Archived - Antiviral therapy for herpes simplex virus in an HIV-positive population

 Download this article as a PDF (209 KB - 8 pages)
Download this article as a PDF (209 KB - 8 pages) Published by: The Public Health Agency of Canada
Issue: Volume 42-2: Update on STIs
Date published: February 4, 2016
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 42-2, February 4, 2016: Update on STIs
Systematic review
Does suppressive antiviral therapy for herpes simplex virus prevent transmission in an HIV-positive population? A systematic review
Smith CR1,2*, Pogany L1, Auguste U1, Steben M3, Lau TTY4
Affiliations
1 Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada, Ottawa, ON
2 Dalla Lana School of Public Health, University of Toronto, Toronto, ON
3 STI Unit, Institut national de santé publique du Québec, Montréal, QC
4 Pharmaceutical Sciences, Vancouver General Hospital, Vancouver Coastal Health, and Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, BC
Correspondence
Suggested citation
Smith CR, Pogany L, Auguste U, Steben M, Lau TTY. Does suppressive antiviral therapy for herpes simplex virus prevent transmission in an HIV-positive population? A systematic review. Can Comm Dis Rep 2016;42-2:37-44. https://doi.org/10.14745/ccdr.v42i02a03
Abstract
Background: Among individuals with genital herpes simplex virus (HSV), co-infection with human immunodeficiency virus (HIV) has been shown to increase the frequency and severity of HSV symptoms, HSV shedding, and risk of HSV transmission.
Objective: To assess whether suppressive antiviral therapy for genital HSV in an HIV-positive population prevents HSV transmission to a susceptible partner.
Methods: A systematic search of the literature was conducted using MEDLINE and EMBASE databases to identify randomized controlled trials published between January 2005 and June 2015. Inclusion criteria were trials written in English or French utilizing suppressive antiviral therapies for HSV. Studies had to report on outcomes related to HSV transmission from HIV-positive populations. Surrogate markers of HSV transmission risk, such as HSV detection and viral load, were also included. Articles underwent a risk of bias assessment, and those with low risk of bias underwent data extraction to complete a narrative synthesis.
Results: This review identified thirteen papers. Only one study directly measured transmission of HSV. The overall transmission rate was <10%, and suppressive antiviral therapy had no significant protective effect (9% transmission rate in the acyclovir group vs. 6% in the placebo group; hazard ratio [HR]: 1.35, 95% CI: 0.83-2.20). The remaining 12 papers addressed surrogate markers of transmission risk: HSV detection and viral load. Suppressive acyclovir appears to be effective in reducing HSV detection among HIV-positive populations, but it does not appear to reduce viral load. Suppressive valacyclovir may be effective in reducing HSV detection and viral load among HIV-positive patients who are antiretroviral therapy (ART)-naïve, but its effect appears to be nullified among those concurrently on ART.
Conclusion: Based on current evidence, suppressive antiviral therapy may reduce HSV detection and viral load, but its impact on HSV transmission is unclear. Clinicians should caution HIV-positive patients with HSV that suppressive therapy may not reduce risk of HSV transmission to susceptible partners.
Introduction
Approximately 14% of Canadian adults tested positive for genital herpes simplex virus (HSV) type 2 in 2009 Footnote 1. HSV is particularly widespread among people with human immunodeficiency virus type 1 (HIV), affecting 50% to 90% of the HIV-positive population Footnote 2.
Genital HSV reactivation has been shown to increase HIV viral load, enhancing risk of HIV transmission and HIV disease progression Footnote 3Footnote 4. In turn, HIV has been shown to increase the frequency and severity of HSV symptoms, HSV shedding, and risk of HSV transmission Footnote 5Footnote 6. Given this interaction between HSV and HIV, prevention of HSV transmission among HIV-positive populations is a significant public health concern. Minimizing rates of HSV co-infection among HIV-positive individuals could prevent increases in HIV viral load, HIV transmission, and HIV progression that characterize HSV and HIV co-infection. Preventing HSV transmission from HIV-positive partners to HIV-negative partners is also important to public health as HSV infection has been estimated to increase the risk of HIV acquisition three-fold Footnote 7.
