Canadian Biosafety Guideline: Human Diagnostic Activities
Download in PDF format
(5.17 MB, 72 pages)
Organization: Public Health Agency of Canada
Published: 2021-06-01
Table of Contents
- Preface
- Abbreviations and Acronyms
- Chapter 1 - Introduction
- Chapter 2 - Risks Associated with Diagnostic Activities
- Chapter 3 - Considerations for Physical Containment
- Chapter 4 - Operational Practices
- Chapter 5 - Decontamination and Waste Management
- Chapter 6 - Glossary
- Chapter 7 - References and Resources
- Appendix A - Licensing Requirements when only Health of Animals Regulations apply
- Appendix B - Proper Hand Hygiene
Preface
The Human Diagnostic Activities guideline was developed by the Public Health Agency of Canada (PHAC) as part of a series of electronic publications that expand upon the biosafety and biosecurity concepts discussed in the current edition of the Canadian Biosafety Handbook (CBH), the companion document to the Canadian Biosafety Standard (CBS). This guideline provides guidance for facilities where human diagnostic activities (i.e., diagnostic testing or laboratory analyses with a human pathogen) are performed. This includes facilities performing activities with pathogens or toxins that are excluded from the Human Pathogens and Toxins Act (HPTA), those that are exempted from requiring a licence, and those conducting controlled activities under a Pathogen and Toxin Licence issued by the PHAC.
In Canada, facilities where Risk Group 2, 3, and 4 human pathogens or toxins are handled and stored are regulated by the PHAC under the HPTA and the Human Pathogens and Toxins Regulations (HPTR). The importation of animal pathogens, infected animals, animal products or by-products (e.g., tissue, serum), or other substances that may carry an animal pathogen or parts thereof (e.g., toxins) are regulated by the PHAC or the Canadian Food Inspection Agency (CFIA) under the Health of Animals Act (HAA) and the Health of Animals Regulations (HAR).
The following figure depicts the document hierarchy used by the PHAC and the CFIA to oversee biosafety and biosecurity operations. Each tier of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards. Acts and regulations are found at the top of the pyramid, as they are the documents that convey the PHAC's and the CFIA's legal authorities. Guidance material and technical pieces are found at the bottom of the pyramid, as they are intended to summarize recommendations and scientific information only.
Figure 1: The Government of Canada's biosafety and biosecurity document hierarchy
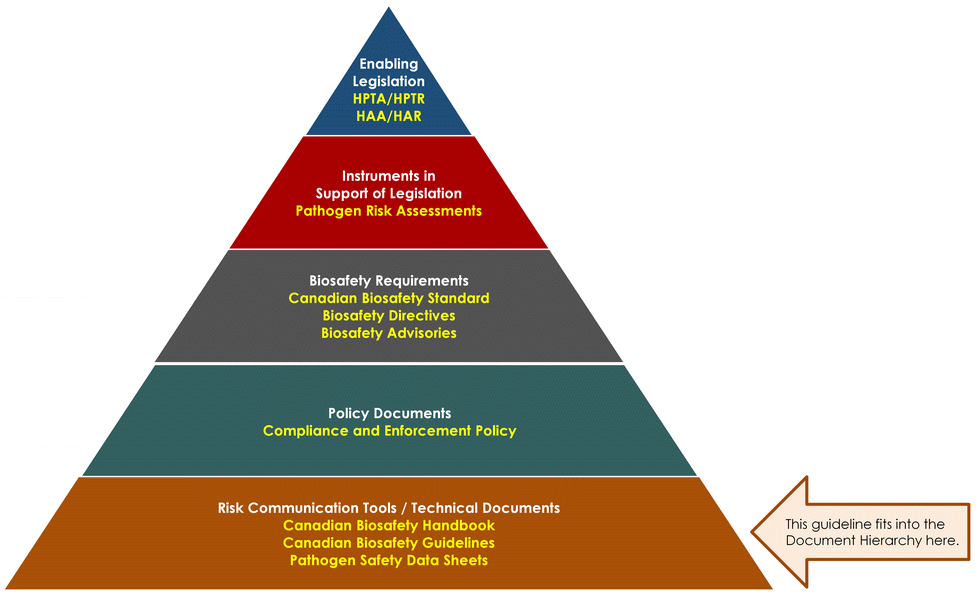
Figure 1 - Text Description
Figure in the form of a pyramid depicting the document hierarchy used by the PHAC to oversee biosafety and biosecurity operations. Each of the five tiers of the pyramid corresponds to a document type, with documents increasing in order of precedence moving upwards.
At the top sits the Enabling Legislation, that is, the HPTA, HPTR, HAA, and HAR, that convey the PHAC's legal authorities. Below the acts and regulations sit Instruments in Support of Legislation, which are the Pathogen Risk Assessments. The next tier down are the Biosafety Requirements, which include the Canadian Biosafety Standard, Biosafety Directives, and Biosafety Advisories. In the second lowest tier are the Policy Documents, which include the Compliance and Enforcement Policy. Guidance material and technical pieces found at the bottom of the pyramid, under the Risk Communication Tools and Technical Documents heading, are intended to summarize recommendations and scientific information only. These include the Canadian Biosafety Handbook, Canadian Biosafety guidelines, and Pathogen Safety Data Sheets.
The Human Diagnostic Activities guideline is continuously evolving and subject to ongoing improvement. The PHAC welcomes comments, clarifications, and suggestions for incorporation into future versions. Please send this information (with references, where applicable) to:
- PHAC e-mail: PHAC.pathogens-pathogenes.ASPC@canada.ca
Abbreviations and Acronyms
- BSC
- Biological safety cabinet
- BSO
- Biological safety officer
- CBH
- Canadian Biosafety Handbook
- CBS
- Canadian Biosafety Standard
- CFIA
- Canadian Food Inspection Agency
- CL
- Containment level (i.e., CL1, CL2, CL3, CL4)
- HAA
- Health of Animals Act
- HAR
- Health of Animals Regulations
- HPTA
- Human Pathogens and Toxins Act
- HPTR
- Human Pathogens and Toxins Regulations
- LAI
- Laboratory-acquired infection/intoxication
- LRA
- Local risk assessment
- PHAC
- Public Health Agency of Canada
- PPE
- Personal protective equipment
- RG
- Risk Group (i.e., RG1, RG2, RG3, RG4)
- SOP
- Standard operating procedure
- SSBA
- Security sensitive biological agent
Chapter 1 - Introduction
The words in bold type are defined in the glossary found in Chapter 6.
Infectious diseases and illnesses caused by pathogens affect millions of Canadians every year. Diagnostic facilities serve a critical function to Canada's health care system by providing essential services that support medical professionals in the diagnosis and treatment of illness or disease in their patients.
In Canada, facilities where controlled activities with Risk Group 2 (RG2), RG3, and RG4 human pathogens, including zoonotic pathogens, or toxins are conducted are regulated by the Public Health Agency of Canada (PHAC) under the Human Pathogens and Toxins Act (HPTA) and the Human Pathogens and Toxins Regulations (HPTR).Footnote 1 Footnote 2 Unless specifically excluded from the HPTA, or exempted from the licensing requirement under the HPTA or the HPTR, these facilities require a Pathogen and Toxin Licence (hereafter, licence) to knowingly conduct controlled activities with a human pathogen or toxin. Controlled activities include possessing, handling or using, producing, storing, permitting access to, transferring, importing or exporting, releasing or otherwise abandoning, and disposing of a human pathogen or toxin.
Regardless of whether a material or activity is excluded from the HPTA or exempted from requiring a licence, the importation and transfer of animal pathogens, infected animals, animal products (e.g., cream, milk, eggs) or by-products (e.g., blood, serum, tissues), or other organisms carrying an animal pathogen or part of one (e.g., toxin) is regulated under the Health of Animals Act (HAA) and the Health of Animals Regulations (HAR) and requires an animal pathogen import permit or transfer authorization issued by the PHAC or the Canadian Food Inspection Agency (CFIA).Footnote 3 Footnote 4
The sections below clarify the types of materials that may be handled in a diagnostic facility that are excluded from the HPTA and the activities involving human pathogens and toxins that are exempted from requiring a licence.
1.1 Human Pathogens and Toxins Excluded from the HPTA
Notwithstanding other exclusion criteria (i.e., a drug in dosage form or a human pathogen or toxin contained in such a drug), the HTPA does not apply to a human pathogen or toxin that is in an environment in which it naturally occurs, provided it has not been cultivated (e.g., cultured) or intentionally collected or extracted (e.g., centrifugation, chromatography) [HPTA 4(a)]. Human pathogens and toxins are considered to be in their natural environment in primary specimens (e.g., blood, plasma, swabs, urine, fecal samples, cerebrospinal fluid, tissue, milk) collected from patients who are infected with a human pathogen or have been exposed to a toxin. Activities with primary specimens (e.g., diagnostic test to identify the cause of an infection) that do not increase the quantity or concentration of the pathogen, such as those designed to detect proteins, antibodies, or nucleic acids are also excluded from the HPTA. There are no legal obligations under the HPTA for facilities where only these activities are conducted; nonetheless, the information provided in this guideline can be used as a reference for best biosafety practices to protect the health and safety of personnel and the community.
1.2 Exemption from HPTA Licensing Requirement for Identification Activities with a Human Pathogen
Under subsection 27(1) of the HPTR, a person who performs diagnostic testing or laboratory analyses with a human pathogen that is not a prion or a prescribed human pathogen (i.e., security sensitive biological agent [SSBA]) does not require a licence, provided that:
-
they do not cultivate (e.g., culture) or otherwise produce a human pathogen.
For example, extraction of nucleic acids from blood or plasma for subsequent polymerase chain reaction (PCR) analysis; or
-
if there is any production, it is done using a sealed container that prevents the pathogen's release and is decontaminated before its disposal or reuse (i.e., the container remains sealed until decontamination has been performed).
For example, inactivating a pathogen in a closed culture vial by a method verified to be effective (e.g., heat treatment, adding chemicals through a septum cap) prior to subsequent manipulations.
Petri dishes or culture tubes may be considered sealed containers if they are inoculated, sealed to prevent the release of the pathogen (e.g., with a sealing film), incubated, examined for growth, and then decontaminated without opening or proceeding to any secondary cultures.Footnote 5 A licence is also not required for individuals who, in the process of carrying out laboratory analyses or diagnostic testing, handle quality control samples or proficiency panels containing infectious RG2 or RG3 human pathogens that mimic primary specimens, and these samples are used to confirm the continued accuracy of diagnostic assays (e.g., to calibrate an instrument, determine the performance of laboratory tests or measurements, monitor a laboratory's continued proficiency).Footnote 5 If activities with human specimens, quality control samples, or proficiency panels are not performed in accordance with the criteria indicated in HPTR 27(1), such activities require a licence allowing controlled activities with the regulated material. When such activities are performed without the appropriate licence, the diagnostic laboratory is considered in inadvertent possession of a regulated material once the pathogen has been identified, which requires notification to the PHAC. As an inadvertent possession can cause an exposure incident, the PHAC must also be notified of a potential exposure incident.
Facilities exempted from the licensing requirement are still regulated under the HPTA, and as such, must take all reasonable precautions to protect the health and safety of the public against the risks posed by activities with human pathogens and toxins [HPTA 6]. These facilities may be subject to inspection by the PHAC to verify whether all reasonable precautions have been taken. The practices outlined in this guideline can be implemented in exempted facilities to demonstrate that reasonable precautions are taken to protect the health and safety of personnel and the community. As best practice, exempted facilities can also demonstrate this requirement by following the applicable physical containment requirements and operational practice requirements specified in the Canadian Biosafety Standard (CBS).Footnote 6 Additional biosafety guidelines from the PHAC are also available to further support exempted facilities.Footnote 7
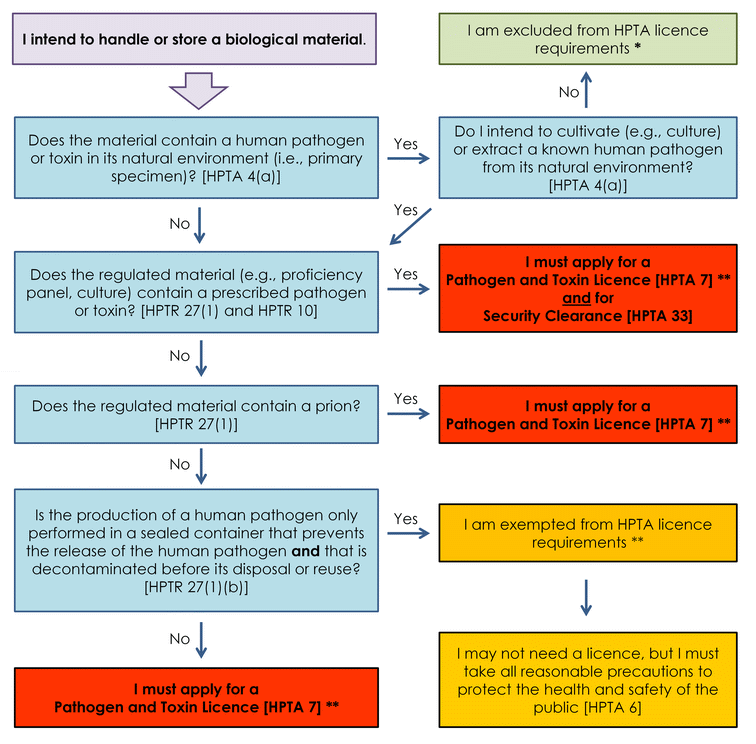
The decision tree presented in Figure 1-1 clarifies when the HPTA and the HPTR are applicable to diagnostic testing activities with a human pathogen, depending on the type of activity conducted within the facility.
