Policy on Listeria monocytogenes in ready-to-eat foods (2023): Principles for controlling L. monocytogenes
Principles for the control of Listeria monocytogenes in ready-to-eat foods
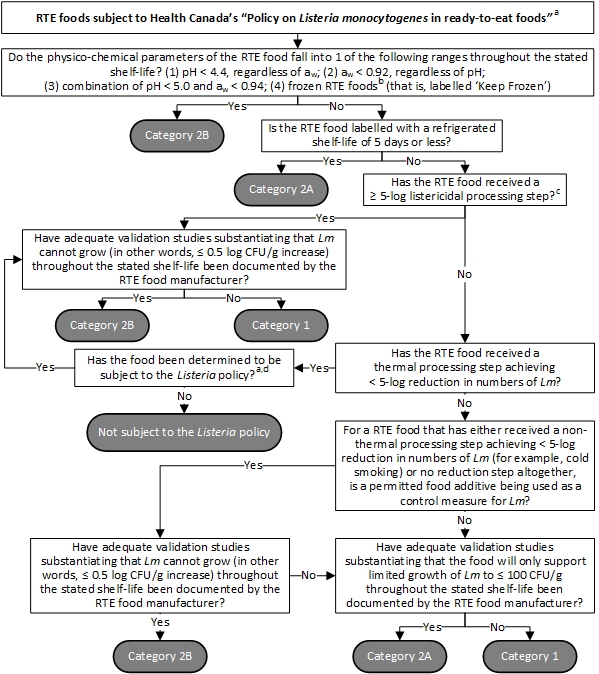
For the purpose of the Listeria policy, RTE foods are classified into 2 categories based on their potential to support L. monocytogenes growth. The decision tree presented in Figure 1 should be followed to determine the category of a RTE food.
On this page
- Category 1 ready-to-eat foods
- Category 2 ready-to-eat foods
- Ready-to-eat foods for vulnerable populations
Demonstrating the ready-to-eat food category (Figure 1) and determining the level of priority for the frequency of process monitoring, environmental sampling and end-product testing
Category 1 ready-to-eat foods
Category 1 RTE foods are those which support the growth of L. monocytogenes under reasonably foreseeable conditions of distribution, storage and use throughout the stated shelf-life. As such, process monitoring, environmental sampling and end-product testing should be conducted more frequently for Category 1 RTE foods as compared to Category 2 RTE foods. Furthermore, the detection of L. monocytogenes in a Category 1 RTE food, as determined by the applicable sampling and testing methodologies specified in the section on Sampling and testing of ready-to-eat foods and Table 1, may trigger a Health Risk 1 concern. Risk management actions may be needed.
Category 2 ready-to-eat foods
Category 2 contains 2 subgroups, for which validation may be necessary to demonstrate that control measures are effective to limit or prevent the growth of L. monocytogenes. For more information on validation, please refer to these relevant publications: Validation of Ready-to-Eat Foods for Changing the Classification of a Category 1 into a Category 2A or 2B Food - in relation to Health Canada's Policy on Listeria monocytogenes in Ready-to-Eat Foods and Listeria monocytogenes Challenge Testing of Refrigerated Ready-to-Eat Foods (Health Canada, 2012a; 2012b). If there is uncertainty regarding the categorization of a RTE food, the relevant regulatory authority should be contacted.
As specified in the section on Sampling and testing of ready-to-eat foods and Table 1, levels of L. monocytogenes exceeding 100 CFU/g in a Category 2 RTE food, as determined by the applicable sampling and testing methodologies, may trigger a Health Risk 2 concern. Risk management actions may be needed.
