Surveillance of ticks and their pathogens, Canada, 2019

 Download this article as a PDF
Download this article as a PDF Published by: The Public Health Agency of Canada
Issue: Volume 48-5, May 2022: Vector-Borne Infections–Part 1: Ticks & Mosquitoes
Date published: May 2022
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 48-5, May 2022: Vector-Borne Infections–Part 1: Ticks & Mosquitoes
Surveillance
Surveillance for Ixodes scapularis and Ixodes pacificus ticks and their associated pathogens in Canada, 2019
Christy H Wilson1, Salima Gasmi2, Annie-Claude Bourgeois1, Jacqueline Badcock3, Navdeep Chahil4, Manisha A Kulkarni5, Min-Kuang Lee4, L Robbin Lindsay6, Patrick A Leighton7, Muhammad G Morshed4,8, Christa Smolarchuk9, Jules K Koffi2
Affiliations
1 Centre for Food-borne, Environmental and Zoonotic Infectious Diseases, Public Health Agency of Canada, Ottawa, ON
2 Centre for Food-borne, Environmental and Zoonotic Infectious Diseases, Public Health Agency of Canada, Saint-Hyacinthe, QC
3 Public Health New Brunswick, New Brunswick Department of Health, Fredericton, NB
4 BCCDC Public Health Laboratory, BC Centre for Disease Control, Vancouver, BC
5 School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON
6 One Health Section, National Microbiology Laboratory Branch, Public Health Agency of Canada, Winnipeg, MB
7 Epidemiology of Zoonoses and Public Health Research Group (GREZOSP), Faculty of Veterinary Medicine, Université de Montréal, Saint-Hyacinthe, QC
8 Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC
9 Analytics and Performance Reporting Branch, Health Standards, Quality and Performance Division, Alberta Health, Edmonton, AB
Correspondence
Suggested citation
Wilson CH, Gasmi S, Bourgeois A-C, Badcock J, Chahil N, Kulkarni MA, Lee M-K, Lindsay LR, Leighton PA, Morshed MG, Smolarchuk C, Koffi JK. Surveillance for Ixodes scapularis and Ixodes pacificus ticks and their associated pathogens in Canada, 2019. Can Commun Dis Rep 2022;48(5):208–18. https://doi.org/10.14745/ccdr.v48i05a04
Keywords: Ixodes scapularis, Ixodes pacificus, surveillance, Borrelia, Anaplasma, Babesia
Abstract
Background: The primary vectors of the agent of Lyme disease in Canada are Ixodes scapularis and Ixodes pacificus ticks. Surveillance for ticks and the pathogens they can transmit can inform local tick-borne disease risk and guide public health interventions. The objective of this article is to characterize passive and active surveillance of the main Lyme disease tick vectors in Canada in 2019 and the tick-borne pathogens they carry.
Methods: Passive surveillance data were compiled from the National Microbiology Laboratory Branch and provincial public health data sources. Active surveillance was conducted in selected sentinel sites in all provinces. Descriptive analysis of ticks submitted and infection prevalence of tick-borne pathogens are presented. Seasonal and spatial trends are also described.
Results: In passive surveillance, specimens of I. scapularis (n=9,858) were submitted from all provinces except British Columbia and I. pacificus (n=691) were submitted in British Columbia and Alberta. No ticks were submitted from the territories. The seasonal distribution pattern was bimodal for I. scapularis adults, but unimodal for I. pacificus adults. Borrelia burgdorferi was the most prevalent pathogen in I. scapularis (18.8%) and I. pacificus (0.3%). In active surveillance, B. burgdorferi was identified in 26.2% of I. scapularis; Anaplasma phagocytophilum in 3.4% of I. scapularis, and Borrelia miyamotoi and Powassan virus in 0.5% or fewer of I. scapularis. These same tick-borne pathogens were not found in the small number of I. pacificus tested.
Conclusion: This surveillance article provides a snapshot of the main Lyme disease vectors in Canada and their associated pathogens, which can be used to monitor emerging risk areas for exposure to tick-borne pathogens.
Introduction
Ixodes scapularis and Ixodes pacificus are tick vectors capable of transmitting several bacterial, viral and protozoan pathogens to humansFootnote 1. Ixodes scapularis populations are increasing in number and distribution in southern central and eastern CanadaFootnote 2Footnote 3Footnote 4. Climate (e.g. increasing temperatures, changes in precipitation) and environmental factors (e.g. changes in land use) contribute to the geographic range expansion of ticks, which can enhance exposure to tick-borne diseases (TBD)Footnote 1Footnote 5Footnote 6Footnote 7. These changes can also create longer seasons for adventitious ticks to become established in new areas and increase human-tick interactionsFootnote 1Footnote 4Footnote 6Footnote 7Footnote 8. Continued expansion of the range of ticks in Canada presents a public health challenge, as awareness of TBD risks and capacity for surveillance and testing must also expand to these areasFootnote 1.
Lyme disease (LD) is the most commonly reported vector-borne disease in Canada, and incidence of reported cases has increased more than 17-fold from 2009 through 2019Footnote 9Footnote 10. The causative agent of LD, Borrelia burgdorferi, is transmitted by I. scapularis in central and eastern Canada and by I. pacificus in British Columbia. Beyond LD, other TBD including anaplasmosis (caused by the bacterium Anaplasma phagocytophilum), babesiosis (caused by the parasite Babesia microti), hard tick-borne relapsing fever (caused by the bacterium Borrelia miyamotoi) and Powassan virus (POWV) disease are emerging as diseases locally acquired within Canada Footnote 1Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15.
Passive surveillance began in the early 1990s in Canada to detect the occurrence of I. scapularis and I. pacificus tick vectors and their infection with B. burgdorferiFootnote 16. Active surveillance has been ongoing since the 2000s to identify areas where vector tick populations are establishing and, as a result, where LD may become endemic (LD risk areas)Footnote 17Footnote 18. This is the first edition of a pan-Canadian annual article summarizing the findings of both passive and active vector surveillance and updating estimates of infection prevalence in ticks. A previous study by Guillot et al.Footnote 19 summarized results of a pan-Canadian study on tick surveillance; however, that study only included active tick surveillance from sentinel sites.
