Polio: For health professionals
To learn about best practices to help prepare for, detect and control poliovirus, and roles and responsibilities for a coordinated response across Canada, consult the:
On this page
- Clinical illness
- Causative agent
- Diagnosis and laboratory testing
- Detection
- Reporting
- Global occurrence
- Domestic occurrence
- Reservoir
- Transmission
- Risk group
- Prevention and control
- Case surveillance
Clinical illness
Polio (poliomyelitis) is a highly infectious vaccine-preventable disease that is caused by the poliovirus. Polio can infect the central nervous system and damage nerve cells that activate muscles.
In approximately 75% of infected people, polio doesn't cause any symptoms and can go unrecognized.
In about 25% of cases, symptoms can include:
- fever
- sore throat
- headache
- feeling unwell
- gastrointestinal symptoms, such as:
- abdominal pain
- nausea
- vomiting
These non-specific symptoms can develop 3 to 6 days after exposure to the virus. Meningitis can also occur in approximately 1% of cases.
In less than 1% of cases, paralysis can occur. Paralysis can be partial or full and is generally asymmetric. It tends to affect the legs more often than the arms and may affect the respiratory muscles. There may also be bulbar involvement affecting the cranial nerves. The time from exposure to onset of paralysis is approximately 7 to 21 days. Paralysis is often permanent, and weakness or paralysis still present 60 days after onset is likely to persist.
Approximately 25 to 40% of adults who contracted paralytic polio during childhood may develop a non-infectious post-polio syndrome 15 to 40 years after initial infection.
Symptoms of post-polio syndrome can include:
- slowly progressive muscle weakness
- loss of muscle function
- muscle atrophy
- pain and fatigue
Muscle weakness can result in breathing and swallowing difficulties.
Causative agent
Poliovirus is an enterovirus.
There are 3 serotypes of poliovirus:
- type 1
- type 2
- type 3
Thanks to the efforts of the Global Polio Eradication Initiative (GPEI), wild type 2 and 3 poliovirus have been eradicated from global circulation since 2015 and 2019, respectively.
There are 2 types of polio vaccines:
- inactivated polio vaccine (IPV)
- oral polio vaccine (OPV) or Sabin vaccine
OPV can cause illness identical to polio in 2 ways:
- OPV consists of live, weakened poliovirus that rarely can cause paralysis in the vaccine recipient or their close contacts (referred to as vaccine-associated paralytic polio or VAPP). The risk of VAPP is estimated at one in 750,000 with the first dose of OPV and one in 2.4 million for all doses of OPV.
- If the strain of poliovirus in the OPV circulates in populations with low vaccine coverage, or replicates in an immunocompromised host, it can undergo mutations and revert to a form that causes paralytic disease indistinguishable from wild polio. This is called vaccine-derived poliovirus (VDPV). If VDPV circulates in the community resulting in several detections, it's referred to as circulating vaccine-derived poliovirus (cVDPV). Genetic sequencing can be used to distinguish the OPV vaccine strains from VDPV. Canada stopped using OPV and began using IPV exclusively as of 1996.
For more information on poliovirus vaccines, see the Poliomyelitis vaccine: Canadian Immunization Guide.
Diagnosis and laboratory testing
National case definition for paralytic poliomyelitis
Investigate all confirmed, probable, and suspected cases of polio as soon as possible according to provincial and territorial guidelines.
Confirmed case
- Clinical illnessFootnote * with laboratory confirmation of infection:
- isolation of polio virus (vaccine or wild-type) from an appropriate clinical specimen or
- detection of polio virus RNA from a clinical specimen
- Or clinical illnessFootnote * in a person who is epidemiologically linked to a laboratory-confirmed case
Probable case
- Clinical illnessFootnote * without detection of polio virus from an appropriate clinical specimen and without evidence of infection with other neurotropic viruses but with one of the following laboratory confirmations of infection:
- significant rise (such as fourfold or greater) in polio IgG titre by any standard serologic assay between acute and convalescent seraFootnote ** or
- positive serologic test for polio IgM antibody in the absence of recent immunization with polio virus-containing vaccineFootnote **
Suspected case
- Clinical illnessFootnote * and no laboratory confirmation of infection (no polio virus detection or serologic evidence), including negative test results and inadequate or no investigation
Detection
Stool is the required clinical specimen for the laboratory investigation and diagnosis of polio. For clinical cases, ensure the collection of at least 2 stool samples (taken at least 24 hours apart and minimum 2 grams per sample) within 2 weeks after symptom onset for viral studies. Stool can be collected up to 6 weeks after symptom onset if not possible to collect earlier. Stool samples are preferred to rectal swabs because they are more sensitive. All samples testing positive for poliovirus or samples collected from a suspected polio or acute flaccid paralysis (AFP) case should be referred to the National Microbiology Laboratory (NML) for confirmatory testing.
