Drug and medical device highlights 2019: Helping you maintain and improve your health

Download the alternative format
(PDF format, 9.7 MB, 97 pages)
Organization: Health Canada
Type: Publication
Published: 2020-05-28
Learn about the new drugs and medical devices that Health Canada approved for sale in Canada, the information we published about potential safety issues, and our other accomplishments in 2019.
Table of contents
- Welcome to our 2019 highlights report
- Message from the Chief Medical Advisor
- Message from the Chief Regulatory Officer
- Drugs for human use
- Medical devices
- Drugs for veterinary use
- Drugs for human use: Life cycle
- Drugs for human use: Approved in 2019
- Medical devices: Life cycle
- Medical devices: Approved in 2019
- Drugs for veterinary use: Life cycle
- Drugs for veterinary use: Approved in 2019
Welcome to our 2019 highlights report
Health Canada helps Canadians maintain and improve their health by providing timely access to safe and effective drugs and medical devices.
In 2019 we welcomed our new Minister, the Honourable Patty Hajdu, to the health portfolio. The Prime Minister's mandate letter to our Minister outlines Health Canada's key priorities to safeguard and improve the health and safety of Canadians.
This year we focussed on two important dimensions of our work, regulatory innovation and international collaboration, to help give Canadians access to new and innovative products. We also continued to improve transparency and reduce administrative burden, while maintaining our high standards of safety, efficacy and quality.
The work we do contributes to improved healthcare outcomes for Canadians. We hope that our annual Highlights report will give you a better understanding of our priorities, and help you learn more about the drugs and medical devices we approved in 2019.
This report describes the new drugs and medical devices that Health Canada approved for sale in Canada, the information we published about potential safety issues and our other accomplishments in 2019.
As with previous years, the Highlights report is divided into three chapters: drugs for human use, medical devices and drugs for veterinary use. This year we have added a "What's New in 2019" section to each chapter, which highlights our key product approvals over the year.
Throughout the report you will also find new "Focus on…" features. These provide a closer look into our scientific work and our international collaboration, and key examples of our work on product safety.
This report provides an overview of our work in 2019. For the most up-to-date information on our activities, see the "Healthy Clicks" sections. We also invite you to follow @GovCanHealth on Twitter to learn about newly approved drugs and medical devices.

Pierre Sabourin,
Assistant Deputy Minister,
Health Canada

Kendal Weber,
Associate Assistant Deputy Minister,
Health Canada
Message from the Chief Medical Advisor
"The only thing that is constant is change". This was as true when Heraclitus, the ancient Greek philosopher, uttered it as it is now.
Science and technology continue to evolve, bringing change to the way we live and the world around us. New therapies such as telehealth devices, 3D printing, gene therapies and health products that use artificial intelligence are entering the global market at an advanced pace. These bring benefits to patients, but also challenges to regulatory organizations like Health Canada. These smart technologies continue to inspire our transformation efforts to ensure that we are ready for the future.
This past year brought forth many innovative products that will help Canadians maintain and improve their health. For example, Health Canada evaluated new medications that are unlike any other approved antibiotics, providing treatment options for hospitalized and critically ill patients with resistant infections. This past year also brought the approval of paradigm-changing cancer treatments that are based on the unique genetic makeup of an individual's tumour, regardless of where in the body it first started. Enabling the use of these treatments means that the health system, including the role of the regulator, must evolve as rapidly as the scientific advancements that give rise to them.
The health system is also evolving. For example, in 2019 we approved several new pediatric formulations for our youngest, and often most vulnerable, Canadians. These formulations mean that healthcare professionals no longer have to rely on the longstanding practice of using medicines approved for adults.
Some challenges to the health system, such as the opioid crisis, are being met with government-wide initiatives. In addition to our work on labelling and warnings for opioids, over the past year we focussed on restricting the marketing and advertising of these medications to ensure that they are used appropriately.
Health Canada recognizes that the challenges we face are not unique to our country. Our efforts such as the modernization of the way we oversee clinical trials and the creation of new pathways to assess novel innovative therapies for Canadians are enriched by the collaboration we have with international colleagues. Health Canada has built strong relationships with regulators worldwide to advance collaboration in our work on new safety standards, and to lead efforts in assessing new innovative products. Sharing work with other regulators maximizes our collective expertise and avoids duplication of effort. For example, collaboration with Australia resulted in the approval of two new anti-cancer drugs in 2019.
The pace of change will only get faster from here. Our efforts to respond to innovation in this interconnected world will prepare us well for the road that lies ahead.

