Pan-Canadian Action Plan on Antimicrobial Resistance: Year 1 Progress Report (June 2023 to May 2024)

Download the alternative format
(PDF format, 16.55 MB, 31 pages)
Organization: Public Health Agency of Canada
Related links
September 2024
Table of contents
- Introduction
- Pillar 1: Research and innovation
- Pillar 2: Surveillance
- Pillar 3: Stewardship
- Pillar 4: Infection prevention and control
- Pillar 5: Leadership
- Look ahead
- Glossary
Introduction
Antimicrobials are essential for preventing and treating infections caused by bacteria, viruses, parasites and fungi in humans, animals and plants/crops. The use of antimicrobials has contributed to the emergence and spread of antimicrobial resistance (AMR), a complex problem which threatens our ability to treat common infectious diseases and to perform life-saving interventions such as chemotherapy, caesarean sections, hip replacements, organ transplantations and other surgeries. In addition, drug-resistant infections can impact the health of animals and plants/crops, reduce farm productivity of the agriculture sectors and threaten food security.
The World Health Organization (WHO) has declared AMR as one of the top ten global health threats. An estimated 4.95 million deaths were associated with drug-resistant bacterial infections in 2019, including 1.27 million deaths directly attributable to bacterial AMR. A One Health approach to AMR is needed, which recognizes that the health of humans, animals, plants/crops and the wider environment are closely linked.
Canada is taking concrete actions to reduce the development and spread of AMR. Federal, provincial and territorial (FPT) governments acknowledge growing and sustained momentum is needed to mitigate AMR and ensure antimicrobials are used prudently and responsibly.
In June 2023, the Government of Canada, in collaboration with provinces and territories, published the Pan-Canadian Action Plan on Antimicrobial Resistance ("Action Plan"). This Action Plan is an ambitious and evergreen five-year (2023-2027) multi-jurisdictional and multi-sectoral plan to collectively combat AMR. The Action Plan is a shared commitment between FPT Ministers of Health and Agriculture that builds on the Pan-Canadian Framework for Action. It is designed to foster collaboration, innovation and effective interventions across Canada and internationally. Ten priority actions are guiding Canada's efforts across five pillars: research and innovation; surveillance; stewardship; infection prevention and control (IPC); and leadership (Figure 1). Along with the Action Plan, a Compendium was also published in June 2023, outlining activities that were underway across FPT governments that advance the priority actions.
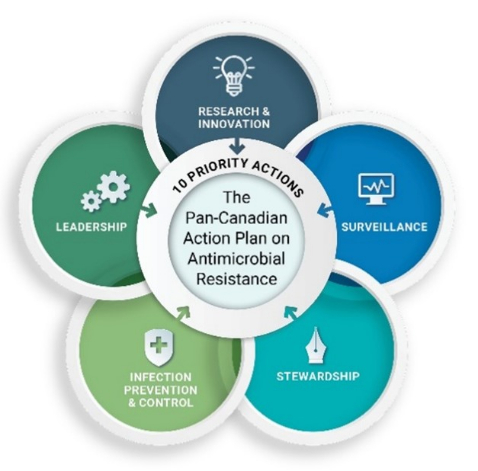
Figure 1: Text description
Figure 1 shows the Pan-Canadian Action Plan on Antimicrobial Resistance as composed of 5 interlocking pillars (Research and Innovation, Surveillance, Stewardship, Infection Prevention and Control, and Leadership) which outline 10 priority actions. The figure demonstrates that actions across pillars are mutually reinforcing and designed as a suite of actions that will together have the greatest impact in the Canadian context.
This Progress Report provides an overview of key FPT activities and milestones from June 1, 2023 to May 31, 2024, that advance the Action Plan. As the five-year plan is in progress, some priority actions are well underway while others are in early stages of implementation. As such, details on implementation progress in this report vary according to each priority action and tend to focus on milestone achievements in Year 1. Future reports will continue to capture progress across priority actions as they are implemented.
Pillar 1: Research and innovation
Priority actions
- Develop and implement economic and/or regulatory incentives to support innovation and facilitate sustainable access to new and existing antimicrobials, diagnostics, and alternatives to antimicrobials.
- Develop a One Health, national research strategy for combatting AMR across all Action Plan pillars.
The Action Plan highlighted the need for multi-disciplinary research and innovation on AMR to support mitigation efforts and improve access to antimicrobials and other health-related products. In the Government of Canada's 2023 budget, Canada committed to develop a pilot project to secure access to new antimicrobials for people in Canada. In response, the Public Health Agency of Canada (PHAC) commissioned the Council of Canadian Academies to provide an assessment of AMR pull incentive options in Canada, which was published in September 2023. This assessment informed the development of the Antimicrobial Economic Incentives Pilot Project that PHAC is undertaking with support from Health Canada (HC) and Public Services and Procurement Canada, as well as engagement with industry and provincial and territorial partners. The pilot is a three-year pull incentive project (expected to start in 2024-2025 fiscal and end in the 2026-2027 fiscal year) to improve access to antimicrobials that address unmet health priority needs for people in Canada. An Unmet Needs Survey was shared with clinicians, pharmacists, public health scientists and experts across Canada from September to November 2023. Results from the Unmet Needs Survey identified priority criteria that should be considered for AMR, the pathogens of priority and the most important drugs needed in clinical practice. The results are being used to inform the pilot design and target priority antimicrobial drugs needed in Canada.
To implement the second Research and Innovation priority action, the Canadian Institutes of Health Research (CIHR) and Agriculture and Agri-Food Canada (AAFC), with PHAC and Environment and Climate Change Canada (ECCC), are developing a National One Health AMR Research Strategy. This strategy will establish a set of AMR research priorities to build scientific knowledge across the five pillars of the Action Plan. Engagement with partners and outreach across the federal government is underway. The Research Strategy will enable increased coordination and collaboration on One Health AMR between partners.
Canada's Genomics Research and Development Initiative on AMR: GRDI AMR One Health
Research and innovation are central to a comprehensive, multi-sectoral approach to mitigating AMR. Genomic approaches (metagenomics, whole-genome sequencing and genomic epidemiology) are pivotal to understanding AMR dissemination through the One Health continuum and to identifying hotspots of AMR transmission to inform strategies to reduce the spread of AMR in Canada. The Genomics Research and Development Initiative (GRDI) supports genomics research to address AMR issues and has provided funding for a second interdepartmental project on AMR (GRDI-AMR2). GRDI-AMR2 (2022-2027) builds on GRDI-AMR1 (2016-2022) to identify targets for action to mitigate AMR in healthcare setting, animals, plants and the environment, and to preserve the effectiveness of antimicrobials. GRDI-AMR2 is currently coordinated by Canadian Food Inspection Agency (CFIA) and Agriculture and Agri-Food Canada (AAFC) with participation from ECCC, Fisheries and Oceans Canada and PHAC in several research activities.