Figure 1-1: Legislative oversight of diagnostic testing activities with a human pathogen under the HPTA and the HPTR
- Figure 1-1 note *
-
HAR requirements may apply if the human pathogen is also an animal pathogen (refer to Appendix A). I must also inform the PHAC without delay of an inadvertent production of a human pathogen or toxin. [HPTA 12(2)]
- Figure 1-1 note **
-
When the human pathogen is an animal pathogen:
- a permit issued by the CFIA is required if the animal pathogen causes an emerging animal disease (EAD) or a foreign animal disease (FAD);
- a Pathogen and Toxin Licence issued by the PHAC that includes a terrestrial animal pathogen permit is required if the material is being imported or has been imported (under the HAR).
Figure 1-1 - Text Description
The figure is a decision tree indicating when diagnostic facilities must apply for a Pathogen and Toxin Licence and when they are excluded from the Human Pathogens and Toxins Act (HPTA) or are exempted from the HPTA licence requirement and do not need to apply for a licence.
A facility where the biological material handled contains a human pathogen or toxin in its natural environment (i.e., primary specimen) and where there is no intent to cultivate (e.g., culture) or extract a known human pathogen from its natural environment is excluded from HPTA licence requirements [HPTA 4(a)]. Nonetheless, HAR requirements may apply if the human pathogen is also an animal pathogen (refer to the figure in Appendix A). The PHAC must also be informed without delay if there is inadvertent production of a human pathogen or toxin.
When the biological material handled contains a human pathogen or toxin that is not in its natural environment or the human pathogen or toxin is in its natural environment (i.e., primary specimen) but there is the intent to cultivate (e.g., culture) or extract it from its natural environment, a Pathogen and Toxin Licence and Security Clearance are required if the regulated material (e.g., proficiency panel, culture) contains a prescribed pathogen or toxin [HPTA 7, HPTA 33, HPTR 27(1), and HPTR 10].
When the biological material handled contains a human pathogen or toxin that is not in its natural environment or the human pathogen or toxin is in its natural environment (i.e., primary specimen) but there is the intent to cultivate (e.g., culture) or extract it from its natural environment, a Pathogen and Toxin licence is required if the regulated material contains a prion [HPTA 7 and HPTR 27(1)].
When the biological material handled contains a human pathogen or toxin that is not in its natural environment or the human pathogen or toxin is in its natural environment (i.e., primary specimen) but there is the intent to cultivate (e.g., culture) or extract it from its natural environment, a Pathogen and Toxin licence is required if the production of the human pathogen is not performed in a sealed container that prevents the release of the human pathogen and can be decontaminated before its disposal or reuse [HTPA 7 and HPTR 27(1)(b)].
When the biological material handled contains a human pathogen or toxin that is not in its natural environment or the human pathogen or toxin is in its natural environment (i.e., primary specimen) but there is the intent to cultivate (e.g., culture) or extract a known human pathogen from its natural environment, a facility may be exempted from HPTA licence requirements if the production of the human pathogen is performed in a sealed container that prevents the release of the human pathogen and can be decontaminated before its disposal or reuse [HPTR 27(1)(b)]. While a facility may not require a licence, all reasonable precautions must be taken to protect the health and safety of the public [HPTA 6].
In all the scenarios in the decision tree, if a facility must apply for a Pathogen and Toxin Licence or is exempted from HPTA licence requirements, and the human pathogen handled is an animal pathogen:
- a permit issued by the CFIA is required if the animal pathogen causes an emerging animal disease (EAD) or a foreign animal disease (FAD);
- a Pathogen and Toxin Licence issued by the PHAC that includes a terrestrial animal pathogen permit is required if the material is being imported or has been imported (under the HAR).
Whether or not regulated under the HTPA and HPTR, pathogens within primary specimens from symptomatic or asymptomatic individuals still pose a biosafety risk to personnel, the community, and the environment. Handling primary specimens as though they contain, at a minimum, an RG2 pathogen will protect against exposure and release.Footnote 8 This can be achieved by handling such samples in a facility that meets the containment level 2 (CL2) requirements specified in the CBS. Should there be reason to believe that a primary specimen may contain a higher risk pathogen, having in place physical containment and operational practices appropriate to the risk associated with the pathogen will protect the safety of personnel and the community (e.g., handling specimens suspected of containing a human RG3 pathogen at CL3).
1.3 Scope
The Human Diagnostic Activities guideline provides guidance for the safe handling of samples collected from humans who may be infected with an RG2, RG3, or RG4 human pathogen. It also provides guidance for managing risks associated with personnel exposure to infectious material that may lead to transmission in the community, and for preventing release of pathogens into the environment.
This guideline is intended for use by all individuals performing diagnostic testing or laboratory analyses with a human pathogen or toxin that is excluded from the HPTA (i.e., that is in an environment in which it naturally occurs), or performing activities that are exempted from the licensing requirement. This guideline can also serve as a reference for individuals conducting controlled activities under a licence issued by the PHAC to help them meet the requirements specified in the CBS.
The information provided in the Human Diagnostic Activities guideline is intended as guidance only to enhance biosafety within facilities where activities for the diagnosis of human infectious diseases are performed, and is not to be interpreted as requirements.
1.4 How to Use the Human Diagnostic Activities Guideline
A detailed list of all abbreviations and acronyms used throughout this guideline is located at the beginning of this document. Each word or term is spelled out upon its first use in the guideline, with the abbreviation immediately following in brackets. After its initial definition, the abbreviation is used exclusively throughout the remainder of the document. A comprehensive glossary of definitions for technical terms is located in Chapter 6 of this document. Words defined in the glossary appear in bold type upon first use in the guideline. The list of references as well as other resources is provided in Chapter 7.
Chapter 2 - Risks Associated with Diagnostic Activities
Biosafety involves the consistent application of safety measures (i.e., physical containment features and operational practices), which are crucial to prevent harm to personnel, the community, and the environment resulting from exposure to, or release of, pathogens and toxins handled within a facility. This is achieved through the implementation of a comprehensive biosafety program that includes elements such as training, medical surveillance, emergency response, and standard operating procedures (SOPs) for safe work practices. The appropriate mitigation measures for a given pathogen, containment zone, work area, or procedure are based on risk assessments, which are the foundation for all components of a biosafety program.
Facilities where laboratory analyses or diagnostic testing involving human pathogens are performed often meet stringent requirements or standards for quality and safety management (e.g., through oversight by external accreditation or certification bodies).Footnote 9 Footnote 10 Footnote 11 These quality control and safety systems often encompass biosafety considerations (e.g., personal protective equipment [PPE], SOPs for safe work practices, adherence to good microbiological laboratory practices and procedures), and as such, these facilities may already meet some of the requirements specified in the CBS.Footnote 6 Footnote 12 Footnote 13
Additionally, routine practices, universal precautions, and standard precautions, which take the approach of treating all specimens of blood, body fluid, or tissue as though they contain a human pathogen, are employed in many diagnostic facilities, further enhancing biosafety within these facilities.Footnote 8 Footnote 14 Footnote 15 Footnote 16
2.1 Inadvertent Possession
The inadvertent possession (including possession resulting from inadvertent production) of a pathogen can occur in any diagnostic laboratory. Where diagnostic testing is conducted in an unlicensed facility, opening a sealed culture container prior to its decontamination is considered an inadvertent possession when an RG2, RG3, or RG4 pathogen has been identified. Inadvertent possession can also result from a controlled activity with an SSBA pathogen or prion. In each of these cases, the activity no longer meets the criteria for the licensing exemption [HPTR 27(1)], and the activity must be reported without delay to the PHAC as an inadvertent possession of a human pathogen [HPTA 12(2)]. Reporting is to occur via the biological safety officer (BSO) or, in unlicensed facilities, via an appropriate internal authority. Additionally, within 30 days, the pathogen must be appropriately disposed of (i.e., decontaminated) or transferred to a facility licensed to handle the pathogen [HPTR 4(1)(f)].
Depending on the situation, opening a sealed culture containing a pathogen with inadequate biosafety precautions (e.g., at a lower containment level, without adequate PPE, outside a biological safety cabinet [BSC]) may also have to be reported to the PHAC as a possible exposure [HPTA 13]. While it is not a requirement at CL2, a record of all individuals who entered the laboratory at the time of an incident will help identify those who may have been at a risk of exposure. Maintaining such a record is particularly important since the identification of the pathogen may not be known for several days after it was handled.
If a licence holder has reason to believe that an incident involving a human pathogen or toxin that is in their possession has, or may have, caused disease in an individual, the licence holder must inform the PHAC without delay and submit all information available [HPTA 13].
2.2 Laboratory-Acquired Infections/Intoxications
Laboratory-acquired infections/intoxications (LAIs) in diagnostic laboratories present a risk to individuals as well as to public health (i.e., via further transmission within the community). Historically, in Canada, the absence of a structured reporting framework has resulted in a lack of precise, and likely underreported, data on LAIs in diagnostic and clinical laboratories.Footnote 17 Identifying the source of LAIs can be difficult due to:
- variable routes of exposure, including direct contact with the pathogen (e.g., percutaneous inoculation, inhalation of aerosols) or indirect contact (e.g., contact between mucous membranes and contaminated items);Footnote 18
- the delay between exposure and manifestation of clinical symptoms or signs of infection (i.e., the incubation period); or
- asymptomatic infection.
Often the direct cause of an LAI remains unknown, indicating that the contributing incident (e.g., containment failure, aerosol generation, contact of contaminated hands with eyes, failure to recognize the pathogen, failure to follow SOPs) went unnoticed.Footnote 19
Based on scientific literature, it is estimated that the incidence of LAIs ranges from 1.4 to 4.0 per 1000 clinical laboratory personnel; approximately 45% of symptomatic LAIs reported between 1979 and 2004 were associated with clinical laboratory activities, and 99% of the infections were of bacterial or viral origin.Footnote 17 In a review of viral infections among laboratory personnel and health care workers, inhalation of infectious aerosols represented the main route of infection for laboratory personnel, most likely due to the predominance of work procedures with the potential of generating infectious aerosols (e.g., centrifugation, pipetting).Footnote 20 Alarmingly, inhalation of infectious aerosols represents a route of infection for viruses that are not naturally transmitted via aerosols or inhalation (e.g., arboviruses, bloodborne viruses, viruses normally transmitted percutaneously).Footnote 17
From the Canadian perspective, the PHAC reported that 46% of all incident notifications received between 2016 and 2019 occurred in hospital laboratories or within the public health sector.Footnote 21 Footnote 22 Footnote 23 Footnote 24 While the majority (n=218) of reported incidents in that period involved exposure only, twenty-one incidents led to suspected (n=16) or confirmed (n=5) LAIs. In total, the incidents reported from 2016-2019 resulted in 539 individuals being exposed, with the two most common routes of exposure being inhalation and inoculation with needles or sharps.
Although the identity of the pathogen causing an infection in a patient seeking medical treatment may be suspected (e.g., based on the clinical presentation, factors such as patient travel and exposure to an infected person), its identity will remain unknown until laboratory analysis or diagnostic testing has been completed. Misidentification (e.g., of an RG3 pathogen as an RG2) during preliminary diagnostic assays may be a contributing factor leading to personnel unknowingly handling pathogens without appropriate precautions (e.g., RG3 pathogen handled at CL2, inadequate PPE). The risks of contracting LAIs in diagnostic facilities can also be associated with personnel errors caused by increased stress, time pressures, and high workload rather than unsafe work practices or inadequate training.Footnote 25 Footnote 26 The continued occurrence of LAIs in diagnostic laboratories highlights the importance of conducting local risk assessments (LRAs) to prevent the exposure of personnel to human pathogens as well as the inadvertent release of pathogens from containment.Footnote 25
2.3 Pathogen Risk Assessments
Microorganisms are classified into one of four risk groups (i.e., RG1, RG2, RG3, RG4) based on the outcome of a pathogen risk assessment, which evaluates the organism's inherent characteristics that contribute to the risk it poses to an individual human or animal, and to public health and the animal population. Risk assessments on well-characterized pathogens have been developed into Pathogen Safety Data Sheets (PSDS) by the PHAC and are available on the Government of Canada website.Footnote 27 In addition, the ePATHogen - Risk group database can be consulted to find risk group classifications of thousands of human and animal pathogens.Footnote 28 The PHAC proactively performs pathogen risk assessments on new and emerging pathogens. When the data obtained from such risk assessments indicate that specific physical containment or operational practices are required to work safely with a new or emerging pathogen, Biosafety Advisories are developed and the information is communicated to regulated parties.
2.3.1 Containment Level
Containment levels describe the minimum physical containment and operational practices that a containment zone (i.e., an identified physical area that meets the requirements for a specified containment level) requires for the safe handling of pathogens or toxins. There are four containment levels ranging from a basic laboratory for work with biological material (i.e., Containment Level 1 [CL1]) to the highly sophisticated facilities for work with the highest risk pathogens (i.e., CL4). The containment level assessment takes into account the pathogen risk assessment and the risks associated with containment zone activities. In general, the risk group of a pathogen is the same as the containment level in which it must be handled (i.e., RG2 pathogens are typically handled at CL2). Many of the physical containment requirements and operational practice requirements at CL3 are aimed at reducing the risks associated with airborne or aerosol-transmitted pathogens. As such, certain activities involving RG3 pathogens not known to be transmissible by inhalation, or activities that are of lower risk for aerosol transmission (e.g., pathogen identification activities), can sometimes be performed at a lower containment level (e.g., CL2). In such cases, the PHAC and the CFIA develop Biosafety Directives to clarify containment requirements.Footnote 29
2.4 Local Risk Assessments
LRAs are site-specific risk assessments that identify hazards for activities with a pathogen or toxin. They are used to identify and quantify risks and determine appropriate mitigation measures (e.g., safe work practices), which are then incorporated into SOPs to control the risk to personnel and to prevent the release of pathogens. LRAs examine all work activities (e.g., procedures with pathogens and toxins), and may support the broader overarching risk assessment. LRAs that are sufficiently generic are able to assess a vast range of potential risks associated with the type of specimen analysed, the pathogens (and their risk groups) that may be encountered, and the diagnostic activities performed.Footnote 30 The PHAC and the CFIA have developed guidance for performing LRAs.Footnote 31
An effective LRA will consider the unique characteristics of the pathogen (e.g., route of infection, resistance to medication, ability to evade available vaccines) and activity-related hazards (e.g., procedures that may generate aerosols) in order to identify effective mitigation measures. Many of the physical containment requirements and operational practice requirements specified in the CBS are intended to prevent the creation of aerosols, contain aerosols, and protect personnel. Taking into consideration activities likely to produce aerosols (e.g., opening liquid cultures, pipetting, centrifuging, vortexing, homogenizing, scraping) can help identify physical and operational measures to mitigate the risk.Footnote 32
2.4.1 Pathogen Identification Activities and Diagnostic Testing Involving Pathogens and Toxins
Diagnostic facilities encounter many different types of specimens and cultures, each presenting unique risks. In addition, procedures performed in a diagnostic facility will influence the associated risk and are taken into account during containment level assessments.
