Canadian Biosafety Guideline - Lentiviral Vectors

Download the alternative format
(PDF format, 2.41 MB, 48 pages)
Organization: Public Health Agency of Canada
Published: 2019-09-15
August 7, 2019
Table of contents
Preface
In Canada, facilities where Risk Group 2, 3, and 4 human pathogens or toxins are handled and stored are regulated by the Public Health Agency of Canada (PHAC) under the Human Pathogens and Toxins Act (HPTA) and Human Pathogens and Toxins Regulations (HPTR). The importation of animal pathogens, infected animals, animal products or by-products (e.g., tissue, serum), or other substances that may carry an animal pathogen or toxin or parts thereof are regulated by the PHAC or the Canadian Food Inspection Agency (CFIA) under the Health of Animals Act (HAA) and Health of Animals Regulations (HAR).
The following figure depicts the document hierarchy used by the PHAC and the CFIA to oversee biosafety and biosecurity operations. Each tier of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards. Acts and regulations are the documents that convey the PHAC’s and the CFIA’s legal authorities, and, therefore, are found at the top of the pyramid. Guidance material and technical pieces are found at the bottom of the pyramid, as they are intended to summarize recommendations and scientific information only.
Figure 1: The Government of Canada’s Biosafety and Biosecurity Document Hierarchy
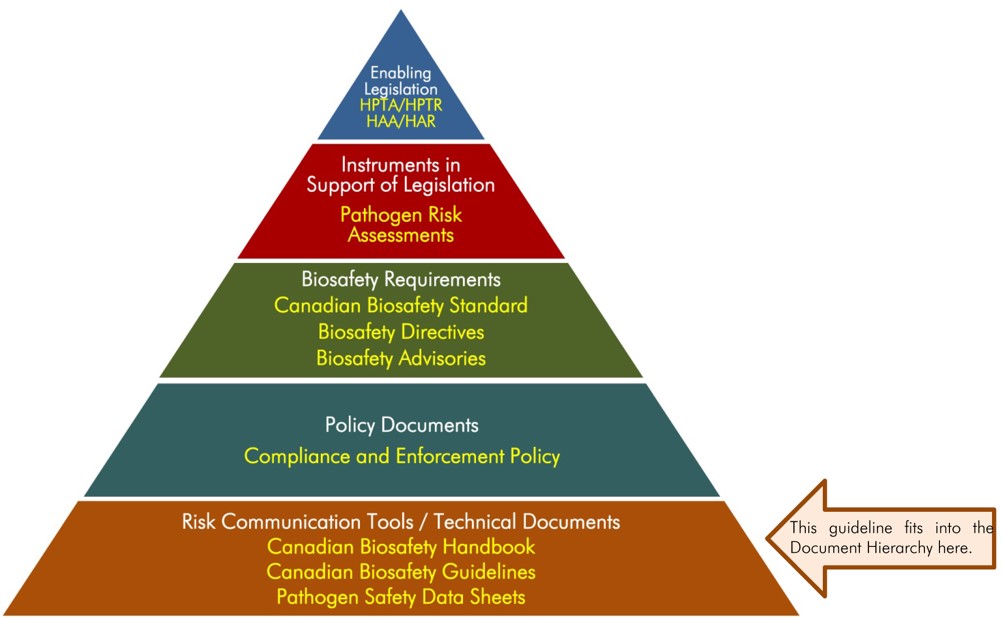
Figure 1: The Public Health Agency of Canada's Biosafety and Biosecurity Document Hierarchy - Text Equivalent
Figure in the form of a pyramid depicting the document hierarchy used by the PHAC to oversee biosafety and biosecurity operations. Each of the five tiers of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards.
At the top sits the Enabling Legislation, that is, the HPTA, HPTR, HAA, and HAR, that convey the PHAC’s legal authorities. Below the acts and regulations sit Instrument in Support of Legislation, which are the Pathogen Risk Assessments. In the next tier down are the Biosafety Requirements, which include the Canadian Biosafety Standard, Biosafety Directives, and Biosafety Advisories. In the second lowest tier are the Policy Documents, which includes the Compliance and Enforcement Policy. Guidance material and technical pieces found at the bottom of the pyramid, under the Risk Communication Tools and Technical Documents heading are intended to summarize recommendations and scientific information only. These include the Canadian Biosafety Handbook, Canadian Biosafety Guidelines, and Pathogen Safety Data Sheets.
The Lentiviral Vectors guideline was developed by the PHAC and the CFIA as part of a series of electronic publications that expand upon the biosafety and biosecurity concepts discussed in the current edition of the Canadian Biosafety Handbook (CBH), the companion document to the Canadian Biosafety Standard (CBS). This guideline describes best practices and recommendations for work involving lentiviral vectors in laboratories and other containment zones.
Thisguideline is continuously evolving and subject to ongoing improvement. The PHAC and the CFIA welcome comments, clarifications, and suggestions for incorporation into the future versions. Please send this information (with references, where applicable) to:
- PHAC e-mail: PHAC.pathogens-pathogenes.ASPC@canada.ca
Abbreviations and Acronyms
- BSC
- Biological safety cabinet
- CBH
- Canadian Biosafety Handbook
- CBS
- Canadian Biosafety Standard
- CFIA
- Canadian Food Inspection Agency
- CL
- Containment Level (i.e., CL1, CL2, CL3, CL4)
- CL2-Ag
- Containment Level 2 large animal containment zone
- DNA
- Deoxyribonucleic acid
- HIV
- Human immunodeficiency virus
- LRA
- Local risk assessment
- LTR
- Long terminal repeats
- MLV
- Murine leukemia virus
- PHAC
- Public Health Agency of Canada
- PPE
- Personal protective equipment
- RCR
- Replication-competent retrovirus
- RG
- Risk Group (i.e., RG1, RG2, RG3, RG4)
- RNA
- Ribonucleic acid
- SOP
- Standard operating procedure
- VSV-G
- Vesicular stomatitis virus glycoproteins
Chapter 1 - Introduction
The words in bold type are defined in the glossary found in Chapter 5.
Lentiviral vectors are retroviruses that are generated in vitro by the multi-plasmid transfection of mammalian cells. The retrovirus particles are harvested from the culture medium and used to stably insert a transgene into the genome of a target cell. These vectors have been engineered so that they are able to infect the target cell, but are not able to replicate following the infection. However, as most lentiviral vectors are derived from the human immunodeficiency virus (HIV), concerns relating to the potential reversion of the vector to a replicative state remain, as do risks associated with the transgene and insertional mutagenesis (e.g. resulting in activation or inactivation of genes).
In some cases, applicable containment requirements for a laboratory or containment zone where pathogens are handled or stored may need to be modified based upon the procedure being performed, or whether the risk associated with a pathogen has changed (e.g., the pathogen has been modified). Lentiviral vectors are generally classified as Risk Group 2 (RG2) human pathogens and RG2 animal pathogens. However, some lentiviral vectors and transgenes may possess unique characteristics that increase their risks, leading to additional or modified biosafety requirements for their safe handling, or to classification as an RG3 pathogen.
1.1 Scope
The Lentiviral Vectors guideline describes biosafety considerations and best practices for conducting a pathogen risk assessment or local risk assessment (LRA) for a containment zone where lentiviral vectors are handled or stored, so that appropriate mitigation measures may be implemented. The guideline presents risk factors and risk mitigation strategies to be considered when performing risk assessments and when establishing biosafety procedures. This guideline is intended to be used in conjunction with the Canadian Biosafety Standard (CBS) and Canadian Biosafety Handbook (CBH)Footnote 1,Footnote 2.
The information provided in this document is meant as guidance only and should not be interpreted as requirements. Regulated parties may choose alternate approaches to meet the requirements specified in the CBS.
1.2 How to use the Lentiviral vectors guideline
A detailed list of all abbreviations and acronyms used throughout this guideline is located at the beginning of this document. Each word or term is spelled out upon first use in the guideline, with the abbreviation immediately following in brackets. After its initial definition, the abbreviation is used exclusively throughout the remainder of the document. A comprehensive glossary of definitions for technical terms is located in Chapter 5; words defined in the glossary appear in bold type upon first use in the guideline. A list of references and other resources is provided in Chapter 6.