Suppressive therapy with acyclovir, famciclovir, or valacyclovir is routinely used in populations co-infected with HSV and HIV. These agents have been shown to reduce HSV detection and viral load, which have been linked to a reduced risk of HSV transmission in immunocompetent populations Footnote 8Footnote 9. Of interest is whether these agents also reduce risk of HSV transmission in HIV-positive populations. The most recent reviews, published in 2007 Footnote 10Footnote 11 but neither were able to find sufficient literature to evaluate HSV transmission in HIV-positive populations.
The objective of this systematic review was to assess randomized controlled trials in the HSV and HIV co-infected population, focusing on the impact of suppressive HSV antiviral therapies on HSV transmission, HSV detection, and HSV viral load.
Methods
Search strategy
A systematic search of MEDLINE and EMBASE databases was conducted to find articles on randomized controlled trials published within the last 10 years; previous reviews included searches for literature published prior to this. The search strategy used MeSH terms for "treatment outcome" and "Herpes simplex virus," along with relevant keywords (see text box).
| # | Search strategy |
|---|---|
| 1 | exp treatment outcome/ |
| 2 | drug efficacy/ or drug effect/ |
| 3 | (effic* or outcome*).mp. |
| 4 | 1 or 2 or 3 |
| 5 | exp *herpes simplex/ or (herpes or HSV1 or HSV2 or HSV-1 or HSV-2 or HSV).tw. |
| 6 | 4 and 5 |
| 7 | limit 6 to (randomized controlled trial and last 10 years) |
Eligibility criteria
Articles were eligible if they were written in English or French and had been published between January 2005 and June 2015 in peer-reviewed journals. Studies needed to report on genital HSV in HIV-positive adults (18 years or older) and describe a pharmacological intervention using a randomized controlled trial design. Articles were only included if the pharmacological comparison evaluated a suppressive treatment with at least one of the three most commonly used anti-HSV oral agents, acyclovir, famciclovir and/or valacyclovir. All eligible articles had to report on HSV transmission, detection, and/or viral load as outcomes. Studies that assessed topical treatments (e.g. gels or creams) were excluded due to their lack of availability outside the scope of clinical trials and the limited efficacy of the treatment. Studies in pregnancy and those that examined episodic treatments were outside the scope of the research question.
Study selection
Titles were screened and excluded if they were not related to HSV or HIV. Articles meeting this basic screening criterion were obtained for full-text review and assessed independently by two authors (CRS, UA) based on the eligibility criteria described above; a review resolved any discrepancies. References from relevant articles were screened and retrieved where appropriate. A formal quality assessment of each article meeting all the eligibility criteria was then performed independently by two authors (CRS, UA), using the Cochrane Collaboration's tool for assessing risk of bias Footnote 12. Studies with a high risk of bias were excluded.
Data extraction
Data were summarized by one author (CRS) in Microsoft Excel and then reviewed for accuracy and completeness by another (UA). Relevant data for each article included the patient population, country of study, pharmacological regimens, follow-up period, antiretroviral therapy (ART) status, CD4 cell count, number of transmissions of HSV (primary outcome), HSV detection and viral load (surrogate markers of transmission risk), effect measures, and adverse events.
The purpose of this review was to provide a narrative synthesis of the literature, so funnel plots and assessment of heterogeneity were not completed. A review protocol was not published for this review.
Results
A total of 492 papers were identified, 485 from databases and 7 through reference searches of relevant articles. Of these, 315 were unique. After screening and eligibility assessment, 14 studies remained to be assessed for risk of bias. One paper was excluded due to a high risk of bias Footnote 13 resulting in 13 papers being included in the systematic review (Appendix 1).
