Guidance Document: Regulatory Requirements for Drug Identification Numbers (DINs)

Download the alternative format
(PDF format, 700 KB, 31 pages)
Organization: Health Canada
Published: May 2019
Date adopted: 2019/05/03
Effective date: 2019/05/03
Administrative changes date: 2025/02/18
Foreword
Guidance documents are meant to provide assistance to industry and health care professionals on how to comply with governing statutes and the Food and Drug Regulations. Guidance documents also provide assistance to staff on how Health Canada mandates and objectives should be implemented in a manner that is fair, consistent and effective.
Guidance documents are administrative instruments not having force of law and, as such, allow for flexibility in approach. Alternate approaches to the principles and practices described in this document may be acceptable provided they are supported by adequate justification. Alternate approaches should be discussed in advance with the relevant program area to avoid the possible finding that applicable statutory or regulatory requirements have not been met.
As a corollary to the above, it is equally important to note that Health Canada reserves the right to request information or material, or define conditions not specifically described in this document, in order to allow the Department to adequately assess the safety, efficacy or quality of a drug. Health Canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented.
| Date | Change | Location (section, paragraph) | Nature of and/or reason for change |
|---|---|---|---|
| May 3, 2019 | This guidance document consolidates and replaces the following policies and guidance documents:
|
Global change | To make it easier to find information relating to DINs by consolidating a number of policies and guidance document into a single document. |
| May 3, 2019 | Addition of the following wording: "(available for immediate use via hospital and retail pharmacies [i.e., the drug is physically on pharmacy shelves])" | 6.4, paragraph 4 | To provide clarification on the concept of availability of a drug on the Canadian market |
| June 19, 2019 | More information on the requirements for submitting a Notification of discontinuation of sale in Section 6.5 of the guidance. | 6.5 | Instructions regarding notification of discontinuation of sale from the manufacturer. |
| Correction to list of submission type Section 6.8 Reissuance of a DIN by Health Canada. | 6.8 | Removal of submission type PDC. | |
| January 14, 2022 | General updates throughout the document based on the process changes and feedback from the internal and external stakeholders | All sections | |
| Minor addition in regards to the issuance of multiple DINs to address safety issues | 6.1.3, paragraph 1 | To provide clarification to the existing practices | |
| Addition of a new paragraph | 6.2, paragraph 2 | A reminder that additional requirements to publicly report drug shortages and discontinuations may apply to some products | |
| Updates to the status descriptions and addition of definition for a Restricted access status | 6.2 | To align and clarify status descriptions | |
| Minor updates throughout Sections 6.3-6.8 | 6.3 to 6.8 | To provide clarification to the existing practices and in regards to the process change (confirmation emails are no longer sent following the processing of the notifications) | |
| Major changes throughout Section 6.10 | 6.10 | To provide additional clarification on filing requirements (including the Regulatory Enrolment Process (REP)) | |
| Minor change to the wording | Appendix A | ||
| Minor change to wording | 5 | Align wording with another section in the document | |
| Minor addition to paragraph | 6.1.1.1 | To provide additional clarification regarding new DINs | |
| May 1, 2024 | Minor revisions to improve clarity | Throughout the document | Revisions to clarify the interpretation of the regulatory requirements associated with a DIN and manufacturer obligations to report to Health Canada |
| Updates to notification types | 2 | To align with updated Regulatory Activity Types and Regulatory Transaction Descriptions | |
| Minor revision to improve clarity | 6.4.1 | To provide additional clarification regarding the Act being referred to | |
| Minor revision to improve clarity | 6.5 | To provide additional clarity regarding information to be submitted (lot number) | |
| Minor change to wording | 6.7 | Align wording with another section in the document | |
| Minor revision to improve clarity | 6.9 | To provide additional clarification regarding the Act being referred to | |
| Updates to Regulatory Activity Type and Regulatory Transaction Description | Table 5 | To align with updated Regulatory Activity Types and Regulatory Transaction Descriptions | |
| July 15, 2024 | Minor change to wording | Throughout the document | To align wording with the Food and Drugs Regulations |
Updates to Regulatory Activity Type and Regulatory Transaction Description |
Table 5 | To align with updated Regulatory Activity Types and control numbers used when submitting notifications | |
| February 18, 2025 | Minor revision to improve clarity | 5 | To guide the reader to consult section 6.6.4.1 for additional clarification on the designation "DIN" on the label of a biocide |
| Addition of new section | 6.6.4 | To provide clarification on cancellation after transitioning to the Biocides Regulations | |
| Addition of new section | 6.6.4.1 | To provide clarification on the difference between the identification number for biocides and the designation "DINs" on the label of a biocide |
On this page
- Introduction
- Purpose
- Scope
- Policy objectives
- Definitions
- Guidance for implementation
- 6.1 DIN assignment
- 6.2 Drug statuses and the Drug Product Database
- 6.3 Market notification
- 6.4 Twelve months without sale notification - dormant status
- 6.5 Discontinuation of sale notification
- 6.6 DIN cancellation
- 6.6.1 Cancellation due to safety issue
- 6.6.2 Cancellation due to failure to provide annual drug notification - C.01.014.6 (2) (a) of the Food and Drug Regulations
- 6.6.3 Cancellation as product is not a drug - C.01.014.6 (1) (c) of the Food and Drug Regulations
- 6.6.4 Cancellation due to a transition under the Biocides Regulations
- 6.7 Required activities following the cancellation of a DIN
- 6.8 Reassignment of a DIN by Health Canada
- 6.9 Commercial exportation
- 6.10 Submitting notifications to Health Canada
- 6.11 Contact us
1. Introduction
When Health Canada authorizes a drug to be marketed in Canada, a Drug Identification Number (DIN) is assigned to the manufacturer and printed on the package labels. A DIN indicates that the evaluation of the drug determined that it met the relevant requirements of the Food and Drugs Act and its Regulations and the drug has a favourable risk/benefit profile. Manufacturers of prescription and non-prescription drugs must obtain a DIN before they are marketed in Canada. Market authorization of a drug may require the additional issuance of a Notice of Compliance (NOC).
The DIN assigned to a drug is unique and serves as a tool to help in the post-market activities of products on the market, such as product identification and verification by health care professionals, recall of products, inspections, and quality monitoring. While the authorization of a drug includes the issuance of a DIN to the manufacturer, the DIN is the property of Health Canada.
