Canadian biosafety guideline: Incident investigation
Download in PDF format
(6.29 MB, 87 pages)
Organization: Public Health Agency of Canada
Published: 2021-08-26
Table of contents
- Preface
- Abbreviations and acronyms
- Chapter 1: Introduction
- Chapter 2: Preparing for an incident investigation
- Chapter 3: Initial response
- Chapter 4: Collection of evidence and information
- Chapter 5: Analysis and identification of root causes
- Chapter 6: Implementation of corrective and preventive measures
- Chapter 7: Evaluation and continual improvement
- Chapter 8: Glossary
- Chapter 9: References and resources
- Appendix A: Biosafety-related incidents
- Appendix B: Incidents reportable to the PHAC
- Appendix C: Example incident scenario involving a student exposed to Staphylococcus aureus
Preface
In Canada, facilities where Risk Group 2, 3, and 4 human pathogens or toxins are handled and stored are regulated by the Public Health Agency of Canada (PHAC) under the Human Pathogens and Toxins Act (HPTA) and the Human Pathogens and Toxins Regulations (HPTR). The importation of animal pathogens, infected animals, animal products or by-products (e.g., tissue, serum), or other substances that may carry an animal pathogen or parts thereof (e.g., toxin) are regulated by the PHAC or the Canadian Food Inspection Agency (CFIA) under the Health of Animals Act (HAA) and Health of Animals Regulations (HAR).
The following figure depicts the document hierarchy used by the PHAC and the CFIA to oversee biosafety and biosecurity operations. Each tier of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards. Acts and regulations are found at the top of the pyramid, as they are the documents that convey the PHAC's and the CFIA's legal authorities. Guidance material and technical pieces are found at the bottom of the pyramid, as they are only intended to summarize recommendations and scientific information.
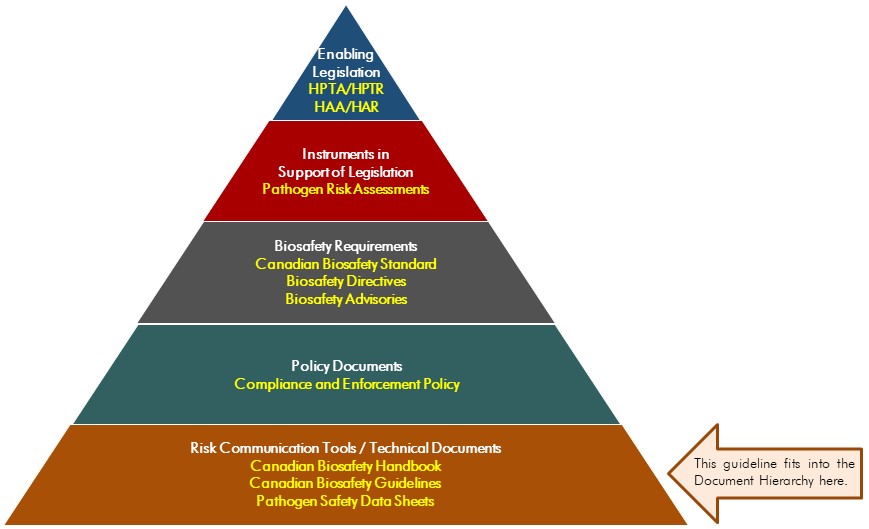
Figure 1 - Text description
Figure in the form of a pyramid depicting the document hierarchy used by the PHAC to oversee biosafety and biosecurity operations. Each of the five tiers of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards.
At the top sits the Enabling Legislation, that is, the HPTA, HPTR, HAA, and HAR, that convey the PHAC's legal authorities. Below the acts and regulations sit Instruments in Support of Legislation, which are the Pathogen Risk Assessments. The next tier down are the Biosafety Requirements, which include the Canadian Biosafety Standard, Biosafety Directives, and Biosafety Advisories. In the second lowest tier are the Policy Documents, which include the Compliance and Enforcement Policy. Guidance material and technical pieces found at the bottom of the pyramid, under the Risk Communication Tools and Technical Documents heading, are intended to summarize recommendations and scientific information only. These include the Canadian Biosafety Handbook, Canadian Biosafety Guidelines, and Pathogen Safety Data Sheets.
Incident Investigation was developed by the PHAC and the CFIA as part of a series of electronic publications that expand upon the biosafety and biosecurity concepts discussed in the current edition of the Canadian Biosafety Handbook (CBH), the companion document to the Canadian Biosafety Standard (CBS). This guideline further elaborates on the incident investigation process introduced in the CBH and serves as a supplementary resource for stakeholders seeking additional information and guidance in the context of responding to biosafety- and biosecurity-related incidents. The information provided in this guideline is meant to assist regulated parties in meeting the requirements specified in the CBS. It should not be interpreted as requirements. In addition, regulated parties may choose to use alternate evidence-based approaches to meet the CBS requirements.
This guideline is continuously evolving and subject to ongoing improvement. The PHAC and the CFIA welcome comments, clarifications, and suggestions for incorporation into future versions. Please send this information (with references, where applicable) to: PHAC.pathogens-pathogenes.ASPC@canada.ca.
Abbreviations and acronyms
- BSC
- Biological safety cabinet
- BSO
- Biological safety officer
- CBH
- Canadian Biosafety Handbook
- CBS
- Canadian Biosafety Standard
- CFIA
- Canadian Food Inspection Agency
- CL
- Containment level (i.e., CL1, CL2, CL3, CL4)
- HAA
- Health of Animals Act
- HAR
- Health of Animals Regulations
- HPTA
- Human Pathogens and Toxins Act
- HPTR
- Human Pathogens and Toxins Regulations
- LAI
- Laboratory-acquired infection/intoxication
- PHAC
- Public Health Agency of Canada
- PPE
- Personal protective equipment
- RG
- Risk group (i.e., RG1, RG2, RG3, RG4)
- SOP
- Standard operating procedure
- SSBA
- Security sensitive biological agent
Chapter 1: Introduction
The words in bold type are defined in the glossary found in Chapter 8.
An incident is an event or occurrence that has the potential to cause harm or damage to personnel, property, the community, or the environment. Any incident that involves pathogens, toxins, or other infectious material (e.g., infected animals or tissues) or that occurs where pathogens or toxins are present (e.g., laboratory, containment zone) has the potential to result in exposure, disease (e.g., infection, intoxication), or release of pathogens or toxins. All incidents, even those seemingly minor, should set in motion the facility's internal incident reporting procedures and incident investigation process, as they may be indicative of failures in equipment, containment systems, procedures, or training. The primary reason to investigate an incident is prevention: finding the cause and taking steps to control or eliminate it can prevent similar events from happening in the future.Footnote 1
1.1 Background
The term "incident" describes both accidents (i.e., an unplanned event that results in exposure, injury, harm, infection, intoxication, disease, or damage) and events or hazardous occurrences that do not result in exposure, injury, harm, infection, intoxication, disease, or damage (i.e., near misses).Footnote 2 Incidents involving pathogens and toxins may include:
- splashes and spills
- exposures (that may or may not cause disease)
- suspected and confirmed laboratory-acquired infections/intoxications (LAIs)
- failures or compromises of containment (i.e., failure of containment systems or devices)
- environmental releases (e.g., improperly treated waste sent to the sewer system)
- animal escapes or
- biosecurity breaches (e.g., theft or intentional misuse of a pathogen or toxin, unauthorized entry)
A summary of commonly documented biosafety- and biosecurity-related incidents is provided for reference in Appendix A.
During the investigation process, information is collected on the events that took place before and during the incident. The primary goal of incident investigation is to determine why and how it happened, as well as any factors that led to the event. The investigation process may also fulfill legal requirements, determine the costs associated with the incident (e.g., estimated costs of repairs, loss of material, productivity, or equipment), and provide an opportunity to self-evaluate compliance with applicable regulations (e.g., conducting an internal audit). Including established protocols for incident investigation and reporting in the facility's emergency response plan and standard operating procedures (SOPs) will help personnel respond to the incident in a timely and appropriate manner. The minimum requirements for incident investigation and reporting in regulated containment zones are specified in Chapter 4 of the Canadian Biosafety Standard (CBS).Footnote 3
This guideline outlines a 5-step model that can assist with the response, investigation, and reporting of biosafety- and biosecurity-related incidents. The model includes a standardized method of analysis to deconstruct the events leading up to and during an incident to explore the factors that contributed to the incident (causal factors) and to uncover the underlying initiating causes (root causes). Subsequently, interventions that target the identified root causes can be developed and implemented to prevent recurrence of the incident. There are numerous resources available that can also be consulted for further information on the development of incident reporting and investigation procedures.Footnote 4Footnote 5Footnote 6Footnote 7
1.2 Scope
The Incident Investigation guideline outlines the general principles of incident investigation that can be followed in response to an incident in a laboratory environment or other work area where pathogens, toxins, or other infectious materials are handled or stored. It expands upon the concepts introduced in the Canadian Biosafety Handbook (CBH).Footnote 8 The 5-step model described in this guideline outlines an approach to collect evidence and information, to identify and analyze the causal factors and root causes of an incident, and, ultimately, to determine corrective and preventive measures. It also supports the collection of information required by the Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA) for any notifiable incident and the identification of the incident root causes (as requested in the PHAC's Biosecurity Portal), and aims to facilitate the incident reporting process when a regulated party is obligated to report to the PHAC or the CFIA. The Canadian Biosafety Guideline - Notification and Reporting Under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal may be consulted for more detailed guidance on how incidents and other notifiable events are reported to the PHAC.Footnote 9
The information provided in this document is presented as recommendations only, and is not to be interpreted as requirements. The 5-step model presented is not the only acceptable or known model by which incidents can be investigated.
Voluntary investigation and reporting of biosafety- and biosecurity-related incidents in facilities not regulated by the PHAC or the CFIA is encouraged; this guideline can be followed in such cases, as long as other applicable legislation is respected. This guideline may also be followed for incidents that do not require mandatory notification of the PHAC or the CFIA, in order to investigate and document all biosafety- and biosecurity-related incidents in a thorough and consistent manner.
1.3 How to use the Incident Investigation guideline
A detailed list of all abbreviations and acronyms used throughout this guideline is located at the beginning of this document. Each word or term is spelled out upon first use, with the abbreviation immediately following in brackets. After its initial definition, the abbreviation is used exclusively throughout the remainder of the document. A comprehensive glossary of definitions for technical terms is located in Chapter 8 of this document. Words defined in the glossary appear in bold type upon first use. A list of references and resources is provided in Chapter 9.
Chapter 2: Preparing for an incident investigation
Incident response and investigation plans and procedures, based on an overarching risk assessment, describe the appropriate steps to be taken should an incident occur. Following established response procedures will improve efficiency, help address the situation in a timely manner, and prevent evidence from being lost or altered (e.g., objects moved or disposed of, poor witness recall).Footnote 10
2.1 Investigation procedures and protocols
This section outlines key considerations for implementing incident investigation procedures and protocols. There exist several standards describing incident investigation planning and response that can be consulted when establishing organizational procedures and protocols.Footnote 4Footnote 5Footnote 6 Additionally, other applicable legislation (e.g., from the province or territory's Ministry of Labour) may be consulted for comprehensive workplace incident investigation policies, protocols, and procedures.
2.1.1 Incident reporting and investigation policy
A high-level incident reporting and investigation policy or code of practice can be considered to establish an internal accountability system for the reporting and investigation of incidents, broadly identify investigation triggers (e.g., based on regulatory requirements, best practices, and injuries), and outline the expectations for personnel. Endorsement by senior management of such a policy or code of practice demonstrates that the organization takes the matter of incident investigation seriously and that senior management is committed to responding to the results of an investigation (i.e., implement necessary mitigation measures). This commitment can also be demonstrated through effective, timely, and open communication processes, which should include considerations for communicating incident investigation results to relevant parties (e.g., internal and external authorities, the surrounding community).
Incident reporting and investigation policies that encourage the reporting of all incidents (including near misses) will help prevent recurrence of similar incidents and lead to a safer work environment.
2.1.2 Emergency response plan
The emergency response plan outlines the pre-determined actions to be taken within a facility or organization in response to laboratory incidents and other emergency situations (e.g., power failure, fire, explosion, flood, earthquake, hurricane). To be effective, the emergency response plan has to be tailored to meet the needs of the organization or facility to which it applies; it should also address the safety of emergency personnel who may enter the containment zone. As a condition of their Pathogen and Toxin Licence (hereafter, "licence") or their animal pathogen import permit, regulated facilities where pathogens or toxins are handled or stored are required to develop and implement an emergency response plan and include a description of the organization's emergency response plan and procedures for incident reporting in the biosafety manual (CBS Matrix 4.1).Footnote 3
2.1.3 Procedures and forms
Having a documented investigation procedure or plan in place supports a timely, efficient, and standardized incident response; this is important to reduce the likelihood of evidence or important information and details being lost or forgotten.Footnote 10 The procedure or plan can include contact information for trained and pre-determined response personnel and investigation team members, contact information of the biological safety officer (BSO), and contact information of the appropriate members of senior management. The incident response can be facilitated by step-by-step instructions that clearly outline the response and investigation process, including:
- how to complete the necessary forms and notifications
- when to report the incident to both internal (i.e., within the organization) and external (e.g., the PHAC, the CFIA, and other regulators) authorities and
- who has the authority or is responsible to liaise with external organizations and authorities
Clearly identifying the roles and responsibilities of key personnel will also help the investigation progress smoothly. In addition, identifying when it is appropriate to end an investigation process will prevent the investigation from stopping prematurely (e.g., the investigation ends only once all possible root causes have been identified for every causal factor).Footnote 11
2.1.4 Reporting and notification of incidents
Thorough institutional incident reporting and investigation policies, protocols, and procedures will specify when and how incidents and associated investigation information are communicated and reported to internal and external authorities and stakeholders. This includes to whom the initial internal incident report will be sent, to whom the final investigation report will be sent, and who in the organization has the authority to communicate with external authorities, such as the PHAC, the CFIA, and local, provincial, and territorial health and safety regulators, as applicable.
2.2 Guiding principles of investigation
The following guiding principles can be reflected in the organization's incident reporting and investigation policy or code of practice and SOPs, and will help investigators reach reasoned, unbiased, and evidence-based conclusions.Footnote 6Footnote 12Footnote 13Footnote 14
- Prevention
The ultimate goal of an incident investigation is preventing future occurrences, achieved through the implementation of corrective and preventive measures that address the identified root causes. - Facts, not blame
The information collected by an investigator is not intended to assign blame or fault to an individual or group of individuals. An investigator is looking for the facts in order to identify and address the root causes and causal factors in processes and systems to prevent future incidents. - Confidentiality and privacy
Safeguarding the confidentiality of information obtained by the investigation team throughout an investigation (e.g., information that may identify persons involved or witnesses) will help maintain the integrity of the investigation. Sharing information regarding incidents on a need-to-know basis, and removing identifiable information (i.e., redacting) will reduce the risks of the persons involved being unnecessarily identified. This can also help encourage personnel to bring forward any relevant information by reducing fear of reprisal. - Thoroughness
A systematic approach that focuses on processes and systems, and not on individuals, with diligent query (i.e., who, what, where, when, why, and how?) can uncover why an event happened and determine all causal factors and associated root causes that may have contributed to the incident. - Objectivity and impartiality
A fair assessment of the collected information and facts is objective and impartial, and free of any personal biases. In some instances, the organization may appoint a third-party consultant to lead or participate in an investigation to maintain impartiality and minimize investigator bias. - Respect and professionalism
An investigation team that conducts an investigation in a professional manner, with integrity, fairness, and diligence, and adheres to the organization's values and ethics code or code of conduct will garner respect and confidence in the investigation process. - Credibility
A credible investigation process is logical, consistent, conducted in a clear and systematic manner, and complete. It also does not contradict itself or leave questions unanswered, it is not biased, it explains all findings, and it outlines any gaps, assumptions, and uncertainties. - Documentation
Carefully documenting all information collected by the investigation team is critical for future reference and analysis, and to maintain the integrity and the credibility of the investigation.