Study characteristics
The characteristics of the 13 studies are summarized in Table 1. Only one of the 13 studies directly measured HSV transmission Footnote 14. The remaining 12 papers focused on surrogate markers of transmission risk, that is, HSV viral detection and/or viral load. Of these, 7 studies addressed both HSV detection and genital HSV viral load Footnote 15Footnote 17Footnote 19Footnote 21Footnote 22Footnote 23Footnote 24 whereas 5 reported on HSV detection alone Footnote 16Footnote 17Footnote 19Footnote 23Footnote 24. All the studies utilized polymerase chain reaction (PCR) assays for both HSV detection and viral load.
The 13 clinical trials included 2,367 HSV and HIV co-infected participants. The majority of trials were conducted on populations in African countries (n = 7), and two were in Peru, two in the United States, and one in Thailand. One trial used a sample that spanned three continents. The majority of studies involved only female participants (n = 9), one used only males, and three included both sexes. Follow-up periods varied from 1 to 24 months. Eight studies included only participants who were ART-naïve, three included only individuals receiving ART, and two did not explicitly state ART status. Most trials either compared suppressive acyclovir (400 mg bid or 800 mg bid) to placebo (n = 7) or suppressive valacyclovir (500 mg bid or 1,000 mg qd) to placebo (n = 5). One study compared suppressive acyclovir (400 mg bid) to high-dose suppressive valacyclovir (1,000 mg bid). None of the studies used famciclovir. All clinical trials were evaluated to be of low risk of bias.
| Authors Year | NumberTable 1 - Note 1 | Country of study | Pharmacological regimen | Follow-up months | HIV treatment | Outcome measure |
|---|---|---|---|---|---|---|
| Mujugira et al.Footnote 14 2013 | 911 males and females | 7 (eastern and southern African countries) | Acyclovir (400 mg bid) versus placebo |
24 | Not receiving Antiretroviral therapy (ART) | HSV-2 transmission |
| Baeten et al.Footnote 15 2008 | 200 females | Peru | Valacyclovir (500 mg bid) versus placebo |
2 | Not receiving ART | Genital HSV-2 detection Genital HSV-2 viral load Adverse effects |
| Cowan et al.Footnote 16 2008 | 125 females | Zimbabwe | Acyclovir (400 mg bid) versus placebo |
3 | Not explicitly stated (however, ART was rarely available at the time of the study) | Genital HSV-2 detection |
| Delany et al.Footnote 17 2009 | 300 females | South Africa | Acyclovir (400 mg bid) versus placebo |
3 | Not receiving ART | Genital HSV-2 detection Genital HSV-2 viral load Adverse effects |
| Dunne et al.Footnote 18 2008 | 67 females | Thailand | Acyclovir (800 mg bid) versus placebo |
1 | Not receiving ART | Genital HSV-2 detection |
| Kim et al.Footnote 19 2010 | 76 males and females | South Africa, Zimbabwe, Zambia, Peru, United States | Acyclovir (400 mg bid) versus placebo |
6 | Not receiving ART | Genital HSV-2 detection Genital HSV-2 viral load |
| Nagot et al.Footnote 20 2007 | 140 females | Burkina Faso | Valacyclovir (500 mg bid) versus placebo |
3 | Not receiving ART | Genital HSV-2 detection Adverse effects |
| Ouedraogo et al.Footnote 21 2006 | 60 females | Burkina Faso | Valacyclovir (500 mg bid) versus placebo |
3 | All patients receiving highly active antiretroviral therapy (HAART) | Genital HSV-2 detection Genital HSV-2 viral load |
| Perti et al.Footnote 22 2013 | 34 males an females | United States | Acyclovir (400 mg bid) versus valacyclovir (1,000 mg bid) |
3 | Not receiving ART | Genital HSV-2 detection Genital HSV-2 viral load Adverse effects |
| Tanton et al.Footnote 23 2010 | 484 females | Tanzania | Acyclovir (400 mg bid) versus placebo |
24 | Not explicitly stated (Free ART became available in regional and district hospitals in the study area during the trial) | Genital HSV-2 detection Genital HSV-2 viral load Adverse effects |
| Tobian et al.Footnote 24 2013 | 96Table 1 - Note 1 females | Uganda | Acyclovir (400 mg bid) versus placebo |
24 | All patients receiving ART | Genital HSV-2 detection Genital HSV-2 viral load |
| Van Wagoner et al.Footnote 25 2015 | 34Table 1 - Note 2 females | United States | Valacyclovir (1,000 mg qd) versus placebo |
6 | All patients receiving ART | Genital HSV-2 detection Adverse effects |
| Zuckerman et al.Footnote 26 2007 | 20 males | Peru | Valacyclovir (500 mg bid) versus placebo |