If wastewater samples are collected that are suspected to contain poliovirus, they must be sent to the NML for testing. All samples (human respiratory and fecal samples, sewage, concentrates and their derivatives) collected at a time and place where poliovirus could be circulating are considered poliovirus potentially infectious materials (PIM). When working with and storing PIM, risk mitigation measures must be followed to prevent facility-related containment breaches, as outlined in the World Health Organization (WHO) PIM Guidance Document (2nd edition). The NML is the only WHO regional reference laboratory for polio in Canada and is subject to the WHO standards and containment certifications to mitigate the risk of facility-related containment breaches.
Learn more about:
Reporting
Polio is nationally notifiable in Canada, and all countries globally. Any confirmed or probable polio case (if the respective case definitions presented above are met) must be reported immediately to the Public Health Agency of Canada (PHAC). All other laboratory-confirmed detections of poliovirus (human and non-human sources) are to be reported to PHAC immediately.
Email us at vpd-mev@phac-aspc.gc.ca and hpoc-cops@phac-aspc.gc.ca within 24 hours of detection.
Cases compatible with the acute flaccid paralysis case definition must be reported to the local public health authority if legislatively required within the jurisdiction.
Notification by PHAC to WHO
Under International Health Regulations (IHR), notification is required for all detections in human or non-human sources of:
- Wild poliovirus (WPV)
- Vaccine-derived polio virus (VDPV), including:
- type 1
- type 2
- type 3
- Sabin and Sabin-like type 2 viruses, from the areas where Sabin OPV2 hasn't been used in the previous 4 months
- Sabin and Sabin-like viruses types 1 and 3 aren't notifiable
As the national IHR focal point, PHAC must meet several requirements under the regulations, including the obligation to urgently notify the WHO.
Note: different types of poliovirus are distinguished based on genetic sequencing. Sabin viruses are the virus in the OPV, while Sabin-like viruses have begun to genetically diverge from the Sabin strain, but to a small degree.
Global occurrence
The World Health Assembly has established the Global Polio Eradication Initiative with the goal of eradicating polio worldwide. This initiative has become the largest international public health effort to date.
Since global eradication efforts began in 1988, the annual global incidence of polio has decreased by over 99%. In the Region of the Americas, the last case of wild poliovirus was detected in August 1991 in Peru. Three years later, in 1994, wild poliovirus was officially declared eliminated in the region, becoming the first WHO region to achieve this distinction.
Countries which have stopped transmission of endemic wild poliovirus can be intermittently affected by importations of wild poliovirus from countries where the virus is still endemic or the emergence and circulation of VDPV. A current list of countries with confirmed cases of wild polio and circulation of vaccine-derived poliovirus can be found on the GPEI website.
The international spread of poliomyelitis has been identified as a Public Health Emergency of International Concern by the WHO since 2014. PHAC works closely with international partners, including the WHO, to monitor polio activity around the world. Canada also supports worldwide efforts to eradicate polio completely.
Domestic occurrence
The incidence of polio in Canada dramatically declined with the introduction of immunization programs in the 1950s (see Figure 1). The last case of wild poliovirus acquired in Canada was in 1977. In 1994, Canada was certified as being free of wild poliovirus by the WHO. Only rare cases of paralytic polio have been reported since Canada and the Region of the Americas were declared to have eliminated endemic wild poliovirus. These rare cases were mainly due to unvaccinated or under vaccinated individuals who were exposed to the virus while travelling abroad in areas where poliovirus transmission occurs and returned to Canada. Alternatively, cases may be reported in unvaccinated or under vaccinated individuals who are exposed to another individual shedding poliovirus following vaccination with OPV. Since 2004, there have been 4 importations of Sabin-like poliovirus and one probable case of VAPP reported in Canada from regions using OPV.
Until polio eradication has been achieved globally, there remains a risk of importation of polio from affected countries into Canada.
