AMR: Neisseria gonorrhoeae in Canada, 2009-2013

 Download this article as a PDF (857 KB - 6 pages)
Download this article as a PDF (857 KB - 6 pages) Published by: The Public Health Agency of Canada
Issue: Volume 41-2: STIs and sexual health awareness month
Date published: February 5, 2015
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 41-2, February 5, 2015: STIs and sexual health awareness month
Surveillance
Antimicrobial resistance to Neisseria gonorrhoeae in Canada: 2009-2013
Martin I1,*, Sawatzky P1, Liu G1, Mulvey MR1
Affiliation
1 National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, MB
Correspondence
DOI
https://doi.org/10.14745/ccdr.v41i02a04
Abstract
Background: Gonorrhea is on the rise in Canada. Treatment has been complicated by the fact that Neisseria gonorrhoeae has acquired resistance to many antibiotics, including penicillin, tetracycline, erythromycin and ciprofloxacin. The emergence of isolates with decreased susceptibilities to the third generation cephalosporins and reports of treatment failures in Canada and around the world are cause for concern.
Objective: To assess the resistance levels of common antibiotics to N. gonorrhoeae and to observe trends in resistance and/or decreased susceptibility to ciprofloxacin, third generation cephalosporins and azithromycin.
Methods: Laboratory surveillance data for N. gonorrhoeae isolates submitted by provincial microbiology laboratories to the National Microbiology Laboratory (NML) from 2009-2013 were compared.
Results: Since 2009, there has been an overall rise in antibiotic-resistant N. gonorrhoeae. In 2013, 24.3% of the isolates were resistant to erythromycin, 18.9% were resistant to penicillin, 33.0% were resistant to tetracycline, and 29.3% were resistant to ciprofloxacin. The percentage of isolates with decreased susceptibility to ceftriaxone (=0.125 mg/L) and/or cefixime (=0.25 mg/L) was 3.9% in 2013. This number represents a decrease from 5.9% in 2012 and 7.6% in 2011. The proportion of azithromycin resistant (MIC =2 mg/L) N. gonorrhoeae isolates increased from 0.4% in 2009 to 1.2% in 2013.
Conclusion: Resistance to erythromycin, penicillin, tetracycline and ciprofloxacin is common. Decreased susceptibility to ceftriaxone and/or cefixime is now almost 4% and azithromycin resistance is emerging but remains low at 1.2%. These results have informed the gonococcal infection treatment recommendations in the Canadian Guidelines on Sexually Transmitted Infections.
Introduction
N. gonorrhoeae,the causative agent of gonorrhea, is the second most commonly reported bacterial sexually transmitted infection in Canada and rates of reported cases have more than doubled between 1997 and 2012Footnote 1.
The treatment and control of gonorrhea is complicated by the ability of N. gonorrhoeae to evolve and develop resistance to many of the antibiotics used to treat itFootnote 2Footnote 3. The emergence of isolates with decreased susceptibilities to the cephalosporinsFootnote 4Footnote 5Footnote 6Footnote 7 and reports of treatment failures in CanadaFootnote 8 and around the world raises the possibility of gonorrhea infections becoming untreatable in the future. The emergence of high-level azithromycin resistant (=256 mg/L) N. gonorrhoeae has been reported internationallyFootnote 9 and isolates with this high level azithromycin resistance have now been identified in Canada.
The number of cultures available for antimicrobial susceptibility testing is on the decline due to the shift from the use of culture to Nucleic Acid Amplification Test (NAAT) for the diagnosis of gonorrhea. This is of concern as N. gonorrhoeae cultures are required for antimicrobial susceptibility testing and some jurisdictions in Canada no longer maintain the capacity to culture this organism. In fact, over 70% of gonococcal infections in Canada are now diagnosed using NAAT and therefore antimicrobial susceptibility data in these jurisdictions are not available. The National Microbiology Laboratory (NML), in collaboration with the provincial laboratories, has been monitoring the antimicrobial susceptibilities of N. gonorrhoeae since 1985.