2.4.1.1 Activities with inactivated biological material
Inactivated biological material means any biological material that has been inactivated using a validated and routinely verified method. Examples of inactivation methods include heat, chemicals, and irradiation. The killing or inactivation process renders the sample free of pathogens and unlikely to be infectious. Inactivation using an effective, validated, and routinely verified method must be performed at the containment level required for the pathogen and may only be performed at a lesser containment level if the culture container remains closed during the inactivation process (e.g., if the culture of an RG3 pathogen was performed in a sealed vessel at CL2, decontamination can also be performed at CL2 provided the vessel remains sealed). Once the material has been inactivated, subsequent activities with the inactivated material are not regulated by the PHAC or the CFIA. Examples of activities with inactivated material include antigen assays, reverse transcriptase assays, and nucleic acid extraction. Activities with material (e.g., DNA) extracted through physical methods (e.g., cell lysis through shearing or French press) are also not regulated by the PHAC or the CFIA when the extracted material is not infectious.
2.4.1.2 Non-propagative identification activities with primary specimens
Primary specimens are biological fluids (e.g., whole blood or components, urine, feces, milk, saliva, sputum, bronchial lavage, cerebrospinal fluid, swabs), cells, or tissues collected directly from humans or naturally exposed animals, or material collected from the environment, generally to identify a pathogen, diagnose a disease, or for monitoring purposes.
Primary specimens generally contain much lower concentrations of pathogens compared to laboratory cultures. As such, primary specimens containing a human pathogen are excluded from the HPTA, unless a human pathogen or toxin present in the specimen has been cultured, or intentionally collected or extracted. While handling primary specimens does not require a licence issued by the PHAC (unless a human pathogen has been cultivated or intentionally collected or extracted from the specimen), the importation or the transfer of primary specimens containing an animal or zoonotic pathogen requires an animal pathogen import permit issued by the CFIA or a licence issued by the PHAC.
An LRA may be used to determine if activities with primary specimens can be safely performed at CL2 (e.g., handling pathogens unlikely to be transmitted via aerosols, using methods unlikely to produce aerosols). Handling all primary specimens as though a suspected pathogen is present (i.e., with the appropriate physical containment and operational practices), and adhering to good microbiological laboratory practices and procedures in work areas where primary specimens are handled will protect against exposure to any pathogen that may be present in the specimen, and the release of pathogens from the laboratory.Footnote 8
Examples of non-propagative identification activities with primary specimens include complete blood count, blood chemistry tests, enzyme-linked immunosorbent assay (ELISA), centrifugation of primary specimens (e.g., to separate plasma, not to pellet a pathogen), and nucleic acid extraction or amplification.
2.4.1.3 Propagative in vitro activities
When a human pathogen is propagated (e.g., cultured) or concentrated from a primary specimen, it is no longer in its natural environment and therefore falls within the scope of the HPTA. Activities involving these pathogens require a licence issued by the PHAC, unless specifically exempted from the licensing requirement [i.e., in accordance with HPTR 27(1)(b)]. The importation or the transfer of cultures of animal pathogens is regulated by the PHAC or the CFIA under the HAA and HAR. If the propagated, concentrated, or isolated pathogen or toxin is an SSBA, additional biosecurity requirements apply and include an HPTA Security Clearance.
Propagative in vitro activities include activities that involve:
- propagating a pathogen by culturing, including stock cultures of clinical isolates or pathogen reference strains, and diagnostic cultures from which a pathogen has been cultivated or intentionally collected or extracted (i.e., the pathogen from the specimen), and processing of such cultures for packaging and distribution to laboratories.
- concentrating a pathogen by various procedures, for example by centrifugation, filtration, or chromatography.
Propagating pathogens increases the concentration and number of organisms, thereby greatly increasing the infectivity of the sample. Propagative in vitro activities with RG2, RG3, and RG4 pathogens and toxins are regulated by the PHAC. When a pathogen in a sample is to be identified (or confirmed) via propagative activities, it is good practice to mitigate risks by performing all activities at the containment level required for the suspected pathogen.
2.5 Additional Considerations for Local Risk Assessments
Certain activities in diagnostic facilities inherently carry a greater risk. Appropriate mitigation measures can be implemented by evaluating the risks associated with these activities through an LRA. The following are examples of hazards that may be encountered during diagnostic activities.
2.5.1 Handling Inactivated Material
Many commonly used laboratory methods are believed to inactivate pathogens. Examples include methods for extracting proteins or nucleic acids, for fixing tissues (e.g., formalin), and for lysing serum samples for antigen or antibody assays. As not all methods will be effective at inactivating all pathogens, it is important that the inactivation method be validated and verified routinely for its effectiveness against the pathogen being handled. Alternatively, the samples could be handled as though they remain infectious and, therefore, activities are conducted at the containment level appropriate for the pathogen.
The ability of a method to inactivate a pathogen must be validated and routinely verified in-house (e.g., by routinely culturing an aliquot of the inactivated material) or based on published best practices (e.g., effectiveness of bleach or lysis method against specific microorganisms). However, published practices should be carefully weighed. Laboratory methods thought to inactivate a broad range of organisms may have limited effect against specific pathogens. For example, material fixed in formalin or embedded within paraffin may remain infectious following the application of a routine inactivation method. This failure of inactivation could be due to the incomplete permeation of a tissue or the presence of a pathogen that is resistant to the effects of the chemical (e.g., prions, spores, Mycobacterium tuberculosis).
2.5.2 Sniffing Culture Plates
While less and less common in the era of automation, sniffing culture plates continues to be practiced among experienced microbiologists due to the characteristic and identifiable odours produced by certain microorganisms.Footnote 33 Footnote 34 This method of identification is strongly discouraged as it has been associated with numerous LAIs, most notably Brucella spp. infections.Footnote 17 Footnote 35 Footnote 36 Footnote 37
2.5.3 Handling Liquid Cultures
Liquid cultures generally contain higher concentrations of pathogens than do primary specimens. As such, they also present a greater risk for spills and aerosol production. Considerations to mitigate the risks associated with liquid cultures include the use of plastic or plastic-coated containers, the use of screwed on vented (filtered) caps, securing flasks in shaking incubators, opening containers only in a BSC, and using incubators with containment features (e.g., seals, HEPA filter).
2.5.4 Storing Samples in Liquid Nitrogen
In addition to the occupational hazards associated with the use of liquid nitrogen, biosafety risks include explosion of cryogenic vials containing a pathogen and contamination of liquid nitrogen by broken, leaking, or improperly decontaminated vials. If liquid nitrogen has seeped into a vial or if the vial remains sealed, the pressure within the vial can be sufficient for it to explode during the thawing process. Vials can be frozen and stored in the vapour phase to prevent this from occurring. When thawing, wrapping vials with gauze or paper and placing them in sealed, heavy-walled containers will contain any material and aerosols dispersed in the event that a tube explodes.
Pathogens contaminating liquid nitrogen can survive and lead to surface contamination of other vials stored in the same cryogenic storage tank. They can also lead to the potential formation of infectious aerosols from boiling liquid nitrogen.Footnote 38 Properly sealing liquid nitrogen-certified cryovials, decontaminating the surface of the vials prior to storage, and storing them in the vapour phase will mitigate the risks associated with the use of liquid nitrogen storage.Footnote 37
2.5.5 Handling Sharps and Needles
While data on LAIs is incomplete, percutaneous incidents represent the most common route of occupational exposure to pathogens among health care workers. Sharps and needles can cause wounds, cuts, or punctures resulting in possible exposure to pathogens.
If possible, it is best to avoid the use of sharps and needles altogether in the laboratory. If there is no appropriate substitute available, precautions can be taken, such as never bending, shearing, breaking, or re-capping needles, or using cut- or puncture-resistant gloves. Placing sharps and needles in puncture-resistant containers designed for sharps that are located close to the point of use will mitigate the risk of incidents during their disposal. Further information regarding sharps containers can be found in the National Standard of Canada published by the Canadian Standards Association (CSA) CSA Z316.6, Sharps Injury Protection - Requirements and Test Methods - Sharps Containers.Footnote 39
2.5.6 Handling Suspected Prions
Prions are infectious proteins that cause a group of progressive neurodegenerative diseases in humans and animals known as transmissible spongiform encephalopathies (TSEs). There are very few documented cases of prion LAIs; however, this may be biased by the long incubation period between the time of infection and symptomatic presentation, which can be up to 30 years.Footnote 40 Footnote 41 The most likely route of transmission of infectious prions to personnel is through accidental inoculation with contaminated instruments, but transmission via exposure of mucous membranes to aerosols (e.g., by inhalation) or splashes may be possible.Footnote 42
Although most infectious prions are classified as RG3 human pathogens, they can be safely handled in a CL2 facility with additional physical containment features and operational practices. Laboratory analyses (e.g., blood counts, blood chemistry, protein levels) with primary specimens that may contain prions are excluded from the HPTA, unless a human pathogen or toxin is cultivated or intentionally collected or extracted from these samples. Nevertheless, the risk of exposure and infection remains, as does the difficulty of decontaminating areas where such specimens are handled. The PHAC's Infection Control Guidelines - Classic Creutzfeldt-Jakob Disease in Canada - Quick Reference Guide 2007 can be consulted for practices to prevent exposure and reduce the risk of contamination.Footnote 43
Prions are very stable in the environment and are resistant to standard decontamination procedures and processes. Efficient decontamination requires high temperatures (e.g., 132°C), strong alkali conditions, or a combination of heat and chemicals. As such, the use of automated equipment for diagnostic activities involving cerebrospinal fluid suspected to contain prions is not recommended due to the extensive procedures required to decontaminate prions.Footnote 44 Footnote 45 A dedicated laboratory area or room, as well as equipment and PPE (preferably disposable), for handling specimens potentially containing prions will minimize the surfaces and materials requiring decontamination.Footnote 46
With the exception of prion specimens that are excluded from the HPTA (i.e., in their natural environment), the handling and storing of prions requires a licence issued by the PHAC and must be in accordance with the applicable requirements specified in the CBS.Footnote 6
2.5.7 Working with certain Laboratory Equipment
In some cases, the equipment used in diagnostic laboratories can increase the risk of exposure, and should be documented in an LRA. Consideration may also be given to the decontamination of liquid waste produced by laboratory equipment, prior to release into the sanitary sewer. The following are examples to consider when assessing equipment used in a facility where diagnostic activities are performed.
2.5.7.1 Matrix-Assisted Laser Desorption-Ionization Time-of-Flight Mass Spectrometry
The use of matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) has improved the speed, accuracy, and cost-effectiveness of bacterial identification in clinical diagnostic laboratories.Footnote 47 However, the increasing use of MALDI-TOF MS has also raised some important biosafety concerns.
The misidentification of a higher risk (i.e., RG3) pathogen as a genetically‑related lower-risk pathogen (i.e., RG2) has resulted in potential laboratory exposures of personnel performing confirmatory testing at CL2.Footnote 48 This misidentification can occur when the reference libraries used for MALDI-TOF MS do not contain the mass spectra of the higher risk pathogens.Footnote 49 The misidentification of genetically‑related pathogens may be avoided by including the mass spectra of higher risk organisms in the spectral library used as reference.Footnote 50 If misidentification of RG3 pathogens as RG2 pathogens remains a possibility, SOPs may be developed, based on LRAs, to mitigate the risks of handling a misidentified RG3 pathogen at CL2.
There is also the potential for incomplete inactivation of pathogens on MALDI-TOF MS target plates. Live cultures applied directly to MALDI-TOF MS target plates as a thin smear may not be completely inactivated by the overlaid matrix solution, particularly in the case of certain sporulating pathogens.Footnote 51 Incomplete inactivation may be of low consequence for an RG2 pathogen handled in a CL2 diagnostic laboratory; however, it becomes a more serious issue when an RG3 pathogen is inadvertently handled at CL2.
2.5.7.2 Automated Analysers
Automated analysers (e.g., for blood counts, blood chemistry, nucleic acid extraction) are commonly used in diagnostic facilities. In order to achieve a high throughput, they often contain parts that move quickly or deliver fluids rapidly, which leads to the potential generation of infectious aerosols. While analysers may have features that contain or minimize the dispersal of any infectious aerosols produced (e.g., closed analyzers), these may not be intended as the sole barrier to limit the risk of exposure, therefore requiring additional precautions for their safe use.Footnote 25 Footnote 37 The ease and method of analyser decontamination is an important biosafety consideration when selecting the appropriate tools for a given task.
2.5.7.3 Vacuum Devices
Devices that create or work under a vacuum (e.g., automated liquid handling devices, plate washers, vacuum pumps) may generate infectious aerosols. Mechanisms, such as in-line filters and disinfectant traps, reduce the risk of pathogen release and internal contamination of the device. The use of plastic-coated or thick-walled flasks can reduce the risk of implosion, though precautions need to be taken to prevent physical damage to equipment subject to pressure differentials (e.g., vacuum flasks, bell jars). The use of a BSC may help contain aerosols generated by activities involving vacuum-assisted aspiration (e.g., aspiration of culture media, use of a plate washer).Footnote 37
2.5.7.4 Shaking Incubators
The motion of a shaking incubator can lead to the production of aerosols. In addition, flasks of liquid cultures may be dropped or fall over in the incubator, leading to spills. Some incubators include containment features, such as seals and HEPA filtration, that prevent contamination of the laboratory and simplify clean-up in the case of a spill or aerosol generation.
