Archived - Updates to the Canadian Immunization Guide

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 44-12: Adult immunization
Date published: December 6, 2018
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 44-12, December 6, 2018: Adult immunization
Overview
What is new in the Canadian Immunization Guide: November 2016 to November 2018
A Fleurant-Ceelen1, M Tunis1, A House1 on behalf of the National Advisory Committee on Immunization (NACI)*
Affiliations
1 Centre for Immunization and Respiratory Infectious Diseases, Public Health Agency of Canada, Ottawa, ON
Correspondence
Suggested citation
Fleurant-Ceelen A, Tunis M, House A, on behalf of the National Advisory Committee on Immunization (NACI). What is new in the Canadian Immunization Guide: November 2016 to November 2018. Can Commun Dis Rep 2018;44(12):331-5. https://doi.org/10.14745/ccdr.v44i12a06
Keywords: Immunization, NACI, Canada, update, vaccine
Abstract
The Canadian Immunization Guide is an online resource that provides evidence-based recommendations on the use of vaccines and vaccine administration practices to health care providers and public health practitioners in Canada. Its contents are based on the most up-to-date recommendations of the National Advisory Committee on Immunization (NACI) and the Committee to Advise on Tropical Medicine and Travel (CATMAT). The Canadian Immunization Guide (CIG) is frequently updated online in response to new evidence and changing product indications. Between November 2016 and November 2018, new and updated recommendations were published for the chapters on Vaccine Administration Practices, Immunization of Immunocompromised Persons, and Immunization During Pregnancy and Breastfeeding and on seven active vaccines (for cholera and traveller’s diarrhea, influenza, hepatitis A, hepatitis B, herpes zoster, human papillomavirus and pertussis), as well as a recent update on measles post-exposure prophylaxis.
Introduction
The National Advisory Committee on Immunization (NACI) has been providing advice on vaccines to governments and health care professionals in Canada and internationally since 1964 Footnote 1. It does this by providing a variety of information products to meet the needs of different audiences. NACI develops detailed and technical products, such as literature reviews and NACI statements, for immunization experts and policy makers. NACI also develops summative and translational products, such as statement summaries in the Canada Communicable Disease Report and updates in the Canadian Immunization Guide (CIG), for front line public health and clinical care. Figure 1 provides an overview of NACI’s production process.
CIG has been providing clinically-relevant information on immunization to front line immunization providers since 1979 Footnote 2. CIG transformed into an evergreen online format in 2012 Footnote 3 and is now updated on an ongoing basis as new recommendations from NACI are completed. It also includes vaccine and related recommendations from the Committee to Advise on Tropical Medicine and Travel (CATMAT). CIG does not address economic and societal considerations related to immunization; however, it does highlight changes in disease epidemiology, safety signals and vaccine supply issues.
CIG is divided into five parts: key immunization information; vaccine safety; vaccination of specific populations; active vaccines; and passive immunization.
The purpose of this update is to provide an overview of the changes that have been made to CIG between November 2016 and November 2018. This includes changes to key immunization information, vaccination of specific populations, active vaccines and measles postexposure prophylaxis.
Figure 1: National Advisory Committee on Immunization: Production process
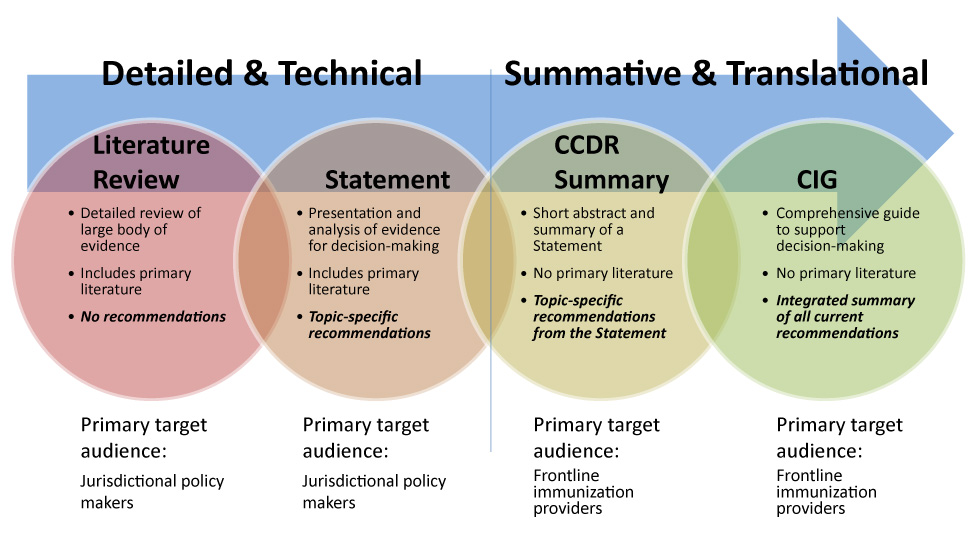
Text description: Figure 1
This figure illustrates the National Advisory Committee on Immunization’s (NACI) production process. On the background, an arrow shows a continuum between the different areas being described. Four types of NACI products are presented: Literature review, Statement, CCDR Summary, Canadian Immunization Guide.