Resistant bacteria have been collected from food and environmental sources and matched with bacteria from Canadian clinics through a One Health continuum approach. Intervention points have been identified, and improved methods for tracking AMR in food and environmental sources have supported the generation of information for clinicians through easy-to-interpret reports, facilitating better clinical decisions. Alternatives to antimicrobials for livestock and poultry, including probiotics, plant bioactives and insect meal, have been validated as viable alternatives, thereby advancing the goal of reducing the use of traditional antimicrobials in livestock production systems. Environmental pollutants such as heavy metals have been shown to promote AMR in contaminated environments and AMR has been shown to persist even after Antimicrobial Use (AMU) ceases in livestock. Both genomic and metagenomic data have been shared in a public repository, contributing to the understanding of AMR in bacteria across the global one-health continuum.
The GRDI-AMR2 One Health project will continue to make strides in addressing AMR through collaborative efforts, innovative research, and strategic interventions. It will produce actionable information that can curtail the movement of AMR across One Health sectors to ensure that all stakeholders have the most modern tools to face this global health threat.
Additional Research and Innovation activities underway include:
- In May 2024, the McGill University Antimicrobial Resistance Centre hosted EDAR7, the leading international conference exploring the environmental dimensions of AMR in a One Health context. This conference was supported by a $250K grant from PHAC and included the participation of multiple federal departments, academic experts and other stakeholders from across Canada and around the world.
- PHAC invested $6.3M in the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), a leading global organization whose mission is to accelerate a diverse portfolio of innovative antibacterial products toward clinical development for human health. This funding aims to strengthen the Canadian AMR research and development ecosystem by increasing the number of Canadian product developers applying to enter the CARB-X portfolio, and to contribute to global pooled efforts to stimulate research and development for the antimicrobial pipeline. CARB-X has provided a detailed report to PHAC with recommendations for Canada based on their research and analyses.
- PHAC dedicated $ 3.2M over five years through the Medical Counter Measures program to pilot a centralized service to provide phage therapy in Canada. Phage therapy is the process of using bacterial viruses to treat infections and is an effective alternative to conventional antimicrobials. This new program, Phage Science, Therapeutics, and Research (PhageSTAR), was funded in 2023 with the lab space and staffing established in 2024. The PhageSTAR program is currently engaged with the Association of Medical Microbiology and Infectious Disease Canada (AMMI) and Health Canada (HC) to develop clinical and regulatory guidelines for providing phage therapy across the country. The goal is to make this centralized service, and the ability to treat cases driven by AMR, available in a limited capacity by the end of 2025. The capacity of the program will then be increased on a yearly basis until the end of March, 2028.
- Canada's Biomanufacturing and Life Sciences Strategy, which was launched in 2021 in support of vaccine, therapeutics and biomanufacturing projects, has included projects that address AMR:
- $49M was invested through the Strategic Innovation Fund toward the Conscience Open Science Drug Discovery Network, announced in October 2023. This network aims to address gaps in the development of drugs and therapeutics, particularly in areas of market failures.
- $750M was invested in a pan-Canadian network of five university-led research hubs. This includes investments to the PRAIRIE Hub for Pandemic Preparedness, which is notable for its One Health research.
- other AMR-relevant projects include: a project at the Eastern Canada Pandemic Preparedness Hub dedicated to using artificial intelligence and other technologies to discover new antibiotics, and to test and produce them in Canada; an international Randomized Control Trial involving the transfer of healthy gut bacteria to help patients combat AMR which was funded by the CIHR AMR Research Initiative through the global collaborative Joint Programming Initiative for AMR (JPIAMR); and a project at Canada's Immuno-Engineering and Biomanufacturing Hub focused on next-generation lipid nanoparticle RNA technology. This technology can be used for the development and manufacture of AMR bacterial pathogen vaccines.
- HC sponsored an Innovation Solutions Canada challenge on AMR diagnostic research and development. Companies selected for Phase 1 and Phase 2 received a total of $ 2,346,237. The two companies funded in Phase 2 presented their final report and held a final progress meeting in 2024.
- Through the Ideation Program (National Program Office) and the Vaccine and Emerging Infections Research Initiative (Human Health Therapeutics Research Center), the National Research Council Canada has invested more than $1M in the development of novel alternatives to antibiotics (such as bacteriophage therapy, nanoimmunobiotics, and antibody-antibiotics conjugate) to support Canadian bacteriophage therapy and Canada's biomanufacturing ecosystem.
- Through Canada's International Development Research Centre (IDRC), an Innovative Veterinary Solutions for Antimicrobial Resistance (InnoVet-AMR) initiative was created to reduce the emerging risk that AMR in animals poses to global health and food security. The $27M program was co-funded by the UK Department of Health and Social Care (UK-DHSC).
- in 2023, a new phase called InnoVet-AMR 2.0 started. This phase funded 14 projects in swine, poultry, ruminants and aquaculture species and covered more than ten diseases using nine different technologies, such as: vaccines, bacteriophages, probiotics, phytochemicals, bacteriocins, polypeptides, nutraceuticals, and two other technologies only applicable to the control of aquaculture pathogens (e.g., nanobubbles and thermoregulation).
- Ontario continued to support Animal Health Laboratories, including the adoption of new diagnostic technologies and work toward standardized reporting for AMR (and other diseases). In addition, the province encouraged and shared research and innovation related to AMR, in collaboration with the University of Guelph Alliance, New Directions Research, Food Safety partners, Canadian Agricultural Partnership and other organizations related to AMR.
- Public Health Ontario (PHO) has established a robust research program aimed at understanding the burden of AMR using provincial laboratory administrative data (Ontario Laboratories Information System - OLIS). Funded by CIHR, the Comprehensive Ontario Microbiology laBoratory Administrative daTa for AntiMicrobial Resistance (COMBAT-AMR) program has generated numerous research outputs this year, advancing knowledge about the prevalence and outcomes of AMR as well as clinician practices related to AMR since its inception in 2018.
Pillar 2: Surveillance
Priority actions
- Expand sources, coverage and integration of AMR and Antimicrobial Use (AMU) surveillance data, including the use of modern laboratory technologies and standardized reporting, to help monitor AMR/AMU across One Health sectors, with specific focus on improving data from the environment; transmission pathways between sectors; and population groups disproportionately impacted by AMR and inappropriate AMU.
- Work with partners to:
- establish baselines and targets for national, provincial and territorial levels of AMR and appropriate AMU in human health; and
- establish baselines, goals and measures of progress for increasing appropriate AMU and reducing AMR in the agriculture and agri-food sectors.
