Interim guidance for the detection of SARS-CoV-2 with the Abbott Panbio COVID-19 antigen rapid test

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 47-01: Foodborne and Animal Contact Disease Outbreaks
Date published: January 2021
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 47-01, January 2021: Foodborne and Animal Contact Disease Outbreaks
Implementation science
Interim guidance on the use of the Abbott Panbio™ COVID-19 Antigen Rapid Test
on behalf of Canadian Public Health Laboratory Network Laboratory Directors Council and the Canadian Public Health Laboratory Network Respiratory Virus Infection Working Group
Correspondence
Suggested citation
Canadian Public Health Laboratory Network Laboratory Directors Council and the Canadian Public Health Laboratory Network Respiratory Virus Infection Working Group. Interim guidance on the use of the Abbott Panbio™ COVID-19 Antigen Rapid Test. Can Commun Dis Rep 2021;47(1):17–22. https://doi.org/10.14745/ccdr.v47i01a04
Keywords: Abbott Panbio, COVID-19, antigen, rapid test, SARS-CoV-2, Canada, public health, guidance
Introduction
This document, prepared December 12, 2020, provides interim guidance on the use of the Abbott Panbio™ COVID-19 Antigen Rapid Test in the context of the Canadian public health system and a coordinated national response to the coronavirus disease 2019 (COVID-19) pandemic.
The Panbio COVID-19 Antigen Rapid Test is used for the qualitative detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen in human nasopharyngeal (NP) swab samples collected from individuals who are suspected of COVID-19 by their healthcare provider. The Panbio COVID-19 Antigen Rapid Test Device functions as a lateral flow assay including both a control and COVID-19 specific test line within a results window. After application of a patient specimen to the test device, the presence of a control line within the results window confirms the validity of the test result while the presence of a test line is interpreted as positive for COVID-19.
It should be noted that while Abbott already markets an antigen rapid test that is in widespread use in the United States (BinaxNOW™ COVID-19 Antigen Card), the antigen test, which has been approved for use and is being marketed in Canada (Panbio COVID-19 Antigen Rapid Test), is manufactured in a different facility. Furthermore, the two versions of the Abbott antigen capture test for COVID-19 differ considerably in their design attributes. As such, performance characteristics may not be the same. Canadian clinical data is required to validate the test that is in distribution nationally and at the time of writing, this data has not yet been adequately collected. Prior to authorization of the Panbio COVID-19 Antigen Rapid Test by Health Canada, the Canadian Public Health Laboratory Network formed a working group to verify performance characteristics of various antigen capture technologies coming to market. At the time of writing of this document, the evaluation and verification of the clinical sensitivity of the Panbio COVID-19 Antigen Rapid Test is ongoing. However, preliminary analytical sensitivity data suggest that the Panbio COVID-19 Antigen Rapid Test will likely have a lower sensitivity when compared with nucleic acid amplification tests, including the Abbott ID NOW™ (Table 1).
| Patient identificationTable 1 footnote b | E-gene Ct | qPCR test location | Adjusted Ct for inputTable 1 footnote c | Approximate number of input copiesTable 1 footnote dTable 1 footnote e | ID NOW result | Panbio result |
|---|---|---|---|---|---|---|
| Patient 1 | CPL | 16 | 22.6 | 1,294,497 | Positive | Positive |
| Patient 2 | CPL | 19 | 25.6 | 271,908 | Positive | Positive |
| Patient 3 | CPL | 19 | 25.6 | 383,421 | Positive | Positive |
| Patient 4 | CPL | 20 | 26.6 | 586,124 | Positive | Positive |
| Patient 5 | NML | 20.4 | 27 | ND | Positive | Positive |
| Patient 6 | NML | 22.2 | 28.8 | ND | Positive | Positive |
| Patient 7 | NML | 22.3 | 28.9 | ND | Positive | Positive |
| Patient 8 | NML | 24.6 | 31.2 | ND | Positive | Negative |
| Patient 9 | CPL | 25 | 31.6 | 16,116 | Positive | Negative |
| Patient 10 | NML | 25.2 | 31.8 | ND | Positive | Negative |
| Patient 11 | CPL | 26 | 32.6 | 1,547 | Positive | Negative |
| Patient 12 | CPL | 26 | 32.6 | 2,428 | Positive | Negative |
| Patient 13 | NML | 27.9 | 34.5 | 3,681 | Positive | Negative |
| Patient 14 | CPL | 30 | 36.6 | 164 | Positive | Negative |
| Patient 15 | NML | 30 | 36.6 | ND | Positive | Negative |
| Patient 16 | NML | 31.6 | 38.2 | 272 | Positive | Negative |
| Patient 17 | CPL | Negative | 0 | 0 | Negative | Negative |
| Patient 18 | CPL | Negative | 0 | 0 | Negative | Negative |
| Patient 19 | CPL | Negative | 0 | 0 | Negative | Negative |
| Pooled | NML | Negative | 0 | 0 | Negative | Negative |
The use of a lower sensitivity test carries risks to clinical and public health decision-making that can only be offset by the extent of possible benefits. Careful consideration must be made regarding where and how the Panbio COVID-19 Antigen Rapid Test is used in order to mitigate the heightened degree of diagnostic uncertainty associated with this technology in comparison with the conventional "gold standard" SARS-CoV-2 diagnostic testing in Canada.