The objective of this report is to summarize the trends in antimicrobial resistance to gonorrhea infections in Canada between 2009 and 2013. It is based on the National Surveillance of Antimicrobial Susceptibilities of Neisseria gonorrhoeae Annual Summary 2013 prepared by the NML, Public Health Agency of Canada (PHAC)Footnote 10.
Methods
Data collection
Provincial public health laboratories submitted a total of 5,518 viable N. gonorrhoeae isolates to the NML for antimicrobial susceptibility testing as part of the passive National Neisseria gonorrhoeae Surveillance Program between 2009 and 2013 (2009, N=913; 2010, N=1,233; 2011, N=1,158; 2012, N=1,031; 2013, N=1,183). N. gonorrhoeae isolates are submitted to the NML when the provincial laboratories identify resistance to at least one antibiotic or if the provincial laboratories do not perform any antimicrobial susceptibility testing. Submission of isolates is voluntary and is not standardized across the country. The overall interpretation of the results is difficult due to the limitations related to the isolates available for testing. Therefore, the total number of isolates cultured in all provinces was used as the denominator to calculate resistance proportion (2009, N=3,106; 2010, N=2,970; 2011, N=3,360; 2012, N=3,036; 2013, N=3,195).
MICs were determined by agar dilutionFootnote 11 and resistance was defined according to the Clinical and Laboratory Standards InstituteFootnote 11 except for erythromycinFootnote 12 and azithromycinFootnote 13. Decreased susceptibility breakpoints for ceftriaxone and cefixime were based on WHO definitionsFootnote 14. Common abbreviations for the different types of resistance have been developed (Table 1).
| Abbreviation | Term in full | Definition |
|---|---|---|
| PPNG | Penicillinase-Producing N. gonorrhoeae | Pen MIC = 2.0 mg/L, ß-lactamase positive, ß-lactamase plasmid (3.05, 3.2 or 4.5 Mdal plasmid) |
| TRNG | Tetracycline Resistant N. gonorrhoeae (plasmid-mediated) | Tet MIC = 16.0 mg/L, 25.2 Mdal plasmid, TetM PCR positive |
| CMRNG | Chromosomal Mediated Resistant N. gonorrhoeae | Pen MIC = 2.0 mg/L, Tet MIC = 2.0 mg/L but = 8.0 mg/L and Ery MIC = 2.0 mg/L |
| Probable CMRNG | Probable Chromosomal Mediated Resistant N. gonorrhoeae | One of the MIC values of Pen, Tet, Ery = 1 mg/L, the other two = 2.0 mg/L |
| TetR | Tetracycline Resistant N. gonorrhoeae (chromosomal mediated) | Tet MIC = 2.0 mg/L but = 8.0 mg/L |
| CipR | Ciprofloxacin Resistant N. gonorrhoeae | Cip MIC = 1.0 mg/L |
| AzR | Azithromycin Resistant N. gonorrhoeae | Az MIC = 2.0 mg/L |
| SpecR | Spectinomycin Resistant N. gonorrhoeae | Spec R = 128 mg/L |
| CxDS | N. gonorrhoeae with decreased susceptibility to Ceftriaxone | Cx MIC = 0.125 mg/L |
| CeDS | N. gonorrhoeae with decreased susceptibility to Cefixime | Ce MIC = 0.25 mg/L |
Results
Figure 1 shows the trends of antimicrobial susceptibilities of N. gonorrhoeae tested in Canada from 2009 to 2013.
