Archived - Toxoplasma gondii: from the Amazon to the Arctic

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 45-7/8: Zoonotic diseases
Date published: July 4, 2019
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 45-7/8, July 4, 2019: Zoonotic diseases
Overview
Toxoplasma gondii: How an Amazonian parasite became an Inuit health issue
SJ Reiling1, BR Dixon1
Affiliation
1 Bureau of Microbial Hazards, Food Directorate, Health Canada, Ottawa, ON
Correspondence
Suggested citation
Reiling SJ, Dixon BR. Toxoplasma gondii: How an Amazonian parasite became an Inuit health issue. Can Commun Dis Rep 2019;45(7/8):183–90. https://doi.org/10.14745/ccdr.v45i78a03
Keywords: toxoplasmosis, marine mammals, fish, climate change, migratory birds
Abstract
Toxoplasma gondii is a protozoan parasite that originated in the Amazon. Felids (mammals in the cat family) are the only definitive hosts. These animals shed large numbers of infectious oocysts into the environment, which can subsequently infect many intermediate hosts, including birds, mammals and, possibly, fish. Human T. gondii seroprevalence is high in some parts of the Canadian Arctic and is associated with adverse health consequences among Inuit population. Since the range of felids does not extend to the Arctic, it is not immediately obvious how this parasite got from the Amazon to the Arctic. The objectives of this overview are to summarize the health impacts of T. gondii infection in Inuit in Canada’s North and to consider how this infection could have reached them. This article reviews the prevalence of T. gondii infection in terrestrial and marine animals in the Canadian Arctic and discusses their potential role in the foodborne transmission of this parasite to humans. Two distribution factors seem plausible. First, felids in more southern habitats may release infectious oocysts into waterways. As these oocysts remain viable for months, they can be transported northward via rivers and ocean currents and could infect Arctic fish and eventually the marine mammals that prey on the fish. Second, migratory terrestrial and marine intermediate hosts may be responsible for carrying T. gondii tissue cysts to the Arctic, where they may then pass on the infection to carnivores. The most likely source of T. gondii in Inuit is from consumption of traditionally-prepared country foods including meat and organs from intermediate hosts, which may be consumed raw. With climate change, northward migration of felids may increase the prevalence of T. gondii in Arctic wildlife.
Introduction
Toxoplasma gondii infection in humans
Toxoplasma gondii is a protozoan parasite that can infect virtually all birds and mammalsFootnote 1. Although this parasite originally evolved in the Amazon region of South AmericaFootnote 2Footnote 3, it now infects an estimated two billion people worldwide, with foci of high prevalence in Latin America, Eastern/Central Europe, the Middle East and South-East Asia and Africa, and lower prevalence in many European countries and both Canada and the United StatesFootnote 4. Humans may become infected via three transmission routes:
- Ingestion of tissue cysts by eating fresh raw meat or organs of an infected intermediate host
- Ingestion of sporulated oocysts, which may persist for months or years in soil or water
- Congenitally, from mother to fetus, if a pregnant woman has acute toxoplasmosisFootnote 5
During the initial infection phase of an intermediate host, including in humans, T. gondii replicates rapidly and spreads throughout the tissues, including the brain (acute toxoplasmosis). In humans, symptoms may be subtle, and otherwise healthy individuals may not notice that they have become infected. Eventually, parasite replication slows down, and the protozoa cluster together in tissue cysts (latent toxoplasmosis). People with latent toxoplasmosis who become immunocompromised may develop reactivated toxoplasmosis, in which the dormant parasites in the tissue cysts will start replicating again. This reactivation can cause severe flu-like symptoms, blurred vision or toxoplasmic encephalitis. Latent toxoplasmosis has also been linked to changes in cell signaling pathways that may lead to neurological disorders including schizophrenia, epilepsy, Alzheimer’s disease and Parkinson’s diseaseFootnote 6Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11. Furthermore, a positive association has been made between T. gondii infection and increased risk-seeking behaviour in humansFootnote 12Footnote 13. Congenital transmission may lead to stillbirth or severe neurological complications.
