Canadian Biosafety Guideline - Containment Level 1: Physical Design and Operational Practices

Download in PDF format
(2.8 MB, 58 pages)
Organization: Public Health Agency of Canada
Cat.: HP45-16/2024E-PDF
ISBN: 978-0-660-70546-0
Pub.: 230823
Published: 2024-04-18
Table of contents
- Preface
- Abbreviations and acronyms
- 1. Introduction
- 2. Physical design features
- 3. Operational practices
- 4. Glossary
- 5. References and resources
Preface
In Canada, the handling or storing of Risk Group 2 (RG2), RG3, and RG4 human pathogens or toxins is regulated by the Public Health Agency of Canada (PHAC) under the Human Pathogens and Toxins Act (HPTA) and the Human Pathogens and Toxins Regulations (HPTR). The handling and storing of imported RG2, RG3 and RG4 animal pathogens or part of one (e.g., toxin), or animals, animal products or by-products (e.g., tissue, serum), or other organisms carrying an animal pathogen or part of one (e.g., toxin) are regulated under the Health of Animals Act (HAA) and the Health of Animals Regulations (HAR). Under the authority of the HAA and the HAR, the PHAC is also responsible for the importation or transfer of terrestrial animal pathogens or part of one (e.g., toxin) in pure culture or in a non-animal matrix (e.g., human, plant, food or environmental sample).
The Canadian Food Inspection Agency (CFIA) is responsible for the regulation of the importation or transfer of CFIA Designated Terrestrial Animal Pathogens (CD-TAP; collective umbrella term used to capture foreign animal diseases, emerging animal diseases, and non-indigenous animal pathogens under CFIA's authority), as well as animals, animal products, and animal by-products (e.g., tissue, serum) that carry a terrestrial animal pathogen or part of one (e.g., toxin), under the authority of the HAA and HAR.
The following figure depicts the document hierarchy used by the PHAC and the CFIA to oversee biosafety and biosecurity operations. Each tier of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards. Acts and Regulations are found at the top of the pyramid, as they are the documents that convey the PHAC's and the CFIA's legal authorities. Risk communication tools and technical documents are found at the bottom of the pyramid, as they are only intended to summarize recommendations and scientific information.Figure 1: Government of Canada's biosafety and biosecurity document hierarchy
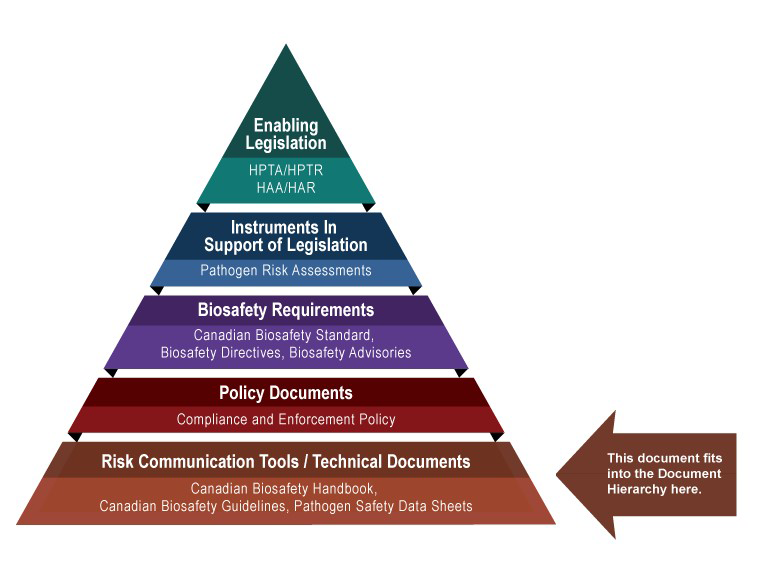
Figure 1: Government of Canada's Biosafety and Biosecurity Document Hierarchy - Text Equivalent
Figure in the form of a pyramid depicting the document hierarchy used by the PHAC to oversee biosafety and biosecurity operations. Each of the five tiers of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards.
At the top sits the Enabling Legislation, that is, the HPTA, HPTR, HAA, and HAR, that convey the PHAC's legal authorities. Below the acts and regulations sit Instruments in Support of Legislation, which are the Pathogen Risk Assessments. The next tier down is the Biosafety Requirements, which include the Canadian Biosafety Standard, Biosafety Directives, and Biosafety Advisories. In the second lowest tier are the Policy Documents, which include the Compliance and Enforcement Policy. Guidance material and technical pieces found at the bottom of the pyramid, under the Risk Communication Tools and Technical Documents heading, are intended to summarize recommendations and scientific information only. These include the Canadian Biosafety Handbook, Canadian Biosafety Guidelines, and Pathogen Safety Data Sheets.
The Containment Level 1: Physical Design and Operational Practices guideline was developed by the PHAC and the CFIA as part of a series of electronic publications that describe biosafety and biosecurity concepts in Canada. This guideline provides risk-based recommendations for facilities handling RG1 biological material, which is either not capable of, or is unlikely to, cause human or animal disease. The Canadian Biosafety Standard (CBS) describes the risk-based requirements for facilities handling regulated materials at Containment Levels 2, 3, or 4.
While the CBS does not specify requirements applicable to the handling and storing of RG1 biological material, it is recommended that RG1 material be handled using safe work practices, and activities be conducted in a laboratory, production or animal work area that incorporates basic containment zone design. This guideline aims to provide stakeholders with support and guidance on how to mitigate risks when working with RG1 biological material that are not subject to the HPTA or HAA. RG1 biological material may pose a low risk to the health of individual humans or animals, and a low risk to public health or animal populations. However, RG1 biological material is not devoid of risk and has the potential to cause infection in some circumstances (e.g., individuals with compromised immune function). As such, it is still important to take reasonable precautions when handling these materials. This guideline describes the general recommendations and considerations for basic laboratory design and the safe handling of RG1 biological material.
Abbreviations and acronyms
- CBS
- Canadian Biosafety Standard
- CD-TAP
- Canadian Food Inspection Agency designated terrestrial animal pathogen
- CFIA
- Canadian Food Inspection Agency
- CL
- Containment Level (i.e., CL1, CL2, CL3, CL4)
- ERP
- Emergency response plan
- HAA
- Health of Animals Act
- HAR
- Health of Animals Regulations
- HEPA
- High efficiency particulate air
- HPTA
- Human Pathogens and Toxins Act
- HPTR
- Human Pathogens and Toxins Regulations
- LRA
- Local risk assessment
- PHAC
- Public Health Agency of Canada
- PM room
- Post mortem room
- PPE
- Personal protective equipment
- RG
- Risk Group (i.e., RG1, RG2, RG3, RG4)
- SOP
- Standard operating procedure
1. Introduction
The words in bold type are defined in the glossary found in section 4.
Containment level 1 (CL1) describes a basic work area designed for the safe handling and storing of Risk Group 1 (RG1) biological material. CL1 design and practices provide the foundation for all containment zones to protect personnel and the environment from exposure to the biological material handled within a facility. Biosafety is primarily achieved through physical design features (e.g., a well-designed, functional laboratory), and a basic level of operational practices (e.g., good microbiological laboratory practices). A containment zone for CL1 activities (hereafter referred to as a CL1 zone) can include the following types of work areas: laboratory work areas, large scale production areas, and animal work areas. Due to the low public and animal health risks of RG1 biological material, the Canadian Biosafety Standard (CBS) does not specify requirements applicable to laboratories or other facilities where activities with RG1 biological material are conducted, nor is a person handling or storing these materials required to obtain a licence or permit under the Human Pathogens and Toxins Act (HPTA) or the Health of Animals Act (HAA).Footnote 1
1.1 Scope
The Containment Level 1: Physical Design and Operational Practices guideline provides comprehensive guidance on best practices for basic work area design and the safe handling of RG1 biological material. These practices encompass the basics of biosafety and biosecurity and serve as starting points for developing the mandatory practices required in higher containment levels as specified in the CBS. Elements provided in this document are presented as recommendations only and may be followed on a voluntary basis, unless otherwise required as a condition of a licence or permit. As described in this guideline, RG1 biological material is not devoid of risk and has the potential to cause infection in some circumstances (e.g., individuals with compromised immune function).
