About antibiotic resistance: Preserving antibiotics now and in the future
Chief Public Health Officer of Canada's Spotlight Report 2019
It is hard to imagine that our grandparents and great-grandparents lived in a world without antibiotics. This was a time when many people died from now-curable infectious diseases. That is not a world we would wish on our children or grandchildren.
And yet, antibiotics are becoming ever less effective, because bacteria can evolve to withstand their effects, a process called antibiotic resistance. Left unchecked, there is risk of losing these medications as an essential life-saving treatment. Although the future is hard to predict, it is estimated that antibiotic-resistant infections could cause 10 million deaths a year globally by 2050, which is more than the current annual worldwide deaths from cancer.Footnote 1
Antibiotic use is an issue not only in human health care but also in animal health as well. Leaders in Canada and around the world are actively joining forces across health and agricultural sectors to tackle antibiotic resistance. For example, Canada's Pan-Canadian Framework for Action on Antimicrobial Resistance, and the soon-to-be released action plan, aims to strengthen Canada's ability to combat the risks of antimicrobial resistance.
This report focuses on antibiotic use and prescribing practices in our communities (or non-hospital settings). This is where 92% of antibiotics prescriptions are written and filled by doctors, nurses, dentists and pharmacists.Footnote 2
The goal of this report is to describe why unnecessary antibiotic use sometimes happens and what we can do about it.
What are antimicrobials?
Antimicrobials are a group of 4 types of medicines that kill microbes or stop their growth.Footnote 3
- Antibiotics are used to treat bacterial infections, such as strep throat and urinary tract infections.
- Antivirals are used against illnesses caused by viruses, such as influenza and HIV.
- Antifungals are used against infections caused by fungi, such as yeast infections.
- Antiparasitics are used against parasites, such as pinworms.
This report focuses on:
- unnecessary antibiotic use
- how it contributes to the problem of antibiotic resistance
- what we can do about it
Unnecessary use of antibiotics
What is considered unnecessary use of antibiotics?Footnote 4
- Taking or being prescribed antibiotics for infections caused by a virus.
- Being prescribed the wrong type, dose or duration of antibiotics.
- Taking antibiotics in ways other than how they were prescribed.
- Taking leftover antibiotics without a prescription or using someone else's antibiotics.
Microbes, good and bad
Every one of us is home to trillions of microscopic organisms that live naturally in our gut, on our skin, and in other areas of our body. This ecosystem of microbes, called the microbiome, helps with digestion, immunity, heart health, and more.Footnote 5 The microbiome is passed down at birth from mother to infant and then changes over time, becoming unique to each person based on diet and environment.Footnote 6 Science is only beginning to discover the multiple effects the microbiome has on human health.
Most of the time, we exist in harmony with our microbiome. Our immune system is active all the time, preventing potentially harmful microbes from causing illness and keeping our microbiome in balance.
For example, Staphylococcus aureus, a bacterium well known to infect humans, normally lives in the noses of about 20% of the population but rarely causes serious illness.Footnote 7 However, sometimes infections do happen. These infections are caused by:
- foreign bacteria passed on from other people, animals, contaminated food or water, or
- the bacteria that already co-exist in our bodies and which find an opportunity to breach our immune defenses
The immune system usually recognizes and fights off harmful microbes in just a few days. Other times, a bacterial infection might require antibiotics. Some people are at higher risk for serious illness, and even death from infections, especially:
- the very young
- the elderly
- people who have a chronic disease or serious injury
For people receiving cancer therapy or who have a weakened immune system, it is crucial that antibiotics remain effective. Before the discovery of antibiotics, people who suffered from serious bacterial infections often died and many medical procedures were unsafe to perform. The discovery of antibiotics and other antimicrobial drugs is considered one of the most important achievements in medical history.Footnote 8 The Golden Age of antibiotics between 1938 and 1952 saw the biggest declines in deaths from infectious diseases.
The way antibiotics kill or stop the growth of bacteria depends on the drug.Footnote 9 While no antibiotic can cure every infection, many antibiotics are multi-purpose (or broad spectrum) and can cure infections caused by many different bacteria, in contrast to more selective (or narrow spectrum) antibiotics.
Some antibiotics, like penicillin and amoxicillin, break down bacterial cell walls so the bacteria burst and die. Other antibiotics, like erythromycin, prevent bacteria from building essential proteins. Certain antibiotics, like daptomycin, are reserved as last-resort options and are used only when other antibiotics have failed to stop an infection. The best type of antibiotic to prescribe depends on the type of bacteria causing the infection, the site and the severity of that infection.