Socioeconomic factors may have a significant impact on human exposure to this parasite. Factors influencing the seroprevalence in humans include proximity to infected domesticated or wild reservoir hosts, access to clean drinking water, urban versus rural lifestyle, types of food consumed, food preparation (raw vs freezing/cooking/drying) and hygiene (washing hands and rinsing fresh produce)Footnote 14.
T. gondii from the Amazon to the Arctic
Toxoplasma gondii evolved in the Amazon rainforestFootnote 2Footnote 3. It is very common in the Amazon region and Indigenous populations of the Amazon River basin have the highest known infection rate worldwide: along the upper Rio Negro, T. gondii seroprevalence is greater than 90%Footnote 15. Despite its worldwide distribution, only in the Amazon is T. gondii characterized by a high level of genetic diversity and the presence of many unique genotypesFootnote 3. Analysis of the gene flow of unique genotypes indicated that a small number of ancestral lineages gave rise to the existing diversity of T. gondiiFootnote 2. The primary hypothesis for the worldwide spread of T. gondii is that shipping traffic facilitated the travel of domestic cats and infected intermediate hosts to other continentsFootnote 1. The parasite reproduces in the small intestine of the felid definitive hosts, and millions of oocysts are shed into the environmentFootnote 5Footnote 14. How T. gondii spread from the Brazilian rainforest to the Canadian Arctic is not known. In this article, the Arctic boundaries are defined as described by the Conservation of Arctic Flora and Fauna (CAFF), which is the biodiversity group of the Arctic Council. The only wild felid that lives in the Canadian North is the Canada lynx, which has a T. gondii seroprevalence of 14%Footnote 16; however, the lynx’s range does not extend north of the treeline (the boreal forest or subarctic). In addition, there are few domestic cats in Canadian Arctic communities. Thus, while the presence of infected felids may explain the spread of T. gondii throughout most of North America, it does not explain the parasite’s presence in the Arctic; and, despite the scarcity of potentially infected felids, T. gondii is still present in a wide variety of Arctic animals.
To complete the parasite’s lifecycle, oocysts that are shed by the felid definitive hosts need to sporulate (Figure 1) and be ingested by intermediate hosts, which are potential prey for felids and which include virtually all warm-blooded animals. Toxoplasma gondii invades the intermediate host’s tissues and disseminates throughout the body, including the brainFootnote 1. However, intermediate hosts do not produce oocysts; thus, the mechanism (or mechanisms) of the geographical spread of T. gondii, in the absence of a definitive host, is still unknown.
Figure 1: Lifecycle of Toxoplasma gondii in Canada’s North