Figure 1: Trends of antimicrobial susceptibilities of Neisseria gonorrhoeae tested in Canada from 2009 to 2013
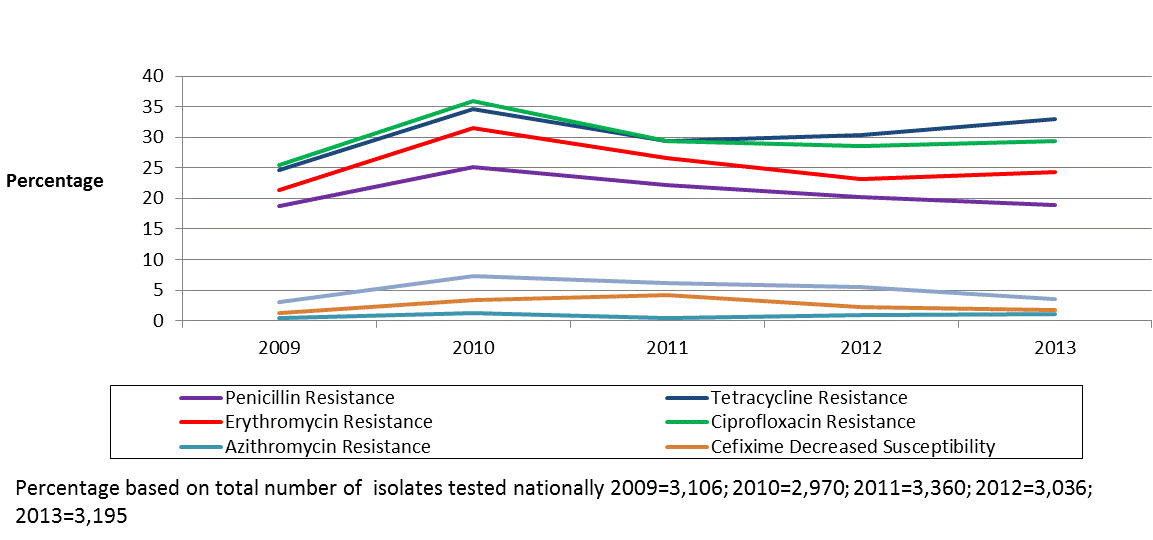
Text description: Figure 1
Figure 1: Trends of antimicrobial susceptibilities of Neisseria gonorrhoeae tested in Canada from 2009 to 2013
| Antimicrobial susceptibilities of Neisseria gonorrhoeae | Year | ||||
|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | |
| Penicillin Resistance | 18.7 | 25.05 | 22.2 | 20.26 | 18.94 |
| Tetracycline Resistance | 24.7 | 34.61 | 29.4 | 30.3 | 32.99 |
| Erythromycin Resistance | 21.3 | 31.52 | 26.6 | 23.12 | 24.32 |
| Ciprofloxacin Resistance | 25.5 | 35.93 | 29.3 | 28.52 | 29.33 |
| Azithromycin Resistance | 0.35 | 1.25 | 0.39 | 0.86 | 1.16 |
| Cefixime Decreased Susceptibility | 1.19 | 3.3 | 4.2 | 2.24 | 1.75 |
| Ceftriaxone Decreased Susceptibility | 3.12 | 7.34 | 6.2 | 5.53 | 3.51 |
Of the 3,195 N. gonorrhoeae isolates cultured in public health laboratories across Canada in 2013, 1,183 presumptively resistant isolates were submitted to the NML. Of these, 1,153 were confirmed to be resistant to at least one antibiotic and 30 were susceptible which translates to 36.1% of all N. gonorrhoeae cases diagnosed by culture as resistant N. gonorrhoeae.
Gender and age data was available for 99.5% of the 2013 isolates tested at the NML. Of these, 83.1% were males ranging from 1 month to 74 years of age. A total of 16.9% of isolates were from females aged 2 to 71 years.