Surveillance is the foundation of Canada's ability to detect, understand and respond to AMR threats across all One Health sectors. Ensuring access to reliable surveillance data improves Canada's ability to reduce the emergence and spread of AMR, enables assessments of the impact of AMU in humans, animals, plants/crops and on AMR trends, allows for potential comparisons with other countries and provides evidence to inform antimicrobial drug approval and post-approval monitoring. Using the data from surveillance makes it possible to create practical, evidence-based policies and programs to address AMR and facilitate prudent and responsible AMU.
Existing and strengthened collaboration across FPT partners and industry sectors have helped close gaps in AMR and AMU surveillance in Canada since the Action Plan was released. For example:
- Participation in AMRNet, a national AMR laboratory surveillance system for human and animal health, has expanded coverage to seven provinces/territories in Canada. This surveillance system captures information on the antimicrobial susceptibility of clinical isolates from human and animals, helping to inform a One Health response to AMR.
- AMR prediction testing of Neisseria gonorrhoeae (GC) expanded from four to eight provinces/territories, focusing on northern and/or remote regions. This type of testing provides:
- valuable information to Indigenous communities for empiric treatment and response to the emergence of drug resistant strains;
- jurisdictions with access to AMR-GC surveillance data where they previously had no data on resistance. This information will help ensure clinicians are implementing appropriate gonorrhea treatments in their jurisdictions.
- Participation in the Enhanced Surveillance of Antimicrobial-Resistant Gonorrhea program has expanded from four to five jurisdictions, providing more details on which populations are affected by gonorrhea to target prevention and treatment approaches. The rate of gonorrhea has been on the rise in Canada and infections are increasingly resistant to antibiotics.
- The Canadian Animal Health Surveillance System (CAHSS), a division of Animal Health Canada (AHC) co-chaired by PHAC Centre for Foodborne, Environmental and Zoonotic Infectious Diseases, houses an AMU/AMR Network. AAFC provided funding to CAHSS (2023-2028) through the Sustainable Canadian Agricultural Partnership with the goal to expand CAHSS's surveillance network of networks and amplify surveillance efforts in alignment with Action Plan objectives. The expanded network is reviewing their work priorities and objectives which will be made publicly available on the CAHSS website once finalized.
National surveillance programs have also improved the timeliness and availability of data. Efforts in Year 1 of the Action Plan implementation include:
- In November 2023, the Canadian Antimicrobial Resistance Surveillance System (CARSS) launched online dashboards on AMR and AMU in both humans and animals. The dashboards present information from multiple surveillance systems and highlight AMR pathogens of concern, such as Methicillin-resistant Staphylococcus aureus, Carbapenemase-producing Enterobacterales and the emerging fungal pathogen Candida auris. CARSS is coordinated by PHAC and integrates data on AMR and AMU in humans and animals from nine different surveillance systems in Canada.
- Coordinated by PHAC, the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) is a national program that collects, analyzes and communicates trends in AMU and AMR for select bacteria from humans, animals and retail meat across Canada in collaboration with federal, provincial and private industry partners. CIPARS updated the existing Veterinary Antimicrobial Sale Reporting (VASR) system dashboard to include recent data up to 2022. HC also made updates through the VASR system to strengthen data reporting which provides a snapshot of medically important antimicrobials (those important in human medicine) available for veterinary use in Canada. This surveillance system is a joint collaboration between PHAC and HC.
- CIPARS launched a new interactive data dashboard on AMU in farmed animals and updated the AMR in Animals and Food dashboards to include the latest 2022 data.
Surveillance advances are making an impact on Canada's ability to enhance outbreak and treatment failure investigations, monitor AMR spread in the food chain, and improve the understanding of AMR's impact on specific populations. These improvements, and others, will strengthen the collaboration with partners and support future efforts to establish baselines, targets, and/or other measures of progress for AMR and AMU.
AMR surveillance and equity
AMR disproportionately impacts certain key human populations and socio-demographics in Canada, including Indigenous Peoples. Inequities in the burdens of AMR are linked to social determinants of health such as access to clean water, safe food, adequate housing, access to relevant and tailored infection prevention tools, and the burden of underlying conditions or infections in a community. Equity analyses of surveillance data allow the identification of communities and populations that bear a disproportionate burden of AMR or of inappropriate AMU, which can be used to improve targeted interventions. The 2023 CARSS Report Executive Summary outlines some examples of disproportionate AMR impacts in Canada.
The Enhanced Surveillance of Antimicrobial-resistant Gonorrhea (ESAG) system
ESAG contributes to a better understanding of antimicrobial-resistant N. gonorrhoeae trends by linking antimicrobial-resistant gonorrhea laboratory data to enhanced epidemiologic and clinical data. ESAG is a collaboration between PHAC and provinces/territories. ESAG data provides:
- gonorrhea treatment failure data;
- clinician adherence to gonorrhea treatment guidelines data;
- antimicrobial-resistant N. gonorrhoeae trends among key populations;
- evidence for public health interventions to minimize the spread of antimicrobial-resistant gonorrhea; and
- information for the treatment recommendations in the Canadian Guidelines of Sexually Transmitted Infections.
Since its inception in 2013, ESAG has seen many improvements. Recently, a new province joined ESAG bringing the number of participating jurisdictions to five. New ESAG reporting and analysis tools have been developed providing a quick turn-around-time for jurisdiction-specific reports. The 2018-2021 ESAG report was recently published (July 2024) and included enhanced analyses such as adherence to PHAC/PT treatment guidelines and analysis by key populations (gay, bisexual and other men who have sex with men, and sex workers) to better inform guidance.
ESAG recently expanded to include molecular AMR surveillance using Nucleic Acid Amplification Testing specimens to improve representativeness requiring the development of new reporting tools. A new ESAG Infobase page is being developed and will be available in 2024. Recruitment of additional jurisdictions is on-going.
Additional Surveillance activities underway include:
- CIPARS hosted three major meetings with stakeholders, including an annual webinar, which included sharing its first whole genome sequencing phenotypic prediction of AMR data for Salmonella isolates from humans, information on gram positive organisms from poultry, chicken layer surveillance data and, for the first time, AMR data on bovine respiratory pathogens. CIPARS is now routinely presenting information from on-farm feedlot cattle and dairy surveillance.
- The Canadian Nosocomial Infection Surveillance Program (CNISP) is actively expanding its network, which currently includes 106 acute care hospitals across ten provinces and one territory. By recruiting additional hospitals, CNISP aims to enhance the quality and representativeness of AMR surveillance data in hospitals.