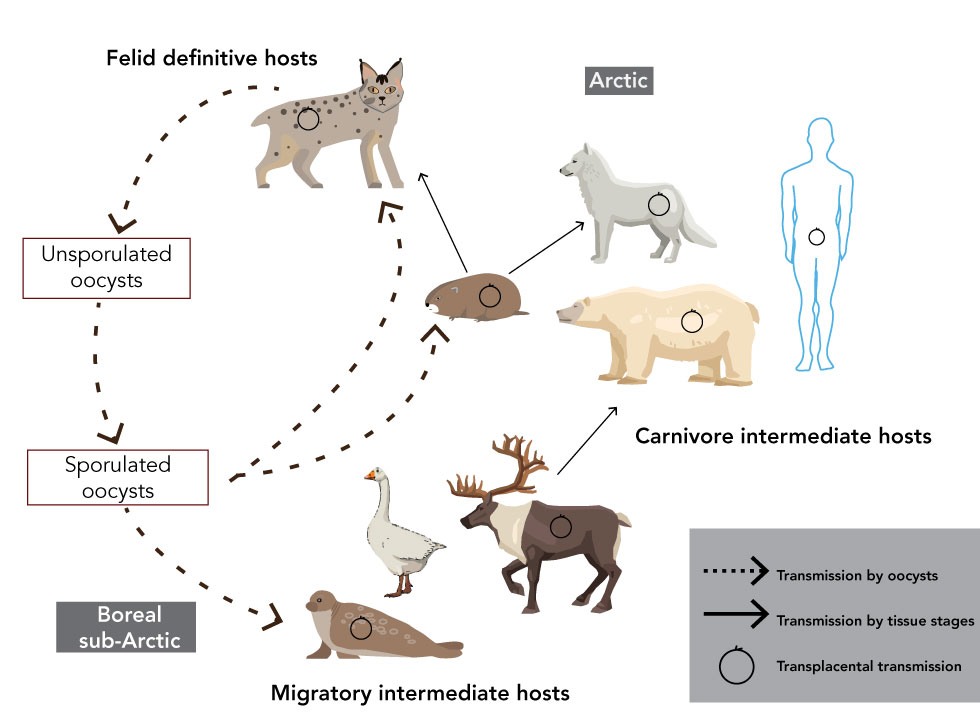
Text description: Figure 1
Figure 1: Lifecycle of Toxoplasma gondii in Canada’s North
Figure 1 is the lifecycle of Toxoplasma gondii in the North. Transmission by oocysts occurs in boreal and sub-Arctic regions where free-ranging definitive hosts (felids) are present. Migratory intermediate hosts (such as Arctic nesting geese, barren-ground caribou, and marine mammals) can become infected through consumption of oocysts when they seasonally migrate into terrestrial or marine sub-Arctic environments contaminated with oocysts. Carnivores in arctic regions become infected through consumption of tissue cysts in migratory intermediate hosts. In all mammalian intermediate hosts, including felids and people, vertical transmission is likely to occur in females infected during pregnancy.
The objective of this review is to highlight the incidence of this parasite in the Canadian Arctic and its impact on Inuit populations, and to consider how this parasite arrived and became endemic in an environment that lacks definitive hosts.
Toxoplasma gondii in the Arctic
T. gondii infection in Canadian Inuit
Toxoplasma gondii infections were first reported in Inuit in the 1980sFootnote 17Footnote 18Footnote 19. More recent studies showed that T. gondii seroprevalence in Inuit in the Canadian North varies greatly depending upon the regionFootnote 17. Toxoplasma gondii seroprevalence in adults in three Canadian Inuit regions was reported at 8% in Nunatsiavut, 28% in Nunavut and 60% in NunavikFootnote 20Footnote 21Footnote 22Footnote 23Footnote 24. There are not enough data to determine whether T. gondii prevalence in Inuit is stable or has changed over the decades.
Traditionally prepared “country foods” have great cultural significance for Inuit and in general are regarded as safe and nutritious for most people. However, it appears that T. gondii infection is related to the harvest and consumption of country foods, especially meat and organs, which may be consumed rawFootnote 19Footnote 25. A correlation between T. gondii seroprevalence and different hunting practices and dietary habits has been debatedFootnote 26Footnote 27Footnote 28Footnote 29. In contrast to Inuit communities, neighboring Cree communities, who usually cook their meat, were found to have a T. gondii seroprevalence of only 5%Footnote 29. It has been demonstrated that either thoroughly cooking meat, or freezing meat for several days, kills the pathogens present in the tissue cystsFootnote 30.
While toxoplasmosis is often asymptomatic in healthy individuals, pregnant women with acute toxoplasmosis are at risk of transmitting the parasite to the developing fetus. In 1987, an outbreak of toxoplasmosis was reported in pregnant women in NunavikFootnote 19. Infection was associated with skinning of animals and consumption of raw caribou meatFootnote 19.