2. Purpose
This guidance document provides:
- assistance on the interpretation of the regulatory requirements associated with a DIN
- guidance to manufacturers on their obligation to accurately report to Health Canada the following notifications for a change of drug status within the required timelines:
- Market notification
- 12 months without sale notification
- Discontinuation of sale notification
3. Scope
This guidance document applies to all drugs that have been assigned a DIN (i.e., human and veterinary drugs, biologics, disinfectants, and radiopharmaceuticals). This document is limited to changes that impact the status of a DIN. This guidance covers the following activities:
- Assignment of a DIN by Health Canada to the manufacturer
- Issuance of a revised Drug Notification Form by Health Canada to the manufacturer
- Filing of market notifications by the manufacturer to Health Canada
- Filing of 12 months without sale notifications by the manufacturer to Health Canada
- Filing of discontinuation of sale notifications by the manufacturer to Health Canada
This document does not cover the following:
- Filing requirements and management of drug submissions and applications
- Reporting of adverse drug reactions
- Reporting of potential or real drug shortages on the third party drug shortages website
- User fees and fees for the right to sell drugs
- Establishment licensing
- Products that have not been assigned a DIN (i.e., medical devices, natural health products, veterinary health products, pest control products, cosmetics, experimental treatments for human and animals, and cannabis for medical purposes regulated under Part 14 of the Cannabis Regulations)
- Annual drug notification process
4. Policy objectives
The policy objectives that guide the regulatory authority for activities relating to DIN assignment and reporting to Health Canada include:
- To protect the health and safety of Canadians from the sale of unsafe, and/or unauthorized drugs
- To provide Canadians with timely, reliable and accurate information on the availability of drugs in Canada
5. Definitions
- Annual Drug Notification Form (ADNF)
- A form intended to assist manufacturers in complying with section C.01.014.5 of the Food and Drug Regulations, which requires that every manufacturer of a drug confirms annually before October 1st that all information previously supplied with regard to that drug is correct.
-
- For more information on the ADNF, consult the Guidance Document - Fees for the Right to Sell Drugs.
- Dossier
- A collection of all regulatory activities throughout the life cycle of a product for a stakeholder.
- Dossier ID
- A code created by Health Canada to uniquely identify the dossier. The dossier ID is also referred to as the Top Level Folder Name. It consists of a lowercase letter followed by six (6) or seven (7) unique numbers depending on the regulatory activity type.
- Discontinue
- When the manufacturer permanently stops the sale of the drug.
- Discontinuation Date
-
If a manufacturer is marketing a drug and decides to permanently discontinue its sale, the date of the discontinuation is the date of the last sale by the manufacturer.
If a manufacturer has temporarily stopped marketing a drug and then subsequently decides to permanently discontinue its sale at a later date, the discontinuation date is the date on which the decision to permanently discontinue the sale was made.
- Drug
-
As defined in Section 2 of the Food and Drugs Act. Includes any substance or mixture of substances manufactured, sold or represented for use in:
- the diagnosis, treatment, mitigation or prevention of a disease, disorder, abnormal physical state or its symptoms, in human beings or animals;
- restoring, correcting or modifying organic functions in human beings or animals; or
- disinfection in premises in which food is manufactured, prepared or kept.
- Drug Identification Number (DIN)
-
A computer-generated 8-digit number assigned by Health Canada to a drug upon market authorization under subsection C.01.014.2 (1) of the Food and Drug Regulations.
It uniquely identifies each drug under the Food and Drug Regulations, sold in a dosage form in Canada, and is located on the package label of prescription and non-prescription drugs that have been evaluated and authorized for sale in Canada.
A DIN uniquely identifies the following characteristics:
- Product Name
- Manufacturer Name
- Active Ingredient(s)
- Strength of Active Ingredient(s)
- Dosage Form
- Route(s) of Administration
- Species (for veterinary drugs only)
Please see section 6.6.4.1 for the distinction between a DIN and the designation "DIN" on the label of a biocide.
- Drug Notification Form (DNF)
-
Is a form issued by Health Canada in accordance with section C.01.014.2 (1) of the Food and Drug Regulations that contains the DIN assigned for a drug, as well as information that is specific to the drug as it has been authorized by Health Canada.
In accordance with section C.01.014.3 of the Food and Drug Regulations, the manufacturer must, within 30 days after the day on which the drug is first sold, date and sign the completed DNF and return it to Health Canada with a statement that the information it contains is correct and with an indication of the date of that first sale.
- Expiration Date of Drug in Dosage Form
-
Means the earlier of:
- the last date a drug would maintain its labelled potency, purity and physical characteristics, or
- the date after which the manufacturer recommends that the drug not be used
The expiration date should be expressed at a minimum as a year and a month.
- Label
-
Includes:
- Labels affixed to the container or packaging of the drug
- Any separate package inserts
- Prescribing Information
- Fact sheets
- Consumer information/patient medication information (i.e., patient leaflets)
- Patient diaries
- Product Monograph, or
- Other material containing information specific to the drug
Package labels generated by the manufacturer may be included in the packaging or supplied to the consumer at the time of dispensing.
- Lot Number
- Any combination of letters and/or figures which can be used to trace a drug being manufactured and/or in distribution.
- Manufacturer
- The person, including an association or partnership, who under their own name or under a trade, design or word mark, trade name or other name, word or mark controlled by them, markets a drug. This is the person or company to which the DIN is assigned. For the purpose of this guidance document, manufacturer may include an agent authorized to act on their behalf.
- Market Notification
- A notification sent by the manufacturer to Health Canada to report the date of first sale for a particular drug, pursuant to section C.01.014.3 of the Food and Drug Regulations.
- New Drug
- As defined in Part C, Division 8, of the Food and Drug Regulations, it means a drug, other than a veterinary health product:
- that contains or consists of a substance, whether as an active or inactive ingredient, carrier, coating, excipient, menstruum or other component, that has not been sold as a drug in Canada for sufficient time and in sufficient quantity to establish in Canada the safety and effectiveness of that substance for use as a drug;
- that is a combination of two or more drugs, with or without other ingredients, and that has not been sold in that combination or in the proportion in which those drugs are combined in that drug, for sufficient time and in sufficient quantity to establish in Canada the safety and effectiveness of that combination and proportion for use as a drug; or
- with respect to which the manufacturer prescribes, recommends, proposes or claims a use as a drug, or a condition of use as a drug, including dosage, route of administration, or duration of action and that has not been sold for that use or condition of use in Canada, for sufficient time and in sufficient quantity to establish in Canada the safety and effectiveness of that use or condition of use of that drug.
- Notice of Compliance
- A notice issued under section C.08.004 or C.08.004.01 of the Food and Drug Regulations to a manufacturer following the satisfactory review of a drug submission for a New Drug.
