Archived - Travel and drug resistant bacteria

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 44-11: Healthcare-associated infections and antimicrobial resistance
Date published: November 1, 2018
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 44-11, November 1, 2018: Healthcare-associated infections and antimicrobial resistance
Overview
Bringing home unwelcome souvenirs: Travel and drug-resistant bacteria
BJ Langford1,2, KL Schwartz1,2,3
Affiliations
1 Public Health Ontario, Toronto, ON
2 St. Joseph’s Health Centre, Toronto, ON
3 Dalla Lana School of Public Health, Toronto, ON
Correspondence
Suggested citation
Langford BJ, Schwartz KL. Bringing home unwelcome souvenirs: Travel and drug-resistant bacteria. Can Commun Dis Rep 2018;44(11):277–82. https://doi.org/10.14745/ccdr.v44i11a02
Keywords: antimicrobial resistance, travel, antimicrobial stewardship, infection prevention and control, medical tourism
Visual Abstract
Visual Abstract

Description for Visual Abstract
GLOBAL TRAVEL INCREASES RISK OF EXPOSURE TO DRUG RESISTANT BACTERIA
Column 1: What is the risk? The most common is resistant Enterobacteriacea that can cause: travellers’ diarrhea, bronchitis/pneumonia, urinary tract infections. Travellers may also become a carrier and pass it on to others
Column 2: Where is the risk? Highest risk is in Asia (especially the south) but also in Africa and the Middle East, the Caribbean and Central America, South America.
Column 3: Best practices. Ask about travel in the past year, especially in those with an infection unresponsive to antibiotics. Educate patients regarding: risk areas, hand hygiene and safe food practices, symptomatic treatment of mild diarrhea, and minimal use of healthcare services.
Reference: Langford BJ, Schwartz KL. Bringing home unwelcome souvenirs: Travel and drug-resistant bacteria. Can Commun Dis Rep 2018;44(11):277−82. https://doi.org/10.14745/ccdr.v44i11a02
Abstract
Antimicrobial resistance poses a significant threat to public health globally and in Canada. Wide regional variability in antimicrobial resistance and ongoing increases in global travel present an important risk for the acquisition and transmission of drug-resistant organisms. Travel from high-income to low- and middle‑income countries, particularly the Indian subcontinent, present the greatest risks for acquiring a drug-resistant Enterobacteriaceae. Risk factors for returning from travel with drug-resistant organisms include seeking medical care while abroad, travellers’ diarrhea and antibiotic use. Health care professionals can play an important role in preventing harm for travellers by counselling patients on the risks of acquiring drug-resistant organisms, appropriate antibiotic prescribing for travellers’ diarrhea and tailored empiric therapy for patients presenting with infection after travel.
Introduction
Antimicrobial resistance (AMR) is a growing problem globally. It is identified as one of the most significant public health threats of our time. In the absence of meaningful intervention, it is estimated that deaths from drug-resistant infections will increase from 700,000 to 10 million annually by 2050, surpassing current cancer rates as the number one cause of death Footnote 1. Moreover, the prevalence of drug-resistant organisms across the globe varies widely. For example, resistance of Escherichia coli to third-generation cephalosporins is much more common in India, at 78%, than in Canada, at 9% Footnote 2.
In this global society, AMR knows no borders. In 2017, the number of passengers using air travel exceeded four billion for the first time; this number is forecast to double by 2036 Footnote 3.
