Expanding roles of Canadian blood suppliers

 Download this article as a PDF
Download this article as a PDF Published by: The Public Health Agency of Canada
Issue: Volume 48-4, April 2022: First Nations Health
Date published: April 2022
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 48-4, April 2022: First Nations Health
Overview
Canadian blood suppliers: An expanding role in public health surveillance?
Sheila F O’Brien1,2, Steven J Drews3,4, Antoine Lewin5,6, Carla Osiowy7, Michael A Drebot7, Christian Renaud5
Affiliations
1 Epidemiology & Surveillance, Canadian Blood Services, Ottawa, ON
2 School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON
3 Microbiology, Canadian Blood Services, Edmonton, AB
4 Diagnostic and Applied Microbiology, Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB
5 Héma-Québec, Montréal, QC
6 Faculté de médecine et des sciences de la santé, Université de Sherbrooke, Sherbrooke, QC
7 National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, MB
Correspondence
Suggested citation
O’Brien SF, Drews SJ, Lewin A, Osiowy C, Drebot MA, Renaud C. Canadian blood suppliers: An expanding role in public health surveillance? Can Commun Dis Rep 2022;48(4):124–30. https://doi.org/10.14745/ccdr.v48i04a02
Keywords: blood donors, surveillance, public health, epidemiology
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic galvanized blood donor seroprevalence studies, which continue to inform public health policy. We propose that the two Canadian blood suppliers, Héma-Québec and Canadian Blood Services, expand their role in public health surveillance in the post-pandemic period. Together blood suppliers have near-national reach, collecting blood donations nearly every day in all larger cities and many smaller municipalities. Blood donors are a healthy subset of the general population. Demographic data, routine infectious disease testing and screening questionnaire data are collected for all donations. Close to one million blood samples per year could be made available for surveillance. With 90% repeat donors, longitudinal sampling is possible. Current blood donor surveillance includes monitoring infectious marker rates in low risk (e.g. HIV, hepatitis C virus) or asymptomatic (e.g. West Nile virus) populations, and ad hoc studies to monitor transfusion-transmissible infections. These include tick-borne infections such as Babesia microti and foodborne infections such as hepatitis E. Canadian Blood Services and Héma-Québec are actively seeking to engage with public health professionals to further develop a role in public health surveillance.
Introduction
Two publicly funded blood services provide Canadians with fresh and fractionated blood products. Héma-Québec serves Québec, and Canadian Blood Services serves the other nine provinces and the three territories. Formed in 1998 in the aftermath of the Commission of Inquiry on the Blood System in Canada (Krever Commission) into transfusion-transmitted HIV, the blood providers operate at arms-length of government to ensure autonomy. The scope of activities of blood providers has further developed into stem cell registries, umbilical cord-blood banking, human milk banking, tissue banking and coordinating organ transplantation (roles vary by blood supplier). Applied research is a high priority and both organizations have independent epidemiology and surveillance departments as well as research and development/innovation departments that primarily focus on blood safety and informing blood service policy.
In this commentary, we discuss the role of the blood services in public health surveillance to date and propose that this role be expanded.
SARS-CoV-2 heralded a new role for blood services
In March 2020, the World Health Organization declared COVID-19 a pandemic. Both blood services initiated and continue to undertake severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serosurveillanceFootnote 1Footnote 2. Blood services around the world capitalized on their infrastructure to quickly start seroprevalence studies to inform public health policy. By June 2020, a short three months after the pandemic was declared, 32 of 48 (67%) countries surveyed had had SARS-CoV-2 seroprevalence studies initiated by blood operatorsFootnote 3Footnote 4. In most cases, the blood service was the only entity able to rapidly collect and test large numbers of blood samples from healthy individuals. In many countries these studies have continued. In the United States, blood operators collaborate with the Centers for Disease Control and Prevention, providing test results routinely.
In Canada, there was early strong engagement of blood operators with public health, public health laboratory networks, mathematical modellers and university partners. The approach by the two blood providers differed somewhat. Over 18,600 donations were tested by Héma-Québec in collaboration with the Ministère de la Santé et des Services sociaux du Québec, and later, with the Government of Canada COVID-19 Immunity Task Force. They carried out three cross-sectional studies including donor-reported infection history and risk factorsFootnote 2. Canadian Blood Services worked with the Government of Canada COVID-19 Immunity Task Force to test cross-sectional samples from nine provinces monthly (over 250,000 samples tested)Footnote 1.