2.2.1 Who should investigate?
Prior to commencing an investigation, an investigation team needs to be established. The size of the team will depend on the size, severity, and complexity of the incident being investigated. The selection of members should take into account the guiding principles.
While in some cases a single individual may be sufficient to conduct the investigation, an investigation team comprised of several individuals may be required for more complex or severe scenarios. The inclusion of the BSO or another internal authority in the investigation team can also help demonstrate senior management's commitment to the investigation process. Investigation teams that include representatives across various levels of personnel within the organization (e.g., management, supervisors, scientists, laboratory staff) may also be more effective, as individuals will approach the investigation with differences in knowledge, perspective, and experience, which helps to prevent bias.Footnote 12
For the investigation to be credible, investigation team members need to be appropriately trained in identifying hazards and conducting risk assessments, experienced in conducting interviews and investigative techniques, and knowledgeable of legal and organizational requirements. Including people who are knowledgeable about the type of work being performed at the time of the incident will also help with the identification of causal factors.Footnote 15 To reduce the potential for conflict of interest and bias, both of which can diminish the integrity of the investigation, it may be preferable for individual(s) involved in the incident (i.e., those who have played a role in the incident or may be affected by it) to not be included in the investigation team. Depending on the nature and complexity of the incident, the investigation team may need the knowledge and expertise of additional individuals, such as health and safety professionals, engineering and maintenance personnel, security personnel (e.g., if the incident involves security matters), legal counsel, and union representatives, if applicable.Footnote 2Footnote 16Footnote 17
2.2.2 Roles and responsibilities
The first step in the investigation is to identify an investigation team leader whose responsibilities could include determining the priorities of the investigation, assigning tasks to other members, setting deadlines, and communicating with senior management and other stakeholders.Footnote 6Footnote 16 Ideally, the investigation team is led by an impartial individual with prior experience in incident investigation and a good understanding of the work activities and procedures related to the incident. The BSO, or biosafety representative, has a specific role with respect to incident investigations, in accordance with CBS and Human Pathogens and Toxins Regulations (HPTR) requirements [CBS Matrix 4.1; HPTR 9(1)].Footnote 18 It is the responsibility of the designated BSO to communicate with the PHAC and the CFIA on behalf of the licence holder, which includes notifying the PHAC of any incidents, as required [HPTR 9(1);Human Pathogens and Toxins Act (HPTA) 13].Footnote 19 Appendix A describes situations that need to be investigated and may require notification to the PHAC or the CFIA. Appendix B describes the situations when a licence holder is required to notify the PHAC without delay. Clearly outlining the roles and responsibilities of the investigation team members in written protocols and procedures allows members to be aware of their specific duties.
2.3 The 5-step model of incident investigation
The 5-step model illustrated in Figure 2-1 is an example of a standard approach for incident investigation. This approach can be followed to identify and analyze causal factors and root causes, which ultimately supports the development, implementation, and communication of corrective and preventive measures. The 5-step model also includes an evaluation step in order to improve or adjust the implementation of measures as well as the investigation process itself. Although the time required to conduct each step of the investigation depends on the type, complexity, and severity of the incident, approximate timelines are provided in this model for general reference. The steps generally proceed in a sequential manner, although elements of each step can overlap. For example, the collection of information (beginning of Step 2) may begin before or at the same time that the notification (end of Step 1) is prepared and submitted to internal or external authorities. Each step will be discussed in the subsequent chapters of this guideline.

Figure 2-1 - Text description
Figure depicting a vertical chevron list detailing the 5-step model of incident investigation. The first chevron represents Step 1, which takes place between 0 and 72 hours. A box at the right of this chevron details the initial response:
- assess the situation; provide first aid and emergency care; control, contain, secure.
- notify the internal and, if applicable, external authority; identify and preserve evidence.
The second chevron represents Step 2, which takes place between 0 and 10 days. A box at the right of this chevron details the collection of evidence and information:
- collect physical evidence, photos, witness accounts, data, documentation, records, logs.
- create a timeline to establish the sequence of events.
The third chevron represents Step 3, which takes place between 0 and 3 weeks. A box at the right of this chevron details the analysis and identification of root causes:
- analyze all the available information to determine the causal factors.
- identify the root causes of each causal factor to explain why the incident occurred.
The fourth chevron represents Step 4, which takes place between 0 and 1 month. A box at the right of this chevron details the implementation of corrective and preventive measures:
- correct the root causes of the incident.
- implement measures to prevent or mitigate the risk of future occurrences.
The fifth and last chevron represents Step 5, which takes place between 3 and 12 months. A box at the right of this chevron details the evaluation and continuous improvement:
- review the implementation of corrective and preventive measures.
- evaluate the effectiveness of corrective and preventive measures in addressing root causes.
Chapter 3: Initial response

Text description
Figure depicting the first chevron of the vertical chevron list illustrated in Figure 2-1, which details the 5-step model of incident investigation.
This chevron represents Step 1, which takes place between 0 and 72 hours. A box at the right of this chevron details the initial response:
- assess the situation; provide first aid and emergency care; control, contain, secure.
- notify the internal and, if applicable, external authority; identify and preserve evidence.
Although a facility's incident investigation process truly begins in Step 2 (Reporting and Notification), the actions undertaken in the initial response (Step 1) are often crucial to support a successful investigation. Personnel involved in Step 1 may or may not be the same people investigating the incident.
The first step taken in response to an incident can be the most critical for an incident investigation. The incident response procedures described in the emergency response plan are initiated the moment an incident is recognized. First aid and emergency care are administered (if required), a preliminary assessment of the incident is conducted, mandatory internal and external reporting occurs, and the formal incident investigation process can begin. Because evidence can disappear and memories fade, it is important that the incident response and investigation processes be initiated as quickly as possible, and stated as such in organizational SOPs.
In general, personnel involved in the initial response will not be members of the incident investigation team, but as the first people on the scene, they are in the best position to assess, control, contain, and secure the scene until first responders arrive (if required) and to prevent evidence being lost or corrupted.Footnote 10
3.1 Securing the scene
Incidents, especially those where someone has been injured, can quickly attract onlookers who could interfere with the emergency response and first aid. Onlookers could also disturb or damage evidence.Footnote 20 While procedures for securing the scene should be included in the emergency response plan to prevent any further injuries or the spread of contamination if a hazardous situation remains, it is also an important component of the investigation to preserve any evidence or information until the investigation team arrives.
Securing the incident scene can be accomplished by cordoning off the area with barricade markers (e.g., safety cones), warning tape, or signs, by locking the door, or by posting a person at the entrance to the affected area to prevent the entry of non-essential or unauthorized individuals.
3.2 Reporting and notification
3.2.1 Internal reporting
The internal reporting of an incident sets in motion the facility's incident investigation process. Facilities regulated by the PHAC and the CFIA are required to develop and maintain documented procedures to define, record, and report incidents involving pathogens or toxins (CBS Matrix 4.1). These procedures describe how incidents are reported internally (e.g., with an incident report form), who to contact (e.g., BSO, health and safety officer, senior management), and how to contact them (e.g., room numbers, phone numbers, email addresses).
The reporting of incidents is a responsibility shared by everyone working in a facility. Personnel are required to immediately inform the appropriate internal authority (e.g., supervisor, laboratory director, BSO) of any incident that may have resulted in an exposure to a human pathogen or toxin, and any disease (i.e., LAI) that may have resulted from an exposure to a human pathogen or toxin in the facility (CBS Matrix 4.2). Likewise, personnel are required to immediately report any incident involving pathogens, toxins, or other regulated infectious material, infected animals, or involving failure of containment systems or control systems to internal authorities (CBS Matrix 4.9).
In addition, any person handling or storing human pathogens and toxins under the authority of a licence issued by the PHAC is required to inform the licence holder or the BSO without delay in the event of any of the following:
- any inadvertent release or production of a human pathogen or toxin [HPTA 12]
- any incident involving a human pathogen or toxin that has, or may have caused disease, in an individual [HPTA 13]
- if a human pathogen or toxin has been stolen or is otherwise missing [HPTA 14]
- any inadvertent possession of a human pathogen or toxin not authorized by their licence [HPTR 4(1)(f)]
- when a shipment of a human pathogen or toxin has not been received within a reasonable time following its expected delivery [HPTR 4(1)(e)]
- when a shipment of a security sensitive biological agent (SSBA) has not been received 24 hours after the expected reception date and time [HPTR 4(2)]
Prompt internal reporting will allow timely follow-up actions to be taken to mitigate any further impacts, such as additional exposures or further spread of contamination, and will also support reporting and notification obligations to external authorities.
3.2.2 Mandatory external reporting
Incident investigation and reporting policies and procedures can specify who within the organization is responsible for notifying external authorities, when notification is mandatory, and how to notify them (e.g., by telephone, email, fax, online). The procedures can also include requirements from applicable federal, provincial, territorial, and municipal regulations. For example:
- When an individual working in a laboratory is exposed to a human pathogen and is later diagnosed with an associated disease or disorder (i.e., a confirmed LAI), the incident must be reported to the PHAC as well as the local occupational health and safety authority, as applicable (e.g., provincial or territorial Ministry of Labour or, for federal employees, the Labour Program of Employment and Social Development Canada).Footnote 21Footnote 22
- Where it is included as a condition of the animal pathogen import permit, incidents involving non-indigenous animal pathogens must be reported to the CFIA.
In accordance with the HPTA, a licence holder is required to notify the PHAC without delay whenever there is reason to believe one of the following laboratory incidents has occurred:
- any incident involving a human pathogen or toxin that has, or may have caused disease, including a confirmed LAI [HPTA 13]
- inadvertent possession, production, or release of a human pathogen or toxin [HPTA 12(1),(2), and HPTR 9(1)(c)(ii)]
- missing, stolen, or lost human pathogen or toxin [HPTA 14], including an SSBA not received within 24 hours of an expected date and time [HPTR 9(1)(c)(iii)]
While licence holders are responsible for notifying the PHAC, they may delegate their reporting role to the designated BSO [HPTR 9(b)]. Incident information is submitted to the PHAC via the online notification and follow-up reporting modules of the Biosecurity Portal.Footnote 23 Additional communication and reporting may be required on a case-by-case basis. The Canadian Biosafety Guideline - Notification and Reporting Under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal can also be consulted for more detailed guidance on how incidents and other notifiable events can be reported to the PHAC.Footnote 9
3.2.3 Involvement of police or jurisdictional authority
The focus of this guideline is investigation in response to inadvertent biosafety-related incidents that generally do not involve criminal intent or anticipated civil litigation; however, these do remain a possibility. If an incident investigation uncovers a potential criminal act, a criminal investigation involving law enforcement (i.e., police or other jurisdictional authority) may be warranted. As soon as an incident appears to involve a possible criminal event, the appropriate law enforcement or jurisdictional authority have to be contacted to expedite the initiation of the criminal investigation. In such a case, it is best to stop the internal investigation as it may compromise evidence required for the criminal or civil investigation. It is important for the investigation team to note that evidence indicating a deliberate or criminal act may have been intentionally concealed to make the incident appear accidental.
Incidents resulting in serious damage, injury, or death may fall under provincial or territorial occupational health and safety legislation and may subsequently be investigated by the appropriate provincial or territorial authority. It may be prudent to involve legal counsel early during the incident investigation if the evidence suggests a potential for a civil lawsuit, or criminal investigation.
3.3 Initiating the formal investigation
The individuals involved in the preliminary assessment of the incident may gather information before the formal investigation team arrives on the scene. Basic information that needs to be documented immediately and will be included in the initial incident report includes:
- the date and time
- who conducted the preliminary assessment
- where the incident occurred
- what pathogens, toxins, or infectious material were involved
- who was involved, including who was injured and witnesses
- the damage that occurred, and
- the actions that have already been taken in response to the incident
The preliminary assessment will help inform the scope of the formal investigation. With this initial information, the investigation team can be assembled and continue to collect information to identify the facts.
Chapter 4: Collection of evidence and information

Text description
Figure depicting the second chevron of the vertical chevron list illustrated in Figure 2-1, which details the 5-step model of incident investigation.
This chevron represents Step 2, which takes place between 0 and 10 days. A box at the right of this chevron details the collection of evidence and information:
- collect physical evidence, photos, witness accounts, data, documentation, records, logs.
- create a timeline to establish the sequence of events.
The evidence and information collected during Step 2 are the basis of any incident investigation. The amount and types of information collected will vary depending on the severity and complexity of the incident, as well as who was involved, the possibility of civil litigation, and whether or not criminal intent may have contributed to the incident. In all cases, a thorough investigation will involve the careful collection of all available information, documenting the facts of the incident, and preserving any related evidence. Fully documenting, investigating, and collecting as much information as possible, no matter how seemingly minor, will help to establish a timeline that describes the sequence of events and the causal factors.Footnote 12
4.1 Collecting evidence and information
The moment the internal authorities are notified, the investigation team can begin collecting evidence and information, including retrieval of information collected during the preliminary assessment and by the emergency response team, which provides the first view of the incident.Footnote 2 The facts about an incident can be determined through physical evidence, interviews, document reviews, or documented information gathered by the investigation team through other means (e.g., circumstantial, anecdotal, and hearsay evidence). To avoid overlooking evidence or having the investigation follow an incorrect path, all evidence must be collected before trying to determine what may have led to the incident.
4.1.1 Physical evidence
Physical evidence is the least disputable type of information that will be collected over the course of an investigation. Taking steps to properly preserve any physical evidence at the site of an incident prevents any transient, fragile, or perishable evidence from being lost, damaged, or contaminated.Footnote 12 Any objects that may be associated with the incident, even those that seem unimportant, need to be collected; the location of where these were found needs to be documented. While the investigation is proceeding, access to the incident scene can be limited to authorized individuals to protect against the removal or disruption (intentional or not) of any evidence. Depending on the incident (e.g., a serious injury, significant damage to equipment, potentially criminal intent), it may be necessary for the incident scene to remain undisturbed until approval has been granted by the appropriate external authority (e.g., inspector or police, if law enforcement have been involved).Footnote 12
Examples of physical evidence can include:Footnote 10Footnote 17
- the location of the incident
- the environmental conditions (e.g., lighting, ventilation, noise levels, general housekeeping, upkeep of the incident area)
- the equipment, parts of equipment, or subassemblies (e.g., rotors), and tools being used
- the location(s) of affected or injured individual(s)
- the details of which personnel were in the laboratory and what tasks were being performed at the time of the incident
- the location(s) of animals or animal cages in animal work areas
- the personal protective equipment (PPE) worn and available
- the safety devices or equipment in use and available
- the samples or specimens, including isolates from the specimens, involved
- documents (e.g., manuals, logbooks, SOPs, laboratory notebooks, inventory lists) and
- a detailed list of all persons who were involved in the incident (e.g., witnesses, victims, emergency responders, supervisors, preliminary assessment team)
The investigation team can capture a clear picture of the incident scene by examining the area before any physical evidence is moved or collected, and documenting (e.g., taking clear notes, photographs, sketches) where each piece of evidence was found or from whom it was collected. If measurements are taken to document the position and orientation of key pieces of evidence, the units of measure and the equipment used need to be documented. Any evidence that may have been disturbed by the emergency responders, or that has been cleaned (intentionally or inadvertently) is also carefully documented. The absence of certain types of evidence at the incident site (e.g., safety devices, warning signs, PPE) also needs to be documented. Samples or specimens, including isolates from the specimens, may need to be collected by the investigation team for further analysis.