Chapter 2 - Description of lentiviral vectors
Lentiviral vector systems are derived from viruses that belong to the genus of lentivirus from the Retroviridae family (Figure 2-1).Footnote 3 Lentiviral vectors are most commonly derived from HIV type 1 (HIV-1).Footnote 4 However, they may also be derived from HIV-2 and non-human lentiviruses, including simian immunodeficiency virus, feline immunodeficiency virus, equine infectious anemia virus, and bovine immunodeficiency virusFootnote 4,Footnote 5,Footnote 6. Lentiviruses are practical vehicles for gene delivery due to their stable integration into the genomes of both dividing and non-dividing cells, as well as their long-term transgene expressionFootnote 2. However, the potential pathogenicity of lentiviral vectors is an important consideration when developing biosafety strategies.
Figure 2-1: Retroviridae phylogenetic tree
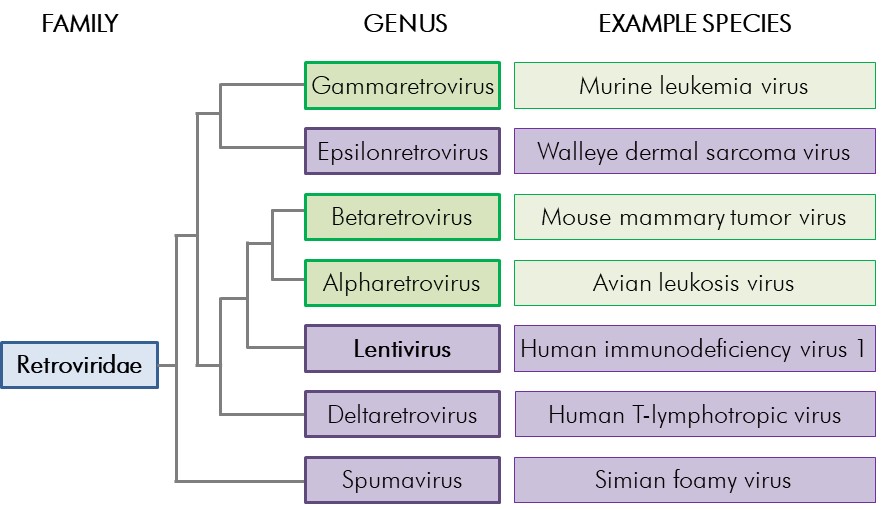
Figure 2-1: Retroviridae phylogenetic tree - Text Equivalent
Figure showing the phylogenetic relationships between the Retroviridae genera. Four of the seven genera, Epsilonretrovirus (e.g., walleye dermal sarcoma virus), Lentivirus (e.g., human immunodeficiency virus 1), Deltaretrovirus (e.g., human T-lymphotropic virus), and Spumavirus (e.g., simian foamy virus) are complex retroviruses. The other three genera presented, Gammaretrovirus (e.g., murine leukemia virus), Betaretrovirus (e.g., mouse mammary tumor virus), and Alpharetrovirus (e.g., avian leucosis virus) are simple retroviruses.
The seven genera within the Retroviridae family are shown; complex retrovirus genera are indicated in purple whereas simple genera are indicated in green. One example species from each of the seven genera is shown. Branch lengths are not meant to represent phylogenetic distance, but are indicated for general reference only.
HIV is the most studied lentivirus, and the predominant primary parent virus from which currently available lentiviral vectors are derived. To overcome the risk of accidental infection, integration into the human genome, and persistent lifelong infection that is associated with HIV pathogenicity, lentiviral vectors are designed to be replication-defectiveFootnote 4. This is accomplished by segregation or removal of genes that are essential for virion replication and packaging from the rest of the HIV genome. The resulting viral particle is capable of infecting a target cell and delivering its transgene, but is incapable of completing the remainder of the viral life cycle to form a replication-competent retrovirus (RCR), the critical step for propagation.
2.1 Retroviruses
The family of Retroviridae is composed of seven genera (Figure 2-1), which consist of single-stranded, enveloped, ribonucleic acid (RNA) retroviruses that are capable of causing disease in humans and animalsFootnote 3. They are broadly classed as simple or complex retroviruses, based on RNA splicing patterns and the presence of accessory genesFootnote 7. The interaction of retroviruses and the host cell surface is highly specific, and is the main determinant of the viral host rangeFootnote 7. After binding and penetrating the host cell, the viral RNA is transcribed into deoxyribonucleic acid (DNA), which is then integrated into the host DNA through processes that use both host- and virally-encoded proteins; following synthesis and assembly, this replication process results in release of infectious virus particles. To perform these tasks, all retroviruses contain the viral genes gag, pol, and env; the gene products and functions of these are summarized in Table 2-1.
| Gene | Products | Function |
|---|---|---|
| gag | Capsid | Protects the core |
| Matrix | Lines envelope | |
| Nuclear capsid | Protects the genome and forms the core | |
| pol | Protease | Essential for Gag protein cleavage during maturation |
| Reverse transcriptase | Reverse transcribes the RNA genome into double stranded DNA | |
| Integrase | Required for integration of the provirus | |
| env | Surface glycoprotein | Outer envelope glycoprotein; major virus antigen |
| Transmembrane protein | Inner component of the mature envelope glycoprotein |
The gag gene encodes proteins that make up the internal structure of the virus including the capsid, the membrane associated matrix, and the nuclear capsid. The pol gene encodes enzymes that are required for transcription, integration of the virus genome into the DNA of the host cell, and viral maturation. The env gene encodes proteins that make up the external portion of the virus including the surface glycoproteins and the transmembrane protein, which form a complex that specifically interacts with target cell receptorsFootnote 7. Long terminal repeats (LTR) at the 5' and 3' end of retroviruses contain elements necessary for gene expression, reverse transcription, and integration into host cell genomes; the RNA packaging signal psi (Ψ) is required for the packaging of RNA into virionsFootnote 3,Footnote 8,
In addition to the essential elements found in simple retroviruses, the complex genera of Retroviridae (i.e., lentivirus, epsilonretrovirus, deltaretrovirus, and spumavirus) have genes that encode for accessory and regulatory proteins, which provide the retroviruses with some control over gene expression and the virus life cycleFootnote 7,. For example, the lentivirus HIV-1 contains the accessory genes nef, vif, vpu, and vpr, which promote the infectivity and pathogenicity of HIV, and the regulatory genes tat and rev, which work in coordination with the LTR for virus replication (Table 2-2)Footnote 7.
| Gene | Products | Function |
|---|---|---|
| nef | Accessory proteins | Enhances virion infectivity |
| vif | Affects infectivity of viral particles | |
| vpu | Enhances virion release | |
| vpr | Enhances virion infectivity | |
| tat | Regulatory proteins | Activates transcription |
| rev | Regulates splicing / RNA transport |
2.2 Evolution of retroviral vector systems
The ability of retroviruses to integrate into host genomes rendered them a potential tool for gene therapy; however, the release of RCRs with disease-causing potential was a significant biosafety concern. In an effort to increase the safety of retroviral vector systems, several iterative developments were directed toward reducing the possibility of replicative competency. As detailed below, this was accomplished by replacing or removing the coding regions of genes essential for virion replication and packaging; resultant retroviral particles retain the ability to infect and deliver the transgene to target cells but are unable to complete the remainder of the viral life cycle, including RCR formation. A further level of safety was achieved by separating the viral components needed to produce the infectious virion into multiple plasmidsFootnote 9.
Early retroviral vector systems were developed from simple retroviruses; these had limited utility since simple retroviruses can only enter the host’s nucleus during the mitotic process and so are only able to integrate into dividing cells. In contrast, complex retroviruses can integrate into both dividing and non-dividing cells due to the additional genome-encoded proteins; as such, lentiviral vector systems are now the predominantly used retroviral vector systems.
2.2.1 Early retroviral vector systems
Initial retroviral vector systems were based on simple retroviruses, such as the murine leukemia virus (MLV; Figure 2-2, panel A), a gammaretrovirus. In these systems, the vector was separated into two plasmids, namely a transfer/vector plasmid and a packaging/helper plasmid (Figure 2-2, panel B). The transfer plasmid included the Ψ and LTR that are required for packaging the transgene into the virion and integrating it into the host genome, respectively. The helper plasmid contained the gag, pol, and env genes that encode the proteins that are necessary for virion packaging; the lack of Ψ in the helper vector prevented the transcribed viral DNA from being incorporated into the virion. The end result was a replication-incompetent virus that included the gene of interest, but lacked any of the viral genes required to complete the viral life cycle within the host cellFootnote 10. Despite the separation of the viral genome that was applied in this retroviral systems, a single recombination event could result in the Ψ signal relocating from the transfer plasmid to the helper plasmid, leading to RCR formationFootnote 10.Thus, use of these early retroviral vector systems requires additional biosafety measures.
