toxins.
2.1.1.3 Biological safety officer
The licence holder or terrestrial animal pathogen import permit holder designates a BSO [HPTA 36(1), Matrix 4.1 of the CBS] to oversee biosafety practices, provide advice and guidance on biosafety program issues, and act as the primary point of contact with the PHAC and the CFIA. The BSO is responsible for the oversight of biosafety and biosecurity practices (and often development and implementation of the biosafety program), and is critical to the organization's biosafety program. The HPTA, HPTR, and CBS are designed to support the BSO's role in enhancing the organization's system of internal accountability by specifying the qualifications, functions, and powers of a BSO. As such, it is essential that the BSO is aware of the scope of their responsibilities and authority. The BSO is the biosafety representative that is responsible, as applicable, to:
- verify the accuracy and completeness of applications for legislative documents;
- communicate with the PHAC and the CFIA;
- promote and monitor compliance with applicable legislation (HPTA, HPTR, HAA, HAR), conditions of licence and terrestrial animal pathogen import permits, the biosafety manual, and standard operating procedures (SOPs);
- assist in the development and maintenance of SOPs and the biosafety manual;
- assist with internal investigations of incidents.
Any individual can be appointed as the BSO, whether they are internal (e.g., technician, safety officer, principal investigator) or external (e.g., a third party contractor) to the organization, as long as they have the appropriate qualifications and can allocate sufficient time and resources to perform their job effectively and efficiently. The qualifications of a BSO include:Footnote 13
- knowledge of microbiology at a level that is appropriate to the risks associated with the pathogens and toxins handled and stored under the licence or terrestrial animal pathogen import permit;
- knowledge of the provisions of the HPTA, HPTR, HAA, HAR, and any applicable federal or provincial/territorial legislation; and
- knowledge of applicable biosafety and biosecurity policies, standards and practices appropriate to the pathogens and toxins handled and stored under the licence or terrestrial animal pathogen import permit.
BSOs can obtain the appropriate knowledge through a combination of education, training, and experience.
A large organization with a complex biosafety program or multiple locations may require a full-time BSO with dedicated support staff (e.g., biosecurity experts/advisors). In other situations, the BSO position may not entail a full-time commitment or a dedicated resource (e.g., a laboratory technician who performs other work functions may be the BSO in a small organization with a simple program intent). When allocating the resources for the biosafety program, senior management can estimate the time required for the position based on the size, complexity, and needs of the organization.
2.1.1.4 Institutional biosafety committee
Though not a requirement, an institutional biosafety committee (IBC) can be established to help in the management of the biosafety program, acting as an independent group to review biosafety and biosecurity issues within the organization. An IBC can vary in size depending on the complexity of the organization, with membership reflecting the diverse occupational areas and scientific expertise of the organization.Footnote 7 The IBC can seek advice from departmental specialists with relevant expertise and may at times solicit assistance from independent experts in associated fields, biosecurity experts, local authorities, and the PHAC or the CFIA. When applicable, IBC members are to communicate with the PHAC or the CFIA through the BSO (CBS Matrix 4.1).
Documented terms of reference may be helpful in clarifying important elements, such as IBC membership, meeting frequency, quorum requirements for decision making, reporting considerations (e.g., frequency and format for reporting to senior management), and consideration for regular communication with the BSO. The terms of reference can also define the scope of the committee's work. For example, the IBC may participate in the development of organizational biosafety and biosecurity policies and codes of practice, and review proposed work involving biological material. Other functions of the committee may include contributing to the development of risk assessments (e.g., through provision of subject matter expertise, review of completed assessments), biosafety and biosecurity review of research proposals (e.g., to assess the dual-use potential of life science research), and mediation in disputes over biosafety matters.
2.1.1.5 Personnel
As the front-line workers, personnel are integral to a successful biosafety program. When personnel are committed to biosafety and biosecurity, they use the appropriate measures available to them (e.g., SOPs, personal protective equipment [PPE], primary containment devices). Personnel are also in a unique position to observe deficiencies and report them to management, but that will only happen if they are aware of and motivated to maintain biosafety and biosecurity and can report issues without fear of reprisal.
While the organization must provide a safe work environment and the equipment needed for work to be performed safely, personnel are responsible for appropriately using the resources provided (e.g., SOPs, PPE) to prevent exposure of personnel, accidental release from containment, and security incidents involving regulated materials or related assets (e.g., sensitive information).Footnote 14 In fact, every person knowingly conducting a controlled activity with a human pathogen or toxin is required to take all reasonable precautions to protect the health and safety of the public against the risks posed by that activity [HPTA 6]; additional obligations for individuals are specified in the HPTA and HPTR.Footnote 13
Participation in biosafety and biosecurity training is intended to prepare personnel for responsible handling of pathogens and toxins and increase awareness of biosafety and biosecurity issues. Facility personnel can inform the BSO, the IBC, and senior management if they believe the resources available are insufficient or could be increased or more effectively allocated to improve biosafety and biosecurity.Footnote 15 Personnel may also contribute to the biosafety program through participation in policy or procedure development, and through active advocacy for safe work practices and a secure work environment.
2.1.2 Biosafety policy
The biosafety policy is the overarching biosafety guidance document for the development of the biosafety program. It lays the foundation for a strong culture of biosafety and biosecurity by demonstrating senior management's commitment to biosafety and biosecurity. The biosafety policy defines the organization's biosafety principles and goals for appropriately managing biosafety and biosecurity risks and establishes the commitment to biosafety and biosecurity by outlining the roles and responsibilities for safety and security. Internally, the policy is tailored to organizational-specific biosafety and biosecurity issues such as containment zone activities, products, and services, the threat landscape and the organization's potential vulnerabilities, and serves to guide personnel on proper biosafety and biosecurity procedures. From an external perspective, the biosafety policy states the organization's commitments to biosafety and biosecurity and meeting performance objectives.Footnote 8
2.1.2.1 Objectives and targets
Objectives and targets define the biosafety and biosecurity priorities of the organization. In addition to legislative, regulatory, and CBS requirements, they take into consideration requirements that apply to the organization as a whole and to specific components (e.g., departments, facilities, functions). When setting objectives and targets, it is important to focus on specific actions that describe how:
- management is committed to appropriately address and manage the risks associated with pathogens, toxins, other infectious material and related assets;
- risks associated with the planned work activities will be identified;
- mitigation measures will diminish the risks;
- biosafety and biosecurity practices and procedures will be implemented effectively;
- practices and procedures will be monitored (e.g., regular, unannounced internal evaluation) to confirm that they are being maintained; and
- personnel will be provided with the appropriate biosafety and biosecurity training (i.e., training program).
Objectives that are SMART (i.e., specific, measurable, achievable, realistic, and time-bound) are more likely to be successful in achieving a goal.Footnote 16 An established action plan can help an organization achieve and review its biosafety program objectives. Depending on the complexity of the program, it may be beneficial to assign responsibilities for individual tasks (e.g., monitoring of procedures) and to include timelines for reviewing objectives.Footnote 7
2.2 Biosafety and biosecurity risk assessments
Planning a biosafety program starts by determining an organization's needs, in accordance with program intent, and the resources required to safely and securely handle and store pathogens and toxins.
