Food labelling coordination: Joint policy statement
Publication date: August 5, 2021
On this page
Introduction
The Food and Drugs Act and the Safe Food for Canadians Act provide a framework that governs the labelling of foods sold in Canada. This framework protects the health and safety of Canadians and supports consumer protection and a fair and secure marketplace. The Canadian Food Inspection Agency (CFIA) and Health Canada share responsibility for food labelling regulations.
CFIA and Health Canada update labelling requirements for food products for several reasons. For example, amendments may be proposed to align requirements with advancements in science to protect the health and safety of Canadians, to align with Codex Alimentarius guidance, to promote harmonization between requirements in different jurisdictions, or to facilitate innovation.
This joint policy statement outlines CFIA and Health Canada's commitment to enhance coordination of changes to food labelling requirements and to establish predictable compliance dates for these changes.
Background
In 2013, CFIA launched the Food labelling modernization initiative and in 2016, Health Canada launched the Healthy eating strategy. In response, industry stakeholders expressed a need for improved coordination and understanding between CFIA and Health Canada, as multiple, sequential label changes introduce unnecessary costs on industry. CFIA and Health Canada have worked together to coordinate these initiatives where possible.
Through Budget 2018, the Government of Canada announced that it would conduct targeted reviews of regulatory requirements and practices that are bottlenecks to innovation and growth. Several industry associations identified the need for concurrent implementation of changes to food labelling requirements.
In the 2019 Agri-food and aquaculture sector regulatory review roadmap, CFIA and Health Canada committed to develop a strategy for coordinating future changes to food labelling requirements, with support from Agriculture and Agri-Food Canada (AAFC). The Roadmap noted that the strategy should consider implementing a:
- Formal interdepartmental coordination process, and
- Regularized cycle for food labelling changes.
An international scan identified the United States as the only country with a predictable labelling cycle for food products.
United States
The United States Food and Drug Administration (US FDA) and the United States Department of Agriculture (USDA) prescribe uniform compliance dates for changes to food labelling regulations. Typically every two years, the US FDA and USDA issue final rules (i.e. regulations) that specify uniform compliance dates for any food labelling requirements finalized in a fixed two-year window. For example, both organizations recently announced January 1, 2024 as the uniform compliance date for labelling changes issued in the calendar years 2021 and 2022. This approach provides industry with a one- to three-year transition period. Special circumstances may justify an exception to this practice, such as matters of safety or economic burden.
According to the US FDA and USDA, the two-year interval allows industry to make orderly adjustments to new labelling requirements without exposing consumers to outdated labels. The practice also allows manufacturers to plan for the use of label inventories and to develop labelling materials that meet the new requirements.
Stakeholder consultation
CFIA and Health Canada conducted a public consultation on a draft policy to confirm that it provides the appropriate level of certainty for, and coordination of, future food labelling changes. The consultation was held over a 60-day period from February 2 to April 3, 2021.
Thirty-two stakeholders, from industry (25), health organizations (3), provincial governments (3) and a consumer advocacy organization (1), provided comments on the draft policy. While most supported the objectives of the draft policy, some expressed concerns about certain aspects. Feedback received throughout the consultation was considered in refining the policy. A summary of comments received and responses is available.
Policy description
Effective date
This policy will apply to all amendments made after August 5, 2021 to the Food and Drug Regulations and Safe Food for Canadians Regulations that mandate a food label change.
Objectives
The objectives of this policy are:
- To provide industry with greater predictability with respect to the compliance dates for food labelling changes, while maintaining CFIA and Health Canada's ability to take timely action to address serious consumer deception and acute risks to health and safety; and
- To establish formal interdepartmental coordination of food labelling initiatives that respects CFIA and Health Canada's existing regulatory development processes and reflects respective mandates.
Scope
This policy applies to all regulatory amendments made pursuant to the Food and Drugs Act or the Safe Food for Canadians Act, that directly or indirectly mandate changes to food labels, including food shipping containers. This includes all amendments published in the Canada Gazette, Part II, as well as documents incorporated by reference in the regulations that are published in CFIA’s notices or Health Canada’s notices.
Amendments that permit, but do not require, a change to food labels would be out of scope. For example, approval of a new food additive by Health Canada, or repeal of an existing requirement to declare that jam "contains pectin" on the main panel by CFIA would be out of scope because label changes would be the result of a business decision rather than a new government requirement.
Compliance dates
The compliance date for a regulation is the date of registration, unless the regulation provides otherwise, including through a transition period or a delayed coming into force date.