Supriya Sharma,
Chief Medical Advisor,
Health Canada
Message from the Chief Regulatory Officer
Canadian laws and regulations underpin the work we do to protect the health and safety of Canadians. Health Canada's regulations enable us to:
- approve clinical trials and approve requests through the Special Access Programme,
- review and approve new drugs and medical devices, and
- monitor drugs and medical devices and take appropriate action throughout their life cycle.
In 2019 we made improvements to the Food and Drugs Act to make sure that Canadians can benefit from new research approaches and highly advanced types of therapeutic products. We proposed an Advanced Therapeutic Products pathway so that we can tailor our regulatory approach for certain products, such as those that rely on artificial intelligence, 3D bio-printing or types of cell technology. We also introduced more efficient and up-to-date ways to regulate clinical trials and classify types of products.
We implemented new regulations to increase our ability to monitor the safety of drugs and medical devices, and provide Canadians more information. Together, these regulations will help Canadians make well-informed decisions about the health products they use.
- Adverse reactions to drugs and medical device incidents account for many emergency visits and hospital admissions in Canada. Hospitals are now required to report serious adverse drug reactions and medical device incidents. Health Canada will monitor these reports and provide healthcare providers and patients with up-to-date information about the safety of their products.
- We now release the clinical information that was provided to us by companies, after we have made our final decision as to whether a drug or medical device can be sold in Canada. This provides Canadians access to clinical data on drugs and medical devices to allow them to make well-informed decisions about their health and that of their families. It will also encourage new research questions and make it easier for the clinical data to be re-analysed independently, both of which could ultimately result in new benefits for Canadians.
- We updated our regulations for medical devices to make them consistent with international standards.
We have also published regulations under the Assisted Human Reproduction Act that will support Canadians who use assisted human reproduction to safely build their families. We strengthened safety standards and now offer the LGBTQ2+ community and single-intended parents more flexibility in building their families through the directed donation process.
In the coming months, we will keep working on modernizing health product regulation for Canadians. We will continue our discussions with stakeholders to ensure our new regulations support innovation and protect Canadians.

David K. Lee,
Chief Regulatory Officer,
Health Canada
Drugs for human use
Drugs for human use: 2019 in brief
Safe, effective and high quality drugs can help Canadians maintain and improve their health. These include vaccines, as well as prescription and non-prescription ("over-the-counter") medicines.
Health Canada evaluates and monitors the benefits and risks of drugs throughout their life cycle, to make sure that the benefits outweigh the potential risks. This is the core of our work.
However, the world in which we work is changing rapidly and so are the drugs we regulate. We must keep pace with advances in science and technology. Two of our key priorities in 2019 focus on these challenges:
- Regulatory innovation: We are developing flexible new regulatory tools that will help us bring novel treatments to Canadians.
- International collaboration: We are working with regulators around the world to help us share information and enhance our scientific knowledge.
The "Drugs for human use: 2019 accomplishments" section describes our progress in these and other areas.
New drugs approved
In 2019 we approved 58 new drugs. These give patients more options for the treatment, prevention and diagnosis of various health conditions.
Thirty-five of the new drugs we approved in 2019 contained medicinal ingredients that have never been approved for sale in Canada, what we call "new active substances". Thirty-four percent of these were approved through an expedited pathway, including those that target specific healthcare needs.
It is also important to bring more choice and more affordable options to Canadians. We approved 129 new generic drugs and 5 new biosimilars in 2019.
In 2019 Health Canada approved four new non-prescription ("over-the-counter") drugs, offering more options to Canadians for treating and managing symptoms of minor ailments.
For a list and description of the new drugs we approved in 2019, go to "Drugs for human use: Approved in 2019". In this section you will also learn how we addressed safety issues that arose for drugs in Canada.
Clinical trials and Special Access Programme
We review applications to allow companies and researchers to conduct clinical trials in Canada. New clinical trials mean Canadians may have access to more innovative treatment options. In 2019, 1089 new clinical trial applications for drugs were approved. Several involved complex, novel, adaptive clinical trial designs, and some trials will facilitate participation of Canadians living in remote areas.
Through our Special Access Programme, we give access to drugs that are not available in Canada. We may grant access to doctors for emergency use or for the treatment of serious or life-threatening conditions. In 2019, 12,314 requests for special access to drugs were authorized. This included drugs for children and drugs for serious infections, as well as cancer treatments.
Surveillance
After we approve a drug for sale in Canada, we continue to monitor and evaluate reports of suspected adverse reactions.
In 2019 we received 1,131,148 reports of adverse reactions to drugs for human use. These come from domestic and international sources. We undertook 1,162 actions related to drugs. These actions can include informing the public and healthcare professionals of new safety information or recommending labelling changes. In the most serious situations, we may remove a drug from the market.
This "Drugs for Human Use" chapter gives you more information about our work in 2019. For up-to-date information about our activities see the "Healthy clicks – Drugs for human use at a glance" section, and follow us on social media.