1.2 Risk Group 1 biological material
For the purposes of this guideline, biological material refers to pathogenic or non-pathogenic microorganisms, proteins, and nucleic acids, as well as any biological matter (e.g., cells, tissues, other specimens) that may contain microorganisms, proteins, nucleic acids, other infectious agents, or parts thereof. Examples include, but are not limited to, bacteria, viruses, fungi, prions, toxins, genetically modified organisms, nucleic acids, tissue samples, diagnostic specimens, environmental samples, live vaccines, and isolates of a pathogen or toxin (e.g., pure culture, suspension, purified spores), regardless of whether or not they are infectious or toxic. RG1 biological material is defined as a microorganism, nucleic acid, or protein that is either:
- a) not capable of causing human or animal disease; or
- b) capable of causing human disease or animal disease, but unlikely to do so.
RG1 biological material capable of causing disease are considered pathogens that pose a low risk to the health of individual humans and animals, and low or no risk to public health and animal populations. However, RG1 pathogens can be opportunistic and may pose a threat to immunocompromised or immunosuppressed individuals (e.g., through medical therapy, pregnancy, diabetes, other conditions). For example, Bacillus subtilis, an RG1 bacterium widely used as a probiotic (i.e., a live bacteria added to food or consumed as a supplement to confer a health benefit to the host), has also been associated with numerous cases of food poisoning and other negative health effects.Footnote 2,Footnote 3,Footnote 4,Footnote 5,Footnote 6
Risk group classification is determined through a pathogen risk assessment, which evaluates the inherent characteristics of a biological agent, including pathogenicity, virulence, communicability, host range, and the availability of effective prophylactic or therapeutic interventions. Risk groups range from RG1 (low individual and low community risk) to RG4 (high individual and high community risk). A full list of pathogen risk factors can be found in the Canadian Biosafety Guideline on Pathogen Risk Assessment.Footnote 7
RG1 biological material is not covered under the HPTA, Human Pathogens and Toxins Regulations (HPTR), HAA, and Health of Animals Regulations (HAR) due to its low risk to human and animal health. Therefore, laboratories and other facilities where only RG1 biological material is handled or stored are not regulated by the Public Health Agency of Canada (PHAC) or the Canadian Food Inspection Agency (CFIA).Footnote 8,Footnote 9,Footnote 10,Footnote 11 Nevertheless, it is important to take reasonable precautions (e.g., good microbiological laboratory practices) when handling these materials. Where there is an increased risk (e.g., immunocompromised individual working with an opportunistic RG1 pathogen), consideration may be given to using containment level 2 (CL2) operational procedures or moving the work into a CL2 zone for additional protection.
If RG1 biological material is modified resulting in an increased risk to personnel or the environment (i.e., increased virulence or pathogenicity, communicability, resistance to a preventive or therapeutic treatment, or toxicity of a toxin), then it becomes regulated material and work is to be stopped. The regulated material must be transferred to a facility of an appropriate containment level that holds a valid Pathogen and Toxin Licence from the PHAC for further activities. Alternatively, work with the regulated material can be put on hold and the regulated material safely transferred to the appropriate containment level (e.g. licensed CL2) until an application can be made for the facility to obtain a Pathogen and Toxin Licence from the PHAC to handle or store a Risk Group 2, 3, or 4 pathogen or toxin.
In the event that modification(s) are made to RG1 biological material associated with a CFIA Designated Terrestrial Animal Pathogen (CD-TAP; collective umbrella term being used to capture foreign animal diseases, emerging animal diseases, and non-indigenous animal pathogens under CFIA's authority) or other biological material that results in an increased risk to animals or an animal community (i.e., increased virulence or pathogenicity, communicability, resistance to a preventive or therapeutic treatment, or toxicity of a toxin), then work with the material is to be stopped, the materials securely stored and the CFIA contacted. The CFIA will evaluate the effects of the modifications and determine what conditions would be necessary to allow work with the material to continue.
1.3 How to use the Containment Level 1: Physical Design and Operational Practices guideline
This guideline describes the general recommendations and considerations for basic work area design and the safe handling of RG1 biological material. These recommended practices are risk- and evidence-based. The physical design features and operational practices for CL1 zones described in this document are considered best practices for work involving RG1 biological material. A local risk assessment (LRA), based on the procedures to be performed and the biological material to be handled, may determine that some of the recommendations can be modified or are not applicable, depending on the situation.Footnote 12
Sections 2 and 3 describe recommended physical design features and operational practices, respectively. The format used in this document is similar to that used in the CBS. Recommendations are grouped by topic into multiple matrices. The explanatory notes, located under the recommendations, provide additional information and clarification on the intent of the recommendation. Different types of work areas for handling biological material are described, including laboratory work areas, large scale production areas, and animal work areas.
A detailed list of all abbreviations and acronyms used throughout this guideline is located at the beginning of this document. Each word or term is spelled out upon first use in the guideline, with the abbreviation immediately following in brackets. After its initial definition, the abbreviation is used exclusively throughout the remainder of the document. A comprehensive glossary of definitions for technical terms is located in section 4 of this document. Terms defined in the glossary appear in bold type upon first use in the guideline. A complete list of references and other resources is provided in section 5. The Canadian Biosafety Guideline series may be consulted for further guidance and details on a variety of biosafety and biosecurity-related topics.Footnote 13
2. Physical design features
For all containment levels, facility design and engineering controls are established to limit the spread of biological material. In CL1 zones, this can be quite basic, and is largely achieved by segregating work areas from surrounding public and administrative areas, and establishing designated spaces where biological material may be handled within a work area. Laboratory work areas for activities with RG1 biological material have no specific physical design features beyond those suitable for a well-designed and functional laboratory space for handling this material (e.g., handwashing sinks, signage). The work areas themselves are designed to be easy to clean and decontaminate. Basic safety, emergency, and security features are integrated to protect personnel, prevent animal escape, and provide a basic level of pest control.
2.1 General physical design features
The basic physical design features recommended below are applicable to any CL1 work area. This includes laboratory work areas, large scale production areas, and animal work areas. Design features specific to large scale production areas incorporate considerations to manage a spill or leak from closed system equipment.
Please note that the following are recommendations only and are not requirements unless otherwise specified by the PHAC or the CFIA.
Outline of section 2.1 General physical design features
Laboratory work areas, large scale production areas, and animal work areas are separated from public and administrative areas by a lockable door.
A door is a physical barrier that protects against the release of biological material by separating the CL1 zone (i.e., "dirty" or contaminated area) from public and administrative areas (i.e., "clean" or uncontaminated areas). Lockable doors provide a basic security barrier to prevent unauthorized access to the CL1 zone and to safeguard the RG1 biological material stored within. If a door serves as a physical barrier encompassing both a "dirty" area and a "clean" area, operational practices can be implemented to limit the spread of contamination. Where large openings provide access to the CL1 zone (e.g., sample receipt counter), closable alternatives (e.g., roll-down door, blinds over counter) can help limit access and contain the release of biological material within the work area in the case of a spill.
Dedicated paper/computer workstations are segregated from workstations where RG1 biological material (e.g., samples, specimens) and animals are handled. Traffic and work flow patterns are established and followed to prevent the spread of contamination.
Clearly identifying areas or surfaces of lower contamination (i.e., "clean") and areas of higher contamination (i.e., "dirty"), and implementing procedures for traffic and work flow patterns to facilitate movement of personnel, equipment, samples, and animals from "clean" to "dirty" areas limits the spread of contamination. Work flow patterns may include having all clean materials (e.g., pipettes, media, flasks) on one side of the workstation and cultures and waste on the other. This can be achieved by separating "clean" spaces dedicated to report writing and other paperwork from areas or surfaces where materials are handled with a physical partition (e.g., splash shield, separate room) or by placing them at a safe distance to minimize the risk of contaminating office supplies (e.g., paper, notebooks) and equipment (e.g., computers) that cannot be easily decontaminated.