Risk assessments are used to identify hazards and to categorize and prioritize the associated risks. They are an integral part of a biosafety program, are based on a combination of science, policy, and expert judgement, and can evolve as the level of scientific understanding progresses. A systematic methodology will yield more consistent outcomes, regardless of who is performing the assessment. Descriptions of probability (e.g., remote, likely, frequent), severity of impact (e.g., minor, moderate, critical), and the organization's risk tolerance level (i.e., acceptable or unacceptable) can be defined and used in the assessments.Footnote 7 Risk assessments also determine whether existing mitigation measures are sufficient, and can identify which additional risk mitigation strategies need to be implemented to reduce risks to an acceptable level.Footnote 7Footnote 17
Several different types of risk assessments are required for separate aspects of the biosafety program, as indicated in Table 2-1.Footnote 18Footnote 19 Risk assessments need to be performed at the start of new programs or projects, when changes occur, following events that may have relevance to biosafety or biosecurity (e.g., incidents, regulatory changes), and as part of a regular review process.Footnote 7
| Type of risk assessment | Scope of risk assessment | Example and description of a hazard /threat assessed through this risk assessment |
|---|---|---|
| Overarching Risk Assessment | A broad assessment that provides a top-down view (i.e., at the organizational level) of biosafety and biosecurity to identify hazards through a systematic review of the spectrum of pathogens and toxins, personnel, locations, and activities planned. Outcomes of this assessment will guide the selection of engineering and administrative controls, operational practices and procedures, and will inform the biosafety program as a whole. | Competence of personnel: Personnel working with pathogens and toxins can range in competence from highly experienced scientists, laboratory supervisors, and technicians to inexperienced new personnel and students in training. A teaching or training organization with high turnover of less experienced personnel, trainees, or students may anticipate different hazards than an organization with established expertise. Individuals who may enter a containment zone include visitors, cleaning staff, maintenance staff, and contractors, all of varying competence. |
| Pathogen Risk Assessment | An evaluation of the risk of harm to humans, animals, and the environment. The inherent characteristics of the pathogen (e.g., pathogenicity, communicability, host range) as well as the risks associated with its handling and storing are considered. | Biological hazards: The pathogens and toxins that are handled or stored within the facility, including any material (e.g., culture supernatant, blood, tissue) or animal that may contain them. |
| Biosecurity Risk Assessment | An evaluation in which the regulated materials, and other related assets (e.g., equipment, animals, sensitive information, personnel, non-infectious material) are defined and prioritized, the likelihood of threats, vulnerabilities, and associated consequences are assessed, and appropriate mitigation strategies are recommended to protect these assets against potential biosecurity events. | Theft, misuse and diversion: The ease with which individuals may gain unauthorized access to a secure area of the building (e.g., containment zone) increases the risk of loss, theft, misuse, diversion, or intentional unauthorized release of biological assets (i.e., regulated materials) and related assets (e.g., equipment, non-infectious material, animals, sensitive information). Unauthorized access can also facilitate biosecurity incidents such as sabotage of critical infrastructure or equipment supporting biocontainment. Understanding the threat landscape (i.e., biosecurity event potential, adversary capabilities and their desired pathogens and/or sensitive information) and the vulnerabilities of the organization can help assess the likelihood of, and the risks associated with, unauthorized access. |
| Local Risk Assessments (LRA) | Site-specific risk assessments that identify hazards based on the biological material used and the activities (e.g., culturing a pathogen, centrifuging, pipetting, entering or exiting a containment zone) to be performed, and inform the development of SOPs for safe work practices. | Laboratory activities: The type of work performed will influence risk. Activity-related hazards can be associated with laboratory manipulations (e.g., pipetting or mixing can produce infectious aerosols), but also with the facility (e.g., entering or exiting a containment zone, donning PPE). |
2.2.1 Identify potential hazards or threats
Identifying all potential hazards related to pathogens and toxins within an organization facilitates an accurate assessment of the risks associated with the planned activities. Examples of the types of hazards that may be considered in the process of biosafety program management and identified through different risk assessments are indicated in Table 2-1.
Comprehensive and efficient hazard identification is conducted by collecting, clearly documenting, and communicating information in such a way that others can easily review the process and resulting outcome.Footnote 20 A standardized methodology or approach can be applied for hazard identification regardless of the nature and complexity of the facility.Footnote 7 The BSO and supporting IBC can complete the hazard identification process using information and guidance from both internal and external experts on safety, security, and biosafety program management. Resources for assessment of hazards may include:
- pathogen-specific information sources (e.g., pathogen safety data sheets [PSDSs], ePATHogen – risk group database);Footnote 21Footnote 22
- completed risk assessments (e.g., pathogen risk assessments, biosecurity risk assessment, LRAs);
- incident investigation reports;
- threat assessments by security experts;
- published information (e.g., peer-reviewed, industry standards); and
- discussion groups with internal and external expertise.
2.2.2 Determine mitigation measures
Various physical controls and operational procedures can mitigate the risks associated with handling and storing pathogens and toxins. Results of risk assessments are used to determine when and what additional mitigation measures are needed to maintain biosafety and biosecurity. Many of the identified mitigation measures will already be in place as a condition specific to the licence or terrestrial animal pathogen import permit (including applicable CBS requirements).
The selection of mitigation measures may be considered in a progressive manner according to their effectiveness, including substitution, engineering controls and other physical solutions, safe work practices (e.g., to prevent generation of aerosols), administrative controls (e.g., training, SOPs), and PPE.Footnote 24 Multiple mitigation methods (e.g., physical, operational, PPE) can be combined to work synergistically to reduce the risks associated with handling or storing pathogens and toxins, and in some cases this provides the redundancy needed to mitigate higher risks (e.g., at CL3, CL4). Examples of mitigation measures for consideration are provided in Table 2-2.
| Mitigation measure | Description of mitigation measure |
|---|---|
| Substitution | Substitution with a lower risk pathogen or toxin (e.g., attenuated strain vs. parental strain), or lower risk equipment (e.g., plasticware vs. glassware) may reduce the dependence on engineering and operational controls and are to be considered if they would yield similar results. In some cases, existing engineering and operational controls may be sufficient if the pathogen, toxin, or equipment were substituted with a lower risk alternative. |
| Physical containment | The physical structure and engineering controls that prevent exposure to pathogens and toxins, and their release from the facility. It includes the structures that make up the containment zone, such as walls, floors, ceilings, anterooms, and animal cubicles. It is also the engineering systems that contribute to physical containment include heating, ventilation, and air conditioning (HVAC) systems, decontamination technologies (e.g., autoclave, effluent decontamination system ), and primary containment devices (e.g., biological safety cabinets [BSCs], ventilated cage racks). |
| Security systems | Systems that prevent intentional and inadvertent unauthorized access to the facility or to defined areas within the facility. These may include physical security (e.g., shatterproof windows, access control system, locked doors) and administrative procedures (e.g., process for obtaining access, identifying the internal authority able to grant access). For higher risk pathogens and toxins, appropriate security may include physical barriers and graded protection (e.g., physical or perceived barriers such as fencing, gates, or landscaping delineating the site perimeter; a sequence of high security doors and access controls, which restrict access to areas leading to pathogens and toxins and computer systems storing dual-use and sensitive information). |
| Administrative oversight | A mechanism put in place in an organization to administratively identify, manage, and control biosafety and biosecurity risks. This includes many of the core elements of the biosafety program, as required in the organization's Plan for Administrative Oversight for Pathogens and Toxins in a Research Setting. Internal oversight of pathogens and toxins, and related assets may be provided by the BSO, IBC, or senior management. |
| Personnel management | The defined procedures implemented during and after the hiring processes for the authorization of personnel to access a containment zone (i.e., only to personnel who meet all requirements), and more importantly, to deny authorization to those who do not meet the necessary requirements. This prevents unauthorized entry into the facility by those who do not meet the minimum requirements. Personnel management may include suitability evaluation, ongoing personnel evaluation and reliability assessment, documented training, and for those handling SSBAs, identifying circumstances that may affect an individual's ability to be issued and maintain a valid HPTA Security Clearance. |
| Work practices and procedures | Procedures for safe work practices (e.g., SOPs for entry and exit, good microbiological laboratory practices, donning and doffing PPE, decontamination, incident reporting), and a system defining document control. SOPs for safe work practices are based on LRAs, and prevent exposures and the release of pathogens and toxins from containment. |
| PPE | Defined procedures for the selection, use, and maintenance of PPE, and systems to facilitate availability (e.g., lab coat acquisition and laundering). In a large animal containment zone, and during some incidents (e.g., a spill of biological material outside of a primary containment device), PPE provides the only barrier between the user and the pathogens and toxins present. |
3. Implement the biosafety program
The size and complexity of an organization will dictate the specifics of its biosafety program. However, there are core elements that, when assembled on a strong foundation of planning and commitment, provide a solid framework for an effective program. Implementation of these elements corresponds to the "do" phase within the PDCA cycle. The core biosafety program elements, detailed below, are more easily standardized across a facility when all levels of the organization are involved in their development.
3.1 Core elements of the biosafety program
Effective implementation and management of the biosafety program requires thorough documentation of all core elements such that they are readily available to senior management. The core elements of the biosafety program include institutional biosafety policies, plans, and practices that contribute to the biosafety program as a whole. The scope of the organization will influence how these are developed and documented. More stringent containment or biosecurity needs, based on risk assessments, will generally lead to more detailed information. These documents can be developed with contributions from individuals with expertise in various fields, such as the IBC, research or technical staff, security experts, and a medical advisor, as necessary.
This collection of documents is the most effective tool for documenting and communicating the objectives of the biosafety program, but only if it is comprehensive, regularly updated, and appropriately communicated to those who need the information. A summary, abridged version, or descriptions, as appropriate can be developed specifically for personnel to increase their awareness of how procedures, processes, and activities relate to each other. An effective tool would provide clear direction on how to meet the objectives and targets of the biosafety program.
This information can be maintained as one comprehensive document (e.g., as a biosafety manual) that applies to all facilities to allow the information to be readily available to all personnel. Alternatively, it can be maintained as one core document containing information that applies to the entire organization, including separate information, for different facilities, departments, or containment zones within the organization (CBS Matrix 4.1) so as not to overwhelm personnel with information they do not need and, in some cases, to which they should not have access (e.g., sensitive information). Links or references to facility- or containment zone-specific elements and SOPs for safe work practices can be provided to those who need them, based on work activities and the containment zones in which they work.Footnote 8 In this way, individual elements in printed form may or may not be housed in a single physical location. For example, in CL3 and CL4 zones, descriptions of the training program and medical surveillance program may be stored outside the containment zone, while SOPs may be kept inside the containment zone where they are needed.
3.1.1 Standard operating procedures for safe work practices
SOPs provide personnel with easy access to the detailed instructions that protect them and the community from the risks posed by the pathogens and toxins handled and stored in the facility. SOPs are specific to the activities (e.g., entry and exit, donning PPE) and work being conducted in the containment zone, and are based on LRAs. Processes for the documentation, review, updates and approval of SOPs are also documented in SOPs.
