Scientific evidence requirements for nutritional adequacy of a preterm infant formula and human milk fortifiers
A guidance document for infant formula and human milk fortifier manufacturers
2021
Table of contents
- 1.0 Introduction
- 2.0 Nutritional adequacy for preterm infant formula or human milk fortifier
- 3.0 Key terms used in preterm infant formula or human milk fortifier assessment
- 4.0 Clinical studies to support the premarket evaluation of preterm infant formula or human milk fortifier
- 5.0 Other studies for preterm infant formula and human milk fortifier
- 6.0 Circumstances that warrant clinical testing and other testing
- 7.0 Post-marketing surveillance
- 8.0 Pre-submission consultations
- 9.0 Abbreviations
- 10.0 References
- Appendix 1. Nutrient requirement template for preterm infant formula or fortifier with human milk (as fed) per 100 kcal
- Appendix 2. Preterm human breast milk composition
1.0 Introduction
In Canada, infant formulas are regulated under the Food and Drugs Act (FDA) and Division 25 of the Food and Drug Regulations (FDR). Manufacturers must submit a premarket submission prior to selling or advertising any new infant formula or human milk fortifier or one that has undergone a major change. A premarket submission must contain detailed information on the composition and manufacturing of the infant formula or human milk fortifier and its packaging and labelling, as well as evidence to establish that the new or changed infant formula or human milk fortifier is safe and nutritionally adequate to promote acceptable growth and development in preterm infants for whom it is intended when consumed according to the directions of use.
This document provides additional guidance to infant formula and human milk fortifier manufacturers on the evidence required to support nutritional adequacy as per the FDR Division 25, sections B.25.046, B.25.048 and B.25.011 (j). Other Health Canada guidance documents such as Guide for the Preparation of Infant Formula and Human Milk Fortifier Premarket Submissions and Good Manufacturing Practices for Infant Formula should also be consulted in conjunction with this document.
Premature infants are a specific, diverse and unique subpopulation. The heterogeneity of their nutritional needs, underlying clinical status at birth, and their immature organs necessitate an individualized approach in terms of feeding, supplementation and clinical management. Preterm infant formula, and human milk fortifier intended for premature infants, are two separate food products having a common goal of providing nutrients to approximate the rate of growth and composition of weight gain for a normal fetus of the same postmenstrual age. It is important to note that the nutritional adequacy of the human milk fortifier is always assessed in combination with preterm human milk, since human milk fortifiers are not nutritionally complete on their own. In clinical practice, if mother's own milk supply is not available or sufficient, term donor milk may be used with a human milk fortifier. However, mother's own preterm milk is recommended (1).
In this document, 'fortified human milk' refers to the combination of human milk fortifier and preterm human milk, which enables the assessment of the nutritional adequacy of the human milk fortifier when compared to international recommendations (refer to Appendix 1).
This guidance document describes the type of evidence required by Health Canada before selling or marketing:
- a new preterm infant formula or human milk fortifier;
- a preterm infant formula or human milk fortifier that has undergone a major change; and
- a preterm infant formula or human milk fortifier to which a new ingredient or novel food has been added.
This document identifies the circumstances that require clinical or other types of testing; identifies the evidence (e.g., clinical, biochemical, animal) that should be submitted for the premarket evaluation of infant formula or human milk fortifier; and aligns the circumstances that require testing with the recommended types of tests.
2.0 Nutritional adequacy for preterm infant formula or human milk fortifier
The assessment of nutritional adequacy of preterm infant formula or human milk fortifier in neonatal intensive care units should include valid, easily obtained, and inexpensive anthropometric measurements and clinical biochemical parameters.
Nutritional adequacy of preterm infant formula or fortified human milk is established when it meets the nutritional goals for the preterm infant and provides nutrients to mimic the rate of growth and composition of weight gain for a normal fetus of the same postmenstrual age while maintaining normal concentrations of nutrients in blood and other tissues. Nutritional adequacy is also established when it reduces the degree of extrauterine or postnatal growth failure that typically occurs by the time of hospital discharge. Recently, experts in the field concluded that a rate of weight gain of 15 to 20 g/kg per day was a reasonable goal for infants 23 to 36 weeks' gestational age, but not beyond (2). They also concluded that 1 cm/week increase in head circumference fits the Fenton Preterm Growth Chart (2013) (3) and Olsen (2010) (4) growth curves well from 24 to 33 weeks' gestational age, and 1 cm/week in length gain from 24 to 32 weeks (2).
A nutritionally adequate preterm infant formula must comply with the compositional requirements set out in the FDR. It is also substantiated by two quality factors which must be met:
- provides a diet which supports physical growth of preterm infants; and
- provides protein of sufficient biological value.
The assessment of the human milk fortifier is based on the composition of the human milk fortifier plus preterm human milk (not fortifier alone). Health Canada has developed a table of nutrient requirements (refer to Appendix 1 and Appendix 2) for preterm infants reflecting the most recent international expert recommendations (5,6,7,8,9). The nutritional profile of the fortified human milk should be within the nutrient ranges recommended by these international experts.
3.0 Key terms used in preterm infant formula or human milk fortifier assessment
3.1 Key terms used in the Food and Drugs Act (FDA) and Food and Drug Regulations (FDR), and internationally
- Clinical trial
An experiment to compare the effects of two or more healthcare interventions. Clinical trial is an umbrella term for a variety of designs of healthcare trials, including uncontrolled trials, controlled trials, and randomised controlled trials (10). A clinical trial is a research study that assigns individuals or groups of participants to one or more health-related interventions and follows them over time to evaluate the effects on health outcomes. It is designed to answer a specific question. Participants receiving the intervention are called the experimental group. The others serve as controls. The effects and clinical significance of the intervention on a health-related outcome are assessed by comparing the two groups. It is the role of institutional review boards to make sure that such studies are ethical and safe. In the case of preterm or low birth weight (LBW) infants, their legally accepted representatives must give informed consent for them to take part.
Manufacturers are encouraged to consult Health Canada's Growth and Tolerance Clinical Trial Protocol – Preterm Infants for more details on the clinical trial protocol for preterm infant formula or human milk fortifier.
- Evidence
Means relevant information gathered from high quality scientific research to address a well-defined question. Evidence should be factual, bias-free, balanced, valid (measuring what it is supposed to be measuring), reproducible and accurate. A clinical growth and tolerance study of preterm infant formula or fortified human milk should include a complete study report with raw data plotted on acceptable growth charts (3). It should include a balanced assessment of the benefits and harms, with emphasis on the study's limitations and generalizability. Evidence from individual studies make up a body of evidence. The evidence must be sufficiently convincing and strong to support a decision to allow for a new preterm infant formula or human milk fortifier or a major change for these products.
