Health Product InfoWatch – October 2021
Download in PDF format
(564 KB, 9 pages)
Health Products and Food Branch
Marketed Health Products Directorate
Health Product InfoWatch Editorial Team
ISSN: 2368-8025
Cat.: H167-1E-PDF
Pub.: 210000
Contents
- Health products mentioned in this issue
- Coronavirus disease (COVID-19)
- Monthly recap
- New information
- Vaccine safety biannual summary: Summary for January 1, 2020 to June 30, 2020
- Product monograph update: CellCept (mycophenolate mofetil)
- Product monograph update: Zyloprim (allopurinol)
- Notice of market authorization with conditions: Lumakras (sotorasib)
- Notice of market authorization with conditions: Pemazyre (pemigatinib)
- Notice of market authorization with conditions: Truseltiq (infigratinib)
- Notice of market authorization with conditions: Zepzelca (lurbinectedin)
- Scope
- Helpful links
- Suggestions?
- Copyright
Health products mentioned in this issue
Pharmaceuticals and biologics
- CellCept (mycophenolate mofetil)
- Gadolinium-based contrast agents
- Glucagon
- Losartan
- Lumakras (sotorasib)
- Mirvala 28
- Pemazyre (pemigatinib)
- Truseltiq (infigratinib)
- Zepzelca (lurbinectedin)
- Zyloprim (allopurinol)
Medical devices
Natural and non-prescription health products
- BlackOxygen Organics Powder
- BlackOxygen Tablets
- Hand sanitizers that may pose health risks
- PURE75 gel hand sanitizer
Coronavirus disease (COVID-19)
For the most up-to-date information on COVID-19, please visit the Government of Canada Coronavirus disease (COVID-19) website Canada.ca/coronavirus, which includes a dedicated section for healthcare professionals, and for the health product industry.
The COVID-19 vaccines and treatments portal provides information for consumers, healthcare professionals and researchers on vaccines and treatments authorized for COVID-19.
For information about adverse events following immunization that individuals have reported after receiving a COVID-19 vaccine in Canada, new safety signals or other safety updates, please visit the COVID-19 vaccine safety in Canada webpage. This page is updated weekly.
Monthly recap of health product safety information
The following is a list of health product advisories, type I recalls and summaries of completed safety reviews published in September 2021 by Health Canada.
BlackOxygen Tablets and BlackOxygen Organics Powder
BlackOxygen Organics recalled all lots of BlackOxygen Tablets (NPN 80106662) and BlackOxygen Organics Powder (NPN 80097385) due to potential health risks. The products were being promoted in ways and for uses that have not been evaluated and authorized by Health Canada.
Advisory – BlackOxygen Tablets and BlackOxygen Organics Powder
Certain hand sanitizers that may pose health risks
Health Canada advised Canadians that certain hand sanitizers were recalled because they either contained ingredients that were not permitted by Health Canada, were not properly labelled, were unauthorized, or were missing important safety information.
Advisory – Certain hand sanitizers that may pose health risks
Contact lenses
This safety review evaluated the risk of limbal stem cell deficiency with the use of contact lenses. Health Canada's review of the available information has found a possible link. Health Canada has updated the It's Your Health article on contact lenses to inform healthcare professionals and contact lens users of this possible risk.
Summary Safety Review – Contact lenses
Gadolinium-based contrast agents
This safety review evaluated the risks of congenital anomalies, stillbirth and neonatal death with the use of gadolinium-based contrast agents (GBCAs) during pregnancy. Health Canada's review of the available information did not establish a link between the use of GBCAs during pregnancy and the risk of congenital anomalies. However, at this time, there is not enough information to rule out a link between the use of GBCAs during pregnancy and the risks of stillbirth and neonatal death. As a precaution, Health Canada will work with the manufacturers of these products to include the risks of stillbirth and neonatal death in the Canadian product monographs for all GBCAs.