- Private Label
- Label of an authorized non-prescription drug sold under the name of a retail store that is neither the manufacturer to whom the DIN was assigned nor the fabricator of the drug.
6. Guidance for implementation
6.1 DIN assignment
Once a drug has been authorized for sale in Canada, Health Canada assigns a DIN under Part C, Division 1 of the Food and Drug Regulations, which permits the manufacturer to market the drug in Canada. For drugs that meet the definition of a new drug under Part C, Division 8 of the Food and Drug Regulations, the drug is required to have a Notice of Compliance (NOC) in addition to a DIN in order to be authorized for sale in Canada.
Prior to June 13, 2018, Schedule C drugs (radiopharmaceuticals) received only a NOC and no DIN. Since the amendments to the Food and Drug Regulations that came into force on June 13, 2018, manufacturers of previously authorized Schedule C drugs have been required to submit an application for a DIN.
- For more information on the assignment of DINs for Schedule C Drugs, consult the Guidance Document: Drug Identification Numbers for Schedule C Drugs (Radiopharmaceuticals and Kits).
The DIN is assigned in the form of a DNF. The DNF contains, in addition to the DIN, information that is specific to the drug as it has been authorized by Health Canada. DNFs are sent by email directly to the manufacturer by Health Canada.
6.1.1 The timing of DIN assignment
6.1.1.1 New DINs
For manufacturers seeking authorization for a drug for human use under Part C, Division 8 of the Food and Drug Regulations, the DIN is assigned prior to issuance of the NOC, when all relevant review streams are completed. However, a drug product cannot be marketed until after NOC is issued and any licensing requirements are met.
For manufacturers seeking authorization for a drug for veterinary use under Part C, Division 8 of the Food and Drug Regulations, the DIN is assigned to the manufacturer when the NOC is granted.
For manufacturers seeking authorization under Part C, Division 1 of the Food and Drug Regulations for drugs for human or veterinary use, no NOC is granted. The DIN, in the form of a DNF, represents the market authorization.
6.1.1.2 Revised Drug Notification Form
Any change to one or more of the drug characteristics as listed in the definition of a DIN (refer to section 5) must be authorized before the DNF can be revised. A submission or application seeking authorization for the proposed changes must be filed.
As a result, for subsequent changes to a drug authorized for human or veterinary use under Part C, Division 8 of the Food and Drug Regulations, the revised DNF with the same sequence of numbers as the original DIN is issued to the manufacturer after the NOC is granted.
For subsequent changes to a drug for human or veterinary use authorized under Part C, Division 1 of the Food and Drug Regulations, a revised DNF with the same sequence of numbers as the original DIN may be issued to the manufacturer. The DNF represents the market authorization, since no NOC is granted for these drugs.
Table 1 below shows the specific changes to characteristics of a drug that would require either the assignment of a new DIN or the issuance of a revised DNF.
| Change in Characteristic | New DIN | Revised DNF with the same sequence of numbers as original DIN |
|---|---|---|
| Product Name | N/A | ✓ |
| Manufacturer Name | N/A | ✓ |
| Active Ingredient(s) | ✓ | N/A |
| Strength of Active Ingredient(s) | ✓ | N/A |
| Dosage Form | ✓ | N/A |
| Route(s) of Administration | ✓ | ✓Footnote * |
| Species (for veterinary drugs only) | N/A | ✓ |
|
||
6.1.2 Assignment of DIN according to product name
A product name for a drug is proposed by the manufacturer so that they may market and advertise that drug. Health Canada reviews each product name as part of a drug submission or an application before a drug is authorized for sale in Canada.
- For information on the assessment of brand names for drug for human use, refer to:
If a manufacturer wishes to market an authorized drug (i.e., a drug that has already been assigned a DIN) under two or more product names, a separate DIN will be assigned for each additional unique product name.
- For more information on the filing of a submission for an additional product name for drugs for human use, refer to the Frequently Asked Questions - Guidance Document for Industry - Review of Drug Brand Names.
- For information on the filing of a submission for an additional product name for veterinary drugs, contact the Veterinary Drugs Directorate at vdd.skmd.so-dgps.dmv.cp@hc-sc.gc.ca.
A new DIN will not be assigned if a manufacturer wishes to market an authorized non-prescription drug with retail specific branding, also known as private label (refer to section 5 for the definition of private label), provided that the product name is the same.
An example of two products that would have the same DIN is provided in Table 2 below.
| Product Information | Product 1 | Product 2 |
|---|---|---|
| DIN | ZZZZZZZZ | ZZZZZZZZ |
| Product Name | Omeprazole | Omeprazole |
| Manufacturer Name | Drug Company | Drug Company |
| Retailer | Drug Store1 | Drug Store2 |
Changes to an authorized label to include or modify retail specific branding elements (e.g., graphics, colour, font, etc.) require a review and authorization by Health Canada before they can be introduced on the market. As a result, an Office of Submissions and Intellectual Property (OSIP) Notification will no longer be accepted for private labels.
- For information on how to submit additional labels for an authorized non-prescription drug for human use, refer to section 5.9 of the Guidance Document: Questions and Answers: Plain Language Labelling Regulations for Non-prescription Drugs.
Health Canada will assign a single DIN for products of varying flavour, colour, and/or fragrance, provided that all other product characteristics (including formulation, route of administration, dosage form, product name, manufacturer's name and labelling - except for the identification of the flavour, colour, and/or fragrance) are identical. As discussed in section 6.1.3 of this document, separate DINs would be assigned for sugar and sugar-free formulations, as well as preservative and preservative-free formulations, and in other cases where differences in formulations may result in safety issues.
The introduction of a new flavour, colour, or fragrance to an already existing product may require review. The Guidance Document - Post-Drug Identification Number (DIN) Changes should be consulted to determine what needs to be filed with Health Canada before implementing any change.
6.1.3 Assignment of multiple DINs to address safety issues
To support Health Canada's ongoing effort to prevent potentially serious medication errors, Health Canada may assign separate DINs for the same concentration but different dose volumes under specific conditions, such as where the entire package represents one dose. This may apply to, but is not limited to, pre-filled pens, auto-injectors of differing dose volumes and pharmacy bulks.
Authorized New Drug Submissions (NDS) and DIN Applications (DINA) involving unit dose pre-filled syringes of the same concentration but offered in syringes of different total volumes will result in the assignment of separate DINs for each of the available total volumes. For example, a product with a strength of 10 mg/ml that comes in a unit dose of 1 ml in a pre-filled syringe and also a unit dose of 2 ml would be assigned two DINs. Health Canada will deal with already marketed drugs on a case-by-case basis.
Future refinements to this policy may be necessary as a result of experience gained.