Evidence for the safety and nutritional adequacy of preterm infant formula or human milk fortifier may come from data on infants, animal models and other data, and should include, but is not limited to:
- clinical trials assessing growth and tolerance of preterm infant formula or fortified human milk (infant studies);
- biochemical tests such as serum albumin, blood urea nitrogen (BUN), calcium, phosphorus, serum alkaline phosphatase, serum electrolytes, blood gases and pH, etc. (the need for such tests depends on the nature of the formulation and would be determined on a case-by-case basis);
- animal studies on protein quality, such as the protein efficiency ratio (PER), which is used to assess if adequate amounts of bioavailable protein are present;
- history of safe use in other countries (complementary evidence);
- expert opinions, e.g., the United States Food and Drug Administration's Generally Recognized as Safe (GRAS) affirmations;
- quality indicators (for example, the amino acid profile as evidence in support of the protein composition, or the fatty acid profile as evidence to support the fat quality); and
- a review of the scientific literature on a new infant formula ingredient or novel food to be added to preterm infant formula or human milk fortifier.
The evidence must be in line with these requirements:
- good manufacturing practices in accordance with the FDA and FDR, as well as the Safe Food for Canadians Act and Safe Food for Canadians Regulations to ensure that all required nutrients and other ingredients are present in the preterm infant formula or human milk fortifier in correct amounts and that the preterm infant formula or human milk fortifier is not contaminated with microorganisms or other substances;
- quality control procedures to ensure that the preterm infant formula or human milk fortifier contains the types and amounts of nutrients that will support infants' physical growth for the entire shelf life of the product;
- minimum and maximum limits set for certain nutrients in the FDR and the international experts nutrient recommendations for preterm infants; and
- published guidelines of authoritative bodies such as the American Academy of Pediatrics/Committee on Nutrition (2020), Tsang RC, et.al. 2005 (7), the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (9), Klein CJ, 2002 (5), Koletzko B, 2014 (6), and WHO (8, 11, 12).
Human Milk Fortifier (FDR B.25.001)
Means a food that:
- includes at least one added vitamin, mineral nutrient or amino acid, and
- is labelled or advertised as intended to be added to human milk to increase its nutritional value in order to meet the particular requirements of an infant in whom a physical or physiological condition exists as a result of a disease, disorder or abnormal physical state
Human milk substitute (FDR B.25.001)
Means any food that is labelled or advertised for use as:
- a partial or total replacement for human milk intended for consumption by infants, or
- an ingredient in a food referred to in paragraph (a)
Infant Food (FDR B.25.001)
Means a food that is labelled or advertised for consumption by infants.
Major Change for a human milk substitute, (FDR B.25.001)
Means, in respect of a human milk fortifier or a human milk substitute, any change of an ingredient, the amount of an ingredient or the processing or packaging of the human milk fortifier or human milk substitute where the manufacturer's experience or generally accepted theory would predict an adverse effect on the levels or availability of nutrients in, or the microbiological or chemical safety of, the human milk fortifier or human milk substitute.
Examples of major changes for a preterm infant formula or human milk fortifier:
- substantial change in processing or packaging of the preterm infant formula or human milk fortifier change of or a new manufacturing facility
- addition of a new macronutrient source (protein, fat, or carbohydrate)
- substantial change in the amount of protein, fat, or carbohydrate
- addition of a new ingredient or novel food
- change in the amount or the source of vitamins or minerals, which, in the manufacturer's experience or generally accepted theory, would predict an adverse effect on:
- the levels or availability of the nutrients, or
- the microbiological or chemical safety
New human milk substitute (Infant Formula) (FDR B.25.001)
Means a human milk substitute that is:
- manufactured for the first time, or
- sold in Canada for the first time, or
- manufactured by a person who manufactures it for the first time.
New infant food ingredient (NIFI)
Is a food substance (e.g., nutritional, microbiological) that:
- is not a novel food and
- is new to infant formula or human milk fortifier in Canada
Novel food (FDR B 28.001)
Means:
- a substance, including a microorganism, that does not have a history of safe use as a food;
- a food that has been manufactured, prepared, preserved or packaged by a process that
- has not been previously applied to that food, and
- causes the food to undergo a major change; and
- a food that is derived from a plant, animal or microorganism that has been genetically modified such that
- the plant, animal or microorganism exhibits characteristics that were not previously observed in that plant, animal or microorganism,
- the plant, animal or microorganism no longer exhibits characteristics that were previously observed in that plant, animal or microorganism, or
- one or more characteristics of the plant, animal or microorganism no longer fall within the anticipated range for that plant, animal or microorganism.
Premarket notification (FDR B 25.046 and B 25.048)
The manufacturer of a new preterm infant formula or human milk fortifier, one that has undergone a major change in composition, manufacturing, packaging, or processing, must notify Health Canada of the intention to sell or advertise for sale the new or changed product (refer to Health Canada's Guide for the Preparation of Infant Formula and Human Milk Fortifier Premarket Submissions).
Preterm Infant
Is defined as a baby born alive before 37 weeks of pregnancy are completed. There are sub-categories of preterm birth; based on gestational age (13) (refer to Table 1).
Classification scheme |
Group |
Definition |
|---|---|---|
Gestational age(13) |
Extreme prematurity/micro-preterm |
< 28 weeks |
Severe prematurity |
28 - < 32 weeks |
|
Moderate to late prematurity |
32 - < 37 weeks |
|
Birth weight(14) |
Low birth weight |
< 2500 g |
Very low birth weight |
< 1500 g |
|
Extremely low birth weight |
< 1000 g |
|
Weight for age(14) |
Small for gestational age (SGA) |
Birth weight < 10th percentile on preterm growth charts |
Appropriate for gestational age (AGA) |
Birth weight ≥ 10th and ≤ 90th percentile on preterm growth charts |
|
Large for gestational age (LGA) |
Birth weight > 90th percentile on preterm growth charts |
Preterm infant formula
Means any food that is represented for use as a partial or total replacement for preterm human milk and intended for consumption by preterm, LBW infants, very low birth weight infants (VLBW) and/or extremely low birth weight infants.
Preterm infant formulas are enriched in calories (approximately 80 kcal/100 ml vs. 67 kcal/100 ml for term infant formula), protein and minerals to help the infant approximate intra-uterine nutrient accretion rates. The calories are provided as protein, fat and carbohydrate. The latter two macronutrients are used to increase energy density. The balance between energy and protein, the limiting macronutrient is critical in determining the type of growth. Compared to unfortified human milk or standard infant formula, preterm infant formula typically contain more protein, sodium, calcium, phosphorus, zinc, copper and vitamins.
Protein quantity and quality
The amount and quality of protein in the preterm infant formula and fortified human milk must be appropriate to their intended use. Protein quality must be demonstrated using existing PER methods (Association of Official Agricultural Chemists or Health Canada's Method FO-1). The PER is currently the best method for demonstrating protein quality for infant formula for young infants (below 6 months of age) and for human milk fortifiers. Methods based on Protein Digestibility Corrected Amino Acid Score (PDCAAS) and Digestible Indispensable Amino Acid Score (DIAAS) are currently not sufficiently developed for use in young infants, especially for plant-based infant formula which usually contain anti-nutritional factors. In addition, the essential and conditionally essential amino acid profile should be in line with the recommended amino acids requirements for preterm and LBW infants as per LSRO 2002 (5).
3.2 Additional terms used in clinical research and practice for preterm infants
- Chronological Age
The age of the infant in weeks from the date of birth without correcting for prematurity (11) (refer to Figure 1).