The objective of this surveillance article is to provide an epidemiologic summary of the main LD vectors in Canada, I. scapularis and I. pacificus, and their associated pathogens, collected through active and passive surveillance systems in 2019. This article will also summarize the prevalence and spatial distribution of tick-borne pathogens.
Methods
Data sources
This article uses two types of surveillance data from six different sources: 1) passive tick surveillance data from the National Microbiology Laboratory (NML) Branch of the Public Health Agency of Canada, the British Columbia Centre for Disease Control and Alberta HealthFootnote 20; and 2) active tick surveillance data from the Canadian Lyme Sentinel Network (CaLSeN), the New Brunswick Department of Health and the University of Ottawa.
Passive tick surveillance
In passive tick surveillance, ticks are collected by the public and submitted to medical clinics, veterinary clinics, or directly to a provincial public health laboratory or other institution (e.g. university laboratory) for species identificationFootnote 16. Location of acquisition, history of travel in the past two weeks, date of collection, level of engorgement, tick instar and host are recorded.
This article focuses on I. scapularis and I. pacificus ticks collected in Canada, although several other tick species were also collected. Ticks with an international location of acquisition, an imprecise location within Canada that could not be geocoded (e.g. province listed only, multiple locations listed) or history of travel were excluded to create a dataset of locally acquired ticks. Over the years, passive tick surveillance programs have been discontinued in different jurisdictions, i.e. Nova Scotia, southwestern Québec (Montérégie) and eastern Ontario; however, the public continues to submit a relatively small number of ticks acquired in these jurisdictions directly to NML.
In 2019, Saskatchewan, Manitoba, Ontario, Québec, Newfoundland and Labrador, New Brunswick, Nova Scotia and Prince Edward Island sent ticks to NML for testing of tick-borne pathogens (A. phagocytophilum, B. burgdorferi and B. microti) using methods described previouslyFootnote 21Footnote 22. Ticks could be submitted singly or in groups of two or more (multiple submission). For laboratory testing, ticks from the same multiple submission were pooled and tested together. In British ColumbiaFootnote 23 and AlbertaFootnote 24, testing was done at provincially funded laboratories on individual ticks for only B. burgdorferi. Ticks are rarely encountered in northern Canada and as a result, formal passive tick surveillance programs for I. scapularis or I. pacificus are not established in the Yukon, Northwest Territories or Nunavut.
Active tick surveillance
Active surveillance involves collection of ticks in the environment through drag sampling or through capture of host mammals that are examined for ticks. This method aims to identify where emerging tick populations are establishingFootnote 4Footnote 18. For this article, only I. scapularis and I. pacificus collected by drag sampling were included for analysis, although several other tick species were also collected.
This article collates data from CaLSeN, the New Brunswick Department of Health and the University of Ottawa. The CaLSeN used standardized methods to conduct dragging in 96 sites across all provincesFootnote 19. The New Brunswick Department of Health and the University of Ottawa used similar dragging methods to visit 73 and 15 sites, respectivelyFootnote 25. Visit date, location of collection (latitude and longitude), tick species and tick instar were recorded for all ticks collected.
Nymphs and adult I. scapularis and I. pacificus were tested for tick-borne pathogens. Ticks collected by CaLSeN and by the province of New Brunswick were tested for A. phagocytophilum, B. microti, B. burgdorferi, B. miyamotoi and POWV (CaLSeN ticks only) at NML using methods previously describedFootnote 19Footnote 21Footnote 22. Ticks collected by the University of Ottawa were tested for A. phagocytophilum, B. burgdorferi and B. miyamotoi with quantitative polymerase chain reaction (qPCR) assays described previouslyFootnote 25 using the flaB gene for B. miyamotoi and including a confirmatory assay targeting msp2 in A. phagocytophilum. Testing for B. microti used a qPCR assay targeted towards the cctη geneFootnote 21.
Analysis
Tick characteristics
For passive tick surveillance, we calculated descriptive statistics for province of acquisition, tick species, instar (larva, nymph, adult male or adult female), level of engorgement (unfed, partially engorged or fully engorged), host (human, dog, cat or other) and month of collection. For active tick surveillance, we calculated descriptive statistics for province of acquisition, tick species and instar (larva, nymph or adult). The probable location of acquisition for ticks was mapped using QGIS (version 3.8.1).
Infection prevalence
For ticks submitted through passive surveillance, maximum likelihood estimates (MLE) of prevalence with 95% confidence intervals (CI) were calculated in Excel (version 16.0) using the PooledInfRate add-in (version 4.0) to account for pooled testingFootnote 26Footnote 27. Co-infection prevalence was assessed among single submissions only to ensure they were true co-infections (two or more pathogens in the same tick). The prevalence of co-infections was calculated as the number of co-infected ticks divided by the total number of ticks tested. Prevalence in active surveillance was calculated in the same manner as all ticks were tested individually.
Results
Passive surveillance tick characteristics
In 2019, there were 10,549 I. pacificus and I. scapularis ticks submitted from all provinces through passive surveillance (Table 1). The majority of ticks (90.0%) were submitted from three provinces: Ontario, Québec, and New Brunswick (Figure 1). The majority of ticks (94.0%) were single submissions, but there were 242 multiple submissions (range: 2–8 ticks). Nova Scotia had the highest proportion of multiple submissions (13.7%; n=7/51).