2 | Not receiving ART | Genital HSV-2 detection Adverse effects |
HSV-2 transmission
HSV-2 transmission was only directly measured in one study, a well-designed trial where 911 sero-discordant heterosexual couples were followed for 24 months Footnote 14. Infected partners were HSV positive and HIV positive (not on ART) and were randomized to either suppressive treatment (acyclovir 400 mg bid) or placebo. Susceptible partners were HSV negative and HIV negative. In this study, suppressive acyclovir did not reduce transmission of HSV when compared with placebo. Transmission occurred in 9% (40/458) of the treatment group and in 6% (28/453) of the placebo group (hazard ratio [HR]: 1.35, 95% confidence interval [CI]: 0.83-2.20). HSV transmission was two times higher from males to females than from females to males.
HSV-2 detection
Twelve studies reported on genital HSV detection. These studies utilized pharmacological regimen and reported the percentage of participants, visits, or swabs/samples that were positive for HSV.
Acyclovir versus placebo
Six clinical trials that reported on HSV detection compared suppressive acyclovir to placebo (Table 2). Five of the six trials used a standard dose of acyclovir (400 mg bid) Footnote 16Footnote 17Footnote 19Footnote 23Footnote 24 whereas one had a higher dose of 800 mg bid Footnote 18. Overall, four of the six studies found a statistically significant effect of suppressive acyclovir treatment on HSV detection; two studies with ART-naïve patients Footnote 17Footnote 18 one with patients who initiated ART at study commencement Footnote 24 and one where ART status was not stated Footnote 16. An 800 mg bid dose appeared to have greater efficacy than 400 mg bid doses. Two of the six trials (both using a 400 mg bid dose) reported null findings--one with ART-naïve participants Footnote 19 and one where ART status was not stated Footnote 23. Only one of the acyclovir studies included both males and females, but this study did not report results by sex.
| Study | Acyclovir dose | Treatment for HIV | Genital herpes simplex virus (HSV) detection | Genital HSV viral loadTable 2 - Note 1 | ||||
|---|---|---|---|---|---|---|---|---|
| Proportion detected: treatment group | Proportion detected: placebo group | Estimate of effect | Treatment group | Placebo group | P value | |||
| Cowan et al., 2008Footnote 16 | 400 mg bid | Not explicitly stated | 10% of visits | 23% of visits | OR = 0.24 (95% CI: 0.12- 0.48) |
- | - | - |
| Delany et al., 2009Footnote 17 | 400 mg bid | Not receiving ART | 33% of patients | 54% of patients | RR = 0.61 (95% CI: 0.46- 0.80) |
Mean = 3.38 | Mean = 3.81 | p = 0.13 |
| Kim et al., 2010Footnote 19 | 400 mg bid | Not receiving ART | 19.4% of patients | 22.5% of patients | Not stated (but p = 0.07) |
Median = 6.50 | Median = 6.90 | p = 0.91 |
| Tanton et al., 2010Footnote 23 | 400 mg bid | Not explicitly stated | 10.9% of patients | 11.8% of visits | OR = 0.90 (95% CI: 0.60- 1.36) |
Mean = 4.16 | Mean = 4.07 | p = 0.73 |
| Tobian et al., 2013Footnote 24 | 400 mg bid | All patients receiving ART | 1.4% of visits | 10.2% of visits | OR = 0.13 (95% CI: 0.04- 0.41) |
Median = 3.52 | Median = 3.57 | p = 0.82 |
| Dunne et al., 2008Footnote 18 | 800 mg bid | Not receiving ART | 1.6% of patients | 42.4% of patients | RR = 0.00 (95% CI:.006- 0.33) |
- | - | - |
Valacyclovir versus placebo
Five clinical trials that reported on HSV detection compared suppressive valacyclovir to placebo (Table 3). Four studies used a 500 mg bid dose Footnote 15Footnote 20Footnote 21Footnote 26 whereas one used 1,000 mg qd Footnote 25. Three studies (all using a 500 mg bid dose) reported a statistically significant protective effect of suppressive valacyclovir on HSV detection Footnote 15Footnote 20Footnote 26 whereas one (also using a 500 mg bid dose) did not Footnote 21. One study (using a 1,000 mg qd dose) did not compare HSV detection between groups because of limited HSV detection in their sample Footnote 25. Of the three studies that reported a significant effect, all were in ART-naïve patients. The two studies that reported a null finding (or could not statistically compare groups) both had participants concurrently on ART. None of the valacyclovir studies sampled both males and females, and thus any differences based on sex could not be reported.