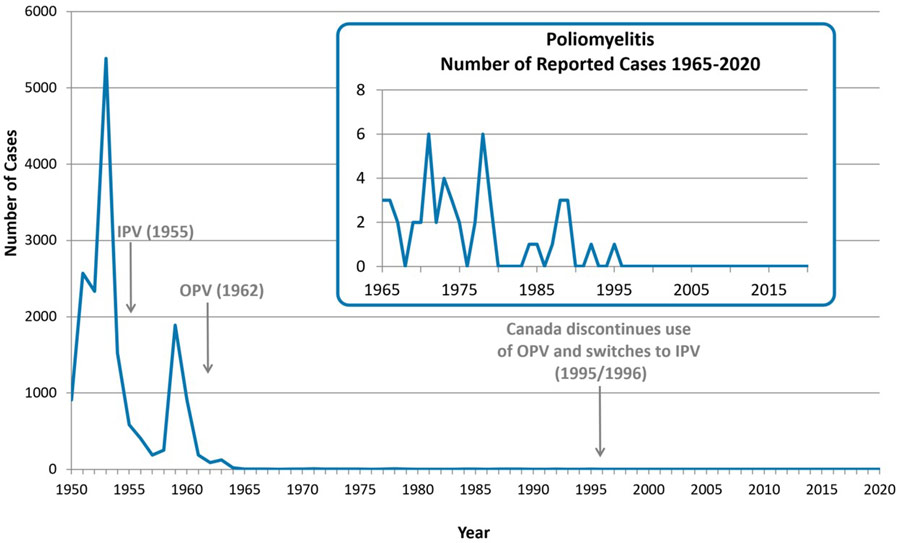
Figure 1 - Text Description
The years in which Canada introduced IPV and OPV are depicted with arrows in 1955 and 1962 respectively. Canada discontinues use of OPV and switches to IPV in 1995/1996.
| Year | Cases |
|---|---|
| 1950 | 911 |
| 1951 | 2568 |
| 1952 | 2334 |
| 1953 | 5384 |
| 1954 | 1526 |
| 1955 | 584 |
| 1956 | 404 |
| 1957 | 185 |
| 1958 | 249 |
| 1959 | 1887 |
| 1960 | 909 |
| 1961 | 188 |
| 1962 | 88 |
| 1963 | 122 |
| 1964 | 19 |
| 1965 | 3 |
| 1966 | 3 |
| 1967 | 2 |
| 1968 | 0 |
| 1969 | 2 |
| 1970 | 2 |
| 1971 | 6 |
| 1972 | 2 |
| 1973 | 4 |
| 1974 | 3 |
| 1975 | 2 |
| 1976 | 0 |
| 1977 | 2 |
| 1978 | 6 |
| 1979 | 3 |
| 1980 | 0 |
| 1981 | 0 |
| 1982 | 0 |
| 1983 | 0 |
| 1984 | 1 |
| 1985 | 1 |
| 1986 | 0 |
| 1987 | 1 |
| 1988 | 3 |
| 1989 | 3 |
| 1990 | 0 |
| 1991 | 0 |
| 1992 | 1 |
| 1993 | 0 |
| 1994 | 0 |
| 1995 | 1 |
| 1996 | 0 |
| 1997 | 0 |
| 1998 | 0 |
| 1999 | 0 |
| 2000 | 0 |
| 2001 | 0 |
| 2002 | 0 |
| 2003 | 0 |
| 2004 | 0 |
| 2005 | 0 |
| 2006 | 0 |
| 2007 | 0 |
| 2008 | 0 |
| 2009 | 0 |
| 2010 | 0 |
| 2011 | 0 |
| 2012 | 0 |
| 2013 | 0 |
| 2014 | 0 |
| 2015 | 0 |
| 2016 | 0 |
| 2017 | 0 |
| 2018 | 0 |
| 2019 | 0 |
| 2020 | 0 |
Reservoir
Humans are the only known reservoir of poliovirus.
Transmission
Transmission of poliovirus occurs predominantly through the fecal-oral route, particularly in areas with poor sanitation (where excreted virus can contaminate food or water). Spread via the respiratory route is also possible, through droplets, but is less frequent. The incubation period is 3 to 6 days with the onset of paralysis in 7 to 21 days. Communicability is greatest around the onset of illness when the virus is present in high concentrations in the throat and feces. The virus, including the vaccine virus if vaccinated with OPV, may be excreted in nasopharyngeal secretions for 1 to 2 weeks. However, poliovirus can continue to be excreted in the feces for 3 to 6 weeks, even among people who don't develop symptoms. Immunocompromised patients (and in rare cases, immunocompetent individuals as well) can excrete poliovirus after infection or OPV vaccination for prolonged periods of time (from greater than 6 months to a number of years).
After vaccination with OPV, the vaccine virus is excreted in stool. If prolonged replication or transmission occurs, the vaccine strain may lose its attenuated (weakening) properties and mutate back into a form that can cause paralysis. This is VDPV and can occur in communities with low immunization coverage, as the virus spreads from one unvaccinated individual to another over a long period of time (about 12 to 18 months). Prolonged replication of virus in immunocompromised individuals can also result in VDPV.
