For health professionals: Rubella
Find out how rubella is monitored.
On this page:
What health professionals need to know about rubella
Rubella is caused by the rubella virus. This virus is an enveloped, positive-sense, single-stranded ribonucleic acid virus. It belongs to the Rubivirus genus of the Togaviridae family.
Rubella is a highly infectious disease. In susceptible pregnant women (inadequately immunized and without a history of infection), it can result in:
- miscarriage
- stillbirth
- congenital rubella infection (CRI)
- congenital rubella syndrome (CRS)
With the introduction of routine rubella vaccination programs in Canada, rubella is no longer a common infection. It is now a rare disease. The National Advisory Committee on Immunization (NACI) recommends immunization against rubella.
Spectrum of clinical illness
The incubation period for rubella is generally 14 to 17 days, but can range from 12 to 21 days.
In children, symptoms are often mild, and up to 50% of infections are subclinical. Symptoms include:
- a transient erythematous rash
- post-auricular and suboccipital lymphadenopathy
- low-grade fever
Since most symptoms are non-specific, rubella can be mistaken for infection caused by other viruses such as:
- parvovirus
- adenovirus
- enterovirus
In adults, rubella is often accompanied by transient polyarthralgia or polyarthritis. Serious complications such as thrombocytopenia, encephalitis and progressive rubella panencephalitis are rare.
Rubella infection during pregnancy often results in CRI of the unborn child. The infection affects all organs of the fetus and results in CRS, causing:
- abnormalities
- miscarriage
- stillbirth
Fetal infection can occur at any stage of pregnancy. The risk of fetal damage following maternal infection is particularly high in the earliest months after conception (85% in the first trimester). The risk decreases as the pregnancy progresses. Fetal abnormalities are rare if infected after 20 weeks of gestation.
Manifestations of CRS can include:
- congenital heart disease
- cataracts, glaucoma or retinitis
- microphthalmia
- cochlear deafness or central auditory imperception
- patent ductus arteriosus or peripheral pulmonic artery stenosis
- encephalitis
- microcephaly
Other manifestations include:
- mental retardation
- autism
- intra-uterine growth retardation
- radiolucent bone disease
- hepatosplenomegaly
- thrombocytopenic purpura
- interstitial pneumonitis
Some manifestations of CRS, including diabetes mellitus, thyroid abnormalities and neurological disorders, may become apparent later in life.
Photos of clinical manifestations of rubella and congenital rubella syndrome
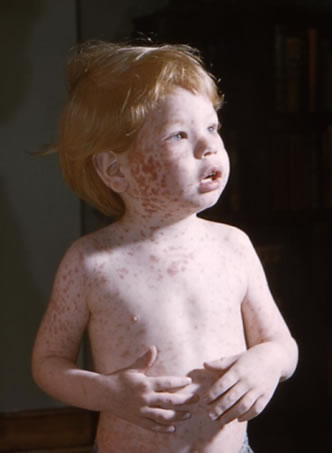
Source: U.S. Centers for Disease Control and Prevention.
Characteristic maculopapular rash for rubella. The rash lasts about 3 days. It usually shows up first on the face and neck. The rash then spreads down the body within 24 hours
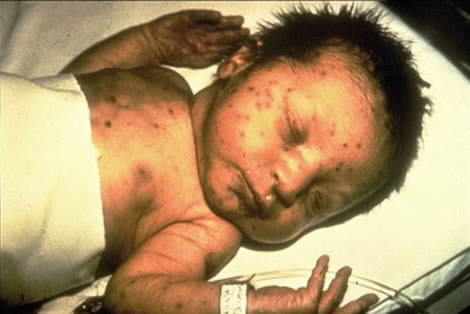
Source: U.S. Centers for Disease Control and Prevention.
Infant with congenital rubella infection. Congenital infection with the rubella virus can affect all organs. This can cause deafness, cataracts, heart defects, microcephaly, mental retardation, bone alterations, liver and spleen damage.
