Tick-borne fever in pregnancy

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 46–10: Laboratory Biosafety
Date published: October 1, 2020
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 46–10, October 1, 2020: Laboratory Biosafety
Case report
A case of tick-borne relapsing fever in pregnancy
John C Lam1, Oscar E Larios1,2, Michael D Parkins1,3, Stephen D Vaughan1
Affiliations
1 Division of Infectious Diseases, Department of Medicine, University of Calgary, Calgary, AB
2 Department of Pathology and Laboratory Medicine, University of Calgary, Calgary, AB
3 Department of Microbiology, Immunology and Infectious Diseases, University of Calgary, Calgary, AB
Correspondence
Suggested citation
Lam JC, Larios OE, Parkins MD, Vaughan SD. A case of tick-borne relapsing fever in pregnancy. Can Commun Dis Rep 2020;46(10):362–4. https://doi.org/10.14745/ccdr.v46i10a09
Keywords: tick-borne relapsing fever, Borrelia hermsii, Ornithodoros, Jarisch-Herxheimer reaction, disease surveillance
Abstract
Tick-borne relapsing fever (TBRF) is an infection caused by Borrelia spirochetes. In North America, Borrelia hermsii is the most common cause for TBRF. This vector-borne disease is transmitted by Ornithodoros hermsi, a soft-bodied tick found in high altitudes in northwestern United States and southwestern Canada. Once bitten by the tick and infected by B. hermsii, episodes of fever alternating with afebrile periods can occur.
A case of TBRF in a pregnant host was complicated by Jarisch-Herxheimer reaction requiring critical care. This case emphasizes the importance of maintaining a high index of suspicion in TBRF. Clinician recognition, diagnosis and treatment of TBRF as well as public awareness of strategies to prevent tick bites should be strengthened.
Introduction
Tick-borne relapsing fever (TBRF) can be challenging to diagnose because of difficulties in isolating the causative bacterium, Borrelia hermsii, in the laboratory. Furthermore, clinicians may not consider TBRF in the differential diagnosis of febrile illnesses as the vector is often unrecognized. In addition, TBRF is a non-reportable illness.
Here, we report a life-threatening case of TBRF in a pregnant individual, discuss some treatment aspects and advocate for active case surveillance by public health officials in areas of high risk.
Written informed consent was obtained from the patient to publish this case report and the accompanying images.
Case
A 30-year-old previously healthy primagravida woman, at 17 weeks’ gestation, presented to hospital in Calgary, Alberta, with a four-day history of fevers, chills and multiple episodes of emesis. Prior to symptom onset, she had spent five days in the Okanagan region of British Columbia on a summer family hiking trip. The entire family stayed in a well-kept air-conditioned house in Vernon, British Columbia, that her extended family regularly inhabited. There was no history of rodent inhabitation or pest control concerns in or around the house.
The patient had had an unremarkable prenatal course, with negative screening serology for syphilis and HIV. There was no exposure history consistent with rat bite fever, leptospirosis or louse-borne relapsing fever. She reported multiple unknown insect bites across her torso, but no rash, during her holiday. On presentation, she was febrile (temperature 38.9°C), hypotensive (blood pressure 85/52) and tachycardic (heart rate 128 beats/minute). The remainder of her examination was otherwise non-contributory.
Initial investigations identified pancytopenia with hemoglobin of 78 g/L, platelets of 27 × 109/L and white blood cells of 3.4 × 109/L and with lymphopenia at 0.1 × 109/L. Metabolic acidosis with pH of 7.21 and lactate of 4.3 mmol/L were also identified. A peripheral blood smear found the presence of spirochetes and a presumptive diagnosis of TBRF was made (Figure 1).