| Province | Number of ticks | Number of single submissionsTable 1 Footnote b | Multiple submissionsTable 1 Footnote b | ||||
|---|---|---|---|---|---|---|---|
| Ixodes scapularis | Ixodes pacificus | Total | Number of submissions | Median number of ticks per submission | |||
| n | Range | ||||||
| British Columbia | 0 | 690 | 690 | 690 | N/ATable 1 Footnote c | N/ATable 1 Footnote c | N/ATable 1 Footnote c |
| Alberta | 55 | 1 | 56 | 56 | N/ATable 1 Footnote c | N/ATable 1 Footnote c | N/ATable 1 Footnote c |
| Saskatchewan | 3 | 0 | 3 | 3 | 0 | N/A | N/A |
| Manitoba | 175 | 0 | 175 | 149 | 8 | 3 | 2–7 |
| OntarioTable 1 Footnote d | 6,857 | 0 | 6,857 | 6,436 | 167 | 2 | 2–8 |
| QuébecTable 1 Footnote d | 1,697 | 0 | 1,697 | 1,618 | 31 | 2 | 2–7 |
| Newfoundland and Labrador | 44 | 0 | 44 | 42 | 1 | 2 | 2 |
| New Brunswick | 941 | 0 | 941 | 868 | 28 | 2 | 2–8 |
| Nova ScotiaTable 1 Footnote e | 72 | 0 | 72 | 44 | 7 | 5 | 2–5 |
| Prince Edward Island | 14 | 0 | 14 | 14 | 0 | N/A | N/A |
| Total | 9,858 | 691 | 10,549 | 9,920 | 242 | 2 | 2–8 |
Figure 1: Ixodes pacificus and Ixodes scapularis ticks submitted through passive tick surveillance, Canada, 2019Figure 1 Footnote aFigure 1 Footnote b
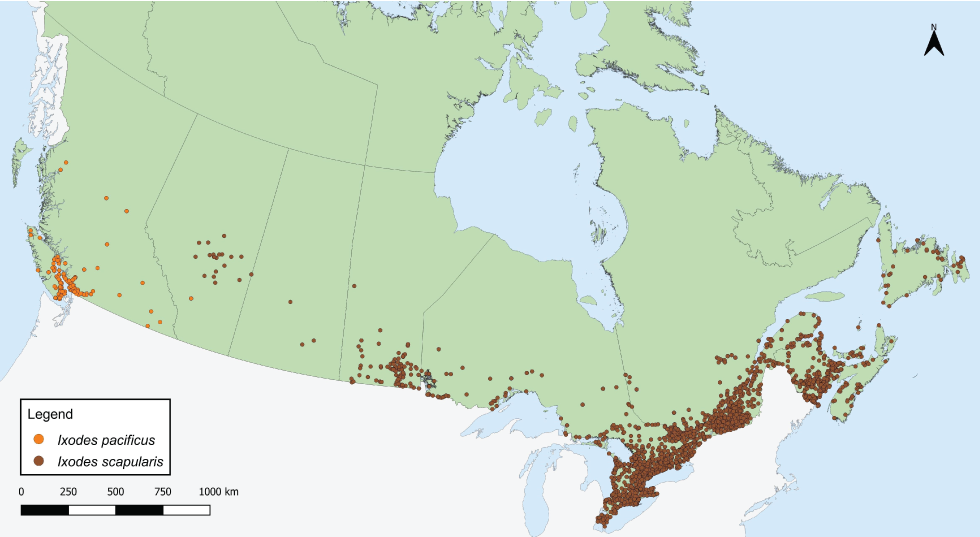
Text description: Figure 1
This map shows the probable location of acquisition of Ixodes scapularis and Ixodes pacificus ticks submitted through passive surveillance. Ixodes pacificus ticks were present in British Columbia and Alberta, while I. scapularis ticks were present in Alberta, Saskatchewan, Manitoba, Ontario, Québec, Newfoundland and Labrador, New Brunswick, Nova Scotia and Prince Edward Island to varying extents.
Data on tick instar, level of engorgement and host were available for 99.9%, 0% and 100% of I. pacificus, respectively. Data on tick instar, level of engorgement and host were available for 99.4%, 99.3% and 99.6% of I. scapularis, respectively. Adult ticks were submitted most frequently, of which most were female (I. scapularis: 89.0%; I. pacificus: 93.8%) (Table 2). Larvae (0.3%; 0.4%) and nymphs (8.1%; 3.3%) were submitted less frequently. Overall, 44.0% of I. scapularis were partially or fully engorged. Humans were the most common host among I. scapularis and I. pacificus (90.3%, 94.4%, respectively), followed by dogs (7.7%, 5.4%, respectively).
| Characteristic | Tick species | |||
|---|---|---|---|---|
| Ixodes scapularis | Ixodes pacificus | |||
| n | % | n | % | |
| Instar | ||||
| Larva | 27 | 0.3 | 3 | 0.4 |
| Nymph | 795 | 8.1 | 23 | 3.3 |
| Adult female | 8,719 | 89.0 | 647 | 93.8 |
| Adult male | 256 | 2.6 | 17 | 2.5 |
| Total | 9,797 | 100 | 690 | 100 |
| Level of engorgementTable 2 Footnote c | ||||
| Fully engorged | 113 | 1.2 | N/A | N/A |
| Partially engorged | 4,188 | 42.8 | N/A | N/A |
| Unfed | 5,485 | 56.0 | N/A | N/A |
| Total | 9,786 | 100 | N/A | N/A |
| Host | ||||
| Human | 8,870 | 90.3 | 652 | 94.4 |
| Dog | 761 | 7.7 | 37 | 5.4 |
| Cat | 119 | 1.2 | 1 | 0.1 |
| OtherTable 2 Footnote d | 72 | 0.7 | 1 | 0.1 |
| Total | 9,822 | 100 | 691 | 100 |
Month of acquisition was available for 99.9% of I. pacificus and 99.4% of I. scapularis. Locally acquired ticks were submitted in every month of the year (Figure 2). Submissions of I. scapularis adults peaked in May and October, while there was a single peak for I. pacificus adults in May. Ixodes scapularis nymph submissions peaked in June and July, while I. pacificus nymph submissions peaked in May.