Both blood operators also worked with clinical trials groups to provide anti-SARS-CoV-2 convalescent plasma products to their studies. Canadian Blood Services also led a smaller seroprevalence study, funded by the Canadian Institutes of Health Research, testing 1,500 donations per month. This linked Canadian Blood Services to collaborators in universities, industry research groups, public health organizations and provincial/national public health laboratories. The data generated, and lessons learned, informed public health policy and guided laboratory practices in provincial and clinical laboratories. In addition, the data and knowledge were shared broadly with other laboratorians and led to further academic collaborations. These linkages continue as serological testing monitors vaccine rollout and antibody concentrations. Both blood suppliers are providing SARS-CoV-2 seroprevalence data to the Secretariat of the Government of Canada COVID-19 Immunity Task Force, housed at McGill University, Montréal, Québec. These data are contributing to analyses to evaluate the seroprevalence of natural infection as well as the impact of vaccine rollout.
Post-pandemic, should blood services in Canada play a role in supporting public health surveillance? Similar questions are being asked in many countries. In Denmark a role for blood donors in public health surveillance was already being implemented pre-pandemicFootnote 5.
Blood donors are a healthy subset of the general population
Blood donors must be 17 years old (18 in Québec) to donate blood, and there are relatively few donors over the age of 72. Prospective donors must complete a detailed health history questionnaireFootnote 6. Those for whom donation is not in their best interests because of their health or who are at risk of infections such as HIV or hepatitis are not eligible. There are also some travel restrictions, including people at risk of tropical infections and those who spent time in the United Kingdom and other areas where they may be at risk for the variant Creutzfeldt-Jakob disease.