- Corrected Age [week or months] (i.e., corrected for prematurity)
Means chronological age reduced by the number of weeks born before 40 weeks of gestation; the term should be used only for children up to 3 years of age who were born preterm (15) (refer to Figure 1).
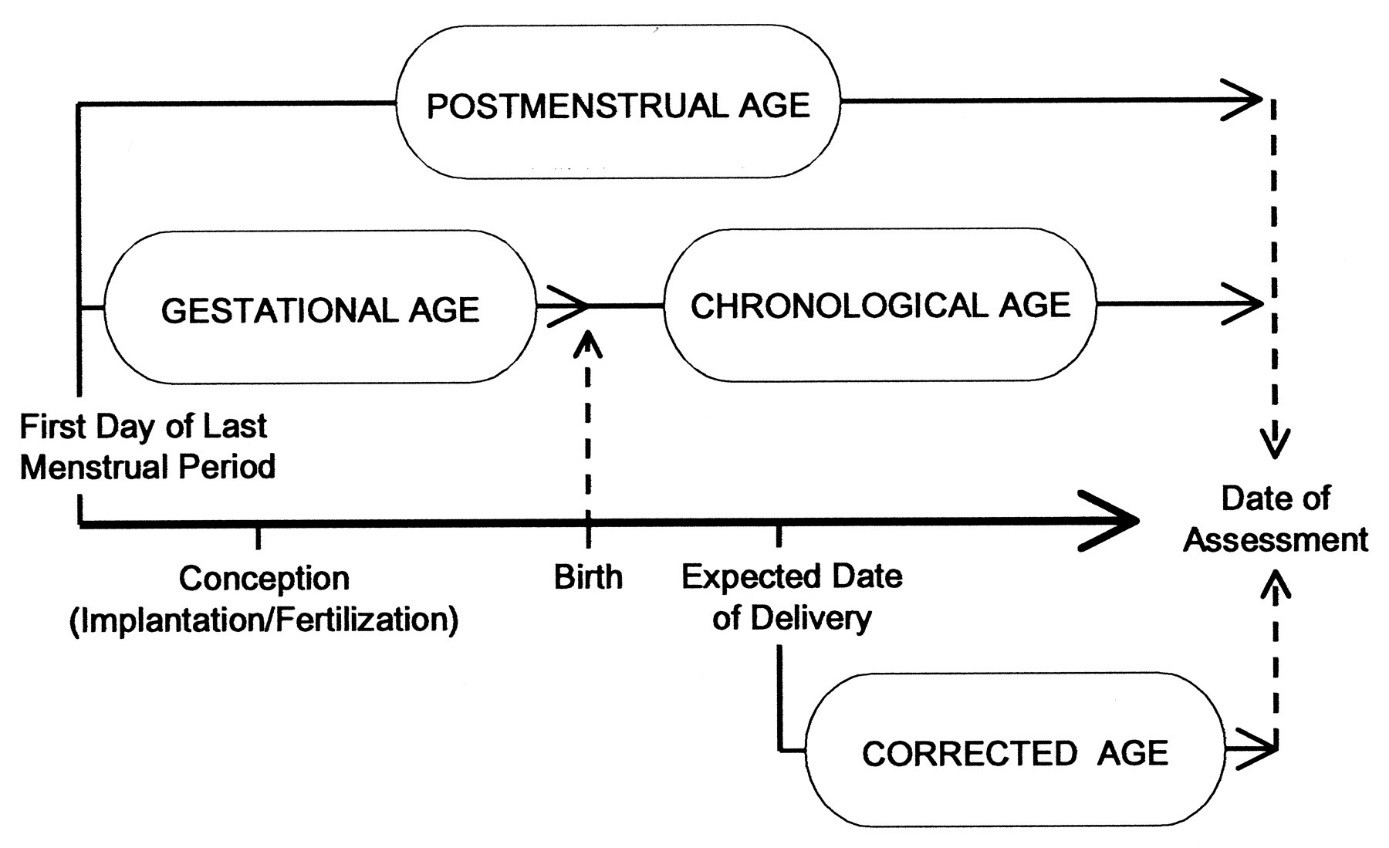
Figure 1 - Text description
A timeline showing the relationship between Postmenstrual age, Gestational age, chronological age and Corrected age, as defined in Section 2.0
- Gestational age
In obstetrics, the age of a fetus, beginning on the presumed first day of the last normal menstrual period (13), (refer to Figure 1).
- Intrauterine growth restriction (IUGR)
Failure to sustain intrauterine growth at expected rates; can be caused by placental insufficiency, infection, malnutrition, etc. (infant may or may not be born prematurely). It describes a fetus that has not reached its growth potential because of genetic or environmental factors (16).
- Late preterm
Infants born between 34-37 weeks gestational age (13).
- Low birth weight (LBW)
Is defined as a weight of less than 2500 g (up to and including 2499 g) irrespective of the gestational age (11).
- Osmolality
Is defined as the concentration of osmoles of solute per kg of solvent, expressed as mOsm/kg. The maximum osmolality recommended by the American Academy of Pediatrics (1976) is 450 mOsm/kg water for infant formula or fortified human milk to minimize the risk of necrotizing enterocolitis (NEC).
- Osmolarity
Is defined as the concentration of osmoles of solute/liter of solvent expressed as mOsm/L; the maximum recommended by the American Academy of Pediatrics is 400 mOsm/L (AAP, 1976).
- Postmenstrual age (PMA)
Indicates the time elapsed between the first day of the last menstrual period and birth (gestational age) plus the time elapsed after birth (chronological age) in weeks and days. This is the preferred term used to describe the age of the preterm infant during the perinatal period and during hospital stay. After the perinatal period, "corrected age" is the preferred term (refer to Figure 1) (15).
- Postnatal growth failure and severe postnatal growth failure
A hospital discharge weight less than the 10th and third percentiles for postmenstrual age, respectively, on the respective growth charts (17).
- Postnatal growth failure
Is a decrease in weight between birth and discharge of more than −2 z-scores using the Fenton Preterm Growth Chart (18).
- Small for gestational age
Means that a fetus or an infant is smaller or less developed than normal for the baby's sex and gestational age, or an infant with birth weight less than the 10th percentile of used preterm growth charts (14).
- The potential renal solute load (PRSL) of infant feedings
Is the sum of dietary nitrogen (expressed as mmol of urea, for example, mg nitrogen divided by 28), sodium, potassium, chloride and phosphorous. The PRSL determines the renal solute load, and, therefore, the osmolar concentration of the urine.
PRSL (mosmol) = N/28 + Na + Cl + K + P
The minimum PRSL for preterm infant formula should be set at 22 mosm/100 kcal to a maximum of 32 mosm/100 kcal for an infant formula containing 81 kcal/100 mL (5).
3.3 Other clinical research and practice terms for use in clinical trials in preterm infants
- Adverse event
An adverse outcome that occurs during or after the use of a drug or other intervention but is not necessarily caused by it (19).
- Catch-up growth
Any improvement in centiles or z-scores. Early catch-up is defined as fast growth in infancy among small newborns and late catch-up is defined as improvement in growth from 1 year of age until adulthood (11).