Figure 1: Wright-Giemsa stained peripheral blood smear showing spirochetes
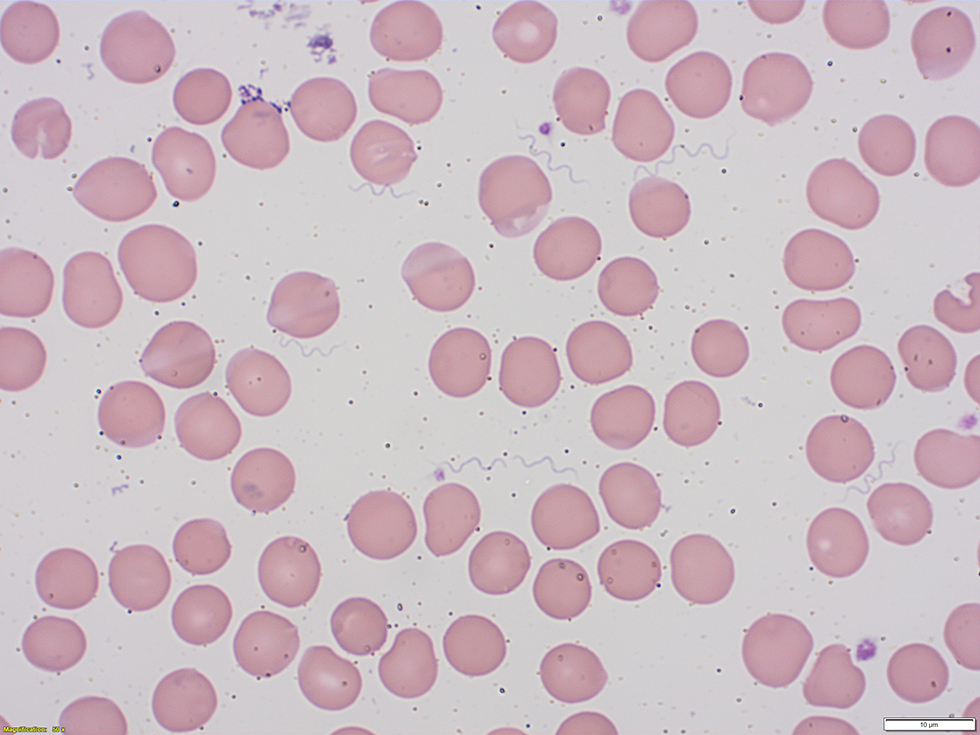
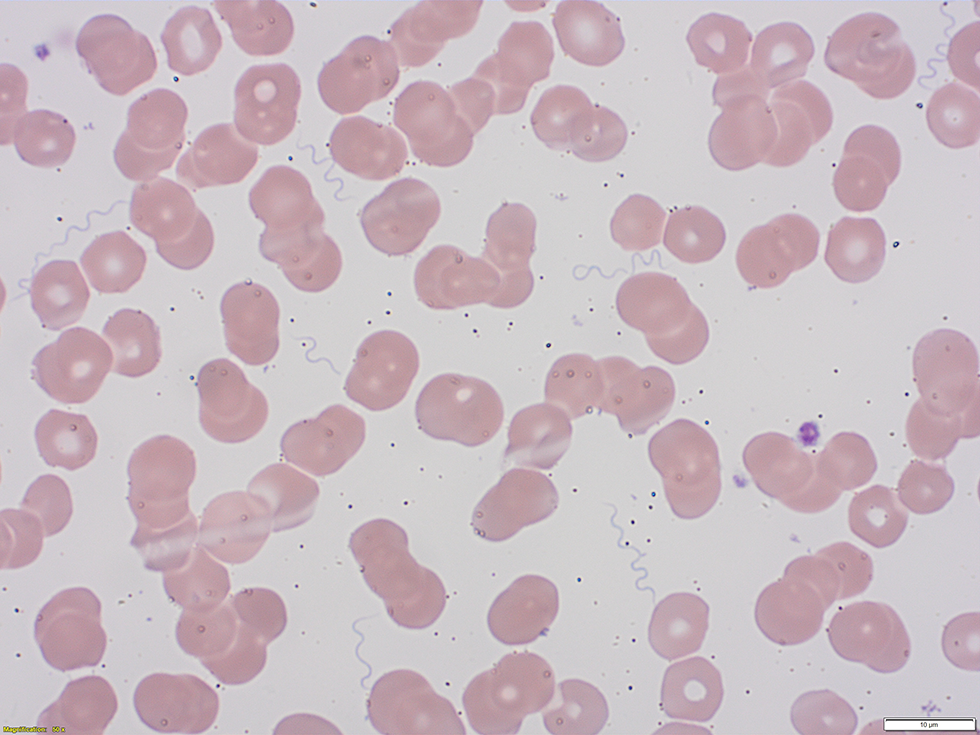
Text description: Figure 1
Figure 1: Wright-Giemsa stained peripheral blood smear showing spirochetes
The figure is a Wright-Giemsa stained peripheral blood smear obtained from the patient. It demonstrates the presence of numerous extracellular spirochete bacteria.