Blood collection sites are in all larger cities, most smaller cities and many towns-most of the more populated areas of Canada. The age, sex and geographic region of donors is comparable to the general population up to 65 years of age (see Figure 1 and Figure 2). Largely excluded are northern regions as well as some rural areas and remote towns. There are also non-represented populations, including long-term care residents and those in detention centres, or people who are less likely to donate because of language barriers.
Figure 1: Percentage of the general population and donors by geographic regionFigure 1 footnote a and age groupFigure 1 footnote b
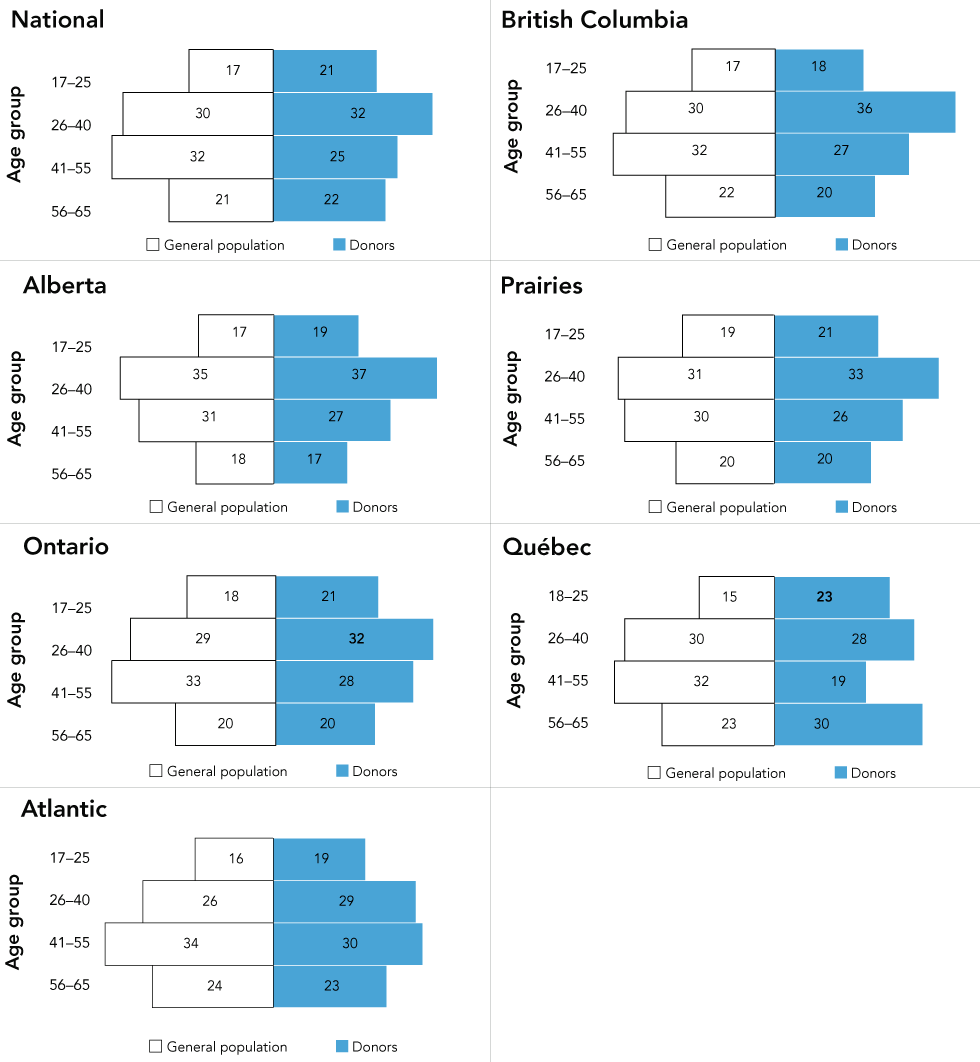
Text description: Figure 1
Percentage of general population (white) and donors (blue) by age group for National (excluding territories), British Columbia, Alberta, Prairies (Manitoba and Saskatchewan), Ontario, Québec and Atlantic (New Brunswick, Nova Scotia, Newfoundland and Labrador and Prince Edward Island). General population and donor age range 17–65 used for all provinces except for Québec (age range 18–65).
This figure shows that the proportions of blood donors by age group are very similar to the general population.
Figure 2: Percentage of general population and donors by geographic regionFigure 2 footnote a and sexFigure 2 footnote b
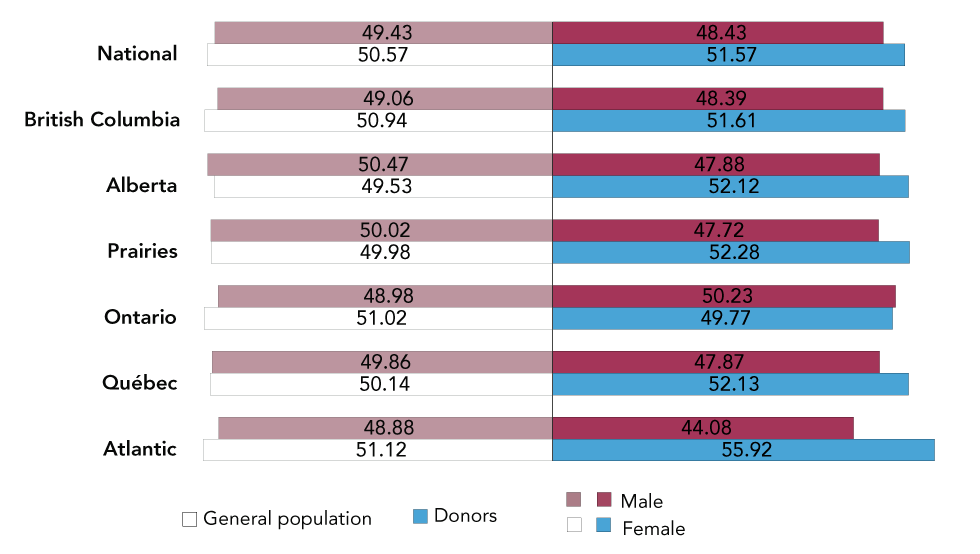
Text description: Figure 2
Percentage of general population (white) and donors (blue) by sex (male, female) for National (excluding territories), British Columbia, Alberta, Prairies (Manitoba and Saskatchewan), Ontario, Québec and Atlantic (New Brunswick, Nova Scotia, Newfoundland and Labrador and Prince Edward Island). General population and donor age range 17–65 used for all provinces except for Québec (age range 18–65).
This figure shows that the proportions of male and female blood donors are very similar to the general population.
While it is true that blood donors consider themselves healthy and self-select to donate, this may also be true of apparently healthy volunteers recruited to participate in studies. SARS-CoV-2 seroprevalence appears to be similar in both the healthy general population and the blood donor populationFootnote 8Footnote 9. Further studies comparing blood donors with the general population are needed to better characterize which segment(s) of the general population donors best represent.