- Control fortified human milk
Means a human milk fortifier authorized in Canada or elsewhere plus human milk.
- Enteral feeding
Administration of any feed into the gastrointestinal tract; it includes intragastric feeding and cup, bottle and breastfeeding (11).
- Fortified human milk
Means human milk fortifier + preterm human milk.
- Investigational fortified human milk
Means the new or changed human milk fortifier plus human milk.
- Oral feeding
Administration of any feed into the oral cavity; it includes cup, spoon, syringe, direct expression, bottle and breastfeeding but not gastric tube feeding (11).
- Recommended rate of weight gain
A goal of up to 20 g/kg per day may be suitable for infants who are between 23 and 36 weeks postmenstrual age (2).
- Serious adverse event (in the context of a clinical trial)
When in the view of either the investigator or sponsor it results in any of the following outcomes: death, a life threatening adverse event, inpatient hospitalization or prolongation of existing hospitalization, a persistence or significant incapacity or substantial disruption of the ability to conduct normal life functions (20).
- Side effect
An adverse event for which the causal relation between the intervention and the event is at least a reasonable possibility (21).
- Stable growing period
The period beginning when the preterm infant is metabolically and clinically stable and growing, ending when the infant reaches 37 weeks of postmenstrual age (11) or hospital discharge.
- Transition period
The period from birth to 7 days when infants are likely to be clinically and metabolically unstable and to lose weight (11).
4.0 Clinical studies to support the premarket evaluation of preterm infant formula or human milk fortifier
In general, clinical studies are used to determine:
- growth and tolerance: safety and ability of the infant formula or fortified human milk to support physical growth;
- bioavailability of selected nutrients and bone minerals (on a case-by-case basis);
- metabolic status and blood biochemistry.
The following types of clinical studies are specifically required to determine the safety and nutritional adequacy of preterm infant formula or human milk fortifier.
4.1 Clinical growth and tolerance study
A clinical growth and tolerance study measures and assesses the following parameters:
- Weight gain rate over a minimum period of at least 28 days or until hospital discharge, expressed as g/kg per day in clinically and metabolically stable growing preterm, LBW or VLBW infants fed the experimental infant formula or fortified human milk as compared to those fed a concurrent control of a preterm infant formula or human milk fortifier (added to human milk for the study) sold in Canada that has been tested and proven safe and nutritionally adequate;
- Rates of gain in length, expressed as (cm/week) and in head circumference, expressed as (cm/week);
- Intake data: average daily intake volume of infant formula or fortified human milk, calories, protein and modular supplements;
- Tolerance data: infant's willingness to consume the infant formula or fortified human milk and tolerate it with minimal gastro-intestinal, respiratory and dermatological symptoms;
- Incidence of adverse events and serious adverse events such as mortality, metabolic acidosis, sepsis, NEC, recorded and assessed daily by medical professionals.
Further details on the above parameters are outlined in Health Canada's Growth and Tolerance Clinical Trial Protocol – Preterm Infants.
4.2 Nutrient bioavailability studies
4.2.1 Serum indices of protein adequacy of the preterm infant formula or fortified human milk
Biochemical markers should complement the anthropometric assessment. Prealbumin, BUN and retinol binding protein (RBP), for example, are used to assess protein status. Low BUN correlates with insufficient protein intake, but high levels may indicate either appropriate amino acid intake, low energy intake relative to protein intake or amino acid intolerance. Pre-albumin and RBP are good markers of current protein status due to their short half-lives but may be affected by factors other than protein nutrition (22).
4.2.2 Serum indices of iron nutritional status for research studies
Iron supplementation in preterm infants is recommended to supply sufficient iron for growth and development without increasing the risk of iron overload. Iron deficiency in early life adversely affects the growth and functioning of multiple organ systems. The effects of iron deficiency on the developing brain are permanent and life-altering. Preterm infants are deprived of the significant iron accretion that occurs in the third trimester of pregnancy and have reduced iron stores at birth compared with term infants (23).
In preterm infants, many non-nutritional factors contribute to iron deficiency. Serum ferritin is commonly used to customize iron supplementation in growing preterm infants; however, inflammatory processes can elevate serum ferritin. The assessment of C-reactive protein simultaneously with serum ferritin is generally used to determine if the ferritin level is a reliable indicator. Hemoglobin, serum iron and total iron-binding capacity are other iron status parameters frequently used in clinical practice.
4.2.3 Metabolic status and blood biochemistry
Due to immature organ systems in preterm infants, they are at increased risk of acid-base imbalance. Metabolic status can be assessed by measuring arterial blood gases, blood pH, urinary pH, protein metabolic markers, serum electrolytes and blood biochemistry at study baseline, mid-point and at completion.
4.2.4 Assessment of bone mineralization
The assessment of bone mineralization by serum levels of calcium, phosphate, and alkaline phosphatase is commonly used. There is insufficient evidence that, individually, these are valid biochemical markers of metabolic bone disease (MBD); however, the combination of high alkaline phosphatase and low phosphate levels is the best biochemical indicator of MBD (22).
4.3 Record of adverse reactions
Accurate and clear information on adverse events and side effects occurring during the study or during a pre-specified follow-up period must be carefully described and reported whether or not it is immediately associated with the study treatment (21).
5.0 Other studies for preterm infant formula and human milk fortifier
Body text In addition to clinical studies and laboratory analyses, animal studies may be required to test the safety of preterm infant formula or human milk fortifier ingredients. This could include measuring the PER which assesses protein quality and can be used to assess adverse interactions between nutrients.
Before new hypoallergenic infant formula are tested in human trials, comprehensive preclinical testing must be conducted in animal models of allergenicity. These trials should assess toxicity, nitrogen balance and predict whether infants allergic to cow's milk proteins will react adversely. Petitioners are encouraged to request a pre-submission consultation as per Section 10 of this document.
6.0 Circumstances that warrant clinical testing and other testing
The recommended testing needed for a new preterm infant formula or human milk fortifier and/or a previously approved preterm infant formula or human milk fortifier, which has undergone a major change is listed below. Note: submission requirements vary on a case by case basis.
6.1 New infant formula
A new infant formula may contain a variety of ingredients, and may be subject to different methods of processing, and/or matrix-interaction among different ingredients, all of which could affect nutrient content, absorption, bioavailability, or adequacy. Therefore, it is essential to demonstrate safety and nutritional non-inferiority/equivalency (or superiority) of a new infant formula compared with that of an infant formula whose nutritional adequacy and safety have been well established (please refer to Health Canada's Growth and Tolerance Clinical Trial Protocol – Preterm Infants.
Studies and data required are:
- clinical growth and tolerance study;
- nutrient bioavailability studies on a case-by-case basis;
- careful reporting of adverse events to assess clinical safety.
6.2 Major changes or modifications of a previously approved preterm infant formula or human milk fortifier
The extent of the assessment or clinical testing that is required for a specific modification to an existing preterm infant formula or human milk fortifier is determined on a case-by-case basis. For example, increasing the protein level, or adding a new ingredient that has never been used in preterm infant formula or human milk fortifier in Canada to an existing preterm infant formula or human milk fortifier, are considered cases where clinical testing would be required.