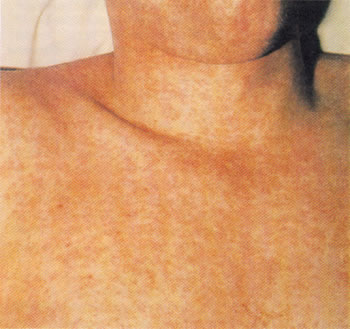
Generalized rubella rash. The diffuse maculopapular rash can be difficult to differentiate from a measles rash and may occur in other viral infections
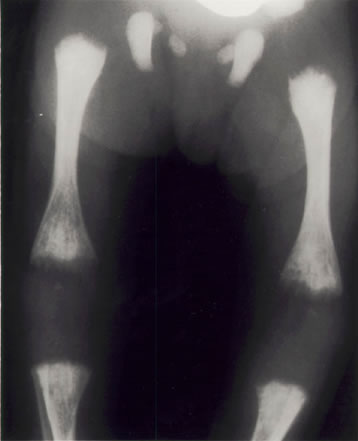
X-ray of the lower limbs in a newborn with congenital rubella syndrome. The ends of the long bones are ragged and streaky in appearance (they look like celery stalks). These changes are due to active rubella infection in the bone.
Prevention and control
Rubella infections can be prevented by immunization. For children up to 12 years of age, NACI recommends immunization with a combined vaccine. This combined vaccine contains measles, mumps and rubella (MMR) or measles, mumps, rubella and varicella (MMRV). One dose of rubella-containing vaccine will provide life-long immunity against rubella. Rubella vaccine is given in a combination MMR or MMRV vaccine. The first dose should be given at 12 to 15 months of age. The second dose of MMR or MMRV vaccine (given for measles and mumps protection) should be given at 18 months of age or any time before the child starts school (at 4 to 6 years).
A single dose of the MMR vaccine should be given to adolescents and adults who do not have:
- documented evidence of receiving a rubella vaccine on or after their first birthday
- laboratory evidence of immunity
- a history of laboratory-confirmed rubella infection
For more information about the vaccine and its recommended use, please refer to the most recent version of the Canadian Immunization Guide.
The main goal of vaccination against rubella is to prevent infection during pregnancy. MMR vaccination is recommended for all non-pregnant adolescents and women of childbearing age without evidence of immunity. This is particularly important for foreign-born individuals and students.
Other priority groups for rubella immunization include:
- people who work with children, such as:
- child care workers
- teachers
- staff in educational settings
- health care workers
- people travelling to rubella-endemic areas
Pregnant women should not receive the vaccine. This is because it is a live attenuated vaccine and there is a theoretical risk of infecting the unborn child. Women of childbearing age are advised to avoid pregnancy for 1 month after vaccination.
All suspected rubella and CRI/CRS cases should be reported as soon as possible to local public health units. Patients should be isolated for 4 days after the appearance of a rash in order to prevent transmission of the virus. All contacts of a suspected rubella case should be identified and classified as susceptible or non-susceptible.
Rubella and CRI/CRS surveillance in Canada
Health professionals in Canada play a critical role in identifying and reporting cases of rubella and CRI/CRS. See the surveillance of rubella section for more information on surveillance in Canada.
For more information
Publications
- Vaccine Preventable Disease Reduction Targets by 2025 (Rubella and Congenital Rubella Syndrome/Congenital Rubella Infection)
- Vaccination Coverage Goals by 2025 (Rubella)
- Measles and Rubella Weekly Monitoring Report
- Highlights from the 2019 childhood National Immunization Coverage Survey (cNICS)
- Vaccine Preventable Disease: Surveillance Report to December 31, 2019 (Rubella section)
- Vaccine Preventable Disease: Surveillance Report to December 31, 2019 (Congenital rubella syndrome and congenital rubella infection section)
- Vaccine Preventable Disease: Surveillance Report to December 31, 2017 (Rubella section) (archived)
- Vaccine Preventable Disease: Surveillance Report to December 31, 2017 (Congenital rubella syndrome and congenital rubella infection section) (archived)
- Vaccine Preventable Disease: Surveillance Report to December 31, 2015 (Rubella section) (archived)
- Vaccine Preventable Disease: Surveillance Report to December 31, 2015 (Congenital rubella syndrome and congenital rubella infection section) (archived)
- Elimination of Measles, Rubella and Congenital Rubella Syndrome in Canada: Documentation and Verification Report
- Seroprevalence of rubella antibodies and determinants of susceptibility to rubella in a cohort of pregnant women in Canada, 2008-2011