4.1.1.1 Taking notes
Effectively documenting and managing the information and evidence collected by the investigation team will demonstrate their commitment to upholding the guiding principles of the investigation (e.g., credibility, thoroughness), and will also facilitate future retrieval and review of the information. Documenting findings in a notebook or an electronic device (e.g., tablet) throughout the investigation can help keep the information together and organized so that it can be used later to recall the facts.Footnote 20 This is particularly useful when incident scenes need to be assessed quickly due to the perishable nature of the evidence or the need for regular operations to resume quickly.Footnote 10 Should details be recalled at a later time, they may be added to the existing documentation; cross-referencing to the original notes may be necessary to effectively manage newly added information.
Good investigation notes:Footnote 20
- are clear, concise, accurate, detailed, and complete
- are legible and easy to understand
- answer "who?", "what?", "when?", "where?", "why?", and "how?" and
- are factual and objective (i.e., do not include the investigator's personal opinions)
4.1.1.2 Photos, video recordings and sketches
Photos, videos, and sketches taken to document the incident scene before anything is moved are a useful and efficient means of capturing details of the physical evidence, including the location, proximity to other pieces of evidence, size, and other characteristics.Footnote 12 They provide a visual record of evidence as it was found after the incident, and their subsequent analysis may reveal information that was initially overlooked.Footnote 12 It may also prove useful for the investigation to verify if video footage (e.g., from security cameras) of the events relating to the incident exist.
The context of the incident can be captured with photos or videos from various perspectives, including the overall scene (i.e., the "bigger picture"), mid-range, close-up shots (e.g., for serial numbers), and from the perspective of any affected individuals.Footnote 6 Scene markers (e.g., tents, flags, or other means to highlight an item of interest) can draw attention to relevant items, and a scale (e.g., a ruler) can be used to indicate size.Footnote 6Footnote 24Footnote 25 It is recommended that investigators obtain permission before photographing any person as part of the investigation process; personal privacy is a right to be respected and may be subject to legal permission requirements in some jurisdictions.Footnote 12
Relevant information (e.g., date, context) related to photos and videos can be documented in a notebook or an electronic device, and cross-referenced to the electronic file. Alternately, printed photos can be labeled (e.g., on the back) to allow relevant information to be linked and more easily found. Backups of photographs and video recordings can be prepared to prevent their loss during the investigation.Footnote 6
Sketches and drawings may also be useful to clarify and supplement an investigator's notes. These diagrams can include details of the location of the scene, the date and time that the diagram was made, and an index or legend that explains what the diagram means.
4.1.2 Witness accounts
Interviewing the people present during the incident (including witnesses) will help the investigation team to better understand how the incident took place.Footnote 6 Witnesses are an important source of information for the investigation, especially when the investigation team is unable to survey the scene immediately after an incident. Depending on the nature of the incident, gathering information from witnesses can be difficult, as witnesses may be under emotional stress or experience fear of reprisal should they divulge all details relating to the incident.Footnote 12
Recall bias can be avoided by collecting details and observations of the incident from the witnesses as soon as possible. Preventing discussion among witnesses until after individual accounts are collected, and obtaining witnesses' accounts independently from other witnesses can also help preserve individual perceptions. It is important that the interviewer clearly states the purpose of the interview and treats the interviewee as an equal.
The likelihood of obtaining reliable information may be improved when witnesses are interviewed in a suitable environment (e.g., a quiet area offering privacy and with little distraction); however, in some cases it can be beneficial to conduct the interview at the scene of the incident.Footnote 13 In more serious cases (e.g., criminal investigations, following an incident that caused severe harm or damage), witnesses may need to provide a written statement.
4.1.3 Documentation
Relevant sources of information and data may be located in various documents, both paper and electronic. Collecting all potentially relevant documents allows for their review at a later time to determine if they provide insight into what may have happened and why.Footnote 12 Documentation can be extensive and may include:Footnote 12Footnote 20
- information about equipment (e.g., user manuals, specifications, maintenance records)
- laboratory notes and results (e.g., logbooks, laboratory notebooks, test results for the work being performed)
- facility- and laboratory-specific information (e.g., policies, biosafety manual, experimental procedures, SOPs, overarching and local risk assessments)
- information about training (e.g., training and competency records, training materials)
- information about regulated material in possession (e.g., pathogen and animal inventory documents, shipping and transfer records)
- regulatory and compliance information (e.g., valid licences and animal import permits, compliance checklists, audit and inspection reports by internal and external authorities, previous incident reports and follow-ups)
- employee attendance records
4.1.4 Preserving evidence
Any evidence and information collected as part of an investigation needs to be appropriately stored and secured, for both security and privacy considerations and to maintain its integrity in case further investigation or analysis is required at a later time. The length of time evidence is stored will depend on the nature of the incident and the investigation. For example, evidence may be stored longer if there is a risk of a lawsuit, or if the investigation becomes a criminal investigation conducted by local law enforcement or other authorities. In scenarios such as these, legal counsel can be consulted for assistance during the investigation. The investigation team may need to consider special arrangements for the safe and secure storage of collected evidence, such as:
- secure storage (e.g., locked cabinets, stored in an area with restricted access) of evidence to prevent tampering
- refrigeration to preserve perishable evidence or samples
- maintenance (e.g., feeding, changing of cages) of any animals involved
4.2 Establishing the incident timeline
Organizing evidence into a timeline that starts with the incident (i.e., the reason for the investigation) is a systematic, simple, and effective way to illustrate the sequence of events that led to an incident. It also helps identify gaps and missing information that may require further investigation. The purpose of the timeline is to identify all actions taken leading up to and following the incident (i.e., the response) and is best achieved by detailing the date and the time (i.e., time stamping) of each event if possible. While all information collected during the investigation can be added to the timeline, it is best to include statements of action (i.e., somebody did something) rather than descriptions of a circumstance or result (e.g., something was missing).Footnote 26Footnote 27 The timeline will include any information associated with the actions that contributed to the incident, although it can be difficult to determine the relevance of details until the timeline is complete. Presenting a preliminary timeline that accurately and adequately describes the incident (i.e., each chronological step is derived logically from the one preceding it) to all the members of the investigation team and establishing a consensus among members provides an opportunity to resolve any missing steps or inconsistencies. It is good practice to review and adjust the incident timeline as more information becomes available. An example of a timeline for the example incident scenario described in Appendix C, which involves the laboratory exposure of a student to Staphylococcus aureus, is illustrated in Figure 4-1.
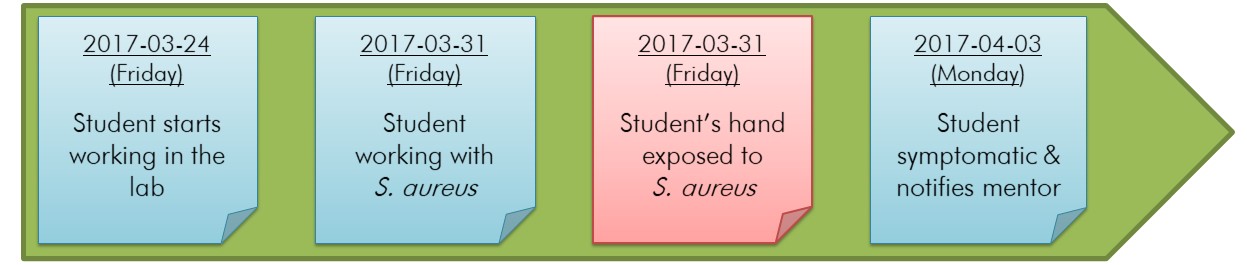
Figure 4-1 - Text description
Figure depicting the timeline for an example incident scenario involving a student exposed to Staphylococcus aureus. Within this horizontal timeline, coloured sticky notes represent specific events related to the incident. The first sticky note represents the first event "Student starts working in the lab" on 2017-03-24 (Friday). The second sticky note represents the second event "Student working with S. aureus" on 2017-03-31 (Friday). The third sticky note represents the exposure event "Student's hand exposed to S. aureus" on 2017-03-31 (Friday). The fourth and last sticky note represents the event "Student symptomatic & notifies mentor" on 2017-04-03 (Monday).
In this figure, four events are indicated, including the exposure event (indicated in the red box).
Although the investigation team may not believe a timeline is necessary when the root causes seem obvious, not creating a timeline can lead to the implementation of corrective and preventive measures that do not address the underlying shortcomings of the biosafety program and fail to prevent recurrences.Footnote 26
4.2.1 Unnoticed exposures resulting in disease
In some cases, the events that led to an incident are not identifiable and it may not be possible to create a full timeline. One example is when a diagnosed disease is suspected to be an LAI resulting from a laboratory exposure that went unnoticed and that may have occurred days or weeks earlier. In such cases, the timeline may be limited to the incident (i.e., the unnoticed laboratory exposure) and the events that followed it (e.g., appearance of symptoms, reporting to internal and external authorities, seeking medical treatment, confirmation of disease). In such investigations, it can be much more difficult to determine the root cause(s) of the incident.
Regardless of when the LAI is identified relative to the exposure, a standard investigation approach can be applied. Two key pieces of information relating to an unnoticed laboratory exposure are the first appearance of the signs and symptoms of a disease (e.g., rash, swelling, fever, malaise) and the incubation period of the pathogen. The incubation period is the time between the occurrence of infection (i.e., moment of laboratory exposure) and the first appearance of signs and symptoms of disease.Footnote 28 The incubation period is known for many human and animal pathogens and is typically indicated as a range (e.g., the incubation period for Bacillus anthracis is within seven days of infection, and usually two to five days). This information can be obtained from various sources, including Pathogen Safety Data Sheets and other online resources, and from medical professionals and health care practitioners.Footnote 29Footnote 30
By knowing when symptoms first appear and counting backwards in time (based on the incubation period), the investigation team can establish a potential time period wherein the exposure was likely to have occurred. With this knowledge, the investigation team can begin establishing a timeline of events for the exposure incident. A standard investigation approach can be used to collect data within this timeframe, including the review of laboratory logs and notebooks relating to activities undertaken before and within this period. Certain types of information and data sources, however, may not be available. For example, rather than collecting physical evidence, which will likely have been disturbed, displaced, removed, or destroyed, the investigation team may focus on reviewing existing SOPs and PPE requirements, and maintenance records for safety equipment, as well as records of personnel compliance with these. Furthermore, if witness accounts are obtained, the likelihood of recall bias increases the longer it takes to obtain them after the laboratory exposure (e.g., greater recall bias if the pathogen has a long incubation period).
The figure below depicts an incident timeline for a hypothetical example where an LAI may have been caused by an unnoticed laboratory exposure. In this example, the laboratory exposure is the incident, and is indicated as having occurred between June 3 and June 6 on the timeline (this is presented as a range, since the precise timing of the incident is unknown). The dates were based on the incubation period of the pathogen (assumed to be four to seven days for this example). Events preceding the incident can be added to the timeline as more evidence and information are collected.
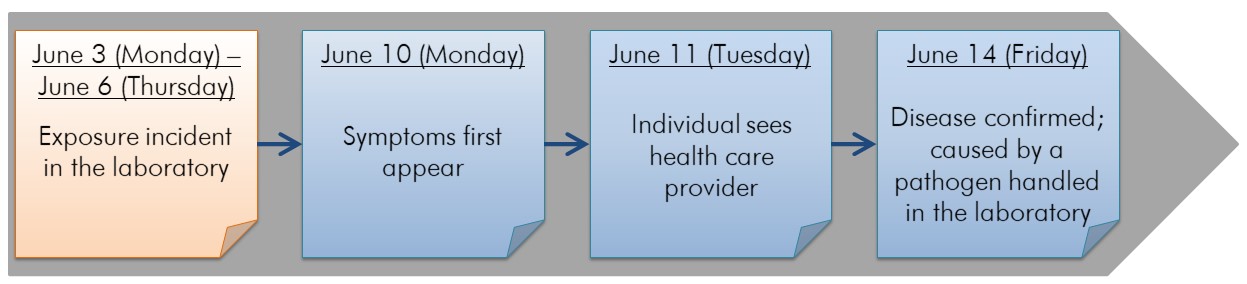
Figure 4-2 - Text description
Figure depicting the timeline for a hypothetical LAI with an unnoticed laboratory exposure. Within this horizontal timeline, there are sticky notes identifying events related to the incident. The first sticky note represents the exposure event "Exposure incident in the laboratory", which most likely occurred between June 3 (Monday) and June 6 (Thursday). The second sticky note represents the event "Symptoms first appear" on June 10 (Monday). The third sticky note represents the event "Individual sees healthcare provider" on June 11 (Tuesday). The fourth and last sticky note represents the event "Disease confirmed; caused by a pathogen handled in the laboratory" on June 14 (Friday).
Once the disease is confirmed, and the incubation period established, the investigation team can map the potential timeframe wherein the exposure was most likely to have occurred. In this example, the incubation period ranges from four to seven days. Based on this information, and the first appearance of symptoms in the affected individual on June 10, the exposure most likely occurred between June 3 and June 6.
Chapter 5: Analysis and identification of root causes

Text description
Figure depicting the third chevron of the vertical chevron list illustrated in Figure 2-1, which details the 5-step model of incident investigation.
This chevron represents Step 3, which takes place between 0 and 3 weeks. A box at the right of this chevron details the analysis and identification of root causes:
- analyze all the available information to determine the causal factors.
- identify the root causes of each causal factor to explain why the incident occurred.
A root cause is the most basic, underlying reason a problem or causal factor exists. Causal factors are errors or failures that describe how the incident happened and how they contributed to the chain of events (e.g., human error, equipment failure) resulting in an incident. A root cause analysis usually starts by identifying general problems, which are further scrutinized until specific, underlying system weaknesses, failures, or gaps that could have prevented the causal factor from existing have been defined.Footnote 26 Root cause analysis (Step 3) is a systematic approach that looks beyond human error to determine the cause of an incident.Footnote 31 Throughout this chapter, the process of root cause analysis will be illustrated using the example incident scenario described in Appendix C, which involves the laboratory exposure of a student to S. aureus.
5.1 Causal factors
Causal factors are the errors, failures, gaps, or weaknesses that, had they been addressed, could have prevented the incident from occurring or mitigated its results. Causal factors will lead to the discovery of root causes, but identifying and addressing only the causal factors of an incident with corrective measures will be ineffective at preventing recurrence of the incident. Defining causal factors during the investigation is important as it:Footnote 26
- begins the root cause analysis process
- provides a greater opportunity to learn from the incident and prevent recurrence, and
- allows the investigation team to separate the incident into smaller components that are easier to analyze
5.1.1 Identifying conditions
The first step of root cause analysis is to identify any conditions that may have contributed to the incident by looking at each event in the incident timeline.Footnote 26 Conditions are circumstances or states surrounding an event that existed before the event and that contribute to an action (e.g., somebody doing something) that led to the incident. They are factual, precise, and quantified, they describe what is known surrounding the event, and they may not be specific to the event. Conditions can often be identified by asking "What was done wrong?" or "Which equipment did not work as intended?" for each event in the timeline.Footnote 26 Multiple conditions can relate to an event, as demonstrated in the timeline illustrated in Figure 5-1.