6.2.1 Energy content
Preterm infant formulas are especially designed for preterm or LBW infants. They are enriched in calories (approximately 80 kcal/100ml) to support nutrient accretion and growth rates that are similar to those intra-uterine.
Preterm infant formula or fortified human milk providing more than 80 kcal/100 ml require a growth and tolerance clinical study. Preterm infant formula or fortified human milk providing high energy density may lead to increased urinary solute loads under increased stress conditions, for example, fever or infectious diarrhea. Therefore, the potential renal solute load of the preterm infant formula or fortified human milk should be provided in the premarket notification. It is also known that human milk fortifier could lead to increased osmolality; therefore, osmolality should be provided in the premarket notifications for the final fortified human milk.
Studies and data required are:
- growth and tolerance study,
- metabolic status,
- potential renal solute load for preterm infant formula and fortified human milk (at different ratios of human milk fortifier with human milk),
- osmolality of the fortified human milk when mixed in different ratios with human milk fortifier,
- markers of hydration status, urine osmolality and urine specific gravity.
6.2.1.1 New energy source
Introduction of a new source of protein, fat or carbohydrate in a preterm infant formula or human milk fortifier will generally require a growth and tolerance study.
6.2.1.2 New protein source
A PER study is required for any new protein source. For example, a new animal milk protein proposed for use in a preterm infant formula or human milk fortifier.
Studies and data required are:
- growth and tolerance study,
- PER determination of any new protein in a preterm infant formula or human milk fortifier,
- amino acid profile of the finished preterm infant formula or fortified human milk,
- biochemical tests: serum albumin, BUN and RBP.
6.2.1.3 New fat source
A new fat source such as an animal fat, vegetable oil or a source of long chain polyunsaturated fatty acids would require the following studies and data:
- growth and tolerance study;
- serum level of calcium and phosphorus serum alkaline phosphatase may be requested;
- fatty acid profile of the finished preterm infant formula or fortified human milk.
6.2.1.4 New carbohydrate source
A new source of carbohydrate (e.g., monosaccharides, disaccharides, polysaccharides or dietary fibre) would require a growth and tolerance study.
6.2.2 Higher protein level
Studies and data required are:
- growth and tolerance study;
- biochemical tests and preterm infant metabolic status; such as serum albumin, BUN, RBP, calcium, phosphorus, serum alkaline phosphatase, serum electrolytes, blood gases and pH (the need for such tests depends on the nature of the formulation and would be determined on a case-by-case basis);
- for human milk fortifier, the use of banked human milk versus the use of the mother's own preterm milk should be taken in consideration when assessing the protein quantity of the final product.
Note: The area of clinical nutrition and optimal growth of preterm and LBW infants is still evolving and advancing. Therefore, if the aim of the study is to demonstrate the superiority of a new test infant formula or human milk fortifier with a higher protein level compared to a commercial control infant formula or human milk fortifier, a superiority design with a predefined superiority margin (ICHE 9) is recommended.
6.2.3 Processing of protein
Any change in the processing of protein (require the following:
- growth and tolerance study;
- amino acid profile of the finished preterm infant formula or fortified human milk;
- PER determination of the finished preterm infant formula, human milk fortifier or fortified human milk.
Additional information required for specific protein sources (e.g., partially, extensively hydrolyzed, amino acid based formulations):
- details of the hydrolysis process;
- enzyme used;
- contents of the enzyme preparation.
6.2.4 Change in protein, fat or carbohydrate mixture
Manufacturers are encouraged to contact Health Canada to discuss recommended testing on the change in protein, fat or carbohydrate mixture (refer to Section 9.0 on how to arrange a consultation).
6.2.5 Change in the amount or source of vitamins or minerals
For Codex approved sources of calcium, phosphorus and other minerals and vitamins, refer to the Codex Alimentarius' List of acceptable vitamin and mineral sources for foods for special dietary use.
For other changes in the amount or source of vitamins or minerals (e.g., increased levels of Vitamin A, Vitamin D, or sodium) studies and data required include:
- growth and tolerance study
- serum levels of vitamins and minerals
6.2.6 New combination of macronutrient sources
New combinations of macronutrient sources that have been used or studied separately in various currently marketed preterm infant formula or human milk fortifier (made by the same manufacturer or a different one) but not together in the same preterm infant formula or human milk fortifier under review require:
- a growth and tolerance study
- blood biochemistry
6.3 Novel food ingredient or new infant food ingredient (NIFI)
A preterm infant formula or human milk fortifier containing a novel food which has been approved, or a NIFI which has been cleared for use must undergo clinical and possibly other testing. It requires the same testing as a new preterm infant formula or human milk fortifier (including the growth and tolerance study), unless such testing has already been conducted in the preterm infants and the data has been submitted for the novel food or new infant formula ingredient assessment.
For further information on the approval process for a novel food or NIFI, the manufacturer should refer to Heath Canada's Guide for the Preparation of Infant Formula and Human Milk Fortifier Premarket Submissions.
7.0 Post-marketing surveillance
A post-marketing (also known as in-marketing) surveillance plan may be considered to support long-term safety (24).
Post-marketing surveillance consists of:
- a monitoring component which includes procedures to monitor for adverse effects once the product has been put on the market;
- a follow-up component which focuses on potential long-term or delayed effects following the period of maximum usage of the product. The length of the follow-up should be determined on a case-by-case basis;
- a biannual or annual written report from the petitioner's medical and scientific committee summarizing the results of its literature review, particularly, those pertaining to the safety and long-term follow-up of any added new ingredient (e.g., Lutein).
8.0 Pre-submission consultations
Manufactures are encouraged to contact Health Canada's Food Directorate Submission Management Information Unit (smiu-ugdi@hc-sc.gc.ca) to request a pre-submission consultation to better prepare for premarket notifications process and submission requirements to demonstrate nutritional adequacy.
9.0 Abbreviations
- BUN
- Blood urea nitrogen
- DIAAS
- Digestible indispensable amino acid score
- FDR
- Food and Drug Regulations
- GRAS
- Generally recognized as safe
- LBW
- Low birth weight
- MBD
- Metabolic bone disease
- NEC
- Necrotising enterocolitis
- NIFI
- New infant food ingredient
- PDCAAS
- Protein digestibility corrected amino acid score
- PER
- Protein efficiency ratio
- PRSL
- Potential renal solute load
- RBP
- Retinol binding protein
- VLBW
- Very low birth weigh
10.0 References
- Moro GE, Arslanoglu S, Bertino E, Corvaglia L, Montirosso R, Picaud JC, Polberger S, Schanler RJ, Steel C, van Goudoever J, Ziegler E. (2015). Human milk in feeding premature infants: consensus statement. Journal of Pediatric Gastroenterology and Nutrition, 61(1), S16-S19.
- Fenton TR, Anderson D, Groh-Wargo S, Hoyos A, Ehrenkranz RA, Senterre T. (2018). An attempt to standardize the calculation of growth velocity of preterm infants—evaluation of practical bedside methods. Journal of Pediatrics, 196,77-83.
- Fenton T and Kim J. (2013). A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatrics. 13(1), 1-13.
- Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. (2010). New intrauterine growth curves based United States data. Pediatrics, 125(2), e214-24.
- Klein CJ. (2002). Nutrient requirements for preterm infant formulas (LSRO). Journal of Nutrition, 132(6 Suppl 1), 1395S-577S.
- Koletzko B, Poindexter B, Uauy R (eds). (2014). Nutritional Care of Preterm Infants: Scientific Basis and Practical Guidelines. World Review of Nutrition and Dietetics, 110.
- Tsang RC, Uauy R, Koletzko B, Zlotkin S. (2005). Nutrition of the preterm infant, Scientific basic and practical guidelines. Digital Educational Publishing, Inc., Cincinnati, OH, USA.
- Tudehope D, Fewtrell M, Kashyap S, Udaeta E. (2013). Nutritional Needs of the Micropreterm Infant. Journal of Pediatrics. 162 (3 Suppl): S72–S80.
- Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, Domellöf M, Embleton ND, Fusch C, Genzel-Boroviczeny O, Goulet O, Kalhan SC, Kolacek S, Koletzko B, Lapillonne A, Mihatsch W, Moreno L, Neu J, Poindexter B, Puntis J, Putet G, Rigo J, Riskin A, Salle B, Sauer P, Shamir R, Szajewska H, Thureen P, Turck D, van Goudoever JB, Ziegler EE; ESPGHAN Committee on Nutrition. (2010). Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 50(1), 85-91.
- Cochrane Collaboration. (2019). Glossary of Cochrane terms. [Online]. Available: https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/SURE-Guides-v2.1/Collectedfiles/source/glossary.html
- World Health Organization (WHO). (2006). Optimal feeding of low-birth-weight infants. [Online]. Available: https://apps.who.int/iris/bitstream/handle/10665/43602/9789241595094_eng.pdf;jsessionid=80736F9 2F581729831081B1CA71D704B?sequence=1
- World Health Organization (WHO). (2011). Guidelines on optimal feeding of low-birth-weight infants in low- and middle-income countries. [Online]. Available: Guidelines on optimal feeding of low birth-weight infants in low- and middle-income countries (who.int).
- World Health Organization (WHO). (2018). Preterm Birth: Fact sheet. [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
- Groh-Wargo S, Thompson M, Cox H (eds). (2000). Nutritional Care for High-Risk Newborns. Precept Press, Inc., Chicago, IL.
- American Academy of Pediatrics, Committee on Fetus and Newborn. (2004). Age terminology during the perinatal period. Pediatrics, 114 (5), 1362-1364.
- Smitten J. (2011). Approach to the child with IUGR/SGA. [Online]. Available: https://learn.pediatrics.ubc.ca/body-systems/neonate/approach-to-the-child-with-iugrsga.
- Horbar J, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, Buzas JS, Bertino E, Gagliardi L, Bellù R. (2015). Weight Growth Velocity and Postnatal Growth Failure in Infants 501 to 1500 Grams: 2000-2013. Pediatrics, 136(1), e84-e92.
- Lee SM, Kim N, Namgung R, Park M, Park K, Jeon J. (2018). Prediction of Postnatal Growth Failure among Very Low Birth Weight Infants. Scientific reports, 8(3729).
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne PC, GØtzsche PC, Lang T for the CONSORT Group. (2001). The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Annals of Internal Medicine, 134(8), 663-694.
- International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. (2016). ICH harmonised tripartite guideline for good clinical practice E6 (R2). [Online]. Available: https://ich.org/page/efficacy-guidelines
- Ioannidis JPA, Evans SJW, Gøtzsche PC, O'Neill RT, Altman DG, Schulz K, Moher D for the CONSORT group. (2004). Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine, 141(10), 781-8.
- Pereira-da-Silva L, Virella D, Fusch C. (2019). Nutritional assessment in preterm infants: a practical approach in the NICU. Nutrients, 11(9), 1999.
- McCarthy EK, Dempsey EM, Kiely ME. (2019). Iron supplementation in preterm and low-birth-weight infants: a systematic review of intervention studies. Nutrition reviews, 77(12), 865-877.
- Institute of Medicine (US) Committee on the Evaluation of the Addition of Ingredients New to Infant Formula. (2004). Infant formula: evaluating the safety of new ingredients. Washington (DC). National Academies Press.
Appendix 1. Nutrient requirement template for preterm infant formula or fortifier with human milk (as fed) per 100 kcal
NOTE: Single value indicates a minimum recommendation. Value in a square bracket has been reported as the calculated value in respective citation.
- MACRONUTRIENTS
| Nutrient | FDR requirements | International expert recommendations | Nutritional profile | Specification | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Unit | ESPGHAN (2010)Reference 1 | Tsang, et. al. (2005)Reference 2 >1000 g BW | LSRO (2002)Reference 3 | WHOReference 4 >1000 g BW (stabilization to term)Footnote a | Koletzko, et.al. (2014)Reference 5 | Min | Max | ||
| Protein | g | 1.8 – 4 | 3.2 – 4.1Footnote b | 2.6 – 3.8 | 2.5 – 3.6 | [2.5 – 3.0] | 3.2 – 4.1 | |||
| Fat | g | 3.3 – 6 | 4.4 – 6.0 | 4.1 – 6.5 | 4.4 – 5.7 | [3.8 – 5.7] | 4.4 – 6.0 | |||
| - LA | g | 0.5 | 0.350 – 1.400 | 0.462 – 1.309 | [0.350 – 1.425]Footnote c | NS | 0.350 – 1.400 | |||
| - ALA | g | NS | 0.050 | NS (1 – 4 E% per kg/day) |
0.077 – 0.228 (1.75 – 4% of total fatty acids) |
NS | 0.050 | |||
| - LA:ALA | - | NS | 5 – 15 | 5 – 15 | 6 – 16 | NS | NS | |||
| - ARA | g | NS | 0.016 – 0.039 | 0.022Reference 3 | [0 – 0.034] Footnote d | NS | 0.032 – 0.041 | |||