Openable windows are equipped with basic pest control.
Basic pest control on windows, especially windows opening directly to the outdoors, can protect against the entry of small-sized animals and insects into the CL1 zone. Preventing the entry and exit of animals and insects protects against the release of biological material outside the CL1 zone. Pest control can be achieved by the installation of screens that are kept in good repair and by closing windows. If no pest control is provided (e.g., no screen, damaged screen), the window can be kept permanently closed (e.g., using tape, screws, nails).
Space is provided for the storage of dedicated personal protective equipment (PPE) that has been worn and may be reused.
Having a dedicated space for PPE that has been worn and may be reused keeps this PPE physically separated from personal clothing (e.g., coats, hats, boots) or unused PPE, which prevents the spread of contamination and the release of biological material to areas outside the CL1 zone. It is best practice for the dedicated space for PPE that has been worn and may be reused to be near the location of donning and doffing. Hooks, lockers, shelves, cubbies, bins, or spaces within dedicated change areas are examples of dedicated storage space for PPE that has been worn and may be reused. An LRA could help determine the location and amount of dedicated space required to store PPE that has been worn and may be reused, prevent cross-contamination, and allow personnel to safely perform exit procedures (e.g., sufficient number of hooks to prevent stacking multiple lab coats one on top of the other).
In accordance with function, surfaces such as floors, ceilings, walls, doors, frames, benchtops, and furniture are:
- a) cleanable;
- b) non-absorbent;
- c) resistant to physical damage;
- d) resistant to damage caused by decontamination procedures and products.
To allow for cleaning and decontamination, cleanable and resistant surface materials and protective finishes (e.g., paint, epoxy) with a compatible non-shrinking sealant provide protection against the stresses associated with the activities performed inside the CL1 zone. Activities may include repeated decontamination (e.g., chemical, gaseous), frequent high pressure washing in animal zones, physical stresses (e.g., impacts, heat, equipment resting on surfaces, animal cages), and scratches. The level of surface protection needed is determined by the function of the CL1 zone and the activities taking place. For example, the floors in a laboratory work area may only require decontamination procedures following incidents (e.g. a spill). Non-absorbent materials may include stainless steel, epoxy resin, or chemical-resistant plastic laminate for benchtops, and urethane or vinyl for stools and chairs.
Surfaces that may come in contact with RG1 biological material are continuous with adjacent and overlapping materials.
The continuity of surfaces (e.g., work surfaces with surface mounted outlets, gas supply, and other services) provides a continuous barrier designed to prevent contaminated liquids and aerosols from depositing onto surfaces that are difficult to access and decontaminate. It also facilitates cleaning and chemical decontamination procedures after a spill, splash, or other type of event that results in contamination of surfaces.
Sinks are provided to facilitate handwashing.
Handwashing prevents the spread of contamination inside and outside the CL1 zone. If sinks are not readily available, an LRA may determine that hands can be decontaminated using an appropriate hand sanitizer and/or washed at an available sink outside the area where activities are conducted.
Design features of large scale production areas prevent the release of fluids containing viable biological material into sanitary sewers or any other route of exit from the facility.
Design features, such as capped or raised floor drains, can be incorporated into large scale production areas to prevent the release of biological material into sanitary sewers or to areas outside the CL1 zone in the event of a leak or a spill. Design features can also include dikes, dams, berms, and pits to contain the largest volume of a leak or a spill of process fluids that can realistically occur based on an LRA.
Where the room does not serve as primary containment for large scale production activities with RG1 biological material, process equipment, closed systems, and other containment devices are designed to prevent the release of RG1 biological material.
Examples of mechanisms that can prevent the release of biological material include installing high efficiency particulate air (HEPA) filters on ports and vents and fully enclosing a primary containment device within a ventilated housing that is exhausted through HEPA filters (e.g., walk-in containment enclosure). Having additional mechanisms (e.g., backup valves) for primary containment devices, process equipment, and closed systems used for large scale production activities provides redundant protection against release of untreated RG1 biological material into the CL1 zone or the sanitary sewer system.
2.2 Additional physical design features for animal work areas
The following physical design features are applicable to CL1 animal work areas, which include rooms where animals are housed, animal cubicles, post mortem rooms (PM rooms), and may also include associated corridors. These best practices build upon the general best practices for laboratory work areas as well as the basic design considerations established by the Canadian Council on Animal Care's Guidelines on Laboratory Animal Facilities.Footnote 14
Please note that the following are recommendations only and are not requirements unless otherwise specified by the PHAC or the CFIA.
Outline of section 2.2 Additional physical design features for animal work areas
Laboratory work areas are located outside of rooms where animals are housed.
Separating laboratory work areas from spaces where animals are housed (i.e., animal cubicles, animal rooms) prevents contamination of work materials not associated with animal work. This can be achieved by designing the animal containment zone to include a laboratory work area for activities that do not directly involve the animals (e.g., preparing samples or inoculants).
Animal cages and rooms where animals are housed or handled are designed to prevent animal escape.
Preventing animal escape is important for protecting personnel and animal safety, as well as preventing the release of biological material or potentially infected animals.
Cold storage area (e.g., cold room) or equipment (e.g., refrigerator, freezer) are provided in or adjacent to the PM room, where the design includes a dedicated PM room, to minimize the decay of animal carcasses during temporary storage.
Cold storage equipment located in, or adjacent to, the PM room is important to protect against the putrefaction of animal carcasses during temporary storage while awaiting necropsy or disposal.
In accordance with function, floors and walls are resistant to damage caused by repeated decontamination and high pressure washing.
The use of appropriate surface (i.e., walls, ceilings, floors) finishes and casework for CL1 animal work areas is necessary to facilitate the maintenance, cleaning, and decontamination of surfaces within the work area. Surface finishes also help protect against the stresses associated with activities routinely performed within the work area, such as repeated decontamination and frequent high pressure washing.
In accordance with function, floors and walls in animal work areas, including PM rooms and corridors, are able to withstand anticipated loads (e.g., heavy animals and caging equipment).
Appropriate floor design (including floor joists, spacing, and trusses) and materials allow flooring to resist damage and withstand the anticipated loads represented by heavy animals and caging equipment, as applicable.
Dedicated cage washing areas are recommended to facilitate cleaning.
The cagewash area is large enough to accommodate the accumulation of dirty equipment throughout the working day and has adequate ventilation to maintain a safe environment conducive to human physical activity and to prevent the spread of vapour and contaminants.
3. Operational practices
Operational practices refer to the administrative controls and procedures in place to prevent personnel exposure and the release of biological material into the environment.
3.1 Good microbiological laboratory practices
The term good microbiological laboratory practices describes a basic set of safe practices and techniques established in microbiology laboratories.Footnote 15,Footnote 16 These practices aim to minimize the spread of contamination generated by the material being manipulated and safeguard the material against contamination from the environment to protect its quality or purity. At the same time, good microbiological laboratory practices provide a basic level of protection to the individual laboratory worker and the environment from the biological material being manipulated.
Good microbiological laboratory practices provide the foundation upon which all biosafety practices at higher containment levels are based. Due to the low level of risk associated with RG1 biological material, it is generally considered safe to conduct most procedures on a benchtop.
Please note that the following are recommendations only and are not requirements unless otherwise specified by the PHAC or the CFIA.
Outline of section 3.1 Good microbiological laboratory practices
Contact of the face or mucous membranes with items contaminated or potentially contaminated is prevented.
Activities such as mouth pipetting, chewing the end of a pencil, eating, drinking, nail biting, applying cosmetics, inserting ear buds, inserting or removing contact lenses, and any other activity involving the potential contact of any item within the CL1 zone (including the hands) with mucous membranes of the eyes, nose, ears, and mouth are prevented to reduce the risk of exposing mucous membranes to items contaminated or potentially contaminated with RG1 biological material.
