Archived - LGV in Quebec

 Download this article as a PDF
Download this article as a PDF Published by: The Public Health Agency of Canada
Issue: Volume 44-2: Sexually transmitted infections
Date published: February 1, 2018
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 44-2, February 1, 2018 Sexually transmitted infections
Surveillance
Lymphogranuloma venereum in Quebec: Re-emergence among men who have sex with men
CA Boutin1, S Venne2, M Fiset2, C Fortin1,3, D Murphy3, A Severini4, C Martineau1,3, J Longtin3, AC Labbé1,3*
Affiliations
1 Département de microbiologie, infectiologie et immunologie, Université de Montréal, Montréal, QC
2 Ministère de la santé et des services sociaux du Québec, Montréal, QC
3 Institut national de santé publique du Québec, Montréal, QC
4 National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, MB
Correspondence
Suggested citation
Boutin CA, Venne S, Fiset M, Fortin C, Murphy D, Severini A, Martineau C, Longtin J, Labbé AC. Lymphogranuloma venereum in Quebec: Re-emergence among men who have sex with men. Can Commun Dis Rep. 2018;44(2):55-61. https://doi.org/10.14745/ccdr.v44i02a04
Abstract
Background: Lymphogranuloma venereum (LGV) is a sexually transmitted infection (STI) caused by Chlamydia trachomatis genotypes L1, L2 and L3. This LGV is associated with significant morbidity and increased risk of HIV transmission. While fewer than two cases per year were reported in Quebec before 2005, LGV emerged in 2005–2006 with 69 cases, followed by a period of low incidence (2007–2012), and subsequent re-emergence since 2013.
Objectives: To describe the incidence of LGV in Quebec and the characteristics of the affected population, including demographics and risk factors, clinical manifestations, laboratory tests, treatments and reinfection rates.
Methods: Descriptive data were collected from the notifiable diseases records through the Institut national de santé publique du Québec (INSPQ) infocentre portal. Questionnaires were obtained through the enhanced surveillance system and transmitted anonymously to the Quebec Ministry of Health. In-depth analysis was performed on cases from 2013 to 2016.
Results: There were 338 cases of LGV over the four–year period in Quebec. All cases were male, excluding one transsexual. Mean age was 41 years. Most lived in Montréal (81%) and were men who have sex with men (MSM; 99%). The majority (83%) reported four sexual partners or more in the last year, met mostly through the Internet (77%) and in saunas (73%). Frequency of sexual intercourse with out–of–province residents decreased in 2013–2016 (27%) compared with 2005–2012 (38%). History of STIs was frequent: 83% were HIV-infected, 81% reported previous syphilis and 78% previous gonorrhea. Recreational drug use was frequent (57%), reaching 71% in 2016. Most cases were symptomatic, a proportion which decreased in 2016 (68%) compared with 2013–2015 (82%; p=0.006). Clinical presentations included proctitis (86%), lymphadenopathy (13%) and ulcer/papule (12%). Reinfections, mostly within two years of first infection, occurred in 35 individuals (10%).
Conclusion: The re-emergence of LGV in Quebec involves an urban subpopulation composed almost exclusively of MSM with STIs, who have a high number of partners and often use drugs.
Introduction
Lymphogranuloma venereum (LGV) is a sexually transmitted infection (STI) caused by Chlamydia trachomatis genotypes L1, L2 and L3 (L1–3). It is associated with anogenital fistula, stenosis formation and lymphatic obstruction, among othersFootnote 1 and increased risk of HIV transmissionFootnote 1Footnote 2Footnote 3. This information was rarely reported in industrialized countries until the early 2000s, but it has since been described in urban settings, mainly among men who have sex with men (MSM). Recent outbreaks occurred in Belgium, France, the Netherlands, the United Kingdom and the United States of AmericaFootnote 2. Over the last decade, LGV emerged in Canada, with sporadic outbreaks mainly in major urban centresFootnote 3Footnote 4Footnote 5.