Third generation cephalosporins
In 2013, according to WHO definitions, 1.8% of isolates were identified as having decreased susceptibility to cefixime and 3.5% were identified as having decreased susceptibility to ceftriaxone. These rates are higher than they were in 2009 (1.2% decreased susceptibility to cefixime and 3.1% decreased susceptibility to ceftriaxone), but lower than in 2011 (4.2% decreased susceptibility to cefixime and 6.2% decreased susceptibility to ceftriaxone). In 2013, 3.9% of isolates were identified with decreased susceptibility to ceftriaxone and/or cefixime decreasing from 5.9% in 2012 and 7.6% in 2011.
Azithromycin
Azithromycin resistant N. gonorrhoeae increased from 0.4% in 2009 to 1.2% in 2013. Between 2009 and 2012, five isolates with high-level azithromycin resistance (MIC =256 mg/L) were identified in Canada. The modal MIC for azithromycin has remained at 0.5 mg/L each year between 2009 and 2012. In 2013, the modal decreased to 0.25 mg/L.
In 2012, seven isolates with combined decreased susceptibility to cephalosporins and resistance to azithromycin were identified (0.2%). In 2013, eight (0.3%) of these isolates were identified. These are the first isolates to emerge in Canada with both decreased susceptibility to cephalosporins and resistance to azithromycin thus threatening the success of currently recommended dual therapy treatment options.
Other antibiotics
The percentage of ciprofloxacin resistant isolates increased from 25.5% in 2009 to 29.3% in 2013. Ciprofloxacin resistance increased from 1.3% in 2000 to a high of 36.0% in 2010. The modal MIC of ciprofloxacin has shifted dramatically from 0.004 mg/L in 2004 to 16.0 mg/L in 2013 (data not shown).
In 2009, 21.3% of isolates were found to be erythromycin resistant. This percentage increased to 31.5% by 2010 and then decreased to 24.3% by 2013. Penicillin resistance increased from 18.7% in 2009, to 25.1% in 2010 and then decreased to 18.9% in 2013. Tetracycline resistance increased from 24.7% in 2009 to 34.6% in 2010 and then decreased to 33.0% in 2013. Of the 5,518 viable isolates tested at NML between 2009 and 2013, none showed resistance to spectinomycin.
In 2013, 13.5% of isolates were classified as Chromosomal mediated resistant N. gonorrhoeae (CMRNG), a slight decrease from the 15.3% identified in 2009. Penicillinase-producing N. gonorrhoeae (PPNG) accounted for 4.3% in 2013, increasing slightly from 2.5% in 2009. Plasmid-mediated tetracycline resistant N. gonorrhoeae (TRNG) increased from 3.2% in 2009 to 8.8% of isolates in 2013 (Figure 2).
Figure 2: Trends in chromosomal and plasmid-mediated antimicrobial resistance in Neisseria gonorrhoeae in Canada from 2009 to 2013
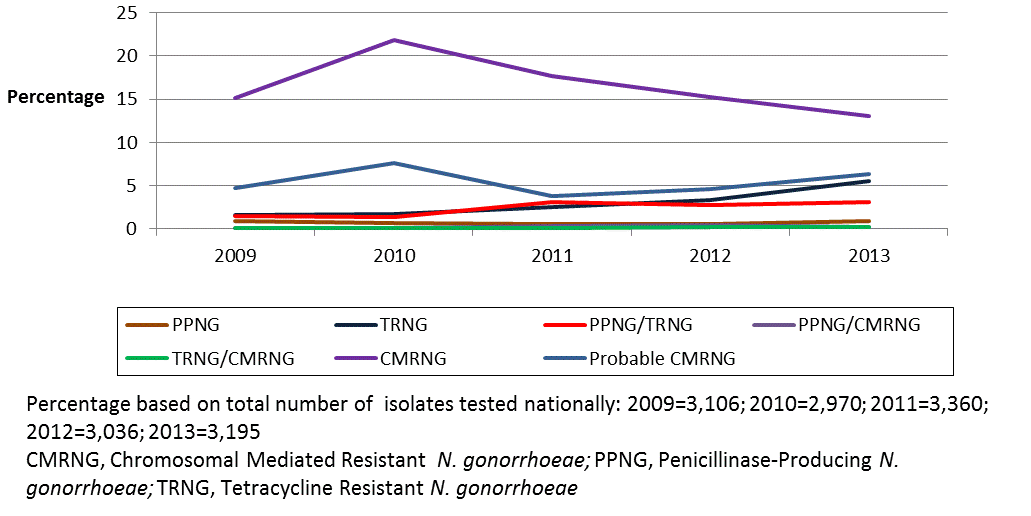
Text description: Figure 2
Figure 2: Trends in chromosomal and plasmid-mediated antimicrobial resistance in Neisseria gonorrhoeae in Canada from 2009 to 2013
| Chromosomal and plasmid-mediated resistance in Neisseria gonorrhoeae | Year | ||||