T. gondii in the absence of definitive hosts
A study from Svalbard, Norway suggested that the role of oocysts in the transmission of T. gondii to Arctic terrestrial animals has been overemphasizedFootnote 31. The Svalbard archipelago is free from any wild or domestic cats, which eliminates the spread of infectious T. gondii oocysts into the environmentFootnote 31. The absence of T. gondii oocysts in Svalbard is supported by findings that non-migratory birds and herbivores were seronegative for T. gondiiFootnote 31. However, carnivores (foxes) were found to be T. gondii-positive. Thus, migrating birds may have introduced T. gondii to Svalbard, and local carnivores were subsequently infected by eating infected prey. Thus, it is possible for T. gondii to be transmitted from one intermediate host to another (e.g. bird to carnivore) without the need of sexual reproduction of the parasite in a felid definitive host. This transmission cycle between multiple intermediate hosts may explain the prevalence of T. gondii in the Arctic, including the Canadian Arctic, especially in non-felid carnivores. This hypothesis is supported by findings that all tested migratory birds and local carnivores in Svalbard were T. gondii-positiveFootnote 31.
Canadian Arctic terrestrial animals
Regardless of the source of infection (environmental oocysts vs tissue cysts from infected prey), numerous mammals and birds in Canada’s North have been reported to have tested positive for T. gondii (Table 1). Birds worldwide have been shown to be susceptible to T. gondii infectionFootnote 31 and in Canada, migratory birds, such as geese, overwinter in areas where felids are common and where infectious T. gondii oocysts are likely to be found in high numbers in the environmentFootnote 32Footnote 33Footnote 34. Toxoplasma gondii has been detected in three tested geese species, with the highest prevalence reported in Ross’s geese (34.5%) and the lowest in Canada geese (5.8%). Of the ptarmigan species tested, only one rock ptarmigan was found to be T. gondii-positive, possibly due to low exposure to oocysts in their arctic, subarctic and alpine tundra habitats.
| Common name (References) | Latin name | Number tested | Number positive | Percent positive |
|---|---|---|---|---|
| Birds | ||||
| Rock ptarmiganFootnote 35 | Lagopus muta | 25 | 1 | 4.0% |
| Willow ptarmiganFootnote 35 | Lagopus lagopus | 24 | 0 | 0.0% |
| Ross’s gooseFootnote 36Footnote 37 | Chen rossii | 357 | 123 | 34.5% |
| Lesser snow gooseFootnote 36Footnote 37 | Chen caerulescens | 354 | 110 | 31.1% |
| Canada gooseFootnote 35Footnote 38 | Branta canadensis | 240 | 14 | 5.8% |
| Mammals | ||||
| Rodents | ||||
| Nearctic brown lemmingFootnote 37 | Lemmus trimucronatus | 84 | 0 | 0.0% |
| Lagomorphs | ||||
| Snowshoe hareFootnote 35 | Lepus americanus | 8 | 0 | 0.0% |
| Arctic hareFootnote 35 | Lepus arcticus | 2 | 0 | 0.0% |
| Ungulates | ||||
| Barren-ground caribouFootnote 39 | Rangifer tarandus groenlandicus | 117 | 43 | 36.8% |
| CaribouFootnote 35 | Rangifer tarandus | 97 | 11 | 11.3% |
| MuskoxFootnote 35Footnote 40 | Ovibus moschatus | 348 | 16 | 4.6% |
| Carnivores | ||||
| Arctic foxFootnote 41 | Vulpes lagopus | 39 | 17 | 43.6% |
| Canada lynxFootnote 16Footnote 35 | Lynx canadensis | 173 | 44 | 25.4% |
| WolverineFootnote 42 | Gulo gulo | 41 | 17 | 41.5% |
| Grey wolfFootnote 35 | Canis lupus | 37 | 7 | 18.9% |
| Black bearFootnote 35Footnote 43 | Ursus americanus | 43 | 16 | 37.2% |
Canadian Arctic rodents and lagomorphs showed no prevalence for T. gondii. Nearctic brown lemmings were negative, as were Arctic hares and snowshoe hares (Table 1). The only route of T. gondii transmission for non-migratory herbivores would be via ingestion of soil, plants or water contaminated with infectious oocysts. The absence of T. gondii prevalence in rodents and lagomorphs in the Canadian Arctic support the hypothesis that non-migratory Arctic herbivores have little to no exposure to infectious T. gondii oocystsFootnote 31.