In Quebec, fewer than two cases per year were reported before a significant rise was noted in 2005 and 2006 with a total of 69 cases. A period of low incidence followed from 2007 to 2012, with a mean of nine cases per year. In 2013, an evolving outbreak started in Montréal.
The classical presentation of LGV is an inguinogenital disease, with an ulcer or papule at the site of inoculation and frequently a unilateral inguinal or femoral lymphadenopathyFootnote 1. Many young patients are now presenting with proctitis or proctocolitis, often mimicking the first manifestations of inflammatory bowel diseaseFootnote 2. Prevalence studies have recently found a higher proportion of asymptomatic cases, representing up to 25–27% of cases in MSMFootnote 6Footnote 7.
In 2014, the Public Health Agency of Canada (PHAC) revised its recommendations to encourage LGV genotyping on positive C. trachomatis specimens among asymptomatic MSM with risk factors, regardless of sampled site this was further updated in 2017 to focus on rectal specimens onlyFootnote 3. Following expert advice from the Institut national de santé publique du Québec (INSPQ)Footnote 8 systematic genotyping of all positive rectal specimens for C. trachomatis (regardless of symptomatology) was implemented in June 2016 (the value of this strategy will be assessed after two years). The INSPQ also provides guidelines on treatment of cases and partnersFootnote 8.
Given the growing epidemic, enhanced surveillance of LGV cases was pursued in Quebec with the goal of implementing targeted public health interventions.
The objective of this article is to describe the Quebec 2013–2016 LGV epidemic and present epidemiologic data including demographic details, risk factors, clinical manifestations, laboratory tests, treatment and reinfection.
Methods
Until 2005, LGV was part of routine surveillance and notifiable to public health authorities. In 2005, PHACFootnote 9 and the Quebec Ministry of Health initiated an enhanced surveillance system for LGV, whereby when a case is notified, key epidemiologic and clinical information are collected by public health nurses who contact the attending physicians or the patients directly. The nurses administer an epidemiologic questionnaire to collect information about patient demographics (age, sex, area of residence), risk factors (number, sex and meeting context of partners; history of sexually transmitted or blood-borne infections [STBBIs]; drug use), clinical presentation, laboratory tests performed and treatment information. In an attempt to prevent missing any data, epidemiologic questionnaires were revised over the surveillance period and medical teams were contacted, if necessary.
For this present surveillance report, descriptive data for all LGV cases in Quebec from 2013 to 2016 were collected from the notifiable diseases database through the INSPQ infocentre portal and enhanced surveillance questionnaires were transmitted anonymously to the provincial Ministry of Health for compilation and analysis. Surveillance definitions changed over time. In 2013–2014, confirmed cases included:
- Patients with proctitis, inguinal/femoral lymphadenopathy or contact with a confirmed LGV case
- Isolation of C. trachomatis or detection by nucleic acid amplification testing (NAAT) from an appropriate clinical specimen and L1–3 genotype documented through DNA sequencing
In 2015, genotyping alone became sufficient to consider a case confirmed and clinical manifestation or contact with a case were no longer required. Probable cases included patients who presented with proctitis or inguinal/femoral lymphadenopathy or had a contact with a confirmed LGV case and had a positive test for C. trachomatis from an appropriate clinical specimenFootnote 10.
Until February 2016, all genotyping was performed at the National Microbiology Laboratory, in Winnipeg, Manitoba. In March 2016, a multiplex polymerase chain reaction (PCR) assay that distinguishes LGV from non-LGV C. trachomatis infectionsFootnote 11 became available at Laboratoire de santé publique du Québec, Sainte-Anne-de-Bellevue, Quebec. Thereafter, only positive LGV samples were sent to the National Microbiology Laboratory for genotype identification by DNA sequencing.
All cases notified in 2013–2016 were investigated to identify any previous episodes since January 1, 2005. A reinfection was defined as a second infection in the same individual occurring at least 90 days after the first episodeFootnote 12.