Summary Safety Review – Gadolinium-based contrast agents
Glucagon
Eli Lilly Canada Inc. recalled one lot (D239382A, expiry May 10, 2022) of Glucagon (DIN 02243297), following a complaint that a vial from this lot was found to be in liquid form rather than powder form. Glucagon normally comes in a powder form with accompanying diluting solution, and should be used immediately after mixing. Other vials in the lot may be affected.
Losartan
Several companies recalled multiple lots of prescription losartan tablets, in 25 mg, 50 mg and 100 mg strengths, after tests found an azido impurity above the acceptable limit. Refer to the Affected Products table in the advisory for information on the recalled lots.
Mirvala 28
Apotex Inc. recalled one lot (LF21272B, expiry 08/2022) of Mirvala 28 (DIN 02410257) as the blister pack may contain a placebo (green) pill in place of an active (white) pill.
PURE75 gel hand sanitizer
Health Canada suspended the product licence for PURE75 gel hand sanitizer (NPN 80098346) due to health risks. The Department tested the product and found that it contained an undeclared impurity, methanol, at elevated levels. The product label is also missing important safety information and directions for use.
Advisory – PURE75 gel hand sanitizer
New health product safety information
The following topics have been selected to raise awareness and, in some cases, to stimulate reporting of similar adverse reactions.
Vaccine safety biannual summary
Health Canada and the Public Health Agency of Canada (PHAC) share the responsibility of monitoring of the safety of vaccines in Canada.
Market authorization holders are required to report serious adverse events following immunization (AEFIs) to the Canada Vigilance Program in Health Canada. The Canada Vigilance Program also receives voluntary reports from healthcare professionals and consumers.
Provincial and territorial public health authorities report AEFIs from publicly-funded vaccine programs to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) in PHAC.
Summary for January 1, 2020 to June 30, 2020
Key messages:
- No new safety signals (potential safety issues) were identified during this period.
- From January 1, 2020 to June 30, 2020, the Canada Vigilance Program received 305 reports of adverse events following immunization for which vaccines were a suspected cause.
This biannual vaccine safety summary includes reports of adverse events following immunization received by the Canada Vigilance Program between January 1, 2020 and June 30, 2020. This summary does not include AEFIs submitted following immunization with COVID-19 vaccines,Footnote a nor does it include AEFIs submitted to CAEFISS. To access summaries published by CAEFISS, please visit the CAEFISS Web site.
- From January 1, 2020 to June 30, 2020, the Canada Vigilance Program received 305 reportsFootnote b of adverse events following immunization for which vaccines were a suspected cause.
- The majority of the reports was received from health professionals through spontaneous reporting.
Figure 1: Total number of reports received by report type
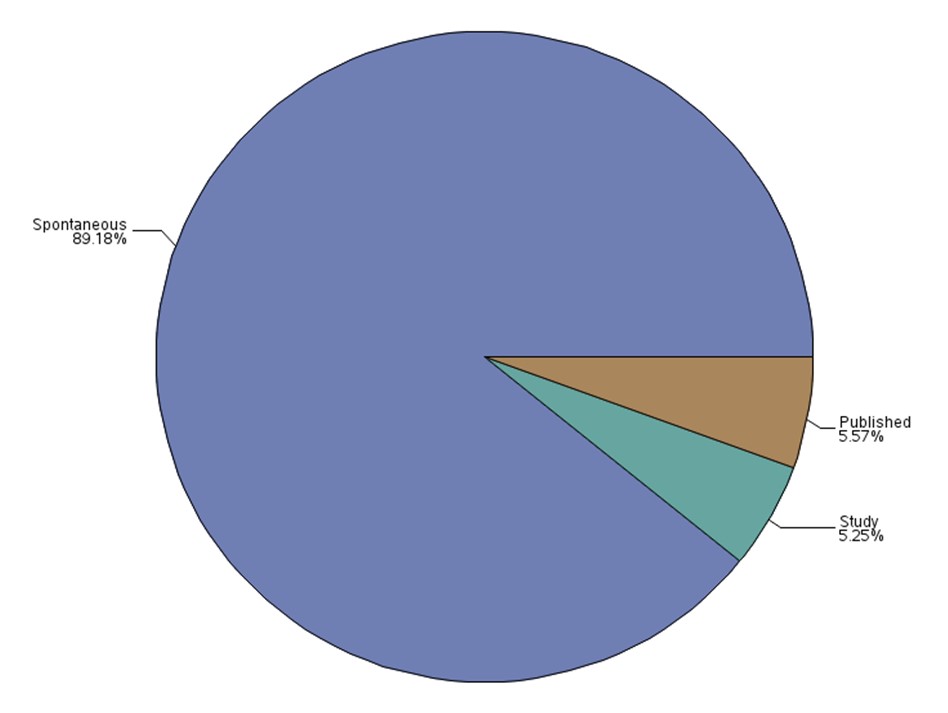
Text description
The figure shows the percentage of the number of reports received by report type.