Incidents can be avoided by appropriately securing flasks prior to beginning the shaking cycle. Plastic flasks or plastic-coated glass flasks prevent breakage if dropped, and the use of vented, filtered caps that can be screwed on tightly is preferred over gauze or foam stoppers that are more easily dislodged. Following incubation, handling the culture in a BSC prevents the release of any aerosols that may remain in the flask.
Chapter 3 - Considerations for Physical Containment
In facilities where diagnostic testing or laboratory analysis is conducted, facility design (e.g., location of laboratory, surface finishes, access control) and provision of biosafety equipment (e.g., BSCs) are carefully considered and established to prevent personnel exposure to potentially infectious material and to limit the spread of pathogens inside and outside the facility. Basic safety, emergency, and security features are also integrated to protect personnel and the community.
As it is recommended that primary specimens be handled as though they contain, at minimum, an RG2 pathogen, good practice would dictate that facilities where these specimens are handled meet the minimum physical containment requirements for CL2 specified in Chapter 3 of the CBS.
3.1 General Physical Design Features
The principal objective of physical design is to provide a barrier or separation between the area where pathogens and toxins are handled and stored and the surrounding areas. Given the diversity of pathogens, specimen types, and activities, there is no single design that is ideal for all diagnostic facilities. The following are physical design feature considerations for diagnostic facilities.
3.1.1 Segregation of Laboratory Work Areas
Segregation of laboratory work areas from public areas (e.g., reception, administrative areas, offices) and dedicated paper and computer workstations can help to prevent the spread of contamination inside and outside the facility. Segregation measures can take the form of physical partitions such as doors or closable and lockable windows at the perimeter of the laboratory. In some cases, segregation of a laboratory work area from a computer or paperwork area within the same room can be implemented by delimiting the areas with tape. Where there is an opening in the wall that is not suitable for a door (e.g., for a sample receipt counter), a lockable roll-down door or other suitable alternative can be used. The ability to close off the laboratory provides a level of security (e.g., to prevent unauthorized access) and is an important barrier in case of spills of infectious materials that can generate infectious aerosols.
In addition, diagnostic activities with certain human pathogens may present an increased risk for laboratory personnel as well as for public health. For example, inhalation is the primary route of infection of certain RG2 pathogens (e.g., some strains of influenza A virus, varicella zoster virus), and some diagnostic procedures have a high probability of generating infectious aerosols. Having a physically separated space designated for higher-risk procedures or pathogens will reduce the risk of exposure for personnel working in surrounding areas and prevent the spread of contamination. Such an arrangement may also limit the need for additional PPE to those in the segregated space.
3.1.2 Materials and Surface Coverings
The use of materials that are resistant to scratches and are non-absorbent will allow for easy cleaning and decontamination of laboratory furniture and surfaces, including chairs, interiors of drawers, cabinets, and shelves. Examples of such materials include stainless steel, epoxy resin, or chemical resistant plastic laminate for benchtops, and urethane or vinyl for stools and chairs. Unfinished wood is porous and can absorb potentially infectious material, making decontamination virtually impossible. In a diagnostic facility, the use of materials such as unfinished wood (e.g., drawers, shelves, slide racks) should be limited to areas where there is little risk of contamination, based on an LRA. Alternatively, porous materials can be sealed to prevent absorption of contaminated liquids.
3.1.3 Handwashing Sinks
Having sinks near the exit(s) of the work area facilitates handwashing by personnel leaving the area, and where sinks are located outside the area, automated measures (e.g., automatic doors) and SOPs will reduce the risks of personnel inadvertently contaminating other surfaces (e.g., door handles).
Handwashing sinks with "hands-free" capability, such as electronic touchless faucets, foot pedals, or elbow-operated taps, prevent contamination of the faucets and sink area that could lead to recontamination of washed hands.
3.1.4 Primary Containment
BSCs are the most common primary containment device used to prevent the release of infectious aerosols generated during laboratory procedures. When used correctly, BSCs provide protection to personnel and the environment from infectious aerosols and aerosolized toxins. BSCs can also protect the material being handled from contamination. Primary containment devices also include customized enclosures for automated equipment (e.g., plate washers, plate readers, cell analyzers, liquid handling robots) that may generate infectious aerosols.
Given the risks of exposure and infection, including from pathogens not normally transmitted via the airborne route, it is good practice to perform any procedure with the potential of creating an infectious aerosol in a BSC. The type and class of BSC selected will depend on its intended use (e.g., whether volatile chemicals or radionuclides will be handled), based on an LRA.
The use of a BSC alone is not sufficient to eliminate the risk of exposure to pathogens and toxins, or their release. Good microbiological laboratory practices and procedures, use of appropriate PPE, and proper use of the BSC, according to SOPs, are essential.Footnote 52 In situations where a standard BSC is not suitable (e.g., for a microscope or specific piece of equipment), a specialized primary containment device can be used.Footnote 53 Examples of primary containment devices other than BSCs include isolators, centrifuges with sealed safety cups or sealed rotors, fermenters, and glove boxes. Risk assessments will guide the selection and use of a primary containment device appropriate for a particular diagnostic activity.
3.1.5 Centrifuges
Commonly used in diagnostic laboratories, centrifuges carry the risk of generating infectious aerosols, in part as a consequence of vials, bottles, or tubes breaking during processing, but also due to possible contamination outside the vial, bottles, or tubes. Sealed centrifuge cups and rotors provide effective containment when maintained. Proper and regular maintenance of centrifuges (e.g., maintaining or replacing O-rings and other seals) and the use of appropriately rated centrifuge tubes will help prevent exposure and release incidents from occurring. The risk of releasing pathogens can also be reduced by unloading sealed safety cups (or rotors) in a BSC.
Chapter 4 - Operational Practices
Operational practices refer to the administrative (e.g., biosafety program management, training) and procedural (e.g., work practices, PPE, decontamination) controls in place to prevent the inadvertent exposure of personnel to pathogens and potentially infectious material, and the release of pathogens from the diagnostic facility.
As it is recommended that primary specimens be handled as though they contain, at minimum, an RG2 pathogen, good practice would dictate that facilities where they are handled meet the minimum operational requirements for CL2 specified in Chapter 4 of the CBS. The following sections briefly describe operational practice considerations to reduce the risks associated with diagnostic activities.
4.1 Safe Work Practices
Following SOPs for safe work practices when handling infectious material during diagnostic activities helps protect personnel and the community from exposure to pathogens. Safe work practices include the proper use and maintenance of laboratory and biosafety equipment (e.g., centrifuges, BSCs), as well as aspects of general maintenance (e.g., tidiness, avoiding clutter) of the area where diagnostic activities are performed.
Well-documented safe work practices and techniques that are made available to all personnel as part of training programs and are included in SOPs demonstrate that management takes safety seriously and helps promote a culture of safety among personnel.
4.1.1 Good Microbiological Laboratory Practices and Procedures
"Good microbiological laboratory practices and procedures" describe a basic set of safe practices and procedures established in microbiology laboratories, which provide the foundation for biosafety at higher containment levels.Footnote 12 Footnote 13 Personnel can apply them to prevent exposure and contamination of samples and the environment in any work area where laboratory-related activities involving potentially infectious material are performed. They encompass aseptic technique, the proper use of PPE (e.g., gloves, aprons, safety glasses), handwashing, and general cleaning and decontamination, to protect workers from infectious material.
4.1.2 Routine Practices, Universal Precautions, and Standard Precautions
Routine practices, universal precautions, and standard precautions are infection prevention practices developed for health care environments to protect individuals from exposure to potential sources of pathogens.Footnote 8 Footnote 14 Footnote 15 Footnote 16 They aim to prevent the transmission of pathogens through occupational contact with primary subjects (e.g., patients, animals), blood (e.g., whole blood, serum, plasma), and other biological samples (e.g., urine, feces, saliva, milk, tissue samples).Footnote 54
Routine practices are based on five major elements: risk assessments, hand hygiene, PPE, environmental controls (e.g., suitable facilities for the disposal of waste, dirty linen, and sharps), and administrative controls (e.g., training, sharps safety program, aseptic technique).Footnote 8 Many of the elements of good microbiological laboratory practices and procedures are common to both universal precautions and routine practices.
Routine practices, universal precautions, and standard precautions take the approach of treating all patients as though they were infected with a pathogen, and specimens collected from patients, such as blood, body fluid, or tissue, as though they contain a human pathogen. Following these practices and precautions will protect personnel and other individuals from exposure and prevent the potential spread or release of pathogens that may be transmitted from symptomatic or asymptomatic humans or animals.Footnote 8
4.1.3 Sample Receipt
Primary specimens received by diagnostic laboratories are generally supplied with information from the requesting clinician. This information (e.g., requested tests, travel history, exposure) can help assess the risk associated with a sample as it should always indicate the type of material (e.g., urine, swab, blood, feces, cerebrospinal fluid, sputum) and may provide an indication of the human pathogens or toxins suspected to be contained in the sample. In some cases, the information may be incomplete and may not give a good indication of the pathogen(s) that may be present.
Given the uncertain conditions that may have been encountered during specimen collection and transportation or movement to the diagnostic facility, and the possibility that information may be missing, handling primary containers as potentially contaminated will reduce the risk of exposure to personnel and prevent laboratory contamination. Examining all received containers for leaks, loose caps, cracks, and visible contamination of the external surface upon arrival and prior to any activities will confirm the integrity of the container and the specimen. A single damaged or leaking container within a parcel can contaminate the entire shipment and potentially lead to exposure or laboratory contamination. Should a leaking or damaged container be received, decontamination of all potentially contaminated containers and material (e.g., test requisition, shipping manifest, packing material) will reduce the possibility of laboratory contamination and exposure. Documentation and notification of the incident to the appropriate internal or external authority (e.g., supervisor, manager, PHAC, CFIA) will guide improvements to SOPs (e.g., for specimen collection, transportation, and movement) and help prevent similar future incidents.Footnote 37 Within Canada, the transportation of dangerous goods, including infectious substances, is regulated by Transport Canada under the Transportation of Dangerous Goods Regulations (TDGR).Footnote 55
Movement of specimens containing a pathogen or toxin within a facility or building (e.g., from the specimen collection or phlebotomy area to the laboratory, from one laboratory to another in a different containment zone or building) using leakproof bags or containers and a cart will prevent the release (e.g., from a leak, drop, or spill) of pathogens or toxins.
4.1.4 Unidirectional Work Flow
Establishing directional traffic and workflow patterns within the facility will facilitate the movement of personnel and materials from "clean" areas (i.e., areas of lower contamination) to "dirty" areas (i.e., areas of higher contamination) in a manner that minimizes the spread of contamination. This can be applied at all levels, from working in the BSC (e.g., clean pipettes and media on one side, waste on the other) to designating dedicated laboratory rooms or spaces (e.g., sample receipt in one room or space, initial processing in another, and culture and post-culture manipulations in a third).
4.1.5 Hand hygiene
Handwashing is the most effective means for preventing the transmission of infection and the spread of contamination inside and outside the facility as it can eliminate all types of pathogens from the surface of the hands. In general, handwashing is performed in accordance with SOPs:
- after completing a task with primary specimens or infectious material;
- when the hands have been contaminated;
- after the removal of gloves; and
- prior to leaving the diagnostic facility.
While gloves provide protection, they can be porous and wear with use. Washing hands after gloves are removed adds a layer of protection against contamination that may have breached the glove barrier or occurred when removing the gloves. Detailed handwashing instructions can be found in Appendix A.
Alcohol-based hand sanitizers are not as effective as handwashing with soap and water and cannot eliminate all types of pathogens.Footnote 56 However, a hand sanitizer may prove to be a suitable alternative where handwashing sinks are not easily accessible to reduce the spread of contamination until the hands can be washed.
4.2 Biosafety Program Management
The development of biosafety policies and a biosafety program is fundamental in implementing safe work practices and improving safety performance in order to prevent exposure to and accidental release of pathogens. A biosafety program is created to mitigate the risks identified by an overarching risk assessment of the facility and its general activities. A comprehensive biosafety program includes SOPs, a biosecurity plan, a medical surveillance program, a training program, an emergency response plan, and a program for housekeeping and maintenance of the facility and equipment.
4.2.1 Standard Operating Procedures
SOPs are documents that provide detailed, step-by-step instructions for a task, and address facility- or activity-specific biosafety issues. They are introduced during training and reviewed prior to performing a procedure for the first time, to refamiliarize personnel with procedures performed infrequently, and whenever the SOP is amended. SOPs may be examined by internal or external auditors and can facilitate evaluation of personnel compliance with program requirements. Storing SOPs where they are easily accessible to all facility personnel, whether in paper or electronic form, will facilitate personnel awareness of, and compliance with, the facility's implemented safe work practices (e.g., use of PPE, entry and exit, waste management).
4.2.2 Medical Surveillance Program
The medical surveillance program aims to prevent and detect employee illnesses resulting from an exposure to a human pathogen or toxin. The medical surveillance program considers the pathogens that may be encountered during diagnostic activities and identifies appropriate mitigation measures. While programs vary depending on the size, structure, and complexity of the facility, components that may be included are pre-placement medical examinations, serum screening or storage, SOPs for post-exposure response (e.g., prophylaxis), and immunization.
A pre-placement medical evaluation can identify any underlying medical conditions (e.g., suppressed immunity, pregnancy, diabetes, liver or kidney disease) that may increase the risk of infection and harm associated with the activities planned. The assessment may take the form of an interview with the institutional occupational health care provider or an analysis of a personal medical history questionnaire. A serum sample can be collected before personnel begin working in the laboratory (and possibly on a scheduled basis thereafter) to determine pre-existing immunity or infection, and in some cases to establish baseline seroreactivity for comparison following a potential exposure (e.g., to detect an increase in antibody titres following laboratory exposure to varicella zoster). The medical evaluation provides an opportunity to inform personnel of all risks associated with the human pathogens that may be handled as part of diagnostic activities, as well as the associated symptoms of disease caused by the pathogens. Given the wide range of pathogens that may be encountered in a clinical diagnostic setting, it may not be possible or practical to advise personnel of all potential pathogens that they may encounter. It may be more reasonable to inform personnel of the symptoms of key concern, for example, when unusual pathogens have been identified in the laboratory (e.g., fungus causing symptoms of pneumonia).