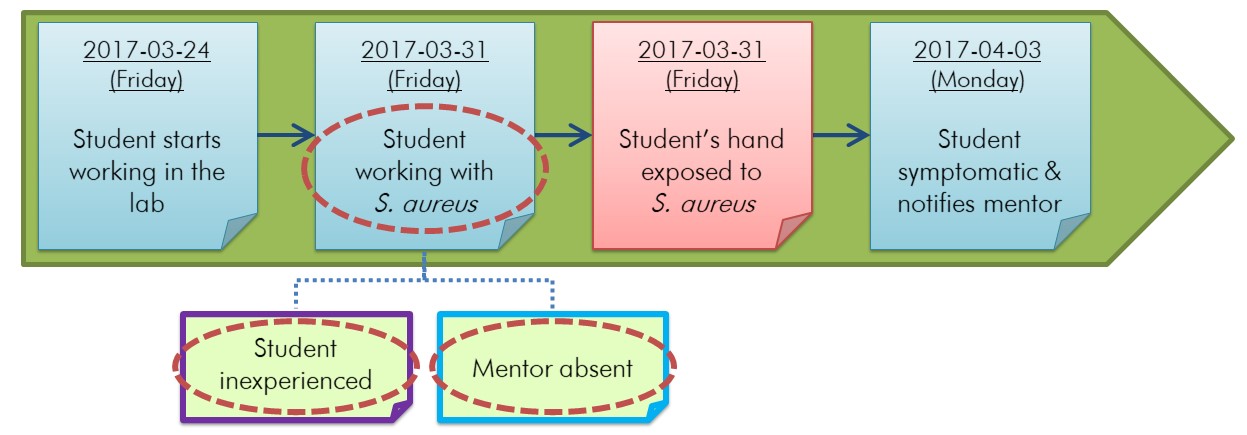
Figure 5-1 - Text description
Figure depicting the timeline in Figure 4-1 for an example incident scenario involving a student exposed to Staphylococcus aureus on which conditions have been identified. In this horizontal timeline, coloured sticky notes represent specific events related to the incident. The first sticky note represents the first event "Student starts working in the lab" on 2017-03-24 (Friday). The second sticky note represents the second event "Student working with S. aureus" on 2017-03-31 (Friday). The third sticky note in the horizontal timeline represents the exposure event itself, more specifically, "Student's hand exposed to S. aureus" on 2017-03-31 (Friday). The fourth and last sticky note in the timeline represents the event "Student symptomatic & notifies mentor" on 2017-04-03 (Monday). Below the horizontal timeline, two sticky notes have been added. These two sticky notes are linked to the second sticky note in the horizontal timeline with blue dotted lines. The sticky note on the left indicates "Student inexperienced" and the sticky note on the right indicates "Mentor absent". In this figure, three conditions have been circled with red dotted lines. These three conditions are "Student working with S. aureus", "Student inexperienced", and "Mentor absent".
This figure builds on the incident timeline depicted in Figure 4-1. In this scenario, three conditions (circled in red) that may have contributed to the incident are identified. Two conditions are linked to one event, and the third condition is the event directly preceding the incident. Details of the example incident scenario can be found in Appendix C.
5.1.2 Related conditions
Most incidents result from a combination of causal factors; any of which could have changed the outcome or impact of the incident. In order to identify these causal factors, the investigation team must determine the event that each identified condition impacts in a factual, precise, and quantifiable manner that refrains from placing blame.Footnote 27 When one condition results in another condition, the investigation team can group these conditions together. An example of grouping related conditions is illustrated in Figure 5-2. In general, each group of related conditions is associated to a distinct human error or failure, but it is acceptable for conditions to be moved or copied to different groups when it is appropriate. If it is not known whether a potential condition existed, it can be worded as a question on which the investigation team can follow up (e.g., Was training provided? Was PPE used? Was the incident reported?). For example, in Figure 5-2, it is not known whether an SOP exists regarding the use of PPE, so the question "Does an SOP for PPE use exist?" was included in the timeline. In some cases, the answer will not be found, and this should be documented as a gap in the investigation.
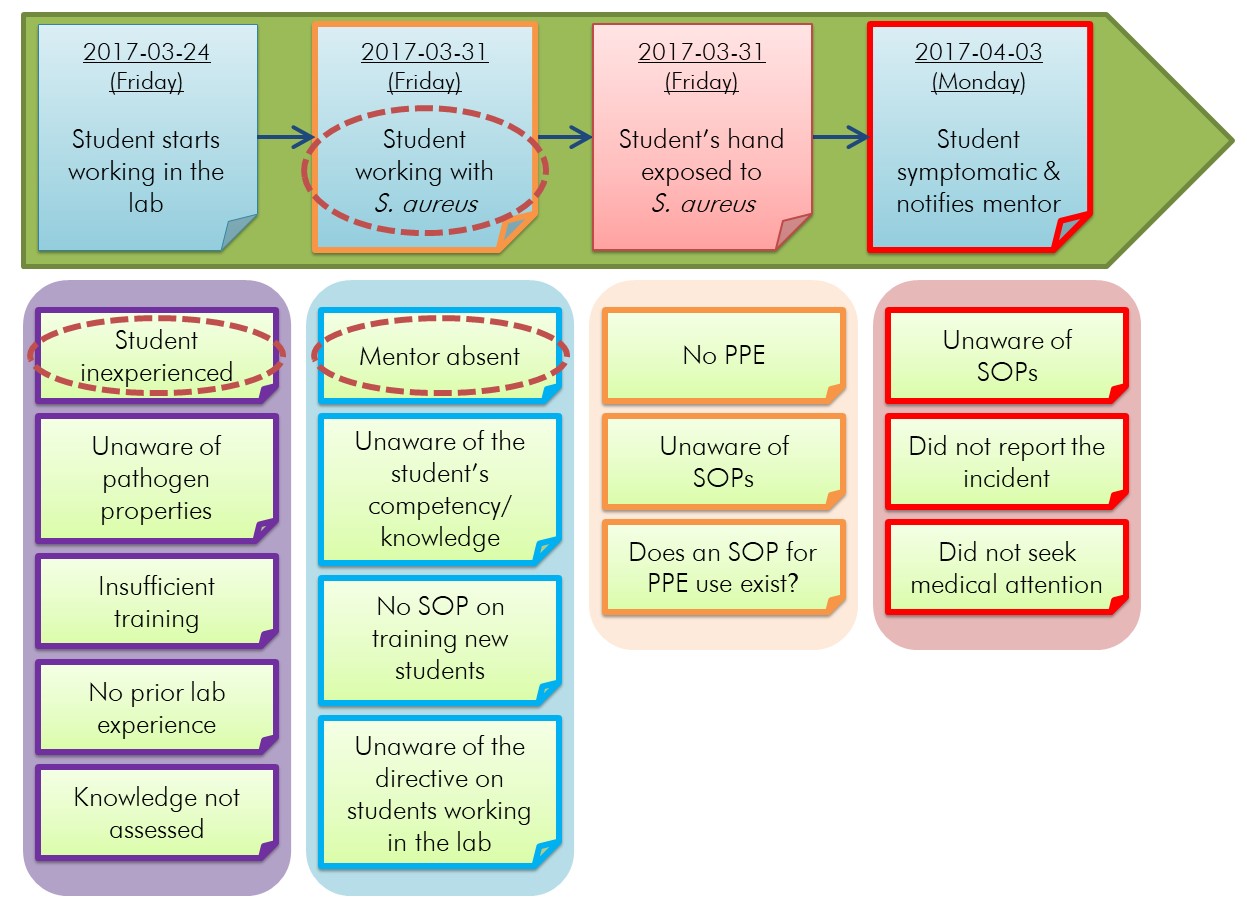
Figure 5-2 - Text description
Figure depicting the horizontal timeline in Figure 4-1 for an example incident scenario involving a student exposed to Staphylococcus aureus under which four boxes have been added. These four boxes are side by side and contain sticky notes lined vertically, each representing a condition. The first box from the left is purple and contains five sticky notes that indicate: "Student inexperienced", "Unaware of pathogen properties", "Insufficient training", "No prior lab experience", and "Knowledge not assessed". The second box is blue and contains four sticky notes that indicate: "Mentor absent", "Unaware of the student's competency/knowledge", "No SOP on training new students", and "Unaware of the directive on students working in the lab". The third box is orange and contains three sticky notes that indicate: "No PPE", "Unaware of SOPs", and "Does an SOP for PPE use exist?". The fourth and last box is red and contains three sticky notes that indicate: "Unaware of SOPs", "Did not report incident", and "Did not seek medical attention". In this figure, three sticky notes have been circled with red dotted lines: "Student working with S. aureus" in the horizontal timeline, "Student inexperienced" and "Mentor absent".
This diagram builds upon the general problems (i.e., conditions) illustrated in Figure 5-1 and the incident timeline illustrated in Figure 4-1. When compared to Figure 5-1, it is evident that additional conditions have been added to the timeline, and related conditions have been grouped together. Groups of related conditions are outlined in matching colours, and grouped into the following broad categories: the student's inexperience in the laboratory (purple), the absence of the mentor (blue), PPE considerations (orange), and post-exposure actions (red). Details of the example incident scenario can be found in Appendix C.
"Over-grouping" conditions can happen when a timeline is not sufficiently detailed and there are too few events represented for the conditions to be well organized. Over-grouping can be resolved by separating an event that is actually made up of more than one action into multiple events on the timeline (e.g., the event "someone moved the box" might become the separate events "someone walked to the box", "lifted the box", "walked to the shelf", and "placed the box on the shelf"). Likewise, adding earlier events that contributed to the incident at the start of the timeline can help elucidate more details in the timeline.Footnote 26
5.1.3 Identifying causality
Identifying causality (i.e., cause-and-effect sequence) between conditions is based on the incident timeline, and involves identifying a specific action which creates a condition that contributes to or results in an event.Footnote 32 In order to identify causality from the related conditions, the investigation team may need to refine and revise the groupings by delving deeper into the collected evidence and information. To determine the logical progression of events, conditions can be reorganized by asking "What happened because of this condition?" and independent conditions can be placed in separate groups. Events and conditions previously marked as problems may be unmarked after discussion; unnecessary information may be removed, and the timeline itself may be revised.
Causality can be established from the incident timeline and each group of related conditions by identifying the first condition, determining which condition contributed to another condition or event, and organizing and reorganizing the conditions to reflect this logical progression. This way, each group of related conditions is arranged logically to identify causality. Once a logical, chronological progression between conditions has been established, independent conditions may be placed in a single group, or a condition that better fits in another grouping may be appropriately relocated. This will help align the problems so that the starting point for the root cause analysis can be identified. Figure 5-3 illustrates an example of how causality can be established for the incident described in Appendix C.
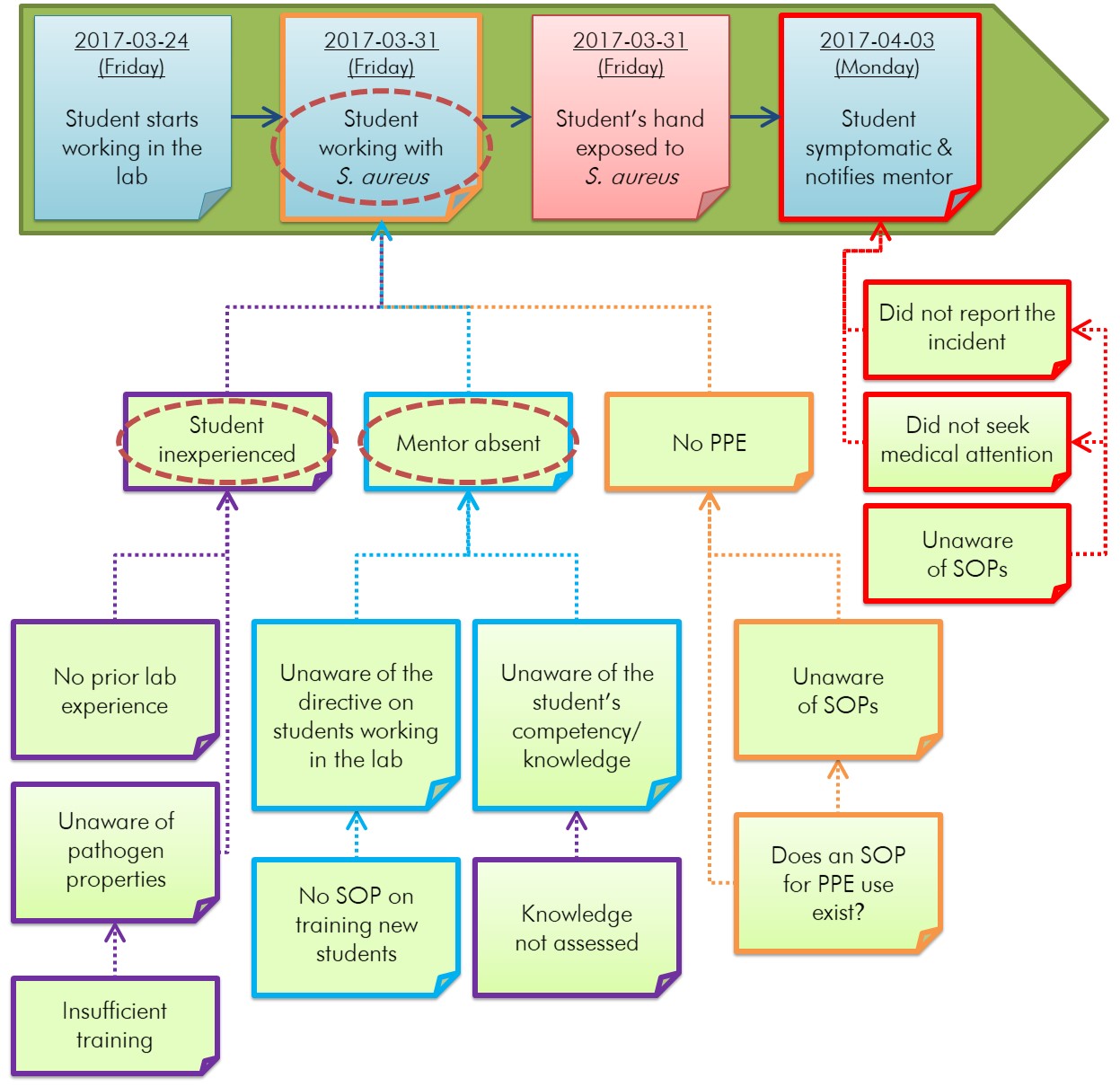
Figure 5-3 - Text description
Figure depicting the horizontal timeline in Figure 4-1 for an example incident scenario involving a student exposed to Staphylococcus aureus. The conditions identified in four boxes in Figure 5-2 have been rearranged into two flowcharts under two events of the horizontal timeline to illustrate the logical progression of events. The flowchart on the left demonstrates that the event "Student working with S. aureus" in the horizontal timeline is directly caused by the conditions "Student inexperienced", "Mentor absent", and "No PPE", which are themselves the result of other conditions. "Student inexperienced" is caused by "No prior lab experience" and "Unaware of pathogen properties". "Unaware of pathogen properties" is caused by "Insufficient training". In the middle of this flowchart, "Mentor absent" is caused by "Unaware of the directive on students working in the lab" and "Unaware of the student's competency/knowledge", which are caused themselves by "No SOP on training new students" and "Knowledge not assessed", respectively. In this flowchart on the right, "No PPE" is caused by "Unaware of SOPs" and "Does an SOP for PPE use exist?". "Unaware of SOPs" is also caused by "Does an SOP for PPE use exist?". The second flowchart demonstrates that the event "Student symptomatic & notifies mentor" in the horizontal timeline is directly caused by "Did not report the incident" and "Did not seek medical attention". Both of these conditions are also caused by "Unaware of SOPs".