In addition to safe work practices applicable to a specific containment zone, there may be organization-wide SOPs (e.g., waste management, equipment maintenance, routine verification of BSCs) that are developed. SOPs may also specify the person responsible for verifying that the maintenance has been completed, and indicate how personnel can verify whether equipment is safe to use (e.g., by identifying the last testing date on BSCs, verifying gauge readings and inward airflow prior to BSC use).
3.1.2 Physical design
A description of the physical design of the containment zones allows for contextualization of SOPs (e.g., where to don PPE, where to wash hands). Depending on the specific type of laboratory work area, a general description may be sufficient, whereas a more detailed representation (with floor plans) may be needed for facilities with more stringent criteria. Using floor plans or "as built" drawings to visually represent the containment zone and adjoining spaces can help personnel to identify key physical features of the space that support containment (e.g., the containment zone perimeter, anterooms, pressure differential monitoring gauges, animal cubicles, post mortem [PM] rooms). Additionally, providing a visual representation (e.g., schematic) of the containment barrier can assist personnel to quickly identify the critical door(s) essential for maintaining containment where inward airflow is provided, and recognize where PPE (e.g., gloves, shoe covers, disposable gowns) dedicated to the area is removed when exiting. In some cases (e.g., part of the facility where SSBA are handled or stored, high containment levels), access to some of the physical design information may be restricted to authorized personnel. To support security mitigation strategies, it is important that detailed information related to physical design is shared on a need-to-know basis.
3.1.3 Biosecurity plan
A biosecurity plan outlines the security measures to be implemented to prevent the loss, theft, misuse, diversion, or intentional unauthorized release of pathogens, toxins, and related assets. All facilities are to have a biosecurity plan that addresses all of the mandatory elements specified in Matrix 4.1 of the CBS.
The complexity and level of detail of the biosecurity plan will depend on the nature of the organization's activities, the biosecurity risks posed by the pathogens and toxins and related assets, including sensitive information within their possession.Footnote 23 For example, a small CL2 facility where only controlled activities with very low dual-use potential involving RG2 pathogens are conducted is unlikely to require the same level of security features as a facility where SSBAs, RG3 or RG4 pathogens are handled. Security features also tend to be more stringent for facilities where work involves animals or is related to vaccine development or production. The complexity of security measures and the level of detail of the biosecurity plan always depend on the results of the biosecurity risk assessment, which is conducted by the organization.
The biosecurity plan may contain sensitive information and access should only be made available to personnel who need it. Redacted biosecurity plans, which omit security sensitive details (e.g., details which point to physical security weaknesses, exact locations of SSBAs) but include biosecurity mitigation strategies (e.g., procedures for reporting suspicious activities or individuals, personnel security screening techniques) can be made available to additional facility personnel who need it. This version can include (or reference) biosecurity-specific SOPs (e.g., access requests) and can be used for personnel training. It can also describe how the dual-use potential of research, including the outputs of this research (e.g., new pathogens, new procedures, information), will be identified, assessed, managed, and controlled, as required in the organization's Plan for Administrative Oversight for Pathogens and Toxins in a Research Setting.Footnote 12 Providing this information to personnel will help them gain a better understanding of the processes involved, as well as their responsibilities and those of others within the organization (e.g., BSO, senior management, IBC). The biosecurity plan can also outline the biosecurity training provided to personnel to identify and report potential threats or incidents. Additional information on biosafety and biosecurity training can be found on the PHAC Training PortalFootnote 24.
3.1.4 Medical surveillance program
The medical surveillance program is intended to prevent, detect and treat illness related to pathogen or toxin exposure. It also provides response mechanisms for early detection and assessment of laboratory-acquired infection or intoxication (LAI) following an exposure to provide early treatment to prevent or reduce the severity of illness, and to prevent secondary transmission in the community. Due to the wide range of pathogens and toxins handled, and the different types of work areas and research taking place in Canada, no single approach to medical surveillance would be appropriate for all facilities. Each medical surveillance program, even the need for one, is to be tailored to the pathogens and toxins that may be encountered and to the needs of the facility, based on the overarching risk assessment and LRAs. The medical surveillance program includes assessment for aspects of personnel health that may affect susceptibility to infection (e.g., immune status) and promotes prevention and treatment of infection (e.g., vaccination, pre-exposure and post-exposure prophylaxis). Overlap and duplication of efforts can be reduced by expanding the program to include aspects of workplace health, such as chemical exposure, allergies (e.g., to latex, animal dander, dust), ergonomic considerations (e.g., physical strain), and excessive noise as part of a comprehensive occupational health and safety program.
Infection with a pathogen or exposure to a toxin does not always quickly lead to symptoms or overt disease. In addition, infections can be symptomatic or asymptomatic. The implementation of medical surveillance practices to identify a seroconversion may allow for the recognition or confirmation of recent or previous infection or disease. Seroconversion can occur following initial infection and clearance of the pathogen, and with certain pathogens (e.g., human immunodeficiency virus [HIV], Mycobacterium tuberculosis, hepatitis C virus, certain fungi, prions), may indicate a post-infection latency period prior to onset of disease.
The confidentiality of medical information is to be protected (e.g., kept with a health professional) to the extent possible and in accordance with institutional policies. For example, should a report to the employer be required following an assessment, the health professional can simply return the decision (e.g., "meets the medical requirements", "does not meet the requirements") to the employer without providing details as to the reasons. Following an exposure incident involving more than one individual, the employer can be informed via an anonymized report that an individual underwent treatment (or not) and the outcome (e.g., infection, no infection, resolved with treatment).
The medical surveillance program may include pre-placement medical evaluations, situations where vaccination may be required, ongoing medical surveillance of personnel, and a plan for post-exposure response and treatment of disease. These are described in the following sections.
3.1.4.1 Pre-placement medical evaluation
The pre-placement medical evaluation provides an opportunity to assess conditions that may affect an individual's susceptibility to infection or disease, or the ability to treat the disease following infection. These may include:
- prior and ongoing medical issues or conditions (e.g., immune suppressant therapy, HIV infection, pregnancy) that may affect susceptibility to infection, or severity of infection should it occur;
- current medications;
- allergies or sensitivities (e.g., to medicines, animals, environmental allergens); and
- prior immunizations or natural infection (e.g., mumps, flu) that may confer immunity to the pathogens handled.
During the pre-placement medical evaluation, consideration can be given to collecting a serum sample for testing to determine pre-existing immunity from prior vaccination or infection. Serum can also be stored long-term to establish baseline seroreactivity for comparison with samples collected following a potential exposure.
Medical requirements are to be clearly documented (e.g., in SOPs). It is recommended that the information be made available to the health professional (e.g., on a form) to clearly inform them of the reason for the assessment (e.g., the pathogens that will be handled) and the points to include in the assessment.
3.1.4.2 Vaccines
Vaccines are regulated biological products administered to induce a protective immune response. There are many commercially available Health Canada authorized vaccines, and the risks and benefits of their use in the work setting can be discussed with a health professional. Most Canadians receive several vaccines in childhood and adolescence to protect them from infection (e.g., diphtheria, polio, tetanus, measles, varicella, human papillomavirus, hepatitis B virus). In some cases, the immunity conferred by vaccination can wane over time (e.g., tetanus, measles). This can be verified with antibody titres and vaccination can be repeated when needed (i.e., "booster shot"), for example, in order to mitigate biorisks.Footnote 25
3.1.4.3 Ongoing medical surveillance
Ongoing medical surveillance (e.g., annual health assessment) provides an opportunity to detect changes in health status that could increase risk of infection or disease (e.g., changes to immune system, pregnancy, new medication, increased stress). It also provides an opportunity to detect unnoticed infections, particularly for pathogens that may cause asymptomatic infection, or that cause latent infection, mild symptoms, or symptoms of common diseases (e.g., common cold, flu). It allows for the infection to be detected and treated before serious disease develops or transmission in the community occurs. For example, most cases of infection with hepatitis C virus are asymptomatic; however, early detection and treatment can prevent serious liver damage that can occur over the course of the infection.
3.1.4.4 Post-exposure response plan
The immediate steps to take following an exposure to a pathogen or toxin may be included as a component of the emergency response plan. SOPs can include pathogen or toxin specific information where it is warranted. An occupational health care provider or practitioner, the IBC, the BSO, the occupational health and safety advisor, and/or an infectious disease specialist may be consulted to identify the optimal response for suspected exposure to different pathogens and toxins, considerations for the types of exposure (e.g., cutaneous, mucosal, inhalation), and arrangements made to have appropriate medical countermeasures and treatments available following an exposure (e.g., antitoxin, post-exposure prophylaxis), particularly when these are not commonly available. A liaison with a local health care facility (e.g., medical centre, hospital) may expedite diagnosis and treatment for higher risk pathogens and pathogens and toxins that are unusual or difficult to diagnose.
3.1.5 Emergency response plan
The emergency response plan prepares personnel to take the appropriate actions in the event of accidents (e.g., spill, damaged PPE), medical emergencies, power failure, loss of containment, or other foreseeable emergency scenarios to prevent an exposure to pathogens and toxins or their release from containment. The response measures are appropriate to the scale and nature of these potential emergency situations, and can include procedures for cleaning, disinfecting, and dressing a wound or exposure site, and can indicate where to seek medical assistance within or outside the organization.