| Report Type | Percentage (%) |
|---|---|
| Published | 5.57 |
| Spontaneous | 89.18 |
| Study | 5.25 |
Figure 2: Total number of reports received by reporter type
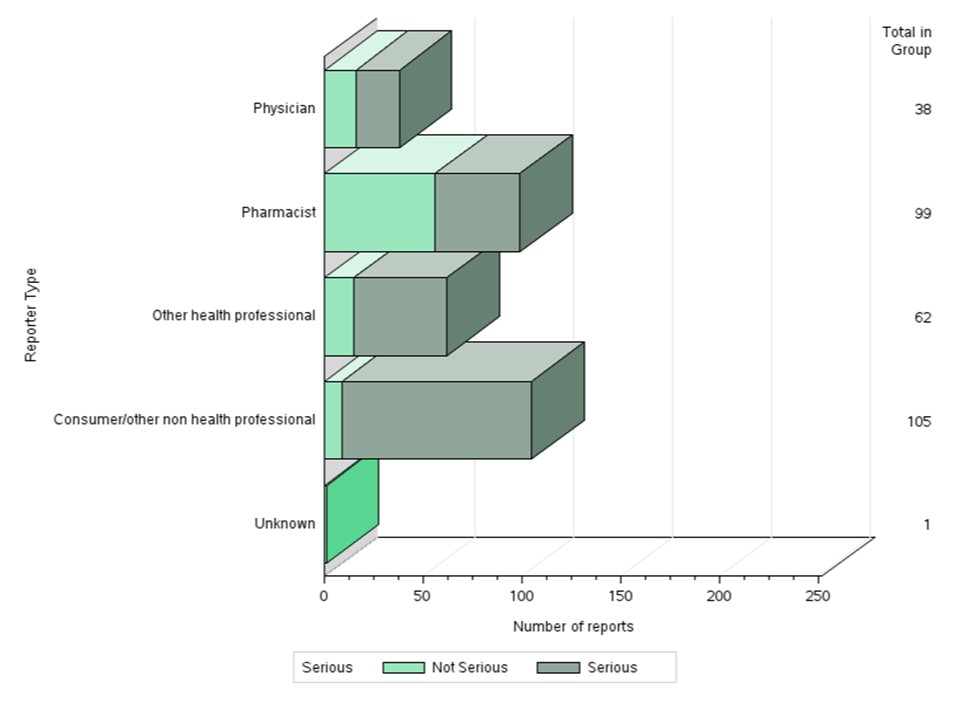
Text description
The figure shows the percentage of the number of reports received by reporter type
| Reporter type | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Consumer or other non health professional | 9 | 96 |
| Other health professional | 15 | 47 |
| Pharmacist | 56 | 43 |
| Physician | 16 | 22 |
| Unknown | 1 | 0 |
- Most of the reports involved females (Figure 3), and the most common age group was adults from 19 to 64 years of age (Figure 4).