| - DHA | g | NS | 0.011 – 0.027 | 0.016Reference 3 | [0 – 0.020]Footnote e | NS | 0.050 – 0.055 | |||
| Carbohydrate | g | NS | 10.5 – 12 | 5.4 – 15.5 | 9.6 – 12.5 | [6.3 – 12.9] | 10.5 – 12 | |||
- VITAMINS AND MINERALS
| Nutrient | FDR requirements | International expert recommendations | Nutritional profile | Specification | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Unit | ESPGHAN (2010)Reference 1 | Tsang, et. al. (2005)Reference 2 >1000 g BW | LSRO (2002)Reference 3 | WHOReference 4 >1000 g BW (stabilization to term)Footnote a | Koletzko, et.al. (2014)Reference 5 | Min | Max | ||
| Vitamin A | IU | 250 – 500 | 1200 – 2467 | 538 – 1364 | 680 – 1267 | 583 – 1250 | 1217 – 3333 | |||
| mcg RE | -- | [360 – 740] | [162 – 410] | [204 – 380] | [175 – 375] | [365 – 1000] | ||||
| Vitamin D | IU | 40 – 100 | 800 – 1000Footnote f per day | 115 – 364 | 75 – 270 | [400 – 800]Footnote f per day | 100 – 350 from milk only | |||
| Vitamin E | IU | 0.6 | [3.0 – 15] | 4.6 – 10.9 | [3.0 – 12] | [5.0 – 10.0] | [3.0 – 15] | |||
| mg α-TE | -- | 2 – 10 | [3.1 – 7.3] | 2 – 8 Footnote g | [3.4 – 6.7] | 2 – 10 | ||||
| Vitamin K | mcg | 8 | 4 – 25 | 6.2 – 9.1 | 4 – 25 | [6.7 – 8.3] | 4 – 25 | |||
| Vitamin C | mg | 8 | 10 – 42 | 13.8 – 21.8 | 8.3 – 37 | [5 – 8] | 18 – 50 | |||
| Thiamine | mcg | 40 | 125 – 275 | 138 – 218 | 30 – 250 | [33 – 42] | 127 – 273 | |||
| Riboflavin | mcg | 60 | 180 – 365 | 192 – 327 | 80 – 620 | [300 – 383] | 181 – 364 | |||
| Vitamin B6 | mcg | 35 | 41 – 273 | 115 – 191 | 30 – 250 | [15]Footnote h | 45 – 273 | |||
| Vitamin B12 | mcg | 0.15 | 0.08 – 0.7 | 0.23 – 0.27 | 0.08 – 0.7 | [0.15]Footnote f | 0.09 – 0.73 | |||
| Niacin | mcg | 250 | 345 – 5000 | 2800 – 4400 | 550 – 5000 | [720] | 900 – 5000 | |||
| Pantothenic acid | mcg | 300 | 300 – 1900 | 900 – 1500 | 300 – 1900 | [667 – 1083] | 450 – 1900 | |||
| Folic acid | mcg | 4 | 32 – 90 | 19 – 45 | 30 – 45 | [50]Footnote f | 32 – 91 | |||
| Biotin | mcg | 2 | 1.5 – 15 | 2.8 – 5.5 | 1.0 – 37 | [1.3] | 1.5 – 15 | |||
| Choline | mg | 12 | 7 – 50 | 11.1 – 25.5 | 7 – 23 | NS | 7.3 – 50 | |||
| Inositol | mg | NS | 4 – 48 | 25 – 74 | 4 – 44 | NS | 4 – 48 | |||
| Taurine | mg | NS | NS | 3.5 – 8.2 | 5 – 12 | NS | NS | |||
| Carnitine | mg | NS | NS | 2.2 – 2.6 | 2 – 5.9 | NS | NS | |||
| Calcium (Ca) | mg | 50 | 110 – 130 | 77 – 200 | 123 – 185 | [134 – 200] | 109 – 182 | |||
| Phosphorus (P) | mg | 25 | 55 – 80 | 46 – 127 | 82 – 109 | [65 – 98] | 55 – 127 | |||
| Ca:P | -- | 1.2 – 2 | NS | NS | 1.7 – 2.0:1 | NS | NS | |||
| Magnesium | mg | 6 | 7.5 – 13.6 | NS | 6.8 – 17 | [4.1 – 8.1] | 7.5 – 13.6 | |||
| Sodium | mg | 20 – 60 | 63 – 105 | 53 – 105 | 39 – 63 | [48 – 77] | 63 – 105 | |||
| Potassium | mg | 80 – 200 | 60 – 120 | 60 – 106 | 60 – 160 | [81 – 114] | 71 – 177 | |||
| Chloride | mg | 55 – 150 | 95 – 161 | 82 – 226 | 60 – 160 | [74 – 118] | 95 – 161 | |||
| Iron | mg | 0.15 | 1.8 – 2.7 | 1.538 – 3.636 | 1.7 – 3.0 | [1.7 – 2.5] | 1.8 – 2.7 | |||
| Zinc | mg | 0.5 | 1.0 – 1.8 | 0.769 – 2.727 | 1.1 – 1.5 | [0.42 – 0.67] | 1.3 – 2.3 | |||
| Copper | mcg | 60 | 90 – 120 | 92 – 136 | 100 – 250 | [58 – 101] | 90 – 210 | |||
| Manganese | mcg | 5 | 6.3 – 25 | 0.5 – 6.8 | 6.3 – 25 | [0.5 – 0.9] | 0.9 – 13.6 | |||
| Iodine | mcg | 5 | 10 – 50 | 7.7 – 54.5 | 6 – 35 | [26 – 53] | 9 – 50 | |||
| Selenium | mcg | NS | 4.5 – 9 | 1.0 – 4.1 | 1.8 – 5.0 | [2.6 – 3.9] | 4.5 – 9 | |||
| Chromium | mcg | NS | 0.027 – 1.12 | 0.08 – 2.05 | NS | 0.04 – 0.08 | 0.027 – 2.045 | |||
| Molybdenum | mcg | NS | 0.27 – 4.5 | 0.23 – 0.27 | NS | 0.16 – 0.32 | 0.27 – 4.5 | |||
| Fluoride | mcg | NS | 1.4 – 55 | NS | 0 – 25 | NS | 1.4 – 55 | |||
- NUCLEOTIDES
| Nutrient | FDR requirements | International expert recommendations | Nutritional profile | Specification | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Unit | ESPGHAN (2010)Reference 1 | Tsang, et. al. (2005)Reference 2 >1000 g BW | LSRO (2002)Reference 3 | WHOReference 4 >1000 g BW (stabilization to term)Footnote a | Koletzko, et.al. (2014)Reference 5 | Min | Max | ||
| Total Nucleotides | mg | NS | NS | NS | NS | NS | NS | |||
| AMP | mg | NS | NS | 0.27 – 0.73 | NS | NS | NS | |||
| CMP | mg | NS | NS | 1.6 – 3.7 | NS | NS | NS | |||
| GMP | mg | NS | NS | 0.03 – 0.54 | NS | NS | NS | |||
| UMP | mg | NS | NS | 0.69 – 0.9 | NS | NS | NS | |||
Abbreviations
- ALA
- Alpha-linolenic acid
- AMP
- Adenosine monophosphate
- ARA
- Arachidonic acid
- BW
- Body weight
- Ca:P
- Calcium to phosphorous ratio
- CMP
- Cytosine monophosphate
- DHA
- Docosahexaenoic acid
- g
- gram
- GMP
- Guanosine monophosphate
- LA
- Linoleic acid
- LSRO
- Life Sciences Research Office
- Max
- Maximum
- mcg
- microgram
- mg
- milligram
- Min
- Minimum
- NS
- None specified
- PUFA
- Polyunsaturated fatty acids
- RE
- Retinol equivalents
- TE
- Tocopherol equivalents
- UMP
- Uridine monophosphate
- WHO
- World Health Organization.
References
- Reference 1
-
Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology, and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition, 2010:50. 85-91.
- Reference 2
-
Tsang RC, Uauy R, Koletzko B, Zlotkin S. Nutrition of the preterm infant, Scientific basic and practical guidelines. Cincinnati, OH: Digital Educational Publishing Inc. 2005.
- Reference 3
-
Klein CJ. Nutrient requirements for preterm infant formulas. Journal of Nutrition, 2002;132:1395S-577S.