6.2 Drug statuses and the Drug Product Database
Once the DIN is assigned or the DIN is assigned and the NOC is issued to the manufacturer, Health Canada publishes information associated with the drug, including the DIN and the status of the drug on the Drug Product Database (DPD). The status of a drug in the DPD is indicative of the availability of the drug on the Canadian market. The manufacturer is required by the Food and Drug Regulations to inform Health Canada of a change in the status of a drug.
Pursuant to sections C.01.014.9 to C.01.014.10 of the Regulations Amending the Food and Drug Regulations (Shortages of Drugs and Discontinuation of Sale of Drugs), some drug authorization holders are required to publicly report drug shortages and discontinuations to the following website: DrugShortagesCanada.ca. For additional information on how to report drug shortages and discontinuation to the website, please consult the Guide to reporting drug shortages and discontinuations.
Figure 1 displays the different statuses that may be assigned to a drug in the DPD. It shows the general order from left to right of how the drug status may change over time.
Figure 1: Possible statuses in general chronological order that may be associated with a drug and that may appear in the DPD
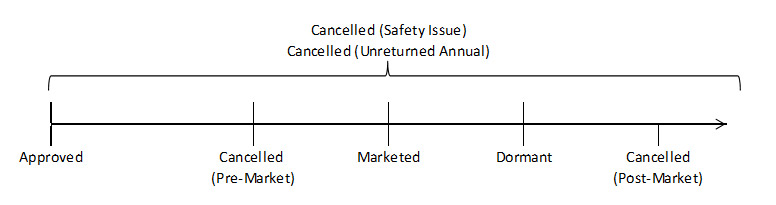
Figure 1 - Text equivalent
Description of Figure 1: Possible statuses in chronological order that may be associated with a drug and that may appear in the Online DPD
Figure 1 displays 5 statuses in chronological order. From left to right: Approved, Cancelled (Pre-Market), Marketed, Dormant, and Discontinued (Post-Market). The statuses Cancelled (Safety Issue) and Cancelled (Unreturned Annual) appear over the top of the chronology to indicate that either of these two events may occur at any time after a DIN has been issued.
The description of each status is summarized below:
- Approved: refers to an active DIN that has been reviewed and authorized for sale in Canada but has not yet been marketed in Canada.
- Cancelled Pre-Market: refers to a DIN that is cancelled before it was ever marketed in Canada. The DIN is no longer considered active.
- Marketed: refers to an active DIN that is currently being sold in Canada.
- Restricted Access: refers to an active DIN where the sale of the corresponding drug product is restricted to federal, provincial and territorial, and municipal government(s), pursuant to C.08.002.02 of the Regulations.
- Dormant: refers to an active DIN that was previously marketed in Canada but for which there have been no sales for a period of at least 12 consecutive months. The drug is still authorized for sale in Canada and could be marketed again.
- Cancelled Post-Market: refers to a DIN that is cancelled further to the discontinuation of the sale by the manufacturer pursuant to Section C.01.014.6 (1) (a) of the Regulations. The DIN is no longer considered active.
- Cancelled (Safety Issue): refers to a DIN that is cancelled under the following paragraphs of the Food and Drug Regulations. In all cases, the DIN is no longer considered active.
- paragraph C.01.014.6 (2) (b) of the Food and Drug Regulations due to failure to provide evidence regarding the safety and effectiveness of a drug, under section C.01.013 of the Food and Drug Regulations
- paragraph C.01.014.6 (2) (c) of the Food and Drug Regulations following the suspension of a Notice of Compliance under section C.08.006
- paragraph C.01.014.6 (3) (a) of the Food and Drug Regulations following the failure to comply with the order issued under section 21.31 of the Food and Drugs Act to conduct an assessment and provide the results
- paragraph C.01.014.6 (3) (b) of the Food and Drug Regulations following the examination of the results of an assessment provided in response to an order issued under section 21.31 of the Food and Drugs Act
- Cancelled (Unreturned Annual): refers to a DIN that is cancelled due to failure to provide the Annual Drug Notification Form pursuant to paragraph C.01.014.6 (2) (a) of the Food and Drug Regulations. The DIN is no longer considered active.
6.3 Market notification
As per section C.01.014.3 of the Food and Drug Regulations, the manufacturer has the obligation to notify Health Canada when they first sell a drug that has been assigned a DIN. A manufacturer must return a completed DNF to Health Canada within 30 days of first selling the drug. The DNF must be filled out, signed, and dated. All pages of the DNF must be returned to Health Canada.
If a manufacturer has been issued a revised DNF (refer to section 6.1.1.2 for more information on revised DNF), they must notify Health Canada when they begin to market the drug with the authorized change. A manufacturer must return a completed DNF to Health Canada within 30 days of selling the drug with new changes. The DNF must be filled out, signed, and dated. All pages of the DNF must be returned to Health Canada. Note that a market notification should not be submitted for the introduction of a new packaging size or new label design unless a new DNF has been issued for this change (see Table 1 in section 6.1.1.2.).
6.3.1 Completing the market notification
A market notification consists of:
- a cover letter
- a completed and signed DNF
- labelling material, when applicable (see Table 3)
The DPD will only be updated to show the status as Marketed or Restricted Access when a market notification is received and deemed accurate and complete.
The sections below outline for the manufacturer:
- how to accurately complete the DNF
- under which circumstances labelling material should be submitted
6.3.1.1 Part I of the Drug Notification Form
Part I of the DNF shows information currently contained in the DPD. It is the responsibility of the manufacturer to verify the information on the DNF when it is received. If any inconsistency is found in the contact information (i.e., mailing address, contact, telephone number, fax number, email address) for the DIN holder, agent or listed importer(s), the manufacturer should contact the Client Information Unit by email at client.information@hc-sc.gc.ca for instructions on how to file updates to company contact information.
Changes to the company name of the DIN holder, brand name, dosage form, route of administration, active ingredient(s), strength(s), and species (for veterinary drugs only) cannot be made on the DNF. Instead, the manufacturer must file a new application or submission.
6.3.1.2 Part II of the Drug Notification Form
Part II of the DNF contains information provided by the manufacturer as part of the market notification.
The following information must be provided in Part II:
- Date the drug was first sold in Canada following:
- the initial authorization; or
- the authorization of a change and for which a revised DNF was issued; or
- its reintroduction on the market (also known as date sales resumed) after a period of at least 12 consecutive months of no sales and for which a 12 months without sale notification was submitted to Health Canada
- Authorized official (title, signature):
- Any person designated by the manufacturer to act on behalf of the manufacturer
- Date the DNF was completed and signed
6.3.1.3 Providing labels with market notification
Under certain circumstances, labelling material is required as part of the market notification.