Data were analyzed for all 338 cases (or cases for which information was available for a specific variable) notified between 2013 and 2016, excluding one transsexual case (man to woman). Data were analyzed with statistical software Epi Info version 7.2.0.1 and Stata 64 (version 10.1). Proportions were compared using the x2 test. The epidemiologic portrait presented here focuses on the 2013–2016 outbreak in Quebec, with parallels to preceding years (i.e. 2005–2012) only where appropriate.
Results
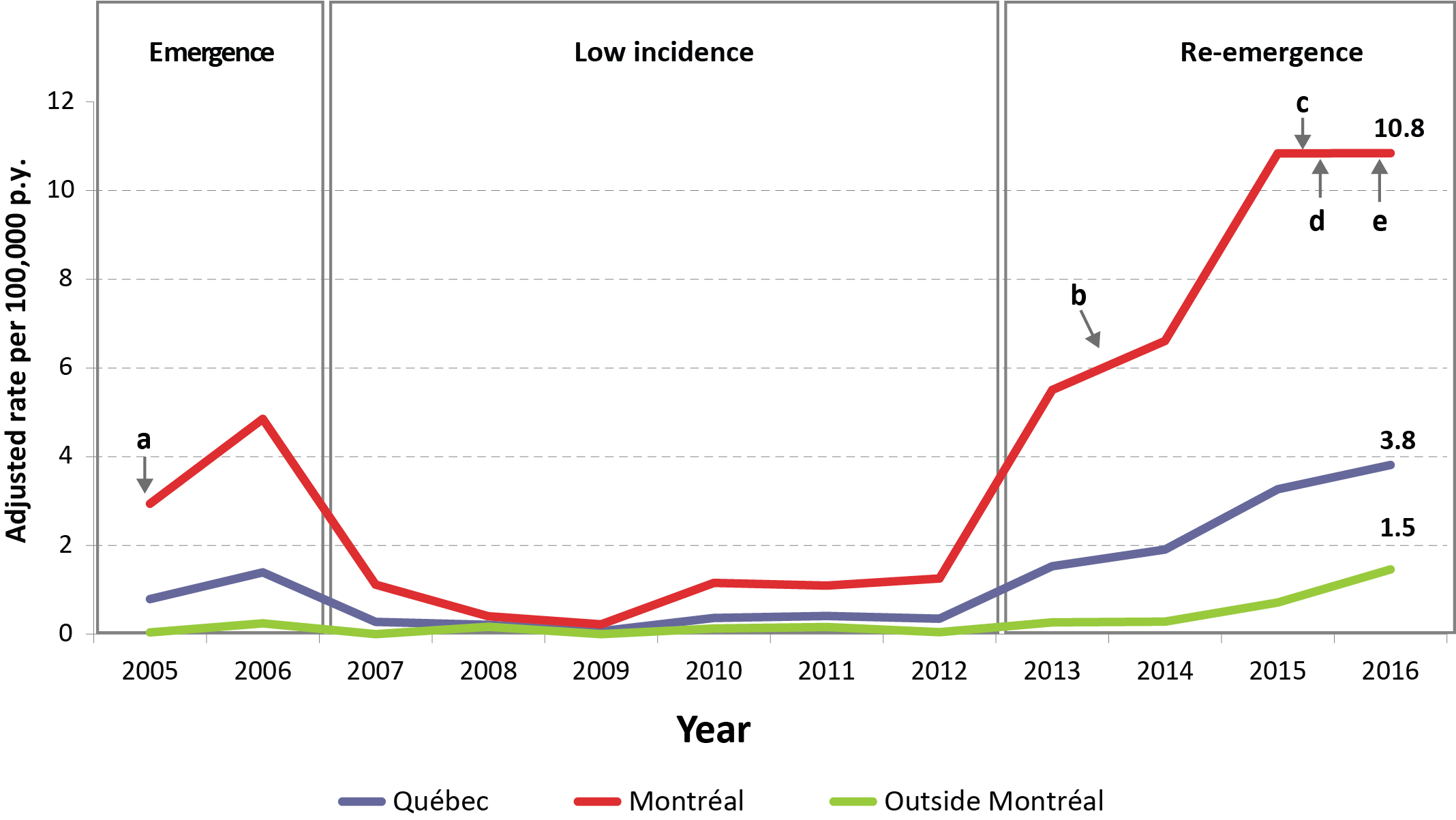
From January 1, 2013, to December 31, 2016, 338 cases (328 confirmed and 10 probable) were reported: 49 in 2013, 61 in 2014, 105 in 2015 and 123 in 2016 (Figure 1).