The T. gondii exposure of ungulates varied between species. Caribou had a T. gondii prevalence of 11.3%, while the subspecies barren-ground caribou had a prevalence of 36.8%. It is unclear why barren-ground caribou were found to have such a high T. gondii prevalence. Muskox had a T. gondii prevalence of only 4.6% (Table 1).
The prevalence of T. gondii in carnivores was high in all species tested, as is to be expected even if the parasite’s prevalence in their prey is relatively low. In Canada, T. gondii prevalence was found to be 43.6% in Arctic foxes, 25.4% in Canada lynxes, 41.5% in wolverines, 18.9% in grey wolves and 37.2% in black bears (Table 1).
Canadian Arctic marine mammals
Most pinnipeds in the Canadian Arctic were positive for T. gondii, including harbour seals (16.4%), ringed seals (10.7%), bearded seals (10.0%), hooded seals (1.7%) and walrus (14.7%) (Table 2). Toxoplasma gondii was not detected in harp seals and more research may be required to determine if different feeding habits protect them from exposure to infected prey.
| Common name (References) | Latin name | Number tested | Number positive | Percent positive |
|---|---|---|---|---|
| Pinnipeds | ||||
| Harbour sealFootnote 26 | Phoca vitulina | 311 | 51 | 16.4% |
| Ringed sealFootnote 26Footnote 35 | Phoca hispida | 896 | 96 | 10.7% |
| Harp sealFootnote 35Footnote 44 | Phoca groenlandica | 113 | 0 | 0.0% |
| Bearded sealFootnote 26 | Erignathus barbatus | 20 | 2 | 10.0% |
| Hooded sealFootnote 44 | Cystophora cristata | 60 | 1 | 1.7% |
| WalrusFootnote 35 | Odobenus rosmarus | 34 | 5 | 14.7% |
| Bears | ||||
| Polar BearFootnote 35Footnote 44Footnote 45Footnote 46Footnote 47 | Ursus maritimus | 599 | 67 | 11.2% |
| Cetaceans | ||||
| BelugaFootnote 35Footnote 48 | Delphinapterus leucas | 69 | 13 | 18.8% |
| Bowhead whaleFootnote 35 | Balaena mysticetus | 2 | 1 | 50.0% |
Polar bears are the only ursines that are considered to be marine mammals because of their dependency on the ocean for food and habitat. Toxoplasma gondii has been detected in polar bears on the Canadian mainland and the Beaufort Sea, with an overall prevalence of 11.2%.
Two Arctic cetacean species have been tested for T. gondii: belugas and bowhead whales (Table 2). Toxoplasma gondii prevalence in belugas in the western Canadian Arctic was found to be 18.8% (Table 2). Of the two bowhead whales tested, one animal was T. gondii-positiveFootnote 35.
T. gondii in Arctic waters
Toxoplasma gondii DNA has been detected in up to 77% of samples of treated and untreated surface water and well water worldwideFootnote 49Footnote 50. In some regions of Canada, increased rainfall has been associated with elevated numbers of T. gondii oocysts in surface watersFootnote 51. Most of Canada’s rivers flow northward; 39% of Canada’s freshwater drains into Hudson Bay and 36% drains into the Arctic OceanFootnote 52. Oocysts that are washed into seawater are known to remain infective for up to two years and may be disseminated with the ocean currentsFootnote 20Footnote 53Footnote 54Footnote 55.