Figure 3: Total number of reports received by gender
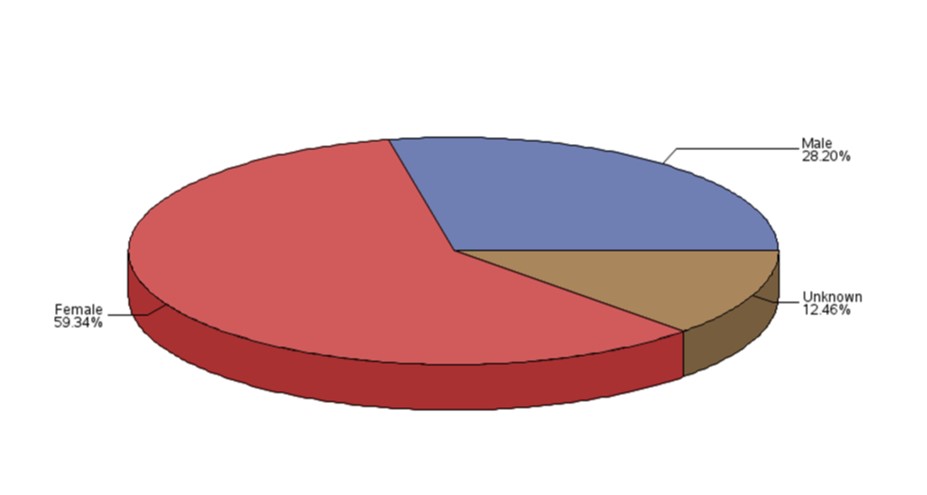
Text description
The figure shows the percentage of the number of reports received by gender.
| Gender | Percentage (%) |
|---|---|
| Female | 59.34 |
| Male | 28.20 |
| Unknown | 12.46 |
Figure 4: Total number of reports received by age group
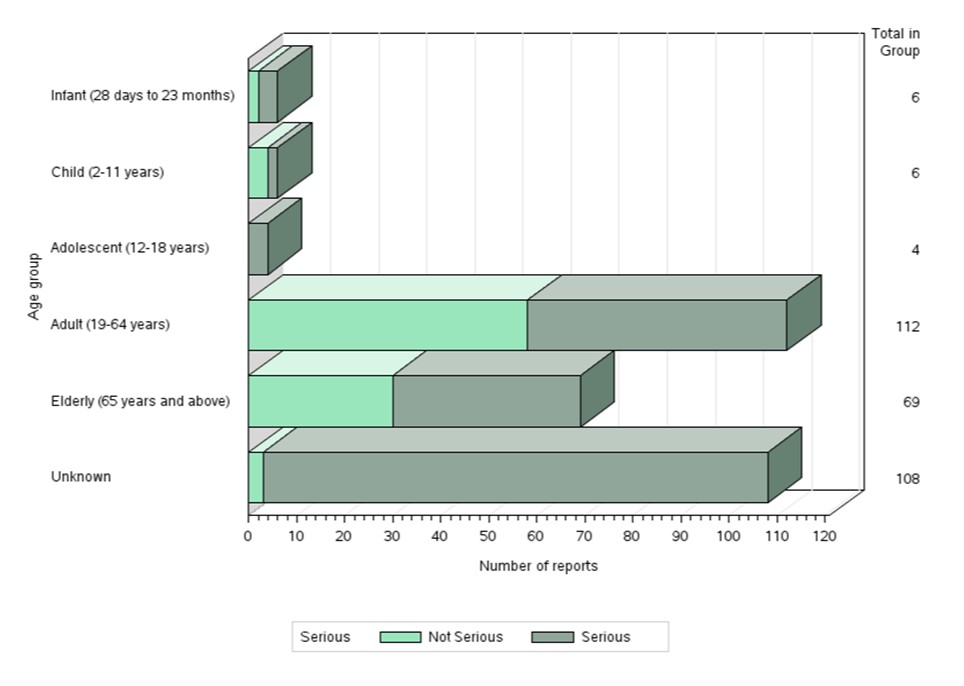
Text description
The figure shows the percentage of the number of reports received by age group.