Magic bullet or double-edged sword?
The effectiveness of antibiotics has led many to view them as "magic bullets," medicines that could treat any infection while leaving the rest of the body unharmed.Footnote 10 As our understanding of these medicines and the complex nature of our microbiome have grown, antibiotics have instead proven to be double-edged swords because they not only kill the bacteria causing the infection, but also destroy the innocent bystanders of the bacterial world.
This can change the balance of the microbiome, and allow foreign microbes to invade or cause an over-growth of native microbes that is harmful. For example, yeast infections from the Candida fungi or diarrhea and bowel ulcers from the bacterium C. difficile, can be unintended consequences of antibiotic use.Footnote 11
Antibiotics are just like any other medication, and while they offer tremendous benefits, they also carry a small risk of allergic reactions, negative interactions with other drugs, and other side effects.Footnote 12 For example, a recognized side effect of amoxicillin is a body rash that occurs if this antibiotic is given to someone who has a viral infection, such as mononucleosis or "mono".Footnote 13
For people with a serious infection, the benefits can far outweigh the risks of antibiotics. It is this low risk that sometimes encourages healthcare providers to prescribe antibiotics when an antibiotic treatment is not needed. Using antibiotics to fight infectious diseases is an important driver of the development of antibiotic resistance, and limiting unnecessary use can help to counter it.
Discovery and the dawn of the Golden Age of antibiotics
The Golden Age of antibiotics followed the chance discovery of penicillin by Dr. Alexander Fleming. In 1928, Dr. Fleming discovered that one of his experiments with the bacterium Staphylococcus had become contaminated with a "white, fluffy growth" which was the mold Penicillium.Footnote 14 To his surprise, he discovered through the microscope that many of the bacteria had died as a result of the mold.Footnote 14 This unexpected observation eventually led to synthesis and manufacture of the antibiotic penicillin in the 1940s.Footnote 8, Footnote 15
Although Dr. Fleming is widely viewed as the discoverer of antibiotics, many early 20th-century scientists were finding ways to tackle the germs infecting the population at that time.Footnote 8 More than a decade before Fleming, German physician Dr. Paul Ehrlich coined the term Zauberkugel (German for "magic bullet") during the development of Salvarsan, an arsenic-based dye designed to target syphilis like a gun's bullet hitting a target.Footnote 16
This discovery was followed by the development of sulfonamides that entered the market by 1935 and dramatically lowered deaths from pneumonia, meningitis, and other infections after childbirth.Footnote 8 Penicillin became available in 1941 for treatment of a wide range of bacterial infections. Further research and development eventually led to the availability of semi-synthetic penicillins, cephalosporins, and carbapenems.
The discovery and development of new antibiotics has slowed considerably over recent decades.Footnote 17 Today fewer than 10 of the 50 leading pharmaceutical companies have active antibiotic development programs, as most new developments are led by specialized small- and medium-sized pharmaceutical companies.Footnote 17
The emergence of antibiotic resistance
Like all living things on earth, bacteria are constantly and quickly evolving in order to survive. Some strains of bacteria have natural genetic traits that protect them against the antimicrobial substances produced by other bacteria and fungi, with which they compete for survival (such as the mold Penicillium from which penicillin was discovered).Footnote 18
We learned how to isolate these substances, make our own, and use them as antibiotics. As we started using more and more antibiotics to fight bacterial infections in people and animals, the problem of antibiotic resistance became apparent. Any time an antibiotic is used, the bacteria with the genetic traits that protect them against the antibiotic will survive and multiply, while those without those genes die.Footnote 19
The surviving bacteria multiply very quickly, in as little as 20 minutes in the case of E. coli bacteria. The next generation of bacteria will now also carry these resistance genes and will also be able to withstand the effect of the antibiotic. Bacteria can even share resistance genes with other bacteria through a process called horizontal gene transfer.Footnote 20
Over time, as populations of bacteria are continually exposed to different antibiotics, they become increasingly resistant to ever more antibiotics until eventually, very few or no effective antibiotic treatments will be left.
