Planning guidance for immunization clinics for COVID-19 vaccines: Clinic planning and operations
On this page
- Clinic planning
- Clinic operations
- De-escalation activities
- Alternative delivery methods
- Evaluation
- Resources
- Appendix 1: Sample immunization clinic list
- Appendix 2: Post-clinic evaluation form for staff and volunteer
- Appendix 3: Client evaluation form
- Endnotes
Clinic planning
Leadership and coordination
Planning and implementation of immunization clinics requires experienced leadership and the coordination of many community groups and individuals. Clinic leadership will need to be established to provide overall management, planning and coordination of clinic operations, as well as knowledge of public health practices and infection prevention and control to prevent the transmission of COVID-19.
A key aspect of leadership and coordination will be to identify areas where collaboration may be required and, where possible, to negotiate agreements in advance (e.g., collaboration with owners of facilities to secure clinic sites, with human resources and unions for rapid hiring and addressing staffing impacts, and with health professional associations for human resources surge capacity). While the Government of Canada has purchased sufficient quantities of some supplies to immunize all Canadians (including syringes, needles, alcohol swabs, bandages, gauze and sharps containers), arrangements will need to be put in place with suppliers for other required supplies.
Collaborations for clinic implementation may include arrangements related to the following:
- Parking (e.g., snow removal and waiver of parking restrictions)
- Transportation of individuals to the clinics (e.g., older adults, people who are home-bound, residents of remote and isolated communities, people with special needs)
- Accessibility of the clinic sites, including removal of physical barriers
- Acquiring, storing and transporting of supplies and biomedical waste
- Security and safety of clinic sites
- Being the sole user of the site, but if that is not possible, coordination with other users to prevent interactions, conflicts and confusion
- Collaboration with school officials and with parents/guardians for implementation of school-based clinics, including for students who are learning remotely.
In developing these collaborations, consider the needs and resources of the community. Planning should consider the diverse needs of the community to be served including age, gender, ability status, culture, language, religious beliefs and other social and demographic factors. It is recognized that each jurisdiction has its own health system infrastructure and planners will need to align clinic plans in accordance with systems that exist in their jurisdiction.
Immunization campaign and clinic planning parameters
Immunization campaign planning begins with determining the number of people to be immunized. The number of people to immunize are then used to plan the number of clinics required and the number of staff and volunteers at each clinic. The following parameters to support planning are outlined below:
- Number of vaccine doses an Immunizer can give per hour (immunization rate)
- Number of Immunizers per clinic
- Duration of each clinic
- Number of clinics per day and per week
When vaccine quantities are limited, the number of doses available and the groups that are eligible to receive the vaccine will also need to be considered when planning immunization clinics.
See Table 2 for a description of the staffing/volunteer roles that are referred to below. Note that the numbers below are rough estimates and suggestions, and may need to be adjusted depending on local circumstances and community needs.
Number of vaccine doses an Immunizer can give per hour (immunization rate)
- On average, if consent forms have already been completed, the vaccine is pre-loaded into the syringes for the Immunizer and the clients continuously flow through the clinic, an Immunizer can give approximately 14 immunizations per hour. If each Immunizer must pre-load their own syringes, the immunization rate is slower at approximately 12 immunizations per hour if no mixing is required and approximately 11 immunizations per hour if mixing is required.
- Although it is ideal for Immunizers to load or pre-load their own syringes, designated Syringe Pre-Loaders may make the clinic run more efficiently and achieve a faster immunization rate. Pre-loading of syringes by designated Syringe Pre-Loaders is most easily performed when only one product, lot and dose are being used at the clinic.
- More experienced immunizers may have faster immunization rates and immunization rates will increase as new immunizers become more familiar with their role.
- In planning appointments, a slower immunization rate could be used for the first few clinics and the immunization rate can be increased for subsequent clinics as experiences are gained.
- Immunizing couples or family units from the same household together may increase the immunization rate (as the consent discussion only needs to happen once for the whole family), although large numbers of young children can slow the immunization rate (need to hold the children and/or respond to their concerns to support them to receive the vaccine).
- Immunizing populations with special needs (e.g., physical or intellectual disabilities) or language or cultural barriers may result in slower immunization rates.
The number of Immunizers depends on the available supports (such as Clinic Leaders) and the size of the clinic space
- An average size clinic has 10-15 Immunizers. Larger clinics with more Immunizers can also be run but require more Clinic Leaders and these clinics may be more challenging to maintain physical distancing and avoid crowding.
- Although normally there could be two Immunizers per table, during COVID-19 only one immunizer per table is recommended to assist with physical distancing. This may limit the number of Immunizers that can fit in a clinic space.
Duration of each clinic
- If possible, consider keeping clinics short, such as six hours (time when the clinic is open for providing immunizations). This helps to reduce fatigue among the staff and volunteers. If longer clinics are needed, consider having two sequential staff/volunteer shifts.
- Staff and volunteers will need to arrive approximately an hour in advance to allow for set-up/organization and orientation.
Number of clinics per day and per week
- The number of clinics per day will depend on the number of staff and sites available and the ability of the infrastructure to support each clinic (i.e., ability to pack for or resupply each clinic, the available cold chain equipment, transportation of vaccine and supplies to the clinic location).
- The number of clinics per week also depends on the number of staff available. Offering clinics on weekends and in the evenings facilitates immunization of those who are working or attending school. If running clinics more than five days per week, it is optimal to provide each staff member with two consecutive days off per week, if possible.
Other planning parameters for staffing
- At a minimum and if possible, it is recommended to have at least two Clinic Leaders at any immunization clinic. Clinics of 10-15 Immunizers should consider having at least three Clinic Leaders and/or Clinic Floaters per clinic, and larger clinics should have additional Clinic Leaders and/or Clinic Floaters.
- If mixing of vials and preloading of syringes is performed by staff other than the Immunizer (i.e., by Syringe Pre-Loaders) consider the following planning parameters:
- If the vaccine requires mixing with an adjuvant or diluent, consider having one Syringe Pre-Loader per three Immunizers.
- If the vaccine does not require mixing, consider having one Syringe Pre-loader for four Immunizers.
- See Table 3 for example of a possible staffing plan, including suggestions about staffing ratios.
Planning for second doses
- As most of the COVID-19 vaccines require a second dose, it is important to make receiving that second dose as easy as possible for the client.
- Ensure that sufficient vaccine is available for the second dose. This can be done by receiving shipments of both doses together in the initial delivery and storing the second dose locally until it is needed, or ensuring that a later shipment will deliver the second dose in time for the second clinic.
- Having clinics for the second dose in the same location and at the same time as the first dose clinic. To avoid confusion and control volumes, the second dose clinic should offer vaccine only to those who received the vaccine at the earlier clinic (21 or 28 days earlier depending on the product). It is recommended that first doses not be offered at a second dose clinic.
Immunization clinic site identification
Identifying appropriate sites for immunization clinics requires selecting sites that meet the needs of the community and support clinic objectives and activities safely and securely. Location, accessibility and amenities should be considered when selecting clinic sites, as outlined in table 1 below. The same clinic sites can be used for the entire duration of the immunization campaign, or different clinic locations may be chosen at different times to facilitate access.
If using school sites for immunization clinics, it is best to run the clinics when the students are not present (i.e., late afternoon or evening, or weekends). As clients often arrive early for clinics, it will be important to ensure that client do not enter the school or interact with students or school staff members if they are present in the building.
| Location | Accessibility | Amenities |
|---|---|---|
|
|
|
Examples of potential clinic sites include:
- schools
- colleges and universities (gyms, auditoriums, cafeterias)
- shopping centres
- trade or convention centres
- city halls
- places of worship
- large vacant stores
- community centres, legions
- arenas
- friendship centres
- nursing stations
In addition, with appropriate equipment (e.g., tents, heaters), outdoor areas such as sports fields and parking lots can be used as potential immunization clinic sites.
When selecting sites, consider challenges that could be encountered and plan accordingly through the use of site visits and clinic implementation exercises, if possible. These challenges can include security issues if the public enters restricted areas, competition for parking spaces with other site users, conflict with other users (e.g. activities in arenas or school gymnasiums), limited storage space, damage to facilities and issues related to malfunctioning of essential systems such as water, electricity, internet connectivity and washrooms. If there is a possibility that clients will need to wait outside during the winter or inclement weather, consider options such as tents and heaters.
Human resources
Clinic staffing
Immunization clinics require many human resources to facilitate effective operations. The number of personnel normally assigned to routine public health immunization programs will be insufficient to respond to COVID-19 immunization requirements, therefore securing additional staff will be necessary.
Additional sources of health care provider staffing for immunization and/or pre-loading syringes may include:
- nursing agencies/ temporary-help agencies
- physicians and nurses who work in the community, health care institutions or facilities
- other health care providers such as paramedics, pharmacists, dentists, midwives
- medical, nursing and pharmacy students
In some jurisdictions, the scope of practice for some of the above providers may preclude their ability to immunize.
The inclusion of non-health care provider public health unit staff, other allied health professionals and volunteers, will help fill the non-health care provider roles required for an effective and efficient clinic. Assignment of activities and requirements for supervision will depend on each jurisdiction's specific regulations and/or policies and the individual's level of competence and experience.
Legislation and regulatory considerations
Immunization clinics utilize a broad range of staff and volunteers, including regulated health professionals. Each jurisdiction will have legislation and regulations governing the practice of health care professionals and delegation of authority within its jurisdiction, which should be considered in clinic staffing and assignment of roles and responsibilities. Additionally, based on jurisdictional requirements, medical directives to delegate the authority to immunize may need to be written, depending on the types of staffing used in the clinic.
Roles and responsibilities
Table 2 provides examples of clinic roles and activities in immunization clinic operations. Depending on the clinic setting and the size of the clinic and resources and needs of a community, some of these roles may be combined or excluded. Clarify which roles and activities will be carried out at headquarters/ the main office of the clinic organizers and which are required on site at the clinic locations. Many of the planning function will require a team approach, although only the lead position is listed below.
| Role | Activities |
|---|---|
| Campaign and clinic planning which can generally be performed at headquarters/main office and not at clinic locations | |
| Immunization campaign leader Role often performed by a director or manager with experience in immunization |
|
| Medical support Role often performed by medical health officer or other physician |
|
| Human resources/scheduling |
|
| Finance |
|
| Logistics Role can be performed by someone with logistical experience who understands procurement, immunization, the organization and the community |
|
| Administrative support |
|
| Epidemiology/data base support |
|
| IT support |
|
| Specialized support functions Role can be performed by a health care provider familiar with infection prevention control or occupational health and safety and cold chain management |
|
| Communications officer |
|
| On-site clinic activity | |
| Parking lot attendants Role can be performed by a volunteer, security guard or non-health care provider staff member |
|
| Security Role can be performed by a hired security guard or provided by the site. |
|
| Clinic leaders Role often performed by managers or senior nurses or other nurses with immunization experience |
|
| Clinic floaters Role often performed by senior nurses or other nurses with immunization experience |
|
| Greeters Role can be performed by a non-health care provider staff member or volunteer; could also be done by a health care provider |
|
| Registration Role often performed by administrative assistants, particularly if being done electronically |
|
| Syringe pre-loaders
Only needed in clinics where pre-loading occurs by non-Immunizers Role could be performed by a nurse, registered practical nurse, pharmacist |
|
| Runners Role could be performed by administrative assistants, non-health care provider staff members or volunteers |
|
| Client flow monitors Role could be performed by volunteers or non-health care provider staff members |
|
| Immunizers Role can be performed by nurses, doctors, paramedics, pharmacists, dentists, registered practical nurses, nursing and medical students, midwives (depending on jurisdictional requirements and legislation) |
|
| Medical support Role can be performed by a physician, nurse practitioner or nurse specifically assigned to this role |
|
| Post-immunization waiting area monitors Role can be performed by health care providers or by non-health care provider staff members or volunteers who inform health care providers if assistance is needed |
|
| Administrative support |
|
| Clinic specialized support Role can be performed by a staff member familiar with infection prevention control or occupational health and safety and cold chain management |
|
| Clinic support (roles that assist clinic staff as needed to efficiently deliver and maintain clinic activities) | |
| Translators / interpreters Trained translators optimal, but can use health care providers or volunteers who speak the required language |
|
| Custodial staff Can be provided by the facility operating the site |
|
| Example of the staffing to vaccinate approximately 1,000 people at a clinic |
|---|
The example provided below is an estimate of the number of staff for the key functions that could be used to vaccinate approximately 1,000 people during an immunization clinic. It assumes that:
These are rough estimates and may need to be adjusted to accommodate how the clinic is operating (e.g., online or onsite registration, pre-loaded syringes or syringes loaded by Immunizers), staff experience, the needs of the community, the size of the clinic site, and the available human resources. Estimated numbers of staff:
|
| Example of the staffing to vaccinate approximately 450 people at a clinic |
The example provided below is an estimate of the number of staff for the key functions that could be used to vaccinate approximately 450 people during an immunization clinic. It assumes that:
These are rough estimates and may need to be adjusted to accommodate how the clinic is operating (e.g., online or onsite registration, pre-loaded syringes or syringes loaded by Immunizers), staff experience, the needs of the community, the size of the clinic site, and the available human resources. Estimated numbers of staff:
|
| Practical tips for remote and isolated communities |
|
Orientation and training
Providing thorough staff orientation and training prior to the first clinic is vital to the effective functioning of immunization clinics. Staff and volunteers should be oriented to relevant administrative requirements such as:
- human resource forms
- scheduling
- time sheets
- who to call regarding shift changes or other questions
- if food and beverages will be provided for breaks
- appropriate clothing and personal protective equipment requirements for clinics
Staff and volunteers should also receive training on general issues related to clinic functioning and their specific roles and responsibilities. See Table 4 for some general issues to cover for all clinic staff.