To the left of the continuum, literature review is described as a detailed and technical product which provides a detailed review of a large body of evidence and which includes primary literature. A Literature review does not include recommendations; its primary target audience is jurisdictional policy makers.
Moving right in the continuum, Statement is described as a detailed and technical product which presents and analyses available evidence for decision-making and which includes primary literature. A statement includes topic-specific recommendations; its primary target audience is jurisdictional policy makers.
Moving further right in the continuum, Canada Communicable Disease Report summary is described as a summative and translational product which present a short abstract and summary of a statement with no primary literature. A CCDR summary includes topic-specific recommendations from the Statement; its primary target audience is frontline immunization providers.
At the right of the continuum, Canadian Immunization Guide is described as a summative and translational product which constitutes a comprehensive guide to support decision-making and which includes no primary literature. The CIG is an integrated summary of all current recommendations; its primary target audience is frontline immunization providers.
Key immunization information
The chapter on Vaccine Administration Practices Footnote 4 was updated. It now has a Needle Selection Guide that emphasizes the importance of selecting needle length for intramuscular injection on a case-by-case basis that includes an assessment of the viscosity of the immunizing agent as well as the recipient’s age, weight and muscle mass. The use of filter needles is not recommended as active ingredients such as adjuvants may be filtered out during the injection process. It notes that injections may be provided through a tattoo or a superficial birthmark; however, injections sites with potentially impaired lymphatic drainage should be avoided. There is a new table that provides immunization pain management strategies for clients of all ages. Regarding the combination of contents of multi-dose vials, health care providers are advised to adhere to jurisdictional or organizational policies.
Vaccination of specific populations
Two chapters were updated: immunization of immunocompromised persons; and immunization during pregnancy and breastfeeding.
Throughout the Immunization of Immunocompromised Persons chapter Footnote 5, tables have been included that outline immunization recommendations by vaccine and primary immunodeficiencies, acquired (secondary) immunodeficiencies, transplant recipients/candidates and HIV-infected persons. New information has been added on defects of innate immunity, criteria for consideration of measles-mumps-rubella and varicella vaccines in those with partial T cell defects, contraindications for live viral vaccines in some types of phagocytic cell defects and immunosuppressive therapy.
The Immunization in Pregnancy and Breastfeeding chapter Footnote 6 was updated to reflect the new recommendation to administer pertussis vaccine during every pregnancy between 27 and 32 weeks. It also clearly states that vaccines containing thimerosal are safe in pregnancy and should be used if indicated. Additional considerations during pregnancy have been added for the administration of Rhesus (Rh) immunoglobulin and other blood products and for the administration of the following vaccines: conjugate quadrivalent meningococcal; meningococcal B vaccine; yellow fever; and Japanese encephalitis.
Active vaccines and passive immunization
Seven active vaccine chapters were updated, along with an update on measles postexposure prophylaxis using immune globulin.