Figure 2-2: Genome of MLV and the evolution to three-plasmid retroviral vector system
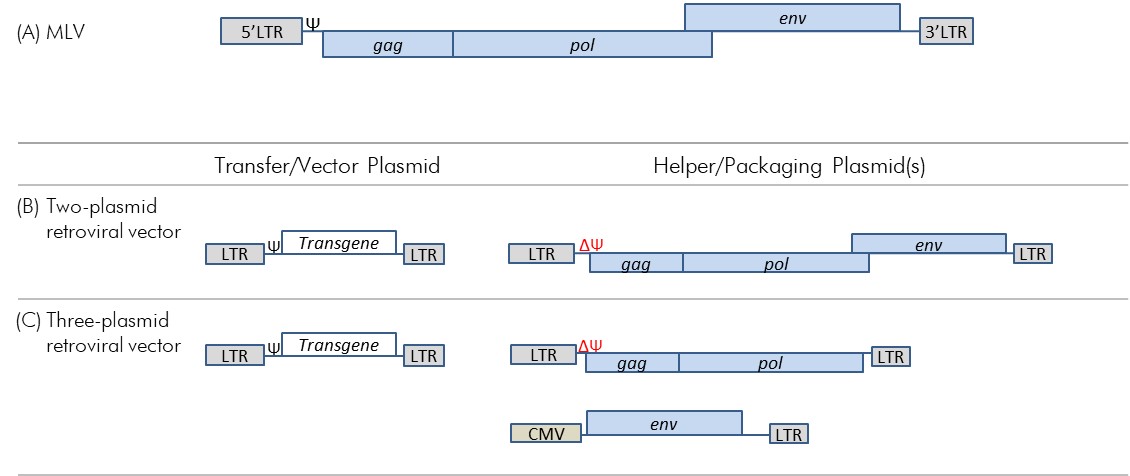
Figure 2-2: Genome of MLV and the evolution to three-plasmid retroviral vector system - Text Equivalent
(A) Figure depicting the organization of MLV genes beginning with the 5’LTR followed in order by the psi signal, gag, pol, and env genes, and ending with the 3’LTR. The env gene slightly overlaps slightly with the pol gene.
(B) Figure depicting the genetic organization of a two-plasmid MLV-based retroviral vector system. This system includes a transfer/vector plasmid and a helper/packaging plasmid. The transfer/vector plasmid includes, in order, a 5’LTR, followed by the psi signal, the transgene, and a 3’LTR. The helper/packaging plasmid includes, in order, a 5’LTR, followed by the gag, pol, and env genes and the 3’LTR. The psi signal has been removed from the helper/packaging plasmid.
(C) Figure depicting the genetic organization of a three-plasmid MLV-based retroviral vector. This system includes one transfer/vector plasmid and two helper/packaging plasmids. The transfer/vector plasmid is identical to (B) (i.e., includes, in order, a 5’LTR, followed by the psi signal, the Transgene, and a 3’LTR). One of the helper/packaging plasmids contains, in order, a 5’LTR, followed by the gag and pol genes, and a 3’LTR ; the psi signal has been removed. The second helper/packaging plasmid contains in order, a CMV promoter, the env gene, and a 3’ LTR.
(A) The MLV genome with common retroviral genes (blue); note that the figure is not to scale and only meant to show the relative location of genes. (B) A two-plasmid retroviral vector system. (C) A three-plasmid retroviral vector system.
A three-plasmid MLV-based retroviral vector system was developed to address the issue of Ψ signal relocation from the transfer plasmid to the helper plasmid (Figure 2-2, panel C)Footnote 11. In this system, the helper plasmid components were divided into two plasmids: one helper (packaging) plasmid contained the gag and pol genes, while the second (envelope) plasmid contained only the env gene. The resulting vector system was as effective at integrating the transgene into dividing cells and at sustaining transgene expression as were earlier vector systemsFootnote 10. Furthermore, since at least two recombination events would be required for Ψ, gag, pol, and env to be located together on the same plasmid, RCR formation was less likely, which improved the biosafety of this system. However, the use of MLV demonstrated poor efficiency of gene transfer in human systems, most likely due to these vectors only being able to integrate into dividing cells and inactivation by the human complement systemFootnote 12,Footnote 13.
2.2.2 First generation lentiviral vector systems
Lentiviral-based vector systems were developed to overcome the deficits of early MLV-based retroviral systems. Unlike simple retroviral vectors, HIV-1 and other lentiviruses are able to infect both dividing and non-dividing cells due to their complex genomes that contain additional regulatory and accessory genes (Figure 2-3, panel A). This broader capacity made lentiviruses promising candidates for gene therapy applications, but their parental origin raised many safety concerns that prevented them from undergoing human testing.
Figure 2-3: Genome of HIV-1 and the evolution to 3rd generation lentiviral vector systems
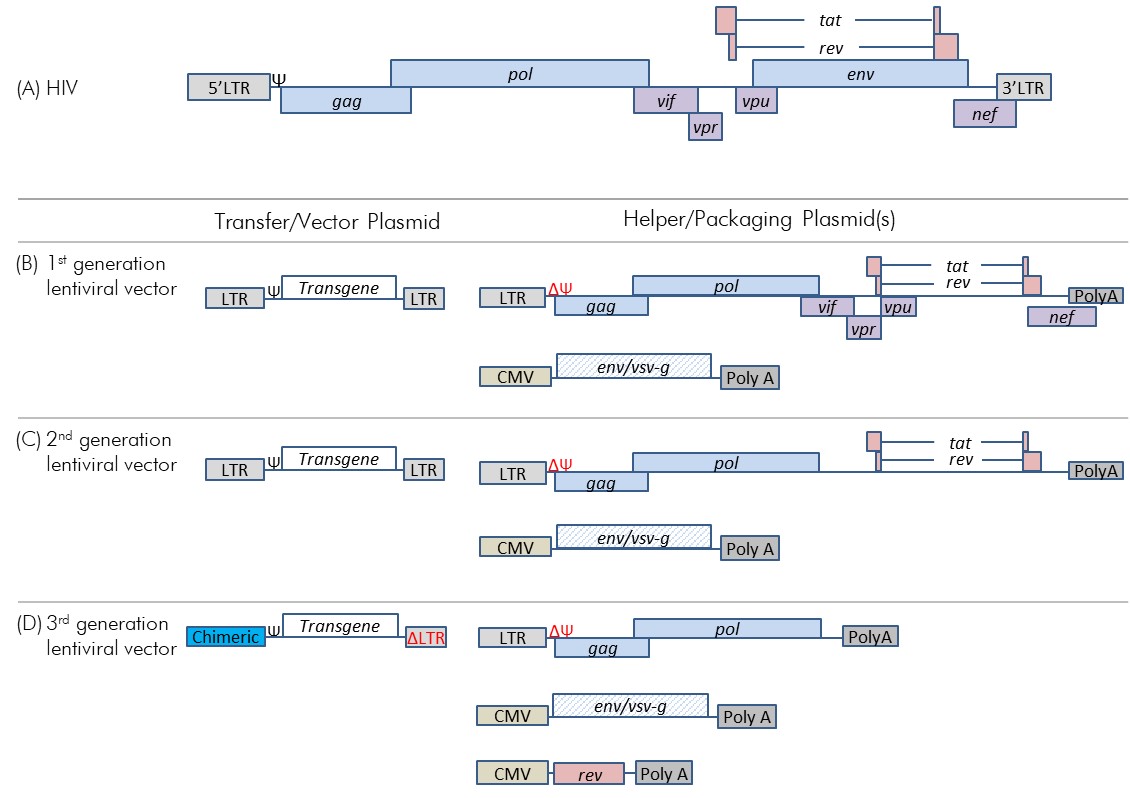
Figure 2-3: Genome of HIV-1 and the evolution to 3rd generation lentiviral vector systems - Text Equivalent
- Figure depicting the genetic organization of HIV genes, which contains the common retroviral genes presented in figure 2-2 (A) (i.e., LTRs, psi signal, and gag, pol, and env genes); however, the gag gene overlaps slightly with pol . HIV-specific accessory gene vif overlaps with the end of pol, vpr overlaps with the end of vif, and vpu overlaps with the beginning of env. Nef overlaps with the end of env and the beginning of the 3’LTR. The regulatory genes tat and rev are splice products consisting of a first part from the vpr/vpu region and a 3’ part from the end of env.