- PHO expanded and updated hospital and community AMU and AMR data within the Ontario Antimicrobial Stewardship (AMS) Program and AMR Comparison Tool. This interactive tool provides AMS, AMU, and AMR data from across Ontario.
- PHO established an operational initiative to strengthen provincial surveillance infrastructure and capabilities to improve access to, comprehensiveness and timeliness of AMR and AMU data using OLIS and other provincial data assets. This will help to better inform provincial AMU/AMR targets, identify areas for targeted interventions including equity considerations and track progress.
- PHO obtained CIHR funding for the "Efficient Facility Feedback of Existing Culture Testing is Able to Mitigate Resistance (EFFECT-AMR) project" which will generate facility-specific antibiograms for hospitals and long-term care homes using OLIS data.
- Activities are underway to strengthen environmental dimensions of Canada's One Health AMR response. For example, PHAC is exploring and enhancing the FoodNet Canada data collection of surface water for AMR and antimicrobial testing. PHAC is working to leverage urban wastewater surveillance established for COVID-19 to investigate population-level AMR and AMU trends through the development of analytical methods to detect AMR and antimicrobials in wastewater. For example, a pilot is being conducted in three Canadian cities to assess correlations between specific AMR targets and select antimicrobials detected using quarterly wastewater samples. PHAC is also working with Indigenous Services Canada (ISC) to implement community-led AMR surveillance in Indigenous communities.
- PHAC gained access to a new source of deidentified primary healthcare electronic medical records in March 2024, through a partnership with the Canadian Primary Care Sentinel Surveillance Network. These data are being used to investigate AMR and AMU trends associated with urinary tract infections (UTIs) in participating family physician clinics across Canada.
- PHAC and CIHR published the third iteration of the living evidence review on The Effects of the COVID-19 pandemic on the Three Core Drivers of AMR in November 2023. This work looked at how COVID-19 impacted AMU and AMR. The report was funded by PHAC and CIHR to examine how AMU, IPC and the use of healthcare related systems impacted the emergence, transmission, and burden of AMR during the COVID-19 pandemic in Canada.
- The Saskatchewan Health Authority Infection Prevention and Control Department and Antimicrobial Stewardship Program launched a provincial surveillance program for UTIs at all long-term care facilities across the province. Data is being collected to determine baseline rates of UTIs and treatment for asymptomatic bacteriuria, with the goal of providing audit and feedback to individual facilities to promote changes in clinical practice and more appropriate antibiotic use.
Pillar 3: Stewardship
Priority actions
- Develop, implement and promote guidelines/standards for appropriate AMU in humans and animals through policy and regulatory initiatives, monitoring and educational interventions/accreditation requirements for health professionals and prescribers.
- Foster understanding of the risks of AMR and the importance of appropriate use of antimicrobials in humans and animals amongst the public, patients and producers through awareness/education campaigns, feedback mechanisms and policy and regulatory initiatives.
AMS is a system-wide approach that recognizes the role of patients, prescribers, producers and the public in promoting appropriate AMU. It includes coordinated interventions designed to promote, improve, monitor and evaluate appropriate AMU to preserve antimicrobial effectiveness while promoting and protecting human and animal health.
FPT partners are strengthening AMS programs across Canada. Forums such as the Western Canada One Health Antimicrobial Stewardship Conference are helping to ensure best practices, lessons learned and new evidence are shared with stewardship stakeholders in human, animal and environmental health sectors. The 2024 conference, hosted by the BC Centre for Disease Control, in collaboration with other partners, follows past conferences hosted by Alberta in 2021 and 2016 and by Saskatchewan in 2019. Additionally, Ontario is supporting and developing AMR/AMU specific continuing education and university curricula, as well as resources for veterinarians and producers through the Ontario Animal Health Network, Ontario Ministry of Agriculture Food and Agribusiness and Rural Affairs website and publications.
Ensuring healthcare prescribers have accessible, reliable and quick access to prescribing guidelines is essential for promoting AMS. Utilizing the WHO AWaRe Antibiotic Book as a reference, Canada is developing national prescribing guidelines for specific infectious syndromes in humans. As such, in May 2024, the federal Minister of Health announced a $840K investment, over three years, to develop and disseminate guidelines to healthcare prescribers at the point-of-care through a digital platform. The Association of Medical Microbiology and Infectious Disease (AMMI) Canada will develop the guidelines and Firstline, a Canadian-based digital technology company, will disseminate the guidelines through a digital platform to ensure healthcare prescribers can access the guidelines at the point-of-care. The prescribing guidelines and digital platform will help optimize prescribing practices in Canada and offer additional utility and flexibility for jurisdictions with similar programs already in place. This investment is an important step forward in ensuring prudent and responsible use of antibiotics, which in turn will contribute to the fight against AMR.
The Stewardship of Antimicrobials by Veterinarians project took place between 2018 and 2023 through funding support from AAFC. This project generated tools to pilot voluntary prescription data collection for cattle, swine and poultry and dispensing data collection, enabling an innovative and viable approach toward the continued establishment of an effective veterinary-based stewardship system.
Ontario: Farmed Animal Antimicrobial Stewardship Initiative (FAAST)
FAAST was initially designed to be a 'one-stop shop' online repository of information for farmed animal owners and their veterinarians on the policy and regulatory changes implemented in 2018 that related to AMU, AMS, AMR and access to antimicrobials. Since then, FAAST has grown to offer general and species-specific evidence-based information on AMR and AMS to veterinarians and producers through a variety of tools and resources, such as videos, podcasts, fact sheets, manuals, case studies and online tutorials, available in both English and French.
From January 2018 to January 2023, the FAAST website traffic and engagement included over 117,000 page views by over 63,000 new users, and over 800 active users per week. From June 2023 to June 2024 there were approximately 57,000 page views by approximately 42,000 users, even with limited active promotion of the website since 2022.
FAAST has a significant opportunity to leverage and promote AMS practices and awareness. Work is currently ongoing to help revitalize and expand the FAAST website to maximize the benefits to all users.
Health Canada: National list of reserve antimicrobial drugs for humans
HC received funding through the Government of Canada's Budget 2021 to develop a national list of reserve antimicrobial drugs ("List") for humans. Using these drugs as last-resort options preserves their effectiveness to ensure they will continue to work when they are needed most.
Canada's reserve List was modelled after the reserve category of the WHO AWaRe classification systems. The List was refined according to Canadian AMU and AMR data and engagements with experts from across Canada. An online public consultation was also conducted in 2023 to seek feedback from healthcare professionals, industry, academia and other stakeholders.
In March 2024, HC published and promoted the List for antimicrobial drugs and notified key stakeholders such as the Council of Chief Medical Officers of Health and AMMI Canada. This List will guide prescribers on which antimicrobial drugs are to be used sparingly to preserve effectiveness.