| Study | Valacyclovir dose | Treatment for HIV | Genital herpes simplex virus (HSV) detection | Genital HSV viral loadTable 3 - Note 1 | ||||
|---|---|---|---|---|---|---|---|---|
| Proportion detected: treatment group | Proportion detected: placebo group | Estimate of effect | Treatment group | Placebo group | P value | |||
| Baetan et al., 2008Footnote 15 | 500 mg bid | Not receiving ART | 3.7% of samples | 22.1% of samples | OR = 0.13 (95% CI: 0.07-0.24) |
Mean = 3.94 | Mean = 4.80 | p = 0.002 |
| Nagot et al., 2007Footnote 20 | 500 mg bid | Not receiving ART | 4.1% of visits | 17.9% of visits | RR = 0.29 (95% CI: 0.14-0.58) |
- | - | - |
| Ouedraogo et al., 2006Footnote 21 | 500 mg bid | All patients receiving highly active antiretroviral therapy (HAART) | 6.6% of visits | 9.8% of visits | OR = 0.37 (95% CI: 0.13-1.05) |
Mean = 3.95 | Mean = 4.98 | p = 0.12 |
| Van Wagoner et al., 2015Footnote 25 | 1,000 mg qd | All patients receiving ART | 3.8% of patients | 12.5% of patients | Not stated | - | - | - |
| Zuckerman et al., 2007Footnote 26 | 500 mg bid | Not receiving ART | 4% of samples | 29% of samples | Not stated (but p < 0.001) |
- | - | - |
Acyclovir versus valacyclovir
One study directly compared suppressive acyclovir (400 mg bid) to a high-dose suppressive valacyclovir group (1,000 mg bid) in ART participants Footnote 22 (Table 4). There was no significant difference in genital HSV detection between groups. Although this study did include males and females, results were not stratified by gender.
| Study | Dose | Treatment for HI | Genital herpes simplex virus (HSV) detection | Genital HSV viral load | ||||
|---|---|---|---|---|---|---|---|---|
| Proportion detected: Acyclovir group | Proportion detected: Valacyclovir group | Estimate of effect | Acyclovir treatment group | Valacyclovir treatment group | P value | |||
| Perti et al., 2013Footnote 22 | Valacyclovir group: 1,000 mg bid Acyclovir group: 400 mg bid |
Not receiving ART | 8.2% of days | 7.8% of days | RR = 0.95 (95% CI: 0.66-1.37) |
Median = 3.0 | Median = 3.0 | p = 0.67 |
HSV-2 viral load
Of the 12 studies that reported on genital HSV detection, 7 also reported the HSV viral load. These studies utilized PCR and reported either mean or median values for log10 copies/mL, with greater values suggestive of a potential increased risk of transmission.
Acyclovir versus placebo
Four clinical trials that compared suppressive acyclovir (400 mg bid) to placebo reported on genital HSV viral load Footnote 17Footnote 19Footnote 23Footnote 24 (Table 2). There were no significant differences in viral load between groups in any of these studies. Two studies had ART-naive participants Footnote 17Footnote 19 one had patients concurrently taking ART Footnote 24 and one did not state ART status Footnote 23. Only one of the acyclovir studies included both males and females, but this study did not report results based on sex.