As possible, develop clinic orientation and training manuals and materials, outlining all aspects of clinic operations. Orientation materials may include the administrative information described above, as well as the following:
- clinic objectives
- clinic roles and responsibilities
- staff and volunteer identification (e.g., name tags, which can indicate if the staff member is bilingual, use of colour-coded badges, vests, or arm bands)
- clinic flow, including diagrams
- client consent
- COVID-19 screening
- determining client eligibility for the particular clinic
- documentation requirements
- medical directives and links to product monographs
- adverse event management (e.g., fainting and anaphylaxis management)
- storage, packaging and transporting of supplies and vaccines, including cold chain management
- biomedical waste disposal
- occupational health and safety and infection prevention and control, including the use of personal protective equipment, recommended immunizations, and the handling of sharps
- management of needle stick injuries
- privacy and confidentiality
- cultural sensitivity and responding to the diverse needs of clients (e.g., older adults, children, people with disabilities, people who speak other languages, cultures with the need for privacy when exposing their skin)
Role specific training materials can also be provided for key roles performed by various staff members. For example, materials for:
- health care providers can describe the roles of Clinics Leader, Clinic Floater, Immunizer, Syringe Pre-Loader, Medical Support and Clinic Specialized Support
- administrative assistants can describe the Registration and Runner roles
- volunteers and other non-health care provider staff members can describe roles of the Parking Lot Attendants, Greeters, Client Flow Monitors and Post-Immunization Waiting Area Monitors
See Table 4 for some training topics for all staff and some of the health care provider roles.
Provide orientation and training through multiple channels and offer numerous opportunities for participants (e.g., online sessions that are self-directed or part of scheduled online meetings; written materials; opportunities to ask questions; group chat functions). Consider using checklists based on the roles the individual will fulfill to ensure that all aspects of orientation and training are covered. If time permits and as needed, consider a dry-run exercise to reinforce training for all roles involved in the clinics, including the management of fainting and anaphylaxis.
If applicable based on jurisdictional requirements, ensure that processes are in place to provide specialized training as required for staff (e.g., immunization certification, infection prevention and control, handling sharps, storage and handling of vaccines, data entry programs, anaphylaxis management, cardio-pulmonary resuscitation (CPR)) in advance of clinic opening. Although specific health care providers at the clinic are designated to manage fainting and anaphylaxis (e.g., Clinic Leader, Clinic Floater, Medical Support), all health care providers should be trained in fainting and anaphylaxis management and have up-to-date CPR (based on provincial/ territorial/ local requirements).
'Just in time' orientation and staff training in advance of beginning the clinic can focus on:
- important features of their role
- areas that are known to cause problems or confusion based on experience at previous clinics
- issues that have been noted from previous clinics, and
- changes in processes from previous clinics
To avoid aggregating of staff members, consider providing this information in advance of the clinic via email or at the clinic individually to each staff member. If staff do need to aggregate for 'just in time' orientation at the clinic, to minimize the risk of COVID-19 infection, ensure physical distancing and that medical masks and eye protection (e.g., face shields) are worn at all times, avoid shouting and keep the sessions as short as possible.
Consider specific support for staff who are new to the immunization clinic such as special attention from the Clinic Leader or Clinic Floater, or a buddy system with a more experienced staff member.
Table 4: Possible topics to be covered during training for all staff and health care providers
For all staff
- The roles and responsibilities of all clinic staff and clinic flow.
- Administrative details such as human resource forms, scheduling, time sheets, who to call regarding shift changes or other questions, if food and beverages will be provided for breaks, appropriate clothing to wear and appropriate footwear.
- Cultural and diversity sensitivity (need for privacy during immunization for some cultures, supporting people who only speak languages other than English or French (depending on the jurisdiction), assisting people with physical or developmental disabilities or mental health concerns, using appropriate and sensitive language).
- Infection prevention and control recommendation and other occupational health and safety issues (e.g., hand hygiene, recommended immunizations for staff and volunteers, the required personal protective equipment and how to properly use the equipment, how to prevent and report needle stick injuries and falls).
- Managing people who do not meet the eligibility criteria, who fail screening for COVID-19 or who do not comply with wearing a mask or physical distancing.
- Managing people who have concerns or complaints or who are upset or angry.
- How to recognize and manage possible abuse (of children, partners, or staff).
- Where to direct requests from the media.
Additional training specific for health care providers (e.g., Immunizers, Syringe Pre-Loaders, Medical Support, Clinic Specialized Support, Clinic Floater, Clinic Leader)
- Information about COVID-19 and the available vaccines to be able to respond to client questions, including questions from those who are hesitant about receiving the vaccine.
- Strategies to manage pain and fear in vaccine recipients, including children.
- How to determine capacity to consent based on age or cognitive functioning.
- How to assist parents in appropriately holding young children for immunization.
- How to seek informed consent, counsel clients and identify contraindications, prepare and administer the vaccine (including appropriate land-marking) dispose of the used needle and syringe, and conduct post-immunization counseling.
- How to perform appropriate documentation for the clinic and the client.
- How to identify and manage a client who may faint, and how to manage fainting and anaphylaxis.
- How to prevent and manage needle stick injuries.
- Proper storage and handling of the COVID-19 vaccines for staff members in specific roles (e.g., Clinic Specialized Support, Clinics Floater, Clinic Leaders), including the appropriate handling of dry ice, if required.
- Refresher on CPR if needed.
Additional training specific for clinic leaders
- How to respond to scenarios such as power outages or other reasons for loss of IT functions.
- How to managing challenging public relations issues (e.g., client not adhering to infection prevention and control requirements, long waits, large crowds, angry clients, client complaints, client injuries).
- Management of media requests and onsite media visits.
- Reporting of adverse events that occurred during the clinic to public health officials.
For additional information on training content and tools for immunizers, see the suggested links below:
- Immunization Competencies for Health Professionals (PDF)
- Education Program for Immunization Competencies
Infection prevention and control
Immunization clinics for COVID-19 vaccines are likely to be taking place while ongoing transmission of COVID-19 is occurring in the community. Preventing transmission of infection at clinic sites is essential. The Public Health Agency of Canada (PHAC) has developed Guidance for influenza vaccine delivery in the presence of COVID-19, which outlines strategies which are also relevant to COVID-19 immunization clinics. Some key infection prevention and control strategies are as follows:
Staff and volunteer immunizations: The following immunizations are recommended for staff and volunteers of immunization clinics (unless contraindicated):
- COVID-19 immunization with the appropriate available vaccine (the first dose can be obtained at the first clinic they work / volunteer at, if it cannot be obtained earlier).
- Consider running an initial clinic just for staff and volunteers who will be working at clinics, which will allow them to receive their immunizations and test out the clinic processes.
- Hepatitis B immunization and measurement of hepatitis B surface antibody titres (if possible to obtain titres)
- For any health care provider or people who may handle sharps containers
- Up-to-date tetanus immunization (if possible)
- Influenza immunization if the clinics are occurring during influenza season
Screening of staff, volunteers and clients for illness/exposure to COVID-19 by the use of signs and active screening (based on provincial / territorial recommendations) before entry into the clinic, either through in-person or telephone questions on arrival. Any online screening done prior to the clinic should be reviewed on entry into the clinic to ensure that there has been no change in health status.
Staff who feel unwell should not attend their shift and should communicate with the Human Resource / Scheduling personnel through pre-arranged methods. Plans should be in place to rapidly find replacements for staff who cannot work due to illness.
Strategies to support physical distancing, such as:
- Scheduling appointments for immunizations. This is an efficient mechanism to prevent crowding while supporting a consistent flow of clients. Appointments can be booked online and via a telephone hotline. Vaccine Information Sheets and Consent Forms can also be provided and completed online.
- Having people wait in their vehicles or outside and being called into the clinic via a phone call or text message at their appointment time.
- Ensuring clinic processes and flow minimize interactions and time in any given area of the clinic. Strategies can include on-line completion of Consent Forms before arrival at the clinic. The clinic should be set up to ensure unidirectional flow of clients.
- Ensuring any seating is physically distanced (at least 2 metres apart). Use tape on the floor to mark the spots were seats should be spaced. See Section on Post-immunization Waiting Period regarding post-immunization waiting strategies.
- At the immunization tables, place seats so that the client sits with their shoulder facing the Immunizer. The client's face should face away from the Immunizer and the immunization table.
- Closely monitoring and re-enforcing physical distancing between clients, staff and volunteers. If clients need to wait in line, use tape or pylons to mark where they should stand so that they remain 2 metres apart.
Infection prevention and control (IPC), including:
- Installing clear plastic barriers at reception areas and between immunization stations, if possible.
- Providing alcohol-based hand rub stations throughout the clinic site, including on entry, at each immunization station and at the exit. Alcohol-based hand rub should be used by Immunizers before and after each immunization and by Syringe Pre-Loaders before touching vaccine vials, needles or syringes, and after other contact with a client. Hands should be washed with soap and water if visibly dirty.
- Set up Immunizer stations so that clients do not touch table surfaces. If table surfaces are touched, advise client to use alcohol-based hand rub and wipe the surface down afterwards with a disinfectant.
- Personal protective equipment for staff and volunteers:
- The use of medical masks and eye protection (e.g., face shields) by all staff and volunteers except if behind a complete physical barrier is recommended. If behind a complete physical barrier, only masks are recommended. Mask can be worn for extended periods during the clinic but should be changed if damp or damaged and after removal (such as after removal for eating).
- Additional personal protective equipment such as eye protection, gowns and gloves should be immediately available to personnel who need to provide first aid or respond to an emergency.
- All staff and volunteers should receive appropriate training regarding the proper use of personal protective equipment, including appropriate donning and doffing of equipment and hand hygiene.
- The only exception to wearing a mask is while eating and drinking. See below for additional information regarding food and beverages at clinics.
- Gloves are not recommended for Immunizers or Syringe Pre-Loaders unless they are administering an oral or intranasal vaccine. Immunizers or syringe pre-loaders with non-intact skin on their hands should discuss their condition with the Clinic Leader; wearing gloves or not working that shift may be options to consider. If gloves are worn due to non-intact skin, they must be changed between each client and hands must be cleaned after gloves are removed.