- Reference 4
-
Tudehope D. et al. Nutritional Needs of the Micropreterm Infant. Journal of Pediatrics, March 2013, Volume 162, Issue 3, Supplement, Pages S72–S80.
- Reference 5
-
Koletzko B, Poindexter B, Uauy R. Nutritional Care of Preterm Infants: Scientific Basis and Practical Guidelines. Karger, 2014:99-120.
Footnotes
- Footnote a
-
Calculated based on the average of the maximum and minimum energy recommendations for the indicated population; 120 kcal/kg/d for stabilization to term.
- Footnote b
-
3.6 – 4.1 g / 100 kcal for infants < 1 kg BW; 3.2 – 3.6 g/100 kcal for infants 1 - 1.8 kg BW
- Footnote c
-
Total fatty acids: 8% (minimum) and 25% (maximum)
- Footnote d
-
A minimum recommendation for ARA was not established. Maximum: 0.6% of total fatty acids with the further stipulation that the ARA:DHA is within the range of 1.5-2.0 : 1
- Footnote e
-
A minimum recommendation for DHA was not established. Maximum: 0.35% of total fatty acids with the further stipulation that the ARA:DHA is within the range of 1.5 – 2.0 : 1
- Footnote f
-
Recommendation given per day, independent of body weight and energy intake
- Footnote g
-
The ratio of vitamin E to PUFA (mg α-TE per g PUFA) should exceed 1.5 (mg α-TE/g PUFA)
- Footnote h
-
Recommendation per gram protein fed
Appendix 2. Preterm human breast milk composition
NOTE: This data is recommended for use to fill the above template, and for standardisation of approach.
| Nutrients | Unit | Preterm Human Milk (per 100 kcal) |
Preterm Human Milk (per 100 mL) |
|---|---|---|---|
| MACRONUTRIENTS | |||
| ProteinReference 1 Footnote a | g | 2.1 | 1.5 |
| FatReference 1 Footnote a | g | 4.9 | 3.5 |
| - LA Reference 2 | g | 0.72 | 0.48 |
| - ALA Reference 2 | g | 0.045 | 0.03 |
| - LA:ALA | -- | 16:1 | 16:1 |
| - ARAReference 2 | g | 0.029 | 0.017 |
| - DHAReference 2 | g | 0.017 | 0.011 |
| Carbohydrate Reference 2 | g | 10.9 | 7.3 |
| VITAMINS AND MINERALS | |||
| Vitamin A Reference 2 | IU | 72 | 48 |
| Vitamin D Reference 2 | IU | 12 | 8 |
| Vitamin E Reference 2 | IU | 0.59 | 0.39 |
| Vitamin K Reference 2 | mcg | 3 | 2 |
| Vitamin C Reference 2 | mg | 6.6 | 4.4 |
| Thiamine Reference 2 | mcg | 13.35 | 8.9 |
| Riboflavin Reference 2 | mcg | 40.5 | 27 |
| Vitamin B6 Reference 2 | mcg | 9.3 | 6.2 |
| Vitamin B12 Reference 2 | mcg | 0.03 | 0.02 |
| Niacin Reference 2 | mcg | 315 | 210 |
| Pantothenic acid Reference 2 | mcg | 345 | 230 |
| Folic acid Reference 2 | mcg | 4.65 | 3.1 |
| Biotin Reference 2 | mcg | 0.81 | 0.54 |
| Choline Reference 3 | mg | 23.7 | 15.8 |
| Inositol Reference 4 | mg | 29.1 | 19.4 |
| VITAMINS AND MINERALS (continued) | |||
| Taurine Reference 2 | mg | 6 | 4 |
| Carnitine Reference 2 | mg | 1.05 | 0.7 |
| CalciumReference 1 Footnote a | mg | 35 | 25 |
| PhosphorusReference 1 Footnote a | mg | 21 | 15 |
| Ca:P | mg-mg | 1.67: 1 | 1.67: 1 |
| Magnesium Reference 2 | mg | 4.95 | 3.3 |
| Sodium Reference 2 | mg | 42 | 28 |
| Potassium Reference 2 | mg | 75 | 50 |
| Chloride Reference 2 | mg | 87 | 58 |
| Iron Reference 2 | mg | 0.135 | 0.09 |
| Zinc Reference 2 | mg | 0.56 | 0.37 |
| Copper Reference 2 | mcg | 57 | 38 |
| Manganese Reference 2 | mcg | 0.54 | 0.36 |
| Iodine Reference 2 | mcg | 26.7 | 17.8 |
| Selenium Reference 2 | mcg | 3.6 | 2.4 |
| ChromiumReference 5 Footnote b | mcg | 0.038 Footnote c | 0.025 Footnote c |
| Molybdenum Reference 5 | mcg | 0.3 | 0.2 |
| Fluoride Footnote c | mcg | NS Footnote d | NS |
| NUCLEOTIDES | |||
| Total Nucleotides | mg | NS | NS |
| AMP | mg | NS | NS |
| CMP | mg | NS | NS |
| GMP | mg | NS | NS |
| UMP | mg | NS | NS |
Abbreviations
- ALA
- Alpha-linolenic acid
- AMP
- Adenosine monophosphate
- ARA
- Arachidonic acid
- BW
- Body weight
- Ca:P
- Calcium to phosphorous ratio
- CMP
- Cytosine monophosphate
- DHA
- Docosahexaenoic acid
- g
- gram
- GMP
- Guanosine monophosphate
- LA
- Linoleic acid
- mcg
- microgram
- mg
- milligram
- NS
- None specified
- UMP
- uridine monophosphate
- WHO
- World Health Organization.
References
- Reference 1
-
Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014 Aug 30;14:216.
- Reference 2
-
Koletzko B, Poindexter B, Uauy R. Nutrtional Care of Preterm Infants. Scientific basis and practical guidelines. Basel, Switzerland: Karger AG; 2014.
- Reference 3
-
Maas C, Franz AR, Shunova A, Mathes M, Bleeker C, Poets CF, Schleicher E, Bernhard W. Choline and polyunsaturated fatty acids in preterm infants' maternal milk. Eur J Nutr. 2017 Jun;56(4):1733-1742.
- Reference 4
-
Moles L, Manzano S, Fernández L, Montilla A, Corzo N, Ares S, Rodríguez JM, Espinosa-Martos I. Bacteriological, biochemical, and immunological properties of colostrum and mature milk from mothers of extremely preterm infants. J Pediatr Gastroenterol Nutr. 2015 Jan;60(1):120-6.
- Reference 5
-
Klein CJ. Nutrient requirements for preterm infant formulas. J Nutr. 2002 Jun;132(6 Suppl 1):1395S-577S. [LSRO-2002]
Footnotes
For Reference #1, 71 kcal/100 mL; for all others, ~67 kcal/100 mL.
- Footnote a
-
Protein, Fat, Calcium & Phosphorus values based on week 2 (from meta-analysis); All other values based on week 1-4
- Footnote b
-
Chromium values based on term human milk used by Life Sciences Research Office (LSRO 2002)
- Footnote c
-
NS due to wide range (likely due to variability of fluoride levels in water)