Figure 2: Number of Ixodes scapularis and Ixodes pacificus ticks submitted through passive surveillance, by month and tick instar, Canada, 2019Figure 2 Footnote aFigure 2 Footnote b
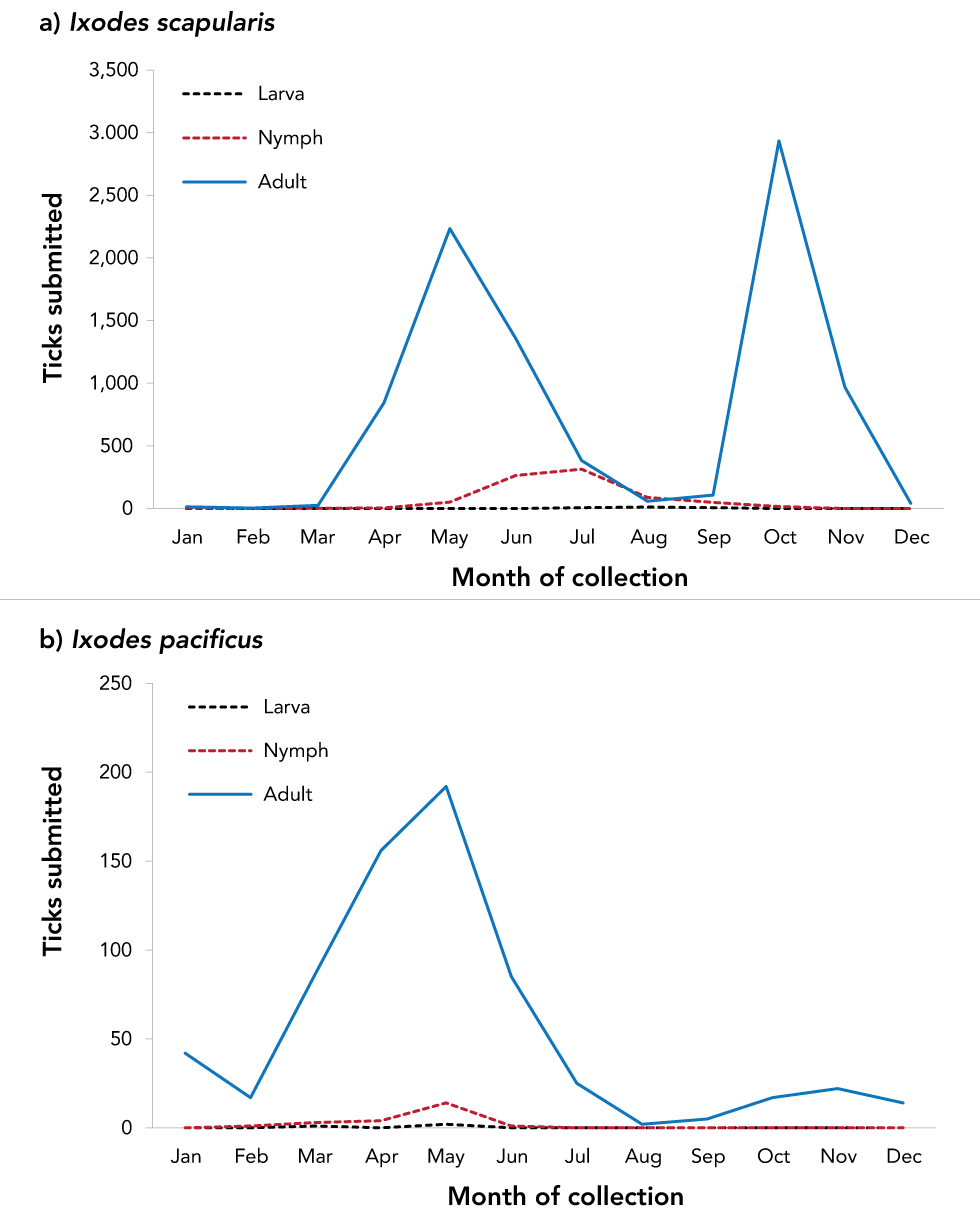
Text description: Figure 2
| Month of collection | Ixodes pacificus | Ixodes scapularis | ||||
|---|---|---|---|---|---|---|
| Larva | Nymph | Adult | Larva | Nymph | Adult | |
| Jan | 0 | 0 | 42 | 0 | 3 | 15 |
| Feb | 0 | 1 | 17 | 0 | 0 | 4 |
| Mar | 1 | 3 | 87 | 0 | 3 | 25 |
| Apr | 0 | 4 | 156 | 0 | 5 | 843 |
| May | 2 | 14 | 192 | 0 | 52 | 2,234 |
| Jun | 0 | 1 | 85 | 1 | 264 | 1,358 |
| Jul | 0 | 0 | 25 | 7 | 314 | 383 |
| Aug | 0 | 0 | 2 | 12 | 88 | 59 |
| Sep | 0 | 0 | 5 | 7 | 49 | 107 |
| Oct | 0 | 0 | 17 | 0 | 16 | 2,934 |
| Nov | 0 | 0 | 22 | 0 | 0 | 970 |
| Dec | 0 | 0 | 14 | 0 | 1 | 43 |
Passive surveillance infection prevalence
Data on laboratory testing were available for 97.4% of I. pacificus and 99.0%–99.5% of I. scapularis, depending on the pathogen. The most prevalent tick-borne pathogen was B. burgdorferi, found in 18.8% of I. scapularis (95% CI: 18.00–19.55), but only 0.3% of I. pacificus (95% CI: 0.05–0.97). Other tick-borne pathogens and co-infections were less prevalent (Table 3).
| Pathogen | Infection prevalence | |||
|---|---|---|---|---|
| Ixodes pacificus | Ixodes scapularis | |||
| Single agent | Maximum likelihood estimateTable 3 Footnote c | |||
| % | 95% CI | % | 95% CI | |
| Borrelia burgdorferi | 0.3 | 0.05–0.97 | 18.8 | 18.00–19.55 |
| Anaplasma phagocytophilum | N/A | N/A | 1.4 | 1.22–1.70 |
| Babesia microti | N/A | N/A | 0.1 | 0.07–0.22 |
| Any of above | 0.3 | 0.05–0.97 | 20.0 | 19.23–20.83 |
| Co-infection | Co-infection rateTable 3 Footnote d | |||
| % | Number co-infected ticks/number ticks tested | % | Number co-infected ticks/number ticks tested | |
| Borrelia burgdorferi + Anaplasmaphagocytophilum | N/A | N/A | 0.28 | 26/9,171 |
| Borrelia burgdorferi + Babesia microti | N/A | N/A | 0.02 | 2/9,171 |
| Anaplasma phagocytophilum + Babesia microti | N/A | N/A | 0.01 | 1/9,171 |
| Any co-infection | N/A | N/A | 0.32 | 29/9,171 |
Prevalence of B. burgdorferi was higher in I. scapularis from multiple submissions (24.5%, 95% CI: 20.64–28.69) than from single submissions (18.5%, 95% CI: 17.71–19.29) (Table 4). Prevalence did not significantly differ by submission type for any other pathogens.