Figure 1: Lymphogranuloma venereum among men: annual adjusted rate per 100,000 person years in Quebec, Montréal and outside Montréal, 2005–2016
Text description: Figure 1
Figure 1: Lymphogranuloma venereum among men: annual adjusted rate per 100,000 person years in Quebec, Montréal and outside Montréal, 2005–2016
| Year | Emergence | Low incidence | Re-emergence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
| Quebec | 0.79 | 1.39 | 0.28 | 0.21 | 0.06 | 0.36 | 0.41 | 0.35 | 1.53 | 1.91 | 3.27 | 3.81 |
| Montréal | 2.94Footnote a | 4.85 | 1.12 | 0.40 | 0.22 | 1.16 | 1.10 | 1.25 | 5.51 | 6.61Footnote b | 10.84 | 10.84Footnote cFootnote dFootnote e |
| Outside Montréal | 0.04 | 0.24 | 0.00 | 0.16 | 0.00 | 0.13 | 0.16 | 0.04 | 0.26 | 0.28 | 0.71 | 1.46 |
Abbreviation: p.y., person-year
|
||||||||||||
Demographic and risk factors
The majority of cases were in the 25–54 year age group (83%) and in Montréal (81%) (Table 1). Most patients were MSM (99%), with 3% (n=7) also having female partners. Half of the patients reported more than 10 sexual partners over the last 12 months (101/205; 49%). Almost all (96%) had a new partner and 84% had anonymous sexual partners. The proportion of cases with more than 20 sexual partners in the last year was higher in Montréal than in the rest of the province (27% vs 3%; p<0.001). Saunas and Internet (including smartphone networking applications) were two important means of meeting partners. Although not statistically significant (p=0.07), fewer patients (27%) had encounters with partners from outside the province in the last year in contrast to preceding years (2005–2012; n=31/81; 38%). Among cases living outside of Montréal, 16/42 (38%) reported partners from Montréal or out of the province.
A high proportion of patients (210/252; 83%) were HIV-seropositive (a considerable augmentation from 2005–2012: 48/82; 59%; p<0.001). Past histories of syphilis (183/226; 81%) and gonococcal (165/211; 78%) infections were also frequently reported. Data on hepatitis B virus (HBV) and hepatitis C virus (HCV) infection were available for only one-third of cases, of which 11% and 10% had a history of past or current infection. The proportion of cases with a history of at least one STBBI was significantly higher in 2013–2016 (286/295; 97%) than in 2005–2012 (95/112; 85%; p<0.001).
Information on drug consumption was available for 241/338 cases, of whom 138 (57%) reported drug use during the past year. Crystal meth (21%), marijuana (20%) and ecstasy (18%) were the most frequently used drugs. A significant rise in crystal meth consumption was observed: 8% (3/38) in 2013, 12% (6/49) in 2014, 25% (19/77) in 2015 and 29% (22/76) in 2016 (test for trend, p=0.008).