One of the big challenges of AMR with respect to travel is infections from drug-resistant Enterobacteriaceae. Enterobacteriaceae are a large family of gram‑negative bacilli that can cause a wide range of infections including those affecting the urinary, respiratory and gastrointestinal tracts. Organisms in this family include E. coli, Klebsiella species, Enterobacter species and Salmonella species. A key mechanism of Enterobacteriaceae antibiotic resistance is the development of beta‑lactamase and carbapenemase enzymes, which hydrolyze beta-lactam antibiotics, rendering them ineffective. Genes for these enzymes are commonly encoded by plasmids, which can transfer between bacterial organisms. Key groups of Enterobacteriaceae resistance are extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL‑PE), and carbapenem-resistant Enterobacteriaceae (CRE). Of growing importance is the plasmid-mediated colistin-resistance gene mcr-1. The ability of bacteria to transfer antimicrobial resistant genes to one another via plasmids poses significant infection control challenges. A rise in Enterobacteriaceae resistance translates to more infection-related mortalities, longer hospital stays and increased costs to the health care system Footnote 4Footnote 5.
The objective of this article is to describe the clinically relevant risk of drug-resistant organisms associated with travel, with a focus on Enterobacteriaceae. For the purposes of this review, travel is defined as movement of people travelling to low- and middle-income countries in Asia, Africa and the Americas, and returning to high-income countries such as Australia, Canada, New Zealand, United States (US) and those in Europe.
What is the risk of bringing home drug-resistant bacteria after travel?
The studies on the risk of travellers acquiring either ESBL‑PE or CRE are sobering and have important considerations to the management of patients in Canada.
Extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL‑PE)
One of the largest studies to evaluate the risk of acquiring a drug-resistant organism during travel focused on the importation of ESBL‑PE to the Netherlands. Through a longitudinal cohort study of 2001 travellers, the authors determined the likelihood of ESBL‑PE colonization before and after the travel period. Of those individuals who did not have an ESBL‑PE prior to travel, 34% acquired an ESBL‑PE while abroad Footnote 6. There was marked variability in the risk of ESBL‑PE colonization associated with the region visited. Travel to southern Asia presented the highest risk, at 75% incidence of colonization, followed by central and eastern Asia (49%), western Asia (43%), southeastern Asia (37%), the Caribbean and Central America (28%), middle and eastern Africa (28%), western Africa (19%), South America (18%) and southern Africa (6%). The median duration of colonization after travel was 30 days (95% confidence interval [CI]: 29–33 days). However, 11.3% remained colonized after 12 months, highlighting the importance of identifying an individual’s travel history within the previous year. Multiple other smaller studies have also evaluated the risk of ESBL‑PE acquisition while travelling Footnote 7. The average risk of ESBL‑PE colonization after travel is 643 per 1,000 travellers from the Indian subcontinent, 340 per 1,000 travellers from Africa and 186 per 1,000 travellers from Central and South America (Figure 1). Although the majority of studies focus on the risk of colonization with drug-resistant organisms, recent travel has been associated with an increased risk of ESBL‑PE urinary tract infections Footnote 8Footnote 9Footnote 10Footnote 11 and bacteremia with ESBL‑PE post-transrectal prostate biopsy Footnote 12.
Figure 1: The number of extended spectrum beta-lactamase (ESBL) producing and carbapenem-resistant Enterobacteriaceae (CRE) per 1,000 travellers by region visited
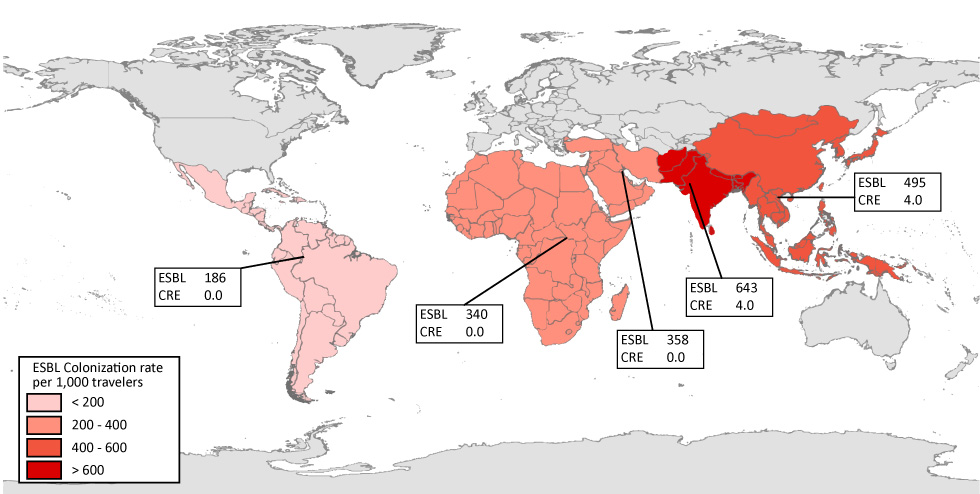
Text description: Figure 1
Figure 1: The number of extended spectrum beta-lactamase (ESBL) producing and carbapenem-resistant Enterobacteriaceae (CRE) per 1,000 travellers by region visited
The number of drug-resistant organisms detected per 1000 healthy travellers. The risk of travellers returning with a drug-resistant bacterial organism varies by region visited and type of organism. The highest risk has been observed in travellers from the Indian subcontinent. ESBL = extended-spectrum beta-lactamase; CRE = carbapenem-resistant. Data for figure based on weighted average of published studies Footnote 7.