| Characteristic | Infection prevalence Maximum likelihood estimate |
|||||||
|---|---|---|---|---|---|---|---|---|
| Borrelia burgdorferi | Anaplasma phagocytophilum | Babesia microti | Any of above | |||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Submission typeTable 4 Footnote b | ||||||||
| Single | 18.5 | 17.71–19.29 | 1.4 | 1.20–1.69 | 0.1 | 0.07–0.22 | 19.7 | 18.92–20.55 |
| Multiple | 24.5 | 20.64–28.69 | 1.7 | 0.89–3.06 | 0.2 | 0.01–0.82 | 26.3 | 22.31–30.70 |
| HostTable 4 Footnote c | ||||||||
| Human | 19.2 | 18.39–20.04 | 1.3 | 1.11–1.59 | 0.1 | 0.07–0.23 | 20.4 | 19.54–21.23 |
| Non-humanTable 4 Footnote d | 14.7 | 12.44–17.13 | 2.6 | 1.68–3.85 | 0.1 | 0.01–0.57 | 16.7 | 14.31–19.29 |
Ixodes scapularis submitted from human hosts had higher prevalence of B. burgdorferi infection (19.2%, 95% CI: 18.39–20.04) than those submitted from non-human hosts (14.7%, 95% CI: 12.44–17.13) (Table 4). However, I. scapularis submitted from non-human hosts had higher prevalence of A. phagocytophilum infection (2.6%, 95% CI: 1.68–3.85) than those submitted from human hosts (1.3%, 95% CI: 1.11–1.59). Both B. burgdorferi-infected I. pacificus ticks were from human hosts.
Tick-borne pathogens were commonly found in ticks submitted from southern Manitoba, northwestern Ontario, southern and eastern Ontario, southern Québec and southern New Brunswick (Figure 3 and Figure 4). Over two-thirds of B. burgdorferi-infected tick submissions were within previously identified LD risk areas (72.1%; n=1,313/1,821) (Figure 3). The majority of multiple submissions came from LD risk areas (76.9%; n=186/242), of which approximately half were infected with B. burgdorferi (51.4%; n=90/175). Newfoundland and Labrador, Nova Scotia and Québec all had higher infection prevalence of B. burgdorferi than the national average for I. scapularis (Table 5). Manitoba had the highest prevalence of A. phagocytophilum and B. microti infection among all provinces.
Figure 3: Ixodes pacificus and Ixodes scapularis ticks submitted through passive surveillance that were infected with Borrelia burgdorferi, Canada, 2019Figure 3 Footnote aFigure 3 Footnote bFigure 3 Footnote c
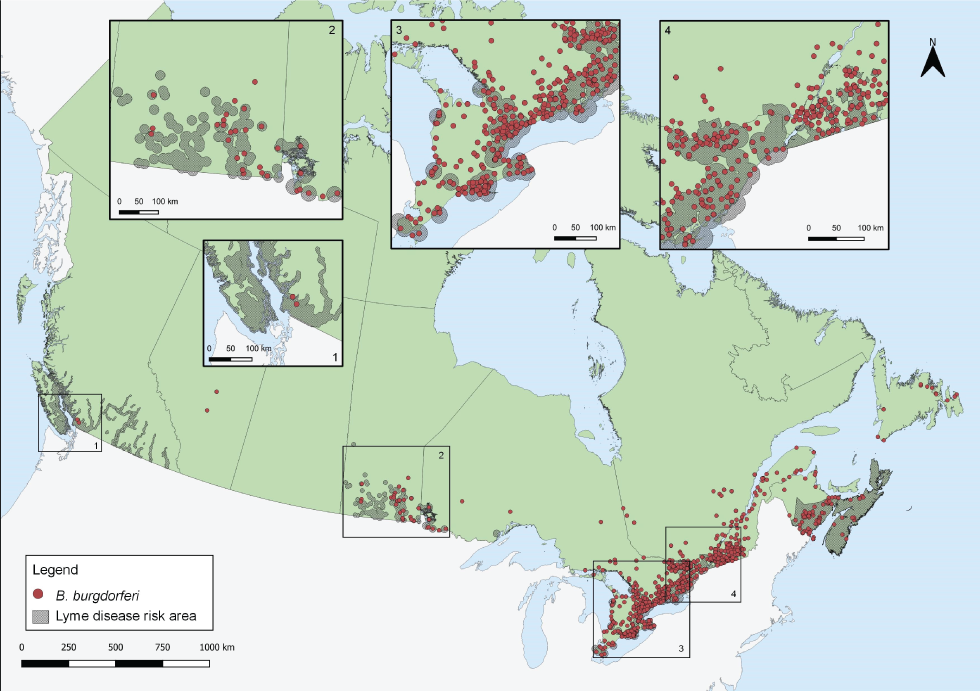
Text description: Figure 3
This map shows the probable location of acquisition of Ixodes pacificus and Ixodes scapularis ticks submitted through passive surveillance that were infected with Borrelia burgdorferi. Infected ticks are found in British Columbia, Alberta, Manitoba, Ontario, Québec, Newfoundland and Labrador, New Brunswick and Nova Scotia to varying extents. Most infected ticks are found in southern Ontario and Québec.