| Age group | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Infant (28 days to 23 months) | 2 | 4 |
| Child (2-11 years) | 4 | 2 |
| Adolescent (12-18 years) | 0 | 4 |
| Adult (19-64 years) | 58 | 54 |
| Elderly (65 years and above) | 30 | 39 |
| Unknown | 3 | 105 |
- There were 208 (68.2%) serious reports. Most of these involved patients with underlying medical conditions and/or concomitant medications, and the serious adverse events were unlikely related to the vaccination.
- The highest number of reports (serious and non-serious) involved herpes zoster vaccines (194 reports) followed by influenza vaccines (38 reports) and pneumococcal vaccines (23 reports).
Figure 5: Total number of reports received by vaccine (some reports may include multiple vaccines)
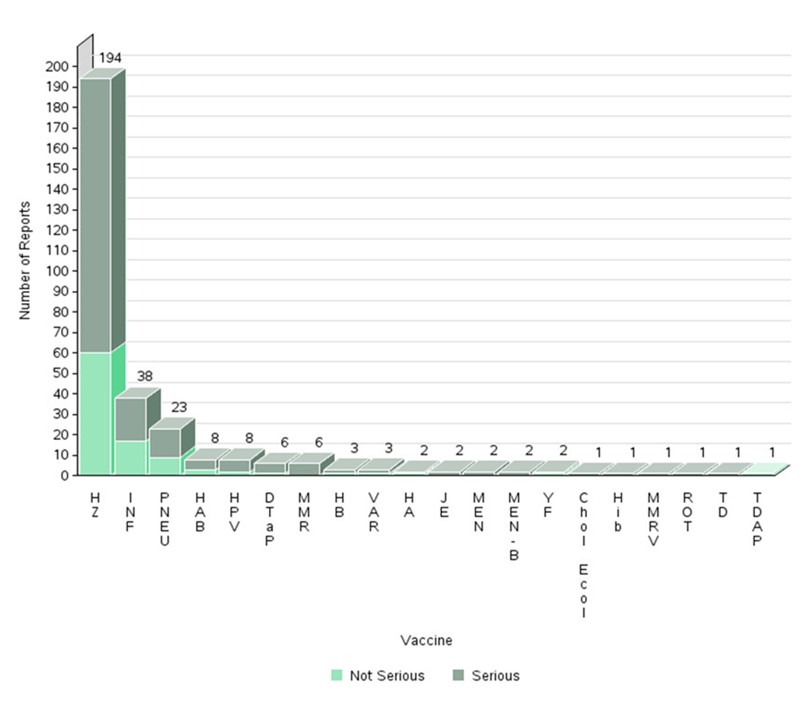
Text description
The figure shows the percentage of the number of reports received by vaccine (some reports include multiple vaccines).
| Vaccine type | Number of non-serious reports | Number of serious reports | |
|---|---|---|---|
| Herpes zoster | HZ | 60 | 134 |
| Influenza | INF | 17 | 21 |
| Pneumococcal | PNEU | 9 | 14 |
| Hepatitis A, B | HAB | 3 | 5 |
| Human papillomavirus | HPV | 2 | 6 |
| Diphteria, tetanus, acellular pertussis | DTaP | 1 | 5 |
| Measles, mumps, rubella | MMR | 0 | 6 |
| Hepatitis B | HB | 1 | 2 |
| Varicella | VAR | 1 | 2 |
| Hepatitis A | HA | 1 | 1 |
| Japanese encephalitis | JE | 0 | 2 |
| Meningococcal | MEN | 0 | 2 |
| Meningococcal B | MEN-B | 0 | 2 |
| Yellow Fever | YF | 1 | 1 |
| Cholera and Enterotoxigenic Escherichia Coli Travellers' Diarrhea | Chol Ecol | 0 | 1 |
| Haemophilus influenza type b | Hib | 0 | 1 |
| Measles, mumps, rubella, varicella | MMRV | 0 | 1 |
| Rotavirus | ROT | 0 | 1 |
| Tetanus, diphtheria (reduced) | TD | 0 | 1 |
| Tetanus, diphtheria (reduced), acellular pertussis | TDAP | 1 | 0 |
- The majority of reports for herpes zoster vaccines were for Shingrix (186). Of the 186 reports for Shingrix, 66 were described as social/digital media cases by the market authorization holder (MAH). None of the social/digital media reports were assessable for relatedness to the vaccine because of the following missing information: doses received (1st or 2nd dose), time to onset, pre-existing and concurrent conditions prior to vaccination, medical history, concomitant medications, corrective treatment, and medical confirmation/test/results of the reported adverse events.