Carbapenem-resistant Enterobacteriacae (CRE)
CRE infections, which have also been increasing around the world, are of particular concern due to the limited treatment options and the high infection-related mortality rates of 40% to 70% Footnote 13Footnote 14. The three main classes of carbapenemase-producing Enterobacteriaceae that confer carbapenem resistance have distinct regional epidemiology Footnote 15:
- Klebsiella pneumoniae carbapenemase (KPC) is the most common carbapenemase-producing Enterobacteriaceae in North America
- OXA-48-like carbapenemases are typical in Turkey and surrounding regions
- New Delhi Metallo-beta-lactamase-1 (NDM-1) was initially associated with those who had received medical care in the Indian subcontinent, but has since been reported in every continent Footnote 16
The Canadian Nosocomial Infection Surveillance Program (CNISP) recently characterized carbapenemase-producing Enterobacteriaceae reported in hospitals across Canada from 2010 to 2014. The incidence was 0.07 cases per 1,000 admissions, with KPC and NDM-1 being the most common. Many of those affected had a history of international travel. India was the most common travel destination with 31% of cases reporting travel to that country within the previous 12 months Footnote 17. However, the risk of acquiring a CRE while travelling is considerably lower than the risk of acquiring an ESBL‑PE (Figure 1).
Colistin-resistance gene, mcr-1
Of further concern is the recently described plasmid-mediated colistin resistance gene mcr-1 which can be co-located with other gram-negative resistance mechanisms. Colistin is one of few antibiotic options for managing CRE; however, it carries significant risk of nephrotoxicity and neurotoxicity. Initially discovered in animal and human clinical isolates in China, mcr-1 has now been reported in clinical isolates globally Footnote 18. A recent Dutch study found that 5% of long-distance travellers had mcr-1 in fecal samples. These travellers had primarily visited southeast Asia or southern Africa Footnote 19.
Travel is also playing a role in the spread of other drug-resistant bacterial organisms such as Salmonella, Shigella and Campylobacter species, which has been reviewed elsewhere Footnote 7.
What are the risk factors for acquiring drug-resistant organisms while travelling?
Health care exposure
Recent health care exposure abroad has been noted as a risk factor for acquiring a drug-resistant organism. In the CNISP evaluation of Canadian patients with CRE, of those with a travel history available, 86% had sought medical care abroad Footnote 17. The association between health care exposure while travelling and drug-resistant organisms has also been observed in a number of European studies Footnote 20Footnote 21Footnote 22Footnote 23Footnote 24Footnote 25Footnote 26Footnote 27. Among those returning home after being hospitalized abroad, colonization with any multidrug-resistant organism (MDRO) ranged from 7% Footnote 21 to 29% Footnote 23. In these studies, MDROs were defined as ESBL‑PE, CRE, other multidrug-resistant gram-negative organisms, methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. The greatest risk was for patients who were transferred directly or repatriated from hospitals abroad compared to those not directly repatriated (odds ratio [OR]=7.4; 95% CI: 2.1–25.2) Footnote 27. Other risk factors include a longer hospital stay abroad Footnote 20Footnote 21; history of a surgical procedure abroad Footnote 22; admission to a high-risk unit (i.e. intensive care unit) Footnote 21; tropical or subtropical country visited (particularly South Asia) Footnote 21Footnote 24; and receipt of antibiotics while hospitalized Footnote 21Footnote 22Footnote 23.