- Figure depicting the gene sequence for the first generation of lentiviral vector. This system consists of a transfer/vector plasmid and two helper/packaging plasmids. The transfer/vector plasmid consists, in order, of a 5’LTR, the psi signal, the transgene, and a 3’LTR. One helper/packaging plasmid is similar to the plasmid depicted in (A); however, the psi signal and env gene have been removed, and the 3’LTR replaced with polyA. The other helper/packaging plasmid contains, in order, a CMV promoter, and env gene pseudotyped to vsv-g, and a 3’ poly A.
- Figure depicting the gene sequence for the second generation of lentiviral vector. This system consists of a transfer/vector plasmid and two helper/packaging plasmids. This system is identical to the one presented in (B), with the exception that the HIV accessory genes (vif, vpr, vpu, and nef) have been removed. The second helper/packaging plasmid is identical to the one presented in (B).
- Figure depicting the gene sequence for the third generation of lentiviral vector. This system consists of a transfer/vector plasmid and three helper/packaging plasmids. The transfer/vector plasmid includes, in order, a chimeric 5’ LTR, the psi signal, and the transgene. Part of the 3’ LTR has been deleted. The first helper/packaging plasmid is similar to the one depicted in (C) (i.e., consists of 5’ LTR, gag, pol, and 3’ poly A); however, the regulatory genes (tat, rev) have been removed. The second helper/packaging plasmid is identical to the second plasmid depicted in (B) (i.e., CMV promoter, env/vsv-g, and 3’ poly A). The third plasmid consists of a CMV promoter, the rev regulatory gene, and a 3’ poly A.
(A) The HIV genome with common retroviral genes (blue) and HIV-specific genes (purple and pink); note that the figure is not to scale and only meant to show the relative location of genes. (B) A first generation three-plasmid lentiviral vector system. (C) A second generation three-plasmid lentiviral vector system. (D) A third generation four-plasmid lentiviral vector system.
The first generation lentiviral vector systems were three-plasmid systems derived from HIV, and included the standard retroviral genes as well as HIV-specific genes (Figure 2-3 panel B).Footnote 14 These first generation lentiviral vector systems were composed of (1) a Ψ–containing transfer plasmid; (2) a packaging plasmid that, in addition to the gag and pol, contains the HIV accessory genes nef, vif, vpu, and vpr and regulatory genes tat and rev; and (3) an envelope plasmid. To increase the tropism, or cellular targets, of the HIV-based lentiviral vector systems, the HIV env gene in the envelope plasmid may be replaced with heterologous glycoprotein genes, a process called pseudotyping; lentiviral vectors are often pseudotyped with genes encoding vesicular stomatitis virus glycoproteins (VSV-G).Footnote 3,Footnote 4, While pseudotyping improves vector stability, altering the tropism of the lentiviral vector to include virtually all mammalian cells increases the inherent risk associated with the lentiviral vectorFootnote 4. Also, since the pseudotyped vector is an animal pathogen as well as a human pathogen, it is subject to regulation by the Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA).
2.2.3 Second generation lentiviral vector systems
To further improve the level of lentiviral vector system safety, the HIV-specific accessory genes (nef, vif, vpu, and vpr) that contribute to pathogenesis were removed from the helper/packaging plasmid in second generation HIV-based vectors (Figure 2-3 panel C). By reducing the number of lentivirus genes from nine to four, the chance of RCR formation was significantly reduced even if multiple recombination events were to occur.
3.2.4 Third generation and higher lentiviral vector systems
Third generation lentiviral vector system developments were directed toward safety improvements and reduction in the risk of RCR formation. The 5' LTR was modified into a chimeric 5’ LTR with a heterologous promoter that is no longer dependent on tat transactivation. Since tat was no longer required, it was removed from the third generation packaging plasmid. In addition, the remaining HIV packaging genes were separated into two packaging plasmids (one with gag and pol, and a second with rev). Along with the transfer and envelope plasmids, the resulting four-plasmid system contains as few as three HIV-specific genes and has significantly reduced RCR formation capability compared with earlier generations (Figure 2-3 panel D). In addition, some third generation systems have a deletion within the 3' LTR in the transfer plasmid, which is transcribed to the 5' LTR during reverse transcription; this results in a self-inactivating vector due to reduced promoter activity. This modification is particularly useful since it addresses biosafety issues that include recombination between the transfer and helper plasmids, insertional oncogenesis due to LTRs, and mobilization of the RCR upon subsequent infection with replication competent HIV-1Footnote 4,Footnote 15.
Developing fourth generation lentiviral vector systems with improved biosafety has been the aim of multiple scientific studies. In one, a transfer plasmid was reconfigured to prevent transcription of some HIV-1 components into the transduced transgene. This was accomplished in part by removing the 5' LTR, and placing RNA signals (e.g., Ψ, Rev-response element) downstream of a self-inactivating 3' LTRFootnote 16. The transduction efficiency of this lentiviral vector system is currently lower than standard technology, so it has yet to be implemented commercially. Separately, the packaging plasmids of a lentiviral vector system were divided into five separate plasmids, in part by separating gag and pol genes, but also by reintroducing the tat gene.Footnote 17 This latter system, as well as several second and third generation systems, remain in use and are available from commercial sources.
While it is highly unlikely, the risk of an RCR forming when using a second or third generation vector system remains. An RCR that contains HIV genes could demonstrate the viral attributes of HIV if it were introduced into a human or animal hostFootnote 18. Monitoring for RCR formation during vector development and manufacturing provides a means to detect its presence before the vector is put to use.
Chapter 3 - Risk assessment
Biosafety involves the consistent application of safety measures (i.e., physical containment features and operational practices) that are crucial to prevent harm to personnel, the community, and the environment that could result from exposure to the pathogens handled within a facility, or their release from containment. The appropriate mitigation measures for a given pathogen, containment zone, work area, or procedure are based on risk assessments, which are the foundation for all components of a biosafety program. This chapter describes considerations for conducting a pathogen risk assessment and an LRA, specific to work with lentiviral vectors.
3.1 Pathogen risk assessment
A pathogen’s risk group is determined through a pathogen risk assessment that evaluates the inherent characteristics that contribute to the pathogen’s risk by considering factors such as pathogenicity, availability of effective preventive and therapeutic treatments, and communicability. The pathogen risk assessment will define the risk group, which helps to determine the appropriate containment level, and is taken into account in LRAs.
Routes of infection for lentiviral vectors are similar to those of HIV and include injection and exposure of mucosal surfaces or broken skin with infectious liquids or aerosols. Exposure to intact skin is not considered a significant risk of infection.Footnote 18, Effective treatment and preventive measures are available for lentiviruses, and the risk of spread of disease caused by these pathogens is low.
The PHAC has determined that lentiviral vectors meet the definition of RG2 human and RG2 animal pathogens. RG2 pathogens are those that pose a moderate risk to the health of individuals or animals, and a low risk to public health. RG2 pathogens are able to cause serious disease in a human or animal but are unlikely to do so. However, under certain conditions (e.g., use of first generation lentiviral vector systems, a modification of the vector, insertion of an oncogenic or toxic transgene), a resultant increased risk of handling these lentiviral vectors may require additional or modified biosafety procedures, or result in a higher risk group classification (i.e., RG3).
Individuals are encouraged to conduct their own pathogen risk assessments on the lentiviral vector system intended for use, particularly when using first generation systems, or when inserting oncogenic or toxic transgenes; completed risk assessments may be submitted to the PHAC for validation. More information on pathogen risk assessments can be found in the Canadian Biosafety Guideline - Pathogen Risk Assessment, and a pathogen risk assessment template is available on the Government of Canada websiteFootnote 19,Footnote 20.