Mask use by clients:
All clients should wear a mask (cloth mask is sufficient unless there are specific jurisdictional requirements) at all times during the clinic (except for children less than 2 years of age, those who cannot tolerate a mask or those who require removal of the mask for medical attention). If a client attends the clinic without a mask, they should be provided with a disposable mask. If the client cannot wear a mask, options include one or more of the following:
- Asking the client to wear a face shield, which may offer some protection from droplet transmission but is inferior to a mask. The face shield should cover the sides of the face and extend below the chin;
- Moving the client through the clinic quickly and asking them to wait in their vehicle (if possible) during the post-immunization waiting period;
- Providing the client with an appointment when there are fewer other clients in the clinic (e.g., before the clinic begins or the first appointment in the day, or at the end of the clinic).
Environmental objects and surfaces:
- Avoid sharing of common objects such as pens and clip boards if possible. If these must be used, ensure appropriate hand cleaning afterwards, and wipe down objects with a disinfectant wipe if possible.
- Clean and disinfect frequently touched surfaces, such as counter tops, railings, and door handles, periodically during the clinic. Ensure washrooms are cleaned and disinfected frequently.
- Avoid table cloths near where client is sitting at the Immunizer tables, so that tables can be wiped down if touched by clients.
Food and beverages at the clinic:
Consuming food and beverages at the clinic may increase the risk of COVID-19 transmission as it requires the removal of masks. Any food or beverage provided by the clinic for staff and volunteers should be individually packaged and provided in a manner to avoid staff and volunteers from congregating. If possible staff and volunteers should eat offsite (e.g., outside or in their vehicles) or if needed, they should eat in large, well-ventilated dedicated staff spaces, maintain a distance of at least 2 metres between individuals, and minimize the number of people in the staff room at any one time (i.e., breaks should be spaced out). Before returning to the clinic area, hands should be cleaned, and eye protection and a new medical mask put on.
Communications
Timely, clear and frequent communication with the public and staff is crucial for the successful implementation and delivery of immunization clinic operations. Important considerations in planning clinic communications include identifying the following:
- Lead spokesperson
- How frequently messages will be communicated
- How messages will be coordinated and conveyed
- Target audiences (languages required for translation of written materials, cultural appropriateness, readability including size of font, reading level of language)
Messages and information can change frequently as new information becomes available. Therefore, communication plans should be flexible and dynamic. Social media channels are an effective way to provide information updates if circumstances change. Where feasible, it is recommended that targeted communication material be prepared in advance and circulated as early as possible before the planned clinic dates.
Public communication (external)
Messages about the pandemic vaccine should be clear, transparent and timely. They should clearly indicate what is known about the vaccines, what is unknown or uncertain, and what is being done to address uncertainties and to monitor safety. Examples of key points to communicate:
- Why vaccine is being offered and expected availability.
- Reassurance that clinics are working hard to get the vaccine to the public in a timely manner and that the vaccine will eventually be offered to all who want it.
- The benefits of receiving the vaccine, both to the individual and the community, including known efficacy and effectiveness data as it becomes available.
- What is known about vaccine safety and how vaccine safety is being monitored, including what to expect and watch for after immunization and how to respond (including the reporting of serious or unexpected adverse events).
- Who is eligible to receive the vaccine and why (e.g., priority groups, age groups, general population).
- Differences between vaccine formulations (if more than one formulation is available), including mechanism of action, efficacy, intended recipients, contraindications, side effects, the need for one or two doses and the interval between doses if two doses are required).
- Clinic locations, hours of operations, parking and directions, including for public transportation.
- Overview of clinic activities to be expected, including:
- the vaccine(s) being offered and if a second dose will be required and when.
- if appointments will be needed and if so, how to make them, and if online consent forms are available. Options should be provided for those who cannot access the information online. Information on cancelling or changing an appointment should be provided.
- COVID-19 screening protocols, including instructions to postpone any clinic visit if symptoms develop or if recent exposure to a person with COVID-19. Local public health officials should be consulted about the appropriate timing for immunization in individuals with symptoms, COVID-19 exposures or who tested positive for COVID-19.
- the need to bring and wear a mask at all times while at the clinic (except if less than 2 years of age or cannot tolerate wearing a mask).
- expected wait times (updated frequently or in real-time while clinics are operating if possible).
- directions to bring appropriate identification and/or a health card (if necessary).
- directions to wear appropriate clothing (short sleeve shirt or shirt that can be rolled up to access the shoulder).
- direction to ensure that they have eaten appropriately during the day to minimize fainting.
- Reinforcement of the need to continue to follow COVID-19 prevention recommendations despite immunization, including physical distancing, wearing a mask and limited contact with others as per the recommendations of public health officials.
In addition to messages for the general public, specific mechanisms should be selected to reach those populations specifically targeted for vaccination at a given point in times (e.g., seniors, long term care facilities, health care providers). Also consider specific communications for health care providers, since even if health care providers are not currently involved in providing immunizations, they are a key source of reliable information for clients making decisions regarding COVID-19 vaccination.
Local emergency services personnel and nearby hospitals should be informed of the dates, times and locations of each clinic, so that medical support can be rapidly available if needed.
| Materials | Medium | Format |
|---|---|---|
|
|
|
Technologies such as social media and instant text messaging make it easier and faster for public frustrations and concerns with immunization clinics to be publicized. These situations should be anticipated and methods to monitor and respond to them should be identified.
For additional tips and facts about communicating, see:
- Canadian Immunization Guide Part 1: Key Immunization Information. Communicating effectively about immunization. August 2016
Clinic staff and volunteer communication (internal)
Methods will be needed to efficiently convey new information to clinic staff and volunteers. Communication with staff and volunteers should be clear and timely to support effective clinic operations. Effective communication practices provide staff and volunteers with people to contact and sources to check to receive information, and lines of communication to relay information, feedback and concerns. See Table 6 for examples of strategies to communicate with staff and volunteers.
Table 6: Methods to communicate with staff and volunteers
Examples of methods to communicate with staff and volunteers
- Regular emails and/or text messages
- Websites used as a repository for shared information
- One-on-one relaying of information before the clinic starts
- Meetings before the clinic opens to the public ('Just in time' training) and debriefs after each shift. If using these approaches, care must be to taken to maintaining physical distancing and wearing masks and eye protection at all times. Shouting should be avoided and meetings should be kept as short as possible.
- Regular teleconferences or webinars
- Regular touch base meetings with Clinic Leaders/ Clinic Floaters to ensure consistency of clinic operations, share ideas and problem solve
Data management
It will be important to identify the methods to collect, manage, store and transport data (e.g. paper and/ or electronic) and to establish appropriate systems to support secure data management, based on jurisdictional legislative and policy requirements. Types of data can include:
- Administrative data such as information on staff and volunteers including contact and banking information (as appropriate); credential verification; staff scheduling; and compensation.
- Clinic-specific data: See Section regarding the information collected on the Daily Clinic Summary.
- Client-specific data: See Section regarding the information collected on the Consent Form.
How client information from the Consent Forms will be captured electronically will need to be determined, including if it will be entered online directly by the client, entered electronically at the clinic by the client or person doing the registration, or entered on paper and then transcribed to a data management system.
Data management systems should easily support the generation of information required for provincial/territorial and/or federal reporting requirements. For the overall campaign, considerations should also be given on how to analyze and report on coverage including numbers vaccinated overall, in the groups targeted for immunization such as those with underlying medical conditions and working in various occupations, and in various sociodemographic groups (age, gender, race) and geographic regions.
Staff training should emphasize the maintenance of privacy and confidentiality and the procedures and policies to respond to any possible breaches. Contingency plans should be in place in case electronic data systems are not working.
Supplies
Non-vaccine clinical supplies
Some clinic supplies will be procured and supplied by the Government of Canada (e.g., syringes, needles, alcohol swabs, bandages, gauze, sharps containers), while other equipment will need to be procured by the province, territory or local jurisdiction or supplied by the immunization site. Appendix 1 offers a suggested list of supplies that can be adapted to jurisdictional needs.
Planning parameters and numbers of people who need to be immunized form the basis for the quantity of supplies needed. Processes and systems will be required to order and receive supplies, monitor inventory and store and pack supplies. Consider a large room where supplies can be stored and organized, with the list of supplies guiding the packing for each clinic. Supplies can be loaded into bins or tubs that are labelled with types of supplies in each one. If operating more than one clinic at a time, consider colour-coding the bins so that the same colored bins go to the same clinic. Additionally processes will be needed transport supplies to each clinic location, to re-supply clinics that remain at the same location over a period of time, and to receive supplies back from clinic sites that are no longer operating. Supplies that remain at the clinic location must be stored securely but be easily accessible to clinic staff. Protocols to replace missing or stolen items on an urgent basis should be developed.
Vaccines: Storage and handling and cold chain maintenance
Special attention will be required for the storage and handling of COVID-19 vaccines, as some COVID-19 vaccines may require storage at ultra-low temperatures (- 80 oC) or freezer temperatures (- 20 oC), while others will required usual + 2 to 8 oC storage, as per manufacturers' specifications. The vaccines requiring storage at ultra-low or freezer temperatures will have specified periods of time where they can be stored at + 2 to 8 oC prior to use. The date and time when these vaccines are put in the refrigerator and when they need to be used by should be marked on the carton, box or paper kept next to or attached to the vial (do not obstruct the vial label with any paper attached to the vial).
If vaccine is being stored at the clinic site overnight, special attention will be required to ensure that this can be done securely. In some clinic settings, equipment may not be available at clinic sites to store vaccine overnight or the location may not be sufficiently secure and therefore arrangements will need to be made to transport vaccines to and from vaccine storage sites, respecting cold chain and transporting requirements for the products and never transporting the vaccine in the trunk of a car. Equipment such as frozen packs, vaccine coolers, insulated bags, dry ice and the personal protective equipment required to manage the dry ice (depending on the product), and thermometers / data loggers will be needed to maintain appropriate cold chain at the clinic site, as well as during transport to and from the clinic.
Protocols will be required for monitoring and recording the vaccine storage temperatures, particularly if the vaccine is being stored in vaccine coolers or insulated bags, instead of in a refrigerator or freezer. Assign a specific staff member (e.g., Clinic Specialized Support, Clinic Floater) to monitor and record vaccine temperatures at specified frequencies, including on arrival and at the end of the clinic and periodically during the clinic, as per jurisdictional requirements.
Vaccines may have specified time frames when they can be kept at room temperature, used once mixed with diluent or adjuvant, used once the vial is punctured and/or when pre-loaded into a syringe. The start time for these time frames should be written down and the paper stuck to or kept right near the product so that the times frames can be closely monitored. The manufacturer may have specific recommendation on what should be marked on the vial. The end time can also be written down; it both times are used, it should be clear which time is the start time and which is the end time. Mixed vaccine vials and pre-loaded syringes that are not being used immediately may need to be stored in an insulated bag or cooler or may be able to be stored at room temperature, based on the manufacturer's recommendations. If stored in an insulated bag or cooler, these should have frozen packs and an appropriate insulating barrier (e.g., bubble wrap, crumpled paper, Styrofoam peanuts) which is positioned so that the vaccine vial or pre-loaded syringes do not touch the frozen packs to prevent freezing the vaccine. If the vaccine remains in the insulated bag for more than an hour, temperature should be monitored and recorded.
Refer to relevant jurisdictional vaccine storage and handling guideline documents or the current National Vaccine Storage and Handling Guidelines for Immunization Providers for information on cold chain management, vaccine storage, temperature monitoring and transportation requirements.