Category 2A ready-to-eat foods
Category 2A: Certain RTE foods in which the growth of L. monocytogenes may be limited to levels not exceeding 100 CFU/g under reasonably foreseeable conditions of distribution, storage and use throughout the stated shelf-life. This category includes (Figure 1):
- RTE foods which are known to occasionally contain low levels of L. monocytogenes and their processing does not involve a heat treatment (based on validation)
- In other words, a RTE food that may have received a non-thermal processing step by the manufacturer achieving less than a 5-log reduction in numbers of L. monocytogenes or no reduction step altogether
Note: The presence of L. monocytogenes in Category 2A RTE foods may be sporadic (for example, the levels could be lower than the method's limit of quantification, but over time may grow to quantifiable levels).
- RTE refrigerated foods with a stated shelf-life of 5 days or less
Note: Information on a food label must not be false or misleading (including, no deception or erroneous impression regarding its safety) and their labelling must comply with section 5 of the Food and Drugs Act (Government of Canada, 2023a). The day of final packaging can be regarded as day 0.
Although these foods can support the growth of L. monocytogenes, such growth is generally limited due to factors such as a short refrigerated shelf-life or the presence of a large population of background microorganisms producing compounds such as bacteriocins and organic acids. As such, process monitoring, environmental sampling and end-product testing could be conducted less frequently for Category 2A RTE foods as compared to Category 1 RTE foods and RTE foods specifically produced for consumption by vulnerable populations (Table 1).
As applicable, manufacturers of Category 2A RTE foods should validate and verify their process to demonstrate that levels of L. monocytogenes are consistently not exceeding 100 CFU/g throughout the foods' stated shelf-life. In order to confirm that a RTE food remains in Category 2A, manufacturers should regularly monitor the food to demonstrate that it continues to meet the specified criteria that justify its categorization as a Category 2A RTE food. In fact, for those specific RTE foods, testing of the RTE food at the beginning of its shelf-life becomes a key process parameter to confirm that the RTE food meets the criteria that were used in the challenge study (i.e., 10 to 30 CFU/g). This is performed to confirm that the concentration of L. monocytogenes, at the beginning of the shelf-life (at time 0), never exceeds the 10 to 30 CFU/g level that was used as an inoculum in the challenge study (Health Canada, 2012a; 2012c).
The RTE food manufacturer should be able to demonstrate that the food meets the criteria of Category 2A. In cases where validation was conducted for categorization, the manufacturer should have documentation of adequate validation studies substantiating that the RTE food will only support limited growth of L. monocytogenes to levels not exceeding 100 CFU/g throughout the stated shelf-life. If insufficient, inadequate or no information exists regarding the Category 2A categorization of the RTE food, the RTE food will be considered as a Category 1 RTE food (that is, a food in which the growth of L. monocytogenes can occur). As such, the sampling and testing methodologies for Category 1 RTE foods, as specified in the section on Sampling and testing of ready-to-eat foods and Table 1, should be used.
Category 2B ready-to-eat foods
Category 2B: RTE foods in which the growth of L. monocytogenes will not occur under reasonably foreseeable conditions of distribution, storage and use throughout the stated shelf-life (CAC, 2009a). This category includes (Figure 1):
- RTE foods for which the pH and aw values are such that they do not support the growth of L. monocytogenes
- pH < 4.4, regardless of aw
- aw< 0.92, regardless of pH
- combination of pH < 5.0 and aw< 0.94
frozen RTE foods (that is, labelled 'Keep Frozen' on the package)
- other RTE foods in which L. monocytogenes does not increase in numbers by more than 0.5 log CFU/g throughout the stated shelf-life (based on validation)
Note: 0.5 log is 2 times the estimated standard deviation (that is, 0.25 log) associated with the experimental enumeration viable counting/plate counts (CAC, 2009a).
Category 2B RTE foods do not support the growth of L. monocytogenes. As such, process monitoring, environmental sampling and end-product testing could be conducted less frequently for Category 2B RTE foods as compared to Category 2A RTE foods, Category 1 RTE foods and RTE foods specifically produced for consumption by vulnerable populations (Table 1). As applicable, Category 2B RTE food manufacturers should regularly monitor the physico-chemical parameters of the food (such as pH and aw), its formulation, and so on to demonstrate that it continues to meet the specified criteria that justify its categorization as a Category 2B RTE food.