Although these studies included travellers with either an elective or emergent reason for hospitalization, this risk is likely applicable to medical tourists, that is, those who travel abroad for the specific purpose of accessing medical care Footnote 28. Global estimates indicate that there are about four million medical tourists annually Footnote 29; a Canadian survey indicates that over 63,000 patients sought medical care abroad in 2016 Footnote 30. Most Canadian medical tourists seek care in the US, followed by low- and middle-income countries in the Americas and Asia Footnote 31, which includes regions with elevated rates of drug-resistance. Given the risk of acquiring drug-resistant organisms while travelling, particularly for individuals who access health care systems abroad, this poses an important and often underestimated risk for those who are considering medical tourism.
Travellers’ diarrhea
Travellers’ diarrhea is caused by ingesting contaminated food or beverages containing bacterial enteropathogens Footnote 32. Depending on the travel location and host factors, the incidence of diarrhea during travel can range from 10% to 40%. In several studies of travellers acquiring drug-resistant organisms abroad, travellers’ diarrhea was noted as a significant risk factor, particularly for acquiring an ESBL‑PE. Travellers’ diarrhea is associated with an approximate 2- to 3-fold increased risk of acquiring an ESBL‑PE abroad Footnote 6Footnote 33Footnote 34. In a study of Finnish travellers, this risk of acquiring an ESBL‑PE was 11% in those without travellers’ diarrhea, 21% in those with travellers’ diarrhea who did not take antibiotics and 37% in those with travellers’ diarrhea who were treated with antibiotics Footnote 33.
Antibiotic exposure
Antibiotics apply selective pressure to the native organisms colonizing the gut, increasing the risk that drug-resistant organisms contracted abroad are incorporated into the microbiome. Treatment with antibiotics has been repeatedly demonstrated to present a risk to travellers. In the previously mentioned Dutch study of travellers who acquired ESBL‑PEs abroad, antibiotic use was associated with a greater than 2-fold risk of acquiring these drug-resistant organisms (OR=2.7; 95% CI: 1.8–4.0) Footnote 6. In a Finnish study, 21% of those who received no treatment, 20% of those treated with the anti-diarrheal medication loperamide alone, 40% of those treated with antibiotics alone and 71% of those treated with both loperamide and antibiotics became colonized with ESBL‑PE Footnote 35.
Among travellers hospitalized abroad, the risk associated with antibiotic treatment was also pronounced, with an 11-fold higher risk of being colonized with an MDRO (OR=10.7; 95% CI: 4.2–27.3) compared to those who did not travel abroad; however, being hospitalized abroad and not receiving an antibiotic was not a risk factor for MDRO colonization in this study Footnote 26. The importance of antibiotic exposure during a travel-associated hospitalization is echoed in a large study in Finland, where risk of colonization with an MDRO was significantly increased in those receiving antibiotics (OR=3.2; 95% CI: 2.3–4.5) Footnote 23. Similarly, a study in the Netherlands found a 2.5- to 3.4-fold increased risk of gram-positive MDRO colonization in those who had been treated with antibiotics while hospitalized abroad Footnote 20.
What can clinicians do to minimize harm in Canadian travellers?