Vaccines stored at ultra-low temperatures (-80 oC) or in a freezer at -20 oC will need to be thawed before use and cannot be refrozen. Manufacturer's instructions should be followed regarding the thawing process in the refrigerator and/or at room temperature. Each vaccine has a limited number of days when they can be maintained at +2 to 8oC before administration. Therefore, sufficient supply to accommodate the anticipated needs of the clinic should be thawed and available at the clinic. The date and time the product was thawed and the date and time which it should be used by should be clearly marked as noted above. Some vaccines may be required to come to room temperature before administering.
A plan should be made to ensure the use of any extra thawed vaccine, which may include using it at a clinic over the next few days, or using it in a congregate living setting or health care provider's office based on current eligibility criteria (assuming is it appropriate to transport the thawed vaccine and it is transported under appropriate cold chain conditions). If thawed vaccine cannot be transported and is approaching the maximum time at refrigerator temperature or the clinic will not be returning to the same site in the near future, consider contingency planning such as a waiting list of people eligible for vaccination who can be called into the clinic on an urgent basis to receive any remaining doses. If that is not possible, consider other approaches to offering the vaccine in the following order: those who are currently eligible; those who are likely to soon become eligible; others as appropriate. It is important not to waste dosages. Decisions that are made to give doses to those who are currently not eligible in order to avoid wastage should be documented.
Non-clinical supplies
Once sites have been selected, it is advisable to identify non-clinical supplies, such as tables, chairs, mats, garbage cans, pylons / stanchions, wheelchairs and electronic equipment that are available on-site for use during clinic activities. This will vary by site. For example, a school may have tables, chairs and mats available for use, while an arena or shopping centre may not, requiring that they be purchased, rented or borrowed. If non-clinical supplies are to be purchased, consider where they will be stored after site closure. Appendix 1 offers a suggested list of electronic, administrative, cleaning and furniture supplies that can be adapted to jurisdictional needs.
Availability and reliability of internet connectivity should also be assessed if that is required for clinic operations, as should locations of power supplies for electronic equipment.
Signage
Clinics should display clear signs with directions to guide clients through clinic stations, so that efficient movement through the immunization process is facilitated. Signs should be clear, in large font and appropriate language, employ features to enhance accessibility and visibility (e.g., high contrast) and where applicable, should use a combination of text and images (e.g. directional arrows). Consider plans and procedures to assist and navigate visually impaired clients. Examples of clinic signs are the following:
- If the clinic is located in a large building, maps and arrows showing directions to clinic area
- Marking entrance and exit points
- Eligibility criteria for the clinic if only specific populations are being vaccinated
- COVID-19 screening questions
- Instructions for clients (e.g., contraindications for the vaccine, need to wait 15 minute after immunization, need to wear a mask and perform hand hygiene)
- Directional arrows to guide people through clinic stations
- Identification of stations (e.g. Registration, Immunization, Post-Immunization Waiting Area, First Aid, washrooms)
Key documents
The following outlines the key documents that may be required in either paper or electronic format. The need for these documents may vary depending on clinic processes. All information sheets and forms for the public should be accessible, clearly written, easy to understand and available in multiple languages based on jurisdictional needs.
- Vaccine Information Sheet: Required to assist with obtaining informed consent. Contains information about: the disease; the vaccine (including who it is indicated for and composition); the benefits and risks of receiving or not receiving the vaccine; contraindications; alternative methods to reduce the risk of acquiring COVID-19 and potential adverse events.
- Consent Form: Used to document consent by the client and receipt of the vaccine by the Immunizer. Contains information such as: name; health card; date of birth; gender; race; various groups targeted for immunization such as those with underlying medical conditions and working in various occupations; phone number; address; email address; screening questions for contraindications and side effects following first vaccine dose (if receiving the second dose of vaccine); brand and generic name of vaccine product received including lot code and expiry date; dose, site and route of vaccine administered; date and time vaccine administered; and name and professional designation of immunizer.
- After-Care Sheet: Used to provide information and advice after immunization. Contains information such as: expected adverse events (e.g., redness at site of injection, sore arm) and how to respond; advice on monitoring for, responding to any serious adverse events (e.g., hives, difficulty breathing, facial swelling); reporting adverse events as per jurisdiction protocols; need for and timing of a second dose (if indicated); need to continue to follow COVID-19 precautions on an ongoing basis (e.g., physical distancing, mask use) as recommended by public health officials.
- Client Immunization Record: Used to record the immunization the client received to provide to the client. Can be paper-based or electronic. Contains information such as: name; data of birth; health card number; vaccine product received and lot number; date of administration; and name of immunizer. The Client Immunization Record can be combined with the After-Care Sheet if appropriate. The client should keep this document in an easily accessible location and bring it with them for their second dose of vaccine; this will help to ensure that the client receives the same vaccine product for both their first and second dose.
- Daily Clinic Summary: Used to record key information for each clinic such as: the date and location of the clinic; names and roles of staff and volunteers at each clinic; vaccine product(s) and lot number(s) administered at the clinic; number of clients immunized; number of missed appointments (if using an appointment system); number of clients who registered but were not vaccinated; incidents that occurred (e.g., needle stick injury, fainting or anaphylaxis); vaccine wastage; supply issues; client/staff feedback; and media interest.
- Medical Directive for Obtaining Consent and Administering Vaccine: A medical directive is a written order that pertains to any patient who meets the criteria outlined in the medical directive. It is required in some jurisdictions to allow a health care provider authorized to perform a controlled act (e.g., immunization) to delegate the performance of that act to another health care provider or group of health care providers. The Medical Directive contains information such as:
- who is delegating the act
- what types of individuals are authorized to performed the controlled act under the medical directive
- what act is being delegated (e.g., obtaining consent for and administering specific COVID-19 vaccines)
- what needs to be in place for the delegation to occur (e.g., training, appropriate equipment, ability to manage anaphylaxis)
- to which clients the medical directive applies and what are the contraindications to immunization
- how the delegated act should be performed
- the documentation and communication required
- how will the medical directive be reviewed
- how will quality be assured under the medical directive
- the signature of the health care provider who is delegating the act and any organizational approvals
- Medical Directive to Manage Anaphylaxis: Similar to above except specific to the management of anaphylaxis, including the use of appropriate medications. Can be accompanied by an Anaphylaxis Medication Quick Reference Dosage Card which summarizes the age and weight-based dosages for the various medications. See the Early vaccine reactions including anaphylaxis chapter of the Canadian Immunization Guide for these dosages.
- Serious Event Form - for clinic use: Used for internal documentation of serious adverse events such as anaphylaxis or fainting with an injury. Contains information such as: the name, address and phone number of the person affected by the event; a description of the event including the date, time and any observers to the event; information on the vaccine product received including the brand name, lot number, date, time, and site of vaccine administration; the management taken (e.g., medications given, details on transfer to a health care facility); follow-up communications within the organization and with the client; the name and phone numbers of staff and volunteers involved, and any other relevant details. The official provincial / territorial adverse events following immunization (AEFI) form should also be completed for all episodes of anaphylaxis or other adverse events as per jurisdictional reporting requirements.
- Incident Report: Used to document an accidents or injuries such as needle stick injuries, fainting with no injury, falls or other occurrences. Contains information such as: the name, address and phone number of the person affected by the event; a description of the event including the date, time and any observers to the event; for needle stick injuries, includes name and contact information for the source patient; the management taken (e.g., medications given, details on transfer to a health care facility, recommended tests); follow-up communications within the organization and with the client; the name and phone numbers of staff and volunteers involved, and any other relevant details.
- Post-Clinic Evaluation Form for Staff and Volunteers: Can be used by staff and volunteers to provide feedback on their experience at the clinic. See Appendix 2 for a possible template. This form can be completed at the end of the clinic or a link to an online version can be sent to the staff and volunteers via email or text.
- Client Evaluation Form: Can be used by the clients to provide feedback on their experience at the clinic. See Appendix 3 for a possible template. This form can be completed in the Post-Immunization Waiting Area or a link to an online version can be sent to the client via email or text.
- Time Sheets: Used to record information regarding the hours staff members worked for the purposes of receiving payment. Contains information such as: the name, phone number and address of the staff member; the date and location of the clinic; the start and stop times the staff member worked; the name and signature of the authorizing Clinic Leader. Volunteers who require recording of their hours may bring their own forms with them, or the time sheet can be used for this purpose if needed.
- Supply/Re-supply List: Used to determine what supplies need to be delivered to a clinic site that is operating over a number of days. Contains a list of all the supplies that are required for the clinic (see Appendix 1) and a column to indicate the quantities needed to re-supply the clinic.
All forms that contain personal or personal health information should conform with jurisdictional information collection and privacy requirements.
Planning considerations for pediatric COVID-19 immunization clinics
The purpose of this section is to assist in planning pediatric COVID-19 immunization clinics that are efficient and physically and emotionally comfortable for children and families. The intended audience for this guidance are public health authorities and personnel who are planning for pediatric vaccine rollout in their jurisdictions. The following provide general guidance for consideration, which should be modified based on specific circumstances.
Pediatric clinic types
The types of pediatric clinics available to families will vary by community. When planning the availability of pediatric vaccines for a community or region, consider the advantages and challenges of different clinic types.
| Clinic types | Advantages | Challenges | Mitigation measures |
|---|---|---|---|
| Community clinics |
|
|
|
| Health care provider (HCP) office |
|
|
|
| School-based clinics while school is operating |
|
|
|
| Pharmacies |
|
|
|
Community-based pediatric clinics
To improve the overall vaccination experience of children and families, special considerations should be taken to adapt the clinic environment and plan the vaccination process to be as child-friendly as possible.
Environment
- Consult Child Life Specialists or others with expertise in child development to equip clinic with strategies to promote coping and minimize stress and anxiety for children and parents/guardians. Budget for additional materials and supplies to adapt the environment for children.
- Aim for a light and child-friendly atmosphere using, for example, colourful decorations and signage. Child-friendly entertainment for pre- and post-immunization waiting rooms may be considered; however, ensure that public health measures are followed and avoid attractions that could promote crowding in large groups.
- Avoid music, which makes it hard to hear and requires people to talk loudly.
- Equip immunization stations with distracting objects or toys for children to hold or play with that can be easily cleaned between clients or are single-use (e.g., stickers, printed colouring pages). When booking their child’s appointment and in electronic communications, parents can be advised of strategies to improve the experience, such as bringing a comfort item (e.g., stuffed toy) or electronic device from home.
- Provide options for privacy such as curtains, cubicle walls, colourful blankets draped or hung from the ceiling, so that children do not see others being vaccinated upon entering the clinic and during their own vaccination.
- Schedule appointments in order to avoid large groups of parents and children in the waiting area.
- Create a private, relaxing space for children who are highly anxious or who have developmental disabilities to wait for their appointment. Consult with a Child Life Specialist or other knowledgeable professional about how to create this type of space.
- Ensure adequate security as per recommendations in the human resources section.
Immunization rate
- Prepare for more human resources and a lower rate of immunizations. Compared to adult clinics, it may take more time for each vaccination to answer parents’ questions and make children feel ready to be vaccinated.
- Support vaccinating siblings together if they are eligible for vaccination, which may speed up the immunization rate.
- Encourage staff to take their time and not to rush during the process.
Training
- See the orientation and training section.
- Specific to the pediatric population, provide training for staff on:
- How to make children comfortable, including using age-appropriate distraction techniques that are available at the clinic.
- How to manage and support children who are highly anxious and/or unwilling to be vaccinated, including children’s rights in these circumstances.
- How to manage and support children with particular developmental and/or emotional needs.
- How to manage situations when the child attends with someone who is not a parent or legal guardian.
- How to identify and respond to issues that may require involvement of child protection services, in consultation with clinic leaders.
- How to respond to adverse events in children.
School-based clinics
Public health authorities may opt to use schools as clinic sites due to the advantages described in Table 7. Planning considerations will depend to some extent on whether clinics are planned for during school hours, outside school hours, or both. School clinics operating outside of school hours, including on weekends, are essentially community clinics that may provide COVID-19 vaccines, and/or other vaccines such as influenza, for other age groups (e.g., siblings and family members) in a familiar, easily accessible location. Please see the section on planning considerations for multi-product vaccination clinics.