Hair that may become contaminated is restrained (e.g., hair tied or clipped back) or covered (e.g., head or beard covers).
Restraining or covering hair (including beards) reduces the risk that hair becomes contaminated through accidental contact with gloves, hands, specimens, containers, equipment, or surfaces, and during activities in rooms where RG1 biological material is handled.
Jewellery that may become contaminated or compromise PPE is removed or covered.
Jewellery (e.g., rings, long necklaces) may become contaminated or impede the cleaning and decontamination of the skin under the jewellery (e.g., during handwashing). Jewellery may also interfere with PPE (e.g., rings and watches may tear gloves). Removing jewellery and leaving it outside the work area also avoids the need to subject jewellery to decontamination procedures prior to removal from the CL1 zone. As a best practice, an LRA may be used to determine whether certain jewellery (e.g., flat band ring, small earrings) may be worn in the CL1 zone if there is minimal risk it could interfere with or damage PPE, become contaminated (e.g., if it is covered by PPE), or it is unlikely to be exposed to contaminated material or aerosols (e.g., the jewellery is in the nose or mouth).
Open wounds, cuts, and scratches are covered in a manner that prevents exposure.
Intact skin provides protection from infection and intoxication. Any breach of the skin (e.g., wound, cut, scratch, graze, rash) may provide a portal of entry for RG1 biological material, and needs to be protected with a bandage or other suitable dressing or cover prior to entry into the CL1 zone. Wounds, cuts, and scratches that occur within the CL1 zone are incidents that need to be reported to the appropriate internal authority. Removal and disposal of the dressing or covering when exiting the CL1 zone helps to prevent the accidental spread of biological material.
Workstations and work areas, including floors, are kept clean with the following minimized:
- a) clutter and obstructions;
- b) materials that are in excess or not required;
- c) materials that cannot be easily decontaminated.
A clean, uncluttered work environment, with few obstructions, allows for the appropriate decontamination of the CL1 zone. It also minimizes slipping, tripping, falling, and collision hazards that could potentially lead to exposure incidents or the spread of contamination. Storing excess materials outside the CL1 zone also protects this material from becoming contaminated and being subjected to decontamination procedures.
Doors and other openings to work areas, including PM rooms, are kept closed.
Doors and other openings (e.g., sample receipt windows) are kept closed to prevent the release of potentially contaminated air (e.g., from aerosolized toxins) from rooms that serve as primary containment (e.g., animal cubicle) or in the event of an incident (e.g., spill), and prevent animal escape. Laboratory materials are stored safely and away from high traffic areas and doors to reduce the risk of an incident.
Access to work areas is granted to authorized personnel and authorized visitors only.
Limiting or restricting access to the CL1 zone, animal room, animal cubicle, PM room, room housing an effluent decontamination system, and to areas with supporting mechanical and electrical services (e.g., electrical panels, mechanical penthouse, heating, ventilation, and air conditioning system control areas) to authorized individuals protects the safety of individuals entering these areas and the security of the materials and assets present. Access authorization provides a mechanism to verify that everyone entering the CL1 zone (e.g., personnel, visitors, maintenance and cleaning staff) meet entry requirements, including having completed the required training (i.e., appropriate level of training based on the training needs assessment for the tasks they are performing).
All personnel, visitors, volunteers, and trainees wear suitable PPE inside the work area.
PPE is exclusively worn and stored in the CL1 work area including:
- shoes that cover the entire foot, with no or low heels;
- PPE, such as lab coats, aprons, gloves, or coveralls;
- protective eyewear, such as goggles, when there is a risk of exposure to splashes;
- full face protection (e.g., face shield) when there is a risk of splashes or flying objects.
Personal clothing and belongings are stored separately from dedicated PPE and separate from work areas.
Storing personal clothing (e.g., outerwear such as coats or scarves, street clothes, undergarments), belongings, and clean PPE separate from dedicated PPE that has been worn in the work area where RG1 biological material is handled (i.e., potentially contaminated) prevents these items from being contaminated. Personal belongings and other items for personal use (e.g., backpack, purse, cell phone) are also kept separate from areas where RG1 biological material is handled or stored. This protects individuals from exposure and prevents the spread of contamination outside the CL1 zone. Personal clothing, belongings, and clean PPE can be stored outside or within the CL1 zone in dedicated change areas. Where personal clothing, belongings, clean PPE, and used PPE are stored within the CL1 zone or within the same anteroom, these can be physically or spatially separated to prevent contamination. For example, placing hooks or lockers on opposite walls, or on each side of the entrance door may be appropriate if standard operating procedures (SOPs) describe how separation is maintained (e.g., one side is reserved for personal clothing and belongings and the other for used PPE) and prevent cross-contamination during exit.
Procedures are performed in a manner that minimizes the risk of producing splashes and aerosols.
Wearing the appropriate PPE (e.g., safety goggles) or modifying procedures can effectively reduce the risk of producing splashes or aerosols. The need to use a primary containment device can be based on LRAs that take into consideration the activities taking place (e.g., likelihood of producing an infectious aerosol), the quantities of material being handled (i.e., large volumes or high concentrations) and the inherent characteristics of the RG1 biological material. An LRA could also determine that highly volatile material (e.g., toxins in powder form, fungal spores) may need to be handled in still air to prevent the spread of contamination.
Personnel wash hands after handling RG1 biological material if gloves are not worn or immediately after removing gloves, and before leaving the work area.
Handwashing is performed according to SOPs immediately before exiting the laboratory work area, animal room, animal cubicle, or PM room to prevent hands from being contaminated upon exit. It includes washing any skin surfaces that may have been exposed to RG1 biological material (e.g., forearms not covered by PPE). An LRA may determine it is safe to remove gloves within an inner room or inner area of the CL1 zone and wash hands at a handwashing sink located near the exit from the CL1 zone. Similarly, it may be acceptable to wash hands at a workstation far from the exit. If sinks are not readily available, an LRA may determine that hands can be decontaminated using a hand sanitizer (confirmed to be effective against the RG1 biological material in use) and hands washed at an available sink outside the CL1 zone.
Dedicated and activity-specific PPE is doffed (i.e., removed) in a manner that minimizes contamination of the skin, hair, and personal clothing, and is stored or disposed of within the work area. Disposable gloves used when handling RG1 biological material are discarded after each use.
Removing dedicated and activity-specific PPE in a particular order and in a manner that prevents the contamination of skin, hair, and personal clothing (as described in SOPs) reduces the potential of creating aerosols and of contaminated PPE coming into contact with unprotected skin, hair, or personal clothing. Reducing such risks protects personnel from exposure. Doffed PPE is stored or disposed of within the CL1 zone to prevent the potential spread of contamination.
Safe work practices for handling sharps are developed and strictly followed to prevent injury and exposure, and include:
- actively avoiding the use of needles, syringes, and sharp and glass objects wherever possible or selecting suitable alternatives (e.g., safety-engineered sharps devices);
- refraining from bending, shearing, breaking, re-capping, or removing needles from syringes;
- collecting and removing sharp objects (e.g., broken glassware) with a brush and dustpan, or tongs;
- discarding used sharps (e.g., scalpel blades, syringes) and other sharp objects (e.g., broken glassware, pipette tips, broken pipettes) in waste containers for sharps that are leak-proof, puncture-resistant, and fitted with lids.
Needles, syringes, and sharp and glass objects can cause punctures or injuries and can potentially result in the injection or inoculation of personnel with biological material. Activities such as bending, shearing, re-capping, or removing needles from syringes create an even greater risk of injury and should be avoided. Where that is not possible, strict adherence to SOPs (e.g., using forceps to bend or recap a needle) can protect personnel. When disposing of sharps, waste containers must be fit for purpose to prevent sharp objects from puncturing the container, which could potentially cause leaks or injuries, and result in exposure of containment zone personnel or individuals handling waste. If sharps waste is to be autoclaved, containers must be able to withstand the temperatures to which they will be exposed.