When developing an emergency response plan, the organization will need to include the contact information of the individual(s) to inform when an incident occurs. Establishing a "chain-of-notification" facilitates notification of the appropriate individuals so they can promptly initiate a response; the chain-of-notification may include supervisors, containment zone and program officials, management, the BSO, and relevant regulatory (e.g., PHAC, CFIA) or public (e.g., police, fire department) authorities.Footnote 26 Well coordinated notification of public authorities can be of particular importance for security-related emergencies such as an active shooter, a cyberattack preventing normal operations, the theft of regulated materials or related assets (sensitive information), the detection of an intruder in the building (e.g., disgruntled former employee), or the presence of potentially hostile activists at the site-perimeter.
3.1.6 Training program
A training program is essential to the effective implementation of the biosafety program and may include education (e.g., learning theoretical knowledge in a classroom, online course), training (e.g., development of specific skills), and supervision. Current and future training needs for the organization are identified through a training needs assessment that is reviewed annually.Footnote 23 Through training, personnel can be made aware of the characteristics of the pathogens in use, the appropriate mitigation measures to prevent exposure and release, and the security measures in place to prevent biosecurity incidents.Footnote 8 This includes initial training, and annual refresher training, on the emergency response plan. Establishing a training program that informs and educates individuals about their responsibilities within the containment zone and the organization is essential to the success of the biosafety program.Footnote 26 Biosafety and biosecurity can only be achieved when all individuals within an organization follow established procedures.
To support and promote compliance, a comprehensive training program highlights the:
- risks associated with work activities;
- means to prevent exposure to hazards;
- importance of complying with biosafety and biosecurity requirements;
- specific roles and responsibilities of personnel; and
- potential consequences of not adhering to SOPs.
It is recommended that the training program consider individual responsibilities (e.g., for managers, supervisors, laboratory personnel) and the existing knowledge and understanding of the subject material by personnel. The biosafety and biosecurity training program can include:Footnote 7Footnote 27
- objectives and goals of the training program;
- how and when to perform training needs assessment(s);
- who is responsible for compiling training material and delivering training sessions;
- when the training sessions are to take place, and their frequency (e.g., for personnel unable to attend a given session, for retraining purposes);
- how the training sessions are to take place (i.e., organization-wide versus site-specific, how the material will be presented);
- to whom specific training sessions are given;
- responsibilities for maintaining training records;
- restrictions on personnel authorization to access a containment zone unsupervised or to prevent them from performing tasks until training is complete; and
- how the effectiveness of training is determined (e.g., evaluations, drills).
An effective training program accounts for all individuals working directly with biological material and individuals who may indirectly come into contact with it (e.g., maintenance, cleaning, security staff). Training can be customized to specific groups of personnel, based on their work in the containment zone (e.g., laboratory technician or technologist culturing a pathogen, maintenance worker replacing lights). Training may also be relevant for personnel responsible for the implementation of mitigation strategies outlined in the biosecurity plan to better understand why specific security measures are in place and must be followed.
3.1.7 Decontamination and waste management
An effective waste management system prevents untreated biohazardous waste (including effluent waste) from entering the general waste stream or sewer system, therefore preventing the release of contaminated material from containment. It is critical for reducing the risk to personnel and preventing greater community exposure to pathogens and toxins. Procedures for decontamination and disposal must comply with all applicable (i.e., federal, provincial or territorial, and municipal) legislation. These can include SOPs for the segregation (e.g., sharps waste, liquid waste, solid waste), labeling, decontamination, validation, verification, and collection process of biohazardous waste generated in the facility.
Waste management considerations to be addressed may include:
- preparation procedures for waste disposal (e.g., collection, bagging);
- segregation of waste and labeling for identification;
- decontamination methods (e.g., incineration, autoclave, chemical disinfectants) for different types of waste generated (e.g., infectious laboratory waste, liquid effluent, sharps, infected animal tissue, non-infectious material);
- movement of contaminated waste outside the containment zone to another location for decontamination (e.g., in a labelled, leak proof container);
- validation of decontamination methods and routine verification; and
- the use of third-party waste disposal companies, as applicable, including verification of their compliance.Footnote 28
3.2 Communication plan
Establishing consistent, credible, and clear communication fosters understanding and cooperative relationships.Footnote 8 Open and transparent communication builds trust within an organization as well as with the surrounding community. A communication plan defines the purpose and approach that the organization will take in communicating biosafety issues both within the organization and externally.Footnote 8 Furthermore, an effective communication plan is designed to address public concerns about the risks associated with the facility and its operation. It includes early and ongoing engagement with all interested parties. Considerations for development of the communication plan may include:Footnote 29
- roles and responsibilities;
- standard templates, formats, or documents;
- procedures to promote internal communication;
- procedures for communicating sensitive or confidential information, including procedures to obtain authorization;
- procedures to manage communication with external parties; and
- an escalation process for resolving communication-based conflicts, concerns, or issues.
Information can be disseminated in many ways (e.g., verbal, physical media [i.e., written or printed], electronic) and may include material posted in public locations, email communication, websites, factsheets, and forums. It is preferable for all formal communication to be documented and stored in an accessible, safe, and secure location.
3.2.1 Internal communication
In addition to training, an efficient internal communication system provides personnel with the information they need regarding the biosafety program, including hazards associated with the organization's operations. Personnel are more likely to be motivated and committed to achieving the biosafety program objectives and goals if they are kept informed on all issues surrounding the biosafety program, including its review and related modifications.Footnote 8Footnote 27
A culture of biosafety and biosecurity can be promoted by being open with information, encouraging questions, and demonstrating a willingness to be self-critical.Footnote 30 Taking a non-punitive approach (e.g., not placing blame, but rather describing incidents in terms of root causes while leaving out personal or identifying information) further motivates personnel to report incidents, unsafe practices, and changes in personnel behaviour (e.g., potential insider threat). Communication between personnel at all levels and functions within an organization is essential to effectively manage the biosafety program. As such, it is recommended that senior management prioritize communication with personnel and encourage communication vertically and laterally between departments and teams.
Regular team, departmental, and interdepartmental meetings (including IBC meetings, and frequent communications with the BSO) facilitate open and active lines of communication and discussion regarding the biosafety program. The frequency of meetings and the method(s) for distributing related information (e.g., agendas, meeting minutes, records of decision) can be outlined in the communication plan. An overview of meeting topics and outcomes can be made available to all personnel so they remain aware of ongoing biosafety and biosecurity activities even if they are not participating in the meetings.
3.2.2 External communication
The communication plan may include procedures for proactively or reactively providing external parties (e.g., public, regulators, funding agencies, media) with information of relevance or interest, including the science taking place in the facility and how it benefits the public; this serves to promote trust and engagement that benefits both the organization and the broader community. An effective communication approach can emphasize the positive aspects of the organization's activities (e.g., development of a vaccine, that a containment zone is a safe and secure place to handle pathogens and toxins). This may include descriptions of mitigation measures as well as reports of internal and external audits and findings when describing hazards.
A well-planned and efficient system for receiving, prioritizing, documenting, and responding to communications from external parties, which quickly directs external correspondence to the appropriate individual or department, facilitates a timely response to external questions and concerns. Promptly and clearly addressing external communication adds to community trust by improving understanding, supporting cooperative professional relationships with external parties, and promoting the organization's professional image. Keeping both the format and the content of external communications consistent, and using simple language while avoiding technical terms and abbreviations will prevent misinterpretation or misunderstanding of the information. Similarly, keeping records of external communications and replies to external inquiries provides a point of reference to establish consistent answers to similar inquiries (i.e., standard lines).Footnote 8Footnote 27
3.3 Records and documentation
Systems for the management of records and documentation can improve efficiency and compliance within the biosafety program. How the organization collects and maintains records and documentation reflects the culture and needs of the organization. For example, the approach to maintaining (and retaining) records and documentation may be influenced by the size of the organization, the program intent, regulatory and legislative requirements, and the complexity of internal processes and systems.
3.3.1 Document management
Documenting planned procedures or activities in writing is crucial for effective day-to-day management of the biosafety program. Documents can be stored in any medium (e.g., paper, electronic) that suits the organization, as long as it is useful, legible, easily understood, and accessible to personnel who need it and, where appropriate, to external parties requesting it. Control of documents may occur by a variety of methods including the development of a standardized process for creating, modifying, and updating documents, and maintaining an efficient document distribution (e.g., to notify personnel of updates or new documents) and retention (e.g., to avoid disposing of required documents) systems.Footnote 8Footnote 27 A document control program is most effective when:
- the contact person and affiliation, where applicable (e.g., team, group, department), or resource for a specific document are easily identified;
- documents are regularly reviewed, updated, and approved by authorized personnel as needed;
- the most current version of each document is easily found and readily available to personnel who require it; and
- obsolete documents are removed or archived, in accordance with retention requirements (CBS Matrix 4.9).