It has been hypothesized that fish could be the missing link between oocysts that end up in the watersheds and infection in marine mammalsFootnote 56. Toxoplasma gondii oocysts have been found in the alimentary tract of a wild fishFootnote 57 and it was shown that oocysts can remain infectious inside a fish’s alimentary tract for several hoursFootnote 58, thereby providing a possible source of infection for apex predators. To date, experimental infection of fish with T. gondii tissue cysts has only been reported in zebrafish and only under tightly controlled conditionsFootnote 57. Toxoplasma gondii has also been reported in a variety of shellfish worldwideFootnote 59, and this may provide another source of infection in marine mammals and humans, although this has not yet been documented and confirmed in the Arctic.
To determine if Arctic fish are a potential source of T. gondii, we tested muscle tissues of 121 freshwater and euryhaline fish from Nunavik for the presence of T. gondii DNA. Fifteen fish (12.4%) tested positive for T. gondii using polymerase chain reaction for DNA amplification, followed by Sanger sequencing. Atlantic salmon and Arctic char had a T. gondii prevalence of 26.7% and 12.0%, respectively. Other fish species that tested positive for T. gondii DNA were lake trout (2.9%) and brook trout (16.7%). Toxoplasma gondii was detected in one sculpin (n=1) but it was not found in pike or lake whitefish, possibly due to low sample size (n=2 and 6, respectively) (Reiling SJ, Boone R, Merks H, Dixon BR. Unpublished data, 2018). While these are preliminary findings, more fish from the Canadian Arctic are currently being analyzed in our laboratory for the presence of T. gondii.
Discussion
There are a number of mechanisms by which T. gondii may have been introduced into the Canadian Arctic. Toxoplasma gondii may have been introduced via migratory birds and mammals that became infected by ingestion of oocysts (which may persist in soil and water in geographical regions where felids are present), or infected prey, in their southern habitats and carried the infection with them to the North. The parasite could then be transmitted from one intermediate host to another in the Arctic, even in the absence of definitive hosts. In addition, predators, such as Arctic foxes, wolverines and grey wolves, showed high T. gondii prevalence, suggesting that carnivory may also be an important route of transmission in the Arctic. Oocysts shed by felids in the south and transported northwards through waterways may be another source of infection in aquatic animals in the Arctic. Until recently, fish had not been known as a potential source for T. gondii infection. However, our preliminary findings suggest that T. gondii may be present in fish in the Canadian Arctic and could be another source of infection in humans and fish-eating mammals.
Environmental factors that increase T. gondii prevalence in animals that are hunted by Inuit for subsistence may pose a growing health threat to Inuit in the Arctic regions of Canada. More research is needed to determine how environmental and socioeconomic changes influence T. gondii prevalence in animals and humans in the Canadian Arctic.
Climate change and warmer temperatures may promote forest growth in regions that were previously too coldFootnote 60Footnote 61Footnote 62. The increasing forest cover could expand the habitat of wild felids, thereby augmenting the release of T. gondii oocysts into the environmentFootnote 20. Higher numbers of oocysts combined with warming temperatures may increase the potential for infection of intermediate hosts, including birds and mammals not yet known to be hosts for T. gondii in the Canadian Arctic. This, in turn, may open up new transmission routes to humans who eat traditionally prepared country foods.
Conclusion
Toxoplasmosis has now spread throughout much of North and South America primarily through felids. Despite the absence of felids, T. gondii has now extended into Canada’s Arctic, and has posed a health risk to Inuit, especially in pregnant women and those with weakened immune systems. The most likely source of T. gondii infection in Inuit is through infected intermediate hosts and the consumption of traditionally prepared country foods including meat and organs which may be consumed raw. Preventing infection by cooking or thoroughly freezing fish, meat, and organs and a better understanding of ongoing zoonotic transmission patterns will help to address this risk.
Authors’ statement
SJR collected and analyzed the data. SJR and BRD wrote, proofread and approved the manuscript.
Conflict of interest
None.
Acknowledgements
We thank A Iqbal and S Lamhoujeb for providing the fish DNA. R Boone and H Merks have provided excellent technical assistance.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC); Visiting Fellowship in Canadian Government Laboratories Program (SJR); and Health Canada (BRD).