This diagram builds upon the incident timeline identifying related conditions illustrated in Figure 5-2. Conditions have been reorganized, grouped, and linked to show the sequence of factors that contributed to an event in the timeline. Some conditions may not be interrelated; however, those that are interrelated (e.g., "knowledge not assessed" led to the mentor being "unaware of the student's competency/knowledge") are linked in sequential order. Details of the example incident scenario can be found in Appendix C.
5.1.4 Identifying causal factors
Identifying and defining causal factors will help align conditions, which allows to identify the starting point for root cause analysis. The goal is to identify the human errors and equipment failures that led to the incident and not to recreate the sequence of events. It is the investigation team's responsibility to determine when the timeline of events, depicting causality amongst conditions, has sufficient detail that causal factors can be identified and defined.
The causal factors are usually the conditions identified at the beginning of a group of related conditions. While causal factors may be human errors and equipment failures, even seemingly simple incidents can require extensive discussion and have multiple causal factors. Figure 5-4 illustrates an example of causal factor identification for the example incident scenario described in Appendix C.
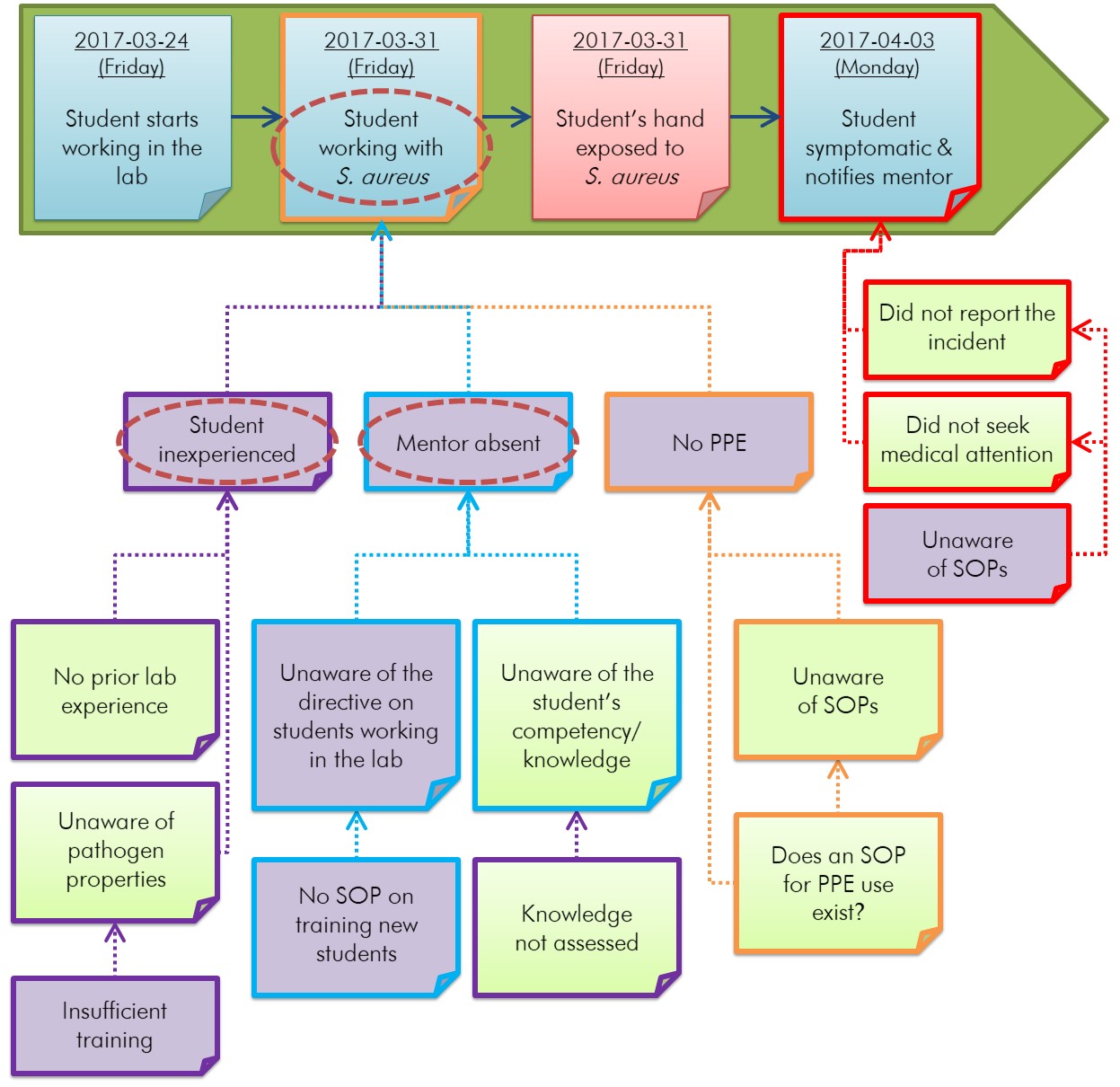
Figure 5-4 - Text description
Figure 5-4 builds upon Figure 5-3. Certain conditions under the horizontal timeline are identified in purple as being causal factors. From left to right, the conditions identified as causal factors are: "Student inexperienced", "Insufficient training", "Mentor absent", "Unaware of the directive on students working in the lab", "No SOP on training new students", "No PPE", and "Unaware of SOPs" (regarding post-exposure actions).
This diagram builds on Figure 5-3. The identified causal factors (purple-filled boxes) are the errors, gaps, failures, and weaknesses that could have prevented the incident from occurring or mitigated its results if they had been addressed. For example, "no PPE" was identified as a causal factor (i.e., a gap), since the use of gloves by the student could have prevented their exposure to S. aureus. Details of the example incident scenario can be found in Appendix C.
5.2 Identifying root cause(s)
Following the identification of the causal factors, each causal factor is analyzed independently to determine its root cause. Root causes are the most basic, underlying reasons a problem exists. Investigations that are focused on placing blame (i.e., cannot look beyond human error) rather than finding root causes are not successful at producing appropriate corrective measures to prevent future incidents.Footnote 31
To be successful and effective, a root cause analysis must be completed in a systematic manner and supported by documented evidence. The root cause analysis may begin with the "Five Whys?" strategy which involves asking "why?" (or "what caused this problem?"), to which the answer prompts another "why?", and so on.Footnote 33 This strategy can provide thought-provoking cues and identify root causes that had not been previously considered such as purchasing controls, training, and equipment operation.Footnote 31 It may also be advisable to question whether or not the incident was isolated or intentional, especially where a biosecurity breach has occurred.
Each causal factor can be analyzed independently using a process of elimination or selection against basic root cause categories, determined by the evidence and information collected. A thorough root cause analysis may point to more than one root cause; the implementation of measures that address all root causes is required to prevent recurrence. Using the process described in the Notification and Reporting Under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal guideline, four of the six basic root cause categories appear to be applicable to the example incident scenario indicated in Appendix C. Once the appropriate root cause categories are selected, they undergo further consideration through a series of triggering questions that determine the root causes.Footnote 31 The following root causes were determined for each root cause category for the incident in Appendix C; these are not comprehensive and serve only as examples:
- Standards, policies, or procedures
- Selected Not known by the user
- Not selected Known but not followed
- Not selected Followed but not correctly
- Not selected Followed but incorrect for the task
- Selected Not implemented but should have been
In the example in Appendix C, the mentor was not aware of a directive regarding students working in the laboratory and the student was not aware of the SOPs relating to incident reporting and post-exposure actions. Additionally, there was no SOP on training new students. Depending on the outcome to the question "Does an SOP for PPE use exist?", which would have been assessed during the investigation, additional root causes could have been identified for this example incident scenario. For example, an SOP may have existed (but was either not followed, or followed incorrectly), or an SOP may not have existed at all.
- Training
- Not selected There was no training for the task related to the incident
- Not selected Training was developed but not implemented
- Not selected Appropriate and sufficient training was available but not completed
- Selected Training was inappropriate or insufficient
- Selected Staff was not qualified or proficient in performing the task related to the incident
In the example in Appendix C, the student was not sufficiently trained by the mentor, which resulted in them not being qualified to work alone with S. aureus in the laboratory.
- Communication
- Not selected There was no method or system for communication
- Selected No communication occurred but should have
- Not selected Communication occurred but was unclear, incorrect, or misunderstood
There were numerous instances in the example incident scenario in Appendix C where communications should have occurred, but did not. For example, effective institutional communications would have made the mentor aware of the directive regarding students working in the laboratory. Similarly, the mentor should have communicated to the student the existence of SOPs regarding incident reporting, and post-exposure actions.
- Management and oversight
- Selected There was no supervision of work related to the incident
- Selected Preparation needs improvement
- Not selected There was no auditing, evaluation or enforcement of standards
- Selected Training lacks auditing, evaluation, or enforcement
- Selected Training needs improvement
With respect to the example incident scenario in Appendix C, there was a clear lack of supervision of the work undertaken by the student at the time of the exposure incident, and insufficient training resulted in the student being unprepared to work unsupervised in the laboratory with S. aureus. Furthermore, there is no evidence that the process for training new students had been evaluated, as such an assessment would likely have identified gaps in the training process.
- Human interaction
This category was ruled out, since none of the causal factors identified for the example incident scenario in Appendix C were associated with human interactions or factors related to work demands or the work environment (e.g., there is no indication that the student was rushing through their work to meet a deadline).
- Equipment
Relating to the example incident scenario described in Appendix C, this root cause category was also ruled out, as it specifically relates to equipment failure (i.e., a piece of equipment failed or did not perform as intended). This category does not include user error (e.g., that PPE was not appropriately used with the equipment).
5.3 Disciplinary measures
Throughout this guideline, it is repeated on several occasions that the goal of the investigation is to determine root causes, and not to lay blame. In the example incident provided in Appendix C, some may feel that the mentor, who was absent and unaware of a directive regarding students, should be to blame and should perhaps be reprimanded; however, doing so would not improve this or future situations. Alternately, had the investigation determined that there had been numerous similar incidents with the same mentor, that this individual continued to ignore requirements to familiarize themselves with directives, and neglected to supervise students, the root cause would relate to Management and Oversight. As such, a potential mitigation measure might be to implement a policy of discipline for ongoing non-compliance with organizational policies and procedures after other measures have been exhausted.
Chapter 6: Implementation of corrective and preventive measures

Text description
Figure depicting the fourth chevron of the vertical chevron list illustrated in Figure 2-1, which details the 5-step model of incident investigation.
This chevron represents Step 4, which takes place between 0 and 1 month. A box at the right of this chevron details the implementation of corrective and preventive measures:
- correct the root causes of the incident.
- implement measures to prevent or mitigate the risk of future occurrences.
In Step 4, corrective and preventive measures that address all of the root causes are identified and implemented to prevent incident recurrence. This can be established in a plan that prioritizes all recommended measures based on their impact (e.g., eliminate immediate hazard or lower the risk [probability and consequence] of recurrence) and the time and resources that will be needed for each measure. The plan can also specify an individual responsible for implementing each measure.
In some organizations or situations, a formal investigation report may be generated to summarize the key findings of the investigation and to outline the investigation team's recommendations for improvement.
6.1 Identifying corrective and preventive measures
Corrective and preventive measures eliminate or mitigate the root causes of an incident, or reduce the likelihood of recurrence, and lessen the consequences if a similar incident does recur. A corrective measure is usually implemented for each root cause identified; however, it is also possible for a single corrective measure to address several root causes. In order for corrective and preventive measures to be effective, they need to be developed in a manner such that they are reasonable and achievable. The immediate, short-term, or long-term measurable implementation can be described in a documented plan. A short-term corrective measure is implemented to quickly address the problem, whereas long-term measures are well-developed and sustainable solutions that aim to correct and prevent recurrence. Clearly identifying the persons responsible for implementing the measures, timelines for completion, and any additional resources needed for implementation will help facilitate the timely and effective implementation of these measures. It will also help senior management prioritize the measures. The investigation team may on occasion seek new members or external expertise to help identify or implement measures.Footnote 27 Additional financial and human resources may also be needed for the implementation of some corrective and preventive measures.Footnote 6
The plan for implementing corrective and preventive measures generally answers the following questions:Footnote 10
- What needs to happen to prevent future incidents?
- What resources are needed?
- Who is responsible for overseeing and implementing the changes?
- Who will follow up and confirm whether the measures have been implemented?
- What will be the long-term plan to improve or maintain corrective and preventive measures?
The proposed corrective and preventive measures for the example incident scenario (detailed in Appendix C) are summarized in Table 6-1.
Root Cause |
Identified Root Causes |
Recommended Corrective and |
Standards, policies, and procedures |
|
|
Training |
|
|
Communication |
|
|
Management and oversight |
|
|
6.2 Final report
Results of the incident investigation can be documented in a final report that provides a record of the evidence, the investigation team's conclusions, and the recommended corrective and preventive measures.Footnote 6Footnote 13 The final report should be communicated to senior management, as this supports their commitment to respond to the results of an investigation. Furthermore, senior management controls resources and is in a position to implement the recommended corrective and preventive measures, which could impact the organization's policies and procedures. The final report may also need to be communicated to external stakeholders such as the PHAC and the CFIA (e.g., if the incident is reportable). Additionally, to support senior management's obligation for transparent communication within the facility, and to the surrounding community (as it relates to the risks posed by the activities conducted within the facility), it may be appropriate to communicate certain aspects of the final report to other relevant individuals.
The incident timeline illustrating how the events unfolded is an important component of the final report, as are the investigation team's recommendations and conclusions.Footnote 2 Evidence (e.g., photographs, diagrams, witness accounts) may also be included to provide context to these recommendations, although confidential information and information pertaining to biosecurity may need to be redacted depending on the audience. Since the purpose of the investigation is to identify issues with processes and systems that led to the incident, the final report should not offer recommendations regarding disciplining a person or persons whose actions may have contributed to the incident.Footnote 2Footnote 20
A comprehensive incident investigation report will answer the following questions:Footnote 10
- When and where did the incident happen?
- What was the sequence of events?
- Who was involved?
- What injuries/damage occurred?
- What are the recommended measures?
A comprehensive incident investigation report will include:Footnote 6 Footnote 13
- a discussion on the evidence and information
- gaps and missing information, if any
- the investigation team's findings and conclusions
- a summary of the identified root causes and causal factors, and
- the proposed plan for implementing corrective and preventive measures,
including the following items:Footnote 17
- the individual(s) responsible for overseeing its implementation
- timelines to implement the measure; and
- any resources required
Chapter 7: Evaluation and continual improvement

Text description
Figure depicting the fifth and last chevron of the vertical chevron list illustrated in Figure 2-1, which details the 5-step model of incident investigation.
This chevron represents Step 5, which takes place between 3 and 12 months. A box at the right of this chevron details the evaluation and continuous improvement:
- review the implementation of corrective and preventive measures.
- evaluate the effectiveness of corrective and preventive measures in addressing root causes.