3.2 Biosafety program management
The development of facility-wide biosafety programs and policies is fundamental in implementing safe work practices and improving safety performance. A biosafety program is created to mitigate the hazards identified by an overarching risk assessment of the facility and its general activities. LRAs, on the other hand, are conducted to identify risks associated with site-specific activities, for which safe work practices are developed and incorporated into SOPs.Footnote 12 Even with RG1 biological material, these risk assessments are recommended in order to identify hazards and develop strategies to mitigate the risks. LRAs for RG1 biological material can be quite simple and, in some scenarios, there may be no significant risk. For example, if there is a risk of contaminating the hands, gloves may be recommended; however, if the material being handled is considered completely safe to handle (e.g., bread mould, yogurt culture), gloves are not required from a biosafety perspective. In contrast, the LRA could identify a risk specific to a particular worker (e.g., an immunocompromised or immunosuppressed individual).
Training aims to provide a rudimentary understanding of biosafety as well as safety in general. To foster a safe work environment and protect workers, it is also important to establish a program to train and educate staff (preferably prior to commencing work with RG1 biological material). This program covers the potential hazards present in their work environment, basic biosafety principles, proper use of PPE and laboratory equipment, as well as appropriate and safe work practices and techniques. In addition, an emergency response plan (ERP) sets out procedures for staff to follow in various emergency situations. Policies on regular inspections of the work area by personnel are important for the timely identification of faults and deterioration of surfaces, installations, and equipment that may put personnel at risk of exposure or cause a release of biological material into the environment. Further information on these topics can be found in the Canadian Biosafety Guideline series.Footnote 13
The size, structure, and complexity of an organization will determine who is responsible for the development and implementation of the biosafety program, including the overarching and local risk assessments. For example, in a larger institution such as a university, the overarching risk assessment is often carried out by senior management in collaboration with the biological safety officer, while the LRA may be conducted by laboratory workers and/or the principal investigator. In smaller organizations working with RG1 biological material, biosafety program and facility management may be a shared responsibility between laboratory workers and administrative personnel.
Please note that the following are recommendations only and are not requirements unless otherwise specified by the PHAC or the CFIA.
Outline of section 3.2 Biosafety program management
A biosafety program that meets the facility's specific biosafety needs is developed, documented, implemented, kept up to date, evaluated, and improved as necessary to oversee safety practices.
A biosafety program allows for the effective implementation and continued application of biosafety practices in accordance with regulatory recommendations and an organization's biosafety policies. The biosafety program is meant to promote a culture of biosafety, to mitigate all risks identified through risk assessments, and to develop plans and programs (e.g., training, incident investigation, validation and verification, creation of SOPs, documentation and records). The biosafety program may be included with, or incorporated into, other safety programs (e.g., occupational health and safety, chemical safety, radiation safety).
A biosafety manual is developed, kept up to date, and communicated to authorized personnel, and includes:
- institutional biosafety policies, programs, and plans;
- appropriate mitigation strategies identified by overarching, local, and biosecurity risk assessments;
- an ERP;
- SOPs for safe work practices for each task involving RG1 biological material, based on the hazards identified by LRAs.
The biosafety manual promotes biosafety and biosecurity among personnel inside and outside the CL1 zone. It can include information on biosafety and biosecurity policies and program intent and can provide personnel with an overarching view of biosafety and biosecurity objectives within the organization. It can also include information specific to a CL1 zone, including a description of the physical design and operation of the CL1 zone and systems to provide context to SOPs (e.g., entry and exit procedures). A communication plan can be implemented to inform personnel of new or updated information, and where it can be found. Information can exist as a single paper-based or electronic document, or as a collection of separate documents. When information related to various biosafety program elements exists across separate documents, a central document (e.g., a table of contents) may allow quick and easy access to all essential information.
The selection of PPE appropriate for a given task, work area, or CL1 zone is determined by an LRA.
Using or wearing dedicated and activity-specific PPE protects individuals from exposure and prevents the release of biological material from the CL1 zone, as determined by an LRA. Examples of PPE include lab coats, aprons, solid-front gowns, coveralls (e.g., for use inside a CL1 large animal work area), eye or face protection (e.g., goggles, face shield) where there is a risk of exposure to splashes or flying objects, and dedicated footwear (e.g., boots, shoes) or additional protective footwear (e.g., boot or shoe covers) where floors may be contaminated (e.g., animal cubicles, PM rooms).
Procedures are in place and include precautions, as determined by an LRA, to prevent a leak, drop, spill, or similar event during storage or the movement of RG1 biological material.
Procedures to prevent leaks, drops, or spills, or to contain the RG1 biological material when these events occur, protect personnel from exposure incidents and prevent the release of RG1 biological material. This includes having procedures for the storage of material (including waste) and movement of material within a building. Procedures (e.g., use of cart, closed containers) are based on LRAs that take into consideration the type of material being moved or stored, the risks associated with the material (e.g., large volumes of material, whether contained in a single vessel or multiple vessels), and the areas in the building where incidents (e.g., collisions, bumps) are more likely to occur.
An ERP, based on an overarching risk assessment and LRAs, is developed and kept up to date. The ERP includes emergency contact information and describes procedures for:
- accidents and incidents;
- medical emergencies;
- chemical and biological spills;
- animal escape (if applicable);
- reporting of incidents to the key internal personnel;
- biosafety and biosecurity incident investigation and follow-up;
- the implementation of measures to mitigate future risks.
A comprehensive ERP enables personnel to respond quickly to emergency situations. It sets out the procedures that personnel need to follow in response to various emergency situations in order to protect their health and safety, to prevent the release or spread of RG1 biological material, and to maintain the security of related assets (e.g., sensitive information) stored inside and outside the CL1 zone. The ERP is reviewed and updated at a frequency determined by the organization (e.g., during a routine biosafety program review, following any changes to the biosafety program).
A training program, based on a training needs assessment, is developed, documented, kept up to date, evaluated, and improved as necessary to educate personnel on all aspects relevant to the work performed and includes:
- SOPs and relevant elements of the biosafety manual;
- potential hazards associated with the RG1 biological material handled;
- necessary precautions to prevent exposure to, or release of biological material;
- correct use and operation of laboratory equipment;
- restraint and handling techniques for work involving animals;
- emergency response procedures.
Personnel are trained and demonstrate knowledge and proficiency in the relevant elements of the established training program before working independently with RG1 biological material.
A training needs assessment can be used when initially developing a training program to determine key components of the training program, including objectives, content, target groups, implementation strategy, and retraining cycles. Additional or refresher training may be developed over time, based on the review process, personnel competence and experience, incidents, or changes in the biosafety program.
A mechanism is in place to prevent, detect, and respond to pest control issues.
The entry and exit of pests (e.g., rodents, insects) may lead to the inadvertent transfer and spread of RG1 biological material outside the CL1 zone. A mechanism (e.g., SOP, device) to detect the presence of pests allows for a prompt response to prevent their entry and exit from the CL1 zone. Where pests may be an issue, mechanisms for basic pest control include fitting windows with properly installed screens that are in good repair, placing traps at strategic locations, and installing door sweeps.
Inspections of the work area are conducted and documented by personnel to identify faults, deficiencies, and deterioration; when found, corrective measures are implemented, and records kept on file.
Faults, deficiencies, or deterioration within a work area include physical elements (e.g., faulty equipment or lighting, cracked or chipped paint or floors, chipped or worn benchtops) and operational elements (e.g., non-adherence to entry procedures). The above may not be noticed by personnel during normal operations (i.e., on a day-to-day basis); therefore, it is best practice to perform inspections of the work area, including surfaces, floors, walls, ceilings, and equipment. When deficiencies are found, corrective measures are implemented in a timely manner. Documenting the processes used (e.g., what was verified and pass/fail criteria) and results serves as evidence that the work area and equipment function as designed and intended, that tests were performed when required (e.g. autoclave validation), and corrective measures were implemented in a timely manner (i.e., what, when and how corrective measures were implemented).