The medical surveillance program also serves to inform personnel of available preventive measures (e.g., vaccinations) or treatments (e.g., antibiotics) against the pathogens handled and stored within the facility, along with the risks and benefits of the measures. The steps to follow in the event of a potential exposure, including appropriate first aid measures, incident reporting, timely post-exposure prophylaxis, and medical treatments are addressed in the emergency response plan and communicated to personnel in SOPs and training.
4.2.2.1 Vaccination
Vaccines are highly regulated complex biological products designed to induce an effective, protective immune response. Commercially available vaccines protect against viral and bacterial pathogens, and can be considered a prophylactic approach to complement existing physical and operational controls where vaccination may mitigate the consequences of a pathogen exposure (e.g., influenza, rabies, hepatitis B). Informing personnel about the availability of vaccines allows them to discuss the risks and benefits with their health care provider, and to make an educated decision on whether or not to be vaccinated prior to commencing work with the pathogen. In some situations, facilities may decide to make vaccination mandatory prior to the handling of certain pathogens.
4.2.3 Training Program
The training program is based on a training needs assessment and encompasses both theoretical and practical approaches, as well as assessment of knowledge and skills (e.g., supervision). It represents an essential element to the success of the biosafety program. The occurrence of incidents is minimized when personnel are aware of the risks associated with the specimens and pathogens they handle, and are knowledgeable about the practices and tools available to protect them.
The review of personnel knowledge and their training progress provides an opportunity for the ongoing assessment of individual understanding of biosafety procedures and adherence to the procedures on which they have received training. Maintaining a record of the training (e.g., completed, expected, required) for all personnel is essential for determining future training needs, including refresher training.
4.2.4 Emergency Response Plan
The emergency response plan outlines the procedures to follow in an emergency and is essential to protect the health of personnel and the community, the property, the environment, and to prevent the release of pathogens and toxins. The emergency response plan is based on the overarching risk assessment and will identify foreseeable emergency scenarios and describe response measures proportional to the scale and nature of the emergency scenario. Emergency situations may include incidents or accidents, medical emergencies, biological spills, power failures, failure of primary containment devices (e.g., BSC), or natural disasters. The emergency response plan may also include contingency plans to continue operations in a safe and secure manner.
Spills are the most common type of laboratory incident with the potential for exposure of personnel to pathogens and toxins, and release from containment. Spills can contaminate surfaces, equipment, samples, and personnel. As such, having SOPs outlining spill response procedures will help personnel to quickly respond to spills in a safe and appropriate manner.
4.2.5 Incident Reporting
The investigation of any incident involving a human pathogen or toxin, including accidents, near misses, and other dangerous occurrences, such as inadvertent production, possession, or release of a pathogen or toxin, LAIs, and missing pathogens or toxins helps identify the root cause(s), which leads to the development of corrective measures to prevent future occurrences. This process is facilitated through the development and maintenance of internal procedures that describe how to define, record, report, and investigate incidents involving infectious material or toxins.
In a licensed facility, the BSO is the primary point of contact for the PHAC and the CFIA, and is also responsible for assisting in incident investigations. The immediate reporting of all incidents involving pathogens or toxins to the appropriate internal authority (e.g., supervisor, BSO) will help establish an appropriate response and expedite the initiation of an investigation. Establishing a non-punitive approach to incident reporting will encourage personnel to report incidents.
In facilities subject to the HPTA (i.e., licensed facilities as well as those exempted from the licensing requirement), reporting incidents (both internally and to the PHAC) is a requirement, as is the timely notification of the PHAC as there is an obligation to inform the Minister [HPTA 12, 13, 14, and 15]. This is meant to improve the timeliness of a public health response should it be needed, and to help maintain the accuracy of information on laboratory exposures and LAIs. While pathogens in their natural environment (e.g., diagnostic specimens) are excluded from the HPTA, it is recommended that any incident in a licensed or unlicensed facility that involves such specimens be reported to the PHAC on a voluntary basis if the identity of the pathogen is known. Even if no infection, exposure, or release has resulted, documenting all incidents (including near misses) allows the information to be used to improve procedures and as a measure of the efficacy of the biosafety program.
4.2.6 Measuring Program Effectiveness
The continual review and improvement of the biosafety program by senior management will help maintain its relevance and effectiveness. This can be achieved through regular review of program reports or by comparing achievements (e.g., reduction in number of incidents) with the program's objectives to identify any deficiencies in the program. Any issues identified will lead to the implementation of improvements to the program. This system is commonly known as a Plan-Do-Check-Act management cycle.Footnote 57
Chapter 5 - Decontamination and Waste Management
The effective decontamination of waste, materials, equipment, and surfaces that have come in contact with potentially infectious material or toxins is fundamental in limiting the spread of contamination beyond the work area and facility. Contaminated waste can be decontaminated on-site using decontamination technologies, or transported to a designated facility for decontamination.
5.1 Decontamination
Decontamination is a key component of containment as it renders materials and surfaces reasonably free of pathogens and toxins and, therefore, safe to handle. Failure to decontaminate equipment or implement appropriate decontamination processes may lead to the release of pathogens and toxins from the facility or the exposure of personnel. Effective decontamination may require disinfection, inactivation, or sterilization depending on the circumstances. Decontamination technologies can function by chemical, thermal, or physical (e.g., washing) means, or a combination of these. The more common decontamination methods are described below.
Chemical disinfectants are commonly used for the decontamination of specimen and sample containers, liquids, room surfaces, equipment that cannot be autoclaved, and spills of infectious material. Disinfectants are less efficient at decontaminating infectious material than sterilization, which completely eliminates all living microorganisms, including bacterial spores. The most commonly used chemical disinfectants are chlorine (e.g., bleach [NaOCl; sodium hypochlorite], chlorine dioxide [ClO2]), alcohol (e.g., 70% ethyl or isopropyl alcohol in water), iodine (e.g., aqueous solutions, tinctures, iodophores), phenolics, quaternary ammonium compounds, and hydrogen peroxide. Many of these chemical disinfectants are used alone or in combination in commercially available disinfectants. The selection of disinfectant is based on its ability to effectively decontaminate the pathogen(s) being handled. Organic load, chemical concentration, contact time, temperature, relative humidity, pH, and stability can have a significant impact on the efficacy of a chemical disinfectant.
Inactivation refers to the destruction of biological activity of a pathogen (e.g., virus, prion) or toxin through heat, chemical, or physical means, and is often used prior to downstream activities taking place outside of the containment zone (e.g., nucleic acid extraction or testing, antigen assays).Footnote 58
Thermal decontamination includes dry heat sterilization, composting, liquid effluent decontamination, incineration, and steam sterilization (e.g., using an autoclave). The most common method used for routine decontamination of laboratory waste is autoclaving. The effectiveness of the process depends on time, temperature, and direct steam contact with the infectious agents. Incineration involves burning at high temperatures and is the only technology capable of handling both non-toxic and toxic biological waste (including cytotoxic waste and volatile compounds).
5.1.1 Decontamination of Prions
Prions are particularly resistant to standard thermal and chemical methods of decontamination including boiling, dry heat, formalin, and alcohol treatment.Footnote 59 These methods can marginally reduce infectivity, but few are highly effective at eliminating infectious prions. An LRA can help to determine the best procedures for clean-up and decontamination in situations where prions are encountered in the diagnostic facility. The safest and surest method of decontamination to eliminate the risk of residual infectivity on contaminated instruments and other materials is to destroy them by incineration.Footnote 37
Combinations of thermal and chemical treatment processes can be used to decontaminate equipment, reusable materials, and waste products not suitable for incineration. Combining both types of processes can achieve greater prion inactivation efficacy than treatment with chemical agents alone. Disposable instruments, PPE, and coverings for work surfaces can be incinerated and avoid the need for less efficient inactivation procedures, which is particularly important as complete inactivation of prions is difficult to achieve.Footnote 37
5.1.2 Validation and Verification of Decontamination
Validation demonstrates that decontamination equipment and methods are effective at decontaminating, inactivating, or eliminating a specific pathogen or toxin, and that the decontamination process is suitable for its intended use and for the given type and quantity of material. For example, the validation of an autoclave cycle can be performed using a representative load (i.e., typical anticipated volume and contents of waste, but consisting of non-contaminated or unused material) and biological indicators placed throughout the load. Validation of commonly accepted methods (e.g., effectiveness of bleach against a species of bacteria) may be based on published best practices.
Verification is the routine monitoring of equipment and processes to confirm they continue to meet the validated parameters. In the case of an autoclave, biological indicators or parametric monitoring devices may be used. For surface decontamination or specimen inactivation, placing an aliquot into culture, according to SOPs, can confirm the absence of growth.
A biological indicator is a standardized population of bacterial spores used to demonstrate effective sterilization conditions in a waste load. Achieving the target level of reduction in viable spores indicates that the decontamination process was effective. Parametric monitoring devices include thermocouples or gauges that capture cycle time, temperature, and pressure to accurately monitor the performance of the decontamination equipment.
5.2 Waste Management
Even after contaminated or biohazardous waste has been effectively decontaminated, it still may not be appropriate to dispose of it in the normal waste stream. Additional waste management considerations or requirements specified by the provincial, territorial, or local (i.e., municipal) authorities may apply and need to be considered when establishing and implementing a waste management program.
Diagnostic facilities are likely to generate both hazardous and non-hazardous solid and liquid waste material, as well as sharps, through their routine activities. The first step in a waste management program is to determine if it is possible to reduce the amount of waste produced, in particular contaminated waste. This can be as simple as minimizing the amount of packaging (e.g., cardboard boxes, packing material) and excess material brought into the diagnostic facility.
Promptly placing all infectious and potentially infectious waste into appropriately labelled, leakproof waste containers will prevent the release of pathogens inside and outside the facility. This will also prevent the release of pathogens during transportation or movement and protect the safety of individuals who handle, clean, and dispose of the waste.
The Canadian Council of Ministers of the Environment (CCME) Guidelines for the Management of Biomedical Waste in Canada describes the recommended minimum practices to follow in the management of biomedical waste, including animal waste, laboratory waste, and sharps waste; however, the CCME guidelines are only enforced where they are adopted by provincial legislation or municipal by-laws.Footnote 60 Local by-laws may be more stringent than the guidelines recommended by the CCME. When developing and implementing a sound waste management program, additional considerations for handling biomedical waste can be found in the standard CSA Z317.10, Handling of Health Care Waste Materials.Footnote 11
In the event that a waste container breaks or leaks, workers handling and disposing of infectious or potentially infectious biological waste may be at risk of exposure to pathogens and toxins. Sharps waste in particular poses a significant risk when mixed with other types of waste, or not properly separated, as it can then become a hidden hazard that can result in sharps-related incidents (e.g., needlestick injuries, inoculation). After use, sharps waste must be safely disposed of directly into a puncture-resistant container in accordance with the standard CSA Z316.6, Sharps Injury Protection – Requirements and Test Methods – Sharps Containers.Footnote 39 Closely following instructions for sharps containers, such as using them according to manufacturer's instructions, keeping the lid securely in place, and never overfilling them so that the lid will remain closed during transport and disposal, will help prevent incidents involving sharps waste.
Infectious and biomedical waste being transported for disposal is regulated in Canada under the TDGR.Footnote 55 In accordance with the TDGR, containers used for the transportation of infectious and biomedical waste must meet requirements of the National Standard of Canada (CAN) published by the Canadian General Standards Board (CGSB) standard CAN/CGSB-43.125, Packaging of Category A and Category B infectious substances (Class 6.2) and clinical, (bio) medical or regulated medical waste.Footnote 61 Biological waste can be stored temporarily prior to disposal. Refrigeration or freezing will help reduce the rate of microbial growth, putrefaction, and smell. Limiting access to storage locations containing potential or confirmed infectious material to authorized personnel will help reduce the risk of incidents resulting from unauthorized access.
Under federal, provincial, and territorial legislation, including the Canadian Environmental Protection Act, 1999, the generator of hazardous waste remains responsible for their waste from "cradle to grave" (i.e., from generation until its final destination).Footnote 62 If an accident happens during transportation away from the facility, the generator of the waste (e.g., the diagnostic laboratory) remains responsible for the waste, even if a third party contractor is enlisted to transport and dispose of the waste; as such, contingency planning in the event of an accident or spill is an important consideration for the waste management program and the emergency response plan (e.g., SOP for spill outside the facility, providing the contractor with contact information of appropriate facility personnel). All workers share the responsibility of practising due diligence at all times with respect to the appropriate handling, treatment, and disposal of any infectious waste generated to prevent release and exposure.
Chapter 6 - Glossary
It is important to note that while some of the definitions provided in the glossary are universally accepted, many of them were developed specifically for the CBS or the Canadian Biosafety Handbook (CBH), and some have been modified to be applicable in the context of the Canadian Biosafety Guideline – Human Diagnostic Activities.
- Administrative area
- Dedicated room or adjoining rooms that are used for activities that do not involve biological material, including infectious material. Examples of administrative areas include offices, photocopy areas, and meeting/conference rooms.
- Aerosol
- A suspension of fine solid particles or liquid droplets in a gaseous medium (e.g., air) that can be created by any activity that imparts energy into a liquid/semi-liquid material.
- Biological material
- Pathogenic and non-pathogenic microorganisms, proteins, and nucleic acids, as well as any biological matter that may contain microorganisms, proteins, nucleic acids, or parts thereof. Examples include, but are not limited to, bacteria, viruses, fungi, prions, toxins, genetically modified organisms, nucleic acids, tissue samples, diagnostic specimens, live vaccines, and isolates of a pathogen (e.g., pure culture, suspension, purified spores).