CFIA and Health Canada will establish compliance dates at a two year interval, beginning on January 1, 2026, which align with the intervals and dates in the United States. These dates are illustrated as flags in the Appendix. It is important to note that the compliance dates for changes to food labelling requirements will not automatically fall to the next available compliance date.
Regulated parties are responsible for ensuring that food products sold in Canada meet regulatory requirements, including those for labelling. CFIA will continue to monitor compliance with applicable regulations using a risk-based enforcement approach which could prioritize inspections at manufacture and import, as well as for food packaged and labelled at retail. These could be prioritized over inspections of other food on retail shelves.
Transition periods
In the United States, the compliance date for a food labelling regulation is typically determined by its publication date, resulting in transition periods of one to three years. In contrast, this policy allows CFIA and Health Canada to tailor the transition period for each food labelling regulation in consultation with stakeholders.
Transition periods for individual labelling regulatory changes will continue to be proposed in the Canada Gazette, Part I for public consultation. Each proposal will propose an appropriate transition period that will be used to conduct the cost-benefit analysis. The precise transition period will be confirmed at Canada Gazette, Part II publication.
To increase predictability for industry, CFIA and Health Canada regulatory amendments for label changes will have a minimum transition period of two years, unless the change addresses serious consumer deception or an acute risk to health and safety (i.e., unless the regulatory amendment is excepted from this policy).
Similarly, transition periods for revisions to applicable documents incorporated by reference will continue to be proposed through departmental and agency notification processes. The precise transition period will be specified in the notice following stakeholder consultation.
Some regulatory proposals may include multiple transition periods due to the complexity of the proposal or potential impacts on small and medium enterprises. In these cases, multiple transition periods for labelling changes could be proposed based on sequential compliance dates. CFIA and Health Canada will continue to consider stakeholder feedback during regulatory development, including in determining an appropriate transition period.
Exceptions
Changes that address serious consumer deception or acute risks to health and safety will not be required to align with the predictable compliance dates. Serious consumer deception (e.g. food fraud) refers to situations where the economic risks to Canadians are high, for an issue of representation, omission, or practice likely to mislead a consumer. Acute risks to human health and safety refer to emerging risks resulting from newly identified harms to human health. The health effects are typically observed in the short term but they could occur over a longer period of time. Flavoured Purified Alcohol and Mechanically Tenderized Beef are recent examples of regulatory amendments that would be considered as addressing acute risks to health and safety.
Exceptions will be determined on a case-by-case basis during regulatory development, in consultation with stakeholders. Should CFIA and/or Health Canada determine that an exception for the proposal to align with the predictable compliance dates is warranted, this decision and a rationale would be included in the proposal's Regulatory Impact Analysis Statement. CFIA and Health Canada will monitor the use of exceptions on an ongoing basis to ensure that they are used appropriately.
Regulatory development
CFIA and Health Canada will continue to advance regulatory proposals through existing organizational processes, as described in policies, guidance and tools related to the Cabinet Directive on Regulation.
Fixed compliance dates every two years does not necessarily mean that CFIA and Health Canada will require labelling changes at each predictable compliance date (i.e., every two years). Rather, this policy establishes a basis for CFIA or Health Canada to identify an appropriate compliance date when food labelling changes would be required. Factors such as scope, complexity and cost will be taken into consideration, in consultation with stakeholders, when determining the appropriate compliance date.
A two-year interval between compliance dates provides greater flexibility for CFIA and Health Canada in consultation with stakeholders, to select a compliance date that results in an appropriate transition period. A longer interval would limit the flexibility and options available, making it challenging to select appropriate compliance dates that account for factors like scope and complexity.CFIA and Health Canada's formal interdepartmental coordination process
CFIA and Health Canada will continue to collaborate when developing food labelling regulatory proposals to align compliance dates, as appropriate.
The Committee on Food Safety (CFS) is an existing interdepartmental committee, chaired by deputy heads of CFIA, Health Canada, AAFC, and the Public Health Agency of Canada. CFIA and Health Canada, will advance a joint list of planned and anticipated food labelling initiatives through the CFS as appropriate to support coordination of regulatory amendments that mandate a food label change. This list may include:- Proposed transition period;
- Anticipated timing for Canada Gazette, Part I publication;
- Anticipated compliance date options;
- Key milestones related to stakeholder engagement, as they become known; and
- Implementation considerations, including the scope and complexity of the proposed changes.
CFS members will consider the list to ensure the initiatives are coordinated in a manner that minimizes unnecessary regulatory burden on industry, while ensuring that Canadians are protected.