Cholera and Enterotoxigenic Escherichia coli (travellers' diarrhea)
Due to the limited benefits associated with this vaccine, the oral cholera vaccine should no longer be routinely recommended to prevent travellers’ diarrhea. CATMAT notes that it may be considered for those who are at highest risk of infection, health complications or serious inconveniences, such as humanitarian workers, health care workers in endemic countries, travellers at high risk of exposure to contaminated water or food, immunocompromised persons and those with chronic illnesses for whom there is an increased risk of serious consequences from travellers’ diarrhea. In addition, CATMAT recommends that all other clients follow hand hygiene, food and water safety practices and consider over-the-counter medication for the management of travellers’ diarrhea Footnote 7Footnote 8.
Influenza
Seasonal influenza vaccine recommendations are updated annually in advance of the influenza season Footnote 9.
Hepatitis A
The recommended dosages for intramuscular immune globulin (IM Ig) as pre- and postexposure prophylaxis for hepatitis A have been increased to reflect new product monograph indications Footnote 10.
Hepatitis B
Based on vaccine immunogenicity and safety data, NACI has revised its recommendation for the dosage of Recombivax HB® for infants (of hepatitis B-negative mothers) to children less than 11 years of age from 0.25 mL to 0.5 mL. For children, previously-received doses of 0.25 mL are still considered valid and do not need to be repeated. For immunocompromised individuals, initial annual monitoring of hepatitis B antibody levels may be considered after primary immunization Footnote 11.
Herpes zoster (shingles)
Following Canadian authorization of the new recombinant herpes zoster vaccine (RZV), Shingrix®, NACI now recommends that RZV should be offered to adults 50 years and older without contraindications, including those who have previously received the live zoster vaccine (LZV), Zostavax®, at least one year prior. NACI recommends that individuals without contraindications who have had a previous episode of herpes zoster may be offered two doses of RZV, at least one year after the last episode. When RZV is contraindicated, unavailable or inaccessible, the previously-approved LZV may still be considered for immunocompetent individuals who are at least 50 years old and who have no contraindications. RZV (but not LZV) may be considered in immunocompromised adults who are at least 50 years old on a case-by-case basis Footnote 12Footnote 13Footnote 14. Two tables have been added to the guidelines that summarize the key considerations for the choice of a herpes zoster vaccine and its administration Footnote 12.
Human papillomavirus
The human papillomavirus (HPV) vaccine, HPV9, is now recommended for immunocompetent males and females who are nine to 14 years old using either a two- or three-dose immunization schedule, while it continues to be recommended using only a three-dose immunization schedule for males and females 15–26 years of age and may be used in those over 26 years of age who are at risk of ongoing exposure. This is similar to HPV2 (females only) and HPV4 vaccines. Any HPV vaccine (HPV 2, HPV4, or HPV9 vaccine) should allow at least 24 weeks between the first and last dose in either a two- or three-dose schedule. Immunocompromised individuals should continue to receive the vaccine on a three-dose immunization schedule with at least 24 weeks between the first and last dose of vaccine Footnote 15.
Pertussis (whooping cough)
Recent evidence suggests that infants can effectively be protected against pertussis (whooping cough) through maternal immunization with the tetanus-diphtheria-acellular pertussis (Tdap) vaccine during pregnancy. The Tdap vaccine is now recommended for every pregnancy between 27 and 32 weeks of gestation. When unique patient considerations preclude vaccination during this period, it is possible to offer the Tdap at any time from 13 weeks to the time of delivery Footnote 16.
Measles
New evidence suggests that the previously recommended dosage of immune globulin (Ig) no longer provides optimal protection for measles postexposure prophylaxis (PEP). For NACI has updated recommendations for Ig PEP dosage, indications and routes of administration Footnote 17 as follows:
- Immunocompetent individuals six months of age and older who have been exposed to measles and who have no contraindications should be offered a measles-mumps-rubella vaccine within 72 hours of the exposure
- If injection volume is not a major concern, infants younger than six months of age should be given IM Ig at a concentration of 0.5 mL/kg, to a maximum dose of 15 mL, administered over multiple injection sites
- If injection volume is not a major concern, infants six to 12 months old who are identified after 72 hours and within six days of measles exposure should receive IM Ig (0.5 mL/kg), to a maximum dose of 15 mL, administered over multiple injection sites
- If injection volume is not a major concern, contacts who are pregnant or immunocompromised can receive IM Ig at a concentration of 0.5 mL/kg, understanding that recipients 30 kg or more will not receive the measles antibody concentrations that are considered to be fully protective
- In cases where injection volume is a major concern or for recipients 30 kg or more, intravenous immunoglobulin (IV Ig) can be provided at a dose of 400 mg/kg Footnote 17; and
- NACI does not recommend that susceptible immunocompetent individuals older than 12 months of age receive Ig PEP for measles exposure due to the low risk of disease complications and the practical challenges of administration for case and contact management
A summary of the updated recommendations on the active vaccines is presented in Table 1.