| Characteristic | 2013–2016 | |
|---|---|---|
| n | % | |
| Age (n=338) | ||
| 15–24 | 23 | 7 |
| 25–34 | 98 | 29 |
| 35–44 | 92 | 27 |
| 45–54 | 83 | 25 |
| >55 | 42 | 12 |
| Demographics (n=338) | ||
| Montréal | 273 | 81 |
| Outside of Montréal | 65 | 19 |
| Sex of partners (n=287) | ||
| Men only | 279 | 97 |
| Men and women | 7 | 2 |
| Women only | 1 | 0.3 |
| History of STBBIsFootnote b | ||
| At least one | 286/295 | 97 |
| HIV | 210/252 | 83 |
| Syphilis | 183/226 | 81 |
| Gonorrhea | 165/211 | 78 |
| HBV | 11/102 | 11 |
| HCV | 11/106 | 10 |
| Number of partners (n=205) in last 12 months | ||
| 1–3 | 34 | 17 |
| 4–10 | 70 | 34 |
| 11–20 | 55 | 27 |
| >20 | 46 | 22 |
| Meeting contextFootnote b | ||
| New partner | 114/119 | 96 |
| Anonymous partners | 82/98 | 84 |
| Sex worker client | 8/191 | 4 |
| Sex worker | 10/196 | 5 |
| Sauna | 136/186 | 73 |
| Internet/Applications | 136/177 | 77 |
| Club/bar | 40/115 | 35 |
| Drug use (n=241) | ||
| At least one | 138 | 57 |
| Crystal meth | 50 | 21 |
| Cannabis | 48 | 20 |
| Ecstasy | 43 | 18 |
| Cocaine | 28 | 12 |
| Poppers | 27 | 11 |
| Speed | 23 | 10 |
| GHB | 12 | 5 |
| Ketamine | 10 | 4 |
| Crack | 5 | 2 |
| Heroine | 1 | 0 |
| IV drug | 10 | 4 |
| Not reported | 13 | 5 |
| Encounter with out-of-province resident (n=208) | ||
| At least one | 56 | 27 |
Clinical manifestations
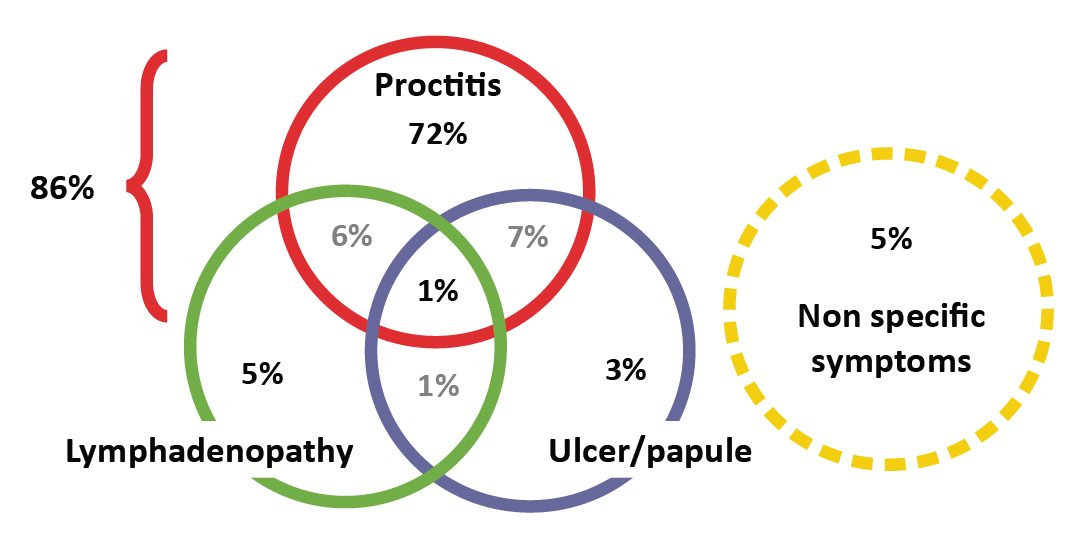
Details about symptoms were available for 303/338 cases. The majority of patients had symptoms at the time of testing (n=237; 78%) (Table 2). The proportion of asymptomatic cases increased from 18% (37/203) in 2013–2015 to 32% (32/100) in 2016 (p=0.01). A minority of patients presenting with symptoms had only unspecific complaints (12/235; 5%). As shown in Figure 2, more than one symptom could occur at the same time. Symptoms included proctitis (86%), ulcer or papule (12%) and lymphadenopathy (13%). Complaints associated with proctitis included bloody stools (69/201; 35%), anal pain (70/201; 34%) and rectal discharge (52/201; 26%).