The licence holder and BSO must be notified in writing if a pathogen risk assessment results in an increase of the lentiviral vector system to RG3 or if experimental evidence indicates that there is a high likelihood that the lentiviral vector will recombine to produce replication competent HIV (HPTR 5a). In such a case, an RG3 pathogen and toxin licence (hereafter referred to as “licence”) issued by the PHAC is required. The licence will specify that all activities with the lentiviral vector system are to be performed in accordance with the Biosafety Directive for Human Immunodeficiency Virus (HIV), Human T-cell Lymphotropic Virus Type 1 (HTLV-1), and Related Simian Retroviruses(i.e., may be performed at Containment Level 2 (CL2) with additional precautions)Footnote 21.
3.1.1 Pathogen risk assessment considerations for lentiviral vectors
The pathogen risk assessment starts by identifying the characteristics that are intrinsic to the pathogen itself and that contribute to its risk. Though not an exhaustive list, the following are considerations for lentiviral vectors:Footnote 4,Footnote 8,Footnote 22,Footnote 23,Footnote 24,Footnote 25
- The nature of the transgene insert. Known oncogenes or genes with high oncogenic or toxic potential may require additional biosafety practices.
- The possibility of alternate routes of infection, depending on the lentiviral vector. Pseudotyping with vsv-g can enable infection by inhalation under some conditions.
- The possibility of recombination during manufacture, which may lead to RCR. Despite the low probability, monitoring vector preparations for the presence of RCR will assess this. Use of third generation systems (i.e., low RCR formation potential) is recommended.
- Following exposure, there is a potential for oncogenesis due to insertional mutagenesis or transactivation of adjacent gene sequences resulting from the integration of viral DNA into the host genome.
- The possible mobilization (i.e., rescue) by wild type virus (e.g., HIV) and spread of mobilized vector particles to non-target cells or tissues (i.e., particularly with vectors that retain the complete LTR).
- The possibility of recombination with wild type virus in exposed HIV-positive individuals or lentivirus-positive animal hosts, whose native virus could recombine with or complement the vector.
- The possibility of productive infection in experimental animals injected with or exposed to RCR-contaminated vectors that could result in vector/virus shedding.
- The potential for lentiviral vectors to become capable of infecting a broader range of human and mammalian cells as a result of pseudotyping of the viral particles or alteration of the viral promoter sequences.
- Despite being replication incompetent, the infectivity of lentiviral vectors remains. Lentiviral vectors are capable of infecting target cells, which can then express the transgene.
The risk group of the vector-transgene combination will determine the appropriate physical containment requirements and operational practice requirements for safely handling the lentiviral vector. Even in cases where the lentiviral vector remains an RG2 pathogen, the risk assessment may identify additional mitigation measures needed for safe handling.
3.2 Local risk assessment
LRAs are site-specific assessments of risks related to activities involving pathogens and toxins. They are used to:
- identify and characterize hazards associated with the activities (i.e., tasks and procedures) being performed with infectious material or toxins;
- assess the risks for each hazard based on the likelihood of incidents occurring (e.g., exposure, release, or loss of pathogens and toxins), and the consequences of those incidents; and,
- develop and implement mitigation measures (e.g., standard operating procedures [SOPs] for safe work practices).
3.2.1 Local risk assessment considerations for lentiviral vectors
Since the most likely routes of laboratory exposure to lentiviral vectors are injection and exposure of mucous membranes, key hazards to consider when performing LRAs for work involving lentiviral vectors include:Footnote 18
- Use of sharps: The primary route of infection of lentiviral vectors is inoculation. The accidental injection of a lentiviral vector could result in HIV-positive serological status, transgene expression (the same is true for other lentiviral vectors and retroviruses), or insertional mutagenesis.
- Handling cell cultures transduced with lentiviral vectors: The integration of lentiviral vectors into the cell genome can have harmful effects on the transduced cells including an increased risk of tumour formation, insertional mutagenesis, and transactivation of neighbouring genes.
- Handling lentiviral vector suspensions: Handling high titre stocks of lentiviral vector suspensions increases the possibility of personnel exposure.
- Aerosol-generating procedures: Indirect contact with droplets produced from aerosol-generating procedures (e.g., centrifugation, pipetting) can result in droplet transmission (i.e., the depositing of infectious agents onto surfaces and subsequent transfer to exposed mucosal surfaces of the recipient).
- Work with animals: Evidence indicates that vectors may be shed from infected animals for over 24 hours post-infection, leading to the possibility of transmission to personnel through direct contact of mucous membranes with infected body fluids.
Table 3-1 outlines some of the factors to consider when performing risk assessments related to the use of lentiviral vectors, and how each factor contributes to increasing or decreasing the overall risk.
| Pathogen Risk Assessment | ||
|---|---|---|
| Lower Risk | Higher risk | |
| Transgene |
|
|
| Vector design |
|
|
| Local Risk Assessment | ||
| Lower Risk | Higher risk | |
| Animals |
|
|
| Production |
|
|
| Manipulations |
|
|
Chapter 4 - Considerations for containment
4.1 Containment level requirements
Based on the pathogen risk assessments completed by the PHAC, it has been determined that all activities with lentiviral vector systems, including propagative in vitro and in vivoactivities, can be safely conducted at CL2 or in a CL2 large animal containment zone (i.e., CL2-agriculture [CL2-Ag]). CL2 is also appropriate in large-scale production areas (>10 L). Applicability of large-scale production area requirements can be determined in consultation with the PHAC and the CFIA on a case-by-case basis. However, under certain conditions (e.g., use of first or second generation lentiviral vector systems) additional operational requirements at CL2 or CL2-Ag may be required to prevent exposure of personnel and the spread of contamination outside the containment zone (e.g., in accordance with the Biosafety Directive for Human Immunodeficiency Virus (HIV), Human T-cell Lymphotropic Virus Type 1 (HTLV-1), and Related Simian Retroviruses).Footnote 26
4.2 Work practices
Operational practices refer to the administrative and procedural controls, including SOPs, in place to prevent the inadvertent exposure of personnel to potentially infectious material, and the release of pathogens and toxins from containment. Personnel are more likely to follow operational practices when they are documented in SOPs and included in personnel training. Operational practice requirements for CL2 and CL2-Ag are specified in Chapter 4 of the CBS.
In areas where lentiviral vectors are handled or stored, safe work practices include the proper use, and maintenance of, laboratory and biosafety equipment (e.g., centrifuges, biological safety cabinets [BSCs]), and the use of good microbiological laboratory practices.
The use of a BSC or other primary containment device will protect personnel against exposure (e.g., via mucous membranes contact) by containing aerosols during procedures that may result in the production of infectious aerosols, or involve high concentrations or large volumes of lentiviral vectors. The use of sealed safety cups or rotors that are unloaded in a BSC will contain aerosols that may be generated when centrifuging lentiviral vectors.
The main risk of transmission of lentiviral vectors to personnel is through accidental inoculation, cuts or punctures with contaminated instruments, and contact with open wounds. Avoiding the use of needles and other sharps will reduce this risk. In the event that the use of sharps is unavoidable, appropriate SOPs can be developed that limit, or offer proper handling instructions regarding the use of sharps so that the instruments are used in a manner that prevents accidental inoculations.4 Safety-engineered sharps and needles that reduce the risk may also be applicable.