3.3.2 Records management
Records provide written and detailed evidence of activities performed or results achieved within the biosafety program. Examples of records include validation records and verification logs, training records, incident investigation reports, and inventories. A system that conforms to existing policies and standards for maintaining records can facilitate preparation for reviews and audits, and can support the organization in meeting regulatory requirements.Footnote 31 The processes outlining record identification, maintenance, and distribution may be described in SOPs.Footnote 8 An efficient record management program:
- supports the maintenance of legible, identifiable, and retrievable records that are traceable to the activity, product, or element of the biosafety program that led to their creation;
- assists in fulfilling document retention requirements;
- supports storage of records in a secure space, with access limited to authorized personnel;
- can be associated with a reporting system, with notifications when there is deviation from set or acceptable limits; and
- can be used for ongoing improvement.
3.3.2.1 Inventory
An inventory system prevents the loss and theft of pathogens and toxins by creating, maintaining, and reconciling a list of the stored biological assets with the material actually present. It may be integrated into a broader inventory system that tracks newly acquired material and the shipping or transfer of material. Maintenance of an inventory of regulated materials in long-term storage (i.e., greater than 30 days) is a required element of a biosafety program (CBS Matrix 4.9). Though the format of the inventory is not prescribed, it is to account for the risk group and location of the material. In addition, the inventory for higher risk pathogens and toxins (e.g., RG3 and RG4 pathogens, SSBA toxins) must specifically identify the pathogen or toxin (e.g., the genus, species, or strain of the pathogen; physical state; quantity present in a facility or specific containment zone) and be sufficiently detailed to allow the detection of any missing or stolen material. For example, the date a pathogen was received, transferred or produced, reference to a purchase order, or the individual who stored the pathogen may facilitate the identification of additional information regarding the pathogen (e.g., culture conditions, passage number, specification information).
Considerations when developing an inventory control system include:
- standardized methods for documenting all (biological) assets in long-term storage in a facility;
- clearly and accurately labelling vessels that contain biological material ;
- a format that is accessible and easy to update by authorized individuals (e.g., written ledger, electronic database, spreadsheet);
- security measures to limit access to authorized individuals;
- augmenting audit frequency/accountability measures for higher-risk pathogens and toxins; and
- policies outlining timeframes for inventory reviews and audits.
Reviews or audits are necessary for verifying that new pathogens or toxins (e.g., obtained from other sources, from new cultures, generated by genetic modification) are captured in the inventory and that material that is no longer stored on the premises (e.g., appropriately transferred, inactivated, destroyed) is removed from the inventory. The frequency of review will depend on the risks associated with the material, results of past audits, the identification of missing material, or related incidents.
4. Ongoing improvement of the biosafety program
An effective biosafety program is agile and can be quickly adapted to address identified weaknesses or gaps. The outputs of the different types of reviews described in this section can identify areas for improvement and recommendations for further action or resources needed.Footnote 7 Additionally, these outputs can indicate aspects of the program that are successful or can be used to guide improvements to other areas of the biosafety program. Any proposed changes will need the approval of senior management, who may prioritize the implementation of mitigation measures in accordance with risk and available human and financial resources.
Biosafety program reviews can include reactionary evaluations of particular program elements (e.g., following an incident), proactive, scheduled reviews of select elements (e.g., audit, inspection), and comprehensive reviews of the entire program. The frequency and scope of such assessments may be influenced by the risks associated with the work involving pathogens and toxins and related assets (i.e., greater risks may warrant more frequent and robust reviews).
4.1 Incident investigation
Following an incident, a thorough investigation will identify the incident's root cause(s) and determine appropriate mitigation measures needed. These can include improvements to existing measures as well as new measures to prevent a recurrence or to reduce the consequences should a similar incident occur. Thorough review of individual incident investigation reports will confirm whether root causes have been identified for all contributing factors, whereas review of reports collectively can identify trends (e.g., common root causes) that may require mitigation at the organizational level. The results from incident investigations can be used to update biosafety program elements (e.g., training program, SOPs, emergency response plans) to prevent or mitigate future occurrences.
4.2 Internal audits and inspections
A review of biosafety program elements may take place during or in response to audits and inspections, which serves to proactively identify hazards, verify compliance with biosafety and biosecurity requirements, and identify deficiencies and areas in need of improvement in an organization's biosafety program. Many organizations implement a cooperative inspection program whereby personnel are responsible for recurrent self-assessments. Such inspection programs are usually combined with less frequent, but more in-depth, inspections by the BSO, members of the IBC, or a third party contactor. The BSO is responsible to report findings of periodic inspections to the licence holder or the terrestrial animal pathogen import permit holder, as applicable (CBS Matrix 4.1). Expectations for the frequency and scope of inspections and audits can be included within the organization's SOPs. Random unannounced inspections and audits serve to verify ongoing compliance with applicable biosafety requirements and procedures, and to identify deficiencies. Random or scheduled audits and inspections related to the biosafety program may include:Footnote 7
- regular informal inspections of work areas and surfaces (e.g., by supervisors, personnel);
- documentation and record reviews;
- inventories;
- incident reports;
- results of self-assessments;
- results of validation and verification logs; and
- SOPs, and evidence of compliance with these;
- equipment maintenance and performance evaluations; and
- facility evaluations.
Establishing a formal assessment process to review and evaluate compliance and the effectiveness of the organization's biosafety program will help the BSO to promptly follow-up on deficiencies, agree on target dates for corrective actions, and verify the implementation of corrective measures. Audits and inspections are meant to promote compliance, and not to lay blame (i.e., identify areas for improvement). The individual performing the audit (e.g., BSO) can work with facility personnel to identify appropriate immediate and long-term measures to correct deficiencies, and to prioritize the implementation of measures based on risk.
Considerations when developing an audit or an inspection program can include:Footnote 7
- the scope of the audit or inspection;
- the frequency of audits and inspections, based on an LRA and compliance history;
- the procedure for audits and inspections;
- how the results of the audit and inspection will be measured and reported; and,
- whether or not unannounced audits and inspections may be performed.
The results of an audit or inspection can be provided to senior management, the licence holder, the terrestrial animal pathogen import permit holder, or the BSO in the form of a written or electronic report. The report can indicate findings (e.g., deficiencies, trends, positive observances) and recommended corrective and preventive actions.Footnote 27 Metrics (e.g., number and severity of non-compliances), and the associated metadata (e.g., trends over time) can be used to track biosafety program performance from year to year, and to evaluate whether biosafety program targets are being met.
4.3 Biosafety program and management review
Planned reviews of the entire biosafety program help maintain the program's suitability, adequacy, and effectiveness.Footnote 7 The organization may choose to develop periodic (e.g., monthly, quarterly, annual, biennial) reports in which accomplishments are assessed against established objectives and targets. The frequency of reviews and reports do not have to correspond. Certain aspects may warrant more frequent reviews (e.g., SOPs, training), particularly when internal inspections and audits identify issues relating to them. On the other hand, a thorough review of the entire biosafety program, which can be very time-consuming, may only be accomplished once or twice in a licence or terrestrial animal pathogen import permit cycle. An external third party may be enlisted to conduct an analysis of the biosafety program to evaluate whether organizational goals and objectives are being met, and to identify any gaps in the program.Footnote 7Footnote 8Footnote 27
The biosafety program review can include information obtained from:Footnote 7Footnote 20
- audits and inspections;
- incident investigations;
- compliance data regarding lagging indicators (e.g., number and severity of non-compliances, number and severity of incidents) and leading indicators (e.g., percentage of personnel who have completed training);
- internal communications, including personnel or IBC suggestions and feedback;
- status of risk assessment activities;
- status of preventive and corrective actions;
- follow-up actions from previous reviews;
- management decisions (e.g., meeting agendas, records of decisions); and
- changes (e.g., organizational, program intent) that could affect the biosafety program.
The full program review addresses broader questions than periodic reviews, including:
- Are the appropriate procedures, processes, and plans in place to meet the objectives and targets outlined in the biosafety policy?
- Is the biosafety program understood and being appropriately communicated, implemented, and maintained within the organization?
- Are hazards being identified and managed effectively?
- Is regulatory compliance achieved?
- Is the inspection process effective?
- Are the SOPs effective?
4.4 Implementation of corrective and preventive measures
To address deficiencies in the biosafety program, both short-term solutions that directly address deficiencies (i.e., corrective measures) and long-term, systemic approaches (i.e., preventive measures) may need to be implemented. Corrective measures address issues or problems (e.g., root causes of an incident) that have led to an incident, whereas preventive measures address identified issues or problems that may lead to an incident.Footnote 32
Key steps in applying appropriate corrective and preventive measures are:Footnote 7
- Identify deficiencies and root causes of incidents.
- Develop measures to prevent the problem(s) from occurring or recurring. Potential solutions may be determined through root cause analysis or similar processes, but may require direction or support from external experts (e.g., workplace investigators, biosafety experts, engineers, subject matter experts).
- Implement the proposed and developed measures. Considerations for implementation include prioritizing the measures needed according to the risk they will mitigate, the threats and likelihood of incidents, and the resources and time required to implement the measures. Short deadlines can be set to implement quick fixes (e.g., update SOPs, repair equipment, provide remedial training) to avoid recurrence of similar incidents, but complex or extensive measures may take a long time to be fully implemented (e.g., new building infrastructure).