| Clinical presentation | 2013–2016 (Total) n=303Footnote a |
2013–2015 n=203Footnote a |
2016 n=100Footnote a |
p value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Asymptomatic | 69 | 23 | 37 | 18 | 32 | 32 | 0.006 |
| Nonspecific symptomsFootnote b only | 12 | 4 | 6 | 3 | 6 | 6 | NS |
| Specific symptom(s)Footnote c | 222 | 73 | 160 | 79 | 62 | 62 | 0.002 |
| Proctitis | 201 | 66 | 141 | 69 | 60 | 60 | NS |
| Lymphadenopathy | 30 | 10 | 23 | 11 | 7 | 7 | NS |
| Ulcer/papule | 29 | 10 | 25 | 12 | 4 | 4 | NS |
Figure 2: Distribution of clinical manifestations among symptomatic lymphogranuloma venereum cases, Quebec, 2013–2016 (n=234)Figure 2 footnote a
Text description: Figure 2
Figure 2: Distribution of clinical manifestations among symptomatic lymphogranuloma venereum cases, Quebec, 2013–2016 (n=234)Figure 2 footnote a
| Distribution of the clinical manifestations, 2013–2016 (n=234) | n | % |
|---|---|---|
| Proctitis only | 169 | 72.2% |
| Proctitis + Ulceration/papule | 16 | 6.8% |
| Proctitis + lymphadenopathy | 13 | 5.6% |
| Lymphadenopathy only | 11 | 4.7% |
| Lymphadenopathy + ulceration/papule | 3 | 1.3% |
| Ulceration/papule only | 7 | 3.0% |
| Ulceration/papule + lymphadenopathy + proctitis | 3 | 1.3% |
| Non-specific only | 12 | 5.1% |
| Total proctitis | 201 | 86.0% |
Laboratory tests and treatment
The number of LGV genotyping tests performed on positive C. trachomatis samples (males and females, all sampling sites, regardless of symptoms) at the Laboratoire de santé publique du Québec are shown in Table 3. Overall, 13% of C. trachomatis samples typable by multiplex PCR assay were genotypes L1–3. Some patients had more than one positive sample for a single episode. An important increase in the number of requests was noted in 2016 as a result of the recommendation to systematically genotype all rectal samples positive for C. trachomatis.
| Year | C. trachomatis positive samples, n | Typable by multiplex PCR assay, n | Non-LGV genotypes, n (%) | LGV genotypes, n (%) | Specific genotype identified by sequencing | ||
|---|---|---|---|---|---|---|---|
| L2b | L2 | Not available | |||||
| 2013 | 424 | 414 | 363 (88%) | 51 (12%) | 49 | 2 | 0 |
| 2014 | 540 | 534 | 475 (89%) | 59 (11%) | 55 | 4 | 0 |
| 2015 | 670 | 656 | 536 (82%) | 120 (18%) | 107 | 4 | 9 |
| 2016 | 1,249 | 1,199 | 1,070 (89%) | 129 (11%) | 124 | 0 | 5 |
| Total | 2,883 | 2,803 | 2,444 (87%) | 359 (13%) | 335 | 10 | 14 |
The reason for requesting testing was known for 302 of the 338 reported cases. The reasons included presence of symptoms (n=221; 73%); screening based on risk factors (n=68; 23%); and contact with a confirmed LGV case (n=12; 4%). DNA sequencing was conducted for 93% (n=314) of cases: 304 were L2b, whereas 10 typed as L2, due to a single nucleotide mutation in the genotyping target of the ompA gene. Retrospective sequencing of the pmpH gene suggests that these cases were due to a variant of the L2b genotype.