In 2014, the publication of the Regulations Amending to the Food and Drug Regulations (Labelling, Packaging and Brand Names of Drugs for Human Use) (Plain Language Labelling Regulations) repealed the requirements in the Food and Drug Regulations to submit copies of marketed labels after a drug is available for sale.
As a result, to determine if a copy of the marketed labels should be submitted with a market notification for a drug, as per the Plain Language Labelling Food and Drug Regulations, refer to Table 3.
| Drug Type | Date that the regulatory activity (e.g., NDS, DINA) containing labels was filed and authorized by Health Canada. | ||
|---|---|---|---|
| Filed before June 13, 2015 |
Filed between June 13, 2015 and June 13, 2017 |
Filed after June 13, 2017 |
|
| Prescription | Yes | No | No |
| Administered or obtained through a health care professional (includes ethical products) | Yes | No | No |
| Non Prescription | Yes | Yes | No |
| Disinfectant | Yes | Yes | Yes |
| Veterinary (Prescription and Non-Prescription) | Yes | No | No |
| Note that when filing a market notification following the reassignment of a DIN, marketed labels are not required. | |||
6.4 Twelve months without sale notification — dormant status
After a drug has been marketed, it is possible that there may be periods where the manufacturer has not sold the drug. If any of these periods reaches 12 consecutive months, the manufacturer is obligated under section C.01.014.71 and subparagraph C.01.014.5 (1) (a) (ii) of Food and Drug Regulations to report the period of no sales to Health Canada. The timing of reporting a period of 12 months without a sale is outlined below in sections 6.4.1 and 6.4.2.
After a complete notification is received and processed, Health Canada updates the status of the drug in the DPD to Dormant. No confirmation email is sent following the processing of the notification. The manufacturer can confirm the status change by consulting the DPD. A drug that is deemed Dormant is still authorized for sale in Canada.
A manufacturer is required to report when 12 months have elapsed without a sale of its drug to Health Canada when the following conditions are met:
- the drug has been assigned a DIN or has been assigned a DIN and an NOC has been issued to the manufacturer;
- the drug has been marketed; and
- the drug has not been sold on the Canadian market for a period of 12 consecutive months
In the case of a drug with no sales due to low market demand (e.g., small patient populations) the manufacturer is still required to report the DIN as Dormant as per section C.01.014.71 and subparagraph C.01.014.5 (1) (a) (ii) of the Food and Drug Regulations. If the manufacturer would like to request that the status of its dormant product remains listed as marketed in the DPD, they may provide a rationale indicating that the following conditions are met:
- the manufacturer maintains an inventory of the drug; and
- the drug is still available for purchase on the Canadian market (available for immediate use via retail pharmacies and hospitals [i.e., the drug is physically on pharmacy shelves]).
In such circumstances, Health Canada may choose to keep the status of the DIN as marketed in the DPD in order to avoid unintended impact on treatment plans. If the manufacturer no longer meets one or both of above noted conditions, it should be reported to Health Canada. If there are some sales, there is no obligation to notify under section C.01.014.71 or subparagraph C.01.014.5 (1) (a) (ii) of the Food and Drug Regulations.
6.4.1 Reporting 12 months without sale within 30 days
Manufacturers of all drugs are encouraged to report 12 months without sale within 30 days as this will allow Health Canada as well as patients, health care practitioners, and other health care stakeholders to have a clear and up to date picture of which drugs are available on the Canadian market.
However, manufacturers of the following drug types for human use are required under section C.01.014.71 of the Food and Drug Regulations to submit a notification to Health Canada within 30 calendar days after a period of 12 consecutive months that a drug has not been sold on the Canadian market by the manufacturer:
- drugs included in Schedules I, II, III, IV or V to the Controlled Drugs and Substances Act
- Prescription drugs
- drugs listed in Schedules C and D to the Food and Drugs Act
- drugs that may be sold without a prescription but are administered only under a practitioner's supervision (e.g., hemodialysis solutions, pre-filled syringes with epinephrine for severe allergic reactions, magnetic resonance imaging (MRI) contrast agents, insulins and vaccines).
The 12 months without sale notification from the manufacturer must be sent in writing on company letterhead. It should clearly identify either the date the product was last sold or the last day of the 12-month period without sale. It should be sent electronically as per section 6.10 below.
The DPD will be only updated to show the status as Dormant when a 12 months without sale notification is received and deemed accurate and complete.
6.4.2 Reporting 12 months without sale for all drugs on the Annual Drug Notification Form
For all marketed drugs which have been assigned a DIN under subsection C.01.014.2 (1) of the Food and Drug Regulations, the manufacturer must indicate, on the Annual Drug Notification Form (ADNF), whether the drug is dormant at the time of filing.
Instructions on how to complete and return the ADNF are included with the ADNF package sent to the manufacturer in June of each year by Health Canada.
Once the ADNF is received by Health Canada, the status of the drug will be updated in the DPD to Dormant. The signature date of the ADNF will be used to determine the date of the Dormant status, if no other date has been provided.
6.4.3 Recommencing sale after being dormant
If the manufacturer restarts the sale of a drug that was previously reported as Dormant, the manufacturer must return, within 30 days after re-starting sale of the drug, a signed and dated DNF. The date that the sale was restarted should be listed on the DNF. The manufacturer should not use the date that the drug was initially marketed. Marketed labels for a drug that is marketed after a period of dormancy are not required with the DNF.
6.5 Discontinuation of sale notification
The manufacturer must submit the discontinuation of sale notification within 30 days after the sale of the drug was discontinued as per section C.01.014.7 of the Food and Drug Regulations. The date of discontinuation is when the manufacturer last sells its drug, not when it is last sold at retail. Health Canada cancels the DIN further to the receipt of the notification as per paragraph C.01.014.6 (1) (a) of the Food and Drug Regulations. The manufacturer remains subject to several obligations for its drug distributed prior to the cancellation of the DIN until the expiration of the last lot distributed or the longest time period referred to in the Food and Drug Regulations. Refer to section 6.7 for more information on required activities following the cancellation of DIN.
The discontinuation of sale notification from the manufacturer must be sent in writing on company letterhead and should contain the following information: the DIN of the product, the date of discontinuation, and, if the product was previously marketed, the lot number and expiry date of the last lot distributed in Canada. The DPD will only be updated to show the status as Cancelled when a discontinuation of sale notification is received and deemed accurate and complete. The discontinuation date provided in the letter will be added to the DPD. If the discontinuation date is not included in the letter, the date of the letter will be used.