The List will be reviewed and revised periodically. Work is also underway to expand this List to a complete AWaRe classification scheme of antimicrobial drugs for human use, with publication expected by March 2027.
Additional Stewardship activities underway include:
- The CFIA and PHAC is supporting the Canadian Academy of Health Sciences to conduct an assessment of AMR and AMU in food-producing animals. This assessment has several objectives including:
- to better understand the links between human health and AMU in food-producing animals, which may ultimately inform interventions in the future;
- to improve understanding of the changes and interventions to AMU in the agriculture sector, which might be achievable in the Canadian context to improve animal health and welfare.
- PHAC is working collaboratively on a project with the primary goal to improve AMS in long-term care homes (nine homes within five provinces) to ensure best practice guidance is being used when testing and treating for UTI. This is done through implementation of a two-pronged intervention:
- Focusing on education for essential care providers (family and friends of residents).
- Facility-level feedback on monthly urine culture rates.
- PHAC is adapting an Australian based pilot digital tool for healthcare professionals in human hospitals to support appropriate AMU; an enhanced protocol is being developed to adjust the tool to the Canadian context, anticipated to be available in the fall of 2024. This digital tool will allow healthcare professionals in hospital settings to enter data related to antimicrobial prescribing, which will then be sent to PHAC to support national surveillance on appropriate AMU in hospital settings.
- Multiple federal, provincial and territorial departments participated in annual World Antimicrobial Awareness Week communications to raise public awareness about AMR and appropriate AMU in humans and animals.
- CIPARS presented the latest data on surveillance of AMU and AMR throughout the food chain in November 2023.
- AHC held a Strategic Planning Workshop with two objectives:
- Develop a leadership structure for farmed animal health sectors, within the One Health context, in response to the actions and goals outlined in the Action Plan.
- Collaborate with industry and government stakeholders within animal health to build a five-year forward plan to support Action Plan implementation.
- PHO continues to participate in World AMR Awareness Week campaigns, including the national #GoBlueforAMR colour campaign that aims to bring awareness to AMR amongst clinicians, policymakers, and the general public.
- Nunavut celebrated World AMR Awareness Week 2023 by distributing daily Practice Pearls to healthcare professionals on topics such as asymptomatic bacteriuria + UTIs, viral prescriptions to reduce antibiotic prescriptions and duration of antimicrobial therapy for common infections.
- Canada is part of the Global Food Animal Residue Avoidance Databank (CgFARAD) which provides information to veterinarians on potential residues in food animals as a result of administering veterinary drugs, including vaccines, to prevent illness or treat sick animals populations. The CFIA provides financial support for the ongoing work of CgFARAD in providing species specific animal health and welfare guidance to veterinarians related to withdrawal times and food safety.
- AAFC, CFIA, HC and PHAC hosted a meeting with the Council of Chief Veterinary Officers to discuss the establishment of baselines and goals to increase the prudent and responsible use of antimicrobials in the agriculture and agri-food sectors.
- HC continues to fund Choosing Wisely, Canada's national Using Antibiotics Wisely campaign, to help clinicians and patients engage in conversations about unnecessary antibiotic use in humans.
- The National Collaborating Centre for Infectious Diseases (NCCID) launched a national webinar series (CAN AMS!) focusing on practical actions that can be undertaken to improve AMS efforts across Canada.
- The Saskatchewan Health Authority Antimicrobial Stewardship Program has initiated the use of procalcitonin, a biomarker produced by the human body to capture inflammation due to bacterial infections, in Regina's Intensive Care Units to help reduce antibiotic use. Additionally, the program provided annual antibiogram updates and clinical guidelines within the Firstline app.
- Saskatchewan has been involved in many educational initiatives for prescribers, students and the public, including: initiating an audit and feedback project to promote AMU for orthopedic surgery patients with beta-lactam allergies; engaging in educational sessions with long-term care staff and residents across the province to promote appropriate management of UTIs and asymptomatic bacteriuria; engaging in a CIHR-funded national research study to provide audit and feedback to high-prescribers of antibiotics in primary healthcare in Saskatchewan; and providing ongoing education sessions and mentorship to health science students and trainees, practitioners, and high school students as well as the general public.
- Saskatchewan worked with appropriate committees to develop a restricted antimicrobial list for the province's acute care facilities and developed a series of documents to support facilities in all sectors of human health (primary care, long-term care and acute care) to meet Accreditation of Canada's Required Organizational Practice standards for AMS.
- PHO is continuing to provide primary care prescribers in Ontario with antibiotic prescribing feedback through Ontario Health and PHO. PHO has explored ways to advance AMS in local public health settings and is continuing to support the implementation of the Urinary Tract Infection Program in long-term care homes through virtual coaching series; this series was attended by over 450 Long-term Care Home staff.
- PHO's antibiotic audit and feedback (AandF) interventions have demonstrated significant impact on reducing unnecessary community antibiotic use in Ontario while being cost-saving. In 2024, PHO published a second randomized controlled trial which included over 5,000 physicians, which found that peer comparison AandF letters reduced overall antibiotic prescribing by 5%, reduced unnecessary prescribing by 11%, reduced antibiotic duration over seven days by 15% and reduced broad spectrum prescribing by 6%. This experience will inform an upcoming national Canadian Antibiotic prescribing feedback initiative (CANBuild-AMR) led by PHO.
- IDRC InnoVet-AMR, along with the International Centre for Antimicrobial Resistance Solutions, funded a study to explore research gaps on the intersection of AMR, gender, human and animal health in order to develop guidance to improve the gender inclusiveness of research and development for AMR innovations. Findings from the study included The Practical Pathways Toolkit, which was developed to offer guidance to researchers on how to better integrate gender in their AMR research.
- HC conducted a Compliance Monitoring Project which provided an opportunity to gain insights on the systems used by Commercial Feed Mills and relevant parties, for the sale, dispensing, and distribution of prescription in-feed drugs and medicated feeds under the Food and Drugs Act and Regulations. Results and recommendations of this project will inform the refinement of policies and guidance, to help the feed industry comply with the requirements around medically important antimicrobials to preserve their effectiveness in the manufacture and sale of medicated feed.
- In March 2024, CFIA completed Public Opinion Research targeting Canadian veterinarians across both food production and companion animal sectors regarding awareness of AMR and AMU as well as IPC options, which will inform future activities under the stewardship pillar of the Action Plan.
- In order to improve long-term access to veterinary care, the Government of Alberta is funding ongoing capital expansion at the University of Calgary to enable the Faculty of Veterinary Medicine to expand from 50 to 100 veterinary students per year starting in 2025 and continuing its investment in the reestablishment of veterinary AMR diagnostic services at the University of Calgary Veterinary Medicine program.