- Biological safety cabinet (BSC)
- A primary containment device that provides protection for personnel, the environment, and the product (depending on BSC class) when working with biological material.
- Biosafety
- Containment principles, technologies, and practices that are implemented to prevent unintentional exposure to infectious material, or its accidental release.
- Biosecurity
- Security measures designed to prevent the loss, theft, misuse, diversion, or intentional release of pathogens, and other related assets (e.g., personnel, equipment, animals).
- Containment level (CL)
- Minimum physical containment and operational practice requirements for handling pathogens or toxins safely in laboratory, large scale production, and animal work environments. There are four containment levels ranging from a basic laboratory (CL1) to the highest level of containment (CL4).
- Contamination
- The undesired presence of infectious material on a surface (e.g., benchtop, hands, gloves), in the environment, or within other materials (e.g., laboratory samples, cell cultures).
- Culture
- The in vitro propagation of microorganisms, tissue cells, or other living matter under controlled conditions (e.g., temperature, humidity, nutrients) to generate greater numbers or a higher concentration of the organisms/cells. In the context of the Canadian Biosafety Guidelines, "cell culture" refers to cells derived from a human or animal source.
- Decontamination
- The process by which materials and surfaces are rendered safe to handle and reasonably free of microorganisms, toxins, or prions; this may be accomplished through disinfection, inactivation, or sterilization.
- Decontamination technology
- Equipment proven by validation to render materials safe to handle and reasonably free of microorganisms, toxins, or prions. Examples include autoclaves, incinerators, tissue digesters, and effluent decontamination systems.
- Diagnostic activities
- Activities (e.g., antibody assay, nucleic acid testing, culture, histology, clinical chemistry) involving primary specimens for the purpose of identifying an infection, intoxication, or disease. These activities are regularly carried out in hospitals and clinical laboratories.
- Disinfection
- Process that eliminates most forms of living microorganisms; disinfection is much less lethal to infectious material than sterilization.
- Emergency Response Plan
- A document outlining the actions to be taken and the parties responsible in emergency situations such as a spill, exposure, release of infectious material, personnel injury or illness, power failure, fire, explosion, or other emergency situations (e.g., flood, earthquake, hurricane).
- Exposure
- Contact with, or close proximity to, infectious material that may result in infection. Routes of exposure include inhalation, ingestion, inoculation, and absorption.
- Facilities
- Structures or buildings, or defined areas within structures or buildings, where biological material is handled or stored. This could include individual research and diagnostic laboratories, large scale production areas, or animal housing zones. A facility could also be a suite or building containing more than one of these areas.
- Good microbiological laboratory practices and procedures
- A basic laboratory code of practice applicable to all types of activities with biological material. These practices serve to protect laboratory personnel and prevent contamination of the environment and the samples in use.
- Incident
- An event or occurrence with the potential of causing injury, harm, infection, disease, or damage. Incidents may include a biological spill, exposure, inadvertent release of infectious material, personnel injury or illness, missing samples or specimens, unauthorized entry, power failure, fire, explosion, flood, or other crisis situations (e.g., earthquake, hurricane). Incidents include accidents and near misses.
- Infectious material
- Any isolate of a pathogen or any biological material that contains human or animal pathogens and, therefore, poses a risk to human or animal health.
- Laboratory work area
- A dedicated room or space inside a facility designed and equipped for in vitro work with biological material.
- Laboratory-acquired infection/intoxication (LAI)
- Infection or intoxication resulting from exposure in areas where pathogens or toxins are handled or stored, or where animals are handled or housed.
- Local risk assessment (LRA)
- Site-specific risk assessment used to identify hazards based on the infectious material in use and the activities being performed. This analysis provides risk mitigation and risk management strategies to be incorporated into the physical design and operational practices of the facility.
- Microorganism
- A cellular or non-cellular microbiological entity, capable of replication or transferring genetic material and that cannot be reasonably detected by the naked human eye. Microorganisms include bacteria, fungi, viruses, parasites, and protozoans, and may be pathogenic or non-pathogenic in nature.
- Movement
- The action of moving (e.g., bringing, carrying, leading, relocating) people, material, or animals from one physical location to another physical location in the same building.
- Operational practices
- Administrative controls and procedures followed in a laboratory work area to protect personnel, the environment, and ultimately the community, from infectious material.
- Overarching risk assessment
- A broad risk assessment that supports the biosafety program as a whole and may encompass multiple laboratory work areas within an institution or organization. Mitigation and management strategies reflect the type of biosafety program needed to protect personnel from exposure and to prevent the release of infectious material.
- Pathogen
- A microorganism, nucleic acid, or protein capable of causing disease or infection in humans or animals. Examples of human pathogens are listed in Schedules 2, 3, 4 and Part 2 of Schedule 5 of the Human Pathogens and Toxins Act, but these are not exhaustive lists. Examples of animal pathogens can be found on the Public Health Agency of Canada's ePATHogen Risk Group Database.
- Personal protective equipment (PPE)
- Equipment and/or clothing worn by personnel to provide a barrier against infectious material being handled, thereby minimizing the risk of exposure. PPE may include, but is not limited to, lab coats, gowns, full-body suits, gloves, protective footwear, safety glasses, safety goggles, masks, and respirators.
- Physical design features
- Engineering controls and facility design characteristics in place to protect personnel, the environment, and ultimately the community, from biological material.
- Primary containment device
- Apparatus or equipment that is designed to prevent the release of pathogens or toxins and to provide a physical barrier between the individual and/or the work environment and the biological material. Examples of primary containment devices include biological safety cabinets, isolators, centrifuges with sealable cups or rotors, process equipment, fermenters, microisolator cages, and ventilated cage racks.
- Primary specimens
- Samples derived directly from a human or animal (e.g., blood, urine, saliva, skin, hair).
- Risk
- The probability of an undesirable event (e.g., accident, incident, inadvertent release) occurring and the consequences of that event.
- Risk group (RG)
- The classification of biological material based on its inherent characteristics, including pathogenicity, virulence, risk of spread, and availability of effective prophylactic or therapeutic treatments, that describes the risk to the health of individuals and the public, as well as the health of animals and the animal population. Examples of RG2, RG3, and RG4 human pathogens can be found in Schedules 2, 3, and 4 (respectively) of the Human Pathogens and Toxins Act and in the ePATHogen risk group database.
- Routine practices
- A comprehensive set of infection prevention control measures that have been developed for use in health care settings, and take the approach of treating all specimens of blood, body fluid, or tissue as though they contain a human pathogen.
- Security sensitive biological agents (SSBAs)
- The subset of human pathogens and toxins that have been determined to pose an increased biosecurity risk due to their potential for use as a biological weapon. SSBAs are identified as prescribed human pathogens and toxins by Section 10 of the Human Pathogens and Toxins Regulations. This means all RG3 and RG4 human pathogens that are in the List of Human and Animal Pathogens and Toxins for Export Control, published by the Australia Group, as amended from time to time, with the exception of Duvenhage virus, Rabies virus and all other members of the Lyssavirus genus, Vesicular stomatitis virus, and Lymphocytic choriomeningitis virus; as well as all toxins listed in Schedule 1 of the Human Pathogens and Toxins Act that are listed on the List of Human and Animal Pathogens and Toxins for Export Control when in a quantity greater than that specified in Section 10(2) of the Human Pathogens and Toxins Regulations. Examples of SSBAs that are more likely to be encountered in a diagnostic laboratory in Canada include Brucella spp. and Francisella tularensis, but there may be regional differences.
- Toxin
- A poisonous substance that is produced or derived from a microorganism and can lead to adverse health effects in humans or animals. Regulated human toxins are listed in Schedule 1 and Part 1 of Schedule 5 in the Human Pathogens and Toxins Act.
- Transportation
- The act of transporting (e.g., shipping or conveyance) pathogens or toxins to another building or location (i.e., different address), within Canada or abroad, in accordance with the Transportation of Dangerous Goods Act and the Transportation of Dangerous Goods Regulations.
- Universal precautions
- Universal precautions are a subset of routine practices specific to bloodborne pathogens transmitted through blood and cerebrospinal, pleural, and amniotic fluids. Routine practices are broader, and aim to protect personnel from exposure to all pathogens as a result of contact with all body fluids, excretions, mucosa, non-intact skin, and potentially contaminated items.
- Validation
- The act of confirming that a method achieves its objective by observing that specific parameters have been met. Examples include using biological indicators to confirm that a given autoclave cycle can decontaminate a representative load of waste, or applying disinfectant to microorganisms on a disk. Validation infers that a method is suitable for its intended purpose. In some cases, the published body of evidence (e.g., for the use of bleach) may be acceptable.
- Verification
- The routine monitoring of equipment and processes to ensure continued efficacy between validations. This includes comparing the accuracy of a piece of equipment to an applicable standard or standard operating procedure (e.g., testing of a Class I biological safety cabinet in accordance with the manufacturer's specifications). Where a method is used to inactivate a pathogen (e.g., for DNA extraction), verification may involve placing an aliquot into culture to confirm no viable microorganisms remain.
- Waste
- Any solid or liquid material generated by a facility for disposal.
- Zoonotic pathogen
- A pathogen that causes disease in both humans and animals, and that can be transmitted from animals to humans and vice versa (i.e., zoonoses). They are considered both human and animal pathogens.
Chapter 7 - References and Resources
- Ae, R., Hamaguchi, T., Nakamura, Y., Yamada, M., Tsukamoto, T., et al. (2018). Update: Dura Mater Graft-Associated Creutzfeldt-Jakob Disease - Japan, 1975–2017. Morbidity and Mortality Weekly Report, 67(9):274-278.
- Aguzzi, A., & Glatzel, M. (2006). Prion infections, blood and transfusions. Nature Clinical Practice Neurology, 2(6):321-329.
- Barkham, T., & Taylor, M. B. (2002). Sniffing Bacterial Cultures on Agar Plates: a Useful Tool or a Safety Hazard? Journal of Clinical Microbiology, 40(10):3877.
- Baron, E. J., & Miller, J. M. (2008). Bacterial and Fungal Infections among Diagnostic Laboratory Workers: Evaluating the Risks. Diagnostic Microbiology and Infectious Disease, 60(3):241-246.
- Bienek, A., Heisz, M., & Su, M. (2017). Surveillance of Laboratory Exposures to Human Pathogens and Toxins: Canada 2016. Canada Communicable Disease Report, 43(11):228-235.
- Block, S. S. (Ed.). (2001). Disinfection, Sterilization, and Preservation (5th ed.). Philadelphia, PA, USA: Lippincott Williams & Wilkins.
- Brandel, J.-P., Vlaicu, M. B., Culeux, A., Belondrade, M., Bougard, D., et al. (2020). Variant Creutzfeldt-Jakob Disease Diagnosed 7.5 Years after Occupational Exposure. The New England Journal of Medicine, 383(1):83-85.
- Broussard, I. M., & Kahwaji, C. I. (2019). Universal Precautions. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Retrieved 10/16, 2019 from: https://www.ncbi.nlm.nih.gov/books/NBK470223/
- Byers, K. B., & Harding, A. L. (2017). Laboratory-Associated Infections. In Wooley, D. P., & Byers, K. B. (Eds.). Biological Safety: Principles and Practices (5th ed., pp. 59-92). Washington, DC, USA: ASM Press.
- CAN/CGSB-43.125-2016, Packaging of Category A and Category B infectious substances (Class 6.2) and clinical, (bio) medical or regulated medical waste. (2016). Gatineau, QC, Canada: Canadian General Standards Board.
- Canadian Centre for Occupational Health and Safety. (2018). OSH Answers Fact Sheets: Routine Practices. Retrieved 03/03, 2021 from http://www.ccohs.ca/oshanswers/prevention/universa.html
- Canadian Council of the Ministers of the Environment. (1992). Guidelines for the Management of Biomedical Waste in Canada. Mississauga, ON, Canada: Canadian Standards Association.
- Canadian Environmental Protection Act, 1999 (S.C. 1999, c. 33).
- Choucrallah, D., Sarmiento, L., Ettles, S., Tanguay, F., Heisz, M., & Falardeau, E. (2019). Surveillance of laboratory exposures to human pathogens and toxins: Canada 2018. Canada Communicable Disease Report, 45(9):244-251.
- Cox, C. R., Jensen, K. R., Saichek, N. R., & Voorhees, K. J. (2015). Strain-level bacterial identification by CeO2-catalyzed MALDI-TOF MS fatty acid analysis and comparison to commercial protein-based methods. Scientific Reports, 5:10470.
- CSA ISO 15190:21, Medical Laboratories - Requirements for Safety. (2021). Toronto, ON, Canada: Canadian Standards Association.
- CSA Z316.6:20, Sharps Injury Protection - Requirements and Test Methods - Sharps Containers. (2020). Toronto, ON, Canada: Canadian Standards Association.
- CSA Z317.10:15 (R2020), Handling of Health Care Waste Materials. (2015). Toronto, ON, Canada: Canadian Standards Association.
- DeMarco, M. (2015). The Lab and CJD: Safe Handling of Infectious Prion Proteins. Retrieved 05/19, 2017 from https://www.aacc.org/publications/cln/articles/2015/november/the-lab-and-cjd-safe-handling-of-infectious-prion-proteins
- Engelkirk, P. G., & Duben-Engelkirk, J. L. (2008). Laboratory Diagnosis of Infectious Diseases: Essentials of Diagnostic Microbiology. Baltimore, MD, USA: Lippincott Williams & Wilkins.
- Evans, M. R., Henderson, D. K., & Bennett, J. E. (1990). Potential for laboratory exposures to biohazardous agents found in blood. American Journal of Public Health, 80(4):423-427.
- Federal Office for the Environment. (2014). Guideline for the use of microbiological safety cabinets when handling human-pathogenic microorganisms (2nd ed.). Bern, Switzerland: Federal Office for the Environment.