- There were 3 reports with an outcome of death. One report involved a male, and 2 were of unknown gender. One was reported in an adult, and 2 did not report age. The reported vaccines were: herpes-zoster vaccine (1), and measles, mumps, and rubella vaccine (2). The information provided in these reports was not sufficient to assess the causal association with the vaccine.
- The most frequently reported adverse events (serious and non-serious) included herpes zoster, vaccination failure, pyrexia, pain, headache, chills, fatigue, rash, pain in extremity, and malaise. These events were mostly reported for Shingrix. Approximately half of the adverse events of vaccination failure and herpes zoster were from social/digital media cases submitted by the MAH. None of the social/digital media reports were assessable for relatedness to the vaccine because of 2 or more of the following missing information: doses received (1st or 2nd dose, or both), time to onset, pre-existing and concurrent conditions prior to vaccination, medical history, concomitant medications, corrective treatment, and medical confirmation/test/results of the reported adverse events.
- No new safety signals (potential safety issues) were identified during this period.
- The benefits of vaccines authorized in Canada continue to outweigh the risks.
- Health Canada, in collaboration with PHAC, will continue to closely monitor the safety of vaccines authorized in Canada.
For additional information, contact the Marketed Health Products Directorate.
Note that because of updated information received by the Canada Vigilance Program, there may be differences in the number of reports and adverse events retrieved at different dates.
Product monograph updates
The following safety labelling updates, which were recently made to Canadian product monographs, have been selected for your awareness. A complete list of safety labelling updates for pharmaceuticals is available on Health Canada's Product monograph brand safety updates page. Canadian product monographs can be accessed through Health Canada's Drug Product Database.
CellCept (mycophenolate mofetil)
The Warnings and Precautions section of the Canadian product monograph for CellCept has been updated with the potential risk of increased severity of COVID-19 in patients treated with CellCept.
Key messages for healthcare professionals:Footnote 1
- Due to the cytostatic effect of CellCept on B- and T-lymphocytes, increased severity of COVID-19 may occur.
- Dose reduction or discontinuation of CellCept in immunosuppression should be considered in cases of clinically significant COVID-19.
Reference:
- Footnote 1
-
Cellcept (mycophenolate mofetil) [product monograph]. Mississauga, ON: Hoffmann-La Roche Ltd.; 2021.
Zyloprim (allopurinol)
The Serious Warnings and Precautions Box, Warnings and Precautions, Adverse Reactions, and Patient Medication Information sections of the Canadian product monograph for Zyloprim have been updated with new safety information concerning a genetic link (HLA-B*5801 allele) to increased risk of serious and life-threatening hypersensitivity reactions.
Key messages for healthcare professionals:Footnote 2
- Zyloprim (allopurinol) should be discontinued immediately at the appearance of a skin rash, as the rash may be, in some instances, followed by dermatological reactions/hypersensitivity syndrome including Stevens Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN) and drug rash with eosinophilia and systemic symptoms (DRESS).