When used appropriately, personal protective equipment (PPE), such as gloves, lab coats, and safety glasses, protect workers from infectious material with which they may come into contact in the course of performing their work. In addition to the PPE required for CL2 and CL2-Ag, as indicated in Matrix 4.4 of the CBS, an LRA may indicate the need for additional PPE. For example, an LRA may determine that a respirator, eye protection, or face shield be worn during procedures likely to generate aerosols.Footnote 4
4.3 Animal work considerations
Pathogen and toxin work performed in vivo (i.e., in living animals) is carried out in an animal containment zone. An animal containment zone refers to a series of co-located animal rooms or animal cubicles, as well as associated corridors and support rooms (e.g., storage and preparation areas) of equal containment level. More detailed information on animal containment zones can be found in the CBS and CBH.Footnote 1,Footnote 2
The delivery of lentiviral vectors or lentiviral vector-transduced cells into an animal must be performed at the appropriate containment level. Animal hosts that are permissive for HIV replication, or those that have been engrafted with human cells, can support replication of infectious HIV, and hence additional precautions may need to be in place. Given the increased hazard posed by animal inoculation, it is important that laboratory personnel handle these materials in a manner that minimizes the risk of incidents, as determined by an LRA. For example, to minimize the risk of self-inoculation, required safety procedures (e.g., animal restraint) may be enhanced with additional precautions and PPE (e.g., puncture-resistant gloves). Where it is not feasible to perform injections in a BSC, additional PPE (e.g., goggles, respirators) may be needed to reduce the possibility of mucosal exposure to aerosols. Following inoculation, disinfecting or cleaning the animal or the site of inoculation will eliminate any lentiviral vector that may remain on the surface of the animal.Footnote 4,Footnote 6
4.3.1 Testing for replication-competent retrovirus
The PHAC’s pathogen risk assessments for lentiviral vector systems clearly indicate that lentiviral vector systems have the potential to form RCR and the potential for oncogenesis following integration. This risk is higher in earlier generation systems; to date, there have been no reports of RCR formation using a third-generation lentiviral vector system. RCR testing during vector manufacture will aid in confirming the presence or absence of RCR; positive detection may negate the vectors subsequent use.Footnote 3,Footnote 27 Contact Health Canada’s Biologics and Genetic Therapies Directorate or the CFIA’s Canadian Centre for Veterinary Biologics for more information on requirements for RCR testing of lentiviral vectors systems for use in humans or animals clinical applications (respectively)Footnote 28,Footnote 29.
It was also found that, following inoculation and until viral clearance, the vector could be shed from infected animals and transmitted to humans through direct contact of mucous membranes with infected body fluidsFootnote 30. Laboratory personnel could also be exposed through accidental injection of the vector preparation. In both cases, the nature of the inserted gene (e.g., oncogene) will be an important consideration to determine the effective physical containment and operational practices that will minimize the risk of exposure. Certain animals cannot support the replication of infectious HIV and are therefore considered to have negligible probability of RCR formation following injection with a lentiviral vector. In such cases, the probability for shedding of RCR is very low.
A risk assessment that considers RCR formation and shedding as well as other factors (e.g., potential exposure of the animal to higher risk pathogens or non-indigenous animal pathogens) may determine that it is permissible to decrease the containment level following vector delivery. For example, if an LRA determines that the likelihood of infection is extremely low or nonexistent and the site of inoculation has been cleaned and the bedding changed, it may be acceptable to move the animal to a lower containment level within a few days following inoculation (the timing of this will be determined by an LRA, and may range from one to seven days).Footnote 6 Similarly, animals that have tested negative for RCR may be moved out of containment a few days following inoculation, provided that the risk assessment determines that there is little to no risk of infection or replication of an infectious virus, and that higher risk pathogens (including non-indigenous animal pathogens) are not handled within the same containment zoneFootnote 4,Footnote 6.
Currently, the most common approach of testing for RCR involves a cell culture based method followed by endpoint detection of RCR components, which can be achieved by polymerase chain reaction (PCR), assays for detection of reverse transcriptase activity, or antigen assaysFootnote 27. Notably, some RCR detection assays use an RG3 positive control, thus increasing the risk of incidents to personnel, and requiring a valid RG3 licence from the PHAC.
4.4 Post-exposure prophylaxis
Prior to working with lentiviral vectors, a post-exposure response plan that addresses the specifics of the planned lentiviral vector system and activities should be in place as a component of the medical surveillance plan.Footnote 18 In case of needlestick or sharps injury with first or second generation lentiviral vectors, prompt administration of post-exposure prophylaxis (e.g., within one hour) will minimize the already low risk of HIV infection.Footnote 18 The risks of transduction with potentially oncogenic or toxic genes, or insertional mutagenesis have not been clinically proven to be prevented by HIV prophylaxis.Footnote 18 However, antiretroviral drugs (e.g., azidothymidine [AZT]) are capable of blocking transduction completely in cell culture.Footnote 8
4.5 Decontamination and waste management
The effective decontamination of waste, materials, equipment, and surfaces that have come in contact with potentially infectious material or toxins is fundamental in limiting the spread of contamination beyond the work area and facility. Decontamination technologies may be provided on site, or contaminated waste can be appropriately packaged and transported to a designated facility for decontamination.
4.5.1 Decontamination of lentiviral vectors
Lentiviral vectors are labile, so surfaces can be easily decontaminated by a variety of chemicals, including 5,000 ppm sodium hypochlorite (about 1/10 dilution of commercial bleach) or 70% ethanol, with a sufficient contact time. If bleach is used, fresh dilutions should be prepared and the residue must be rinsed off with water to prevent corrosion of surfaces (e.g., stainless steel).Footnote 31 Liquid waste can be decontaminated by autoclaving or mixing with bleach to a final concentration of 5,000 ppm sodium hypochlorite and leaving for at least 30 minutes. Solid waste can be autoclaved or incinerated.Footnote 5
Decontaminating (and labelling as decontaminated) all materials that may have been in contact with lentiviral vectors prior to removal from the containment zone will prevent contaminated material from leaving the containment zone. If the material is to be decontaminated off-site, it can be placed in a closed, labelled, leak-proof container that has been surface decontaminated.
Chapter 5 - Glossary
Most of the following list is derived from the CBS and the CBH. It is important to note that while some of the definitions provided in the glossary are universally accepted, many of them were developed specifically for the CBS or the CBH; therefore, some definitions may not be applicable to facilities that fall outside of the scope of the CBS and the CBH. A comprehensive list of terms and their definitions can be found in the Glossary in Chapter 24 of the CBH.
- Animal cubicle
- A room or space designed to house an animal (or animals) where the room itself serves as primary containment. These spaces are used to house large-sized animals (e.g., livestock, deer), or small-sized animals that are housed in open caging (i.e., not primary containment caging).
- Animal pathogen
- Any pathogen that causes disease in animals, including those derived from biotechnology. In the context of the CBS, "animal pathogen" refers only to pathogens that cause disease in terrestrial animals; including those that infect avian and amphibian animals, but excluding those that cause disease in aquatic animals and invertebrates.
- Animal room
- A room designed to house animals in primary containment caging. These spaces are used to house only small-sized animals (e.g., mice, rats, rabbits).
- Biological safety cabinet (BSC)
- A primary containment device that provides protection for personnel, the environment and the product (depending on BSC class), when working with biological material.
- Biosafety
- Containment principles, technologies, and practices that are implemented to prevent unintentional exposure to infectious material and toxins, or their accidental release.
- Containment
- The combination of physical design parameters and operational practices that protect personnel, the immediate work environment, and the community from exposure to biological material. The term "biocontainment" is also used in this context.
- Containment level (CL)
- Minimum physical containment and operational practice requirements for handling infectious material or toxins safely in laboratory, large scale production, and animal work environments. There are four containment levels ranging from a basic laboratory (CL1) to the highest level of containment (CL4).
- Containment zone
- A physical area that meets the requirements for a specified containment level. A containment zone can be a single room (e.g., CL2 laboratory), a series of co-located rooms (e.g., several non-adjoining but lockable CL2 laboratory work areas), or it can be comprised of several adjoining rooms (e.g., CL3 suite with dedicated laboratory areas and separate animal rooms, or animal cubicles). Dedicated support areas, including anterooms (with showers and "clean" and "dirty" change areas, where required), are considered to be part of the containment zone.
- Good microbiological laboratory practices
- A basic laboratory code of practice applicable to all types of activities with biological material. These practices serve to protect workers and prevent contamination of the environment, and the samples in use.
- Handled or stored
- "Handling or storing" pathogens, toxins, or infectious material includes possessing, handling, using, producing, storing, permitting access to, transferring, importing, exporting, releasing, disposing of, or abandoning such material. This includes all controlled activities involving human pathogens and toxins specified in Section 7(1) of the HPTA.
- Incident
- An event or occurrence with the potential of causing injury, harm, infection, intoxication, disease, or damage. Incidents can involve infectious material, infected animals, or toxins, including a spill, exposure, release of infectious material or toxins, animal escape, personnel injury or illness, missing infectious material or toxins, unauthorized entry into the containment zone, power failure, fire, explosion, flood, or other crisis situations (e.g., earthquake, hurricane). Incidents include accidents and near misses.
- In vitro
- Latin for "within glass"; describes experimentation involving components of a living organism within an artificial environment (e.g., manipulation of cells in petri dish), including activities involving cell lines or eggs.