Blood service capacity for surveillance
There are some important strengths of blood services in public health surveillance. Between Canadian Blood Services and Héma-Québec, there is near-national reach in terms of daily blood collection. From each of the annual 1.2 million donations, an extra ethylenediaminetetraacetic acid (EDTA; an anticoagulant) tube of blood is collected. About 20% of these donations are used for testing, which is essential to be able to release the blood product, but this leaves about 950,000 samples that could be made available for surveillance. An important advantage of using blood donations for surveillance is that about 90% of donors donate repeatedly. These donors can form a cohort for on-going monitoring. Donors return according to their own preference and the interval between donation may vary unlike research cohort participants.
Hemoglobin levels are measured before each donation. Data including demographic variables (e.g. age, sex, postal code, and ethnicity), current medications, recent vaccinations and recent travel history are collected via the routine donor history questionnaireFootnote 6. Currently, it is not possible to add more research questions to the donor history questionnaire, but electronic surveys could be sent within days of collecting samples. A recent survey of donor HIV risk factors to assess compliance with screening questions achieved a response rate of about 33% from the 40,000 donors invited to participate. Both blood operators also have the infrastructure, staffing and protocols to safely collect large volumes of plasma (>250 mL) from donors in a safe and controlled manner.
Examples of blood service surveillance relevant to public health
All blood donations are tested for HIV, hepatitis C virus, and hepatitis B virus using a nucleic acid test (NAT) and serology and human T-lymphotropic virus and syphilis using serology. West Nile virus (WNV) is tested seasonally using NAT, and Trypanosoma cruzi is tested in at-risk donors using serology (see Table 1). Repeat reactive specimens also undergo confirmatory testing where available. Depending on the positive assay target, specimens may also be sent to the National Microbiology Laboratory for nucleic acid sequencing and strain analysis. Positive results are reported to public health authorities where required by law.
| Infection | Markers |
|---|---|
| HIV | Antibody |
| Nucleic acid | |
| Hepatitis B virus | Hepatitis B surface antigen |
| Antibody to hepatitis B core antigen | |
| Nucleic acid | |
| Hepatitis C virus | Antibody |
| Nucleic acid | |
| West Nile virus | Nucleic acid |
| Human T-lymphotropic virus | Antibody |
| Trypanosoma cruzi (Chagas disease, at-risk donors only) | Antibody |
| Treponema pallidum | Antibody |
Blood donors are a population who believe that they are not at risk and have replied in the negative to a battery of risk questions. Nevertheless, about 40 people per year test positive for hepatitis C virus and hepatitis B virusFootnote 10Footnote 11. These responses can provide insight into infected individuals with no apparent self-declared risk and may be useful in determining the potential benefit of screening low-risk populations. Testing for human T-lymphotropic virus and T. cruzi provides insight into these rare, non-reportable infections. Given the rise in diseases of despair blood donor screening may also shed light on sexual and high-risk behavioural networks that are not readily apparent to public health investigatorsFootnote 12Footnote 13.
Historically, both blood establishments have played important roles in monitoring emerging infectious diseases. By 2003, in response to the emergence of WNV in Canada, both blood services were testing blood donations and monitoring incidence with the West Nile Virus Task Force. Public health surveillance of WNV identifies symptomatic individuals who seek medical assistance, whereas blood donors will be initially screened with a WNV NAT either early stage or asymptomatic, unlike in public health laboratories. Blood operators may also send early WNV NAT-positive samples to the National Microbiology Laboratory or the Laboratoire de santé publique du Québec for molecular characterization.
As a result of these unique screening and testing approaches, in some years the first WNV infection of the season is identified in a blood donor. In Québec, the 2012 WNV data were used to estimate the underreported rate of neurologic WNV at between 26% and 37.5%Footnote 14. This was used to inform physician education materials, which subsequently shown (or demonstrated) to have improved case identification. Additional studies utilizing WNV-positive donor samples may involve the identification and characterization of viral genetic variants and the possible incursion of new lineages into the countryFootnote 15Footnote 16. Finally, it is important to note that the WNV NAT is actually a broadly reactive assay for the Japanese encephalitis serocomplex and adds an additional level of surveillance for Japanese encephalitis, Kunjin virus, Murray Valley encephalitic virus, Saint Louis encephalitis virus and Usutu virusFootnote 17.