The final stage of the incident investigation (Step 5) takes place after the investigation has concluded and involves reviewing the success of the implemented corrective and preventive measures, as well as the review of the investigation procedure itself to identify opportunities for improvement. The incident investigation procedures and protocols can follow the quality management system framework cycle of planning, implementing, measuring, and improving (or the "Plan-Do-Check-Act cycle", as described by the International Organisation for Standardization [ISO]).Footnote 34
7.1 Measuring success
In order for any management system to be effective, its performance must be measured against intended goals and objectives. This corresponds to the "Check" phase in the "Plan-Do-Check-Act" cycle. Evaluating the success of the corrective and preventive measures usually occurs after the investigation has concluded and the investigation team has disbanded.Footnote 27 Identifying the individuals responsible for monitoring and evaluating the implementation of measures in the final incident investigation report or implementation plan will help ensure that the measures are fully implemented in a timely manner. Incident investigation reports and implementation plans can also be reviewed in consultation with senior management and personnel to assess the success of the investigation outcomes and recommendations. A thorough review of the investigation outcomes can be conducted at planned intervals after an investigation is completed and may be delegated to a health and safety officer, BSO, or health and safety committee members, depending on the nature of the incident.Footnote 6
Questions that can be answered as part of the review may include, but are not limited to:Footnote 34
- Have the recommended corrective and preventive measures been implemented?
- Have the recommended corrective and preventive measures been implemented within the specified timeframes?
- Have similar incidents occurred since?
- Are the current measures effective and sustainable?
- Do the current measures need periodic replacement or improvement?
- Do additional corrective measures need to be implemented to prevent recurrence?
- Are personnel aware of and following the corrective and preventive measures in place?
- Is additional training required, and has it been added to the training program?
- Have all applicable legal requirements been met?
In the event that corrective and preventive measures cease to mitigate identified root cause(s), or more incidents occur due to the same root cause(s), a thorough and comprehensive review needs to take place. The reasons for incident recurrence need to be identified in order to develop additional corrective and preventive measures or a more robust implementation plan.
7.2 Making improvements
Opportunities for improvement of the incident investigation process may be identified through the review of incident investigation reports and incident trends, and following consultation with facility personnel and senior management. Trend analysis of all incidents over a period of time can help identify patterns and previously unseen problems that could lead to future incidents.Footnote 20 A regular review of organizational incident investigation policies and processes should be undertaken by senior management at a predetermined frequency (e.g., quarterly, semi-annually, annually) and following any significant incident.
A comprehensive review of a facility's policies and processes will help address broad questions, such as:
- Are the current tools working?
- Are the appropriate procedures and protocols in place to meet the objectives?
- Are the procedures adequately communicated to, and understood by, personnel?
- Do the procedures need to be updated?
- Can the procedures be adapted in response to changes?
- Are adequate resources available to support all steps of the incident investigation?
Any identified deficiencies can be addressed through additional mitigation measures.
Chapter 8: Glossary
It is important to note that while some of the definitions provided in the glossary are universally accepted, many of them were developed specifically for the CBS, or the CBH; therefore, some definitions may not be applicable to facilities that fall outside of the scope of the CBS.
- Accident
- An unplanned event that results in exposure, injury, harm, infection, intoxication, disease, or damage.
- Animal pathogen import permit
- A permit issued by the Public Health Agency of Canada or the Canadian Food Inspection Agency for the importation into Canada of: animal pathogens or toxins; animals, animal products, animal by-products, or other organisms carrying an animal pathogen or part of one; under Section 51(a) and (b) of the Health of Animals Regulations.
- Biological safety officer (BSO)
- An individual designated for overseeing the facility's biosafety and biosecurity practices.
- Biosafety
- Containment principles, technologies, and practices that are implemented to prevent unintentional exposure to pathogens and toxins, or their accidental release.
- Biosecurity
- Security measures designed to prevent the loss, theft, misuse, diversion, or intentional release of pathogens, toxins, and other related assets (e.g., personnel, equipment, non-infectious material, animals).
- Causal factor
- Factor that led directly to the incident, or led to more severe consequences. Identifying and correcting the root causes that allow this factor to exist will help prevent similar incidents from occurring in the future.
- Causality
- A cause-and-effect sequence, based on the incident timeline, in which a specific action creates a condition (problem) that contributes to or results in an event occurring.
- Community
- Encompasses both human (i.e., the public) and animal populations.
- Conditions (problems)
- Problems that describe states or circumstances and not events, actions, or occurrences. Conditions are passive rather than active.
- Containment
- The combination of physical design parameters and operational practices that protect personnel, the immediate work environment, and the community from exposure to biological material.
- Containment system
- Dedicated equipment that functions to provide and maintain containment. This includes, but is not limited to, primary containment devices (e.g., biological safety cabinets [BSCs]), heating, ventilation, and air conditioning (HVAC) and control systems, and decontamination systems (e.g., autoclaves).
- Containment zone
- A physical area that meets the requirements for a specified containment level. A containment zone can be a single room (e.g., CL2 laboratory), a series of co-located rooms (e.g., several non-adjoining but lockable CL2 laboratory work areas), or it can be comprised of several adjoining rooms (e.g., CL3 suite with dedicated laboratory areas and separate animal rooms, or animal cubicles). Dedicated support areas, including anterooms (with showers and "clean" and "dirty" change areas, where required), are considered to be part of the containment zone.
- Disease
- A disorder of structure or function in a living human or animal, or one of its parts, resulting from infection or intoxication. It is typically manifested by distinguishing signs and symptoms.
- Emergency response plan
- A document outlining the actions to be taken and the parties responsible in emergency situations such as a spill, exposure, release of pathogens or toxins, animal escape, personnel injury or illness, power failure, fire, explosion, or other emergency situations (e.g., flood, earthquake, hurricane).
- Exposure
- Contact with, or close proximity to, pathogens or toxins that may result in infection or intoxication, respectively. Routes of exposure include inhalation, ingestion, inoculation, and absorption.
- Facility
(plural: facilities) - A structure or a building, or a defined area within a structure or a building, where pathogens or toxins are handled or stored. This could include individual research and diagnostic laboratories, large scale production areas, or animal housing zones. A facility could also be a suite or building containing more than one of these areas.
- Handling or storing
- "Handling or storing" pathogens, toxins, or infectious material includes possessing, handling, using, producing, storing, permitting access to, transferring, importing, exporting, releasing, disposing of, or abandoning such material. This includes all controlled activities involving human pathogens and toxins specified in Section 7(1) of the Human Pathogens and Toxins Act.
- Incident
- An event or occurrence with the potential of causing exposure, injury, harm, infection, intoxication, disease, or damage. Incidents can involve infectious material, infected animals, or toxins, including a spill, exposure, release of pathogens or toxins, animal escape, personnel injury or illness, missing pathogens or toxins, unauthorized entry into the containment zone, power failure, fire, explosion, flood, or other crisis situations (e.g., earthquake, hurricane). Incidents include accidents and near misses.
- Infectious material
- Any isolate of a pathogen or any biological material that contains human or animal pathogens and, therefore, poses a risk to human or animal health.
- Intoxication
- A substance-induced disorder or disease resulting in a symptomatic or asymptomatic condition or other physiological change resulting from an exposure (i.e., ingestion, inhalation, inoculation, or absorption) to a toxin produced by or isolated from a microorganism. This includes a similar response from exposure to a synthetically produced microbial toxin.
- Laboratory
- An area within a facility or the facility itself where biological material is handled for scientific or medical purposes.
- Laboratory-acquired infection/intoxication (LAI)
- Infection or intoxication resulting from exposure to infectious material, infected animals, or toxins being handled or stored in the containment zone.
- Licence
- An authorization to conduct one or more controlled activities with human pathogens or toxins issued by the Public Health Agency of Canada under Section 18 of the Human Pathogens and Toxins Act.
- Near misses
- Events or other hazardous occurrences that did not result in exposure, injury, harm, infection, intoxication, disease, or damage.
- Pathogen
- A microorganism, nucleic acid, or protein capable of causing disease or infection in humans or animals. Examples of human pathogens are listed in Schedules 2 to 4 and in Part 2 of Schedule 5 of the Human Pathogens and Toxins Act but these are not exhaustive lists. Examples of animal pathogens can be found by visiting the Canadian Food Inspection Agency website.
- Personal Protective Equipment (PPE)
- Equipment and clothing worn by personnel to provide a barrier against pathogens or toxins, thereby minimizing the risk of exposure. PPE may include, but is not limited to, lab coats, gowns, full-body suits, gloves, protective footwear, safety glasses, safety goggles, masks, and respirators.
- Release
- The discharge of pathogens or toxins from a containment system.
- Risk
- The probability of an undesirable event (e.g., accident, incident, breach of containment) occurring and the consequences of that event.
- Root cause
- The most basic, underlying reason or factor that led to an incident occurring.
- Root cause analysis
- A systematic approach that looks beyond human error to determine the root causes of an incident.
- Security sensitive biological agents
(SSBAs) - The subset of human pathogens and toxins that have been determined to pose an increased biosecurity risk due to their potential for use as a biological weapon. SSBAs are identified as prescribed human pathogens and toxins by Section 10 of the Human Pathogens and Toxins Regulations. This means all Risk Group 3 (RG3) and RG4 human pathogens that are in the List of Human and Animal Pathogens and Toxins for Export Control, published by the Australia Group, as amended from time to time, with the exception of Duvenhage virus, Rabies virus and all other members of the Lyssavirus genus, Vesicular stomatitis virus, and Lymphocytic choriomeningitis virus; as well as all toxins listed in Schedule 1 of the Human Pathogens and Toxins Act that are listed on the List of Human and Animal Pathogens and Toxins for Export Control when in a quantity greater than that specified in Section 10(2) of the Human Pathogens and Toxins Regulations.
- Senior management
- The ultimate authority responsible for delegating appropriate biosafety authority. Senior management is responsible for ensuring that adequate resources are available to support the biosafety program, to meet legal requirements, and that biosafety concerns are appropriately prioritized and addressed.
- Standard operating procedure (SOP)
- A document that standardizes safe work practices and procedures for activities with pathogens and toxins in a containment zone, as determined by a local risk assessment.
- (Microbial) Toxin
- A poisonous substance that is produced or derived from a microorganism and can lead to adverse health effects in humans or animals. Human toxins are listed in Schedule 1 and Part 1 of Schedule 5 in the Human Pathogens and Toxins Act.
Chapter 9: References and resources
Aliyu, M. (date unknown). Common Laboratory Accidents and Causes in Secondary Schools of Zaria
Environ. Retrieved 11/28, 2017 from
http://www.globalacademicgroup.com/journals/pristine/COMMON%20LABORATORY
%20ACCIDENTS%20AND%20CAUSES%20IN%20SECONDARY%20SCHOOLS%20OF%20ZARIA%20ENVIRON.pdf
American Society of Safety Professionals. (2017). Understanding the Top 5 Laboratory Hazards. Retrieved 04/27, 2021 from https://www.assp.org/news-and-articles/2017/10/22/understanding-the-top-5-laboratory-hazards
Association of Workplace Investigators and Canadian Association of Workplace Investigators. (2020). Guiding Principles for Conducting Workplace Investigations. Retrieved 04/27, 2021 from https://cdn.ymaws.com/www.awi.org/resource/resmgr/files/publications/AWI-Guiding-Principles-Broch.pdf
Bloch, M. C. (2008). Guide to Conducting Workplace Investigations. Retrieved 10/02, 2017 from http://www.corporatecompliance.org/Portals/1/Users/169/29/60329/Workplace_Investigations_Guide.pdf
BS ISO 45001:2018, Occupational Health and Safety Management Systems. Requirements with Guidance for Use. (2018). London, UK: British Standards Institution.
Buys, J. R., & Clark, J. L. (1995). Events and Causal Factors Analysis. Scientech, Inc.: Idaho Falls, ID, USA. Retrieved 11/06, 2017 from https://www.iosh.com/media/2053/events-and-casual-factors-chiltern-january-2017.pdf
Byers, K. B., & Harding, A. L. (2017). Laboratory-Associated Infections. In Wooley, D. P., & Byers, K. B. (Eds.). Biological Safety: Principles and Practices (5th ed., pp. 59-92). Washington, DC, USA: ASM Press.
CAN/CSA-ISO 9001:16 (R2020), Quality Management Systems - Requirements. (2016). Toronto, ON, Canada: Canadian Standards Association.
Canadian Centre for Occupational Health and Safety. (2017). OSH Answers Fact Sheets - Canadian Government Departments Responsible for OH&S. Retrieved 10/18, 2017 from https://www.ccohs.ca/oshanswers/information/govt.html
Canadian Centre for Occupational Health and Safety. (2017). OSH Answers Fact Sheets - Incident Investigation. Retrieved 05/24, 2017 from https://www.ccohs.ca/oshanswers/hsprograms/investig.html
Canadian Centre for Occupational Health and Safety. (2017). OSH Answers Fact Sheets - Laboratory Technician and Technologist. Retrieved 07/18, 2017 from https://www.ccohs.ca/oshanswers/occup_workplace/labtech.html
CEN Workshop 31 - Laboratory biosafety and biosecurity. CEN Workshop Agreement (CWA) 15793:2011, Laboratory biorisk management. (2011). Brussels, Belgium: European Committee for Standardization.
Charles, R., Hood, B., DeRosier, J. M., Gosbee, J. W., Bagian, J. P., et al. (2017). Root Cause Analysis and Actions for the Prevention of Medical Errors: Quality and Improvement and Resident Education. Orthopedics, 40(4):e628-e635.
Charles, R., Hood, B., DeRosier, J. M., Gosbee, J. W., Li, Y., et al. (2016). How to perform a root cause analysis for workup and future prevention of medical errors: a review. Patient Safety in Surgery, 10(20).
Coelho, A. C., & García Díez, J. (2015). Biological Risks and Laboratory-Acquired Infections: A Reality That Cannot be Ignored in Health Biotechnology. Frontiers in Bioengineering and Biotechnology, 3(56).
Collins, C. H., & Kennedy, D. A. (1999). Laboratory-Acquired Infections. In Collins, C. H., & Kennedy, D. A. (Eds.). Laboratory-Acquired Infections: History, Incidence, Causes and Preventions (4th ed., pp. 1-97). Oxford, UK: Butterworth-Heinemann.
CSA Z1000:14 (R2019), Occupational Health and Safety Management. (2014). Toronto, ON, Canada: Canadian Standards Association.
CSA Z1005-17, Incident Investigation. (2017). Toronto, ON, Canada: Canadian Standards Association.
Gandsman, E. J., Aaslestad, H. G., Ouimet, T. C., & Rupp, W. D. (1997). Sabia Virus Incident at Yale University. American Industrial Hygiene Association Journal, 58(1):51-53.
Government of Canada. (2013). Accident Investigation: A Responsibility to be Taken Seriously! Available from https://www.canada.ca/en/employment-social-development/services/health-safety/reports/accident.html
Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition
Government of Canada. (2016). Canadian Biosafety Handbook (2nd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/handbook-second-edition.html
Government of Canada. (2017). Notification and Reporting Under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal. Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
Government of Canada. Biosecurity Portal. Available from https://biosecurity-portal.hc-sc.gc.ca
Hawkes, N. (1979). Smallpox Death in Britain Challenges Presumption of Laboratory Safety. Science, 203(4383):855-856.
Health and Safety Executive. (2013). RIDDOR - Reporting of Injuries, Diseases and Dangerous Occurrences Regulations 2013. Retrieved 11/28, 2017 from https://www.hse.gov.uk/riddor/
Health and Safety Executive. (date unknown). How to deal with an exposure incident. Retrieved 04/05, 2017 from https://www.hse.gov.uk/biosafety/blood-borne-viruses/how-deal-exposure-incident.htm
Health of Animals Act (S.C. 1990, c. 21).