CFIA and Health Canada will continue to make their forward regulatory plans (FRPs) available online, independent of this policy. FRPs are lists of planned or anticipated regulatory initiatives, including those that mandate food labelling changes that each organization intends to propose or finalize within a two-year period, or potentially a longer time period. FRPs are updated at minimum annually. Effort will be made to publish target compliance dates for food labelling initiatives in the FRPs once the policy is in effect.
Roles and responsibilities
The Directorate leads in CFIA and Health Canada (Executive Director, Food Safety and Consumer Protection, CFIA and Director General, Food, Health Canada) play key roles in determining when a regulatory amendment that necessitates a food label change is required. They also identify emerging issues and ensure communication with the other government department with a view to developing a joint list of planned and anticipated food labelling initiatives.
The CFS will review the joint list of initiatives and, when appropriate, suggest amendments to the list.
Disclaimer
This document does not constitute part of the FDA, the SFCA or their respective regulations. In the event of any inconsistency or conflict between the FDA, SFCA or regulations and this document, the FDA, SFCA and/or the regulations take precedence. This document is an administrative document that is intended to enhance predictability of future food labelling compliance dates and facilitate compliance by regulated parties with the FDA, SFCA, the regulations and the applicable administrative policies.
Glossary
Acronyms
- AAFC
- Agriculture and Agri-food Canada
- CFIA
- Canadian Food Inspection Agency
- CFS
- Committee on Food Safety
- FDA
- Food and Drugs Act
- FRP
- Forward Regulatory Plan
- PHAC
- Public Health Agency of Canada
- SFCA
- Safe Food for Canadians Act
Terms
- Coming-into-force date
- Date a regulation takes effect and becomes enforceable. Typically fixed by the date of registration or a later date that is specified in the regulations.
- Compliance date
- Date by which products sold in Canada must comply with the new regulations. The compliance date may be, but is often not, the same as the coming-into-force date.
- Incorporation by Reference
- Drafting mechanism that allows a document or list to be made a part of the regulations without having to replicate its content in the regulations.
- Incorporated documents subject to this policy are those in which any future changes do not need to follow the regular regulatory amendment process (also known as an ambulatory or open reference).
- Publication date
- Date a regulation is published in the Canada Gazette, Part II. Regulations are published within 23 days after their registration.
- Registration date
- Date a regulation is officially registered by the Privy Council Office. Typically regulations come into force on the day they are registered, unless the enabling statute or the regulations themselves specify another commencement date.
- Transition period
- Period beginning with the registration date and ending with the compliance date.
- During the transition period, regulated parties can comply with either the status quo or the new regulations.
Appendix: Predictable compliance date approach
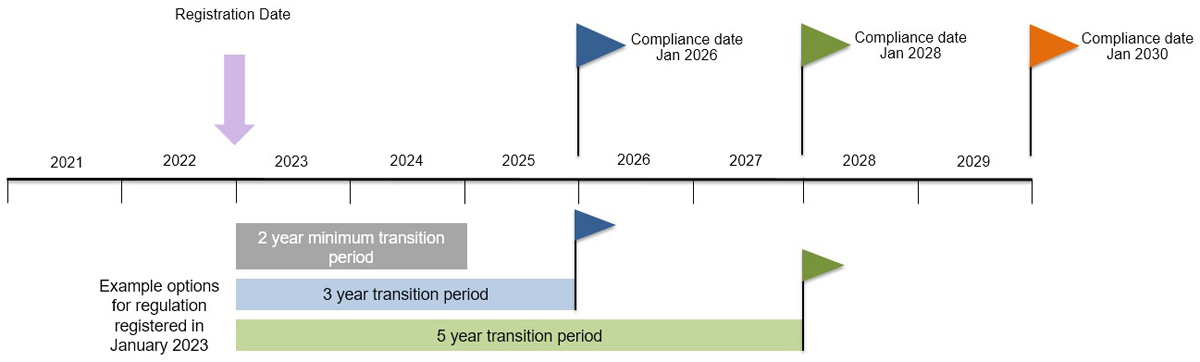
Text description
A timeline that shows the calendar years 2021 to 2029. Predictable compliance date options are shown with flags in January 2026, 2028 and 2030.
For an example regulation registered in January 2023, following a two-year minimum transition period, regulators could choose a compliance date of January 2026 or January 2028. A compliance date of January 2026 would result in a three-year transition period, and a compliance date of January 2028 would result in a five-year transition period.