Primary containment devices used for large scale production activities are visually inspected to confirm their integrity.
Primary containment devices (e.g., process equipment, closed systems, sealed centrifuge cups and rotors) are tested to confirm they operate as intended to prevent the release of RG1 biological material, and exposure incidents resulting from equipment failure. This assessment includes tests for HEPA filters when they are present. HEPA filters that cannot be tested (e.g., small in-line filters, inaccessible HEPA filters) can be replaced regularly according to a replacement schedule for which the frequency of replacement is based on manufacturer's specifications and LRAs. Visual inspection of small equipment may be performed more routinely than larger equipment (e.g., visually inspecting O-rings in centrifuge buckets).
3.3 Decontamination and waste management
The effective decontamination of waste, materials, equipment, and surfaces that have come in contact with RG1 biological material is fundamental in limiting the spread of contamination beyond the work area and facility. To ensure effective decontamination, it is important to select a method for disinfection that is fit for purpose. Scientific literature (e.g., peer-reviewed journal articles) is referenced in order to determine the appropriate disinfectant, decontamination and verification methods for the specific RG1 biological material present in the facility.
In Canada, chemical products used as disinfectants on environmental surfaces and inanimate objects require pre-market assessment by Health Canada and a Drug Identification Number (DIN).Footnote 17 Health Canada must be provided with sufficient information to support the safety, efficacy, and quality of a disinfectant when used in accordance with the label's recommended conditions of use before market authorization can be granted.Footnote 17 Examining a commercial disinfectant's label will provide information on the biological material it is effective against and proper use instructions.
Bleach is an example of a broad spectrum disinfectant (or an ingredient of one) that is generally effective against the majority of RG1 biological material. A 10% dilution of bleach (i.e., 1 in 10 dilution, or 5,000 parts per million [ppm] sodium hypochlorite), prepared fresh to maintain disinfectant activity and applied for an appropriate contact time, is sufficient for surface decontamination of most RG1 biological material. Rinsing work surfaces with water after the application of bleach (for an appropriate amount of time to effectively disinfect) will reduce the occurrence of pitting in some surface materials (e.g., stainless steel).
Solvents, detergents, and alcohols may also be suitable disinfectants for use, depending on the biological material and the work being performed. For example, alcohol (e.g., 70% ethanol or isopropanol, in water) is often used as an alternative to bleach as it is generally effective against vegetative bacteria, mycobacteria, and enveloped viruses. However, it is not effective against bacterial or fungal spores.Footnote 18,Footnote 19 Contact time and concentration of an alcohol solution are critical factors for its effectiveness as a disinfectant since it can quickly evaporate; therefore, procedural and storage conditions are designed to account for this.Footnote 20 It is critical to follow the authorized labelling instructions provided with the product to attain the necessary disinfection levels.
Please note that the following are recommendations only and are not requirements unless otherwise specified by the PHAC or the CFIA.
Outline of section 3.3 Decontamination and waste management
Gross contamination is removed from surfaces and equipment prior to their decontamination and is disposed of in accordance with SOPs.
Organic material (e.g., feces, blood, bedding, feed) may inactivate or impede certain surface decontamination methods (e.g., chemical disinfectants, ultraviolet radiation). Removing gross contamination by physical methods such as scraping, brushing, and wiping from surfaces, equipment, and other items will allow for their effective decontamination. It is important that tools used to physically remove gross contamination do not damage surfaces or equipment.
After work with RG1 biological material, and after any spills or splashes, surfaces are cleaned and decontaminated using effective disinfectants or neutralizing chemicals at a frequency determined by an LRA.
Decontaminating surfaces (e.g., work surfaces, floors, walls) using disinfectants or neutralizing chemicals effective against the RG1 biological material in use minimizes the risks of exposure when it is performed at an appropriate frequency based on an LRA. An LRA may determine some surfaces need to be decontaminated more routinely based on certain activities taking place (i.e., risk of contamination). For example, cleaning and decontaminating animal cubicles, PM rooms, and the "dirty" corridor whenever they are grossly contaminated and at the end of each experiment prevents the spread of contamination, which protects personnel from exposure. SOPs can be developed to establish the frequency of decontamination of specific surfaces.
Disinfectants and neutralizing chemicals effective against the RG1 biological material are available, used in the work area, and routinely verified (e.g., by methods recommended in peer-reviewed publications).
Disinfectants and neutralizing chemicals available inside the work area allow for decontamination of surfaces (e.g., benchtops, containers) and prompt response to biological spills when used in accordance with their authorized labelling instructions. Neutralizing chemicals used for a given toxin may not be effective against others. Similarly, a disinfectant that is effective against one microorganism may not be effective against others. In addition, other factors that may influence their effectiveness include the type of biological material being decontaminated (e.g., virus, bacteria, toxin), its state (e.g., vegetative, spore form), the organic load (i.e., amount of organic material such as soil, bedding, litter, feed, or manure present on a surface or in a suspension), and the shelf life of the disinfectant (both the concentrated and working solutions). As such, it is best practice to label disinfectants with the expiry date or date of preparation if not in their original container, and to always use them according to the instructions on the authorized Canadian label.
Contaminated and potentially contaminated liquids are decontaminated prior to release into sanitary sewers.
Decontamination of liquids using a validated and routinely verified method prior to discharge into sanitary sewers prevents the release of biological material. It is best practice to decontaminate potentially contaminated liquids leaving a CL1 zone prior to release into sanitary sewers as liquids handled within the CL1 zone are at risk of becoming contaminated during experimental procedures.
Solid and liquid waste, clothing, PPE, equipment, and other items that may be contaminated are:
- a) decontaminated prior to disposal or removal from the work area, and in the case of equipment, prior to maintenance or repair;
- b) placed in closed, labelled, and leak-proof secondary containers that have been surface decontaminated for movement or transport to another area for decontamination.
Decontamination of RG1 biological material and potentially contaminated items such as PPE (e.g. gloves), waste, clothing, and equipment prior to disposal or removal from the CL1 zone, or prior to testing, maintenance or repair of equipment is important to prevent the release of RG1 biological material from the CL1 zone. If contaminated material is removed from the CL1 zone in a closed, labelled and leak-proof secondary container for subsequent decontamination at a location outside the CL1 zone, it is best practice to decontaminate the surface of the secondary container to protect the safety of individuals who handle, clean, and dispose of it. An LRA could be used to determine if decontamination of the surface of the secondary container is needed, taking into account the likelihood for the outside of the secondary container to be contaminated. An example of a leak-proof secondary container for waste is an autoclave bag within a solid impact-resistant container that is labelled and kept closed.
3.4 Animal work considerations
Additional biosafety concerns exist for work involving animals. Animals may harbour microorganisms that are pathogenic to humans as part of their normal flora, creating additional risks to personnel that include exposure to RG1 pathogens from infected animals or animal products containing RG1 pathogens, and injury due to bites, scratches, or kicks. Thus, it is important to develop safe work practices to minimize animal stress and protect personnel from exposure or injury.
Please note that the following are recommendations only and are not requirements unless otherwise specified by the PHAC or the CFIA.
Outline of section 3.4 Animal work considerations
Procedures (e.g., restraint and handling techniques) are used to minimize the risk of personnel injury, including scratches, bites, kicks, crushing injuries, and accidental self-inoculation.
Since animals can behave unpredictably, and different species give rise to different risks, using restraint and handling techniques appropriate to the species or animal (i.e., based on an LRA) provides both personnel and animal safety, especially when the animal is diseased or has been exposed to RG1 biological material.
Caging and cubicles that house animals are labeled to identify any RG1 biological material in use or other potential risks to personnel.
Identifying the RG1 biological material to which an animal has been exposed on the label of the primary containment caging or animal cubicle housing the animal is critical for personnel to be aware of the risks associated with the animal and to properly contain the material present during procedures with the animal. This is particularly important when more than one RG1 biological material may be in use.