Valacyclovir versus placebo
Two studies that compared suppressive valacyclovir (500 mg bid) to placebo reported on genital HSV viral load Footnote 15Footnote 21 (Table 3). One study found valacyclovir significantly reduced viral load in ART participants Footnote 15. The other did not find a significant difference between groups in a population concurrently on highly active ART Footnote 21. Neither study sampled both sexes so differences based on sex could not be noted.
Acyclovir versus valacyclovir
Compared to high-dose suppressive valacyclovir (1,000 mg bid), suppressive acyclovir (400 mg bid) demonstrated no significant difference in viral load among ART participants Footnote 22 (Table 4). Although this study did include males and females, results were not stratified by sex.
Adverse events
Acyclovir versus placebo
Of the seven clinical trials comparing acyclovir to placebo, only two reported on adverse events Footnote 17Footnote 23 both trials noted comparable rates in acyclovir and placebo groups. Adverse events included bacterial infections unrelated to treatment, HIV-related events, and malaria.
Valacyclovir versus placebo
Of the five studies comparing valacyclovir to placebo, four reported on adverse events. Two studies reported no serious adverse events, without going into any further detail Footnote 15Footnote 26. The remaining two studies had similar rates of adverse events in valacyclovir and placebo groups Footnote 20Footnote 25. One of these studies summarized frequencies of adverse events, with the most common being headaches (29% and 40%, in the valacyclovir group and placebo group, respectively), hypersensitivity reactions (15% and 21%), fatigue (15% and 25%), and nausea (16% and 10%) Footnote 20.
Acyclovir versus valacyclovir
The study comparing acyclovir to high-dose valacyclovir reported two adverse events related to high-dose valacyclovir Footnote 22. One participant developed urticaria and two participants with depression developed exacerbations.
Discussion
In our systematic review, only one study directly examined HSV transmission from an HIV-positive population and found no significant difference between suppressive acyclovir and placebo. Although this study had a large sample size and a lengthy follow-up period, confirmation of this finding is needed. Most studies included in this review focused on surrogate markers of HSV transmission risk, which included HSV detection and viral load. Overall, suppressive acyclovir appears to be successful in reducing HSV detection among HIV-positive populations, but it does not effectively reduce viral load. Given variations in acyclovir absorption Footnote 27 this contrary finding between HSV detection and HSV viral load may not be surprising. The effect of suppressive valacyclovir may be linked to ART status. Although this observation was based on a small sample of studies, it appears that ART may nullify the otherwise significant impact of valacyclovir therapy on HSV detection and viral load. However, this result should be interpreted with caution, as it could be confounded by the fact that individuals eligible for ART may be at a more advanced stage of HIV. In addition, the studies included in this review were not designed to specifically evaluate the impact of ART.
This systematic review provides updated information on a topic important to public health. Although previous reports assessed the impact of suppressive antiviral treatment on HSV and HIV co-infection, they were unable to specifically address HSV transmission in this population. One of the main strengths of our review is that it summarizes recent literature with a low risk of bias. In addition, all studies utilized PCR for HSV detection and viral load, overcoming the limitations of culture, which may vary more widely between studies. Based on our evaluation of the published literature, when prescribing suppressive antiviral treatment for those co-infected with HSV and HIV, clinicians should clearly articulate that treatment may not necessarily reduce risk of HSV transmission to an un-infected partner.
Further work is needed to confirm whether higher doses of acyclovir have a greater impact on viral load, and whether valacyclovir is only effective in ART populations. In addition, whereas resistance to acyclovir and valacyclovir is extremely low among immunocompetent populations, the resistance rate is approximately 5% in the HIV-positive population in North America Footnote 27Footnote 28. Further studies among patients with HSV and HIV are needed to address the impact of the notable level of resistance in this specific population.