- Record and monitor the effectiveness of these measures to address the underlying issues within the organization.
An action plan can be developed with realistic timelines for the implementation of corrective and preventive measures, along with timelines on how and when the effectiveness of the new measures will be verified.Footnote 8
4.5 Managing change
Improving the biosafety program will involve some degree of change. This may include minor modifications to SOPs, the implementation of new SOPs, and remodeling, renovating, and repurposing a containment zone. Changes may be reactionary, for example resulting from an incident, the introduction of a new pathogen, or externally imposed requirements (e.g., legislative changes). They may also be proactive, for example when seeking to improve the safety and security margins, efficiency, or effectiveness, or simply keeping the biosafety program up to date and pertinent. Regardless of the nature of the changes made, improvement of one aspect of the biosafety program may affect another. Using standardized methods and procedures (e.g., SOPs) to direct the efficient handling of all changes to the biosafety program or its components will minimize the impact of the change. Effective change management will include:
- a process to keep the BSO and senior management informed of any planned changes to allow appropriate actions to be taken (e.g., approval process, informing the PHAC or the CFIA when required);
- an assessment of the benefit of change versus potential impact on other areas of biosafety and biosecurity. A seemingly beneficial change to one aspect (e.g., simplifying entry to the containment zone to increase work efficiency) may have unexpected detrimental effects to another (e.g., increased biosecurity risks);
- an established approval process;
- an implementation plan for the change. This may include updating or developing new SOPs, planning a shutdown for renovations or installation of new equipment, or the addition of a new pathogen; and
- a process to communicate the plan to personnel, including when the change will come into effect and ensuring training is conducted and documented when applicable.
4.6 Ongoing improvement
The PDCA process is a continuous cycle, and once established, does not have a beginning or an end. At completion of a full cycle, the newly applied actions initiate the beginning of the next cycle; this progression is important to continually adapt and improve the biosafety program. Ongoing effort and support of the biosafety program from all levels within the organization are necessary to continue the forward momentum and maintain the relevance of the program so that it continues to meet the organization's commitment to biosafety and biosecurity.
5. Glossary
While some of the definitions provided in the glossary are universally accepted, many of them were developed specifically for the CBS, the CBH, or the Canadian Biosafety Guideline: Biosafety Program Management; therefore, some definitions may not be applicable to facilities that fall outside of the scope of the CBS.
| Term | Definition |
|---|---|
| Accident | An unplanned event that results in injury, harm, or damage. |
| Animal cubicle | A room or space designed to house an animal (or animals) where the room itself serves as primary containment. These spaces are used to house large-sized animals (e.g., livestock, deer) or small-sized animals that are housed in open caging (i.e., not primary containment caging). |
| Anteroom | A room, or series of rooms, inside the containment zone, used to separate "clean" areas from "dirty" areas (i.e., areas with a lower risk of contamination from those with a higher risk of contamination), for personnel, materials, and animal entry/exit across the containment barrier, and for entry to/exit from animal rooms, animal cubicles, and post mortem rooms. The negative differential air pressures in containment zones where inward airflow is required can be more effectively maintained through the presence of an anteroom. An anteroom may also provide appropriate space at the points of entry/exit to don, doff, and store dedicated and additional personal protective equipment, as required. |
| Authorized personnel | Individuals who have been granted unsupervised access to the containment zone by an internal authority (e.g., the containment zone director, biological safety officer, another individual to whom this responsibility has been assigned). Access is dependent on personnel completing training requirements and demonstrating proficiency in the standard operating procedures, as determined to be necessary by the facility. |
| Biocontainment | See "Containment". |
| Biological material | Pathogenic and non-pathogenic microorganisms, proteins, and nucleic acids, as well as any biological matter that may contain microorganisms, proteins, nucleic acids, other infectious agents, or parts thereof. Examples include, but are not limited to, bacteria, viruses, fungi, prions, toxins, genetically modified organisms, nucleic acids, tissue samples, diagnostic specimens, environmental samples, live vaccines, and isolates of a pathogen or toxin (e.g., pure culture, suspension, purified spores). |
| Biological safety cabinet (BSC) | A primary containment device that provides protection for personnel, the environment, and the product (depending on the BSC class) when working with biological material. |
| Biological safety officer (BSO) | An individual designated for overseeing the facility's biosafety and biosecurity practices. |
| Biosafety | Containment principles, technologies, and practices that are implemented to prevent unintentional exposure to pathogens and toxins, and their accidental release. |
| Biosafety manual | A collection of facility-specific documents that describe the core elements of a biosafety program (e.g., biosecurity plan, training, personal protective equipment) that are applicable to the containment zone. The information can exist as a single paper or electronic document or as a collection of documents. |
| Biosafety program | A program that describes institutional plans and policies that facilitate the safe handling and storing of regulated materials, and prevent their release from the containment zone. Core elements of a biosafety program include, but are not limited to, a biosafety manual, a comprehensive training program, a medical surveillance program, an emergency response plan, standard operating procedures, and a biosecurity plan. |
| Biosecurity | Security measures designed to prevent the loss, theft, misuse, diversion, or intentional release of regulated materials, and other related assets (e.g., personnel, equipment, non-infectious material, animals, sensitive information). |
| Biosecurity event | A biosecurity act involving or related to regulated materials, sensitive information, or equipment that could cause disease or harm to people, animals, or both, as well as to the organization. |
| Biosecurity risk assessment | A risk assessment in which the regulated materials, and other related assets (e.g., equipment, animals, sensitive information, personnel, non-infectious material) are defined and prioritized, the likelihood of threats, vulnerabilities, and associated consequences are assessed, and appropriate mitigation strategies are recommended to protect these assets against potential biosecurity events. |
| Community | Encompasses both human (i.e., the public) and animal populations. |
| Containment | The combination of physical design parameters and operational practices that protect personnel, the immediate work environment, and the community from exposure to biological material. The term "biocontainment" is also used in this context. |
| Containment barrier | The physical structures or barriers that create a boundary between "clean" and "dirty" areas or between areas of lower contamination and higher contamination (e.g., between the laboratory work areas, large scale production areas, animal rooms, animal cubicles, or post mortem rooms, and outside that containment area). The containment barrier itself is created by the walls, doors, floors, and ceilings of a room that physically enclose the areas within containment, as well as inward airflow at critical doors (where inward airflow is required). |
| Containment level (CL) | Minimum physical containment and operational practice requirements for handling regulated materials safely in laboratory, large scale production, and animal work environments. There are four containment levels ranging from a basic laboratory (i.e., CL1) to the highest level of containment (i.e., CL4). |
| Containment zone | A physical area that meets the requirements for a specified containment level (CL). A containment zone can be a single room (e.g., a CL2 laboratory), a series of co-located rooms (e.g., several non-adjoining but lockable CL2 laboratory work areas), or it can be comprised of several adjoining rooms (e.g., a CL3 suite with dedicated laboratory areas, and separate animal rooms or animal cubicles). Dedicated support areas, including anterooms with showers and "clean" and "dirty" change areas where required, are considered to be part of the containment zone. |
| Controlled activities | Any of the following activities referred to in subsection 7(1) of the Human Pathogens and Toxins Act: possessing, handling or using a human pathogen or toxin; producing a human pathogen or toxin; storing a human pathogen or toxin; permitting any person access to a human pathogen or toxin; transferring a human pathogen or toxin; importing or exporting a human pathogen or toxin; releasing or otherwise abandoning a human pathogen or toxin; or disposing of a human pathogen or toxin. |
| Critical door | Any door of a containment zone, animal cubicle, or post mortem room where inward airflow is required. |
| Decontamination | The process by which materials and surfaces are rendered safe to handle and reasonably free of microorganisms, toxins, or prions; this may be accomplished through disinfection, inactivation, or sterilization. |
| Decontamination technology | Equipment proven by validation to render materials safe to handle and reasonably free of microorganisms, toxins, or prions. Examples include autoclaves, incinerators, tissue digesters, and effluent decontamination systems. |
| Deficiency | An observation of non-conformity with the applicable requirements of the Canadian Biosafety Standard, a Biosafety Directive, the Human Pathogens and Toxins Act, Human Pathogens and Toxins Regulations, Health of Animals Act, or Health of Animals Regulations. |
| Disease | A disorder of structure or function in a living human or animal, or one of its parts, resulting from infection or intoxication. It is typically manifested by distinguishing signs and symptoms. |
| Disinfectant | A chemical agent capable of eliminating viable biological material on surfaces or in liquid waste. Effectiveness can vary depending on the properties of the chemical, concentration, shelf life, and contact time. |