Collaborate with school administrators and community
- As far as possible in advance, liaise with school boards to establish expectations, roles and responsibilities of both school authorities and public health, with respect to clinic implementation. Discuss the need for security at each school-based clinic.
- Liaise with school representatives about clinic scheduling to maintain continuity of essential school functions and minimize educational disruption. Determine if parents would be allowed to come to the school to support their child during the vaccination process if desired.
- Work with school representatives to determine appropriate locations in the school, flow of the clinic, and how students will travel to and from the clinic and their class while maintaining public health measures.
- Identify non-clinical equipment (e.g., child-sized chairs, mats, tables, signage) at the site that can be used for the clinic, and what needs to be purchased, rented, or borrowed from other facilities.
Communicate with parents and children about vaccination options
- Develop a communication strategy to address parents’ questions and concerns and promote the return of consent forms (e.g., via email, newsletters, a virtual “town hall” meeting). Collaborate with school board or local school officials to determine appropriate communication mechanisms. Ensure that positive vaccine-related imagery is used in all communications.Footnote 2
- Consider offering parents the option to attend the school-based clinic if desired.
- Provide advice to parents whose children may need extra support during the vaccination process. Parents of these children may choose to attend the school-based clinic with their child if that is an option. Alternatively, parents of these children may choose to bring their child to another location in the community where they can attend with their child.
- Fear of pain and needles is common and should be taken seriously to support the child through the current process. A positive experience with the first COVID-19 vaccination supports receipt of subsequent doses and future immunization experiences. Pain management strategies that can be employed at school-based clinics should be communicated with parents in advance so that the child can come prepared (such as bringing objects that can provide distraction or comfort to the child, or using topical anesthetic cream). For more information, visit kidsinpain.ca.
Get informed consent
- See the key documents section for general information and sample vaccine information sheets, consent forms and after care sheets. Specific to school-based clinics consider:
- Ensure vaccine information sheet and consent forms are simple and clear and contain the appropriate information for the parent/guardian to provide informed consent.
- Consider asking for the child’s weight on the consent form and the date of the weight in case this information is needed for epinephrine dosing in the event of an anaphylactic reaction.
- Provide mechanisms for parents to contact the health unit if they have questions (e.g., phone, email or text) on the information sheet.
- Ensure multiple means of contacting the parent/guardian and an appropriate alternate are provided on the consent form in case of an emergency.
- Ensure written materials are available in languages appropriate to the school, including vaccine information sheets, consent forms, after care sheets and signage.
- Develop a system for checking consent forms ahead of the clinic date or start time so that clinic staff can ensure that there are no contraindications or precautions and verify or clarify consent form information with parents/guardians.
- Consider ways to reduce reliance on paper consent forms carried by children to increase response rate (e.g., communicating directly with parents by email with secure electronic consent forms).
- Consider if a system for following up with parents/guardians who do not respond regarding consent forms is appropriate.
- For older children/adolescents, plan for scenarios in which children want to be immunized without parental consent. Know the regulations in your jurisdiction about informed consent of minors.
Logistics
- Order vaccine and clinic supplies based on returned (or anticipated) consent forms with extra in case of last minute consents.
- Ensure an adequate number of staff and volunteers based on anticipated number of children to vaccinate.
- Plan transportation to and from the school for equipment and vaccines.
- Provide education and training for school staff and volunteers so that they are prepared to assist with clinics, maintain clinic flow, and get children from the class and return them to the class.
- Children can return to class after the vaccination to be observed by the teacher for the 15 minute post-immunization period (children should avoid going outside for recess or lunch during that period). Teachers, other school staff and volunteers should know to return to the clinic with any child who is feeling unwell post-immunization and how to obtain clinic staff if needed to respond to an emergency.
- Consider what processes will be required to identify each student correctly, particularly for younger children (e.g., identified by the teacher, holding or wearing paper with their name, asking each child to provide their name).
- Plan to securely handle and store personal health information (e.g., consent forms), and consider human resources needed to manually enter data into a database or immunization registry (if applicable).
- Conduct a debrief to discuss successes and challenges of school-based clinic implementation and document an improvement plan for future immunization clinics at the site.
Pre-immunization processes
- Promote pre-registration to avoid large groups and long wait times within the clinic.
- At time of booking their child’s appointment, parents should be provided information about pain management and comfort options that require planning (e.g., over-the-counter topical anesthetic including how and when to apply it in advance of vaccination, bringing familiar items like stuffed toys or electronics). For more information, visit kidsinpain.ca.
Immunization processes
- Train immunizers in all applicable vaccine administration practices outlined in the Canadian Immunization Guide.
- Prepare or make available communication guides and frequently asked questions and answers, to help immunizers answer parents’ and children’s questions about COVID-19, and the benefits and risks of vaccination using plain language.
- Use pain management techniques.
- Use strategies to promote coping and improve the vaccination experience (e.g., the CARD system – Comfort, Ask, Relax, Distract).
- Refer families to alternate clinic options where they exist if the vaccination cannot be completed at that visit (e.g., clinics at children’s hospitals that offer extra support for those with needle phobia, general anxiety, or behavioural or sensory needs).
- If a child 5 to 11 years of age inadvertently receives a 30 microgram dose of Pfizer-BioNtech Comirnaty, or an adolescent 12 years of age inadvertently receives at 10 microgram dose of Pfizer-BioNtech Comirnaty, refer to the guidance on managing COVID-19 vaccine administration errors or deviations.
Post-immunization waiting period
- For infection prevention and control purposes, encourage children not to congregate during the post-immunization waiting period.
- Consider child friendly activities that comply with COVID-19 infection prevention and control guidelines as outline in the Environment section above.
- A token of congratulation for being vaccinated may be considered (e.g., stickers, toys); however avoid providing food (unless it is wrapped to go) to prevent clients removing their masks to eat inside the clinic. Ensure any food that is wrapped to go does not contain allergens such as peanuts.
Planning considerations for multi-product immunization clinics
The purpose of this section is to assist in planning multi-product immunization clinics to safely and effectively administer multiple vaccine products at the same clinic location (i.e., COVID-19 and influenza vaccines, or different COVID-19 vaccine products and doses for multiple age groups). The following provides general guidance for consideration, which should be modified based on specific circumstances.
Concurrent vaccination campaigns in fall 2021/winter 2022
There will be multiple concurrent vaccination campaigns for adults and children in the fall 2021 and winter 2022 period.
| Campaign type | Population | Vaccine types |
|---|---|---|
| Primary series adolescent/adult COVID-19 vaccines | Adolescents and adults (12 years and over) | COVID-19 (Pfizer-BioNTech Comirnaty, Moderna Spikevax and much smaller quantities of AstraZeneca Vaxzevria and Janssen Jcovden [Johnson & Johnson]) |
| Additional/ booster doses of adolescent/adult COVID-19 vaccines | Adolescents and adults (12 years and over) – depending on jurisdictional eligibility criteria | COVID-19 (Moderna Spikevax, Pfizer-BioNTech Comirnaty) |
| Primary series pediatric COVID-19 vaccines | Children (5 to 11 years of age)Footnote * | COVID-19 (Pfizer-BioNTech Comirnaty for children 5 to 11 years oldFootnote **) |
| Seasonal influenza vaccine | Children, adolescents and adults (6 months of age and older) | Influenza vaccines (various products available including age-specific products) |
|
||
Recommendation from the National Advisory Committee on Immunization (NACI) on concomitant vaccine administration
- Adolescents and adults (12 years of age and over): NACI has recommended that for adolescents and adults the COVID-19 vaccine can be given at the same time as, or any time before or after any other non-COVID-19 vaccines.
- Children (5 to 11 years of age): As a precaution, NACI has recommended that COVID-19 vaccines for children 5 to 11 years of age should not routinely be given at the same time as any other non-COVID-19 vaccines, unless advised to do so by the child’s healthcare provider. The suggested waiting period of 14 days before or after the administration of another vaccine is to avoid possible confusion between adverse events should they arise post-vaccination.
Multi-product immunization clinics
Multi-product immunization clinics are clinics in which multiple vaccine products are available at the same location. The types of multi-product clinics available to the public will vary by community. When planning the availability of vaccines for a community or region, consider the advantages and challenges of multi-product clinics and the various ways multi-product immunization clinics can be organized.
Advantages
- More convenient for the public
- May increase coverage for all vaccines being offered
- Requires fewer health human resources than separate clinics
Challenges
- The operation of each clinic is more logistically complex
- Increased risk of administration errors, with potential health consequences for the individual and risk of reducing public confidence in mass vaccination campaigns and vaccination more broadly
- Multiple vaccination campaigns at the same site may lead to public confusion
Clinic organization
Multi-product immunization clinics may be organized in several ways, and each comes with considerations around safety, convenience, and clinic flow.
| Organizational structure | Description | Pros and cons |
|---|---|---|
| Completely separate vaccination model: The clinic is divided into separate areas for each vaccine. |
|
|
Hybrid model:
|
|
|
Multiple vaccines by same vaccinator model:
|
|
|
Planning considerations
At multi-product immunization clinics, care must be taken to ensure that each client receives the appropriate product(s). Clinic planning should consider precautions that can be taken through clinic design and administrative controls such as policies and procedures.
Clinic design
- Colour code all items associated with each vaccine being administered, including consent forms, labels for vaccines, trays, line-up area, etc. Examples include the following:
- Pfizer-BioNTech Comirnaty adult/adolescent formulation (for age 12 and older): purple
- Pfizer-BioNTech Comirnaty pediatric formulation (for ages 5 to 11): orange
- Moderna Spikevax adult/adolescent formulation (for age 12 and older): brown
- Influenza quadrivalent vaccine (e.g., for age 6 months and older): blue
- Influenza older adult vaccine (e.g., for age 65 and older): grey
- Separate dilution and drawing-up stations for different products in distinct areas of the clinic.
Administrative controls to prevent errors
- Provide comprehensive staff training and refreshers as needed, including orientation and educational materials and overviews and touch bases before the start of each clinic.
- Optimize the use of experienced staff, especially in the early days of the campaign.
- Pair new vaccinators with more experienced vaccinators.
- If vaccinators are to be giving more than one product, use most experienced vaccinator for this process.
- Ensure a clinic lead or lead nurse is available to respond to staff questions at all times during the shift. Encourage questions and clarifications.
- Keep diluting/drawing up areas completely separate for each product and dosage. Assign staff to dilute and draw up only one product and one dosage per shift, if possible.
- Accountability checks should be in place to ensure staff are aware of and follow all vaccine administration policies and procedures, such as signing a daily “quality assurance” check list that summarizes important information, any recent changes in processes, and available resources and assistance.
- Do not give through-put goals which could result in immunizers rushing the administration process or taking short-cuts. Encourage immunizers to take as much time as needed.
- Provide job-aids for quick reference such as a clear table showing vaccines available at the clinic, eligibility criteria, brand name/trade name of each product and dosages, colour-coding used at the clinic, and a glossary of terms.
- Develop policies and procedures to manage errors and actively encourage reporting of errors or near-errors so that:
- Appropriate client follow-up can be initiated as needed. See Quick reference Guide on Use of COVID-19 Vaccines: Managing COVID-19 vaccine administration errors and deviations.
- Improvements to processes, policies and procedures can be made.
- These instances can be used as learning opportunities for staff.
Pre-immunization processes
- In community/mass immunization clinic settings, pre-loading of syringes can support timely and efficient vaccine administration.
- Considerations for pre-loading include the following:
- Pre-loading is lowest risk when a single vaccine product with a single dose is being provided to all clients at the clinic.
- If more than one vaccine type or dose is being offered at a clinic, the diluting/preloading stations should be completely separate for each product and dose and staff should be assigned to only one station per shift.
- In clinics where more than one vaccine or dosage is offered, pre-filled syringes should be labelled with the name and the dose of the vaccine product, lot number, expiry date, the initials of the preparer and the time that it should be used by, and colour coding for easy visual identification. In other clinics with only one product and dose, the information can be placed on the syringe or in the basket.