Pillar 4: Infection prevention and control
Priority actions
- Increase effective implementation of infection prevention measures, particularly for populations disproportionately impacted by AMR such as remote, northern and isolated communities, First Nations, Inuit and Métis populations, long-term care residents, and hospitalized patients by developing, updating and promoting uptake of guidelines/best practices for human health.
- Support the increased implementation of enhanced IPC, biosecurity and food safety protocols across the agriculture and agri-food sectors, prioritizing sound animal husbandry, access to veterinary care, and access to additional health and nutritional aids to promote animal health.
Adhering to IPC measures across all One Health sector settings is essential to mitigate the impact of AMR and decrease the transmission of AMR pathogens to populations in vulnerable situations. IPC measures are the practices and procedures designed to prevent and protect from the spread of infections within different settings and the community. These measures are critical for protecting patients, workers, and the general public. Addressing information gaps, increasing uptake of IPC guidelines and advancing knowledge sharing are critical strategies to ensure best practices are held across all One Health sectors.
Ontario Ministry of Health: Infection Prevention and Control (IPC) Hubs
The Ontario Ministry of Health established local networks of Infection Prevention and Control (IPC) Hubs across the province in the Fall 2020 to provide a formal and coordinated pathway for IPC leads in congregate living settings (CLSs) including long-term care homes, retirement homes, homes serving adults with developmental disabilities, and a host of other settings (e.g., shelters) to access IPC expertise and support. The Hubs offer support to implement foundational IPC best practices, so as to position them to be successful in the prevention and management of current and future IPC concerns.
IPC Hubs delivered IPC education, training, coaching, mentoring, networking opportunities and other supports to assist CLSs in their efforts to establish robust IPC programs that are tailored to their specific settings. In 2023-2024, IPC Hubs delivered close to 45,000 total services (remote and onsite) with approximately 10,000 services delivered onsite.
The Ministry is engaging IPC Hubs and working with partner ministries in a process to refine the model with a view to building on its core strengths. The overarching goal is to establish a sustainable IPC infrastructure that will be available to support CLSs in their IPC practices and, in the future, be available to pivot to address emerging and urgent issues across the broader public health system.
Additional IPC activities underway include:
- CFIA updated and developed the National Biosecurity Standards and Biosecurity Principles for both plants/crops and animals to support industry implementation of infection prevention.
- HC and CFIA developed and implemented policies and guidance material to allow the addition of Veterinary Health Products (VHPs) in livestock feeds prior to the completion of the CFIA Feed Modernization Regulatory project. The new Feeds Regulations 2024 were registered on June 17, 2024, and published in the Canada Gazette, Part 2 on July 3, 2024. These regulatory changes will allow VHPs to be added to livestock feed. VHPs, which include ingredients such as vitamins and minerals, are health products with general wellness claims intended to bolster animal health and reduce the need to treat animals with antimicrobials.
- CFIA continues to work with HC to consult and develop guidance that will clarify the classification and registration requirements (i.e., feed vs. veterinary drug). CFIA ensures that safe and efficacious innovative products that support animal health such as gut modifiers (e.g., probiotics), mycotoxin detoxifiers and acid-based products are available to livestock producers and will continue to evaluate, consult on and register feed ingredients and products.
- The CFIA's Canadian Centre for Veterinary Biologics continues to facilitate access to antimicrobial alternatives through the approval of veterinary vaccines which help keep animals healthy and reduce AMU.
- Between July 1, 2023 and June 30, 2024, the CFIA has consulted on or approved over 20 feed innovative products including gut modifiers, mycotoxin detoxifiers and feed mitigants and approved 17 new veterinary biologics for use in Canada.
- Through the Sustainable Canadian Agricultural Partnership (2023-2028), AAFC provides funding support for the continued improvement and expansion of existing on-farm food safety programming, which includes considerations for biosecurity, product quality and animal care, with specific attention on AMR/AMU, such as the Dairy Farmers of Canada's proAction®assurance program. AAFC also supports industry-led research through the AgriScience Program (i.e., Clusters Component) to develop new approaches to reduce AMU for the major livestock and poultry sectors in Canada. Nationally, disease planning and response to Highly Pathogenic Avian Influenza as well as the risk of introduction of African Swine Fever and Foot and Mouth Disease resulted in enhanced biosecurity efforts, which provided benefits related to prevention of infection and disease spread on farms.
- Indigenous Services Canada (ISC) supported communities in the development and implementation of Communicable Disease Control (CDC) and Health Emergency Management programs to address the health needs of Indigenous communities. ISC also assisted communities with improving their preventative health strategies including enhanced PPE train-the-trainer sessions, vaccination programs and building more capacity for in-community health services to promote equitable access to testing for infectious diseases.
- PHO expanded IPC knowledge by supporting front-line practitioners in long term care and acute care sectors (as well as other settings) to make improvements to various IPC programs. PHO's efforts include the publication of new long-term care focused resources to address the growing concern of antimicrobial resistant organisms, including risk-based screening guidance and a Candida auris case management algorithm.
- PHO published a number of IPC checklists, new hand hygiene resources, and new online learning modules for the environmental cleaning role. In future months, PHO will publish a completely redesigned IPC online learning training package for those working in healthcare settings.
- To ensure the effective dissemination of IPC deliverables, such as guidelines, interim recommendations, infographics, and webinars, PHAC is employing tailored communication strategies to targeted audiences. PHAC leveraged existing stakeholder relationships to develop a communication strategy with FPT partners by conducting key informant interviews to gather insights, feedback, and to identify gaps and needs in the national IPC guidance landscape. Through PHAC's Healthcare-Associated Infection Prevention and Control, the team:
- developed a comprehensive plan for a strategic multimodal approach to ensure effective and timely dissemination of IPC knowledge products through social media, webinars, and up to date guidelines and infographics to partners and stakeholders; and
- is developing a plan to monitor and evaluate the effectiveness of its communication strategy, as well as the implementation of IPC guidelines and other key deliverables.
- The National Advisory Committee on Infection Prevention and Control (NAC-IPC) aids in developing evidence-based guidelines, offering technical advice on emerging infectious diseases, identifying research priorities, working to promote public health, respond to health emergencies and enhance intergovernmental collaboration to combat healthcare-associated infections and AMR. In the past year, members of the NAC-IPC contributed to the following organism specific guidance, which is now being finalized prior to publication:
- Candida auris Infection Prevention and Control in Canadian Healthcare Settings; and
- Carbapenemase-Producing Enterobacterales Infection Prevention and Control in Canadian Healthcare Settings.