The emergence of antibiotic resistance is not new. As early as 1924, 4 years before the discovery of penicillin, researchers had already identified a strain of syphilis that was resistant to Salvarsan, the drug used to treat the disease at that time.Footnote 21, Footnote 22 In the years following, many forewarned of the emergence of antibiotic resistance, including Dr. Alexander Fleming, the discoverer of penicillin. True to these warnings, the first cases of penicillin-resistant bacterial infections were observed in 1945.Footnote 8 By the mid-1950s, 90% of tested strains of Staphylococcus aureus were resistant to penicillin.Footnote 23
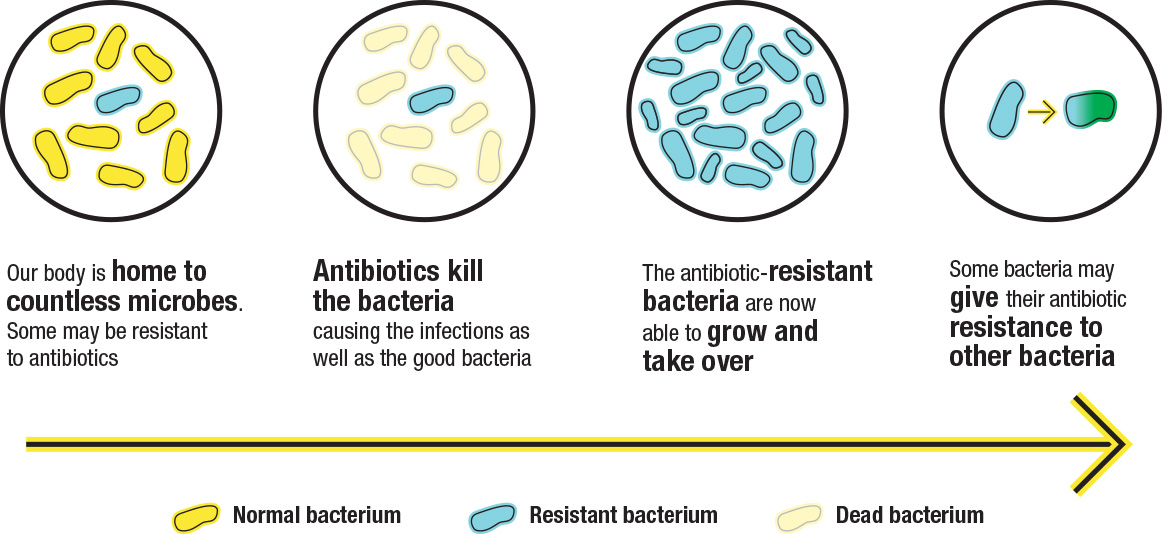
Figure 1 - Text description
The figure shows a common sequence of events to demonstrate how antibiotic resistance happens:
- Our body is home to countless microbes. Some may be resistant to antibiotics.
- Antibiotics kill the bacteria causing the infections as well as the good bacteria.
- As a result, the antibiotic-resistant bacteria are able to grow and take over.
- Some types of bacteria may give their resistance to other bacteria.
Antibiotic resistance today
Many Canadians know someone who has been impacted by antibiotic-resistant infections. Antibiotic resistance makes it harder to fight infections, leading to more and longer hospital stays, which increase health care costs. It is estimated that 1 in 16 Canadians admitted to hospital will develop an infection from a resistant superbugFootnote 24 and that 7 in 10 Canadians worry about the risk of infection at hospitals and healthcare facilities.Footnote 25
Progress has been made in Canada, and rates of antibiotic resistance are lower than in many other countries in the world. For example, worldwide, the rate of infections caused by Enterobacteriaceae resistant to a last line of defence class of antibiotics called carbapenems has increased over time, but in Canada, it has remained low and relatively stable.Footnote 2
That said, there has been a 5-fold increase in bacteria resistant to carbapenems found in people who do not have an infection, suggesting that these bacteria are becoming more common in Canada. The rate of methicillin-resistant Staphylococcus aureus infections (often called MRSA) acquired in hospital decreased slightly between 2012 and 2017, but the rate of MRSA infection acquired in the community increased over that time period.
Similarly, while hospital associated C. difficile infection rates declined in previous years, C. difficile infection rates acquired in the community remained stable since 2015. Rates of resistant gonorrhoea infections, one of the most commonly sexually transmitted diseases in Canada, have also risen over the past decade. Now, more than 50% of gonorrhoea infections are due to bacteria that are resistant to at least one antibiotic.Footnote 26

Figure 2 – Text description
The figure lists national trend data for selected antibiotic-resistant infections or bacteria in the community:
- The rate of methicillin-resistant Staphylococcus aureus infections (often called MRSA) acquired in the community increased by 60% between 2012 and 2017.
- The rate of C. difficile infections acquired in the community remained stable since 2015.
- Resistant gonorrhoea infection rates rose over the past decade. Now more than 50% of all gonorrhoea infections are resistant to at least 1 antibiotic.
- A 5-fold increase was observed in people carrying the bacteria resistant to carbapenems, which are amongst the most powerful antibiotics that exist.