Procedures are designed and carried out to minimize the creation of aerosols and are conducted separately from the area where animals are housed.
Certain procedures that are used to expose animals to RG1 biological material (e.g., inoculation, surgery, necropsy), or that involve animals that have been exposed (e.g., sample collection, surgery and necropsy techniques) have the potential for creating aerosols. Procedures that prevent the creation of aerosols and the dissemination of dust and other particulates containing RG1 biological material protect personnel from exposure and prevent the spread of contamination. For example, it may be preferable to use a low-pressure water spray or other similar method during initial cleaning procedures for animal cubicles or PM rooms instead of a pressure washer that is more likely to aerosolize RG1 biological materials. Procedures can also include avoiding inoculation, surgical, and necropsy techniques that have the potential for creating aerosols or implementing procedures to mitigate the risks associated with these techniques (e.g., using a downdraft or backdraft station).
Animals and carcasses are moved into, within, and out of the animal work area in a manner that prevents animal escape and the spread of contamination.
Live animals are safely and securely moved into, within, and out of the CL1 zone (e.g., using restraint techniques) to prevent animal escape and the spread of contamination within (e.g., in a "clean" corridor) or outside the CL1 zone. Procedures may include using primary containment caging with filters, or the use of gates and restraints. Removing animal carcasses in a manner that prevents the spread of contamination helps prevent exposure to, or release of RG1 biological material. Examples include packaging animal carcasses in labelled, leak-proof, and impact-resistant containers that have been surface decontaminated. Large animal carcasses can also be divided into smaller portions prior to packaging in order to facilitate movement.
Animal work areas, including PM rooms and associated corridors, when present, are decontaminated at a frequency determined by an LRA, and when grossly contaminated.
Removing gross contamination, cleaning with soap and water, and decontaminating surfaces (e.g., work surfaces, floors, walls) using disinfectants or neutralizing chemicals effective against the RG1 biological material in use minimizes the risks of exposure when it is performed at an appropriate frequency that can be based on an LRA. An LRA may determine some surfaces need to be decontaminated more routinely based on certain activities taking place (i.e., risk of contamination). For example, decontaminating animal cubicles, PM rooms, and the "dirty" corridor whenever they are grossly contaminated and at the end of each experiment prevents the spread of contamination, which protects personnel from exposure. SOPs can be developed to establish the frequency of decontamination of specific surfaces.
4. Glossary
It is important to note that the definitions provided in this glossary may differ from universally accepted definitions or those published in the CBS. Terms identified with an asterisk (*) have been specifically adapted from the CBS definitions for use within the context of the Canadian Biosafety Guideline – Containment Level 1: Physical Design and Operational Practices.
- Accident
- An unplanned event that results in injury, harm, or damage.
- Administrative area *
- A dedicated room or adjoining rooms that are used for activities that do not involve biological material. Administrative areas do not require any containment equipment, systems, or operational practices. Examples of administrative areas include offices, photocopy areas, and meeting/conference rooms.
- Aerosol
- A suspension of fine solid particles or liquid droplets in a gaseous medium (e.g., air) that can be created by any activity that imparts energy into a liquid or semi-liquid material.
- Animal cubicle
- A room or space designed to house an animal (or animals) where the room itself serves as primary containment. These spaces are used to house large-sized animals (e.g., livestock, deer) or small-sized animals that are housed in open caging (i.e., not primary containment caging).
- Animal room
- A room designed to house animals in primary containment caging. These spaces are used to house only small-sized animals (e.g., mice, rats, rabbits).
- Animal work area
- A room or space dedicated to housing or conducting activities with animals.
- Authorized personnel
- Individuals who have been granted unsupervised access to the containment zone by an internal authority (e.g., the containment zone director, biological safety officer, another individual to whom this responsibility has been assigned). Access is dependent on personnel completing training requirements and demonstrating proficiency in the standard operating procedures, as determined to be necessary by the facility.
- Biological material
- Pathogenic and non-pathogenic microorganisms, proteins, and nucleic acids, as well as any biological matter that may contain microorganisms, proteins, nucleic acids, other infectious agents, or parts thereof. Examples include, but are not limited to, bacteria, viruses, fungi, prions, toxins, genetically modified organisms, nucleic acids, tissue samples, diagnostic specimens, environmental samples, live vaccines, and isolates of a pathogen or toxin (e.g., pure culture, suspension, purified spores).
- Biological safety officer (BSO)
- An individual designated for overseeing the facility's biosafety and biosecurity practices.
- Biosafety *
- Containment principles, technologies, and practices that are implemented to prevent unintentional exposure to biological material, or their accidental release.
- Biosafety program *
- A program that describes institutional plans and policies that facilitate the safe handling and storing of biological materials, and prevent their release from the containment zone. Core elements of a biosafety program include, but are not limited to, a biosafety manual, a comprehensive training program, a medical surveillance program, an emergency response plan, standard operating procedures, and a biosecurity plan.
- Biosecurity *
- Security measures designed to prevent the loss, theft, misuse, diversion, or intentional release of biological material and other related assets (e.g., personnel, equipment, animals, sensitive information).
- Biosecurity risk assessment *
- A risk assessment in which the biological material, and other related assets (e.g., equipment, animals, sensitive information, personnel) are defined and prioritized, the likelihood of threats, vulnerabilities, and associated consequences are assessed, and appropriate mitigation strategies are recommended to protect these assets against potential biosecurity events.
- Closed system
- Equipment, apparatus, or process system designed to contain biological material and prevent its release into the surrounding environment (e.g., the containment zone).
- Containment
- The combination of physical design parameters and operational practices that protect personnel, the immediate work environment, and the community from exposure to biological material. The term "biocontainment" is also used in this context.
- Containment level (CL) *
- Minimum physical containment and operational practice requirements for handling biological material safely in laboratory, large scale production, and animal work environments. There are four containment levels ranging from a basic laboratory (i.e., CL1) to the highest level of containment (i.e., CL4).
- Containment zone *
- A physical area that meets the requirements for a specified containment level. A containment zone can be a single room, a series of co-located rooms, or it can be comprised of several adjoining rooms. Dedicated support areas, including anterooms with showers and "clean" and "dirty" change areas where required, are considered to be part of the containment zone.
- Contamination *
- The undesired presence of biological material on a surface (e.g., benchtop, hands, gloves) or within other materials (e.g., laboratory samples, cell cultures).
- Decontamination
- The process by which materials and surfaces are rendered safe to handle and reasonably free of microorganisms, toxins, or prions; this may be accomplished through disinfection, inactivation, or sterilization.
- Disease
- A disorder of structure or function in a living human or animal, or one of its parts, resulting from infection or intoxication. It is typically manifested by distinguishing signs and symptoms.
- Disinfectant
- A chemical agent capable of eliminating viable biological material on surfaces or in liquid waste. Effectiveness can vary depending on the properties of the chemical, concentration, shelf life, and contact time.
- Emergency response plan (ERP) *
- A document outlining the actions to be taken and the parties responsible in emergency situations such as a spill, exposure, release of biological material, animal escape, personnel injury or illness, power failure, or other emergency situations.
- Emerging animal disease (EAD)
- A new infectious disease resulting from the evolution or change of an existing pathogenic agent; a known infectious disease spreading to a new geographic area or population; or a previously unrecognized pathogenic agent or disease diagnosed for the first time which may have a significant impact on animal health, as determined by the Canadian Food Inspection Agency.
- Exposure *
- Contact with, or close proximity to, biological material that may result in infection or intoxication. Routes of exposure include inhalation, ingestion, inoculation, and absorption.
- Facility *
- Structures or buildings, or defined areas within structures or buildings, where biological material is handled or stored. This could include individual research and diagnostic laboratories, large scale production areas, or animal zones. A facility could also be a suite or building containing more than one of these areas.