- If pre-filling syringes in a clinic, clinic leads and runners should communicate to decide when pre-loading should stop to avoid wastage of all products being used at the clinic.
- Pre-loading is lowest risk when a single vaccine product with a single dose is being provided to all clients at the clinic.
- Vaccine products intended for separate administration should never be combined in a syringe by immunizers.
Immunization processes for concomitant administration of COVID-19 and other vaccine(s)
- Ensure that informed consent has been attained for each vaccine.
- Ensure that individuals are screened for contraindications and precautions for each vaccine, as these vary somewhat by product.
- If multiple vaccines are administered to an individual at a single visit, administer each injection in a different injection site using separate injection equipment.Footnote 3
- Whenever possible, separate anatomical injection sites (i.e., left and right deltoid muscles) should be used.
- If multiple injections in the same deltoid muscle are required, the injection sites should be separated by at least 2.5 cm (1 inch). In individuals where there is insufficient deltoid muscle mass, the anterolateral thigh muscle can be used.Footnote 4
- If the same immunizer is giving more than one vaccine, establish a policy such that vaccinators always give the vaccines in the same order (e.g., COVID-19 vaccine is given first and in the left arm, influenza vaccine is given second and in the right arm).
- Record the site of administration of each vaccine, so that if an injection site reaction occurs, the associated vaccine can be identified.
- If a child 5 to 11 years of age inadvertently receives a 30 microgram dose of Pfizer-BioNtech Comirnaty, or an adolescent 12 years of age inadvertently receives a 10 microgram dose of Pfizer-BioNtech Comirnaty, refer to the guidance on managing COVID-19 vaccine administration errors or deviations.
Post-immunization waiting period
- The duration of the waiting period (e.g., 15 minutes or 30 minutes) should be determined by immunizers for each individual based on precautions to any of the vaccines that they have received concomitantly.
- Adverse events following immunizations (AEFI), such as anaphylaxis or serious or unexpected AEFIs, are reported as per jurisdictional protocols for AEFI reporting. The AEFI form can be retrieved from respective jurisdictions or the Public Health Agency of Canada (PHAC) website. All vaccines received prior to the AEFI should be reported on the form.
Clinic operations
Immunization clinic set-up and flow
Clinic setup will vary by site capacity and room layout but nonetheless should have a logical unidirectional flow. As much as possible, it is recommended that a standard clinic layout be used to avoid confusion among rotating staff. An example of how an immunization clinic can be set-up is provided in Figure 1.
Figure 1: Immunization clinic set-up
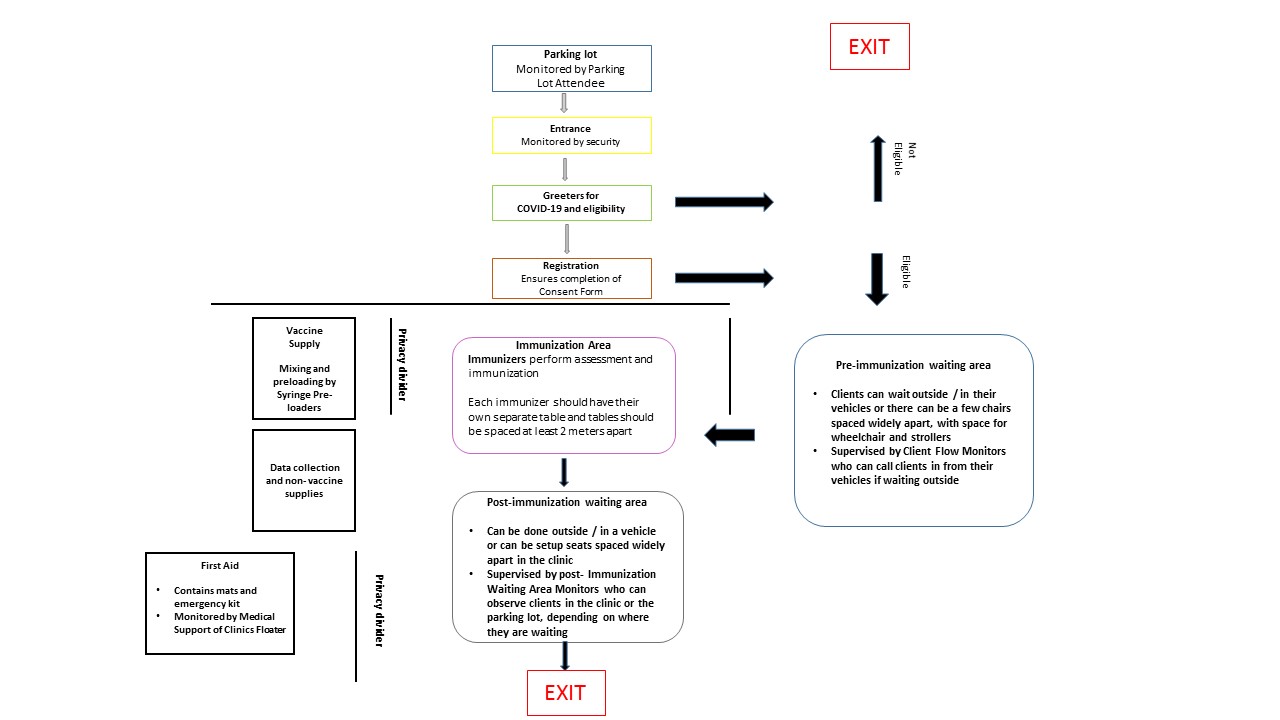
Figure 1 - Text description
The figure depicts a sample clinic setup with unidirectional flow to each of the clinic stations.
- Parking Lot:
- Parking lot is monitored by a parking lot attendee
- Entrance:
- Entrance area is monitored by security
- Greeters: Screen for COVID-19 and eligibility of receiving vaccine
- If not eligible, proceed to designated Exit which is separated from the flow to the rest of the clinic
- Registration station
- Ensures completion of the Consent Form
- If eligible, proceed to Pre-Immunization Waiting Area
Privacy divider separates Registration station from the rest of the clinic stations
- Pre-immunization waiting area: Clients may wait outside or in their vehicles. Inside, a few chairs for seating can be set up spaced widely apart, with room for wheelchairs and strollers. The area is supervised by Client Flow Monitors who can call clients in from their vehicle if waiting outside.
- Immunization Area: Separated by a privacy divider for:
- Vaccine supply
- Data collection and non-vaccine supplies
- Post-immunization Waiting Area
- Can be done outside or in vehicles. If inside clinic seats should be spaced widely apart.
- Needs to be supervised by Post-Immunization Waiting Area Monitors who can observe clients inside or outside.
- For those inside, after the waiting period is over, proceed to Exit #2.
- First Aid station: Separated from 7, the Post-Immunization Waiting Area, by a privacy divider
Pre-immunization processes
Pre-scheduling appointments/ pre-registration before attending the clinic
To prevent transmission of COVID-19 at immunization clinics, it will be essential to minimize crowding and ensure physical distancing. Strategies to assist with appropriately spacing clients and minimizing crowding on arrival at the clinic include:
- Online and phone appointments and registration: Clients use a designated website or phone number with accessibility features to schedule a date, time and clinic location for their appointment. Vaccine Information Sheets and Consent Forms can also be provided and completed online. Texts, email or phone can be used to inform clients of any changes in appointments or delays at the clinic.
- Invitations: Clients are invited to the clinic by postal code, school attended or alphabetically by family name.
- Wristbands or ticket number: At the clinic entrance or after on-site registration, clients who are eligible for immunization are assigned a number on a wristband or ticket with the predicted time the client should return to the clinic, or their cell phone number is taken and they are texted or called when they can return to the clinic.
- Block appointments: Set aside blocks of time for specific groups arriving together (groups of older adults, students, those from remote and isolated communities).
Parking lot
Parking Lot Attendants guide flow and manage the parking lot. To further prevent lines and crowding on arrival at the clinic, consider having clients call or text when they arrive at the clinic and wait in their vehicle or outside until they receive a call or text to enter the clinic. Clients without a cell phone can advise the clinic that they have arrived in person and provide their license number and location of their vehicle, so that staff can notify them when they can proceed to enter the clinic. After receiving their vaccine, there may also be an option for clients to wait in their vehicles for the post-immunization waiting period.
Line-ups
If lining up is required before entering the clinic, clients should wear a mask (unless unable to do so) and maintain physical distancing. Tape on the ground can help indicate places to stand that are at least 2 metres apart, with assistance provided for clients who are blind or partially sighted. The Greeters or other clinic staff (such as the Clinic Floater) should monitor the lines, and provide clients with updates regarding waiting times and answer questions. Appropriate seating and arrangements (such as moving them through the clinic more quickly) should be provided for those clients who cannot tolerate standing in line for long periods (e.g., older clients, people with disabilities, families with young children). If there is a possibility that clients will need to wait outside during the winter or inclement weather, consider options such as tents and heaters.
Clients may show-up at clinics in advance of the start of the clinic. Strategies should be considered to manage this line-up including beginning the clinic earlier / as soon as everything is in place and organized to begin, and having the maximum number of Immunizers available at the start of the clinic.
Greeting and screening
Clients are welcomed to the clinic by the Greeters. For appointment-based clinics, the Greeters determine if the client has an appointment and if the client fits the COVID-19 vaccine eligibility criteria for the particular clinic. Screening for COVID-19 is performed as per the clinic and jurisdiction's screening protocol. Signage with the eligibility criteria and COVID-19 screening questions can assist with these processes. Clients who fail the COVID-19 screening should return for immunization at a later date, as per recommended local public health protocols, and should be advised to seek medical attention as appropriate. If they need to speak with a health care provider at the clinic, this should be done away from others and with the client wearing a mask. Clients who pass the screening and are eligible for immunization are directed to the Registration area, or can be directed to appropriate clinic staff if they have medical questions.
Registration
In the Registration area, staff confirm that the client is able to attend the clinic (e.g., has an appointment if the clinic is appointment-based, and meets the eligibility criteria if the vaccine is only being offered to some populations). If required to attend the clinic, a health card or other form of identification is requested from the client.
Prior to providing informed consent and completing the Consent Form, clients must receive specific information and have the opportunity to ask questions to a regulated health care provider. The specific information is usually provided by having the client read a Vaccine Information SheetSheet (see Section on Key documents). If clients have difficulty reading or understanding the Vaccine Information Sheet or Consent Form, they should be referred to a health care provider (e.g., Clinic Leader, Clinic Floater, Medical Support, Clinic Specialized Support, translator).
To expedite the clinic registration process, it is optimal for the Vaccine Information Sheet to be provided online prior to arriving at the clinic and for the Consent Form to be completed online. Those without online access could be directed to an accessible telephone line where the information required for informed consent can be provided and the questions required for the Consent Form can be obtained; the online form would be completed by the staff on the phone. Alternatively, the Vaccine Information Sheet can be provided in paper form at the clinic and the information for the Consent Form entered electronically by the client or staff at the clinic, or a paper-based Consent Form can be completed with subsequent entry into an electronic system. If electronic documentation methods are used, it is advisable to have hard copies of forms on hand in case of equipment/software failure, connectivity issues or power outages.
Health care providers at the clinic should be consulted if there are concerns about the capacity of the individual to provide informed consent (unaccompanied child or an adult who may not be capable of understanding the information and making an informed choice). Health care providers at the clinic should also be consulted if the client has contraindications to immunization or medical questions.
Attention should be paid to ensure that all personal and personal health information cannot be overheard and that written and electronic information is appropriately safeguarded.
Practical tips for remote and isolated communities
- Consider having strategies in place to address individuals who present to clinic sites from outside of the community (e.g., individuals from neighbouring communities and off-reserve First Nations).
- Consider arranging home visits for those unable to attend immunization clinics (e.g., those who are homebound).