Figure 4: Ixodes scapularis ticks submitted through passive surveillance that were infected with Anaplasma phagocytophilum, Babesia microti and co-infections, Canada, 2019Figure 4 Footnote aFigure 4 Footnote b
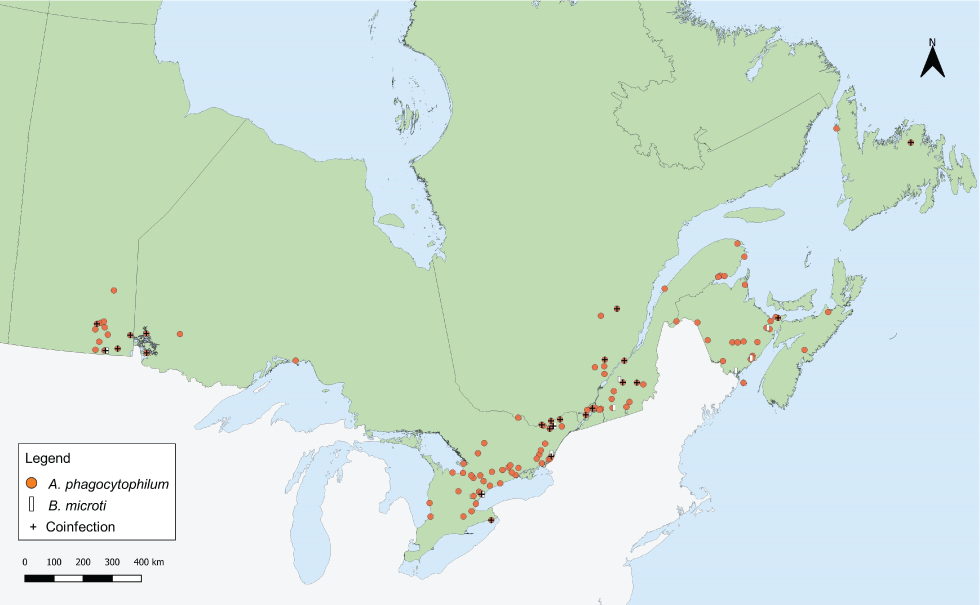
Text description: Figure 4
This map shows the probable location of acquisition of Ixodes scapularis ticks submitted through passive surveillance that were infected with Anaplasma phagocytophilum, Babesia microti or a co-infection with 2 of the following: A. phagocytophilum, Borrelia burgdorferi and B. microti. Ticks infected with A. phagocytophilum were found in Manitoba, Ontario, Québec, Newfoundland and Labrador, New Brunswick and Nova Scotia. Ticks infected with B. microti were found in Manitoba, Ontario, Québec and New Brunswick. Ticks with co-infections were found in Manitoba, Ontario, Québec, Newfoundland and Labrador and New Brunswick.
| Province | Infection prevalence Maximum likelihood estimate |
|||||
|---|---|---|---|---|---|---|
| Borrelia burgdorferi | Anaplasma phagocytophilum | Babesia microti | ||||
| % | 95% CI | % | 95% CI | % | 95% CI | |
| Ixodes pacificus | ||||||
| British Columbia | 0.3 | 0.05–0.97 | N/A | N/A | N/A | N/A |
| Ixodes scapularis | ||||||
| AlbertaTable 5 Footnote b | 5.5 | 1.45–14.01 | N/A | N/A | N/A | N/A |
| Saskatchewan | 0.0 | 0–56.15 | 0.0 | 0–56.15 | 0.0 | 0–56.15 |
| Manitoba | 18.3 | 12.94–24.68 | 10.4 | 6.38–15.81 | 2.4 | 0.78–5.63 |
| Ontario | 18.3 | 17.37–19.22 | 0.9 | 0.73–1.18 | 0.1 | 0.02–0.14 |
| Québec | 24.2 | 22.18–26.30 | 1.9 | 1.32–2.63 | 0.1 | 0.02–0.39 |
| Newfoundland and Labrador | 29.5 | 17.63–44.01 | 4.6 | 0.82–14.28 | 0.0 | 0–8.02 |
| New Brunswick | 12.8 | 10.80–15.10 | 2.6 | 1.70–3.74 | 0.3 | 0.08–0.87 |
| Nova Scotia | 26.2 | 15.38–39.82 | 3.9 | 0.70–12.31 | 0.0 | 0–6.82 |
| Prince Edward Island | 0.0 | 0–21.53 | 0.0 | 0–21.53 | 0.0 | 0–21.53 |
| Total | 18.8 | 18.00–19.55 | 1.5 | 1.22–1.70 | 0.1 | 0.07–0.22 |
Active surveillance tick characteristics
In active surveillance, I. scapularis and I. pacificus were found at 78 of 184 surveillance sites (range of ticks found: n=0–130). Ixodes scapularis (n=1,156) were found in Manitoba, Ontario, Québec, New Brunswick, Nova Scotia and Prince Edward Island, while I. pacificus (n=10) were found in British Columbia. Regarding the instar, 51.5% (n=601/1,166) of ticks were identified as nymphs, 29.5% (n=344/1,166) were adults and 19.0% (n=221/1,166) were larvae.
Active surveillance infection prevalence
Data on laboratory testing were available for 100% of I. pacificus collected and 73.8%–98.3% of I. scapularis nymphs and adults collected, depending on the pathogen. No tick-borne pathogens were found in I. pacificus (Table 6). In I. scapularis, B. burgdorferi was identified in 26.2% of ticks tested and in four provinces: Ontario, Québec, New Brunswick, and Nova Scotia. Anaplasma phagocytophilum was identified in the same four provinces in 3.4% of I. scapularis. Borrelia miyamotoi and POWV were found in 0.5% or fewer. Figure 5 shows the locations of ticks with tick-borne pathogens collected in active surveillance.