- In vivo
- Latin for "within the living"; describes experimentation conducted within the whole living organism (e.g., studying the effect of antibiotic treatment in animal models).
- Large animal containment zone (LA zone)
- Animal containment zone comprised of two or more co-located or adjoining rooms of equal containment level where animals are housed in animal cubicles (i.e., the room itself provides the primary containment). An LA zone may include, for example, large-sized animals, such as livestock or deer, housed in cubicles or, cubicles where small-sized animals, such as mice or raccoons, are housed in open caging (i.e., not primary containment caging). Post mortem rooms, where present, are considered to be part of an LA zone.
- Local risk assessment (LRA)
- Site-specific risk assessment used to identify hazards based on the infectious material or toxins in use and the activities being performed. This analysis provides risk mitigation and risk management strategies to be incorporated into the physical containment design and operational practices of the facility.
- Non-indigenous animal pathogens
- A pathogen that causes an animal disease listed in the World Organization for Animal Health's OIE-Listed diseases, infections and infestations (as amended from time to time) and that is exotic to Canada (i.e., foreign animal disease agents that are not present in Canada), or any other animal disease agent that is not indigenous to Canada, as determined by the CFIA. These pathogens may have serious negative health effects to the Canadian animal population.
- Oncogene
- A mutated form of a gene involved in normal cell growth whose activation is associated with the conversion of normal cells into cancer cells.
- Operational practice requirements
- Administrative controls and procedures followed in a containment zone to protect personnel, the environment, and ultimately the community, from infectious material or toxins, as outlined in Chapter 4 of the CBS.
- Pathogen
- A microorganism, nucleic acid, or protein capable of causing disease or infection in humans or animals. Examples of human pathogens are listed in Schedules 2 to 4 and in Part 2 of Schedule 5 of the HPTA,but these are not exhaustive lists.
- Pathogenicity
- The ability of a pathogen to cause disease in a human or animal host.
- Pathogen risk assessment
- The determination of the risk group and appropriate physical containment requirements and operational practice requirements needed to safely handle the infectious material or toxins in question.
- Personal protective equipment (PPE)
- Equipment and/or clothing worn by personnel to provide a barrier against infectious material or toxins, thereby minimizing the risk of exposure. PPE may include, but is not limited to, lab coats, gowns, full-body suits, gloves, protective footwear, safety glasses, safety goggles, masks, and respirators.
- Physical containment requirements
- Physical barriers in the form of engineering controls and facility design used to protect personnel, the environment, and ultimately the community, from pathogens or toxins, as outlined in Chapter 3 of the CBS.
- Primary containment device
- Apparatus or equipment that is designed to prevent the release of infectious material or toxins and to provide primary containment (i.e., provide a physical barrier between the individual and/or the work environment and the biological material). Examples of primary containment devices include biological safety cabinets, isolators, centrifuges with sealable cups, process equipment, fermenters, microisolator cages, and ventilated cage racks.
- Risk group (RG)
- The classification of biological material based on its inherent characteristics, including pathogenicity, virulence, risk of spread, and availability of effective prophylactic or therapeutic treatments, that describes the risk to the health of individuals and the public as well as the health of animals and the animal population.
Chapter 6 - References and Resources
- Amado, R. G., & Chen, I. S. Y. (1999). Lentiviral vectors — the promise of gene therapy within reach? Science, 285(5428): 674-676.
- Cockrell, A. S., Ma, H., Fu, K., McCown, T. J., & Kafri, T. (2016). A trans-lentiviral packaging cell line for high-titer conditional self-inactivating HIV-1 vectors. Molecular Therapy, 14: 276–284.
- Canadian Food Inspection Agency. Licensing Requirements for Veterinary Biologics – Overview. Retrieved 05/16, 2019 from http://www.inspection.gc.ca/animals/veterinary-biologics/guidelines-forms/4-5e/eng/1318508906578/1318509047147
- Coffin, J. M., Hughes, S. H., & Varmus, H. E. (1997). Retroviruses. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press.
- Curiel, D. T., & Douglas, J. T. (2005). Cancer Gene Therapy (pp.467). Totowa, NJ, USA: Humana Press.
- Debyser, Z. (2003). Biosafety of lentiviral vectors. Current Gene Therapy, 3(6): 517-525.
- Escors, D., & Breckpot, K. (2010). Lentiviral vectors in gene therapy: their current status and future potential. Archivum Immunologiae et Therapie Experimentalis, 58(2): 110-119.
- Evans, J. T., & Garcia, J. V. (2000). Lentivirus vector mobilization and spread by human immunodeficiency virus. Human Gene Therapy, 11(17): 2331-2339.
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.) Ottawa, ON, Canada: Government of Canada. Retrieved 22/10, 2018 from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines.html
- Government of Canada. (2016). Canadian Biosafety Handbook (2nd ed.). Ottawa, ON, Canada: Government of Canada. Retrieved 22/10, 2018 from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines.html
- Government of Canada. (2018). Canadian Biosafety Guideline on Pathogen Risk Assessment. Retrieved on 22/10, 2018 from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance/pathogen-risk-assessment.html
- Government of Canada. (2019). Biosafety Directive for Human Immunodeficiency Virus (HIV), Human T-lymphotropic Virus (HTLV), and Related Simian Retroviruses. Retrieved 07/22, 2017 from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosafety-directives-advisories-notifications/human-immunodeficiency-virus-hiv-human-t-cell-lymphotropic-virus-type-1-htlv-1-and-related-simian-retroviruses.html
- Government of Canada. Biologics and Genetic Therapies Directorate. Retrieved 05/16, 2019 from https://www.canada.ca/en/health-canada/corporate/about-health-canada/branches-agencies/health-products-food-branch/biologics-genetic-therapies-directorate.html
- Government of Canada. Pathogen Risk Assessment Template. Retrieved 22/10, 2018 from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/pathogen-risk-assessment-template.html
- Health of Animals Act (S.C. 1990, c.21).
- Health of Animals Regulations (C.R.C., c.296).
- Human Pathogens and Toxins Act (S.C. 2009, c.24).
- Human Pathogens and Toxins Regulations (SOR/2015-44).
- Johnson, L. G., Olsen, J. C., & Boucher, R. C. (2000). Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Therapy, 7(7): 568-574.
- Kappes, J. C., & Wu, X. (2001). Safety considerations in vector development. Somatic Cell and Molecular Genetics, 26(1-6): 147-158.
- Matrai, J., Chuah, M. K., & VandenDriessche, T. (2010). Recent advances in lentiviral vector development and applications. Molecular Therapy: The Journal of the American Society of Gene Therapy, 18(3): 477-490.
- Miller, D. G, Adam, M. A., & Miller, A. D. (1990). Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Molecular Cell Biology, 10(8): 4239‑4242.
- Nakagawa, T., & Hoogenraad, C. C. (2011). Lentiviral transgenesis. Methods in Molecular Biology, 693: 117-142.
- Naldini, L., Blömer, U., Gallay, P., Ory, D., Mulligan, R. et al. (1996). In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science, 272(5259): 263-267.
- Oregon Health and Science University. (2010). Lentiviral Biosafety Manual. Retrieved 02/01, 2018 from http://www.ohsu.edu/xd/about/services/integrity/policies/upload/Template-Lentivirus-Biosafety-Manual.doc
- Pauwels, K., Gijsbers, R., Toelen, J., Schambach, A., Willard-Gallo, K. et al. (2009). State-of-the-art lentiviral vectors for research use: risk assessment and biosafety recommendations. Current Gene Therapy, 9(6): 459-474.
- Reuter, J. D., Fang, X., Ly, C. S., Suter, K. K., & Gibbs, D. (2012). Assessment of hazard risk associated with the intravenous use of viral vectors in rodents. Comparative Medicine, 62(5): 361-370.
- Schlimgen, R., Howard, J., Wooley, D., Thompson, M., Baden, L. R., et al. (2016). Risks associated with lentiviral vector exposures and prevention strategies. Journal of Occupational and Environmental Medicine, 58(12): 1159-1166.
- Soneoka, Y., Cannon, P. M., Ramsdale, E. E., Griffiths, J. C., Romano, G., et al. (1995). A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Research, 23(4): 628-633.
- Takeuchi, Y., Cosset, F. L., Lachmann, P. J., Okada, H., Weiss, R. A., & Collins, M. K. (1994). Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. Journal of Virology, 68: 8001-8007.