Limitations of this review include the potential for publication bias and the exclusion of non-randomized controlled trials. Within the clinical trials, sample sizes, population characteristics, types of ART, follow-up periods, and reporting methods varied. Although most studies required participants to have a CD4 count higher than 200 cells/µL, there was variation within studies. Dosing regimens for antivirals also varied. In addition, biological measures of adherence were not used in any study; thus, reported compliance may have been inaccurate. It is also important to note that all of the studies addressing detection and viral load were powered on HIV-related outcomes, rather than the outcomes of interest in this review. As a result, it is possible that in some cases, sample sizes may have been too small to detect a difference in HSV detection or viral load. Lastly, the majority of studies were set in developing countries, with heterosexual populations; the generalizability of these results to a Canadian context and to populations of men who have sex with men may be limited.
Conclusion
Although suppressive antiviral therapies may reduce HSV detection and viral load in those with HIV co-infection, their impact on HSV transmission needs to be confirmed. When prescribing suppressive antivirals for patients with HSV and HIV co-infection, clinicians should caution patients that suppressive therapy may not reduce risk of HSV transmission to susceptible partners.
Acknowledgements
The authors would like to thank Shalane Ha for her work as a third reviewer for eligibility screening and risk of bias assessment, and Portfolio Librarian Ella Westhaver for her assistance with the systematic search and retrieval of literature. The authors would also like to thank Margaret Gale-Rowe, Karen Timmerman, Jun Wu and Cathy Latham-Carmanico for their helpful comments on this review.
Conflict of interest
There are no conflicts of interest to declare.
Funding
No funds were received for this study.
Appendix 1: Flowchart showing selection of randomized control trials for review
Appendix 1: Flowchart showing selection of randomized control trials for review
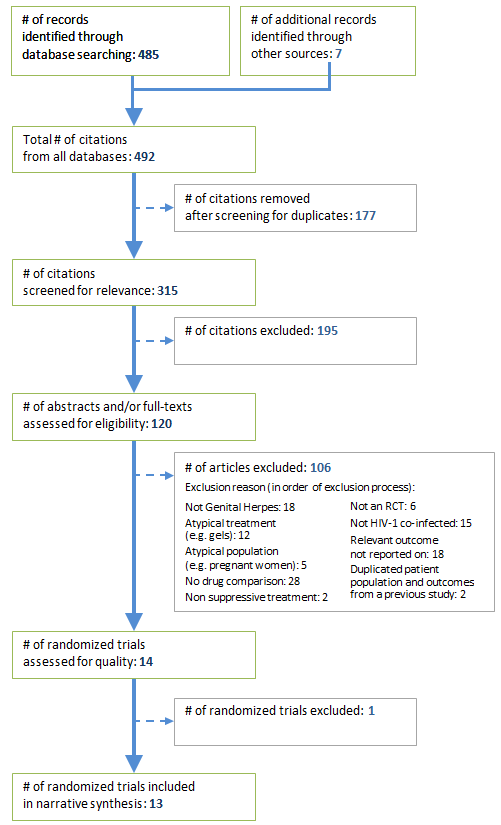
Text description: Appendix figure
Appendix 1: Flowchart showing selection of randomized control trials for review
In total, 492 articles were identified from the search; 485 through database searches and 7 through reference searches of relevant articles. After removal of duplicates, 315 articles remained and were screened for relevance to HSV and/or HIV. One-hundred and ninety-five articles were excluded for not indicating HIV or HSV in their titles. The remaining 120 articles were examined for eligibility, and 106 were excluded. Reasons for exclusion included non-genital HSV (n=18), the use of an atypical treatment (n=12), the use of an atypical population (e.g. pregnant women) (n=5), a lack of drug comparison (n=28), not including suppressive treatment (n=2), not using a randomized controlled trial design (n=6), no HIV-1 co-infection (n=15), lack of reporting on relevant outcomes (n=18), and duplicated patient population and outcomes reported from a previous study (n=2). Fourteen articles were then assessed for risk of bias, and 1 study was excluded. The remaining 13 studies were included in the qualitative analysis.