Treatment with penicillin G at four million units intravenously every four hours was commenced. Two hours after penicillin initiation, the patient developed chills and worsened hypotension (blood pressure 70/50) despite administration of 6 L of crystalloid resuscitation. This is characteristic of a Jarisch-Herxheimer reaction. The patient was subsequently transferred to the intensive care unit for closer monitoring. Her hypotension resolved within one day, and pancytopenia improved within the week. She was discharged to complete a 14-day course of intravenous penicillin G. Molecular testing of blood by 16S ribosomal polymerase chain reaction confirmed B. hermsii as the causative pathogen.
At 40 weeks, the patient delivered a healthy infant.
Discussion
B. hermsii is a spirochete implicated in TBRF. B. hermsii commonly occurs in mountainous regions of North, Central and South America. In Canada, approximately 50 cases have been reported over the last two decadesFootnote 1. TBRF is transmitted via the night-biting soft tick Ornithodoros hermsiFootnote 2. O. hermsi are found in southern British Columbia and northwestern United States, preferring coniferous forests at 450–2,450 m occupied by rodents such as tree squirrels and chipmunksFootnote 3. O. hermsi live in the nests of rodent reservoirs and feed nocturnally. In the absence of rodents, O. hermsi will feed on humans.
Infected individuals present with characteristic recurrent three-day fevers punctuated by week-long periods of being afebrile. Episodes of fevers and tachycardia, known as the “chill phase,” are followed by the “flush phase,” during which transient hypotension and drenching sweats occur. The acuity of the patient’s presentation is likely linked to the relative immunosuppression of pregnancy, based on reports of increased severity in pregnant patientsFootnote 4. The potential for poor neonatal outcomes is well documentedFootnote 5.
Microscopy may be useful in diagnosing TBRF because the spirochetes associated with B. hermsii are clearly visible, particularly during febrile episodes. Peripheral blood smears stained with Wright-Giemsa stain are positive for the presence of extracellular spirochetes in about 70% of patients, particularly during the flush phaseFootnote 6. Although molecular testing can be used to confirm spirochete species, testing turnaround time is lengthy, and it is not appropriate to wait for results prior to initiating therapy. Similarly, serological testing performed weeks after infection confirms presence of appropriate antibody response but is of little use in the acute management of the illness.
TBRF during pregnancy is rare, and considerations of drug therapy and neonatal consequences are important. Oral doxycycline or intravenous beta-lactams are suitable therapies, but intravenous beta-lactams are preferable in pregnancy because of the teratogenicity of tetracyclinesFootnote 7. Jarisch-Herxheimer reaction, characterized by chills, fevers and hypotension, can develop within 24 hours in patients treated for spirochetal infections. Jarisch-Herxheimer reaction has been documented in upwards of 50% of patients treated for TBRFFootnote 8Footnote 9. Thrombocytopenia associated with acute TBRF poses risks of preterm labour and spontaneous abortion. Cases of placental transmission to the neonate have also been reportedFootnote 4.
Because TBRF is not a reportable disease in Canada, it is not known whether distribution of B. hermsii and incidence of TBRF is similar to a decade ago. Over 80% of patients will not develop a rash from a nighttime painless bite or exhibit the characteristic fever syndrome. As such, the burden of TBRF in southwestern Canada is likely underestimatedFootnote 10. Case surveillance and reporting may improve systemized approach to diagnosis and greater clinician awareness of this disease.
Although rare, TBRF can have severe sequelae and be fatalFootnote 11. Enhanced public awareness for TBRF may lead to a more concerted effort to prevent TBRF by reducing rodent habitats, contacting pest control for chemical treatment of rodent-infested areas and educating people to apply topical repellants (e.g. permethrin) when sleepingFootnote 12. Active case surveillance could be considered by public health officials in areas of high risk.
Geographic distribution of O. hermsi may expand in Canada with predicted changes in climateFootnote 13.
Conclusion
This case illustrates TBRF as a life-threatening complication of pregnancy in the absence of the typical exposure in a rustic dwelling.
Surveillance data would be useful for characterizing the epidemiology of this probable underdiagnosed infection in Canada.
Authors’ statement
All authors were involved in the management of the patient. JCL wrote the initial draft of the manuscript and all authors contributed to its revision. SDV oversaw manuscript preparation and revisions. All authors read and approved the final manuscript.
A copy of the written consent is available for review by the Editor-in-Chief of Canada Communicable Disease Report.
Competing interests
None.
The case was presented in part at the Clinical Grand Rounds, European Congress of Clinical Microbiology & Infectious Diseases 2019, Amsterdam Noord, the Netherlands.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