Pillar 5: Leadership
Priority actions
- Build on existing One Health AMR governance structures to create a "network of networks" with inclusive representation to support action plan implementation and share progress and lessons learned within and across the five pillars of action, prioritizing strengthened FPT, First Nations, Inuit and Métis collaboration to co-develop AMR actions.
- Increase Canada's contributions to global efforts to advance key bilateral and multilateral commitments by prioritizing:
- Generating improved data/evidence on AMR/AMU and strengthening surveillance systems and data standards
- Expanding efforts to support low- and middle-income countries by advancing equitable access, stewardship and IPC initiatives
Domestic stakeholders expect FPT governments to mobilize efforts across sectors to implement the Action Plan. The Action Plan prioritized building on existing networks where possible to coordinate and inform implementation. Existing FPT governance tables have been leveraged to provide collaborative domestic leadership and support across all priority actions. This includes a renewed FPT AMR Steering Committee, comprised of senior level officials from human health, animal health and agriculture/agri-food sectors. The AMR Advisory Group has also continued to provide advice on multiple areas of Action Plan implementation. Membership of this group consists of experts from various sectors and disciplines, such as the biotechnology and pharmaceutical industry, health professionals, animal health organizations and academia. In parallel, existing stakeholder networks and organizations, such as AHC and the NCCID, have supported engagement with stakeholders from diverse One Health sectors and disciplines to identify implementation priorities and approaches. Continued efforts to leverage existing stakeholder networks and organizations where possible will be crucial moving forward.
Globally, the Government of Canada continues to engage with partners in both bilateral and multilateral fora. These fora have proved to be key opportunities for Canada to advocate for its whole-of-government international AMR objectives. Collaborations with other countries and international organizations is required and essential to address AMR. Throughout 2023-2024, the Government of Canada has supported several international organizations and initiatives, including investing in the JPIAMR – a global organization and platform supported by 29 countries to address AMR within a One Health approach. The efforts of the organization are to fund research that fill the gaps on AMR as well as supply countries with guidance on how to align their AMR research both nationally and internationally. Additionally, Canada holds the co-chair position on the Global AMR Research and Development Hub, which was launched in May 2018 to create a sustainable ecosystem for the research and development of the solutions, tools, products and strategies needed to fight the ongoing impacts of AMR.
Given the number of international events in 2024, including the United Nations General Assembly (UNGA) High-Level Meeting (HLM) on AMR in September 2024, it is widely acknowledged that it is a crucial year for the international community to come together to address AMR. To support this, Canada has been an active participant in various negotiations, provided technical advice and guidance to partners, helped bolster the development of new initiatives and attended various international events that allowed for the strengthening of existing relationships and the development of new collaborative partnerships with both public and private sector institutions. Additionally, Canada participated in the negotiation of and ultimately co-sponsored the AMR Resolution which was tabled at the World Health Assembly in May 2024, as well as the negotiation process for the political declaration for the UNGA HLM on AMR. Going forward, Canada will continue to collaborate with international partners to ensure momentum is maintained and will engage with PT governments in an appropriate, timely manner when international initiatives impact areas of PT jurisdiction.
Nova Scotia AMR Action Plan
Nova Scotia identified a need for a structured and collaborative plan to address AMR. Building on initial work done in 2019, and investments in IPC and hospital-based AMS in 2020, an AMR Action Plan was introduced in June 2024. The intended audience includes government-based decision makers and funders, as well as those providing leadership and oversight in human health, animal health, and agri-food sectors.
The AMR Action Plan was developed with subject matter experts from the above-mentioned sectors and modeled off Canada's Action Plan following the pillars of action and guiding principles of one health, equity and collaboration.
Governance of the AMR Action Plan is through a steering committee chaired by the provincial Chief Veterinary Officer and the Chief Medical Officer of Health, and four pillar working groups. Each working group will align with the Action Plan actions to address both human and animal health needs.
To facilitate collaboration, engagement and further development and implementation of the Action Plan, an AMR symposium will be held in September 2024.
The AMR Action Plan is an evergreen document that will be reviewed and revised on an ongoing basis through the steering committee, working groups and collaborative networks.
Additional Leadership activities undertaken include:
- Participation and engagement in a number of international initiatives such as:
- G20 and G7 Health and Development Partnership's AMR Legislator Initiative, which was established to develop recommendations for parliamentarians to accelerate action to address AMR.
- the Multistakeholder Partnership Platform for AMR coordinated by the Quadripartite, a group consisting of the Food and Agriculture Organization of the United Nations (FAO), the United Nations Environment Programme, the WHO and the World Organization for Animal Health (WOAH). This platform aims to accelerate global action to address AMR by providing opportunities for all sectors (government, academic, public, etc.) for collaboration on solutions to addressing AMR.
- the Transatlantic Taskforce on Antimicrobial Resistance (TATFAR), which includes a taskforce of government experts from Canada, the European Union, United States of America, United Kingdom, and Norway who work across several areas related to AMR such as surveillance, risk assessment, communication, innovation and collaboration. Canada contributed to a joint publication with other members of the TATFAR regarding different approaches taken by member countries/regions for reporting AMU/sales data for antimicrobials intended for use in animals. This report will help stakeholders better understand surveillance data reported by different countries or regions.
- Canada participates in the standard setting process for WOAH and actively comments on new and revised chapters for the Terrestrial and Aquatic Animal Health Codes. In May 2024, Canada worked collaboratively with the WOAH Region of the Americas and QUADS Alliance (Australia, New Zealand, the USA, the UK and Canada) partners to determine positions on the WOAH Terrestrial Code Chapter 6.10 Responsible and Prudent Use for Antimicrobials in Veterinary Medicine. Canada's WOAH Delegate participated in WOAH's 91st General Session where a revised version of Chapter 6.10 was adopted for inclusion in the WOAH Terrestrial Animal Health Code.
- experts from Canada provide technical expertise to the WOAH as members of its Terrestrial Animal Health Standards Commission (May 2024 -May 2027) and AMR Working Group (starting in February 2024) which work to support WOAH Members through the development and revision of standards and guidance on animal health standards including those for AMU and AMR.
- AAFC, CFIA, HC and PHAC jointly hosted a meeting with provinces/territories and the animal industry sectors to discuss international activities on AMR. The objective of the meeting was to discuss activities leading up to the UNGA HLM on AMR in September 2024, given that goals were proposed as part of the zero draft. The topics included a review of international pressures, a description of various types of goals, considerations needed to establish goals, and a discussion of experiences in other countries.
- Additionally, PHAC, HC and the NCCID hosted a parallel event with human health stakeholders in advance of the UNGA HLM on AMR, to get their views and perspectives on priorities for Canada in the international space for human health, and how Canada could leverage the global AMR community to address this growing threat.