- Federal Office for the Environment. (2015). Safety Measures in Medical Microbiology Diagnostic Laboratories (2nd ed.). Bern, Switzerland: Federal Office for the Environment.
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition
- Government of Canada. (2016). Canadian Biosafety Handbook (2nd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/handbook-second-edition.html
- Government of Canada. (2017). Canadian Biosafety Guideline – Local Risk Assessment. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
- Government of Canada. (2018). Biosafety directives and advisories. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosafety-directives-advisories-notifications.html
- Government of Canada. (2018). Canadian biosafety guidelines. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
- Government of Canada. ePATHogen – Risk Group Database. Available from https://health.canada.ca/en/epathogen
- Government of Canada. Pathogen Safety Data Sheets. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment.html
- Health and Safety Executive. (2005). Biological agents: Managing the risks in laboratories and healthcare premises. United Kingdom: Health and Safety Executive.
- Health Canada. (2009). Guidance document - Human-Use Antiseptic Drugs. Ottawa, ON, Canada: Health Canada.
- Health of Animals Act (S.C. 1990, c. 21).
- Health of Animals Regulations (C.R.C., c. 296).
- Human Pathogens and Toxins Act (S.C. 2009, c. 24).
- Human Pathogens and Toxins Regulations (SOR/2015-44).
- ISO 15189:2012, Medical laboratories – Requirements for Quality and Competence. (2012). Geneva, Switzerland: International Organization for Standardization.
- ISO 9001:2015, Quality Management Systems – Requirements. (2015). Geneva, Switzerland: International Organization for Standardization.
- Leunda, A., Van Vaerenbergh, B., Baldo, A., Roels, S., & Herman, P. (2013). Laboratory activities involving transmissible spongiform encephalopathy causing agents: Risk assessment and biosafety recommendations in Belgium. Prion, 7(5):420-433.
- Lien, A., Abalos, C., Atchessi, N., Edjoc, R., & Heisz, M. (2020). Surveillance of laboratory exposures to human pathogens and toxins, Canada 2019. Canada Communicable Disease Report, 46(9):292-298.
- Lim, D. (2003). Microbiology (3rd ed.). Dubuque, IA, USA: Kendall/Hunt Publishing Company.
- Noble, M. A. (2015). Prevention of Laboratory-Acquired Infections. In Jorgensen, J. H., Pfaller, M. A., Carroll, K. C., Funke, G., Landry, M. L., et al. (Eds.). Manual of Clinical Microbiology (11th ed., pp. 169-182). Washington, DC, USA: ASM Press.
- Occupational Safety and Health Administration and American Biological Safety Association Alliance. Principles of Good Microbiological Practice. Retrieved 03/03, 2021 from https://absa.org/wp-content/uploads/2017/01/PrinciplesGoodMicroPracticesFactSheet.pdf
- Patel, R. (2015). MALDI-TOF MS for the Diagnosis of Infectious Diseases. Clinical Chemistry, 61(1):100-111.
- Pedrosa, P. B. S., & Cardoso, T. A. O. (2011). Viral infections in workers in hospital and research laboratory settings: a comparative review of infection modes and respective biosafety aspects. International Journal of Infectious Diseases, 15(6):e366-e376.
- Pike, R. M. (1979). Laboratory-associated infections: incidence, fatalities, causes, and prevention. Annual Review of Microbiology, 33:41–66.
- Pomerleau-Normandin, D., Heisz, M., & Su, M. (2018). Misidentification of Risk Group 3/Security Sensitive Biological Agents by MALDI-TOF MS in Canada: November 2015–October 2017. Canada Communicable Disease Report, 44(5):110-115.
- Pomerleau-Normandin, D., Heisz, M., & Tanguay, F. (2018). Surveillance of laboratory exposures to human pathogens and toxins: Canada 2017. Canada Communicable Disease Report, 44(11):297-303.
- Public Health Agency of Canada. (2007). Infection Control Guidelines – Classic Creutzfeldt-Jakob Disease in Canada – Quick Reference Guide 2007. Retrieved 09/10, 2019 from https://www.canada.ca/en/public-health/services/infectious-diseases/nosocomial-occupational-infections/creutzfeldt-jakob-disease/infection-control-guidelines.html
- Public Health Agency of Canada. (2012). Hand Hygiene Practices in Healthcare Settings. Ottawa, ON, Canada: Public Health Agency of Canada.
- Public Health Agency of Canada. (2016). Exemptions from the Licensing Requirements of the Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/human-pathogens-toxins-act/exemptions-licensing-requirements-human-pathogens-toxins-act-human-pathogens-toxins-regulations.html
- Public Health Agency of Canada. (2017). Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings. Ottawa, ON, Canada: Public Health Agency of Canada.
- Sewell, D. L. (1995). Laboratory-Associated Infections and Biosafety. Clinical Microbiology Reviews, 8(3):389-405.
- Singh, K. (2009). Laboratory-Acquired Infections. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America, 49(1):142-147.
- Stuart, D. G. (1999). Primary Containment (Chapter 3). Journal of the American Biological Safety Association, 4(1):6-16.
- Tedder, R. S., Zuckerman, M. A., Goldstone, A. H., Hawkins, A. E., Fielding, A., et al. (1995). Hepatitis B transmission from contaminated cryopreservation tank. Lancet, 346(8968):137-140.
- Tracz, D. M., Tober, A. D., Antonation, K. S., & Corbett, C. R. (2018). MALDI-TOF mass spectrometry and high-consequence bacteria: safety and stability of biothreat bacterial sample testing in clinical diagnostic laboratories. Journal of Medical Microbiology, 67(3):341-346.
- Tracz, D. M., Tyler, A. D., Cunningham, I., Antonation, K. S., & Corbett, C. R. (2017). Custom database development and biomarker discovery methods for MALDI-TOF mass spectrometry-based identification of high-consequence bacterial pathogens. Journal of Microbiological Methods, 134:54-57.
- Transportation of Dangerous Goods Regulations (SOR/2001-286).
- United Kingdom Department of Health, Engineering and Science Advisory Committee into the Decontamination of Surgical Instruments Including Prion Removal (ESAC‑Pr). (2008). New Technologies Working Group Report on Prion Inactivating Agents. London, UK: Department of Health.
- United States Centers for Disease Control. (1987). Recommendations for Prevention of HIV Transmission in Health-Care Settings. Morbidity and Mortality Weekly Report, 36(2):1S-18S.
- United States Centers for Disease Control and Prevention. (2012). Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories - Recommendations of a CDC-convened, Biosafety Blue Ribbon Panel. Morbidity and Mortality Weekly Report, 61(1):1-101.
- United States Centers for Disease Control and Prevention. (2020). Show Me the Science – When & How to Use Hand Sanitizer in Community Settings. Retrieved on 03/03, 2021 from http://www.cdc.gov/handwashing/show-me-the-science-hand-sanitizer.html
- United States Department of Labor. (2001). Occupational Safety and Health Standards: 1910.1030 - Bloodborne Pathogens. Washington, DC, USA: United States Department of Labor.
- World Health Organization. (2000). WHO infection control guidelines for Transmissible Spongiform Encephalopathies: report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. Geneva, Switzerland: World Health Organization.
- World Health Organization. (2020). Laboratory Biosafety Manual (4th ed.). Geneva, Switzerland: World Health Organization.
Appendix A - Licensing Requirements when only Health of Animals Regulations apply
The decision tree presented in Figure A-1 clarifies HAR requirements when activities are excluded from HPTA licence requirements. This decision tree figure can help determine whether a licence or permit issued under the HAR is required.
Figure A-1: Legislative oversight of diagnostic testing activities with a human pathogen under the HAR
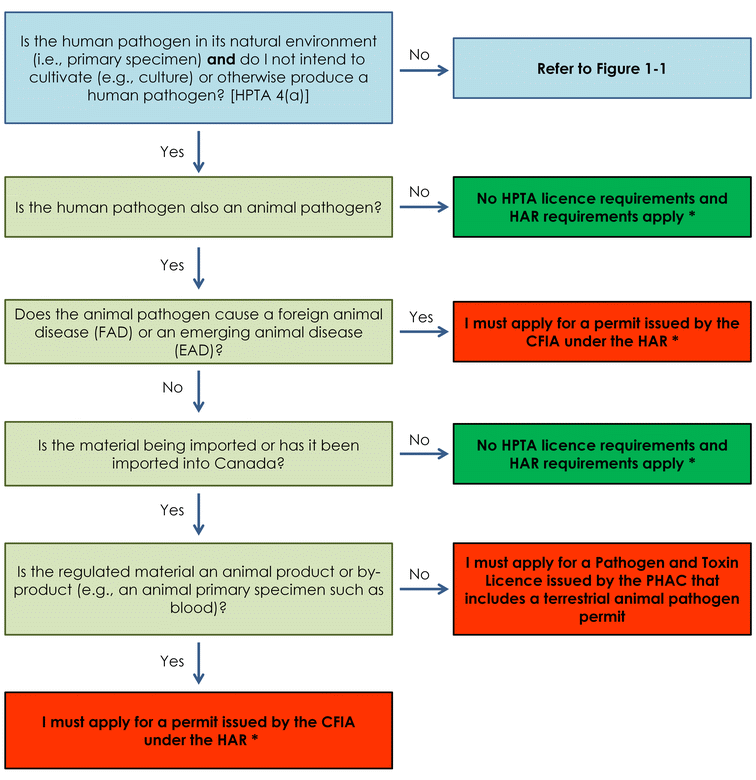
- Figure A-1 note *
-
I must inform the PHAC without delay if there is inadvertent production of a human pathogen or toxin. [HPTA 12(2)]
Figure A-1 - Text Description
The figure is a decision tree indicating when diagnostic facilities must apply for a Pathogen and Toxin Licence or a permit issued by the CFIA under the HAR and when they do not need a licence as HPTA licence requirements and HAR requirements do not apply.
When the biological material handled contains a human pathogen that is not in its natural environment or there is the intent to cultivate (e.g., culture) or otherwise produce a human pathogen, licence requirements are described in Figure 1-1 of this guideline.
When the biological material handled contains a human pathogen in its natural environment (i.e., primary specimen), there is no intent to cultivate (e.g., culture) or otherwise produce a human pathogen, and the human pathogen is not an animal pathogen, there are no HPTA licence requirements and HAR requirements that apply [HPTA 4(a)]. Nonetheless, the PHAC must still be informed without delay if there is inadvertent production of a human pathogen or toxin [HPTA 12(2)].
When the biological material handled contains a human pathogen in its natural environment (i.e., primary specimen), there is no intent to cultivate (e.g., culture) or otherwise produce a human pathogen, and the human pathogen is an animal pathogen that causes a foreign animal disease (FAD) or an emerging animal disease (EAD), a permit issued by the CFIA under the HAR is required. Furthermore, the PHAC must be informed without delay if there is inadvertent production of a human pathogen or toxin [HPTA 12(2)].
When the biological material handled contains a human pathogen in its natural environment (i.e., primary specimen), there is no intent to cultivate (e.g., culture) or otherwise produce a human pathogen, the human pathogen is an animal pathogen that does not cause a foreign animal disease (FAD) or an emerging animal disease (EAD), and the material is not being imported or has not been imported into Canada, there are no HPTA licence requirements and HAR requirements that apply. Nonetheless, the PHAC must still be informed without delay if there is inadvertent production of a human pathogen or toxin [HPTA 12(2)].
When the biological material handled contains a human pathogen in its natural environment (i.e., primary specimen), there is no intent to cultivate (e.g., culture) or otherwise produce a human pathogen, the human pathogen is an animal pathogen that does not cause a foreign animal disease (FAD) or an emerging animal disease (EAD), the material is being imported or has been imported into Canada, and the regulated material is an animal product or by-product (e.g., an animal primary specimen such as blood), a permit issued by the CFIA under the HAR is required. Furthermore, the PHAC must be informed without delay if there is inadvertent production of a human pathogen or toxin [HPTA 12(2)].
When the biological material handled contains a human pathogen in its natural environment (i.e., primary specimen), there is no intent to cultivate (e.g., culture) or otherwise produce a human pathogen, the human pathogen is an animal pathogen that does not cause a foreign animal disease (FAD) or an emerging animal disease (EAD), the material is being imported or has been imported into Canada, and the regulated material is not an animal product or by-product (e.g., it is a human primary specimen such as blood), a Pathogen and Toxin Licence issued by the PHAC that includes a terrestrial animal pathogen permit is required.
Appendix B - Proper Hand Hygiene
Handwashing is the most common method for decontaminating the hands and the most effective means for preventing the transmission of infection. Handwashing, using soap and clean running water is an effective way to remove visible soil and organic material and eliminate all types of pathogens from the surface of the hands.
Proper Handwashing (Soap and Water)Footnote 63
- Wet hands under running water.
- Use enough soap to lather all surfaces of the hands, including fingers, fingertips, between fingers, palms, backs of hands and thumbs, base of thumbs, and if a ring is worn, on and under the ring.
- Rub the palms and backs of each hand vigorously, interlocking and interlacing fingers to ensure fingers and thumbs are rubbed to remove visible soil and organic material for 15 to 30 seconds.
- Rinse hands thoroughly under running water with the fingers pointing downward.
- Dry hands thoroughly by patting with a single-use towel.
- Use paper towels to turn off manual faucets to prevent recontaminating hands in the process.
- The complete handwashing procedure (going to sink, wetting hands, applying soap, lathering, rinsing, and drying) takes 40 to 80 seconds.
Considerations on the Use of Alcohol-based Hand Sanitizers
- Hand sanitizers containing alcohol or other active ingredients such as chlorhexidine gluconate, as described in the Guidance Document - Human-Use Antiseptic Drugs and the Food and Drug Act cannot eliminate all types of pathogens and are not as effective as handwashing with soap and water, particularly when hands are visibly dirty or greasy.Footnote 56 Footnote 64
- To prevent the spread of contamination, a hand sanitizer that has been demonstrated to be effective against the pathogen(s) or toxin(s) in use in the facility may be an alternative where handwashing sinks are not readily accessible. In this instance, handwashing should follow as soon as a suitable handwashing sink is available.