Prescribed treatment was in line with guidelines (doxycycline for 21 days) (3,8) for 77% of cases (221/288), and appropriate treatment was more often initially prescribed in 2016 than in 2013–2015 (74% vs 61%; p=0.03) (Table 4).
| Treatment | 2013–2016 (Total) n=288Footnote a |
2013–2015 n=194Footnote a |
2016 n=94Footnote a |
p value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Appropriate treatmentFootnote b | 221 | 77 | 144 | 74 | 77 | 82 | NS |
| At first prescription | 189 | 66 | 119 | 61 | 70 | 74 | 0.03 |
| At second prescription | 32 | 11 | 25 | 13 | 7 | 7 | NS |
| Inappropriate treatment | 67 | 23 | 50 | 26 | 17 | 18 | NS |
Reinfections
Of the 35 individuals who were repeaters (338 cases occurred in 303 individuals), that is, were reinfected more than 90 days after an episode, 10 (29%) had had at least one previous documented episode before 2013, whereas 25 had had their first episode in 2013–2016Footnote 12. Most reinfections (75%) occurred within two years of first infection (from 108 days to 10.5 years). The LGV repeaters were more likely than those who had had only one episode to be living in Montréal (31/35 [89%] vs 210/268 [78%]; p=0.13). Compared with individuals who had a single episode, repeaters were also more likely to be HIV-infected (29/30 [97%] vs 155/193 [80%]; p=0.03); to report a past history of syphilis (27/27 [100%] vs 132/173 [76%]; p=0.004); and to have used recreational drugs during the past 12 months (17/22 [77%] vs 106/194 [55%]; p=0.04).
Discussion
Incidence of LGV, which occurs within a core group of MSM living in an urban setting, has been increasing in Quebec over the last four years. The MSM population is characterized by a significant past history of STBBIs, especially HIV, a high number of sexual partners and frequent drug use. Our data show a high prevalence of proctitis as the presenting symptom, with very few classical inguinogenital diseases. The majority of the cases were diagnosed as L2b genotypes, a variant isolated as far back as the 1980s in San FranciscoFootnote 13. A number of studies of LGV outbreaks across Europe reported a population of high-risk MSM similar to what we found in the current Quebec outbreakFootnote 2. A large percentage of men are HIV and/or hepatitis C positive and very few cases, if any, are detected in heterosexual men or women. Asymptomatic cases have been detected, but the vast majority of cases present with symptoms of rectal infectionFootnote 14Footnote 15Footnote 16Footnote 17Footnote 18Footnote 19.
The LGV rate remains relatively low in comparison with other common STIs including non-LGV C. trachomatis infections. In 2015, among males aged 15–75 years in Quebec, the annual incidence rates of infectious syphilis (17.2/100,000 person-year) and gonococcal infection (69.4/100,000 person-year) were significantly higher than those of LGV (2.6/100,000 person-year)Footnote 20. As LGV is thought to be more often symptomatic than syphilis or gonococcal infection, it could be speculated that those who become infected are more likely to seek medical attention and get treatment, restricting transmission. For now, LGV is also confined to a smaller subgroup of MSM, while gonococcal and non-LGV C. trachomatis infections also involve the heterosexual population. However, the incidence prior to 2014 was likely underestimated given the exclusion of asymptomatic patients in previous case definitions. The higher proportion of asymptomatic cases in 2016 can also be partly explained by the systematic genotyping of all positive C. trachomatis rectal specimens, which started in June 2016 in Quebec.
The presented clinical manifestations are in line with recent literature reporting proctitis and proctocolitis as the most common presentationFootnote 2Footnote 21. Although systematic genotyping for LGV is currently performed in Quebec only for rectal specimens, there is some evidence suggesting that extrarectal LGV (pharyngeal and urethral infections) could be a potential contributor to the ongoing outbreak. In 2014, van Rooijen et al.Footnote 22 collected pharyngeal swabs from MSM and found that 1% were C. trachomatis positive; of these, 53% did not have concomitant anogenital infection. The implication of pharyngeal as well as urethral LGV in transmission of the epidemic strain remains unclear. Rectal infections have been shown to be far more common in various LGV prevalence studiesFootnote 23Footnote 24Footnote 25Footnote 26. It is thus arguable that extrarectal screening would not be a cost-effective measure.