- Serious and life-threatening allopurinol hypersensitivity reactions have been observed and can manifest in many different ways, including maculopapular exanthema, hypersensitivity syndrome (also known as DRESS) and SJS/TEN. If such reactions occur at any time during treatment, allopurinol should be discontinued immediately. Rechallenge should not be undertaken in patients with hypersensitivity syndrome and SJS/TEN. Extra vigilance for signs of hypersensitivity syndrome or SJS/TEN/DRESS is required and the patient should be informed of the need to stop treatment immediately at the first appearance of symptoms.
HLA-B*5801 allele
- The HLA-B*5801 allele has been shown to be associated with an increased risk of developing allopurinol related serious hypersensitivity syndromes, including SJS and TEN. The frequency of the HLA-B*5801 allele varies widely between ethnic populations: up to 20% in Han Chinese population, 8-15% in the Thai, about 12% in the Korean population and 1-2% in individuals of Japanese or European origin.
- Screening for HLA-B*5801 should be considered before starting treatment with allopurinol in patient subgroups where the prevalence of this allele is known to be high. Chronic kidney disease may increase the risk in these patients additionally.
- In case that no HLA-B*5801 genotyping is available for patients with Han Chinese, Thai or Korean descent, the benefits should be thoroughly assessed and considered to outweigh the possible higher risks before starting therapy. The use of genotyping has not been established in other patient populations.
- If the patient is a known carrier of HLA-B*5801 (especially in those who are from Han Chinese, Thai or Korean descent), allopurinol should not be started unless there are no other reasonable therapeutic options and the benefits are thought to exceed risks.
- SJS/TEN can still occur in patients who are found to be negative for HLA-B*5801 irrespective of their ethnic origin.
Reference:
- Footnote 2
-
Zyloprim (allopurinol) [product monograph]. Vaughan (ON): AA Pharma Inc.; 2021.
Notice of market authorization with conditions
A Notice of Compliance with Conditions (NOC/c) is a form of market authorization with conditions granted to a product on the basis of promising evidence of clinical effectiveness following review of the submission by Health Canada. Communicating a NOC/c is intended to raise awareness on the details of the drug and the nature of authorization granted.
Healthcare professionals are encouraged to report to Health Canada any adverse reactions suspected of being associated with marketed health products, including drugs authorized under the NOC/c policy.
The content of these notices reflects current information at the time of publication. Conditions associated with the NOC/c will remain until they have been fulfilled and authorized by Health Canada, in accordance with the NOC/c policy. For the most up-to-date information, consult Health Canada's NOC database.
Lumakras (sotorasib): Authorization with conditions
Health Canada has issued a Notice of Compliance, under the NOC/c policy, for Lumakras (sotorasib), tablets, 120 mg. Lumakras is indicated for the treatment of adult patients with Kirsten rat sarcoma viral oncogene homolog (KRAS) G12C mutated locally advanced (not amenable to curative therapy) or metastatic non-small cell lung cancer who have received at least one prior systemic therapy. Patients should be advised about the conditional market authorization for this indication.
For the complete prescribing information and information available for the patients/caregivers, please consult the Lumakras Canadian product monograph. The product monograph can be accessed through Health Canada's Drug Product Database, the Amgen Canada Inc. website or by contacting Amgen Canada Inc. at 1-866-502-6436. Contact the company for a copy of any references, attachments or enclosures.
Pemazyre (pemigatinib): Authorization with conditions
Health Canada has issued a Notice of Compliance, under the NOC/c policy, for Pemazyre (pemigatinib), tablets, 4.5 mg, 9 mg, and 13.5 mg. Pemazyre is indicated for the treatment of adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement. Patients should be advised about the conditional market authorization for this indication.
For the complete prescribing information and information available for the patients/caregivers, please consult the Pemazyre Canadian product monograph. The product monograph can be accessed through Health Canada's Drug Product Database, the Incyte Corporation website or by contacting Incite Corporation at 1-833-309-2759. Contact the company for a copy of any references, attachments or enclosures.