- Templeton, N. S. (2009). Gene and Cell Therapy: Therapeutic Mechanisms and Strategies (3rd ed. pp.215). Boca Raton, FL, USA: CRC Press.
- United States Department of Health and Human Services, Food and Drug Administration. (2006). Guidance for industry – supplemental guidance on testing for replication competent retrovirus in retroviral vector-based gene therapy products and during follow-up of patients in clinical trials using retroviral vectors. Retrieved 7/24, 2017 from http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm078723.pdf
- United States National Institutes of Health. (2006). Biosafety considerations for research with lentiviral vectors – recombinant DNA advisory committee guidance document. Retrieved 7/21, 2016 from https://osp.od.nih.gov/wp-content/uploads/2013/12/Lenti_Containment_Guidance.pdf
- Vink, C. A., Counsell, J. R., Perocheau, D. P., Karda, R., Buckley, S. M. K., et al. (2017). Eliminating HIV-1 packaging sequences from lentiviral vector proviruses enhances safety and expedites gene transfer for gene therapy. Molecular Therapy, 25(8): 1790-1804.
References
- Footnote 1
-
Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.) Ottawa, ON, Canada: Government of Canada. Retrieved 22/10, 2018 from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines.html
- Footnote 2
-
Government of Canada. (2016). Canadian Biosafety Handbook (2nd ed.) Ottawa, ON, Canada: Government of Canada. Retrieved 22/10, 2018 from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines.html
- Footnote 3
-
Escors, D., & Breckpot, K. (2010). Lentiviral vectors in gene therapy: their current status and future potential. Archivum Immunologiae et Therapiae Experimentalis, 58(2): 107-119.
- Footnote 4
-
Pauwels, K., Gijsbers, R., Toelen, J., Schambach, A., Willard-Gallo, K., et al. (2009). State-of-the-art lentiviral vectors for research use: risk assessment and biosafety recommendations. Current Gene Therapy, 9(6): 459-474.
- Footnote 5
-
Nakagawa, T., & Hoogenraad, C. C. (2011). Lentiviral transgenesis. Methods in Molecular Biology, 693: 117-142.
- Footnote 6
-
United States National Institutes of Health. (2006). Biosafety considerations for research with lentiviral vectors – recombinant DNA advisory committee guidance document. Retrieved 7/21, 2016 from https://osp.od.nih.gov/wp-content/uploads/2013/12/Lenti_Containment_Guidance.pdf
- Footnote 7
-
Coffin, J. M., Hughes, S. H., & Varmus, H. E. (1997). Retroviruses. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press.
- Footnote 8
-
Debyser, Z. (2003). Biosafety of lentiviral vectors. Current Gene Therapy, 3(6): 517-525.
- Footnote 9
-
Amado, R. G., & Chen, I. S. Y. (1999). Lentiviral vectors — the promise of gene therapy within reach? Science, 285(5428): 674-676.
- Footnote 10
-
Templeton, N. S. (2009). Gene and Cell Therapy: Therapeutic Mechanisms and Strategies (3rd ed. pp.215). Boca Raton, FL, USA: CRC Press.
- Footnote 11
-
Soneoka, Y., Cannon, P. M., Ramsdale, E. E., Griffiths, J. C., Romano, G., et al. (1995). A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Research, 23(4): 628-633.
- Footnote 12
-
Miller, D. G., Adam, M. A., & Miller, A. D. (1990). Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Molecular Cell Biology, 10(8): 4239-4242.
- Footnote 13
-
Takeuchi, Y., Cosset, F. L., Lachmann, P. J., Okada, H., Weiss, R. A., & Collins, M. K. (1994). Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. Journal of Virology, 68: 8001-8007.
- Footnote 14
-
Naldini, L., Blömer, U., Gallay, P., Ory, D., Mulligan, R. et al. (1996). In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science, 272(5259): 263-267.
- Footnote 15
-
Matrai, J., Chuah, M. K., & VandenDriessche, T. (2010). Recent advances in lentiviral vector development and applications. Molecular Therapy: The Journal of the American Society of Gene Therapy, 18(3): 477-490.
- Footnote 16
-
Vink, C. A., Counsell, J. R., Perocheau, D. P., Karda, R., Buckley, S. M. K., et al. (2017). Eliminating HIV-1 packaging sequences from lentiviral vector proviruses enhances safety and expedites gene transfer for gene therapy. Molecular Therapy, 25(8): 1790-1804.
- Footnote 17
-
Cockrell, A. S., Ma, H., Fu, K., McCown, T. J., & Kafri, T. (2016). A trans-lentiviral packaging cell line for high-titer conditional self-inactivating HIV-1 vectors. Molecular Therapy, 14: 276–284.
- Footnote 18
-
Schlimgen, R., Howard, J., Wooley, D., Thompson, M., Baden, L. R., et al. (2016). Risks associated with lentiviral vector exposures and prevention strategies. Journal of Occupational and Environmental Medicine, 58(12): 1159-1166.
- Footnote 19
-
Government of Canada. (2018). Canadian Biosafety Guideline on Pathogen Risk Assessment. Retrieved on 22/10, 2018 from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance/pathogen-risk-assessment.html
- Footnote 20
-
Government of Canada. (2018). Pathogen risk assessment template. Retrieved on 22/10, 2018 from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/pathogen-risk-assessment-template.html
- Footnote 21
-
Government of Canada. (2017). Biosafety Directive for Human Immunodeficiency Virus (HIV), Human T-lymphotropic Virus (HTLV), and Related Simian Retroviruses. Retrieved 07/22, 2017 from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosafety-directives-advisories-notifications/human-immunodeficiency-virus-hiv-human-t-cell-lymphotropic-virus-type-1-htlv-1-and-related-simian-retroviruses.html
- Footnote 22
-
Curiel, D. T., & Douglas, J. T. (2005). Cancer Gene Therapy (pp.467). Totowa, NJ, USA: Humana Press.
- Footnote 23
-
Kappes, J. C., & Wu, X. (2001). Safety considerations in vector development. Somatic Cell and Molecular Genetics, 26(1-6): 147-158.
- Footnote 24
-
Johnson, L. G., Olsen, J. C., & Boucher, R. C. (2000). Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Therapy, 7(7): 568-574.
- Footnote 25
-
Evans, J. T., & Garcia, J. V. (2000). Lentivirus vector mobilization and spread by human immunodeficiency virus. Human Gene Therapy, 11(17): 2331-2339.
- Footnote 26
-
Government of Canada. (2017). Biosafety Directive for Human Immunodeficiency Virus (HIV), Human T-lymphotropic Virus (HTLV), and Related Simian Retroviruses. Retrieved 07/22, 2017 from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosafety-directives-advisories-notifications/human-immunodeficiency-virus-hiv-human-t-cell-lymphotropic-virus-type-1-htlv-1-and-related-simian-retroviruses.html
- Footnote 27
-
United States Department of Health and Human Services, Food and Drug Administration. (2006). Guidance for industry – supplemental guidance on testing for replication competent retrovirus in retroviral vector -based gene therapy products and during follow-up of patients in clinical trials using retroviral vectors. Retrieved 7/24, 2017 from http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm078723.pdf
- Footnote 28
-
Government of Canada. Biologics and Genetic Therapies Directorate. Retrieved 05/16, 2019 from https://www.canada.ca/en/health-canada/corporate/about-health-canada/branches-agencies/health-products-food-branch/biologics-genetic-therapies-directorate.html
- Footnote 29
-
Canadian Food Inspection Agency. Licensing Requirements for Veterinary Biologics – Overview. Retrieved 05/16, 2019 from http://www.inspection.gc.ca/animals/veterinary-biologics/guidelines-forms/4-5e/eng/1318508906578/1318509047147
- Footnote 30
-
Reuter, J. D., Fang, X., Ly, C. S., Suter, K. K., & Gibbs, D. (2012). Assessment of hazard risk associated with the intravenous use of viral vectors in rodents. Comparative Medicine, 62(5): 361-370.
- Footnote 31
-
Oregon Health and Science University. (2010). Lentiviral Biosafety Manual. Retrieved 02/01, 2018 from http://www.ohsu.edu/xd/about/services/integrity/policies/upload/Template-Lentivirus-Biosafety-Manual.doc