These guidelines are meant to be updated periodically as more information is available regarding test sensitivity and specificity in the overall context of infection with SARS-CoV-2.
While this document as currently written is specific for the Abbott Panbio, many of these guidelines may also be applied to any less sensitive molecular and rapid antigen-based tests that are approved for use in the future.
Key messages
- Health Canada provided approval for use of the Panbio COVID-19 Antigen Rapid Test (October 2020).
- The Intended Use for this assay is outlined in the Panbio COVID-19 Antigen Rapid Test kit insert and states the following:
"The Panbio COVID-19 Antigen Rapid Test Device is an in vitro diagnostic rapid test for the qualitative detection of SARS-CoV-2 antigen in human nasopharyngeal swab specimens from individuals who meet COVID-19 clinical and epidemiological criteria. Panbio COVID-19 Antigen Rapid Test Device is for professional use only and is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection. The product may be used in laboratory and non-laboratory environments that meet the requirements specified in the Instruction for Use and local regulation. The test provides preliminary test results. Negative results don't preclude SARS-CoV-2 infection and they cannot be used as the sole basis for treatment or other management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. The test is not intended to be used as a donor screening test for SARS-CoV-2."
- Clinical performance of the Panbio COVID-19 Antigen Rapid Test must continue to be carefully monitored due to the anticipated low sensitivity of the assay.
- The performance of the assay should be verified in the field before recommending its use. This is critical since data obtained from pre-market evaluations cannot adequately account for anticipated variability in training or in the quality of sample collection that follows its use in a broader population, and particularly, in point-of-care (POC) situations.
- The Panbio COVID-19 Antigen Rapid Test requires the collection of an NP swab. This test may be less acceptable for serial testing of populations, particularly in low-risk and asymptomatic individuals, as compared with other tests (i.e. Abbott ID NOW, which, in addition to NP swabs, can also be operated with throat or nasal swabs).
- The "in-field" performance characteristics of the Panbio COVID-19 Antigen Rapid Test is still under evaluation in Canada; however, data about the performance of the Panbio COVID-19 Antigen Rapid Test assay in the United States suggest that the tests have lower sensitivity but comparable specificity to laboratory-developed tests and commercial nucleic acid amplification tests.
- Although the rapid nature and the ease of use of the Panbio COVID-19 Antigen Rapid Test makes it suitable for POC applications, the performance characteristics described above combined with the incidence of infection within the population being tested must be considered when interpreting the results.
- Indications for testing (e.g. symptomatic versus asymptomatic, outbreak vs non outbreak, congregated settings vs general population) are also an important consideration in use of this technology.
- In discussion with provincial and territorial laboratory directors, careful consideration regarding the use of this test must be in place.
- At this time, until further data is collected, because of the decreased sensitivity, all negatives should be considered preliminary negatives.
- Owing to an expected higher rate of false negatives (relative to conventional nucleic acid amplitude testing), it is recognized that reflexive laboratory-based testing of preliminary negatives from the Panbio COVID-19 Antigen Rapid Test (depending on its proposed use) will likely introduce an additional burden to reference laboratories already facing enormous testing volumes. The utility of lab retesting using a more sensitive method must take into account the initial indication for testing.
- This document outlines scenarios where the Panbio COVID-19 Antigen Rapid tests may prove useful, should the expected performance characteristics be confirmed.
Current approach to SARS-CoV-2 testing in Canada
Since the emergence of SARS-CoV-2, testing has been a key pillar of Canada's response to the pandemic. The broad use of testing, as part of an array of public health measures, contributed to a flattening of the epidemic curve in the spring of 2020, demonstrating the value of testing as a part of the COVID-19 response. To date, testing has relied on molecular (i.e. reverse transcription polymerase chain reaction; RT-PCR) testing performed on a NP or alternate respiratory sample collected by a health care professional. This testing method currently remains the gold standard for detecting SARS-CoV-2 infection in Canada.
Considerations for the use of the Panbio COVID-19 Antigen Rapid Test
Notwithstanding the difference in the performance profile, other features of the Panbio COVID-19 Antigen Rapid Test (including, but not limited to, faster turnaround time, lower per-test cost, ability to deliver testing in some jurisdictions by non-healthcare professionals and on a more frequent basis) suggest that it could have an important role to play in the next phase of the pandemic response.
It is critically important to understand the timing of specimen collection in relation to symptom onset, since the lower sensitivity of the test is not expected to be uniform over the course of infection. Data suggest that viral shedding may begin 2-3 days before symptoms appear, peaking around the time of symptom onset and then declining gradually over time Footnote 1 Footnote 2. During the first five days of symptom onset, viral loads are most likely to be above the limit of detection for the Panbio COVID-19 Antigen Rapid Test, although the time post-symptom onset still needs to be carefully considered. It is also important to understand test performance relative to the time since a potential exposure (i.e. the number of days after exposure that one might expect to have viral loads that can be optimally detected with the Panbio COVID-19 Antigen Rapid Test) when used for rapid contact tracing.
It is important for public health, microbiology and infectious disease experts to identify the scenarios whereby the use of the Panbio COVID-19 Antigen Rapid Test may further strengthen the public health response by 1) expanding access to testing beyond existing indications and 2) increasing capacity for detection of SARS-CoV-2. Furthermore, establishing mechanisms to allow the results from a new POC test to be efficiently input into the public health system is critical (see Reporting of results and quality control section below).
Balancing test sensitivity against other considerations
The intrinsic performance characteristics of the Panbio COVID-19 Antigen Rapid Test are not the only factors that determine its utility. The final interpretation of a test must take into account the performance parameters, prevalence of infection, predictive values and intended use of the test result. Therefore, the tolerance for sensitivity and specificity thresholds will vary based on the reason for testing and the expected action that would follow either a positive or a negative result.
In scenarios where critical decisions and actions rely on a test result (e.g. a symptomatic resident in a long-term care home or a patient in the Intensive Care Unit who requires immediate treatment), the recommended test would be the most accurate test. At the time of writing, the indicated (best) test would be RT-PCR performed on a NP sample or lower respiratory tract samples in those with evidence of pneumonia. However, there may be circumstances where a rapid POC test would be permissible and would enhance testing capacity to support the public health response, particularly when the demand for RT-PCR testing exceeds laboratory capacity, is otherwise unavailable or in situations where a symptomatic individual may otherwise be lost to follow-up.
Proposed use of the Panbio COVID-19 Antigen Rapid Test
One strategy to reduce the sensitivity gap of a technology would be to use repeat serial testing. However, this may not be feasible with the Panbio COVID-19 Antigen Rapid Test. This technology specifically requires the use of a NP swab, which may limit its utility and uptake owing to the uncomfortable nature of the patient specimen collection and the requirement for collection by a healthcare professional. In low prevalence, low-risk settings, serial repeat testing with the Panbio COVID-19 Antigen Rapid Test may not be ideal. This may be particularly relevant in settings involving a paediatric population (daycares, schools, sport teams).
There are, however, specific situations that the Panbio COVID-19 Antigen Rapid Test might be considered as a suitable option: when infection is present (whether symptomatic or asymptomatic) within a community; symptomatic testing in congregated settings; symptomatic testing in Northern, remote and isolated (NRI) communities; and asymptomatic community-based surveillance in the general population.
Infection is prevalent within a community
The Panbio COVID-19 Antigen Rapid Test could be used to test individuals when the prevalence of infection is high within a community and the access to timely RT-PCR testing is significantly limited (Figure 1). Positive results could be considered preliminary (presumptive) positive and actioned immediately because of the increased positive predictive value in these settings. Public health action (isolation, contact tracing) should be implemented immediately while laboratory-based PCR tests are conducted to confirm results.
Figure 1: Scenario 1-symptomatic testing when infection is prevalent within a community
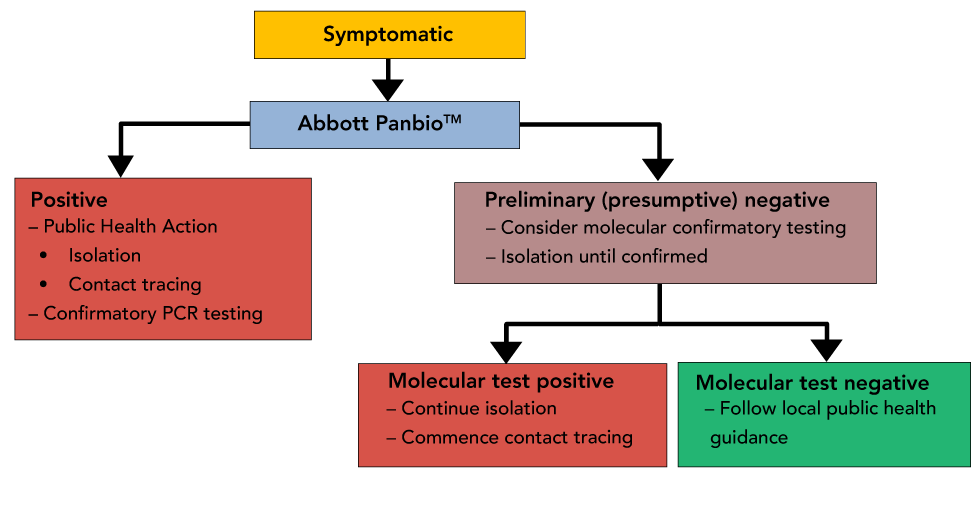
Text description: Figure 1
Figure 1: Scenario 1-symptomatic testing when infection is prevalent within a community
Decision Tree representing suggested public health actions, reporting, and requirement for retesting of symptomatic patients when infection is prevalent within a community (for example, during an outbreak). Due to the higher positive predictive value during an outbreak, a positive result could be considered a true positive, although the need for retesting would be left to provincial/territorial guidance.
One must take into consideration if an individual who receives a negative result from the Panbio COVID-19 Antigen Rapid Test is symptomatic or asymptomatic, as all negative results are considered to be "preliminary (presumptive) negative".
Scenario 1-symptomatic testing within a community: It is recommended that symptomatic individuals who receive preliminary (presumptive) negative results be re-tested and maintained in isolation until confirmatory laboratory-based RT-PCR testing results are available. The flow diagram in Figure 1 depicts one possible approach to testing; however, algorithms are likely to vary across different provinces/territories depending on local factors, including stage of pandemic wave and health system experience with the Panbio assay.
NRI communities face additional barriers to accessing timely test results due to transportation time required to deliver a specimen to a testing laboratory. Given the importance of accurately identifying new cases in NRI communities in order to prevent spread in the face of limited healthcare resources, RT-PCR testing is the recommended test for these settings. The use of the Panbio COVID-19 Antigen Rapid Test may be helpful in NRI communities where access to laboratory-based testing services and rapid results are unavailable or difficult to access.
Scenario 2-asymptomatic testing within a community: The reflexive re-testing of asymptomatic individuals who receive preliminary (presumptive) negative results must take into consideration the burden that will be placed on already overwhelmed laboratory-based testing systems (Figure 2).
Figure 2: Scenario 2-asymptomatic testing when infection is prevalent within a community
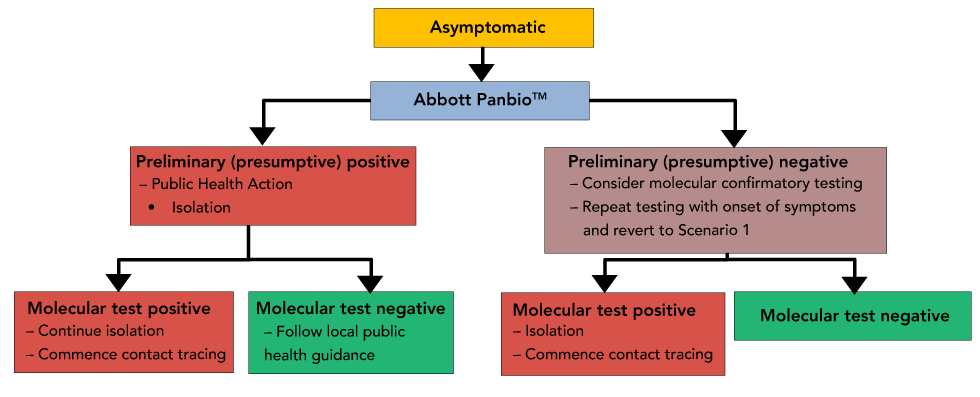
Text description: Figure 2
Figure 2: Scenario 2-asymptomatic testing when infection is prevalent within a community
Decision Tree representing suggested public health actions, reporting and retesting of asymptomatic patients when infection is prevalent within a community. In this scenario, a positive Panbio result would require retesting by a more sensitive molecular method.
Testing in congregate settings
Scenario 1-symptomatic testing within a congregate setting: While the use of a less sensitive test would not be recommended for the exclusive management of an outbreak, testing of symptomatic individuals and direct contacts with the Panbio COVID-19 Antigen Rapid Test can be a useful tool for the early identification of possible outbreaks in congregate settings (e.g. long-term care and correctional facilities, large processing plants, workers in remote mine settings, homeless shelters) (Figure 3). Testing can be part of suspected outbreak identification and investigation where patients can be tested rapidly on site if faster preliminary results will help inform and expedite public health action (triage of patients and contact tracing). All POC antigen tests should be followed up with an in-lab PCR test when done in the setting of an outbreak. This may be particularly relevant in situations where a symptomatic individual may otherwise be lost to follow-up (i.e. homeless shelter).
Figure 3: Scenario 1-symptomatic testing in congregate settings
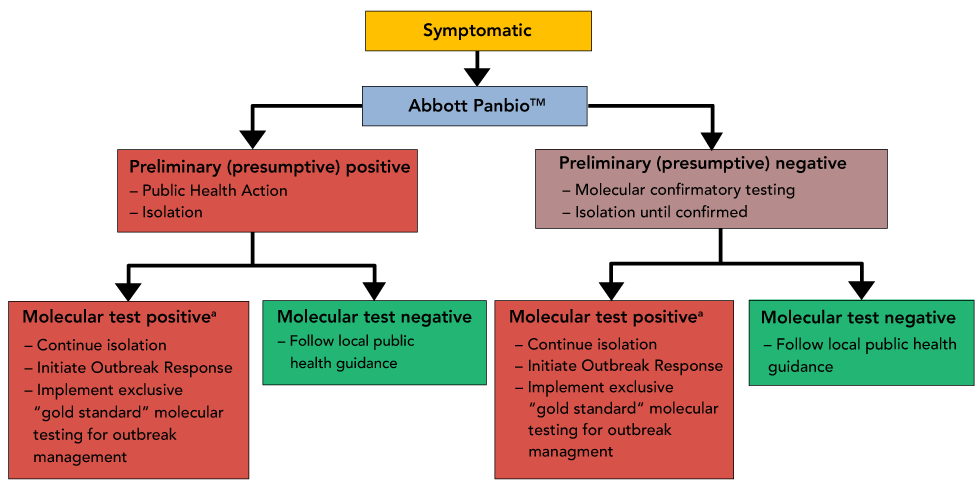
a Single molecularly-confirmed positive case within residents or staff initiates the Outbreak Response
Text description: Figure 3
Figure 3: Scenario 1-symptomatic testing in congregate settings
Decision Tree representing suggested public health actions, reporting and retesting of symptomatic patients in a congregated/high-risk setting. In this scenario, both positive and negative Panbio results would require reflexive retesting due to the increased consequences of both false negative and false positive results.
Here the intended use of a POC test is for monitoring infection in individuals who may not otherwise be able to be tested with the same frequency due to challenges with testing capacity. Due to the potential reduction in pre-test probability of a positive result, the test would have to be confirmed using a laboratory-based nucleic acid amplification test. This requirement for confirmation is to reduce the potential for negative factors associated with a false positive test (e.g. unnecessary removal from work, stigma that may be associated with infection).
Scenario 1A-symptomatic testing in isolated northern communities: Panbio COVID-19 Antigen Rapid Tests could be used to screen all individuals in NRI communities presenting with one or more COVID-19 symptoms (within five days of symptom onset) as a means of providing real-time surveillance of a potential COVID-19 outbreak and to expedite public health actions. Due to anticipated delays in the return of laboratory results, two NP swabs would always be collected when a patient first presents for care. One NP swab would subsequently be tested on the Panbio COVID-19 Antigen Rapid Test while the second NP swab would be reflexively sent for testing by a gold standard molecular method (at a reference laboratory or at a site using the GeneXpert Xpert™ Xpress SARS-CoV-2 molecular test). In this scenario, all Panbio COVID-19 Antigen Rapid Test results (both positive and negative) would be considered preliminary/presumptive until molecularly confirmed. Presumptive negative results would require symptomatic individuals to continue self-isolation until results were confirmed negative by reference testing, while a presumptive positive result would allow for immediate public health actions that could significantly benefit community members at increased risk of severe illness from COVID-19 (i.e. over 65 years of age or underlying medical conditions). If a Panbio COVID-19 Antigen Rapid Test is confirmed as positive by a molecular reference method, the NRI community would initiate an outbreak response that could include ongoing Panbio screening but would also need to incorporate gold standard molecular testing for effective outbreak management.
Scenario 2-asymptomatic testing in congregate settings: Monitoring of asymptomatic individuals who are at risk of introducing infection into high-risk settings could be considered. Modelling data suggest that testing protocols that incorporate repeated and frequent re-testing of asymptomatic individuals could be effective Footnote 3. The one caveat is that there may be resistance by individuals to undergo repeat NP swab collections due to discomfort. The need for a healthcare professional to obtain NP swabs, combined with a decreased sensitivity of the Panbio COVID-19 Antigen Rapid Test, suggest that this technology may have less utility for the repeat serial testing of asymptomatic individuals in the absence of a known outbreak or in a high prevalence setting. At this time, the market authorization for the Panbio COVID-19 Antigen Rapid Test from Health Canada-Medical Devices Bureau is focussed exclusively on symptomatic testing in the early phase of disease, so the use of the test in a monitoring context will require careful clinical validation. The frequency of repeat testing has not yet been defined.
Asymptomatic community-based surveillance in the general population
There is an abundance of data highlighting the asymptomatic spread of SARS-CoV-2. Up to 40% of all transmissions occurring in the general population, even with hand hygiene, mask use and social distancing, appear to be due to silent or asymptomatic transmission events. Widespread testing of individuals in the general population will provide a better understanding of the extent of asymptomatic spread and prevalence of the infection in the general population and may also help to destigmatize testing for COVID-19. Similar to recent know-your-status programs for sexually transmitted blood-borne infections, widespread screening and the knowledge that is gained from it can help normalize COVID-19 testing and help to inform and reduce behaviours associated with transmission. Widespread community-based testing of asymptomatic individuals must take into consideration the impact this testing may have on both the healthcare and laboratory systems, ensuring that health care and laboratory resources can remain focussed on the needs of high-risk and symptomatic individuals. As such, community-based testing for COVID-19 will likely require novel approaches to sample collection such as using non-regulated, non-healthcare professionals who are trained to provide testing on site. Tested individuals would either be able to receive results on site or have results returned to them via text or email in a timely manner.
Figure 4 summarizes the steps to be taken for asymptomatic community-based surveillance in the general population.