Immunization processes
Vaccine preparation
Optimally only one vaccine product (and one dose volume) is provided at each immunization clinic. If more than one vaccine is being provided at a clinic, care must be taken to ensure the client receives the appropriate product, depending on eligibility criteria or if receiving their second dose. Consider dividing the clinic into separate areas for each vaccine if more than one product is being utilized.
Ideally, vaccine should be drawn up for each client at the time of immunization. However, in immunization clinic settings, pre-loading of syringes supports timely and efficient vaccine administration. Pre-loading is particularly applicable when a single vaccine with a single dose is being provided to all clients at the clinic, or less optimally, if distinct areas of the clinic can be allocated to each vaccine, with separate Syringe Pre-Loaders and Immunizers for each area.
To support pre-loading of syringes, the following should be considered:
- Prior agreement on professional accountability if different individuals will pre-load and administer the vaccine
- Data on the stability of pre-loaded product for a specified time period and recommendations from the manufacturer
- Maintenance of cold chain requirements for the product (see Section on Vaccines - Storage and Handling and Cold Chain Maintenance)
The area of the clinic where the vaccine mixing and pre-loading is occurring should be located so that clients cannot easily come near that area and cannot see the mixing and pre-loading process.
Mixing with diluent or adjuvant: If a vaccine requires mixing with a diluent or adjuvant, this is optimally done by one Syringe Pre-Loader dedicated to this task. Mixing should be performed using aseptic technique and as per the manufacturer's recommendation and the Canadian Immunization Guide, Vaccine administration practices chapter. The staff member should ensure that once the mixing has occurred, the used diluent or adjuvant bottle is promptly discarded into the sharps container to avoid confusing the various bottles. The bottle with the mixed vaccine should be marked with the date and time of mixing. The mixed product should be used within the manufacturer's recommended timeframe. Care should be taken not to mix more than the number of vials needed to complete the clinic.
Loading of syringes: Drawing the vaccine into the syringe should be done using aseptic technique and as per the manufacturer's recommendation and the Canadian Immunization Guide, Vaccine administration practices chapter. Syringe Pre-Loaders or Immunizers should prepare only the necessary amount of pre-loaded vaccine to support a continuous flow of immunizations, using pre-loaded syringes as soon as possible after pre-loading or as per manufacturer's requirements. If using Syringe Pre-Loaders, Runners deliver pre-loaded syringes from the Syringe Pre-Loaders to the Immunizers and should closely monitor how many pre-loaded syringes are at the Immunizers' stations, especially as the clinic is nearing the end. Runners should communicate with the Syringe Pre-Loaders and Clinic Leads to decide when pre-loading should stop so that no pre-loaded syringes are wasted. Any vial that has been punctured but not completely used during the clinic should be marked with the date and time of first puncture and the date it should be used by, to ensure it is used within the timeframe recommended by the manufacturer. If the vaccine must be used right away, see strategies to avoid wastage.
Vaccine administration
Once registration is complete, clients can proceed to be immunized. They can be directed to the next Immunizer by the person directing client flow. The Immunizer can signal that they are able to see the next client by waving or signaling with a flag. In areas where services are offered in English and French, the Clinic Flow Monitor should direct the client to an Immunizer who speaks their preferred language.
Before vaccine administration: Before vaccine administration the Immunizer should confirm that they have the correct consent form for the client (e.g., by requesting the client's name and date of birth), conduct a pre-immunization assessment, pursuant to relevant jurisdictional professional regulations and policies, which may include determining: the client's health status, the client's understanding of the information provided at registration and ability to provide informed consent, confirmation that there are no contraindications/precautions to immunization (including for the second dose, if there were any serious adverse effects after the first dose that would require assessment before receiving the second dose), and answering any remaining questions the client may have.
Immunizers should be watchful for clients who appear very anxious, pale or sweaty. Clients with these features or a history of fainting when receiving previous immunizations or medical procedures should be immunized lying down on a mat in the First Aid area or in a reclining chair to prevent fainting and injury.
Clients who need privacy to expose their skin can also be taken to the First Aid area or other private space for vaccination.
Immunization: Immunization should be performed as per the manufacturer's recommendation and the Canadian Immunization Guide, Vaccine administration practices chapter, including hand hygiene and appropriate land-marking. Techniques to decrease immunization injection pain are outlined by age in this chapter.
Before administering the vaccine, the Immunizer should visually inspect the vaccine for any discoloration or particulates and ensure that the appropriate dose is in the syringe.
After immunization: After immunization, the safety engineered mechanism on the needle should be activated, and the needle and syringe discarded promptly into the sharps container. Used needles should never be re-capped, and used needles and syringes should never be placed on the immunization table to avoid accidental re-use.
Clients who appear faint, pale, sweaty or weak should be encouraged to remain seated with their head down to prevent fainting and injury. If needed, they can be assisted to lie down on a mat in the First Aid Area. They should only proceed to the Post-Immunization Waiting Area when they have fully recovered.
Documentation: The Immunizer should complete the appropriate documentation in the client's electronic record or on the Consent Form (see Section for the content of the Consent Form). The client should also be provided with a record of their immunization including the brand name of the product received, the date of administration and the name and professional designation of the immunizer.
The client should be advised of the post-immunization waiting period and protocol, and provided with an After-Care Sheet and counselled on its content, including the importance of receiving the second dose of the COVID-19 vaccine and when and where to receive this dose (see Section for content of the After-Care Sheet and counseling information).
Post-immunization waiting period
After immunization, it is recommended that clients be kept under observation in the clinic for at least 15 minutes to monitor for immediate vaccine reactions (i.e., fainting and anaphylaxis). Thirty minutes is safer when there is a specific concern about possible vaccine allergy.
The Post-Immunization Waiting Area could be a potential location where clients may be too close together. Clients should be encouraged to remain at least 2 metres apart at all times in this area with their masks on. Those who cannot wear a mask should be places as far apart from others as possible or should wait outside or in their vehicle (if possible). Chairs should be spaced at least 2 metres apart and the floor should be marked with tape to indicate the position of the chairs. The area should be monitored either by a health care provider, or a volunteers who informs health care providers if assistance is needed. The waiting period also provides an opportunity during which clients can complete an evaluation of their clinic experience (see Appendix 3 for a possible Client Evaluation Form).
The National Advisory Committee on Immunization (NACI) has developed Recommendations on the Duration of the Post-vaccination Observation Period for Influenza Vaccination during the COVID-19 Pandemic. Some of these criteria can be applied to COVID-19 vaccines. While the first NACI criteria related to influenza vaccine was that the individual has been previously vaccinated with that vaccine, for the first dose of COVID-19, no one will have been previously vaccinated. However, it may still be possible to have people wait outside of the clinic or in their vehicles providing that they:
- Feel well after the immunization and have no history of fainting after medical procedures.
- Are with a responsible adult who can monitor them for 15-30 minutes after immunization.
- Remain on the clinic grounds or in the parking lot and have direct access or telephone access to the clinic if they need assistance.
- Do not operate a motorized vehicle or other form of transportation (e.g., bicycle, skateboard, rollerblades, scooter) or machinery for a minimum of 15-30 minutes after immunization as advised by the Immunizer.
If clients are waiting outside or in their vehicles after immunization, Post-Immunization Waiting Area staff should circulate in this area so they can respond to issues and concerns that may arise. Clients can be advised to honk their horn if they need assistance.
Management of adverse events
Though very rare, anaphylaxis can occur following immunization and must be managed quickly and appropriately. Fainting, which may or may not be accompanied by brief seizure activity, occurs more commonly, and also requires prompt management. A separate First Aid area of the clinic with mats, and optimally with a privacy divider, should be designated for first aid and the management of fainting and anaphylaxis.
Although specific health care providers at the clinic are designated to manage fainting and anaphylaxis (i.e., Clinic Leaders, Clinic Floaters, Medical Supports), all health care providers should be trained in fainting and anaphylaxis management and have up to date cardio-pulmonary resuscitation (CPR) certifications (if possible based on provincial/territorial/local requirements).
Emergency kits to manage anaphylactic reactions must be readily available and easily accessible by staff at the clinic. Protocols must be in place for maintenance of kits including verifying that all the supplies are still present and none of the medications have expired (epinephrine auto-injectors can have short shelf lives). There should be at least two kits at each clinic location which are located in consistent locations in the clinics (at least one of which is in the First Aid area). A recommended list of items in an anaphylaxis kit is available in the Canadian Immunization Guide: Part 2 - Vaccine Safety. A plan should be in place to rapidly replenish a kit should it be used.
Clinic procedures to manage anaphylaxis or injury after fainting should follow jurisdictional protocols and should include:
- emergency telephone numbers
- Medical Directive to Managing Anaphylaxis, which can be accompanied by an Anaphylaxis Medication Quick Reference Dosage Card
- a clear plan for patient transport to a health care facility
- communication protocols
- appropriate documentation on a Serious Event Form or Incident Report, which includes space to record all actions and observations during the management of the event, and
- follow-up with the client
Clinic procedures for managing fainting and anaphylaxis along with the medical directive and quick reference card should be readily available in the emergency kits. Juice and snacks should be available for clients who feel weak or faint.
Adverse events following immunizations (AEFI), such as anaphylaxis, are reported as per jurisdictional protocols for AEFI reporting. The AEFI form can be retrieved from respective jurisdictions or the Public Health Agency of Canada (PHAC) website.
Debriefing after serious events at the clinic will offer support to staff and volunteers who were involved and provide opportunities to learn from the event and improve processes.
De-escalation activities
Site closure
Some immunization clinic sites are transient and may be taken down and/or moved frequently, while other sites offer clinics for prolonged periods from the same location. Planning and procedures for site take down and closure should be in place. Staff and volunteers should be made familiar with shutdown procedures and their specific roles. Bins labelled according to the material that should be packed into them helps packing to occur in an organized manner.
Examples of shutdown activities to consider for fixed sites that have remained in the same location are as follows:
- Communication with the public, staff and collaborating partners about the clinic end date
- Identification of how remaining supplies will be stored and managed (i.e. inventory tracking)
- Proper disposal of sharps containers
Discussion of cleaning protocols with site owners and procedure to identify, report and manage any damage to the facility:
- Follow-up procedures for clients if subsequent doses of vaccine are required when the site is no longer operating
- Financial tracking and payments
Alternate delivery methods
Although the focus of this document is larger immunization clinics, there are other possible locations and methods to deliver COVID-19 vaccines which include:
- Community health care providers' offices (physicians', nurse practitioners' or public health offices)
- Pharmacies
- Workplace clinics (including in health care settings)
- Facility-based administration for institutional or congregate living settings (e.g., hospitals, long term care homes, retirement homes, shelters, group homes, correctional facilities)
- Mobile vans
- Home visits, including door-to-door clinics
- Walk-up, drive through or parking lot clinics
Some of the processes outlined in this document may also apply to immunization in these other locations. Specific considerations for outdoor clinics (i.e., walk-up, drive through or parking lot clinics) include:
- Choosing a location that is accessible, safe and will not expose the workers to vehicle traffic or fumes.
- Ensuring the location protects workers and equipment from inclement weather.
- Providing appropriate infection control and personal protective equipment for Immunizers, and having clients wear a mask.
- For immunizations of clients in vehicles, ensuring the immunizer provides the vaccine from outside the vehicle with the window rolled down. The immunizer must have appropriate access to the client to support proper land-marking to avoid shoulder injuries.
- Sufficient space for clients to wait at least 15 minutes after immunization before leaving the clinic location. If waiting in their vehicles, there should be sufficient space in the parking lot.
- Ensuring that the cold chain can be appropriately maintained for the vaccine.