The RTE food manufacturer should be able to demonstrate that the food meets the criteria of Category 2B. In cases where validation was conducted for categorization, the manufacturer should have documentation of adequate validation studies substantiating that L. monocytogenes cannot grow throughout the stated shelf-life. If insufficient, inadequate or no information exists regarding the Category 2B categorization of the RTE food, the RTE food will be considered as a Category 1 RTE food (that is, a food in which the growth of L. monocytogenes can occur). As such, the sampling and testing methodologies for Category 1 RTE foods, as specified in the section on Sampling and testing of ready-to-eat foods and Table 1, should be used.
Frozen ready-to-eat foods
RTE food manufacturers should be able to demonstrate the RTE food category and determine the level of priority for the frequency of process monitoring, environmental sampling and end-product testing. For frozen RTE foods, manufacturers should consider the intended consumers (for example, vulnerable populations); the information specified on the label of the package pertaining to thawing times and temperatures, as well as any refrigerated shelf-life after thawing; and process controls, that is Critical Control Points (CCPs) pertaining to the food's formulation. Information on a food label must not be false or misleading (including, no deception or erroneous impression regarding its safety) and their labelling must comply with section 5 of the Food and Drugs Act (Government of Canada, 2023a). If there is uncertainty regarding the categorization of a RTE food, the relevant regulatory authority should be contacted.
Furthermore, frozen Category 2B RTE foods that are not labelled with information pertaining to thawing times and temperatures, or refrigerated shelf-life after thawing, but will be thawed before direct consumption should be monitored and sampled more frequently as compared to frozen Category 2B RTE foods that are consumed directly in the frozen state.
Manufacturers of second-generation RTE foods should consider developing supply-chain controls (for example, a formal agreement with the supplier(s), Certificate of Analysis) in order to have greater confidence that the ingredients to be used in second-generation RTE foods are safe and suitable for use. Examples of such situations involving frozen RTE foods are described below.
- 1. When initially frozen RTE foods are used as ingredients in other RTE foods, these second-generation RTE end-products, as intended to be sold to consumers, also need to be categorized appropriately, since they could fall into any of the categories described previously, that is Category 1, 2A or 2B.
- For example, if a secondary manufacturer uses a frozen Category 2B RTE smoked fish to make a RTE refrigerated smoked fish mousse that supports the growth of L. monocytogenes and has a refrigerated shelf-life greater than 5 days, this mousse would be considered a Category 1 RTE food.
- For example, if a secondary manufacturer uses a frozen Category 2B RTE smoked fish to make a RTE refrigerated smoked fish mousse that supports the growth of L. monocytogenes and has a refrigerated shelf-life greater than 5 days, this mousse would be considered a Category 1 RTE food.
- 2. When an initially frozen RTE food is repackaged and intended by the secondary manufacturer to be stored under refrigeration, these second-generation RTE end-products also need to be categorized appropriately, since they could fall into any of the categories described previously, that is Category 1, 2A or 2B.
There may be situations involving frozen RTE foods where the manufacturer's intended storage condition is not met. The following situations should trigger an assessment to determine if they represent a health concern. Risk management actions may be needed.
- 3. A frozen RTE food that has been temperature-abused prior to reaching retail. In this situation, the growth of L. monocytogenes may have occurred.
- 4. A finding of L. monocytogenes in a frozen RTE food that is labelled 'Keep Frozen' on the package, but is otherwise thawed for sale at retail.
- As specified in the section on Purpose and scope, retail food businesses that sell food directly to consumers are not subject to the Listeria policy. Nevertheless, all businesses that sell food in Canada are subject to all relevant provisions of the Food and Drugs Act, including sections 4 and 7 (Government of Canada, 2023a).