- In 2023-2024, AHC received $174.9K in federal funding to help strengthen domestic AMR governance across animal health and agriculture partners and sectors with the goal to support AMR coordination and sharing of information and to advance Action Plan implementation across all pillars. The Canadian Animal Health AMU/AMR Leadership Team will provide strategic guidance in the agri-food and animal health sectors by providing leadership and coordination in relation to AMR/AMU stewardship in animal agriculture in the Canadian context. This Leadership team is dedicated to effective, transparent and ongoing communication with stakeholders and will engage in initiatives to raise awareness about its activities. Through collaboration with One Health and One Welfare stakeholders, this team will actively engage in the evolving landscape of AMR in its global interconnection with animal, human, and environmental health.
- At the request of authorities in Malaysia, Canada conducted a technical consultation on AMR with Malaysia facilitated by a global development consulting company. The CFIA led three virtual policy workshops, with PHAC, HC and AAFC participating. The technical consultations included Canadian activities on AMU and AMR surveillance in animals/food, control measures for disease and AMR, AMR risk assessments and stewardship activities.
- Canada also contributed to global efforts through improving data and strengthening surveillance systems. Canada:
- provided annual data via CFIA, from CIPARS, to the WOAH's ANIMUSE global database on antimicrobials intended for use in animals and to the WHO's Global Antimicrobial Resistance and Use Surveillance System (GLASS) on AMR in human Salmonella.
- applied international methodologies for CIPARS, CNISP and CARSS human, animal and retail meat surveillance and reporting where possible and applicable. For example, CIPARS methodology for the analysis of antimicrobial sales data and farm AMU data is based upon guidance from the WOAH and the European Medicines Agency, while the CARSS and CNISP methodology for reporting to the GLASS is based on guidance from the WHO.
- supported the development of new international surveillance initiatives by providing technical expertise for the development of the FAO InFARM system. Canadian experts engaged with the Quadripartite organizations, in particular, WHO, WOAH and FAO, throughout the year regarding technical aspects of integrated surveillance and One Health AMR research.
- PHO was invited by the US Centers for Disease Control to participate in an international meeting to identify key strategies for Health Department-Led Stewardship Efforts resulting in PHO's contributions being included within the newly developed Core Elements of Antibiotic Stewardship for Health Departments guidance issued by the CDC.
Look ahead
Partners in Action Plan implementation are continuing to advance priority actions across all pillars. Looking ahead to the second year of Action Plan implementation planned activities across Canada include, but are not limited to:
Research and innovation:
- Launch of the three-year incentivization project to improve access to antimicrobials that address unmet health priority needs for people in Canada.
- Continue to finalize a National One Health AMR Research Strategy to improve coordination of research efforts, identify research areas and build a program of work that responds to stakeholder needs across the five pillars of the Action Plan.
Surveillance:
- Expansion of surveillance networks to identify emerging threats such as a strategy on environmental surveillance which is currently being developed and will be released in 2025.
- Development of a revised list of priority pathogens to guide AMR surveillance, which is anticipated to be released in Spring 2025.
Stewardship:
- HC will update Canada's Categorization of Antimicrobial Drugs Based on Importance in Human Medicine to inform priority setting so stakeholders have the most current information available to support the development of veterinary education programs and inform decisions about AMU.
- Continue the development and distribution of national guidelines and education for AMU, education of healthcare professionals for appropriate AMU, patients and others to ensure stewardship and sharing of the latest resistance data.
- CFIA will continue to facilitate access to alternatives to using antimicrobials by way of pre-market authorizations of innovative feed products and veterinary vaccines which helps keep animals healthy and reduce AMU.
- ISC is working with Indigenous partners, through an Antimicrobial Stewardship Steering Committee, to coordinate AMS activities and to support the development of strategies and capacity that reduce AMR across Indigenous communities within Canada.
Infection prevention and control:
- CFIA will support farmers and veterinarians through the development of biosecurity guidance, education modules on medicated livestock feed and information kits with materials for veterinarians that can be shared with clients on AMR and antimicrobial alternatives.
- ISC's National Infection Prevention Control Program will be inclusive of AMR. Although currently in its preliminary stages of development, this program will assist regional staff as they work collaboratively with provinces and territories and Indigenous partners to ensure AMR education is being implemented and practiced.
- Ontario will continue to support practitioners through the publication of a new organization risk assessment tool and an updated IPC checklist for clinical office settings, and in the home and community care sector.
Across all pillars, Canada is strengthening domestic and global leadership on AMR in collaboration with stakeholders across One Health sectors. Progress will be monitored and reported in future progress reports.
Glossary
- AAFC
- Agriculture and Agri-Food Canada
- AandF
- Audit and Feedback
- AHC
- Animal Health Canada
- AMMI
- Association of Medical Microbiology and Infectious Disease
- AMR
- Antimicrobial Resistance
- AMU
- Antimicrobial Use
- AMS
- Antimicrobial Stewardship
- CAHSS
- Canadian Animal Health Surveillance System
- CARB-X
- Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator
- CFIA
- Canadian Food Inspection Agency
- CgFARD
- Global Food Animal Residue Avoidance Databank
- CIHR
- Canadian Institutes of Health Research
- CIPARS
- The Canadian Integrated Program for Antimicrobial Resistance Surveillance
- CNISP
- Canadian Nosocomial Infection Surveillance Program
- ECCC
- Environment and Climate Change Canada
- ESAG
- Enhanced Surveillance of Antimicrobial-resistant Gonorrhea
- FAAST
- Farmed Animal Antimicrobial Stewardship Initiative
- FAO
- Food and Agriculture Organization of the United Nations
- FPT
- Federal, Provincial and Territorial
- GLASS
- Global Antimicrobial Resistance and Use Surveillance System
- GRDI
- Genomics Research and Development Initiative
- IDRC
- International Development Research Centre
- IPC
- Infection Prevention and Control
- ISC
- Indigenous Services Canada
- HC
- Health Canada
- JPIAMR
- Joint Programming Initiative on AMR
- NCCID
- National Collaborating Centre for Infectious Disease
- OLIS
- Ontario Laboratories Information System
- PHAC
- Public Health Agency of Canada
- PHO
- Public Health Ontario
- WHO
- World Health Organization
- WOAH
- World Organization for Animal Health
- TATFAR
- Transatlantic Taskforce on Antimicrobial Resistance Group
- UNGA HLM
- United Nations General Assembly High-level Meeting
- UTI
- Urinary Tract Infection
- VASR
- Veterinary Antimicrobial Sales Reporting
- VHP
- Veterinary Health Products