Health of Animals Regulations (C.R.C., c. 296).
Hodkiewicz, T. S. (2012). Pre-planning for incident investigations. Safety+Health, 185(3). Retrieved 10/05, 2017 from https://www.safetyandhealthmagazine.com/articles/pre-planning-for-incident-investigations-2
Human Pathogens and Toxins Act (S.C. 2009, c. 24).
Human Pathogens and Toxins Regulations (SOR/2015-44).
Infrastructure Health and Safety Association. (2020). Accident Investigation & Reporting. Retrieved 04/27, 2021 from https://www.ihsa.ca/resources/accident_investigation.aspx
Jucan, G. (date unknown). Root Cause Analysis for IT Incidents Investigation. Retrieved 11/06, 2017 from http://hosteddocs.ittoolbox.com/gj102105.pdf
Lien, A., Abalos, C., Atchessi, N., Edjoc, R., & Heisz, M. (2020). Surveillance of laboratory exposures to human pathogens and toxins, Canada 2019. Canada Communicable Disease Report, 46(9):292-298.
Namvar, O., & Ordonez, M. (2016). Incident Investigation and Reporting [PowerPoint slides]. Retrieved 10/17, 2017 from http://safetymanagementeducation.com/teaching-resources/teaching-modules/IncidentInvestigationAndReportingModule.pptx
National Collaborating Centre for Infectious Diseases. (2016). Glossary of Terms for Infectious Disease Modelling: A Proposal for Consistent Language. Retrieved 04/25, 2019 from https://nccid.ca/publications/glossary-terms-infectious-disease-modelling-proposal-consistent-language/
National Forensic Science Technology Center. (2013). A Simplified Guide To Crime Scene Photography. Retrieved 10/26, 2017 from http://www.forensicsciencesimplified.org/photo/Photography.pdf
National Safety Council. (2017). How to Conduct an Incident Investigation. Retrieved 10/04, 2017 from http://www.nsc.org/JSEWorkplaceDocuments/How-To-Conduct-An-Incident-Investigation.pdf
Occupational Health and Safety Agency for Healthcare (OHSAH) in British Columbia. (2008). Incident Investigations: Participant's Guide. Retrieved 10/10, 2017 from http://www.phsa.ca/Documents/Occupational-Health-Safety/GuideIncidentInvestigationsParticipantGuide.pdf
Ogren, M. (2003). An Accident Waiting to Happen? The Scientist, 17(2):30-33.
Organisation for Economic Co-operation and Development. (2003). OECD Guiding Principles for Chemical Accident Prevention, Preparedness and Response: Guidance for Industry (including Management and Labour), Public Authorities, Communities and other Stakeholders (2nd ed.). Paris, France: OECD Publications. Retrieved 01/22, 2020 from https://www.oecd.org/env/ehs/chemical-accidents/guiding-principles-chemical-accident-prevention-preparedness-and-response.htm
Public Health Agency of Canada. (2011). Pathogen Safety Data Sheets: Infectious Substances - Bacillus anthracis. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/bacillus-anthracis-material-safety-data-sheets-msds.html
Public Health Agency of Canada. Food Safety - Fact Sheets. Available from https://www.canada.ca/en/public-health/services/food-safety/fact-sheet.html
Sharma, S. K., Saini, N., & Chwla, Y. (2005). Hepatitis B Virus: Inactive carriers. Virology Journal, 2(82).
Staggs, S. (2014). Crime Scene and Evidence Photography - Camera and Lighting. Retrieved 10/26, 2017 from http://www.crime-scene-investigator.net/csp-cameraandlighting.html
United Kingdom Health Protection Agency. (2006). Eye of the Needle: United Kingdom Surveillance of Significant Occupational Exposures to Bloodborne Viruses in Healthcare Workers. London, UK: Health Protection Agency. Retrieved 11/28, 2017 from https://webarchive.nationalarchives.gov.uk/20140714113638/http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1205394781623
United States Centers for Disease Control and Prevention. (1998). Fatal Cercopithecine herpesvirus 1 (B Virus) Infection Following a Mucocutaneous Exposure and Interim Recommendations for Worker Protection. Morbidity and Mortality Weekly Report, 47(49):1073-1076, 1083.
United States Centers for Disease Control and Prevention. (2011). Fatal Laboratory-Acquired Infection with an Attenuated Yersinia pestis Strain --- Chicago, Illinois, 2009. Morbidity and Mortality Weekly Report, 60(7):201-205.
United States Centers for Disease Control and Prevention. (2014). Human Typhimurium Infections Linked to Laboratory Exposure, 2014. Retrieved 11/23, 2017 from https://www.cdc.gov/salmonella/typhimurium-labs-06-14/index.html
United States Centers for Medicare and Medicaid Services. (date unknown). Five Whys Tool for Root Cause Analysis. Retrieved 04/27, 2021 from https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/FiveWhys.pdf
United States Centers for Medicare and Medicaid Services. (date unknown). Guidance for Performing Root Cause Analysis (RCA) with Performance Improvement Projects (PIPs). Retrieved 04/27, 2021 from https://www.cms.gov/medicare/provider-enrollment-and-certification/qapi/downloads/guidanceforrca.pdf
United States Department of Health and Human Services. (2020). The Stages of HIV Infection. Retrieved 04/27, 2021 from https://hivinfo.nih.gov/understanding-hiv/fact-sheets/stages-hiv-infection
United States Department of Labor, Occupational Safety and Health Administration. (2015). Incident [Accident] Investigations: A Guide for Employers. Retrieved 07/24, 2017 from https://www.osha.gov/dte/IncInvGuide4Empl_Dec2015.pdf
Van Noorden, R. (2013). Safety survey reveals lab risks. Nature, 493(7430):9-10.
Washington State Department of Labor & Industries. (2009). Accident Investigation Basics: How to do a workplace accident investigation [PowerPoint slides]. Retrieved 04/27, 2021 from https://wisha-training.lni.wa.gov/training/presentations/AccidentInves.pps
Weinstein, R. A., & Singh, K. (2009). Laboratory-Acquired Infections. Clinical Infectious Diseases, 49(1):142-147.
Willemarck, N., Van Vaerenbergh, B., Descamps, E., Brosius, B., Dai Do Thi, C., et al. (2015). Laboratory-Acquired Infections in Belgium (2007-2012): An Online Survey. Brussels, Belgium: Institut Scientifique de Santé Publique. Retrieved 11/28, 2017 from https://www.bioveiligheid.be/sites/default/files/2015_willemarck_lai_report_belgium_2007_2012_final.pdf
Wooley, D. P., & Byers, K. B. (Eds.). Biological Safety: Principles and Practices (5th ed.). Washington, DC, USA: ASM Press.
Wurtz, N., Papa, A., Hukic, M., Di Caro, A., Leparc-Goffart, I., et al. (2016). Survey of Laboratory-Acquired Infections Around the World in Biosafety Level 3 and 4 Laboratories. European Journal of Clinical Microbiology & Infectious Diseases, 35(8):1247-1258.
Appendix A: Biosafety-related incidents
Activities involving pathogens and toxins in laboratories and other containment zones carry an inherent risk of exposure due to the nature of the work and the pathogens and toxins involved, and may lead to injuries, and in rarer cases, even deaths.Footnote 35Footnote 36Footnote 37Footnote 38Footnote 39Footnote 40 Internationally, there are limited and variable requirements governing the reporting of laboratory incidents involving biological agents, and these are generally limited to bloodborne viruses.Footnote 41Footnote 42 In Canada, the Laboratory Incident Notification Canada (LINC) surveillance system has been implemented by the PHAC as part of a larger comprehensive national biosafety and biosecurity program to better protect the Canadian public from the risks posed by human pathogens and toxins. This program has resulted in the development of annual reports describing the number and the type of incidents occurring in licensed facilities in Canada, which are legally required to report all laboratory incidents, as well as those reporting on a voluntary basis. For example, in 2019, the majority (62%) of incidents resulting in exposure or LAI occurred in CL2 laboratories.Footnote 35
Incidents include accidents (i.e., unplanned events resulting in injury, harm, or damage), events deliberately instigated and other hazardous occurrences that do not always result in an LAI. Examples of documented laboratory-related incidents include:Footnote 43Footnote 44Footnote 45Footnote 46
- spills, splashes, or leaks of hazardous liquids
- inhalation and ingestion of hazardous liquids (e.g., droplets, aerosols, airborne infectious particles)
- direct contact of skin (i.e., absorption) or mucosal membranes (e.g., ingestion) with hazardous materials and contaminated surfaces
- fires and explosions from flammable materials and electronic equipment
- burns and scalds from hot equipment
- slips, trips, and falls
- cuts and lacerations from broken glass or other sharp objects
- needlestick injuries or other inoculation with a contaminated sharp object (e.g., scalpel blade) and
- animal bites and scratches
The minimum requirements for incident investigation and reporting in regulated containment zones are specified in the CBS. Records of incidents involving pathogens, toxins, other regulated infectious material, infected animals, or losses of containment are to be kept on file for a minimum of 10 years. The following non-exhaustive tables list incident scenarios that need to be investigated whether or not reporting of the incident to the PHAC or the CFIA is required.
| Incident | Reference |
Inadvertent release of a human pathogen or toxin. |
HPTA 12(1) |
Inadvertent possession and inadvertent production of a human pathogen or toxin. |
HPTA 12(2) HPTR 9(1)(c)(ii) |
Exposure to a human pathogen or toxin that has, or may have, caused disease. |
HPTA 13 |
Missing or stolen human pathogen or toxin. |
HPTA 14 |
More detail and examples of each type of incident are provided in Appendix B.
| Incident |
Failure of containment barrier seals (e.g., biological sealing flange or gasket made of flexible material surrounding the body of a barrier autoclave or other seal on the containment barrier). |
Failure of non-shrinking sealants (e.g., silicone, polyurethane, or polyether caulking) used to seal barrier penetrations such as conduits, plumbing, and wiring. |
Failure of door interlocks or alarms on pass-through technologies on the containment barrier (e.g., double-door barrier autoclaves, pass-through chambers, dunk tanks, barrier cage washers, feed chutes). |
Failure of inward airflow, where applicable. |
Failure of heating, ventilation, and air conditioning (HVAC) systems, controls, or alarms. |
Failure or compromise of backdraft protection, high efficiency particulate air (HEPA) filters, or HEPA filter housings. |
Failure of deep seal traps and plumbing. |
Failure of BSCs or other primary containment devices. |
Puff-back from class II B2 BSCs, where present. |
Loss of containment integrity in large scale process equipment, closed systems, or other primary containment devices. |
Failure of large scale equipment controls or alarms. |
Failure of primary containment caging or ventilated enclosures. |
Failure or improper operation of decontamination technologies (e.g., autoclave, effluent decontamination system) or controls. |
Failure of positive-pressure suits. |
| Incident | Reference |
Medical emergencies, fires, chemical or biological spills (small/large; inside/outside BSC and centrifuge), power failure, animal escape, and natural disasters. |
CBS |
Possession or disposal of an animal or thing that was imported in contravention of the HAA or the HAR. |
HAA 15(1) |
Movement of an imported animal pathogen or part of one that retains its pathogenicity (e.g., a toxin) from the place or places specified in the animal pathogen import permit to any other place, unless under and in accordance with that permit or another permit issued for that purpose. |
HAR 51.1(a) |
Introduction of an imported animal pathogen or part of one that retains its pathogenicity (e.g., toxin) into an animal unless under and in accordance with that permit or another permit issued for that purpose. |
HAR 51.1(b) |
| Incident | Reference |
Biosecurity incidents and emergencies described in the biosecurity plan. |
CBS |
Access to a containment zone by unauthorized person(s). |
CBS |
Access to a containment zone in which controlled activities with RG3 or RG4 pathogens or regulated toxins are authorized by person(s) who are not authorized. |
CBS |
Access to a part of a facility in which controlled activities with SSBAs are authorized by person(s) that do not hold a valid HPTA security clearance issued by the PHAC, or who are not accompanied and supervised by a person who holds an HPTA security clearance. |
HPTA 33 HPTR 24 CBS |
Failure to remove an individual's authorization to access a part of a facility where SSBAs are present and accessible when the individual is no longer in need of access to that part of the facility or when the individual no longer holds a valid HPTA security clearance. |
CBS |
Unauthorized access to supporting mechanical and electrical services for the containment zone. |
CBS |
Unauthorized access to areas housing an effluent decontamination system. |
CBS |
Appendix B: Incidents reportable to the PHAC
Licence holders are required by the HPTA and the HPTR to notify the PHAC without delay in the event that any of the following incident scenarios occur.Footnote 18Footnote 19 While licence holders may delegate reporting responsibilities to the designated BSO, the licence holder remains ultimately responsible under the HPTA. Notifications to the PHAC can be made via the online submission of a notification report through the Biosecurity Portal.Footnote 9
B.1 Inadvertent release [HPTA 12(1)]
An inadvertent release refers to a release that is unscheduled, unintentional, or unplanned and includes any leaking, spraying, depositing, dumping, or vaporizing (i.e., generating aerosols) pathogens or toxins. Reporting to the PHAC is mandatory when the release occurs outside of the designated containment zone. An example of an inadvertent release is unknowingly sending improperly decontaminated waste (i.e., that still contains viable pathogens) to the sewer system or to a landfill.
Any person working in a licensed facility who has reason to believe that an incident involving an inadvertent release of a human pathogen or toxin has occurred is required to inform the licence holder without delay [HPTA 15]. Notification must be provided to the PHAC without delay when there is reason to believe that a human pathogen or toxin has been released inadvertently from a licensed facility [HPTA 12(1)].
B.2 Inadvertent possession [HPTR 4(1)(f)] and inadvertent production [HPTA 12(2)]
Inadvertent possession occurs when a person unexpectedly or unintentionally comes into possession of a human pathogen or toxin without the appropriate licence issued by the PHAC. An example of an incident involving inadvertent possession of a pathogen is the receipt of a package containing a human pathogen or toxin that is not authorized by the facility's licence as a result of a shipping error from the vendor. Notification of inadvertent possession can be performed by any individual aware of an unauthorized possession taking place, or aware of a human pathogen or toxin being handled or stored in a location where it is not authorized by a licence.
Inadvertent production refers to any method of production (e.g., manufacturing, cultivating, developing, reproducing, synthesizing, converting, refining, or altering of physical or chemical properties) of a human pathogen or toxin that is unplanned or unintentional and is not authorized by the licence under which activities are taking place. An example of an inadvertent production of a pathogen is the presence of a mutated viral or bacterial strain resulting from unplanned recombination events during the course of an experiment.
Any person aware of inadvertent production or inadvertent possession taking place is required to immediately notify the BSO or the licence holder without delay [HPTA 15 and HPTR 4(1)(f)]. After being informed, the BSO or licence holder is required to inform or notify the PHAC without delay of all occurrences of unauthorized and inadvertent possession or production of a human pathogen or toxin [HPTR 9(1)(e), HPTR 9(1)(c)(ii), and HPTA 12(2)].