| Dual-use potential | Qualities of a pathogen or toxin, scientific method, intellectual property, or other related asset that allow it to be either used for legitimate scientific applications (e.g., commercial, medical, or research purposes), or intentionally misused to cause harm or disease. Examples of assets with dual-use potential include pathogens or toxins that could be used as a biological weapon (i.e., for bioterrorism), a method that facilitates propagation of such pathogens in a non-traditional laboratory setting, or the discovery that a certain mutation results in resistance to all available treatments. |
| Effluent decontamination system | Equipment connected to the drain plumbing used to decontaminate, through heat and/or chemical means, the liquid waste (i.e., effluent) produced in a containment zone prior to release into sanitary sewers. |
| Emergency Response Plan (ERP) | A document outlining the actions to be taken and the parties responsible in emergency situations such as a spill, exposure, release of regulated materials, animal escape, personnel injury or illness, power failure, or other emergency situations. |
| Exposure | Contact with, or close proximity to, pathogens or toxins that may result in infection or intoxication, respectively. Routes of exposure include inhalation, ingestion, inoculation, and absorption. |
| Facility | Structures or buildings, or defined areas within structures or buildings, where regulated materials are handled or stored. This could include individual research and diagnostic laboratories, large scale production areas, or animal housing zones. A facility could also be a suite or building containing more than one of these areas. |
| Good microbiological laboratory practices | A basic laboratory code of practice applicable to all types of activities with biological material. These practices serve to protect workers and prevent contamination of the environment and the samples in use. |
| Handling or storing | "Handling or storing" regulated materials includes possessing, handling, using, producing, storing, permitting access to, transferring, importing, exporting, releasing, disposing of, or abandoning such material. This includes all controlled activities involving human pathogens and toxins specified in subsection 7(1) of the Human Pathogens and Toxins Act. All tenses and variations of "handling or storing" are also used in this context. |
| Hazard | A source of potential damage, harm, or adverse effects. In the context of biosafety, examples include objects (e.g., sharps, needles), materials (e.g., pathogens, toxins), animals (e.g., bites, scratches), and situations (e.g., containment system failure). |
| Human Pathogens and Toxins Act (HPTA) Security Clearance | An authorization following verification of an individual's background and reliability status issued by the Public Health Agency of Canada under section 34 of the HPTA. |
| Importing | The activity of bringing (e.g., transferring, transporting) regulated materials into Canada from another country or jurisdiction. In the context of the Canadian Biosafety Standard, this term does not apply to any activity to which the Transportation of Dangerous Goods Act, 1992 applies. |
| In vivo | Latin for "within the living"; describes experimentation conducted within the whole living organism (e.g., studying the effect of antibiotic treatment in animal models). |
| Incident | An event or occurrence that has the potential of causing injury, harm, infection, intoxication, illness, disease, or damage. Incidents include accidents and near misses. |
| Infectious material | Any isolate of a pathogen or any biological material that contains human or animal pathogens and therefore, poses a risk to human or animal health. |
| Inspection | Actions undertaken for the purpose of verifying whether an organization is in compliance with the Human Pathogens and Toxins Act, Human Pathogens and Toxins Regulations, Health of Animals Act, and/or Health of Animals Regulations, or for the purpose of preventing non-compliance. |
| Inventory | A list of (biological) assets associated with a containment zone identifying regulated materials in long-term storage (i.e., beyond 30 days) both inside and outside the containment zone. |
| Inward airflow | Air that always flows from areas of lower containment or lower contamination risk to areas of higher containment or higher contamination risk, as the result of a negative air pressure differential within the containment zone created by a ventilation system. Inward airflow protects against the release of airborne pathogens, infectious aerosols, and aerosolized toxins into "clean" areas. |
| Laboratory | An area within a facility or the facility itself where biological material is handled. |
| Laboratory-acquired infection/intoxication (LAI) | An infection or intoxication resulting from exposure to pathogens, infectious material, infected animals, or toxins being handled or stored in the containment zone. |
| Laboratory work area | An area inside a containment zone designed and equipped for in vitro activities (e.g., for research, diagnostics, and teaching purposes). |
| Large animal containment zone (LA zone) | An animal containment zone comprised of one or more co-located or adjoining rooms of equal containment level where animals are housed in animal cubicles (i.e., the room itself serves as primary containment). An LA zone may include, for example, large-sized animals, such as livestock or deer, housed in cubicles, or cubicles where small-sized animals, such as mice or raccoons, are housed in open caging (i.e., not primary containment caging). Post mortem rooms, where present, are considered to be part of an LA zone. |
| Large scale production | Production or pre-production activities, including in vitro cultures, that involve large volumes of regulated materials. Large scale activities differ from laboratory- or bench-scale activities based on the equipment used as they are typically performed in fermenters, bioreactors, and other closed systems. |
| Legislative document | Legislative documents issued by the Public Health Agency of Canada include the following:
Legislative documents issued by the Canadian Food Inspection Agency apply to non-indigenous terrestrial animal pathogens, terrestrial animal pathogens in animals, animal products or by-products, or other organisms, and animals naturally or experimentally infected or intoxicated with a terrestrial animal pathogen. These include the following:
|
| Licence | See "Pathogen and Toxin Licence". |
| Local risk assessment (LRA) | A site-specific risk assessment used to identify hazards based on the regulated materials in use and the activities being performed. This analysis informs risk mitigation and risk management strategies, which are to be incorporated into the physical containment design and operational practices of the facility. |
| Long-term storage | In the context of the Canadian Biosafety Standard, the possession of regulated materials beyond 30 days of receipt or creation. |
| Medical surveillance program | A program designed to prevent and detect personnel illness related to exposure to regulated materials. The focus of the program is primarily preventive, but provides a response mechanism through which a potential infection or intoxication can be identified and treated before serious injury or disease occurs, and to reduce the potential of disease spread within the community. |
| Microorganism | A cellular or non-cellular microbiological entity that cannot be reasonably detected by the naked eye, and is capable of replication or transferring genetic material. Microorganisms include bacteria, fungi, viruses, and parasites, and may be pathogenic or non-pathogenic in nature. |
| Movement | The action of moving (e.g., bringing, carrying, leading, relocating) people, material (including regulated materials), or animals from one physical location to another physical location in the same building. This can include movement within the same containment zone, to a different containment zone, or to another location within the same building. |
| Non-compliance | A state of non-conformity with legislative requirements (e.g., Human Pathogens and Toxins Act, Human Pathogens and Toxins Regulations, Health of Animals Act, Health of Animals Regulations, conditions of licence and terrestrial animal pathogen import permit). |
| Overarching risk assessment | A broad risk assessment that supports the biosafety program as a whole and may encompass multiple containment zones within an organization. The overarching risk assessment identifies hazards, risks, and mitigation strategies for the proposed activities involving regulated materials. Mitigation and management strategies reflect the type of biosafety program needed to protect personnel from exposure and to prevent the release of regulated materials. |
| Pathogen | A microorganism, nucleic acid, protein, or other infectious agent that is transmissible and capable of causing disease or infection in humans or animals. Classified human and animal pathogens can be found on the Public Health Agency of Canada's ePATHogen – Risk Group Database. |
| Pathogen and Toxin Licence | An authorization issued by the Public Health Agency of Canada:
"Licence" is also used in this context. |
| Pathogen risk assessment | An evaluation of the inherent characteristics of a biological agent (i.e., microorganism, protein, nucleic acid, or biological material containing parts thereof), which determines its risk group classification. A pathogen risk assessment involves the analysis of four key risk factors, including pathogenicity (i.e., infectivity and virulence), pre- and post-exposure measures, communicability, and impact on the animal population (i.e., host range, natural distribution, and economic impact). |
| Pathogenicity | The ability of a pathogen to cause disease in a human or animal host. |
| Personal protective equipment (PPE) | Equipment and/or clothing worn by personnel to provide a barrier against biological material, thereby minimizing the risk of exposure. PPE may include, but is not limited to, lab coats, gowns, full-body suits, gloves, protective footwear, safety glasses, safety goggles, masks, and respirators. |
Post mortem room |
A room within the containment zone where necropsies and dissections are conducted on animals outside a primary containment device. |
| Primary containment device | Apparatus or equipment that is designed to prevent the release of regulated materials, and to provide primary containment (i.e., provide a physical barrier between the regulated materials and the individual or the work environment). Examples include biological safety cabinets, isolators, centrifuges with sealable cups or rotors, process equipment, fermenters, bioreactors, microisolator cages and ventilated cage racks. |
| Prion | A small proteinaceous infectious particle generally considered to be responsible for causing a group of neurodegenerative diseases in humans and animals known as transmissible spongiform encephalopathies. |
| Program intent | A description of the planned work to be performed in a containment zone. This includes, but is not limited to, the scope of work (e.g., diagnostic, teaching, research, large scale production, in vitro work, in vivo work), a list of pathogens and toxins to be handled or stored, a list of animal species to be involved in in vivo work with pathogens and toxins in the containment zone, and a list of procedures that may create aerosols. |