- Applying hand sanitizer to wet hands will dilute the active ingredient and may render it ineffective.
- Following the manufacturer's instructions, which includes rubbing all hand surfaces and avoiding wiping or drying hands with paper towels before the product has dried, will allow for the appropriate contact time and more effective decontamination of the hands.
- Alcohol-based hand sanitizers are flammable and may alter the porosity of gloves. Allowing them to completely dry prior to contact with an oxygen-rich environment and prior to putting on gloves will avoid these issues.
- Hand wipes that are impregnated with soap, antimicrobials, or alcohol are not alternatives to alcohol-based or other active ingredient hand sanitizers for hand antisepsis as they are not effective at decontaminating hands.
References
- Footnote 1
-
Human Pathogens and Toxins Act (S.C. 2009, c. 24).
- Footnote 2
-
Human Pathogens and Toxins Regulations (SOR/2015-44).
- Footnote 3
-
Health of Animals Act (S.C. 1990, c. 21).
- Footnote 4
-
Health of Animals Regulations (C.R.C., c. 296).
- Footnote 5
-
Public Health Agency of Canada. (2016). Exemptions from the Licensing Requirements of the Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/human-pathogens-toxins-act/exemptions-licensing-requirements-human-pathogens-toxins-act-human-pathogens-toxins-regulations.html
- Footnote 6
-
Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition
- Footnote 7
-
Government of Canada. (2018). Canadian biosafety guidelines. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
- Footnote 8
-
Public Health Agency of Canada. (2017). Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings. Ottawa, ON, Canada: Public Health Agency of Canada.
- Footnote 9
-
ISO 15189:2012, Medical laboratories – Requirements for Quality and Competence. (2012). Geneva, Switzerland: International Organization for Standardization.
- Footnote 10
-
CSA ISO 15190:21, Medical Laboratories - Requirements for Safety. (2021). Toronto, ON, Canada: Canadian Standards Association.
- Footnote 11
-
CSA Z317.10:15 (R2020), Handling of Health Care Waste Materials. (2015). Toronto, ON, Canada: Canadian Standards Association.
- Footnote 12
-
Occupational Safety and Health Administration and American Biological Safety Association Alliance. Principles of Good Microbiological Practice. Retrieved 03/03, 2021 from https://absa.org/wp-content/uploads/2017/01/PrinciplesGoodMicroPracticesFactSheet.pdf
- Footnote 13
-
World Health Organization. (2020). Laboratory Biosafety Manual (4th ed.). Geneva, Switzerland: World Health Organization.
- Footnote 14
-
Canadian Centre for Occupational Health and Safety. (2018). OSH Answers Fact Sheets: Routine Practices. Retrieved 03/03, 2021 from http://www.ccohs.ca/oshanswers/prevention/universa.html
- Footnote 15
-
Broussard, I. M., & Kahwaji, C. I. (2019). Universal Precautions. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Retrieved 10/16, 2019 from: https://www.ncbi.nlm.nih.gov/books/NBK470223/
- Footnote 16
-
United States Centers for Disease Control. (1987). Recommendations for Prevention of HIV Transmission in Health-Care Settings. Morbidity and Mortality Weekly Report, 36(2):1S-18S.
- Footnote 17
-
Byers, K. B., & Harding, A. L. (2017). Laboratory-Associated Infections. In Wooley, D. P., & Byers, K. B. (Eds.). Biological Safety: Principles and Practices (5th ed., pp. 59-92). Washington, DC, USA: ASM Press.
- Footnote 18
-
Sewell, D. L. (1995). Laboratory-Associated Infections and Biosafety. Clinical Microbiology Reviews, 8(3):389-405.
- Footnote 19
-
Pike, R. M. (1979). Laboratory-associated infections: incidence, fatalities, causes, and prevention. Annual Review of Microbiology, 33:41–66.
- Footnote 20
-
Pedrosa, P. B. S., & Cardoso, T. A. O. (2011). Viral infections in workers in hospital and research laboratory settings: a comparative review of infection modes and respective biosafety aspects. International Journal of Infectious Diseases, 15(6):e366-e376.
- Footnote 21
-
Bienek, A., Heisz, M., & Su, M. (2017). Surveillance of Laboratory Exposures to Human Pathogens and Toxins: Canada 2016. Canada Communicable Disease Report, 43(11):228-235.
- Footnote 22
-
Pomerleau-Normandin, D., Heisz, M., & Tanguay, F. (2018). Surveillance of laboratory exposures to human pathogens and toxins: Canada 2017. Canada Communicable Disease Report, 44(11):297-303.
- Footnote 23
-
Choucrallah, D., Sarmiento, L., Ettles, S., Tanguay, F., Heisz, M., & Falardeau, E. (2019). Surveillance of laboratory exposures to human pathogens and toxins: Canada 2018. Canada Communicable Disease Report, 45(9):244-251.
- Footnote 24
-
Lien, A., Abalos, C., Atchessi, N., Edjoc, R., & Heisz, M. (2020). Surveillance of laboratory exposures to human pathogens and toxins, Canada 2019. Canada Communicable Disease Report, 46(9):292-298.
- Footnote 25
-
Noble, M. A. (2015). Prevention of Laboratory-Acquired Infections. In Jorgensen, J. H., Pfaller, M. A., Carroll, K. C., Funke, G., Landry, M. L., et al. (Eds.). Manual of Clinical Microbiology (11th ed., pp. 169-182). Washington, DC, USA: ASM Press.
- Footnote 26
-
Evans, M. R., Henderson, D. K., & Bennett, J. E. (1990). Potential for laboratory exposures to biohazardous agents found in blood. American Journal of Public Health, 80(4):423-427.
- Footnote 27
-
Government of Canada. Pathogen Safety Data Sheets. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment.html
- Footnote 28
-
Government of Canada. ePATHogen – Risk Group Database. Available from https://health.canada.ca/en/epathogen
- Footnote 29
-
Government of Canada. (2018). Biosafety directives and advisories. Available from https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/biosafety-directives-advisories-notifications.html
- Footnote 30
-
Health and Safety Executive. (2005). Biological agents: Managing the risks in laboratories and healthcare premises. United Kingdom: Health and Safety Executive.
- Footnote 31
-
Government of Canada. (2017). Canadian Biosafety Guideline – Local Risk Assessment. Available from https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/guidance.html
- Footnote 32
-
Federal Office for the Environment. (2015). Safety Measures in Medical Microbiology Diagnostic Laboratories (2nd ed.). Bern, Switzerland: Federal Office for the Environment.
- Footnote 33
-
Barkham, T., & Taylor, M. B. (2002). Sniffing Bacterial Cultures on Agar Plates: a Useful Tool or a Safety Hazard? Journal of Clinical Microbiology, 40(10):3877.
- Footnote 34
-
Engelkirk, P. G., & Duben-Engelkirk, J. L. (2008). Laboratory Diagnosis of Infectious Diseases: Essentials of Diagnostic Microbiology. Baltimore, MD, USA: Lippincott Williams & Wilkins.
- Footnote 35
-
Singh, K. (2009). Laboratory-Acquired Infections. Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America, 49(1):142-147.
- Footnote 36
-
Baron, E. J., & Miller, J. M. (2008). Bacterial and Fungal Infections among Diagnostic Laboratory Workers: Evaluating the Risks. Diagnostic Microbiology and Infectious Disease, 60(3):241-246.
- Footnote 37
-
United States Centers for Disease Control and Prevention. (2012). Guidelines for Safe Work Practices in Human and Animal Medical Diagnostic Laboratories - Recommendations of a CDC-convened, Biosafety Blue Ribbon Panel. Morbidity and Mortality Weekly Report, 61(1):1-101.
- Footnote 38
-
Tedder, R. S., Zuckerman, M. A., Goldstone, A. H., Hawkins, A. E., Fielding, A., et al. (1995). Hepatitis B transmission from contaminated cryopreservation tank. Lancet, 346(8968):137-140.
- Footnote 39
-
CSA Z316.6:20, Sharps Injury Protection - Requirements and Test Methods - Sharps Containers. (2020). Toronto, ON, Canada: Canadian Standards Association.
- Footnote 40
-
Brandel, J.-P., Vlaicu, M. B., Culeux, A., Belondrade, M., Bougard, D., et al. (2020). Variant Creutzfeldt-Jakob Disease Diagnosed 7.5 Years after Occupational Exposure. The New England Journal of Medicine, 383(1):83-85.
- Footnote 41
-
Ae, R., Hamaguchi, T., Nakamura, Y., Yamada, M., Tsukamoto, T., et al. (2018). Update: Dura Mater Graft-Associated Creutzfeldt-Jakob Disease - Japan, 1975–2017. Morbidity and Mortality Weekly Report, 67(9):274-278.
- Footnote 42
-
Aguzzi, A., & Glatzel, M. (2006). Prion infections, blood and transfusions. Nature Clinical Practice Neurology, 2(6):321-329.
- Footnote 43
-
Public Health Agency of Canada. (2007). Infection Control Guidelines – Classic Creutzfeldt-Jakob Disease in Canada – Quick Reference Guide 2007. Retrieved 09/10, 2019 from https://www.canada.ca/en/public-health/services/infectious-diseases/nosocomial-occupational-infections/creutzfeldt-jakob-disease/infection-control-guidelines.html
- Footnote 44
-
DeMarco, M. (2015). The Lab and CJD: Safe Handling of Infectious Prion Proteins. Retrieved 05/19, 2017 from https://www.aacc.org/publications/cln/articles/2015/november/the-lab-and-cjd-safe-handling-of-infectious-prion-proteins
- Footnote 45
-
World Health Organization. (2000). WHO infection control guidelines for Transmissible Spongiform Encephalopathies: report of a WHO consultation, Geneva, Switzerland, 23-26 March 1999. Geneva, Switzerland: World Health Organization.
- Footnote 46
-
Leunda, A., Van Vaerenbergh, B., Baldo, A., Roels, S., & Herman, P. (2013). Laboratory activities involving transmissible spongiform encephalopathy causing agents: Risk assessment and biosafety recommendations in Belgium. Prion, 7(5):420-433.
- Footnote 47
-
Patel, R. (2015). MALDI-TOF MS for the Diagnosis of Infectious Diseases. Clinical Chemistry, 61(1):100-111.
- Footnote 48
-
Pomerleau-Normandin, D., Heisz, M., & Su, M. (2018). Misidentification of Risk Group 3/Security Sensitive Biological Agents by MALDI-TOF MS in Canada: November 2015–October 2017. Canada Communicable Disease Report, 44(5):110-115.
- Footnote 49
-
Cox, C. R., Jensen, K. R., Saichek, N. R., & Voorhees, K. J. (2015). Strain-level bacterial identification by CeO2-catalyzed MALDI-TOF MS fatty acid analysis and comparison to commercial protein-based methods. Scientific Reports, 5:10470.
- Footnote 50
-
Tracz, D. M., Tyler, A. D., Cunningham, I., Antonation, K. S., & Corbett, C. R. (2017). Custom database development and biomarker discovery methods for MALDI-TOF mass spectrometry-based identification of high-consequence bacterial pathogens. Journal of Microbiological Methods, 134:54-57.
- Footnote 51
-
Tracz, D. M., Tober, A. D., Antonation, K. S., & Corbett, C. R. (2018). MALDI-TOF mass spectrometry and high-consequence bacteria: safety and stability of biothreat bacterial sample testing in clinical diagnostic laboratories. Journal of Medical Microbiology, 67(3):341-346.
- Footnote 52
-
Federal Office for the Environment. (2014). Guideline for the use of microbiological safety cabinets when handling human-pathogenic microorganisms (2nd ed.). Bern, Switzerland: Federal Office for the Environment.
- Footnote 53
-
Stuart, D. G. (1999). Primary Containment (Chapter 3). Journal of the American Biological Safety Association, 4(1):6-16.
- Footnote 54
-
United States Department of Labor. (2001). Occupational Safety and Health Standards: 1910.1030 - Bloodborne Pathogens. Washington, DC, USA: United States Department of Labor.
- Footnote 55
-
Transportation of Dangerous Goods Regulations (SOR/2001-286).
- Footnote 56
-
United States Centers for Disease Control and Prevention. (2020). Show Me the Science – When & How to Use Hand Sanitizer in Community Settings. Retrieved on 03/03, 2021 from http://www.cdc.gov/handwashing/show-me-the-science-hand-sanitizer.html
- Footnote 57
-
ISO 9001:2015, Quality Management Systems – Requirements. (2015). Geneva, Switzerland: International Organization for Standardization.
- Footnote 58
-
Block, S. S. (Ed.). (2001). Disinfection, Sterilization, and Preservation (5th ed.). Philadelphia, PA, USA: Lippincott Williams & Wilkins.
- Footnote 59
-
United Kingdom Department of Health, Engineering and Science Advisory Committee into the Decontamination of Surgical Instruments including Prion Removal (ESAC-Pr). (2008). New Technologies Working Group Report on Prion Inactivating Agents. London, UK: Department of Health.
- Footnote 60
-
Canadian Council of the Ministers of the Environment. (1992). Guidelines for the Management of Biomedical Waste in Canada. Mississauga, ON, Canada: Canadian Standards Association.
- Footnote 61
-
CAN/CGSB-43.125-2016, Packaging of Category A and Category B infectious substances (Class 6.2) and clinical, (bio) medical or regulated medical waste. (2016). Gatineau, QC, Canada: Canadian General Standards Board.
- Footnote 62
-
Canadian Environmental Protection Act, 1999 (S.C. 1999, c. 33).
- Footnote 63
-
Public Health Agency of Canada. (2012). Hand Hygiene Practices in Healthcare Settings. Ottawa, ON, Canada: Public Health Agency of Canada.
- Footnote 64
-
Health Canada. (2009). Guidance document - Human-Use Antiseptic Drugs. Ottawa, ON, Canada: Health Canada.