- If the drug has never been marketed (i.e., Approved status in the DPD), the status of the drug will be updated on the DPD to Cancelled (Pre-Market).
- If the drug was marketed or previously marketed (e.g., Marketed or Dormant status in the DPD), the status of the drug on the DPD will be updated to Cancelled (Post-Market). The expiry date of the last lot distributed in Canada, the lot number, and the date of discontinuation of sale of the product must be provided by the manufacturer and will be posted on the DPD. Where applicable, manufacturers should provide an explanation as to why the expiry date and/or lot number is not included in the notification.
No confirmation email is sent following the processing of the notification. The manufacturer can confirm the status change by consulting the DPD.
Drugs can only be cross-referenced to other already authorized drugs if their DINs are not cancelled. In addition, for drugs that have entered into a licensing agreement under the administrative pathway, following the cancellation of a DIN by a licensor, associated licensees should refer to section 2.5.4.1 of the Guidance Document Administrative Processing of Submissions and Applications: Human or Disinfectant Drugs to understand the impact on their respective DINs.
6.5.1 Discontinuing after being dormant
After notifying Health Canada of the 12-month period without a sale, a manufacturer may decide that they will not resume marketing of the drug on the Canadian market. When the manufacturer has not actively sold its drug (i.e., they have stopped selling its drug but have not yet made the decision to stop sales permanently) and then decides to discontinue its sale, the discontinuation date is the date on which they make the decision to discontinue its sale. In the case where a DIN has been listed as Dormant, the date of discontinuation should fall on a day after the date the DIN was reported to be dormant.
6.6 DIN cancellation
There are many reasons that Health Canada can initiate the cancellation of a DIN. These reasons are outlined in section C.01.014.6 of the Food and Drug Regulations.
6.6.1 Cancellation due to safety issue
6.6.1.1 Cancellation due to concerns regarding safety and efficacy - C.01.014.6 (2) (b) of the Food and Drug Regulations
The cancellation of the DIN may be initiated by Health Canada when a manufacturer fails to provide Health Canada with sufficient evidence regarding the safety and efficacy of the drug for its recommended use.
Following the determination that a DIN cancellation is warranted, Health Canada will subsequently update the status of the drug on the DPD to Cancelled (Safety Issue).
6.6.1.2 Cancellation following the suspension of a Notice of Compliance - C.01.014.6 (2) (c) of the Food and Drug Regulations
As outlined by paragraph C.08.002 (1) (c) of the Food and Drug Regulations, no person can market a drug with a suspended NOC. This prohibition on sale applies to the manufacturers and to all other parties such as wholesalers, retailers, pharmacists and medical practitioners and is effective on the date the NOC is suspended.
Following the suspension of the NOC, Health Canada may cancel the DIN in accordance with paragraph C.01.014.6 (2) (c) of the Food and Drug Regulations.
Further to the decision to cancel the DIN, Health Canada will subsequently update the status of the drug on the DPD to Cancelled (Safety Issue). The NOC Database will be updated to indicate that the NOC has been suspended.
6.6.1.3 Cancellation following the failure to comply with the order to conduct an assessment and provide the results - C.01.014.6 (3) (a) of the Food and Drug Regulations
To enable Health Canada to regulate a drug more efficiently and effectively, Health Canada has the authority to order the manufacturer to conduct assessments, compile information, conduct tests or studies or monitoring of experience in respect of the drug and provide Health Canada with the results under section 21.31 of the Food and Drugs Act.
- For more information on Health Canada's authority to require assessments under section 21.31 of the Food and Drugs Act and authority to require test, studies, etc., refer to the following: Guide to authorities under the Protecting Canadians from Unsafe Drugs Act (Vanessa's Law) (power to require and disclose information, power to order a label change and power to order a recall).
If the manufacturer fails to comply with the order under section 21.31 of the Food and Drugs Act to provide the requested information, Health Canada may cancel the DIN in accordance with paragraph C.01.014.6 (3) (a) of the Food and Drug Regulations.
Further to the decision to cancel the DIN, Health Canada will subsequently update the status of the drug on the DPD to Cancelled (Safety Issue).
6.6.1.4 Cancellation following the examination of the results of an assessment — C.01.014.6 (3) (b) of the Food and Drug Regulations
If following the assessment of the information provided in response to an order under section 21.31 of the Food and Drugs Act (as described in section 6.6.1.3), it is determined that the risks to injury or health outweigh the benefits, Health Canada may cancel the DIN in accordance with paragraph C.01.014.6 (3) (b) of the Food and Drug Regulations.
Further to the decision to cancel the DIN, Health Canada will subsequently update the status of the drug on the DPD to Cancelled (Safety Issue).
6.6.2 Cancellation due to failure to provide annual drug notification - C.01.014.6 (2) (a) of the Food and Drug Regulations
Every year, manufacturers must provide a signed copy of their Annual Drug Notification Form (ADNF) to Health Canada. The ADNF serves as an attestation that all the information previously provided by the manufacturer with respect to the drug is correct and provides any related updates to Health Canada.
Each year before the 1st day of October, Health Canada will contact all manufacturers who have failed to return a signed copy of their ADNF to remind them of their regulatory obligations.
If, by the 1st day of October, the ADNF has not been received by Health Canada, as per section C.01.014.5 of the Food and Drug Regulations, Health Canada may initiate the cancellation of the DIN(s). A written final notice will be provided to the manufacturer to inform them that their DIN(s) will be cancelled in accordance with paragraph C.01.014.6 (2) (a) of the Food and Drug Regulations and that they can no longer market the drug as per C.01.014 (1) of the Food and Drug Regulations.
Health Canada will subsequently update the status of the drug on the DPD to Cancelled (Unreturned Annual).
6.6.3 Cancellation as product is not a drug - C.01.014.6 (1) (c) of the Food and Drug Regulations
The cancellation of the DIN is initiated by Health Canada when it is determined that the product is not a drug under the Food and Drug Regulations.
In this situation, Health Canada will explain to the manufacturer in writing that the product is being reclassified and will no longer be regulated as a drug under the Food and Drug Regulations.
Health Canada will provide the manufacturer with the date on which the drug status will be changed and the DIN cancelled. If applicable, information will be provided on the relevant regulations the product will be subject to in order for the product to be marketed in Canada.
Following the reclassification of the product, Health Canada will cancel the DIN and remove the product from the DPD.
6.6.4 Cancellation due to a transition under the Biocides Regulations
Disinfectants authorized for sale under the Food and Drug Regulations will be allowed a transition period of four (4) years from the coming-into-force date of May 31st, 2025, to obtain a market authorization under the Biocides Regulations. At the time of the issuance of the biocide market authorization, Health Canada will update the status of the transitioned product(s) on the DPD to Cancelled (Transitioned to Biocides).