| Regulated material | In the context of the Canadian Biosafety Standard, regulated material includes:
|
| Release | The discharge of regulated materials from a containment system or containment zone (e.g., resulting from leaking, spraying, depositing, dumping, vaporizing). |
| Risk | The probability of an undesirable event (e.g., accident, incident, breach of containment) occurring and the consequences of that event. |
| Risk group (RG) | The classification of a biological agent (i.e., microorganism, protein, nucleic acid, or biological material containing parts thereof) based on its inherent characteristics, including pathogenicity, virulence, communicability, and the availability of effective prophylactic or therapeutic treatments. The risk group describes the risk to the health of individuals and the public, as well as the health of animals and the animal population. |
| Root cause | The most basic, underlying reason or factor that led to an incident occurring. |
| Scientific research | As defined in section 1 of the Human Pathogens and Toxins Regulations, the following types of systematic investigation or research that are carried out in a field of science or technology by means of controlled activities:
|
| Security sensitive biological agents (SSBAs) | The subset of human pathogens and toxins that have been determined to pose an increased biosecurity risk due to their potential for use as a biological weapon. SSBAs are identified as prescribed human pathogens and toxins by section 10 of the Human Pathogens and Toxins Regulations (HPTR). This means all Risk Group 3 (RG3) and RG4 human pathogens that are in the List of Human and Animal Pathogens and Toxins for Export Control , published by the Australia Group, as amended from time to time, with the exception of Duvenhage virus, Rabies virus and all other members of the Lyssavirus genus, Vesicular stomatitis virus, and Lymphocytic choriomeningitis virus; as well as all toxins listed in Schedule 1 of the Human Pathogens and Toxins Act that are listed on the List of Human and Animal Pathogens and Toxins for Export Control when in a quantity greater than that specified in subsection 10(2) of the HPTR. |
| Senior management | The ultimate authority responsible for delegating appropriate biosafety authority. Senior management is responsible for ensuring that adequate resources are available to support the biosafety program, to meet legal requirements, and that biosafety concerns are appropriately prioritized and addressed. |
| Standard operating procedure (SOP) | A document that standardizes safe work practices and procedures for activities with regulated materials in a containment zone, as determined by a local risk assessment. Examples of SOPs include experimental protocols, entry and exit procedures, decontamination protocols, and emergency response procedures. |
| Terrestrial animal pathogen | A microorganism, nucleic acid, protein, or other infectious agent that is transmissible and capable of causing disease or infection in terrestrial animals; including those derived from biotechnology. These include pathogens that cause disease in avian and amphibian animals, but exclude those that only cause disease in invertebrates and aquatic animals. This also includes terrestrial animal pathogens or part of one (e.g., toxin) present on or in animal products, animal by-products, or other organisms. |
| Terrestrial animal pathogen import permit | A permit issued under paragraph 51(a) and (b) of the Health of Animals Regulations by the Public Health Agency of Canada or the Canadian Food Inspection Agency for the importation into Canada of terrestrial animal pathogens or part of one (e.g., toxin); or animals, animal products, animal by-products (e.g., tissue, serum), or other organisms carrying a terrestrial animal pathogen or part of one (e.g., toxin). |
| (Microbial) Toxin | A poisonous substance that is produced by or derived from a microorganism and can lead to adverse health effects in humans or animals. Human toxins are listed in Schedule 1 and Part 1 of Schedule 5 in the Human Pathogens and Toxins Act. |
| Training needs assessment | An evaluation performed to identify the current and future training needs of the facility (i.e., organization, containment zone), including training, refresher training, and retraining, and to identify gaps in the current training program. |
| Transfer | A change in possession of regulated materials between individuals from the same or different facilities (i.e., the movement from the place or places specified in the licence or terrestrial animal pathogen import permit to any other place). |
| Validation | The act of confirming that a method achieves its objective and is suitable for its intended purpose through the provision of objective evidence. This can be achieved by observing that specific conditions have been met (e.g., using biological indicators, chemical integrators, or parametric monitoring devices placed in challenging locations within the load to confirm that a given autoclave cycle can decontaminate a representative load of waste). |
| Verification | The routine monitoring of equipment and processes to confirm continued efficacy between validations (e.g., testing the performance of an autoclave using biological indicators, viewing airflow gauges to confirm fan function in a biological safety cabinet). Verification includes comparing the accuracy of a piece of equipment to an applicable standard or standard operating procedure. |
| Waste | Any solid or liquid material generated by a facility for disposal. |
6. References
- Footnote 1
-
Human Pathogens and Toxins Regulations (SOR/2015-44).
- Footnote 2
-
Health of Animals Regulations (C.R.C., c. 296).
- Footnote 3
-
Government of Canada. (2010). Containment Standards for Facilities Handling Aquatic Animal Pathogens (1st ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.inspection.gc.ca/animals/aquatic-animals/imports/pathogens/facilities/eng/1377962925061/1377963021283
- Footnote 4
-
Government of Canada. (2007). Containment Standards for Facilities Handling Plant Pests (1st ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.inspection.gc.ca/plants/plant-pests-invasive-species/biocontainment/containment-standards/eng/1412353866032/1412354048442
- Footnote 5
-
New Substances Notification Regulations (Organisms) (FSOR/2005-248).
- Footnote 6
-
Government of Canada. Biosafety directives and advisories. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosafety-directives-advisories-notifications.html
- Footnote 7
-
CEN Workshop 55 - CEN Workshop Agreement (CWA) 16393:2012, Laboratory biorisk management – Guidelines for the implementation of CWA 15793:2008. (2012). Brussels, Belgium: European Committee for Standardization.
- Footnote 8
-
Burnett, L. C. (2006). Biological safety program management. In Fleming, D. O., & Hunt, D. L. (Eds.). Biological safety: Principles and practices (4th ed., pp. 405-415). Washington, DC, USA: ASM Press.
- Footnote 9
-
ISO 14001:2015, Environmental management systems – requirements with guidance for use. (2015). Geneva, Switzerland: International Organization for Standardization.
- Footnote 10
-
Government of Canada. (2022). Canadian Biosafety Standard (3rd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/third-edition.html
- Footnote 11
-
Perkins, D., Danskin, K., & Rowe, A. E. (2017). Fostering an International Culture of Biosafety, Biosecurity, and Responsible Conduct in the Life Sciences. Science & Diplomacy, 6(3).
- Footnote 12
-
Government of Canada. Public Health Agency of Canada. (2015). Plan for A dministrative Oversight for Pathogens and Toxins in a Research Setting – Required Elements and Guidance. Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/licensing-program/plan-administrative-oversight-pathogens-toxins-a-research-setting-required-elements-guidance.html
- Footnote 13
-
Human Pathogens and Toxins Act (S.C. 2009, c. 24).
- Footnote 14
-
Canada Occupational Health and Safety Regulations (SOR/86-304).
- Footnote 15
-
Red River College. (2019). Biological Safety Manual. Retrieved 12/15, 2022 from https://www.rrc.ca/wp-content/uploads/sites/36/2019/12/Biosafety-Manual-2019.pdf
- Footnote 16
-
Doran, G.T. (1981). There's a S.M.A.R.T. way to write management's goals and objectives. Management Review, 70(11):35-36.
- Footnote 17
-
Government of Canada. (2018). Canadian Biosafety Guideline: Local Risk Assessment. Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
- Footnote 18
-
Government of Canada. (2018). Canadian Biosafety Guideline: Pathogen Risk Assessment. Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
- Footnote 19
-
Government of Canada. (2018). Canadian Biosafety Guideline: Conducting a Biosecurity Risk Assessment. Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
- Footnote 20
-
United States Department of Labor. (Date unknown). Hazard identification and assessment. Retrieved 12/22, 2022 from https://www.osha.gov/safety-management/hazard-Identification
- Footnote 21
-
Government of Canada. Pathogen safety data sheets. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment.html
- Footnote 22
-
Government of Canada. ePATHogen – risk group database. Available from https://health.canada.ca/en/epathogen
- Footnote 23
-
Government of Canada. (2016). Canadian Biosafety Guideline: Developing a Comprehensive Biosecurity Plan. Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
- Footnote 24
-
Government of Canada. PHAC Training Portal. Available from https://training-formation.phac-aspc.gc.ca/
- Footnote 25
-
Government of Canada. Canadian Immunization Guide. Available from https://www.canada.ca/en/public-health/services/canadian-immunization-guide.html
- Footnote 26
-
United States Department of Health and Human Services, United States Centers for Disease Control and Prevention, & United States National Institutes of Health. (2020). Biosafety in Microbiological and Biomedical Laboratories (6th ed.). Washington, DC, USA: United States Government Printing Office.
- Footnote 27
-
ISO 14004:2016, Environmental management systems – General guidelines on implementation. (2016). Geneva, Switzerland: International Organization for Standardization.
- Footnote 28
-
CSA Z317.10:15, Handling of health care waste materials. (R2020). Toronto, ON, Canada: Canadian Standards Association.
- Footnote 29
-
Project Management Docs. (Date unknown). Communications Management Plan Template. Retrieved 12/15, 2022 from http://www.projectmanagementdocs.com/project-planning-templates/communications-management-plan.html#axzz5LjAzhyVV
- Footnote 30
-
Pentella, M. A. (2017). Components of a Biosafety Program for a Clinical Laboratory. In Wooley, D. P., & Byers, K. B. (Eds.). Biological safety: Principles and practices (5th ed., pp. 687-694). Washington, DC, USA: ASM Press.
- Footnote 31
-
Laserfiche. (Date unknown). What's the Difference Between Document and Records Management? Retrieved 12/15, 2022 from https://www.laserfiche.com/ecmblog/whats-the-difference-between-document-and-records-management/
- Footnote 32
-
Quality Progress. (2005). Corrective vs. Preventive Action. Retrieved 01/05, 2023 from http://asq.org/quality-progress/2005/03/problem-solving/corrective-vs-preventive-action.html