See map of global wild poliovirus type 1 and circulating vaccine-derived poliovirus cases.
Risk group
Polio infections are more common in children under 5 years of age. However, any person who is not immune to poliovirus, regardless of age, can become infected including those who are unvaccinated or under vaccinated. Unvaccinated or under vaccinated persons who travel to endemic or outbreak countries are also at higher risk.
Prevention and control
Polio can be prevented through immunization. The National Advisory Committee on Immunization recommends routine childhood immunization against polio. IPV was introduced in Canada in 1955 and OPV in 1962. Vaccine programs in Canada switched from OPV to IPV in 1996. OPV is no longer recommended or available in Canada.
Poliomyelitis vaccines used in Canada contain the 3 serotypes of wild poliovirus and are available as a trivalent IPV or in combination vaccines. IPV is very effective at preventing paralysis.
- Routine immunization of children is recommended at 2, 4, 6, and 12 to 23 months of age (generally given at 18 months of age) with a booster dose at 4 to 6 years of age. A dose of IPV isn't required at 6 months of age for a complete primary series of polio vaccine. However, it's acceptable to give the additional dose of IPV at 6 months of age for convenience of administration in combination with DTap and Hib or DTap, Hib and HB.
- Similar to children, vaccination of adults is recommended to prevent the introduction and circulation of polio. Primary immunization is indicated for non-protected adults.
- Re-immunization of some individuals at high risk of poliovirus exposure (such as those travelling to, or planning to work in areas that have wild polio or vaccine-derived polio outbreaks or individuals with other exposure risks) may be required. Children with a complete primary series don't require additional doses of IPV vaccine before travelling. For adults at increased risk of exposure to polio, a single lifetime adult booster dose of IPV-containing vaccine is recommended. WHO has recently endorsed use of IPV to respond to outbreaks in countries that use only IPV for routine childhood immunization.
For more information on poliovirus vaccines, see the Poliomyelitis vaccine: Canadian Immunization Guide.
For additional information on travellers, please refer to:
Case surveillance
Polio is a nationally notifiable disease in all provinces and territories, with cases reported to provincial or territorial departments of health. As such, a national case definition for polio has been developed.
Basic epidemiological data on polio since 1924 can be found on the Notifiable Diseases Online platform.
To ensure that there continues to be no polio in Canada, PHAC conducts routine surveillance of AFP, one of the characteristics of polio. AFP involves the acute onset of paralysis in one or more limbs and can occur for various reasons such as neurological conditions or botulism.
Like polio, AFP is also nationally notifiable and a national case definition for AFP has been developed. However, not all provinces and territories include AFP in their provincial or territorial notifiable disease lists. For this reason, PHAC, in collaboration with the Canadian Paediatric Society, conducts enhanced surveillance of AFP in children less than 15 years of age. By actively seeking out cases of AFP in Canada and ruling out the presence of polio, Canada can provide ongoing evidence that the country remains polio free.
Data on cases of AFP are collected from the following surveillance programs, managed by the Canadian Paediatric Society:
- Canadian Paediatric Surveillance Program (CPSP): A surveillance network that gathers data from over 2,500 paediatrician and paediatric subspecialists each month to monitor rare diseases and outcomes in Canadian children.
- IMPACT (Immunization Monitoring Program, ACTive): A paediatric hospital-based active surveillance network for adverse events following immunization, vaccine failures and selected infectious diseases that are, or will be, vaccine preventable.
AFP surveillance results are published annually in the CPSP results publications and are also reported to the Pan American Health Organization on a biannual basis.
Related links
Surveillance
Polio guidelines and recommendations
- Guidance for the response and management of a poliovirus event or outbreak in Canada
- Global Polio Eradication Initiative: Tools, protocols and guidelines for polio outbreak response
- Federal, provincial, territorial public health response plan for biological events
- Canadian Paediatric Surveillance Program: Acute flaccid paralysis study protocol (PDF version)
- Protocol for the investigation of acute flaccid paralysis and suspected paralytic poliomyelitis (PDF version)
- Pan American Health Organization: Responding to a poliovirus event and outbreak
Lab processes and biosecurity
- Canada's roles and progress in poliovirus containment
- Canada Human Pathogens and Toxins Act
- World Health Assembly: Poliomyelitis: containment of polioviruses (PDF version)
- World Health Organization: Global action plan for poliovirus containment (PDF version)
- World Health Organization: Public health management of facility related exposure to live polioviruses (PDF version)