On June 1st, 2029, the day following the end of the four (4)-year transition period, any remaining DINs assigned to disinfectants will no longer be valid. Health Canada will update the status of these DINs to Cancelled.
- For more information on biocides, consult the Guidance on Biocides.
- For more information on the transition period, consult the Guidance on the transition of disinfectants and surface sanitizers to the Biocides Regulations.
6.6.4.1 Biocide Identification Number and the designation "DIN" on the label of a biocide
Section 31 (1) (d) of the Biocides Regulations allows the designation "DIN" to be used preceding the 8-digit biocide identification number on the label of a biocide. These numbers are not the same as Drug Identification Numbers (DINs) as biocides are not regulated under the Food and Drug Regulations.
Any questions in regards to these products, including identification numbers for biocides, should be directed to the Natural and Non-prescription Health Products Directorate (NNHPD) at nnhpd-dpsnso@hc-sc.gc.ca.
6.7 Required activities following the cancellation of a DIN
When a DIN is cancelled under section C.01.014.6 of the Food and Drug Regulations, no further sales may be made by the manufacturer since C.01.014 (1) of the Food and Drug Regulations prohibits a manufacturer from marketing a drug without a DIN.
To not create undue burden on industry, Health Canada may allow other parties in the downstream chain of distribution such as wholesalers, retailers, pharmacists and medical practitioners to continue to market or distribute the remaining drug after the DIN is cancelled, if:
- the expiry date of the lot has not passed; and
- the cancellation of the DIN was not due to health or safety reasons
If the DIN is cancelled, the drug can no longer be commercially imported into Canada.
If Health Canada becomes aware of any risk or non-compliance with respect to a drug with a cancelled DIN, Health Canada will take appropriate actions to mitigate the risk in accordance with the Compliance and Enforcement Policy (POL-0001).
The following scenarios are provided to illustrate some of the required activities that should be undertaken by a manufacturer following a DIN cancellation. Manufacturers should consult the regulations for the full details on their obligations.
Scenario 1: Safety updates to product monograph
Even if the DIN has been cancelled, the manufacturer should continue to file the appropriate submission(s) in order to incorporate additions or other changes to the Product Monograph related to safety (particularly with respect to contraindications, warnings and precautions, or adverse reactions) that may be necessary as a result of newly available information until all the lots of the drug that exist on the market have expired.
Scenario 2: Adverse reaction reporting
The requirements for manufacturers to maintain records of adverse drug reactions as per section C.01.020 of the Food and Drug Regulations and for wholesalers and distributors to keep records as per section C.02.022 of the Food and Drug Regulations are still applicable after the DIN has been cancelled. Although the manufacturer is not obliged to report any new cases of adverse reactions received following the drug discontinuation, Health Canada strongly encourages the reporting of all serious adverse reactions and may request the provision of this information. Additional information on these reporting requirements for drugs for human use can be found in the Reporting Adverse Reactions to Marketed Health Products - Guidance Document for Industry.
Scenario 3: Sale, record keeping and reporting
A manufacturer discontinues the sale of a drug on February 28th and informs Health Canada on the same day. Health Canada cancels the DIN. However, the drug is currently on the market and the expiry date of the last lot is December 30th.
Wholesalers, retailers, pharmacists and medical practitioners may continue to market or distribute the drug until December 30th if there were lots of the drug which were already in their possession before the DIN was cancelled. However, an importer cannot continue to import a drug with a cancelled DIN for commercial use.
The wholesalers, distributors and importers are still responsible for all the record keeping requirements of the Food and Drug Regulations, including C.02.022 of the Food and Drug Regulations, for all the lots of the drug that existed on the market, including the ones after the DIN was cancelled (i.e., between February 28th and December 30th). Under section C.02.022 of the Food and Drug Regulations, records shall be retained for 1 year after the expiration date of the last lot unless the establishment license specifies some other period.
Scenario 4: Labelling update
In the case where a manufacturer cancels a DIN associated with a particular strength of a drug (e.g., Drug X, 10 mg) while marketing the DINs associated with other strengths of the same drug (e.g., Drug X, 20 mg, 40 mg, 80 mg), the manufacturer is required to file a submission to remove the information related to the cancelled DIN from the labelling (i.e., product monograph, package insert, prescribing information, etc.) after the expiry of all the lots of the drug available on the market.
6.8 Reassignment of a DIN by Health Canada
Under specific circumstances, Health Canada may reassign the same sequence of numbers as the original DIN for the same drug after it has been cancelled.
The manufacturer must contact the DIN Issuance Unit within OSIP in order to determine the requirements for an application or submission to market the drug again. Requests should be sent to the DIN Issuance Unit within OSIP using the template in Appendix A.
The information received from the manufacturer will be forwarded to the appropriate review bureau to determine what type of submission, if any, (e.g., S(A)NDS, DIN(A)(B)(D)(F)) should be filed in order to reassign the same sequence of numbers as the original DIN associated to the drug. The applicable submission fee and review timelines, if any, will apply.
At the discretion of Health Canada, the filing of a submission may be required for the reissuance of a DIN.
The drug for which the manufacturer is seeking a DIN reassignment is subject to all current regulatory requirements, which may include filing of safety and labelling updates, as well as a look-alike sound-alike (LASA) brand name assessment. If a potential LASA issue arises, the drug for which the manufacturer is seeking a DIN reassignment may require a change to its brand name. Refer to Guidance Document for Industry - Review of Drug Brand Names for more information on brand name assessments.
If the information provided by the manufacturer is acceptable, a DNF with the same sequence of numbers as the original DIN will be sent to the manufacturer. The status of the DIN in the DPD will be updated to Approved. The manufacturer will be required to notify Health Canada once the product is marketed as per section C.01.014.3 of the Food and Drug Regulations. Refer to section 6.3 above for more information on market notification.
6.9 Commercial exportation
When a manufacturer stops the sale of a drug for consumption in Canada but continues to market and export the drug, the exportation with or without invoking section 37 of the Food and Drugs Act will determine whether the sale of the drug is considered to be discontinued in Canada.
- For more information on section 37 of the Food and Drugs Act, consult the document Intention to Invoke Section 37 of the Canada Food and Drugs Act for Products Being Exported.
6.9.1 Exportation without invoking Section 37
To export a drug without invoking section 37 of the Food and Drugs Act, manufacturers require, among other things, to hold a DIN and/or NOC since these types of commercial exportations are usually considered sales in Canada.