Ready-to-eat foods for vulnerable populations
RTE food businesses should consider their business model and client expectations as they pertain to food for vulnerable populations (for example, food being sold or marketed to such special groups of consumers or institutions). Hence, RTE foods specifically produced for consumption by vulnerable populations are those foods for which food businesses have specified consumption by people with weakened immune systems, pregnant people or adults ages 60 and over (for example, RTE foods that will be consumed in a hospital setting, convalescent care centres, long-term care facilities) in the product description of their HACCP plan or PCP. These RTE foods should be monitored and sampled at a greater frequency as compared to foods produced for consumption by the general population (CAC, 2020) (Table 1). Furthermore, other specific control measures (see the section on Process control and Appendix C) may be taken for these foods as the presence of L. monocytogenes in such foods represents an increased health concern for these populations.
Irrespective of category, the detection of L. monocytogenes in RTE foods specifically produced for consumption by vulnerable populations (as specified in the section on Sampling and testing of ready-to-eat foods and Table 1) may trigger a Health Risk 1 concern, and not a Health Risk 2 concern, given the significantly increased susceptibility of vulnerable populations in acquiring foodborne listeriosis (see the section on Scientific basis for the policy). In such situations, risk management actions may be needed.
As per the definition of RTE foods (see the section on Application of the policy), certain refrigerated or frozen processed foods labelled on the package with validated cooking instructions would be excluded from the Listeria policy. However, as vulnerable individuals have an increased susceptibility to infection with L. monocytogenes, additional recommendations, as described in the section on Requirements and best practices for foods excluded from the policy, should be taken into account.
- If there is uncertainty regarding whether a food is subject to the Listeria policy, the relevant regulatory authority should be contacted.
- To categorize a frozen RTE food, manufacturers should take into consideration the information specified on the label of the package pertaining to thawing times and temperatures, as well as any refrigerated shelf-life after thawing; and process controls, that is Critical Control Points (CCPs) pertaining to the food's formulation. If there is uncertainty regarding the categorization of a RTE food, the relevant regulatory authority should be contacted.
- For the purpose of the Listeria policy, a '≥ 5-log listericidal processing step' represents a validated treatment by the RTE food manufacturer that achieves a minimum 5-log reduction in numbers of L. monocytogenes.
- For example, this could be processed foods which have a cooked appearance (but are not fully cooked).
Figure 1- Text description
Figure 1 consists of a series of questions to help determine the categorization of ready-to-eat foods that are subject to Health Canada's "Policy on Listeria monocytogenes in ready-to-eat foods", referred to as the Listeria policy. To begin, if there is uncertainty regarding whether a food is subject to the Listeria policy, the relevant regulatory authority should be contacted.
Thereafter, if the physico-chemical parameters of the ready-to-eat food fall into 1 of the following 4 ranges throughout the stated shelf-life, this ready-to-eat food should be categorized as Category 2B. That is, (1) pH less than 4.4, regardless of water activity; (2) water activity less than 0.92, regardless of pH; (3) combination of pH less than 5.0 and water activity less than 0.94; or (4) frozen ready-to-eat foods that are labelled 'Keep Frozen'. To categorize a frozen ready-to-eat food, manufacturers should take into consideration the information specified on the label of the package pertaining to thawing times and temperatures, as well as any refrigerated shelf-life after thawing; and process controls, that is Critical Control Points or CCPs, pertaining to the food's formulation. If there is uncertainty regarding the categorization of a ready-to-eat food, the relevant regulatory authority should be contacted.
If the physico-chemical parameters of the ready-to-eat food do not fall into 1 of those 4 ranges throughout the stated shelf-life and the ready-to-eat food is labelled with a refrigerated shelf-life of 5 days or less, this ready-to-eat food should be categorized as Category 2A.