| Province | Infection prevalence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anaplasma phagocytophilum | Babesia microti | Borrelia burgdorferi | Borrelia miyamotoi | Powassan virus | ||||||
| Number positive tick/number tick tested | % | Number positive tick/number tick tested | % | Number positive tick/number tick tested | % | Number positive tick/number tick tested | % | Number positive tick/number tick tested | % | |
| Ixodes pacificus | ||||||||||
| British Columbia | 0/10 | 0 | 0/10 | 0 | 0/10 | 0 | 0/10 | 0 | 0/10 | 0 |
| Ixodes scapularis | ||||||||||
| Manitoba | 0/3 | 0 | 0/3 | 0 | 0/3 | 0 | 0/3 | 0 | 0/3 | 0 |
| Ontario | 14/406 | 3.5 | 0/397 | 0 | 126/410 | 30.7 | 1/410 | 0.2 | 0/188 | 0 |
| Québec | 2/141 | 1.4 | 0/141 | 0 | 28/141 | 19.8 | 1/141 | 0.7 | 0/141 | 0 |
| New Brunswick | 8/194 | 4.1 | 0/194 | 0 | 41/194 | 21.1 | 3/194 | 1.6 | 0/194 | 0 |
| Nova Scotia | 7/169 | 4.1 | 0/169 | 0 | 46/169 | 27.2 | 0/169 | 0 | 1/169 | 0.6 |
| Prince Edward Island | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 |
| Total | 31/915 | 3.4 | 0/906 | 0 | 241/919 | 26.2 | 5/919 | 0.5 | 1/697 | 0.1 |
Figure 5: Ixodes scapularis and Ixodes pacificus ticks with associated pathogens collected through active surveillance, Canada, 2019Figure 5 Footnote aFigure 5 Footnote b
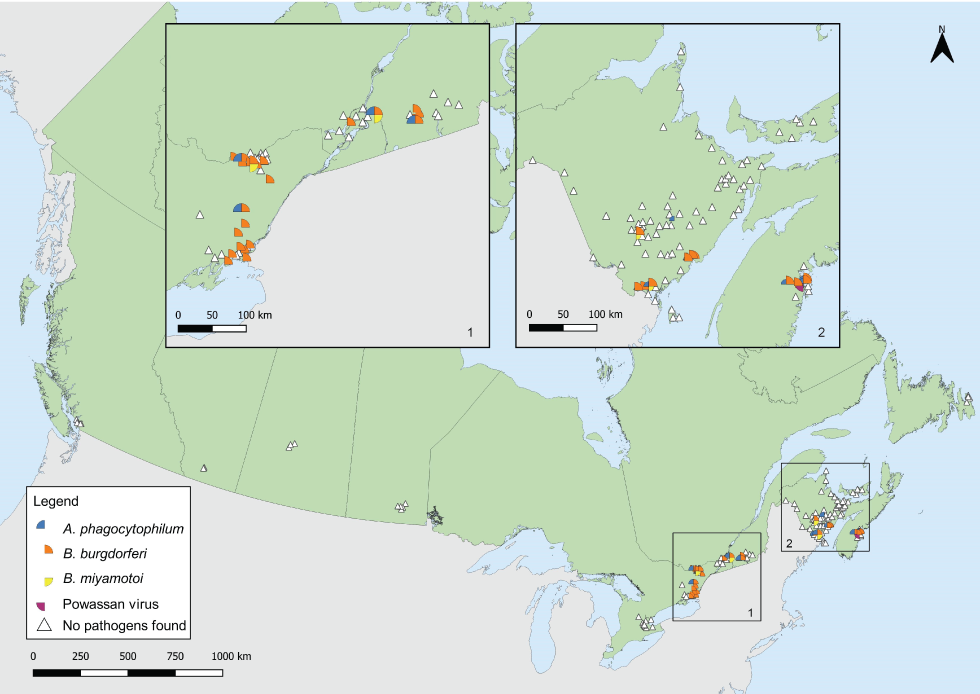
Text description: Figure 5
This map shows the locations where Ixodes scapularis and Ixodes pacificus ticks infected with Borrelia burgdorferi, Anaplasma phagocytophilum, Borrelia miyamotoi or Powassan virus were found in active surveillance. Borrelia burgdorferi and A. phagocytophilum were found in ticks in Ontario, Québec, New Brunswick and Nova Scotia. Borrelia miyamotoi was found in ticks in Ontario, Québec and New Brunswick. Powassan virus was found in a tick in Nova Scotia. All ticks found in British Columbia, Manitoba and Prince Edward Island through active surveillance were negative for all pathogens. No ticks were found in Alberta, Saskatchewan and Newfoundland and Labrador.
Discussion
In 2019, 9,858 I. scapularis and 691 I. pacificus were submitted in passive surveillance in eight provinces. Of these, 20.0% of I. scapularis and 0.3% of I. pacificus were infected with at least one of the tick-borne pathogens tested, including B. burgdorferi, A. phagocytophilum or B. microti. Active surveillance identified four tick-borne pathogens among I. scapularis collected in four provinces, and no tick-borne pathogens among the few I. pacificus collected.
In passive surveillance, one I. pacificus with no travel history was identified in Alberta, outside of British Columbia where reproducing populations are known to be established. Ixodes pacificus have been found in the province before on migratory birdsFootnote 29, or from human or animal hosts mostly associated with travelFootnote 20.
Ticks were submitted through passive surveillance in every month, highlighting the potential year-round risk (depending on location and weather) of exposure to ticks, which may or may not be infected with tick-borne pathogen(s). Ixodes spp. ticks, for example, were often found in western Canada in the winter but were rarely infectedFootnote 23. The single peak of I. pacificus tick submissions in the spring has historically been observed in British ColumbiaFootnote 23 and in the western United StatesFootnote 30, as nymphs and adults are both active during the cooler spring monthsFootnote 31. Bimodal peaks for adult I. scapularis in late spring and autumn have been previously observed in central and eastern CanadaFootnote 3Footnote 16Footnote 32, and are consistent with adult I. scapularis activity in a 3 to 4-year lifecycle extended, in part, by cooler spring temperaturesFootnote 31Footnote 33. Nymphs of both species, which are most implicated in LD transmissionFootnote 34, peak during the late spring to summer months when LD onset in humans also peaksFootnote 9.