In the case of emerging tick-borne pathogens, in 2013 the first Babesia microti-positive public health case was reported in Manitoba, but no B. microti NAT or antibody-positive donations were identified from about 14,000 donations testedFootnote 18Footnote 19Footnote 20. In 2018, of 50,000 donations Canada-wide, there was one NAT-positive donation in Manitoba, and in a subset of 14,000 donations from the geographic region spanning Manitoba to Nova Scotia, four antibody-positive donations were identified, all in southwestern OntarioFootnote 21. In 2019, a donor who felt unwell after donating was found to be B. microti-positive. An investigation that involved Canadian Blood Services, the National Microbiology Laboratory, an additional reference laboratory and two provincial public health laboratories found that transfusion-transmitted babesiosis had not occurredFootnote 20. Thus, two of the three known endemic NAT-positive cases were found in blood donors, suggesting that B. microti has gained a foothold in Canada. Blood donor studies can potentially evaluate infections and document exposures from other emerging tick-borne and arthropod-borne infections such as Borrelia burgdorferi, Anaplasma phagocytophilum, Powassan virus and Eastern equine encephalitis virus. This type of surveillance is important in the context of climate change and expanding vector habitats.
Hepatitis E was evaluated in two national studiesFootnote 22Footnote 23. In the first study of 14,000 donors tested, no hepatitis E virus NAT-positive donations were identified, but 5.6% were antibody positive. In the second study of about 50,000 donations tested with a more sensitive NAT assay, 1 in 4,615 tested positive for hepatitis E viral RNA. This was one of the largest hepatitis E virus studies carried out in Canada.
Strengths and limitations of blood donors for public health surveillance
The near-national reach of blood services' daily collections and laboratory capacity can be leveraged to rapidly survey pathogens at a relatively low cost. Importantly, blood services cross jurisdictional boundaries and have streamlined decision-making processes. For national surveillance activities, there are substantial advantages over other sources of healthy individuals, for example, patient testing and pregnancy screening programs, which are generally local rather than national.
Both blood services conduct and enable research with the oversight of external research ethics committees that follow the guidance outlined in the Tri-Council Policy Statement: Ethical Conduct for Research Involving HumansFootnote 24. Blood services also undertake unique lookback processes (investigating recipients of a test-/disease-positive donor) and traceback processes (investigating donations and donors from a disease-/test-positive recipient) for blood recipients or donors with a suspected blood-borne infectionFootnote 20Footnote 25. These processes could be leveraged to support further active surveillance.
The potential disadvantages of using blood donors as a data source are that some segments of the population are underrepresented, such as those living in rural areas, older adults, people with serious illnesses and those with risk factors for transfusion-transmissible diseases. Furthermore, children are not eligible to donate blood, and anthropometric measurements and biologic samples such as urine are not currently available.
Future directions
More research is needed to understand how the donor population differs from the general population. Increased collaboration between blood services and provincial and federal public health departments will help initiate new research projects. A potential role of blood services in the surveillance of vaccine-preventable infections is being explored, and an expanded role in vector-borne infection surveillance would be a natural extension of blood service surveillance. Héma-Québec has established a biobank specifically for COVID-19 projects, and Canadian Blood Services has stored samples from the SARS-CoV-2 seroprevalence study. Larger (not project-specific) biobanks are under consideration by both blood services. Methods for collecting more health and lifestyle data through questionnaires are being explored as are ways to link donor data to health registries for research. The value of biobanks will be amplified as more detailed information about donors is collected, increasing potential applications to public health surveillance and research.
Conclusion
The emergence of SARS-CoV-2 highlights the value of blood services to leverage operational capacity for rapid implementation of large-scale nationwide serosurveillance. Together Canadian Blood Services and Héma-Québec have near-national reach of a healthy adult population. Blood donations are collected daily and longitudinal sampling is possible. Demographic data, routine infectious disease testing information and screening questionnaire data such as current medications, recent vaccinations and travel history are collected from all donors. Avenues by which the blood services can contribute to public health surveillance post-pandemic are being actively explored. Potential areas include serosurveillance of vaccine-preventable infections, lookback and traceback investigations and monitoring for emerging vector-borne pathogens.
Authors' statement
All authors (SFO, SJD, AL, CO, MAD, CR) conceptualized and revised this paper. SFO drafted the paper.
Competing interests
None.
Acknowledgements
We thank Samra Uzicanin for assistance with the figures.
Funding
This work was supported by Canadian Blood Services and Héma-Québec.