If the physico-chemical parameters of the ready-to-eat food do not fall into 1 of those 4 ranges throughout the stated shelf-life, it is not labelled with a refrigerated shelf-life of 5 days or less, and it has received a 5-log or more listericidal processing step, then, this ready-to-eat food should be categorized as Category 2B if adequate validation studies have been documented by the ready-to-eat food manufacturer to substantiate that L. monocytogenes cannot grow, in other words, a less than or equal to 0.5 log colony forming unit per gram increase, throughout the stated shelf-life. For the purpose of the Listeria policy, a '5-log or more listericidal processing step' represents a validated treatment by the ready-to-eat food manufacturer that achieves a minimum 5-log reduction in numbers of L. monocytogenes. Alternatively, the ready-to-eat food should be categorized as Category 1 if adequate validation studies have not been documented by the ready-to-eat food manufacturer to substantiate that L. monocytogenes cannot grow, in other words, a less than or equal to 0.5 log colony forming unit per gram increase, throughout the stated shelf-life.
If the physico-chemical parameters of the ready-to-eat food do not fall into 1 of those 4 ranges throughout the stated shelf-life, it is not labelled with a refrigerated shelf-life of 5 days or less, it has not received a 5-log or more listericidal processing step, but it has received a thermal processing step achieving less than a 5-log reduction in numbers of L. monocytogenes, it should be determined whether this food is subject to the Listeria policy. If there is uncertainty regarding whether a food is subject to the Listeria policy, the relevant regulatory authority should be contacted. For example, this could be processed foods which have a cooked appearance, but are not fully cooked. In that case, the food is not considered to be subject to the Listeria policy. If the food is subject to the Listeria policy and adequate validation studies have been documented by the ready-to-eat food manufacturer to substantiate that L. monocytogenes cannot grow, in other words, a less than or equal to 0.5 log colony forming unit per gram increase, throughout the stated shelf-life, this ready-to-eat food should be categorized as Category 2B; otherwise, this ready-to-eat food should be categorized as a Category 1.
If the physico-chemical parameters of the ready-to-eat food do not fall into 1 of those 4 ranges throughout the stated shelf-life, it is not labelled with a refrigerated shelf-life of 5 days or less, it has not received a 5-log or more listericidal processing step, and it has not received a thermal processing step achieving less than a 5-log reduction in numbers of L. monocytogenes, then it is a ready-to-eat food that has either received a non-thermal processing step achieving less than a 5-log reduction in numbers of L. monocytogenes, for example, cold smoking, or no reduction step altogether. If no permitted food additive has been used as a control measure for L. monocytogenes in its manufacturing, this ready-to-eat food should be categorized as Category 2A if adequate validation studies have been documented by the ready-to-eat food manufacturer to substantiate that the food will only support limited growth of L. monocytogenes to less than or equal to 100 colony forming units per gram throughout the stated shelf-life; otherwise, this ready-to-eat food should be categorized as Category 1.
If the physico-chemical parameters of the ready-to-eat food do not fall into 1 of those 4 ranges throughout the stated shelf-life, it is not labelled with a refrigerated shelf-life of 5 days or less, it has not received a 5-log or more listericidal processing step, and it has not received a thermal processing step achieving less than a 5-log reduction in numbers of L. monocytogenes, then, it is a ready-to-eat food that has either received a non-thermal processing step achieving less than a 5-log reduction in numbers of L. monocytogenes, for example, cold smoking, or no reduction step altogether. If a permitted food additive has been used as a control measure for L. monocytogenes in its manufacturing, and adequate validation studies have been documented by the ready-to-eat food manufacturer to substantiate that L. monocytogenes cannot grow, in other words, a less than or equal to 0.5 log colony forming unit per gram increase, throughout the stated shelf-life, this ready-to-eat food should be categorized as Category 2B. Alternatively, if adequate validation studies have not been documented by the ready-to-eat food manufacturer to substantiate that L. monocytogenes cannot grow, but adequate validation studies have been documented by the ready-to-eat food manufacturer to substantiate that the food will only support limited growth of L. monocytogenes to less than or equal to 100 colony forming units per gram throughout the stated shelf-life, this ready-to-eat food should be categorized as Category 2A; otherwise, this ready-to-eat food should be categorized as Category 1.