Compared to most recent estimates, infection prevalence of B. burgdorferi among I. pacificus ticks in British Columbia (0.3%) was consistent with annual rates from 2002 to 2018 between 0.1 and 0.4%Footnote 23. In Manitoba, infection prevalence among I. scapularis was lower (18.3%) than the 2018 minimum infection rate of 20.7%Footnote 35. In Ontario, infection prevalence among I. scapularis increased to 18.3% from the 2011–2017 rate of 15.8%Footnote 36. In Québec, infection prevalence also increased to 24.2% from 17.6% in I. scapularis adults from 2009 to 2015Footnote 37. Inter and intra-provincial variability in annual prevalence is influenced, however, by annual variation in weather, effort of surveillance, history of established vector populations and habitat suitability.
Prevalence of I. scapularis being infected with at least one of the tick-borne pathogens tested was higher in multiple submissions than single submissions. As multiple submissions are indicators of tick establishment in a given areaFootnote 38, this suggests higher infection prevalence among established tick populations.
Over two-thirds of B. burgdorferi-infected ticks had probable locations of acquisition within LD risk areas. The LD risk areas are identified by the provinces using the methods described in the 2016 national LD case definitionFootnote 28 and are regularly updated to incorporate new surveillance data. Borrelia burgdorferi-infected ticks collected outside of these known LD risk areas may be adventitious ticks, brought to these areas by migratory birds or terrestrial hostsFootnote 18. Public health authorities and clinicians should be aware that risk of exposure to infected ticks exists outside of known LD risk areas. Increasing the collaborative effort of active surveillance can support the timely recognition of new LD risk areas. Promptly identifying and removing ticks, regardless of their locality of acquisition, can prevent transmission of tick-borne pathogens.
In active surveillance, there was geographic variability in infection prevalence, similar to findings from passive surveillance. Conducting standardized and consistent active surveillance across the country can help identify new LD-risk areas and detect other emerging tick-borne pathogens in known LD-risk areas, thereby informing local risk of exposure to TBD.
While B. burgdorferi was the most prevalent tick-borne pathogen in both passive and active surveillance, A. phagocytophilum, B. microti, B. miyamotoi and POWV were also detected. All provinces, however, had lower infection prevalence than hyper-endemic areas in the northeastern United States. For example, I. scapularis adults collected in Maine through passive surveillance had B. burgdorferi, A. phagocytophilum and B. microti infection prevalence of 42.4%, 11.1% and 6.5%, respectivelyFootnote 39.
Ongoing climate and environmental changes affect TBD risk in a variety of ways, by altering populations of ticks and their animal hosts, as well as increasing human exposure to ticksFootnote 1. As current projections predict an increased risk of TBD from expansion of Ixodes spp. habitat in the futureFootnote 1Footnote 5Footnote 40, continued surveillance can monitor changes in tick distribution and infection prevalence. More studies are also needed to understand the emergence and ecology of other tick-borne pathogens across Canada, which may differ from B. burgdorferi, for example, in their enzootic transmission cyclesFootnote 41.
Strengths and limitations
This inaugural article combining active and passive tick surveillance presents a national snapshot of tick vectors and their emerging associated pathogens. By integrating the two types of surveillance, the strengths and weaknesses of the individual systems are complemented. Whereas active surveillance is resource-intensive and therefore limited in geographic scope, passive surveillance programs can be implemented on a larger geographic scale; however, passive surveillance lacks specificity as it often collects adventitious ticks seeded by migratory birds, especially ticks collected from companion animal hosts which readily acquire ticks from the environmentFootnote 18Footnote 38.
There are several limitations to this study. Provincial passive surveillance programs, and the effort and timing of active surveillance, vary across Canada due to resource limitations or logistics. Passive tick surveillance has been discontinued or limited to specific hosts in several regions. Further, passive tick surveillance can be limited by public awareness, and geographic or host-specific biases in tick submissionsFootnote 3Footnote 42Footnote 43. Not all active surveillance conducted in Canada in 2019 was included in this study; data from the many groups that conduct active surveillance, which includes university researchers, Indigenous communities and local or provincial public health units, was not all available. These limitations lead to underestimating the number of ticks, which affects the accuracy of infection prevalence. Lastly, it may be inappropriate to pool data from multiple active and passive surveillance systems due to differences in methodology between sources.
Conclusion
Passive and active surveillance identified both I. scapularis and I. pacificus across Canada in varying amounts depending on location, including some ticks which were infected with tick-borne pathogen(s). Both passive and active tick surveillance have utility in signalling and confirming new LD risk areas, which can be used to inform public health authorities where environmental risk for LD occurs. This information is used to communicate the local risk of LD and TBD to the public as well as to healthcare workers. Continued surveillance will be crucial for monitoring any expansion of areas at risk of exposure to ticks and tick-borne pathogens, and to appropriately target public health interventions such as education and awareness campaigns towards at-risk areas.
Authors' statement
CW — Formal analysis, visualization, writing–original draft, writing–review and editing
SG, AB, JK — Conceptualization, supervision, writing–review and editing
JB, NC, MK, ML, PL, RL, MM, CS — Writing–review and editing
Competing interests
None.
Acknowledgements
We thank all those involved with tick collection and testing at regional, provincial and national levels, including members of the public who submitted ticks. Yann Pelcat created the Lyme disease risk area shapefile in Figure 3. Drag sampling in New Brunswick was a collaboration between the New Brunswick Department of Health; the New Brunswick Department of Agriculture, Aquaculture and Fisheries; and the University of New Brunswick.
Funding
This study was supported by Public Health Agency of Canada (PHAC). Passive surveillance from British Columbia was supported by the BC Centre for Disease Control Foundation. Active surveillance conducted by the Canadian Lyme Sentinel Network (CaLSeN), which is part of the Canadian Lyme Disease Research Network, was funded by the Canadian Institutes of Health Research. The Institut national de santé publique du Québec (INSPQ) and Ministère de la Santé et des Services sociaux (MSSS) funded data collection in 12 sampling sites in Québec as part of annual surveillance activities. Active surveillance conducted by the New Brunswick Department of Health and the University of Ottawa were funded by PHAC.