| Vaccine-preventable disease | Previous recommendation | New recommendation |
|---|---|---|
| Cholera and travellers’ diarrhea | Not routinely recommended for travellers | May be considered for those who are at highest risk of infections, complications or inconveniences |
| Influenza | New seasonal recommendations are issued every year in preparation of the upcoming influenza season | |
| Hepatitis A | For protection lasting less than three months IM Ig is 0.02 mL/kg of body weight If protection is required for three months or longer, 0.06 mL/kg of body weight should be administered and repeated every six months |
IM Ig standard dose with a dosage of 0.1 mL/kg is recommended for household and institutional hepatitis A case contacts For travellers to high risk areas, prophylactic doses are as follows:
|
| Hepatitis B | Recombivax HB® dosage for children 0–10 years old of hepatitis B negative mothers: 0.25 mL | Recommended dosage for Recombivax HB increased to 0.5 mL |
| Herpes zoster (shingles) | LZV (Zostavax®) is recommended for adults 50 years and older without contraindication |
|
| Human papillomavirus | HPV9 vaccine recommended using a three-dose schedule, compared to HPV2 and HPV4 vaccine which may be used in a two- or three-dose schedule in some populations |
|
| Pertussis (whooping cough) |
Tdap vaccine should be offered to pregnant women during pertussis outbreaks | Tdap vaccine should be offered to every woman during every pregnancy, ideally between weeks 27 and 32 of gestation to protect infants |
| Measles | Dosage: when indicated, IM Ig at a concentration of 0.25 mL/kg should be administered or 0.5 mL/kg for immunocompromised individuals Populations: IM Ig provided to susceptible individuals of all ages presenting between 72 hours and six days post-exposure; and provided to infants under six months of age, pregnant women, or immunocompromised individuals presenting anytime up to six days postexposure |
|
Summary and conclusion
CIG continues to provide practical, evidence-based recommendations, based on the advice provided by NACI and CATMAT, to health care professionals to inform front line immunization practices. Summaries of changes are highlighted in Canada Communicable Disease Report from time to time. There is also a list of the changes made to CIG available online, and this list is updated in close to real time Footnote 18. Notices of new NACI recommendations, statements, NACI updates and updates to CIG chapters are also available by subscribing to NACI and CIG mailing lists Footnote 19.
Authors’ statement
AFC — Writing original draft, review and editing
MT — Review and editing
AH — Review and editing
Conflict of interest
None.
Acknowledgements
NACI members: C Quach (Chair), W Vaudry (Vice-Chair), N Dayneka, S Deeks, P DeWals, V Dubey, R Harrison, M Lavoie, M Salvadori, B Sander, C Rotstein, N Sicard, R Warrington
Liaison representatives: J Brophy (Canadian Association for Immunization Research and Evaluation), E Castillo (Society of Obstetricians and Gynaecologists of Canada), A Cohn (Centers for Disease Control and Prevention, United States), T Cole (Canadian Immunization Committee), J Emili (College of Family Physicians of Canada), C Mah (Canadian Public Health Association), D Moore (Canadian Paediatric Society), A Pham-Huy (Association of Medical Microbiology and Infectious Disease Canada)
Ex-officio representatives: K Barnes (National Defence and the Canadian Armed Forces), G Charos (Centre for Immunization and Respiratory Infectious Diseases [CIRID], Public Health Agency of Canada [PHAC]), J Gallivan (Marketed Health Products Directorate, Health Canada [HC]), J Pennock (CIRID, PHAC), R Pless (Biologics and Genetic Therapies Directorate, HC), T Wong (First Nations and Inuit Health Branch, HC)
Funding
The work of NACI is supported by the Public Health Agency of Canada.