Evaluation
Evaluations of immunization campaigns and clinics are important as they provide processes to document the response and identify areas for improvement. Ideally, an evaluation plan and evaluation tools should be developed ahead of time, and should include opportunities for staff, volunteers and clients to provide input on their experiences. Evaluation tools should be administered and analyzed throughout the response, in order to make adjustments in real time, not just at the end of the campaign. A variety of evaluative processes can be used, including:
- review of clinic data collection tools such as the Daily Clinic Summary
- review of information reported on daily debriefing by staff after each clinic (which can be done via text, email, online, or if needed, in-person with the appropriate precautions). Appendix 2 provides a template for a Post-Clinic Evaluation Form for Staff and Volunteers.
- review of client evaluations conducted during the waiting period at clinics or online after the clinic (see Appendix 3 for possible template for a Client Evaluation Form)
- review of Serious Event Forms and Incident Reports
- client, staff and volunteer surveys
- formal staff and volunteer debrief sessions at the end of the campaign
Gathering and analyzing all evaluations should be completed in a timely fashion to ensure that nothing is lost. A written summary report including the processes used in running the clinics, quantitative summary data (e.g., numbers of clinics, numbers vaccinated, numbers of adverse events), evaluation outcomes and lessons learned will support future clinic planning.
Resources
Some resources to help with planning immunization clinics, including drive-in immunization clinics, are as follows:
- Public Health Agency of Canada. Guidance for influenza vaccine delivery in the presence of COVID-19.
- Centers for Disease Control and Prevention. Guidelines for Large-scale influenza vaccination clinic planning. 2015 Dec 16.
- Centers for Disease Control and Prevention. Checklist of best practices for vaccination clinics held at satellite, temporary or off-site locations.
- Australia NSW Health. Drive-in Immunisation Clinics. Advice for providers during COVID-19 response. 4 May 2020.
Some key resources to assist with immunization practices are as follows:
- Public Health Agency of Canada. Canadian immunization guide.
- Public Health Agency of Canada. Immunization competencies for health professionals.
- Public Health Agency of Canada. National Vaccine Storage and Handling Guidelines for Immunization Providers.
Appendix 1: Sample immunization clinic list
When determining the clinic supply requirements, consider the estimated number of clients that could be immunized per day (see Section: Immunization Clinic Planning Parameters).
| Item | Quantity | Cost | Supplier | Comments |
|---|---|---|---|---|
| Clinic supplies | ||||
Needles and syringes for:
|
- | - | To be supplied by the National Emergency Strategic Stockpile (NESS) for COVID-19 response | - |
| Alcohol swabs | - | - | To be supplied by the NESS for COVID-19 response | - |
| Adhesive bandages | - | - | To be supplied by the NESS for COVID-19 response | - |
| Cotton balls or gauze | - | - | To be supplied by the NESS for COVID-19 response | - |
| Disposable non-latex gloves (assorted sizes) (note: not recommended for immunizing unless skin is not intact) | - | - | - | - |
| Paper cups | - | - | - | - |
| Table covers | - | - | - | - |
| Alcohol-based hand rub (sufficient for each immunizer table, registration desks, entrance, exit, waiting areas) | - | - | - | - |
| Surgical/ medical masks (for staff and if needed, for clients who do not have a mask) | - | - | - | - |
| Face shields | - | - | - | - |
| Tissue boxes | - | - | - | - |
| Disposable gowns | - | - | - | - |
| Paper towels | - | - | - | - |
| Paper bags (lunch size) | - | - | - | - |
| Hypoallergenic tape | - | - | - | - |
| Disinfectant wipes | - | - | - | - |
| Disinfectant solution | - | - | - | - |
| Sharps containers (of appropriate sizes) | - | - | To be supplied by the NESS for COVID-19 response | - |
| Biohazard waste boxes | - | - | - | - |
| Biohazard yellow bags | - | - | - | - |
| Insulated coolers and bags | - | - | - | - |
| Frozen packs | - | - | - | - |
| Maximum-minimum thermometers | - | - | - | - |
| Blood pressure cuff (child and adult) | - | - | - | - |
| Stethoscope | - | - | - | - |
| Adrenalin (epinephrine) 1:1000 or Epi-pens | - | - | - | - |
| Flashlight | - | - | - | - |
| Pediatric pocket mask with one way valve | - | - | - | - |
| Adult pocket mask with one way-valves | - | - | - | - |
| Wheelchair if possible (particularly if the clinic will remain at the same location for a period of time) | - | - | - | - |
| Carry bags/totes | - | - | - | - |
| Numbers for clients in waiting lines | - | - | - | - |
| Table numbers for immunizing stations | - | - | - | - |
| Flags for immunizers to indicate that they are ready for the next client | - | - | - | - |
| Small boxes with no lids for the Runners to carry pre-loaded syringes from the Syringe Pre-loader to the Immunizer | - | - | - | - |
| Water bottles | - | - | - | - |
| Face cloths for clients who feel faint | - | - | - | - |
| Juice boxes for clients who feel faint | - | - | - | - |
| Snacks for clients who feel faint | - | - | - | - |
| Administrative supplies | ||||
| Pens (quantity will depend on whether a paper based system is being used) | - | - | - | - |
| Clipboard (quantity will depend on whether a paper based system is being used) | - | - | - | - |
| Paper, including paper for signs | - | - | - | - |
| Scissors | - | - | - | - |
| Highlighter | - | - | - | - |
| Transparent tape and masking tape | - | - | - | - |
| Rubber bands | - | - | - | - |
| Stapler and staples | - | - | - | - |
| Batteries | - | - | - | - |
| Replacement ink cartridges | - | - | - | - |
| Large envelopes | - | - | - | - |
| Date stamps | - | - | - | - |
| Identification badges | - | - | - | - |
| Measuring tape to measure distant for furniture | - | - | - | - |
| Tape to stick on floors to space out furniture | - | - | - | - |
| Forms | ||||
| Vaccine Information Sheet | - | - | - | - |
| Consent Forms | - | - | - | - |
| After-Care Sheet | - | - | - | - |
| Client Immunization Record | - | - | - | - |
| Daily Clinic Summary | - | - | - | - |
| Medical Directive for Obtaining Consent and Administering Vaccine | - | - | - | - |
| Medical Directive to Manage Anaphylaxis, including the Anaphylaxis Medication Quick Reference Dosage Card | - | - | - | - |
| Serious Event Forms - for clinic use | - | - | - | - |
| Incident Reports | - | - | - | - |
| Adverse Event Following Immunization - official provincial/territorial or national AEFI reporting form | - | - | - | - |
| Post-Clinic Evaluation Form for Staff and Volunteers | - | - | - | - |
| Client Evaluation Form | - | - | - | - |
| Time Sheets | - | - | - | - |
| Supply / Re-Supply Lists | - | - | - | - |
| Signage | ||||
| Maps and directional arrows | - | - | - | - |
| Entrance and exit signs | - | - | - | - |
| Eligibility criteria for the clinic (if indicated) | - | - | - | - |
| COVID-19 screening questions | - | - | - | - |
| Instructions for clients (e.g., contraindications for the vaccine, need to wait 15 minute after immunization, need for masks and hand hygiene) | - | - | - | - |
| Names of stations (e.g., Registration, Immunization, Pre-Immunization Waiting Area, Post-Immunization Waiting Area, First Aid, Washrooms) | - | - | - | - |
| List of other clinics in the area and/or on that day (if needed) | - | - | - | - |
| Other resources | ||||
| Orientation and training manuals, electronic or laminated with job descriptions | - | - | - | - |
| Incident reports | - | - | - | - |
| Product monographs laminated or online | - | - | - | - |
| Canadian Immunization Guide online or printed copies of relevant sections | - | - | - | - |
| Electronic supplies | ||||
| Laptops with privacy screens | - | - | - | - |
| Printer (as needed) | - | - | - | - |
| Server (as needed) | - | - | - | - |
| Fax machine (as needed) | - | - | - | - |
| Cellular phones | - | - | - | - |
| Internet access | - | - | - | - |
| Photocopier (as needed) | - | - | - | - |
| Extension cords | - | - | - | - |
| Furniture (as required) | ||||
| Chairs | - | - | - | - |
| Tables | - | - | - | - |
| Cots/mats for First Aid Area | - | - | - | - |
| Physical barriers for infection prevention and control | - | - | - | - |
| Privacy dividers including for First Aid Area | - | - | - | - |
| Garbage cans | - | - | - | - |
Modified from the Peterborough County-City Health Unit, Pandemic Influenza Plan - Appendix A Mass Vaccination Plan
Appendix 2: Post-clinic evaluation form for staff and volunteers
Download in Word format the Post-clinic evaluation form.
- Date of clinic:
- Location of clinic:
- What was your role in the immunization clinic?
- Was this your first time participating in a larger immunization clinic?
- Yes
- No
- Was the training you received for immunization clinics adequate? If no, what additional training would you require?
- Yes
- No
- Did you understand your role and responsibility? If no, what would improve your understanding?
- Yes
- No
- Was your supervisor accessible when you needed him/her? If no, what could improve accessibility?
- Yes
- No
- Was the chain of communication clear? If no, what could improve this?
- Yes
- No
- Were you made aware of any changes and updates in clinic activities? If no, what could improve this?
- Yes
- No
- Did you feel that the infection prevention and control and occupational health and safety measures were adequate at your clinic? If no, do you have suggestions for improvement?
- Yes
- No
- How would you rate your clinic site overall?
- Excellent
- Good
- Okay
- Poor
Explain the factors that contributed to your rating.
- What was the greatest personal challenge faced during your time in the immunization clinic?
- In your opinion, what were the challenges for your clinic site?
- What went well for your site?
- Do you have any suggestions to improve the operations of immunization clinics?
Appendix 3: Client evaluation form
Please complete the following evaluation form to guide us on the steps we need to take to improve future immunization clinics.
The comments you provide will be anonymous and only used to identify areas for improvements and practices that worked best. We thank you in advance for taking the time to complete this form.
Download in Word format the Client evaluation form.
- Date of clinic
- Location of clinic
- Gender
- Male
- Female
- Prefer not to say
- Prefer to self-describe
- Age
- 5-11
- 12-17
- 18-24
- 25-29
- 30-39
- 40-49
- 50-59
- 60-69
- 70+
- City of residence:
- Did you receive an immunization today?
- Yes
- No
- Did you bring anyone else with you today to receive an immunization? Check all that apply.
- No, just myself
- Children in my care
- Spouse
- Older adult
- Other (please specify)
- How did you hear about the clinic? Check all that apply.
- Newspaper
- Website
- Poster
- Social media
- Radio/Televisions
- Health care provider/ public health official
- Co-worker/friend/family
- Other
- Did you find that you had enough information about the vaccine before you received it?
- Yes
- No
- If no, are there other things you would like to know about the disease or vaccine?
- Was the location and set-up of the clinic site appropriate for your needs?
- Yes
- No
- If no, what would have been a more appropriate location and/or set-up?
- What influenced you the most to get your vaccine today? Check all that apply.
- Concern for my health
- Concern for the health of others
- Advised by my health care provider
- Advised by public health officials
- Convenient location
- Convenient time
- It was free
- Think it is the right thing to do to control the pandemic
- Other (please specify)
- What was the approximate time you spent waiting to receive your immunization?
- The wait time was:
- Shorter than I expected
- About the time I expected
- A bit longer than expected, but reasonable
- Unreasonably long
- Were you satisfied with the care you received at the clinic?
- Yes
- No
- If no, please specify your concerns
- Were you satisfied with the precautions to protect you from exposure to COVID-19 at the clinic?
- Yes
- No
- If no, please specify your concerns
- What did you like about the clinic?
- What did you not like about the clinic?
- Do you have any suggestions to improve the clinic?
Endnotes
- 1
-
https://canvax.ca/brief/cardtm-system-patient-centred-care-tool-ease-pain-and-fear-during-school-vaccinations
- 2
-
For example, see SELF Magazine x American Academy of Pediatrics Vaccine Photo Project, featuring free photos for download: https://www.flickr.com/photos/selfmagazine/albums/72157710332198661
- 3
-
https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html#a7.7
- 4
-
https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-8-vaccine-administration-practices.html#p1c7a3c