|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | 2012 | 2013 | |
| PPNG | 0.9 | 0.71 | 0.54 | 0.59 | 0.88 |
| TRNG | 1.64 | 1.72 | 2.56 | 3.39 | 5.48 |
| PPNG/TRNG | 1.54 | 1.41 | 3.1 | 2.70 | 3.13 |
| PPNG/CMRNG | 0.09 | 0.1 | 0.27 | 0.40 | 0.25 |
| TRNG/CMRNG | 0.06 | 0.07 | 0.12 | 0.20 | 0.19 |
| CMRNG | 15.19 | 21.82 | 17.68 | 15.28 | 13.08 |
| Probable CMRNG | 4.67 | 7.64 | 3.75 | 4.61 | 6.35 |
Discussion
The evolution of antimicrobial resistance in gonorrhea is complex and the emergence and spread of resistant isolates is a recognized global public health threat. Surveillance and monitoring of the antimicrobial susceptibilities of N. gonorrhoeae will continue to inform efforts to mitigate the impact of antimicrobial resistance in gonorrhea and guide therapeutic recommendations.
Reports of cefixime treatment failures and the observed MIC creep between 2001 and 2010 for both cefixime (from 0.016 mg/L to 0.125 mg/L) and ceftriaxone (from 0.016 mg/L to 0.063 mg/L) led to gonorrhea treatment changes. In 2011, the Canadian STI Guidelines issued updated recommendations for the use of combination gonorrhea therapy with 250 mg ceftriaxone intramuscularly and azithromycin 1 g orally as the first-line regimen in men-who-have-sex-with men (MSM) and in pharyngeal infectionsFootnote 15.
Since the 2011 changes to gonorrhea treatment recommendations in Canada there has been a decrease in the proportion of isolates with elevated MICs to the cephalosporins. In 2011, 7.6% of isolates exhibited decreased susceptibility to ceftriaxone and/or cefixime according to the WHO definition. This decreased to 5.9% in 2012 and further declined to 3.9% of isolates tested in 2013.
Fortunately, dropping rates of reduced cefixime susceptibility are also being seen elsewhere. For example, the US reported declines to decreased cefixime susceptibility from 3.9% in 2010 to 2.9% in the first half of 2012Footnote 16. The UK reported the prevalence of isolates with decreased cefixime susceptibility dropped from 17.1% in 2010 to 10.8% in 2011Footnote 17.
Enhancing surveillance to include linked epidemiological and laboratory data will assist with the limitations in the current passive surveillance system regarding data representativeness and interpretation. These improvements to the gonococcal surveillance program are expected with the Enhanced Surveillance of Antimicrobial Resistant Gonorrhea (ESAG) program beginning in 2014.
Acknowledgements
The full report was prepared by the National Microbiology Laboratory and the Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada. Its publication would not have been possible without the collaboration of all provinces and territories through the Canadian Public Health Laboratory Network (CPHLN), whose continuous contribution to National Neisseria gonorrhoeae Surveillance Program is greatly appreciated.
Conflict of interest
None
Funding
This work was supported by the Public Health Agency of Canada.
Page details
- Date modified: