Health Product InfoWatch, June 2020

Download the alternative format
(PDF format, 668 KB, 9 pages)
Health Products and Food Branch
Marketed Health Products Directorate
Health Product InfoWatch Editorial Team
ISSN: 2368-8025
Cat.: H167-1E-PDF
Pub.: 200000
Contents
- Health products mentioned in this issue
- Coronavirus disease (COVID-19)
- Did you know? List of Drugs for Exceptional Importation and Sale
- Announcement: Statements on vaccine confidence by the International Coalition of Medicines Regulatory Authorities
- Monthly recap
- New information
- Scope
- Helpful links
- Suggestions?
- Copyright
Health products mentioned in this issue
Pharmaceuticals and Biologics
- Axid (nizatidine)
- Cabometyx (cabozantinib)
- Caprelsa (vandetanib)
- Cisatracurium Omega Multi-Dose (cisatracurium besylate injection)
- Convalescent plasma to treat COVID-19
- Fresenius Propoven 2% (propofol 20 mg/mL)
- Iclusig (ponatinib)
- Inlyta (axitinib)
- Ketamine Hydrochloride Injection
- Lenvima (lenvatinib)
- Nexavar (sorafenib)
- Norvasc (amlodipine)
- Ofev (nintedanib)
- Rocuronium Bromide Injection distributed by Avir Pharma Inc.
- Rocuronium Bromide Injection distributed by SteriMax Inc.
- Salbutamol Aldo-Union inhalers
- Stivarga (regorafenib)
- Sutent (sunitinib)
- Teva Salamol CFC-Free (salbutamol sulfate) Inhaler
- Vascular endothelial growth factor receptor tyrosine kinase inhibitors
- Votrient (pazopanib)
Medical Devices
Natural and Non-prescription Health Products
- Hand sanitizers, disinfectants, household cleaning products and bleaches
- Ibuprofen-containing products
- Triton Hand Sanitizer
Other
Coronavirus disease (COVID-19)
For the most up-to-date information on COVID-19, please visit the Government of Canada Coronavirus disease (COVID-19) Web site Canada.ca/coronavirus, which includes a dedicated section for healthcare professionals, and for the health product industry.
Did you know? List of Drugs for Exceptional Importation and Sale
Due to an unprecedented demand and urgent need for access to drugs during the COVID-19 pandemic, the Minister of Health signed an Interim Order to allow certain foreign drugs, approved by another regulator and with similar high quality and manufacturing standards to those required for Canadian approved products, to be imported and sold in Canada. An Interim Order is one of the fastest mechanisms available to address large-scale public health emergencies, without following the usual regulatory processes.
To access the list of these designated drugs please visit the List of Drugs for Exceptional Importation and Sale. The list is continuously updated as new foreign drug products are allowed to be imported.
Announcement: Statements on vaccine confidence by the International Coalition of Medicines Regulatory Authorities
Health Canada is a member of the International Coalition of Medicines Regulatory Authorities (ICMRA). This executive-level initiative of regulatory authorities comprises 29 regulators with the World Health Organization as an observer. It provides strategic leadership to address current and emerging regulatory and safety challenges in human medicines.
To help address the public health challenge posed by vaccine hesitancy, ICMRA members have published two statements about confidence in vaccine safety and effectiveness:
- ICMRA statement about confidence in vaccine safety and effectiveness (for healthcare professionals)
- ICMRA statement about confidence in vaccines (for the general public)
Monthly recap of health product safety information
The following is a list of health product advisories, type I recalls as well as summaries of completed safety reviews published in May 2020 by Health Canada.
Akwaton International Multipurpose Wipes
Health Canada advised Canadians to stop using Akwaton International Multipurpose Wipes. This product has not been authorized by Health Canada and may cause skin irritation or an allergic reaction, especially in vulnerable populations such as children. Health Canada has requested a stop sale and advertising of the unauthorized product to Canadians.
Advisory – Akwaton International Multipurpose Wipes
Axid (nizatidine)
Two lots of Axid (nizatidine) were recalled from the Canadian market, as they contain a nitrosamine impurity, N-nitrosodimethylamine (NDMA). The NDMA levels in the 2 recalled lots are not higher than acceptable limits at this time, but they could increase with time.
Cisatracurium Omega Multi-Dose (cisatracurium besylate injection)
There is an increased demand and shortage in Canada for cisatracurium besylate injection as a result of the COVID-19 pandemic. To maintain a continued supply of cisatracurium besylate injection in Canada, Health Canada has authorized a temporary colour change of the ferrule (metal seal) from teal to red for Cisatracurium Omega Multi-Dose 20 mg/10 mL (2 mg/mL) vials. This is a temporary change that will remain in place until additional inventory is received by Omega. Healthcare professionals are advised that there is a potential risk of errors resulting from inadvertent selection and administration of neuromuscular blockers.
Convalescent plasma trial to treat COVID-19
Health Canada is expediting the review of any submissions related to COVID-19 and has authorized a nation-wide clinical trial (CONCOR-1 study) on the use of convalescent plasma to treat COVID-19. A list of all the COVID-19 clinical trials authorized by Health Canada, including this one, is available on Health Canada’s Web site, Vaccines and treatments for COVID-19.
Advisory – Convalescent plasma trial to treat COVID-19
Fresenius Propoven 2% (propofol 20 mg/mL)
There is an increased demand and shortage of propofol injection in Canada as a result of the COVID-19 pandemic. Health Canada has added Fresenius Propoven 2% in 100 mL vials to the List of Drugs for Exceptional Importation and Sale. Fresenius Propoven 2% (propofol 20 mg/mL) contains twice the concentration of propofol compared to ALL currently authorized and marketed propofol injection products (propofol 1%, 10 mg/mL) in Canada. Healthcare professionals are advised that dosing calculations need to be modified to reflect the propofol concentration (20 mg/mL).
Health Professional Risk Communication – Fresenius Propoven 2% (propofol 20 mg/mL)
Hand sanitizers, disinfectants, household cleaning products and bleaches
Health Canada warned Canadians about the risks of improperly using hand sanitizers, disinfectants, household cleaning products and bleaches, and reminded them to always read and follow the directions on product labels. Authorized hand sanitizers have an eight-digit Drug Identification Number (DIN) or Natural Product Number (NPN) on the label and are listed on the List of Hand Sanitizers Authorized by Health Canada. Authorized hard-surface disinfectants will have an eight-digit DIN on the label and are listed on the List of Disinfectants for Use Against COVID-19.
Advisory – Hand sanitizers, disinfectants, household cleaning products and bleaches
Ibuprofen-containing products
This safety review evaluated the risk of necrotising fasciitis associated with ibuprofen containing-products used in children with chickenpox infection. Health Canada's review of the available information did not find a link. Health Canada will continue to monitor the safety of ibuprofen.
Summary Safety Review – Ibuprofen-containing products
Ketamine Hydrochloride Injection
There is an increased demand for ketamine hydrochloride injection in Canada as a result of the COVID-19 pandemic. Health Canada has added US-labelled Ketamine Hydrochloride 500 mg/5 mL (100 mg/mL concentration) to the List of Drugs for Exceptional Importation and Sale. US-labelled Ketamine Hydrochloride Injection (100 mg/mL) contains 10 times or 2 times higher concentration compared to Canadian-labelled ketamine hydrochloride injection products (10 mg/mL or 50 mg/mL, respectively). Healthcare professionals are advised that dosing calculations need to be modified to reflect the higher ketamine concentration (100 mg/mL).
Health Professional Risk Communication – Ketamine Hydrochloride Injection
Respirator masks
Health Canada determined that certain respirators, which have failed to demonstrate a 95% filtration rate, may pose a health and safety risk to users, when used in a setting that requires 95% filtration (such as healthcare settings). Health Canada has asked importers and distributors that may have imported respirators that do not meet performance standards to notify their customers and relabel products to indicate that while these masks may not meet the standards required for frontline healthcare workers, they could be used as face masks in settings where a 95% filtration is not needed. The products are not being removed from the market.
Rocuronium Bromide Injection distributed by Avir Pharma Inc.
There is an increased demand and shortage of Rocuronium Bromide Injection in Canada as a result of the COVID-19 pandemic. Health Canada has added US-labelled Rocuronium Bromide Solution for Injection 50 mg/5 mL (10 mg/mL) to the List of Drugs for Exceptional Importation and Sale. The US-labelled Rocuronium Bromide Injection does not have a red ferrule (metal seal on vial). Instead, this product has a yellow ferrule. There is a potential risk of errors resulting from inadvertent selection and administration of neuromuscular blockers.
Rocuronium Bromide Injection distributed by SteriMax Inc.
There is an increased demand and shortage of Rocuronium Bromide Injection in Canada as a result of the COVID-19 pandemic. Health Canada has added Sterimax’s US-labelled Rocuronium Bromide Injection 50 mg/5 mL (10 mg/mL) vials to the List of Drugs for Exceptional Importation and Sale. The US-labelled Rocuronium Bromide Injection does not have a red ferrule (metal seal on vial). Instead, this product has a yellow ferrule. There is a potential risk of errors resulting from inadvertent selection and administration of neuromuscular blockers.
Health Professional Risk Communication – Rocuronium Bromide Injection distributed by SteriMax Inc.
Salbutamol Aldo-Union inhalers
There is an increased demand and shortage of salbutamol metered-dose inhalers (MDIs) in Canada as a result of the COVID-19 pandemic. Health Canada has added Spanish-labelled Salbutamol Aldo-Union metered-dose aerosol (100 mcg salbutamol /metered dose) to the List of Drugs for Exceptional Importation and Sale. While the label text of the imported salbutamol is in Spanish, a sticker will be added to the carton label including the distributor name, contact information and unique bar code.
Health Professional Risk Communication – Salbutamol Aldo-Union inhalers
Teva Salamol CFC-Free (salbutamol sulfate) Inhaler
There is an increased demand and shortage of salbutamol metered-dose inhalers (MDIs) in Canada as a result of the COVID-19 pandemic. Health Canada is allowing the importation of a limited supply of Teva’s UK-labelled salbutamol inhaler (100 mcg per metered dose) to help alleviate the current shortage of this product.
Health Professional Risk Communication – Teva Salamol CFC-Free (salbutamol sulfate) Inhaler
Triton Hand Sanitizer
Some units of Triton Hand Sanitizer containing technical-grade ethanol were distributed and sold without the required risk information on the label. This hand sanitizer contains technical-grade ethanol, which Health Canada recently authorized on a temporary basis for use in hand sanitizers in Canada to help address the increased demand in response to COVID-19. Fluid Energy Group Ltd. has contacted all of its customers to request that they stop selling the mislabelled product and is working to correct the labelling issue for any remaining product in stock.
Advisory – Triton Hand Sanitizer
Unauthorized COVID-19 test kits
Health Canada advised Canadians not to buy home diagnostic test kits, including mail-in sample collection kits, claiming to diagnose or detect COVID-19 because they may provide inaccurate or false results. These products have not been authorized by Health Canada. Health Canada has authorized COVID-19 test kits intended for use only by healthcare professionals or trained operators.
Advisory – Unauthorized COVID-19 test kits
New health product safety information
The following topics have been selected to raise awareness and, in some cases, to stimulate reporting of similar adverse reactions.
Vaccine safety biannual summary
Health Canada and the Public Health Agency of Canada (PHAC) share the responsibility of monitoring of the safety of vaccines in Canada.
Marketing authorization holders are required to report serious adverse events following immunization to the Canada Vigilance Program in Health Canada. The Canada Vigilance Program also receives voluntary reports from healthcare professionals and consumers.
Provincial and territorial public health authorities report adverse events following immunization (AEFIs) from publicly-funded vaccine programs to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) in PHAC to monitor the safety of immunization programs.
Report for July 1, 2019 to December 31, 2019
Key Messages:
- No new safety signals (potential safety issues) were identified during this period.
- From July 1, 2019 to December 31, 2019, the Canada Vigilance Program received 597 reportsFootnote * of adverse events following immunization for which vaccines were a suspected cause.
This biannual vaccine safety summary includes reports of adverse events following immunization received by the Canada Vigilance Program between July 1, 2019 and December 31, 2019. To access reports published by the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS), please visit the CAEFISS Web site.
- From July 1, 2019 to December 31, 2019, the Canada Vigilance Program received 597 reportsFootnote * of adverse events following immunization for which vaccines were a suspected cause.
- The largest proportion of the reports was from spontaneous reporting (Figure 1). The source of the reports is presented in Figure 2.
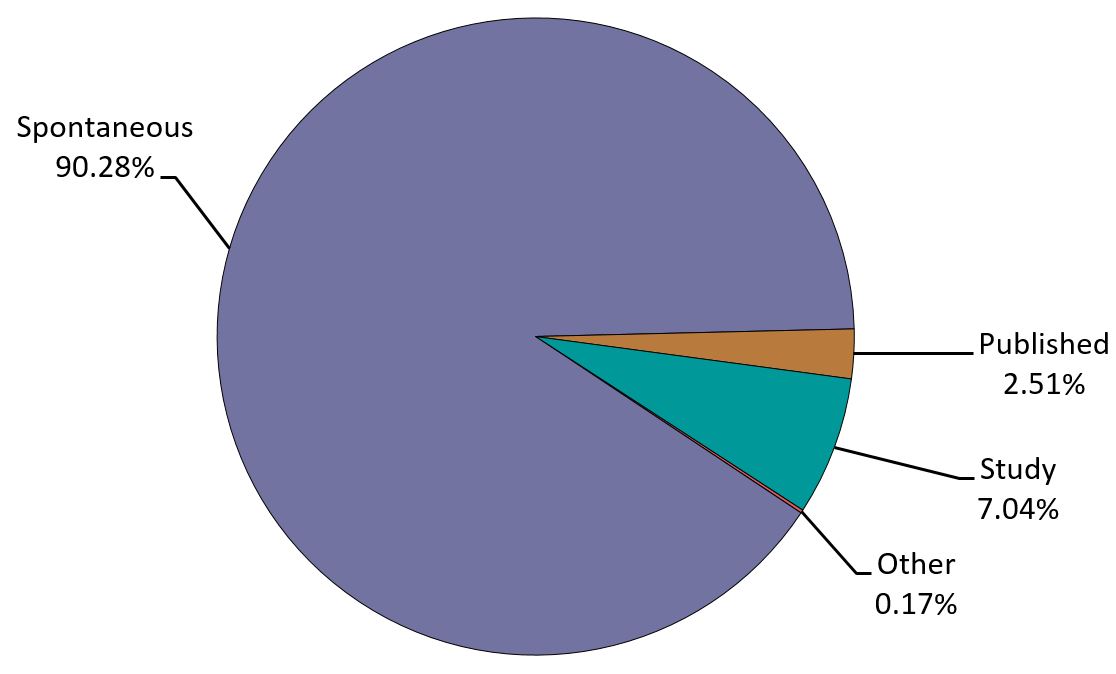
Figure 1 - Text description
The figure shows the percentage of immunization reports received from July 1 to December 31, 2019, by type of report.
| Report Type | Percentage (%) |
|---|---|
| Other | 0.17 |
| Published | 2.51 |
| Spontaneous | 90.28 |
| Study | 7.04 |
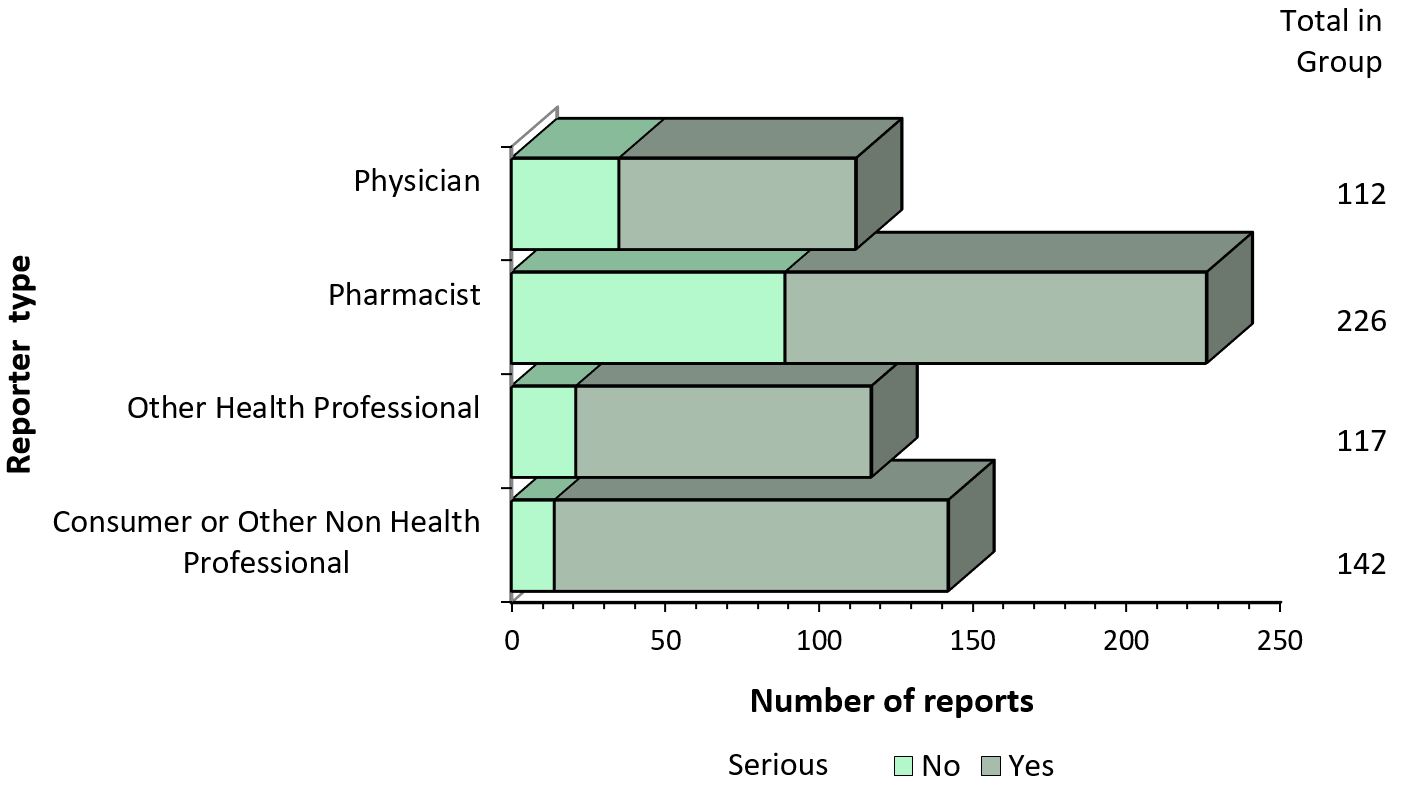
Figure 2 - Text description
The figure shows the total number of reports received from July 1 to December 31, 2019, by type of originating reporter and severity.
| Originating reporter | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Physician | 35 | 77 |
| Pharmacist | 89 | 137 |
| Other Health Professional | 21 | 96 |
| Consumer Or Other Non Health Professional | 14 | 128 |
- Most of the reports involved females (Figure 3), and the most common age group was adults from 19 to 64 years of age (Figure 4).
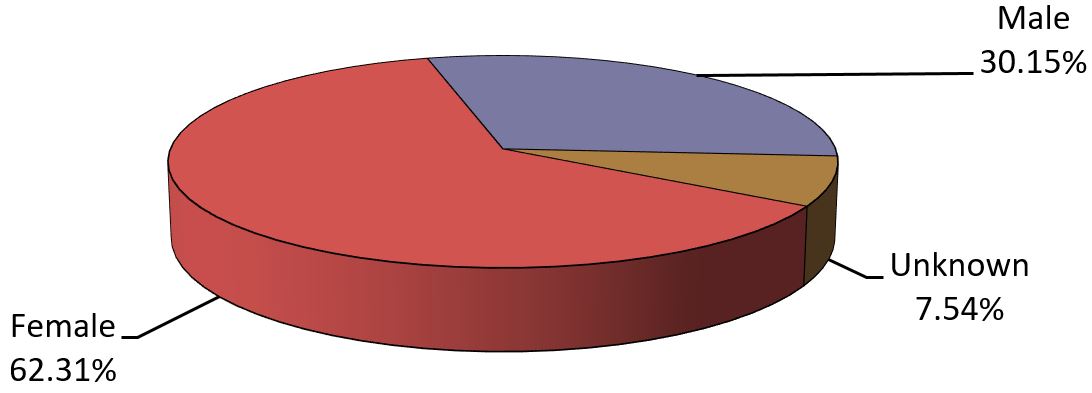
Figure 3 - Text description
The figure shows the percentage of immunization reports received from July 1 to December 31, 2019, by gender.
| Sex | Percentage (%) |
|---|---|
| Female | 62.31 |
| Male | 30.15 |
| Unknown | 7.54 |
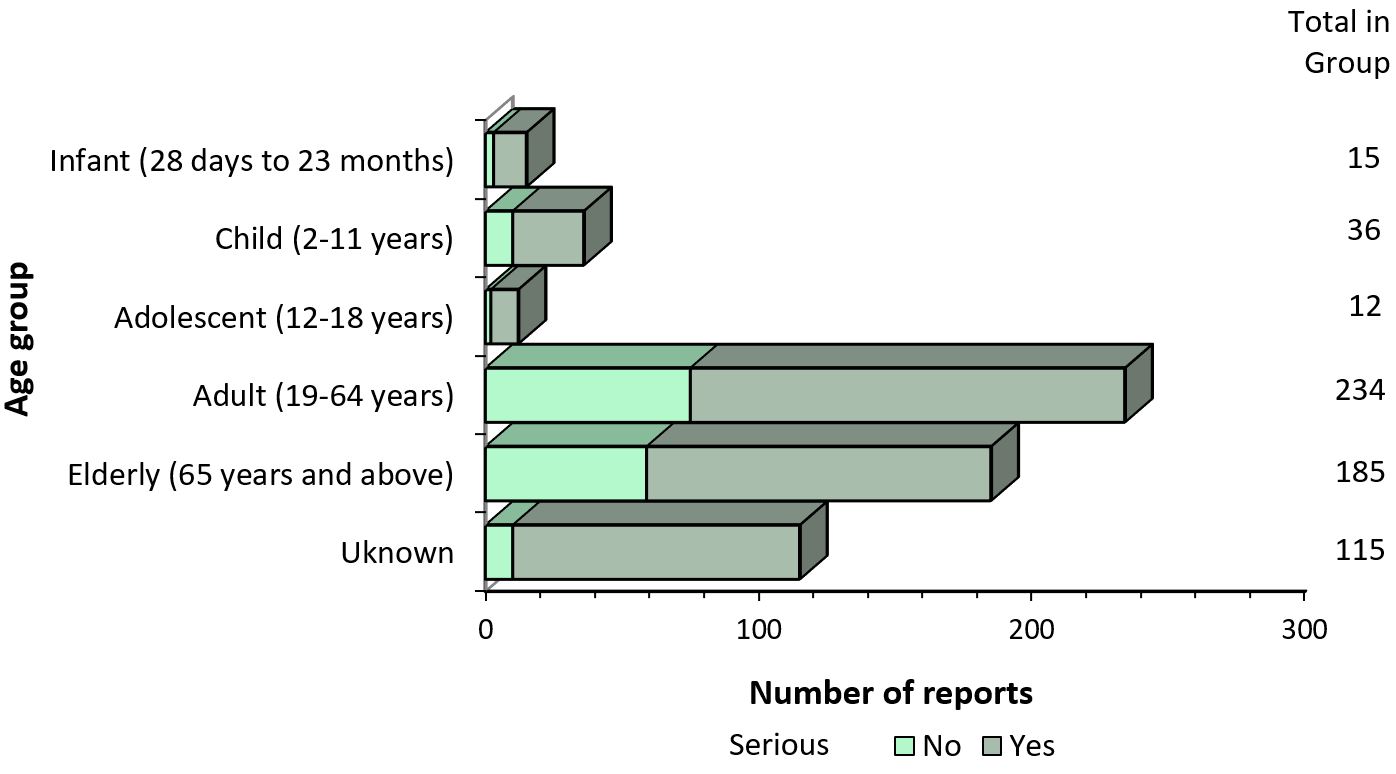
Figure 4 - Text description
The figure shows the total number of reports received from July 1 to December 31, 2019, by age group and severity.
| Age | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Infant (28 days to 23 months) | 3 | 12 |
| Child (2-11 years) | 10 | 26 |
| Adolescent (12-18 years) | 2 | 10 |
| Adult (19-64 years) | 75 | 159 |
| Elderly (65 years and above) | 59 | 126 |
| Unknown | 10 | 105 |
- There were 438 (73%) serious reports. Most of these involved patients with underlying medical conditions and/or concomitant medications, and the serious adverse events were unlikely related to the vaccination.
- The highest number of reports (serious and non-serious) involved herpes zoster vaccines (321 reports), followed by influenza vaccines (125 reports) and pneumococcal vaccines (73 reports). Some reports include multiple vaccines (Figure 5).
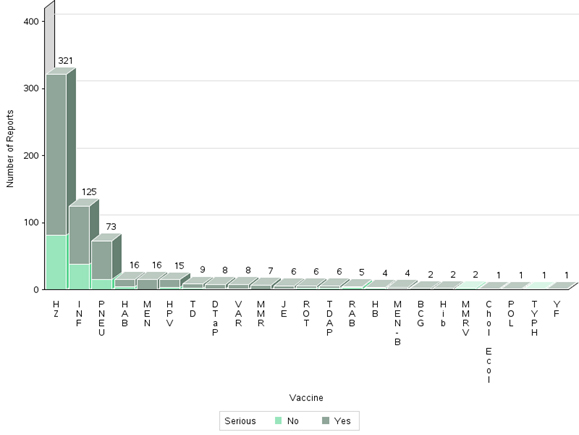
Figure 5 - Text description
The figure shows the total number of reports received from July 1 to December 31, 2019, by vaccine type and severity.
| Vaccine Type | Number of non-serious reports | Number of serious reports |
|
|---|---|---|---|
| Bacille Calmette-Guérin | BCG | 0 | 2 |
| Cholera and Enterotoxigenic Escherichia Coli Travellers' Diarrhea | Chol Ecol | 0 | 1 |
| Diphtheria, tetanus, acellular pertussis | DTaP | 1 | 7 |
| Hepatitis A | HA | 0 | 0 |
| Hepatitis A, B | HAB | 5 | 11 |
| Hepatitis B | HB | 2 | 2 |
| Haemophilus influenzae type b | Hib | 0 | 2 |
| Human papillomavirus | HPV | 4 | 11 |
| Herpes zoster | HZ | 81 | 240 |
| Influenza | INF | 38 | 87 |
| Japanese encephalitis | JE | 1 | 5 |
| Meningococcal | MEN | 0 | 16 |
| Meningococcal B | MEN-B | 1 | 3 |
| Measles, mumps, rubella | MMR | 0 | 7 |
| Measles, mumps, rubella, varicella | MMRV | 2 | 0 |
| Pneumococcal | PNEU | 15 | 58 |
| Poliomyelitis | POL | 0 | 1 |
| Rabies | RAB | 4 | 1 |
| Rotavirus | ROT | 2 | 4 |
| Tetanus, diphtheria (reduced) | TD | 3 | 6 |
| Tetanus, diphtheria (reduced), acellular pertussis | TDAP | 2 | 4 |
| Typhoid | TYPH | 1 | 0 |
| Varicella | VAR | 1 | 7 |
| Yellow Fever | YF | 0 | 1 |
- The majority of reports for herpes zoster vaccines (321 reports) included Shingrix (301 reports). Of these 301 reports with Shingrix, 52 were described as social/digital media cases retrieved by the marketing authorization holder (MAH). None of the social/digital media reports were assessable for the relatedness to the vaccine because of missing information.
- There were 5 reports with an outcome of death. One was male, 1 was female and 3 were of unknown gender. Age was not provided in 2 reports. In the remaining 3 reports, 1 was elderly, 1 was an adult and 1 was an adolescent. The reported vaccines were: influenza vaccine (2), human papillomavirus vaccine (1), pneumococcal vaccine (1) and varicella-zoster virus vaccine (1). The information provided in these reports was not sufficient to assess the causal association with the vaccine.
- The most frequently reported adverse events (serious and non-serious) included vaccination failure, herpes zoster, pyrexia, pain and malaise. These were mostly reported for Shingrix. Approximately half of the adverse events of vaccination failure and herpes zoster were from social/digital media cases submitted by the MAH. None of the social/digital media reports were assessable for the relatedness to the vaccine because of 2 or more of the following missing information: doses received (first or second dose or both), time to onset, pre-existing and concurrent conditions prior to vaccination, medical history, concomitant treatments, corrective treatment, and medical confirmation/test/results of the reported adverse events.
- No new safety signals (potential safety issues) were identified during this period.
- The benefits of vaccines authorized in Canada continue to outweigh the risks.
- Health Canada, in collaboration with the Public Health Agency of Canada (PHAC), will continue to closely monitor the safety of vaccines authorized in Canada.
For additional information, contact the Marketed Health Products Directorate.
Note that because of updated information received by the Canada Vigilance Program, there may be differences in the number of reports and adverse events retrieved at different dates.
Product monograph updates
The following safety labelling updates, which were recently made to the Canadian product monograph, have been selected for your awareness. A complete list of safety labelling updates for pharmaceuticals is available on Health Canada's Product monograph brand safety updates page. Canadian product monographs can be accessed through Health Canada's Drug Product Database.
Norvasc (amlodipine)
The Contraindications, Warnings and Precautions, and Consumer Information sections of the Canadian product monograph for Norvasc have been updated with new information and contraindications.
Key messages for healthcare professionals:Footnote 1
- Norvasc is now contraindicated in patients with:
- obstruction of the outflow tract of the left ventricle (e.g., high grade aortic stenosis)
- haemodynamically unstable heart failure after acute myocardial infarction
- In an amlodipine long-term, placebo-controlled study in patients with severe heart failure (NYHA class III and IV), the reported incidence of pulmonary edema was higher in the amlodipine treated group than in the placebo group.
Reference
- Footnote 1
-
Norvasc (amlodipine) [product monograph]. Kirkland (QC): Upjohn Canada ULC; 2020.
Vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR TKIs): Cabometyx (cabozantinib), Caprelsa (vandetanib), Iclusig (ponatinib), Inlyta (axitinib), Lenvima (lenvatinib), Nexavar (sorafenib), Ofev (nintedanib), Stivarga (regorafenib), Sutent (sunitinib) and Votrient (pazopanib)
The risks of artery dissection and aneurysm have been included in the Warnings and Precautions, Post-Market Adverse Drug Reactions, and Consumer Information sections of the Canadian product monographs for the class of VEGFR TKIs.
Key messages for healthcare professionals:Footnote 2Footnote 3Footnote 4Footnote 5Footnote 6Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11
- Serious cases of artery dissection have been reported in patients using VEGFR TKIs, with or without hypertension.
- Artery aneurysm (including rupture) has been reported in association with VEGFR TKIs.
References
- Footnote 2
-
Cabometyx (cabozantinib) [product monograph]. Mississauga (ON): Ipsen Biopharmaceuticals Canada Inc.; 2019.
- Footnote 3
-
Caprelsa (vandetanib) [product monograph]. Mississauga (ON): Sanofi Genzyme, a division of sanofi-aventis Canada Inc.; 2020.
- Footnote 4
-
Iclusig (ponatinib) [product monograph]. Cambridge (MA): ARIAD Pharmaceuticals, Inc.; 2019.
- Footnote 5
-
Inlyta (axitinib) [product monograph]. Kirkland (QC): Pfizer Canada ULC; 2020.
- Footnote 6
-
Lenvima (lenvatinib) [product monograph]. Mississauga (ON): Eisai Limited; 2019.
- Footnote 7
-
Nexavar (sorafenib) [product monograph]. Mississauga (ON): Bayer Inc.; 2020.
- Footnote 8
-
Ofev (nintedanib) [product monograph]. Burlington (ON): Boehringer Ingelheim (Canada) Ltd.; 2020.
- Footnote 9
-
Stivarga (regorafenib) [product monograph]. Mississauga (ON): Bayer Inc.; 2020.
- Footnote 10
-
Sutent (sunitinib) [product monograph]. Kirkland (QC): Pfizer Canada ULC; 2019.
- Footnote 11
-
Votrient (pazopanib) [product monograph]. Dorval (QC): Novartis Pharmaceuticals Canada Inc.; 2020.
Scope
This monthly publication is intended primarily for healthcare professionals and includes information on pharmaceuticals, biologics, medical devices and natural health products. It provides a summary of key health product safety information published in the previous month by Health Canada, as well as a selection of new health product safety information meant to raise awareness. New information contained in this issue is not comprehensive but rather represents a selection of clinically relevant items warranting enhanced dissemination.
Reporting Adverse Reactions
Canada Vigilance Program
Telephone: 1-866-234-2345
Fax or mail: Form available on MedEffect Canada
For more information on how to report an adverse reaction, visit the Adverse Reaction and Medical Device Problem Reporting page.
Helpful links
- MedEffect™ Canada
- Recalls and Safety Alerts Database
- New Safety and Effectiveness Reviews
- Canada Vigilance Adverse Reaction Online Database
- Drug Product Database
- Medical Devices Active Licence Listing
- Licensed Natural Health Products Database
- The Drug and Health Product Register
- Drug Shortages Canada
- Annual trends for adverse reaction case reports and medical device problem incidents
- Stop Illegal Marketing of Drugs and Devices
Suggestions?
Your comments are important to us. Let us know what you think by reaching us at HC.infowatch-infovigilance.SC@canada.ca
Health Canada
Marketed Health Products Directorate
Address Locator 1906C
Ottawa ON K1A 0K9
Telephone: 613-954-6522
Fax: 613-952-7738
Copyright
© 2020 Her Majesty the Queen in Right of Canada. This publication may be reproduced without permission provided the source is fully acknowledged. The use of this publication for advertising purposes is prohibited. Health Canada does not assume liability for the accuracy or authenticity of the information submitted in case reports.
Adverse reactions (ARs) to health products are considered to be suspicions, as a definite causal association often cannot be determined. Spontaneous reports of ARs cannot be used to estimate the incidence of ARs because ARs remain underreported and patient exposure is unknown.
Due to time constraints relating to the production of this publication, information published may not reflect the most current information.