Truseltiq (infigratinib): Authorization with conditions
Health Canada has issued a Notice of Compliance, under the NOC/c policy, for Truseltiq (infigratinib), capsules, 25 mg and 100 mg. Truseltiq is indicated for the treatment of adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with a fibroblast growth factor receptor 2 (FGFR2) fusion or other rearrangement. Patients should be advised about the conditional market authorization for this indication.
For the complete prescribing information and information available for the patients/caregivers, please consult the Truseltiq Canadian product monograph. The product monograph can be accessed through Health Canada's Drug Product Database, the QED Therapeutics, Inc. website or by contacting QED Therapeutics, Inc. at 1-905-477-4553. Contact the company for a copy of any references, attachments or enclosures.
Zepzelca (lurbinectedin): Authorization with conditions
Health Canada has issued a Notice of Compliance, under the NOC/c policy, for Zepzelca (lurbinectedin for injection), lyophilized powder, 4 mg/vial. Zepzelca is indicated for the treatment of adult patients with Stage III or metastatic small cell lung cancer who have progressed on or after platinum-containing therapy. Patients should be advised about the conditional market authorization for this indication.
For the complete prescribing information and information available for the patients/caregivers, please consult the Zepzelca Canadian product monograph. The product monograph can be accessed through Health Canada's Drug Product Database, the Jazz Pharmaceuticals Canada Inc. website or by contacting Jazz Pharmaceuticals Canada Inc. at 1-800-520-5568. Contact the company for a copy of any references, attachments or enclosures.
Scope
This monthly publication is intended primarily for healthcare professionals and includes information on pharmaceuticals, biologics, medical devices and natural health products. It provides a summary of key health product safety information published in the previous month by Health Canada, as well as a selection of new health product safety information meant to raise awareness. New information contained in this issue is not comprehensive but rather represents a selection of clinically relevant items warranting enhanced dissemination.
Reporting Adverse Reactions
Canada Vigilance Program
Telephone: 1-866-234-2345
Fax or mail: Form available on MedEffect Canada
For more information on how to report an adverse reaction, visit the Adverse Reaction and Medical Device Problem Reporting page.
Helpful links
- MedEffect™ Canada
- Recalls and Safety Alerts Database
- New Safety and Effectiveness Reviews
- Canada Vigilance Adverse Reaction Online Database
- Drug Product Database
- Medical Devices Active Licence Listing
- Licensed Natural Health Products Database
- The Drug and Health Product Register
- Drug Shortages Canada
- Stop Illegal Marketing of Drugs and Devices
- List of drugs for exceptional importation and sale
- Drug and vaccine authorizations for COVID-19: List of authorized drugs, vaccines and expanded indications
- Reported side effects following COVID-19 vaccination in Canada
Suggestions?
Your comments are important to us. Let us know what you think by reaching us at infowatch-infovigilance@hc-sc.gc.ca
Health Canada
Marketed Health Products Directorate
Address Locator 1906C
Ottawa ON K1A 0K9
Telephone: 613-954-6522
Fax: 613-952-7738
Copyright
©2021 Her Majesty the Queen in Right of Canada. This publication may be reproduced without permission provided the source is fully acknowledged. The use of this publication for advertising purposes is prohibited. Health Canada does not assume liability for the accuracy or authenticity of the information submitted in case reports.
Adverse reactions (ARs) to health products are considered to be suspicions, as a definite causal association often cannot be determined. Spontaneous reports of ARs cannot be used to estimate the incidence of ARs because ARs remain underreported and patient exposure is unknown.
Due to time constraints relating to the production of this publication, information published may not reflect the most current information.
- Footnote a
-
For information about adverse events following immunization that individuals have reported after receiving a COVID-19 vaccine in Canada, please visit the COVID-19 vaccine safety in Canada webpage.
- Footnote b
-
Glossary of Fields in the Canada Vigilance Adverse Reaction Online Database