A significant improvement in adequate first prescribed treatment has been noted in 2016, possibly following educational efforts towards physiciansFootnote 3Footnote 8. The province-wide genotyping of positive rectal C. trachomatis specimens implemented in 2016 could have contributed to improvements in case management by shortening diagnostic delays, from a mean of 30 days in 2014Footnote 9 to 12 days in 2016 (data not shown).
A specific subpopulation seems to be at greater risk of reinfection with higher rates of HIV infection, in line with potentially riskier sexual behaviours as shown in other reportsFootnote 27Footnote 28. In an attempt to describe this specific subpopulation, Rönn et al.Footnote 12 found repeaters to be more likely to be infected by HIV and HCV, and to have a concomitant gonococcal infection compared with patients with a single episode of LGV. It remains unclear if serosorting, the strategy by which MSM have unprotected sexual encounters with seroconcordant partners, has a role to play in nurturing the present outbreak, as hypothesized for other STIs with higher incidence within this specific groupFootnote 29Footnote 30.
The national enhanced LGV surveillance, conducted between 2004 and 2012, received 170 case reports from provincial and territorial health authorities (including 104 confirmed and 66 probable cases)Footnote 5. Confirmed cases were reported from Quebec, Ontario, Alberta and British Columbia; probable cases were reported from these provinces as well as one from Nova ScotiaFootnote 5.
Strengths and limitations
This study provides recent surveillance data on LGV in Quebec with a high number of cases. Description of risk factors contributes to understanding the current outbreak and its sexual network. Limitations include the descriptive nature and lack of standardization of the questionnaires, both over time and across the different administrative regions, as well as some missing information. Data collecting forms have since been revised and medical teams contacted to allow better standardization. Information regarding HIV follow-up (i.e. treatment, viral load, CD4, etc.) were unfortunately not available.
Conclusion
The re-emergence of LGV in Quebec involves a core group of MSM with history of STIs, most being HIV-seropositive, with multiple partners and substantial drug use. Sporadic transmission outside of Montréal and relatively frequent reinfections highlight the potential for a further spread; this is of particular significance given the associated morbidity. The transmission among HIV-infected patients is of concern given the implication of unprotected sexual encounters within the LGV-affected population as well as the increased risk of HIV transmission associated with inflammation of rectal mucosa seen in LGV proctitisFootnote 1Footnote 2Footnote 3. Enhanced surveillance helps monitor and better describe this subgroup in order to tailor public health actions to reduce the risk of LGV transmission. A clinical tool for LGV was released in October 2017 to assist medical teams with LGV screening, diagnosis, treatment, follow-up and partners' medical careFootnote 31. The systematic genotyping of rectal specimen positive for C. trachomatis proved useful at identifying asymptomatic patients. An analysis of the cost effectiveness of such a strategy would inform future public health actions.
Author's statements
CAB – Writing – original draft, review and editing, visualization
SV – Conceptualization, methodology, validation, formal analysis, investigation, writing – review and editing, project administration
MF – Conceptualization, methodology, software, validation, formal analysis, data collection and curation, investigation, writing – review and editing, project administration
DM – Methodology, investigation, data collection and curation, writing – review and editing
CM – Methodology, writing – review and editing
AS – Genotyping by direct sequencing, writing – review and editing
JL – Resources, writing – review and editing, supervision
CF – Writing – review and editing
ACL – Conceptualization, formal analysis, validation, writing – review and editing, visualization, supervision
Conflict of interest
None.
Acknowledgements
The authors thank all public health nurses, collaborating physicians, regional public health directorates, the Ministère de la santé et des services sociaux du Québec, Bureau de Vigie sanitaire and the Direction de la lutte contre les ITSS(SLITSS) for the collection and processing of clinical data. We would also like to thank Suzanne Gibbons and Vanessa Zubach at the Viral Exanthemata and STDs laboratory (National Microbiology Laboratory) as well as Martine Morin and Lyne Desautels for the implementation of the multiplex PCR assay at the Laboratoire de santé publique du Québec and laboratory testing of specimens.
Funding
This epidemiologic study was supported by the provincial public health organizations that participated in data collection and analysis.