In this case, the exported drug is not considered to be discontinued and manufacturers are not required to send a notification of discontinuation of sale to Health Canada. The product will continue to appear in the DPD with the status Marketed and the manufacturer remains subject to post-market obligations.
6.9.2 Exportation under Section 37
If the sale of a drug which was destined for consumption in Canada is stopped, but the manufacturer continues to export under section 37 of the Food and Drugs Act, the drug is considered discontinued in Canada.
In this case, manufacturers will be required to send a discontinuation of sale notification to Health Canada. Once Health Canada receives the notification and deems it accurate and complete, Health Canada will update the status of the drug on the DPD to Cancelled (Post-Market).
6.10 Submitting notifications to Health Canada
For Health Canada to be able to process notifications received from manufacturers in a timely manner, all required documents must be provided, completed accurately, and submitted in the proper format.
6.10.1 Document requirements
| Activity | DNF | Label | Cover Letter |
|---|---|---|---|
| Market Notification | Required | Refer to Table 3 | Required |
| Recommencing Sale After Being Dormant | Required | Not required | Required |
| 12 Months without Sale Notification | Not required | Not required | Required |
| Discontinuation of Sale Notification | Not required | Not required | Required |
| Request for DIN reassignment | Not required | Not required | Not required - sent by email (see Appendix A) |
When submitting a notification or a DIN reassignment request to Health Canada, the manufacturer must provide all required documents and information (see Table 4 for required documents). If the provided documents are incomplete or the required information is missing or incorrect, the notification will be placed on hold. Once Health Canada has received all required information and documents, the notification will be processed.
6.10.2 Format and filing instructions
Market notifications, 12 months without sale notifications, discontinuation of sale notifications, and requests for DIN reassignment must all be sent electronically. Refer to Table 5 below for information on transmission methods.
Human drugs and disinfectants
Market notifications, 12 months without sale notifications and discontinuation of sale notifications must be provided to the Office of Submissions and Intellectual Property (OSIP) via the Common Electronic Submissions Gateway (CESG) using Regulatory Enrolment Process (REP):
1. For eCTD transaction as indicated in:
- Guidance Document: Preparation of Regulatory Activities in eCTD Format available on the filing submissions electronically information page; and
- Regulatory Enrolment Process (REP) Guidance Document available on the REP information page.
2. For non-eCTD transaction as indicated in:
- Guidance Document: Preparation of Regulatory Activities in the Non-eCTD Format available on the filing submissions electronically information page; and
- Regulatory Enrolment Process (REP) Guidance Document available on the REP information page.
Veterinary drugs
The market notification, the 12 months without sale notification and discontinuation of sale notification for veterinary products are accepted via email.
For dossiers previously filed using the REP, these notifications must be filed in non-eCTD format via the CESG using the REP as indicated in:
- Guidance Document: Preparation of Regulatory Activities in the Non-eCTD Format available on the filing submissions electronically information page; and
- Regulatory Enrolment Process (REP) Guidance Document available on the REP information page.
For all human, veterinary drugs and disinfectants: DIN reassignment requests must be filed via email. Refer to Appendix A for request template.
| Notification type | Regulatory Activity Type | Regulatory Transaction Description | Transmission method |
|---|---|---|---|
| Market notification | Match the regulatory activity type for the control number the DNF has been issued | Drug Notification Form | Drugs for human use and disinfectants: Mandatory via CESG. Drugs for veterinary use: Accepted via email or via CESG if the product has been assigned a dossier ID and is using the REP. |
| 12 months without sale notification | Always use the most recently approved (EU)(A)NDS (CV) or DIN(A)(B)(D)(F). If the DIN was assigned under S(A)NDS, use the regulatory activity type of the (EU)(A)NDS (CV) to which it is a supplement. |
Notification for Interruption of Sale (DIN Dormant) | Drugs for human use and disinfectants: Must be sent via CESG if the product has been assigned a dossier ID. Otherwise, email will be accepted. Drugs for veterinary use: Accepted via email or via CESG if the product has been assigned a dossier ID and is using the REP. Always file under the control number of the most recently approved (EU)(A)NDS (CV) or DIN(A)(B)(D)(F). If the DIN was assigned under S(A)NDS, use the control number of the (EU)(A)NDS (CV) to which it is a supplement. For older drug products with control numbers in the following format "#HN#########" or if there is no control number associated with the most recently approved (EU)(A)NDS (CV) or DIN(A)(B)(D)(F), file under control number "000000". |
| Discontinuation of sale notification | Always use the most recently approved (EU)(A)NDS (CV) or DIN(A)(B)(D)(F). If the DIN was assigned under S(A)NDS, use the regulatory activity type of the (EU)(A)NDS (CV) to which it is a supplement. |
Discontinuation of Sale Notification | Drugs for human use and disinfectants: Must be sent via CESG if the product has been assigned a dossier ID. Otherwise, email will be accepted. Drugs for veterinary use: Accepted via email, or via CESG if the product has been assigned a dossier ID and is using the REP. Always file under the control number of the most recently approved (EU)(A)NDS (CV) or DIN(A)(B)(D)(F). If the DIN was assigned under S(A)NDS, use the control number of the (EU)(A)NDS (CV) to which it is a supplement. For older drug products with control numbers in the following format "#HN#########" or if there is no control number associated with the most recently approved (EU)(A)NDS (CV) or DIN(A)(B)(D)(F), file under control number "000000". |
| DIN Reassignment request | N/A | N/A | Send by email (see Appendix A) |
6.11 Contact Us
Any questions in regards to the information or requirements in this guidance document should be directed to the OSIP's DIN Issuance Unit at din@hc-sc.gc.ca.
Appendix A Template email - Request for DIN reassignment
To: din@hc-sc.gc.ca
Subject: Request for DIN reassignment for [Product Name - DIN XXXXXXXX]
Hello,
I am requesting that Health Canada reassign DIN XXXXXXXX for [Product Name].
The following information is provided to assist Health Canada in determining whether the filing of a submission or application is needed to reassign the DIN(s).
- Were there any changes in formulation since the approval of the last submission and/or since the discontinuation of the drug? Y/N
- Were there any manufacturing changes since the approval of the last submission and/or since the discontinuation of the drug? Y/N
- Is the product available in other jurisdiction(s) (e.g., United States Food and Drug Administration)? Y/N
- Were there any labelling changes since the approval of the last submission and/or since the discontinuation of the drug? Y/N
- Have there been any unexpected adverse events reported domestically or internationally? Y/N
- Why was the DIN discontinued?
I certify that the information provided is true and accurate.
Yours sincerely,
[Authorized signature]