B.3 Exposure that has or may have caused disease [HPTA 13]
Exposure incidents include:
- confirmed cases of LAI (i.e., resulting from exposure to a pathogen or a toxin)
- any disease in an individual that may be linked to a pathogen or toxin handled or stored within the facility
- any incident where there is any reason to believe that an individual had contact with or was in close proximity to a pathogen or toxin and that an infection or intoxication may occur as a result
In some cases, symptoms of disease may not occur immediately after initial exposure (e.g., human immunodeficiency virus [HIV] infection) or may never develop (e.g., inactive carriers following hepatitis B virus infection); these infected individuals may inadvertently infect others.Footnote 47Footnote 48
Exposures in a containment zone that may lead to infection can occur via:
- inhalation (e.g., breathing in infectious aerosols or aerosolized toxins)
- ingestion (e.g., contact of mouth with contaminated hands or materials)
- percutaneous inoculation (e.g., subcutaneous contamination by puncture, needlestick, animal bite or scratch)
- absorption (e.g., entry through direct skin, eye, or mucous membrane contact)
Personnel working in a containment zone are required to immediately inform the appropriate internal personnel or authority (e.g., supervisor, laboratory director, BSO) of any incident that may have resulted in an exposure or any disease that may have been caused by an exposure in a facility (CBS). In CL4 facilities, supervisors may also contact their personnel with unexpected work absences to determine whether the absence may be related to a possible exposure incident. Any person working in a licensed facility who has reason to believe that an incident involving a human pathogen or toxin has caused, or may have caused disease in an individual is obligated to inform the licence holder without delay [HPTA 15]. Notifications must be provided to the PHAC without delay when an incident involving a human pathogen or toxin has caused, or may have caused, disease in an individual [HPTA 13].
More information on notification following exposure events can be found in the Notification and Reporting Under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal guideline.Footnote 9
B.4 Missing or stolen pathogen or toxin [HPTA 14]
Missing pathogens or toxins could be the result of a targeted security breach, improper inventory control, failure to follow labelling and general laboratory management procedures, or as a result of delays or errors in shipments.
Any person who discovers that a human pathogen or toxin has not been received within the expected timeframe must make reasonable efforts to locate it and inform the BSO of the situation without delay [HPTR 4(1)(e)]. Any person who discovers that an SSBA has not been received within 24 hours of the expected time must make reasonable efforts to locate it and inform the BSO of the situation without delay [HPTR 4(2)]. Moreover, any person working in a licensed facility who has reason to believe that an incident involving a stolen or otherwise missing human pathogen or toxin has occurred is obligated to inform the licence holder without delay [HPTA 15]. Notification must be provided to the PHAC without delay when there is reason to believe that a human pathogen or toxin that was in his or her possession has been stolen or is otherwise missing from a licensed facility [HPTA 14]. In addition, the licence holder must take reasonable measures to locate the missing pathogen or toxin.
Appendix C: Example incident scenario involving a student exposed to Staphylococcus aureus
On Friday March 31, 2017 at 1:00 pm, a high school student "A" (male) experienced an accidental exposure while working with a laboratory strain of a risk group 2 (RG2) bacterium, Staphylococcus aureus (S. aureus). The student had started working in the laboratory on March 24 (seven days prior), and was assigned to work under the direct supervision of a mentor. Since there was no clear procedure on how to train a new student, the mentor gave him a brief introduction on his project involving S. aureus, provided an overview of the laboratory techniques involved, and showed him the binder containing all SOPs used in the laboratory. "A" also spent a few days shadowing the mentor. As "A" seemed to have understood all the information presented, the mentor let "A" handle a bacterial culture while he tended to other tasks. At this point, the mentor was unaware of existing directives requiring students to wear gloves when handling all specimens and culture plates.
While attempting to place a cover on an agar dish inoculated with a live culture of S. aureus, "A"'s hand came into contact with the culture. "A" immediately washed his hands with antibacterial soap followed by alcohol-based hand sanitizer. The mentor did not observe the incident and only became aware of the possible LAI when the student recounted the incident two days later and reported pain, swelling, and redness in his hand. Once informed, the mentor notified the laboratory supervisor (licence holder representative), who called the local hospital's urgent response line. The on-call responding physician prescribed the antibiotic cephalosporin and advised the student to contact his family doctor for continued follow-up.
The supervisor then completed an incident report for the BSO who then filed it with the local and national authorities. In addition, "A"'s educational institute was notified of the incident. The BSO also submitted a notification report to the PHAC as described in the Canadian Biosafety Guideline - Notification and Reporting Under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal.Footnote 9
Two days after the antibiotic was prescribed and symptoms were reduced, the on-call physician aspirated the student's wound and ordered culture susceptibility testing. Testing results confirmed that the student was infected with the same strain of the bacteria with which he had been working. The mentor properly disposed of the plate according to relevant SOPs.
References
- Footnote 1
-
Infrastructure Health and Safety Association. (2020). Accident Investigation & Reporting. Retrieved 04/27, 2021 from https://www.ihsa.ca/resources/accident_investigation.aspx
- Footnote 2
-
Canadian Centre for Occupational Health and Safety. (2017). OSH Answers Fact Sheets - Incident Investigation. Retrieved 05/24, 2017 from https://www.ccohs.ca/oshanswers/hsprograms/investig.html
- Footnote 3
-
Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition
- Footnote 4
-
BS ISO 45001:2018, Occupational Health and Safety Management Systems Requirements with Guidance for Use. (2018). London, UK: British Standards Institution
- Footnote 5
-
CSA Z1000:14 (R2019), Occupational Health and Safety Management. (2014). Toronto, ON, Canada: Canadian Standards Association.
- Footnote 6
-
CSA Z1005-17, Incident Investigation. (2017). Toronto, ON, Canada: Canadian Standards Association.
- Footnote 7
-
CEN Workshop 31 - Laboratory biosafety and biosecurity. CEN Workshop Agreement (CWA) 15793:2011, Laboratory biorisk management. (2011). Brussels, Belgium: European Committee for Standardization.
- Footnote 8
-
Government of Canada. (2016). Canadian Biosafety Handbook (2nd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/handbook-second-edition.html
- Footnote 9
-
Government of Canada. (2017). Notification and Reporting Under the HPTA and HPTR Using the Reporting Module of the Biosecurity Portal. Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
- Footnote 10
-
Washington State Department of Labor & Industries. (2009). Accident Investigation Basics: How to do a workplace accident investigation [PowerPoint slides]. Retrieved 04/27, 2021 from https://wisha-training.lni.wa.gov/training/presentations/AccidentInves.pps
- Footnote 11
-
Organisation for Economic Co-operation and Development. (2003). OECD Guiding Principles for Chemical Accident Prevention, Preparedness and Response: Guidance for Industry (including Management and Labour), Public Authorities, Communities and other Stakeholders (2nd ed.). Paris, France: OECD Publications. Retrieved 01/22, 2020 from https://www.oecd.org/env/ehs/chemical-accidents/guiding-principles-chemical-accident-prevention-preparedness-and-response.htm
- Footnote 12
-
United States Department of Labor, Occupational Safety and Health Administration. (2015). Incident [Accident] Investigations: A Guide for Employers. Retrieved 07/24, 2017 from https://www.osha.gov/dte/IncInvGuide4Empl_Dec2015.pdf
- Footnote 13
-
Association of Workplace Investigators and Canadian Association of Workplace Investigators. (2020). Guiding Principles for Conducting Workplace Investigations. Retrieved 04/27, 2021 from https://cdn.ymaws.com/www.awi.org/resource/resmgr/files/publications/AWI-Guiding-Principles-Broch.pdf
- Footnote 14
-
Bloch, M. C. (2008). Guide to Conducting Workplace Investigations. Retrieved 10/02, 2017 from http://www.corporatecompliance.org/Portals/1/Users/169/29/60329/Workplace_Investigations_Guide.pdf
- Footnote 15
-
Occupational Health and Safety Agency for Healthcare (OHSAH) in British Columbia. (2008). Incident Investigations: Participant's Guide. Retrieved 10/10, 2017 from http://www.phsa.ca/Documents/Occupational-Health-Safety/GuideIncidentInvestigationsParticipantGuide.pdf
- Footnote 16
-
Hodkiewicz, T. S. (2012). Pre-planning for incident investigations. Safety+Health, 185(3). Retrieved 10/05, 2017 from https://www.safetyandhealthmagazine.com/articles/pre-planning-for-incident-investigations-2
- Footnote 17
-
National Safety Council. (2017). How to Conduct an Incident Investigation. Retrieved 10/04, 2017 from http://www.nsc.org/JSEWorkplaceDocuments/How-To-Conduct-An-Incident-Investigation.pdf
- Footnote 18
-
Human Pathogens and Toxins Regulations (SOR/2015-44).
- Footnote 19
-
Human Pathogens and Toxins Act (S.C. 2009, c. 24).
- Footnote 20
-
Namvar, O., & Ordonez, M. (2016). Incident Investigation and Reporting [PowerPoint slides]. Retrieved 10/17, 2017 from http://safetymanagementeducation.com/teaching-resources/teaching-modules/IncidentInvestigationAndReportingModule.pptx
- Footnote 21
-
Canadian Centre for Occupational Health and Safety. (2017). OSH Answers Fact Sheets - Canadian Government Departments Responsible for OH&S. Retrieved 10/18, 2017 from https://www.ccohs.ca/oshanswers/information/govt.html
- Footnote 22
-
Government of Canada. (2013). Accident Investigation: A Responsibility to be Taken Seriously! Available from https://www.canada.ca/en/employment-social-development/services/health-safety/reports/accident.html
- Footnote 23
-
Government of Canada. Biosecurity Portal. Available from https://biosecurity-portal.hc-sc.gc.ca
- Footnote 24
-
National Forensic Science Technology Center. (2013). A Simplified Guide To Crime Scene Photography. Retrieved 10/26, 2017 from http://www.forensicsciencesimplified.org/photo/Photography.pdf
- Footnote 25
-
Staggs, S. (2014). Crime Scene and Evidence Photography - Camera and Lighting. Retrieved 10/26, 2017 from http://www.crime-scene-investigator.net/csp-cameraandlighting.html
- Footnote 26
-
Buys, J. R., & Clark, J. L. (1995). Events and Causal Factors Analysis. Scientech, Inc.: Idaho Falls, ID, USA. Retrieved 11/06, 2017 from https://www.iosh.com/media/2053/events-and-casual-factors-chiltern-january-2017.pdf
- Footnote 27
-
United States Centers for Medicare and Medicaid Services. (date unknown). Guidance for Performing Root Cause Analysis (RCA) with Performance Improvement Projects (PIPs). Retrieved 04/27, 2021 from https://www.cms.gov/medicare/provider-enrollment-and-certification/qapi/downloads/guidanceforrca.pdf
- Footnote 28
-
National Collaborating Centre for Infectious Diseases. (2016). Glossary of Terms for Infectious Disease Modelling: A Proposal for Consistent Language. Retrieved 04/25, 2019 from https://nccid.ca/publications/glossary-terms-infectious-disease-modelling-proposal-consistent-language/
- Footnote 29
-
Public Health Agency of Canada. (2011). Pathogen Safety Data Sheets: Infectious Substances - Bacillus anthracis. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/bacillus-anthracis-material-safety-data-sheets-msds.html
- Footnote 30
-
Public Health Agency of Canada. Food Safety - Fact Sheets. Available from https://www.canada.ca/en/public-health/services/food-safety/fact-sheet.html
- Footnote 31
-
Charles, R., Hood, B., DeRosier, J. M., Gosbee, J. W., Li, Y., et al. (2016). How to perform a root cause analysis for workup and future prevention of medical errors: a review. Patient Safety in Surgery, 10(20).
- Footnote 32
-
Jucan, G. (date unknown). Root Cause Analysis for IT Incidents Investigation. Retrieved 11/06, 2017 from http://hosteddocs.ittoolbox.com/gj102105.pdf
- Footnote 33
-
United States Centers for Medicare and Medicaid Services. (date unknown). Five Whys Tool for Root Cause Analysis. Retrieved 04/27, 2021 from https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/FiveWhys.pdf
- Footnote 34
-
CAN/CSA-ISO 9001:16 (R2020), Quality Management Systems - Requirements. (2016). Toronto, ON, Canada: Canadian Standards Association.
- Footnote 35
-
Lien, A., Abalos, C., Atchessi, N., Edjoc, R., & Heisz, M. (2020). Surveillance of laboratory exposures to human pathogens and toxins, Canada 2019. Canada Communicable Disease Report, 46(9):292-298.
- Footnote 36
-
Byers, K. B., & Harding, A. L. (2017). Laboratory-Associated Infections. In Wooley, D. P., & Byers, K. B. (Eds.). Biological Safety: Principles and Practices (5th ed., pp. 59-92). Washington, DC, USA: ASM Press.
- Footnote 37
-
Coelho, A. C., & García Díez, J. (2015). Biological Risks and Laboratory-Acquired Infections: A Reality That Cannot be Ignored in Health Biotechnology. Frontiers in Bioengineering and Biotechnology, 3(56).
- Footnote 38
-
United States Centers for Disease Control and Prevention. (2014). Human Typhimurium Infections Linked to Laboratory Exposure, 2014. Retrieved 11/23, 2017 from https://www.cdc.gov/salmonella/typhimurium-labs-06-14/index.html
- Footnote 39
-
United States Centers for Disease Control and Prevention. (2011). Fatal Laboratory-Acquired Infection with an Attenuated Yersinia pestis Strain --- Chicago, Illinois, 2009. Morbidity and Mortality Weekly Report, 60(7):201-205.
- Footnote 40
-
Hawkes, N. (1979). Smallpox Death in Britain Challenges Presumption of Laboratory Safety. Science, 203(4383):855-856.
- Footnote 41
-
Health and Safety Executive. (2013). RIDDOR - Reporting of Injuries, Diseases and Dangerous Occurrences Regulations 2013. Retrieved 11/28, 2017 from https://www.hse.gov.uk/riddor/
- Footnote 42
-
United Kingdom Health Protection Agency. (2006). Eye of the Needle: United Kingdom Surveillance of Significant Occupational Exposures to Bloodborne Viruses in Healthcare Workers. London, UK: Health Protection Agency. Retrieved 11/28, 2017 from https://webarchive.nationalarchives.gov.uk/20140714113638/
http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1205394781623 - Footnote 43
-
Collins, C. H., & Kennedy, D. A. (1999). Laboratory-Acquired Infections. In Collins, C. H., & Kennedy, D. A. (Eds.). Laboratory-Acquired Infections: History, Incidence, Causes and Preventions (4th ed., pp. 1-97). Oxford, UK: Butterworth-Heinemann.
- Footnote 44
-
Canadian Centre for Occupational Health and Safety. (2017). OSH Answers Fact Sheets - Laboratory Technician and Technologist. Retrieved 07/18, 2017 from https://www.ccohs.ca/oshanswers/occup_workplace/labtech.html
- Footnote 45
-
American Society of Safety Professionals. (2017). Understanding the Top 5 Laboratory Hazards. Retrieved 04/27, 2021 from https://www.assp.org/news-and-articles/2017/10/22/understanding-the-top-5-laboratory-hazards
- Footnote 46
-
Aliyu, M. (date unknown). Common Laboratory Accidents and Causes in Secondary Schools of Zaria Environ. Retrieved 11/28, 2017 from http://www.globalacademicgroup.com/journals/pristine/COMMON%20LAB
ORATORY%20ACCIDENTS%20AND%20CAUSES%20IN%20SECONDARY%20SCH
OOLS%20OF%20ZARIA%20ENVIRON.pdf - Footnote 47
-
United States Department of Health and Human Services. (2020). The Stages of HIV Infection. Retrieved 04/27, 2021 from https://hivinfo.nih.gov/understanding-hiv/fact-sheets/stages-hiv-infection
- Footnote 48
-
Sharma, S. K., Saini, N., & Chwla, Y. (2005). Hepatitis B Virus: Inactive carriers. Virology Journal, 2(82).