- Foreign animal disease (FAD)
-
A disease that appears in the World Organisation for Animal Health Listed Diseases (as amended from time to time) that is not considered indigenous to Canada, as determined by the Canadian Food Inspection Agency (CFIA); or any CFIA-regulated Reportable Disease that does not exist in Canada for which the CFIA has an established response strategy; or any other disease which after due consideration is designated as such by the Minister of Agriculture and Agri-Food. Pathogens causing an FAD may also have serious negative health effects on Canadian animal populations.
- Good microbiological laboratory practices
- A basic laboratory code of practice applicable to all types of activities with biological material. These practices serve to protect workers and prevent contamination of the environment and the samples in use.
- Gross contamination
- The accumulation of organic material (e.g., bedding, feed, excrement, blood, tissues) on a surface that can be removed by physical methods, such as scraping, brushing, and wiping.
- Handling or storing *
- "Handling or storing" biological material includes possessing, handling, using, producing, storing, permitting access to, transferring, importing, exporting, releasing, disposing of, or abandoning such material. All tenses and variations of "handling or storing" are also used in this context.
- Hazard
- A source of potential damage, harm, or adverse effects. In the context of biosafety, examples include objects (e.g., sharps, needles), materials (e.g., pathogens, toxins), animals (e.g., bites, scratches), and situations (e.g., containment system failure).
- High efficient particulate air (HEPA) filter
- A pleated mechanical air filter capable of filtering 99.97% of airborne particles 0.3 µm in diameter, the most penetrating particle size. Due to the effects of impaction, diffusion, and interception, HEPA filters are even more efficient at trapping and retaining particles that are either smaller or larger than 0.3 µm in diameter.
- Incident
- An event or occurrence that has the potential of causing injury, harm, infection, intoxication, illness, disease, or damage. Incidents include accidents and near misses.
- Laboratory
- An area within a facility or the facility itself where biological material is handled.
- Laboratory work area
- An area inside a containment zone designed and equipped for in vitro activities (e.g., for research, diagnostics, and teaching purposes).
- Large scale production area *
- A room or space where production or pre-production activities, including in vitro cultures, that involve large volumes of biological material are conducted. Large scale activities differ from laboratory- or bench-scale activities based on the equipment used as they are typically performed in fermenters, bioreactors, and other closed systems.
- Local risk assessment (LRA) *
- A site-specific risk assessment used to identify hazards based on the biological material in use and the activities being performed. This analysis informs risk mitigation and risk management strategies, which are to be incorporated into the physical containment design and operational practices of the facility.
- Mechanism
- A physical or operational measure.
- Microorganism
- A cellular or non-cellular microbiological entity that cannot be reasonably detected by the naked eye, and is capable of replication or transferring genetic material. Microorganisms include bacteria, fungi, viruses, and parasites, and may be pathogenic or non-pathogenic in nature.
- Movement *
- The action of moving (e.g., bringing, carrying, leading, relocating) people, material (including biological material), or animals from one physical location to another physical location in the same building. This can include movement within the same containment zone, to a different containment zone, or to another location within the same building.
- Operational practices
- Administrative controls and procedures followed in a containment zone to protect personnel, the environment, and ultimately the community, from biological material.
- Overarching risk assessment *
- A broad risk assessment that supports the biosafety program as a whole and may encompass multiple containment zones within an organization. The overarching risk assessment identifies hazards, risks, and mitigation strategies for the proposed activities involving biological material. Mitigation and management strategies reflect the type of biosafety program needed to protect personnel from exposure and to prevent the release of biological material.
- Pathogen
- A microorganism, nucleic acid, protein, or other infectious agent that is transmissible and capable of causing disease or infection in humans or animals. Classified human and animal pathogens can be found on the Public Health Agency of Canada's ePATHogen – Risk Group Database.
- Pathogenicity
- The ability of a pathogen to cause disease in a human or animal host.
- Personal protective equipment (PPE) *
- Equipment and/or clothing worn by personnel to provide a barrier against biological material thereby minimizing the risk of exposure. PPE may include, but is not limited to, lab coats, gowns, full-body suits, gloves, protective footwear, safety glasses, safety goggles, masks, and respirators.
- Physical design features
- Physical barriers in the form of engineering controls and facility design used to protect personnel, the environment, and ultimately the community from biological material.
- Post mortem room (PM room)
- A room within the containment zone area where necropsies and dissections are conducted on animals outside a primary containment device.
- Primary containment *
- The first level of physical barriers designed to contain biological material, and prevent their release. This is accomplished by the provision of a device, equipment, or other physical structure situated between the biological material and the individual, the work environment, or other areas within the containment zone. Examples include biological safety cabinets, glove boxes, and microisolator cages. In animal cubicles, the room itself serves as primary containment, and personal protective equipment serves as primary protection against exposure.
- Primary containment caging *
- Animal caging serving as a primary containment device to prevent the release of biological material. Examples include ventilated filter-top cages and ventilated microisolator cage rack systems, with or without HEPA filters.
- Primary containment device *
- Apparatus or equipment that is designed to prevent the release of biological material, and to provide primary containment (i.e., provide a physical barrier between the biological material and the individual or the work environment). Examples include biological safety cabinets, isolators, centrifuges with sealable cups or rotors, process equipment, fermenters, bioreactors, microisolator cages and ventilated cage racks.
- Prion
- A small proteinaceous infectious particle generally considered to be responsible for causing a group of neurodegenerative diseases in humans and animals known as transmissible spongiform encephalopathies.
- Process equipment
- Specific equipment used to carry out a manufacturing procedure involving biological material. This term is generally used to describe equipment used in large scale processes (e.g., industrial fermentation equipment, bioreactors).
- Regulated material
-
In the context of the Canadian Biosafety Standard, regulated material includes:
- human pathogens and toxins (under the Human Pathogens and Toxins Act and Human Pathogens and Toxins Regulations);
- terrestrial animal pathogens (under the Health of Animals Act [HAA] and Health of Animals Regulations [HAR]);
- terrestrial animal pathogens in animals, animal products, animal by-products, or other organisms (under the HAA and HAR).
- Release *
- The discharge of biological material from a containment system or containment zone (e.g., resulting from leaking, spraying, depositing, dumping, vaporizing).
- Risk
- The probability of an undesirable event (e.g., accident, incident, breach of containment) occurring and the consequences of that event.
- Risk group (RG)
- The classification of a biological agent (i.e., microorganism, protein, nucleic acid, or biological material containing parts thereof) based on its inherent characteristics, including pathogenicity, virulence, communicability, and the availability of effective prophylactic or therapeutic treatments. The risk group describes the risk to the health of individuals and the public, as well as the health of animals and the animal population.
- Standard operating procedure (SOP) *
- A document that standardizes safe work practices and procedures for activities with biological material in a containment zone, as determined by a local risk assessment. Examples of SOPs include experimental protocols, entry/exit procedures, decontamination protocols, and emergency response procedures.
- (Microbial) Toxin
- A poisonous substance that is produced by or derived from a microorganism and can lead to adverse health effects in humans or animals. Human toxins are listed in Schedule 1 and Part 1 of Schedule 5 in the Human Pathogens and Toxins Act.
- Training needs assessment
- An evaluation performed to identify the current and future training needs of the facility (i.e., organization, containment zone), including training, refresher training, and retraining, and to identify gaps in the current training program.
- Validation
- The act of confirming that a method achieves its objective and is suitable for its intended purpose through the provision of objective evidence. This can be achieved by observing that specific conditions have been met (e.g., using biological indicators, chemical integrators, or parametric monitoring devices placed in challenging locations within the load to confirm that a given autoclave cycle can decontaminate a representative load of waste).
- Verification
- The routine monitoring of equipment and processes to confirm continued efficacy between validations (e.g., testing the performance of an autoclave using biological indicators). Verification includes comparing the accuracy of a piece of equipment to an applicable standard or standard operating procedure.
- Virulence
- The degree or severity of a disease caused by a pathogen.
- Waste
- Any solid or liquid material generated by a facility for disposal.