Figure 4: Asymptomatic community-based surveillance in the general population
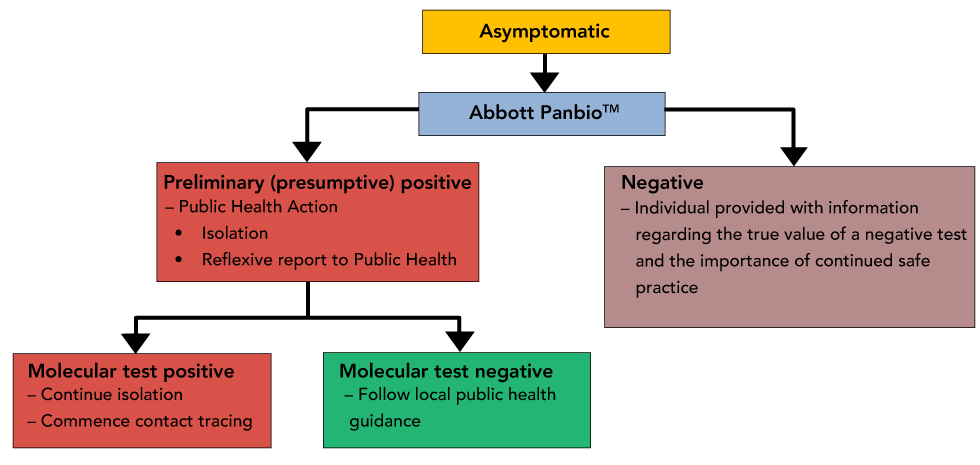
Text description: Figure 4
Figure 4: Asymptomatic community-based surveillance in the general population
Decision Tree representing suggested public health actions, reporting and retesting of asymptomatic patients for surveillance of the general population. In this scenario, a negative Panbio result would not require reflexive testing owing to the likelihood of overwhelming an already strained laboratory testing program.
In the case of a negative result, text messages can also include information about the limitations of a negative result and reinforce public health measures such as continued vigilance/attention to symptoms. Negative results would not require reflexive testing as it would likely overwhelm an already strained laboratory-based testing program.
In the case of a positive result, the individual would be told to self-isolate and be appropriately linked to provincial/territorial public health systems for confirmatory testing and follow up (i.e. contact tracing). Information can be provided in parallel to public health to expedite effective interventions.
Reporting of results and quality assurance
The use of the Panbio COVID-19 Antigen Rapid Test will most likely occur outside of a laboratory environment. The current anticipated market authorizations are expected to require oversight of the testing procedure by a trained healthcare professional. It will be essential that a mechanism and guidance for reporting of results (particularly positive results) into the public health system and/or laboratory system is established to ensure appropriate data capture and quality control, and to support public health action.
It is critical that quality assurance practices be considered when implementing POC testing, regardless of the perceived simplicity of the test. Where POC testing is implemented outside a hospital environment, sites are recommended to partner with local accredited laboratories for ongoing guidance and oversight. The laboratory director and partnering laboratories will guide sites to ensure important quality assurance practices are in-place.
Examples of quality assurance practices that must be considered:
- Training and ongoing authorization of staff who will perform POC testing
- Initial and ongoing reagent validation prior to clinical use
- Quality control practices for regular monitoring of test performance
- Proficiency testing to monitor overall testing practices at a site
- Troubleshooting issues with tests and/or devices
- Reporting of results
Critical scientific questions
The state of the science continues to evolve daily as unprecedented global investment in research and development continues. Despite this, there remains a number of critical questions to inform the use of new tests such as the Panbio COVID-19 Antigen Rapid Test and sample types.
- How do these tests perform in "real life" situations?
- Many submissions for regulatory approval have used simulated samples to evaluate tests. This creates uncertainty about the true performance when applied to actual patients. There must be a verification of performance by comparing the real life performance of intended use in the field compared to the traditional nucleic acid amplification methodology.
- How frequently is testing required to close the sensitivity gap?
- This requires understanding of the dynamics of the test over time. It will be important to determine the frequency of testing to best mitigate the risk of cases being missed due to the lower sensitivity of the Panbio COVID-19 Antigen Rapid Test.
- At what threshold of community transmission is repeat testing in specific environments beneficial?
Conclusion
This document provides interim guidance on the use of the Abbott Panbio COVID-19 Antigen Rapid Test in the context of the Canadian public health system and a coordinated national response to the coronavirus disease. These guidelines are meant to be updated periodically as more information is available regarding test sensitivity and specificity in the overall context of infection with SARS-CoV-2, 2019 (COVID-19) pandemic.
Competing interests
None.
Acknowledgements
The Canadian Public Health Laboratory Network (CPHLN) input is derived from the CPHLN Laboratory Director's Council and Respiratory Virus Infection Working Group (ReVI). We would like to thank members of the CPHLN Secretariat, including A MacKeen and T Kuschak for coordinating the document synthesis.
Funding
None.