By understanding the risk of AMR associated with travel, health care professionals will be better able to implement approaches to improve management and reduce transmission of drug-resistant organisms as well as educate the public to make informed decisions (Figure 2). Opportunities for clinicians include:
- counselling patients, pretravel, on the risk of acquiring a drug-resistant organism, tailored to the patient’s itinerary and specific region of travel (Figure 1)
- counselling patients on the risks of unplanned health care exposure abroad; minimizing the risk through pretravel immunizations and counselling on how to prevent travellers’ diarrhea and avoid high risk activities
- counselling patients on the risks of medical tourism, tailored to the patient’s itinerary and specific region of travel (Figure 1)
- when considering a prescription for anticipatory travellers’ diarrhea prior to travel, given the risk of acquiring an ESBL‑PE, understanding that recent guidelines encourage supportive care only for mild travellers’ diarrhea and that antibiotic prophylaxis for travellers’ diarrhea is indicated only in select patients at high risk for complications Footnote 36; and
- considering recent travel (within the past 12 months) when selecting empirical antimicrobial therapy for patients who have a severe infection (patients who have travelled to Asia, particularly the Indian subcontinent, should be considered at very high risk for drug-resistant Enterobacteriaceae)
Figure 2: Opportunities to manage risk of acquiring drug-resistant organisms from travelling
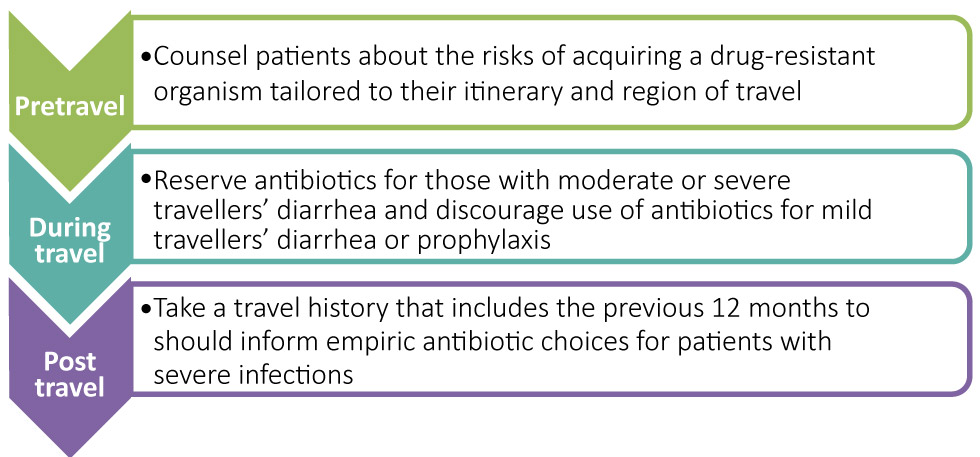
Text description: Figure 2
Figure 2: Opportunities to manage risk of acquiring drug-resistant organisms from travelling
Figure 2 is an image of three areas where clinicians can implement approaches to improve and educate the public to make informed decisions regarding travel:
Post travel – Travel history within the previous 12 months should inform empiric antibiotics choices for patients with severe infections
During travel – Antibiotics should be reserved for those with moderate or severe travellers’ diarrhea and discouraged as prophylaxis to reduce the risk of acquiring a drug-resistant organism
Pretravel – Counsel patients about the risk of acquiring a drug-resistant organism tailored to their itinerary and region of travel
Conclusion
Travelling abroad carries a significant risk for acquiring a drug-resistant organism. Asia and the Indian subcontinent in particular present the greatest risks for acquiring an ESBL‑PE or CRE. Medical care, travellers’ diarrhea and antibiotic use abroad further increase the risks for travellers. Health care professionals can play an important role in reducing the risk for travellers through counselling, appropriate antibiotic prescribing and tailored empirical therapy for patients presenting with severe infections who have travelled recently.
Conflict of interest
None.
Acknowledgements
We would like to thank Sean Marshall from Public Health Ontario for creating the map figure.