John Patrick Stewart,
Director General,
Therapeutic Products,
Health Canada

Celia Lourenco,
Director General,
Biologics and Genetic Therapies,
Health Canada

Manon Bombardier,
Director General,
Non-Prescription Drugs,
Health Canada

Marc Mes,
Director General,
Marketed Health Products,
Health Canada
Drugs for human use: What's new in 2019
In 2019 Health Canada approved 58 new drugs, including 5 new biosimilars. More detail is available in the section "Drugs for human use: Approved in 2019".
Alimentary tract and metabolism
Antiinfectives for systemic use
Antineoplastic and immunomodulating agents
Blood and blood forming organs
Cardiovascular system
Dermatologicals
Genito urinary system and sex hormones
Musculo-skeletal system
Nervous system
Respiratory system
Sensory organs
Systemic hormonal preparations, excluding sex hormones and insulins
Various
Drugs for human use: 2019 accomplishments
Regulating innovative drugs
Scientific and technological advances have led to new ways to diagnose, treat and monitor patients. For example, technologies like gene editing mean that products can be personalized to an individual patient.
In 2019 Health Canada announced it would develop new regulations that are agile and that will support innovative new therapies. Health Canada met with key stakeholders across the country and around the world to better understand the changes we are seeing. We also discussed the challenges that stakeholders face in bringing innovative new products to market. Health Canada published a "What we heard" document that describes the key findings from these discussions.
As part of our ongoing Regulatory Innovation initiative, we are focussing on how we oversee clinical trials, approve complex new therapies and use foreign reviews and decisions.
Changing how we regulate clinical trials
Clinical trials are usually the first step in developing new drugs. As products evolve, so do clinical trials. We need clinical trial regulations that are more flexible so that new drugs can be developed and tested. The level of oversight should be based on the potential risk of the trial.
Health Canada will put in place regulations to allow for new types and designs of clinical trials. For example, people who live in remote areas may be able to participate in trials through the use of virtual technology. This approach will improve our alignment with regulators around the world. It will also encourage more sponsors to conduct clinical trials in Canada.
Creating a flexible approach for approving complex new therapeutic products
Some therapeutic products are so novel, complex and distinct that they do not fit within our current regulations. We need a way to approve these products that is flexible and based on their potential risk.
Health Canada is proposing a new pathway for these "Advanced Therapeutic Products". Using this pathway, we will collaborate with stakeholders to create regulatory requirements that can be tailored to a specific product. It will allow us to manage these products throughout their life cycle, and still maintain our high standards for patient safety.
Using foreign reviews and decisions
Health Canada has a process in place where we may use information provided by other regulatory agencies. We are now looking into ways we can build on this, to help increase access to drugs for Canadians. Health Canada is also exploring opportunities to share work with our international partners according to our respective expertise.
We are also exploring using decisions of certain regulators to approve certain drugs that would meet an unmet medical need. For example, this could help bring some drugs that are currently accessed through the Special Access Programme to the broader Canadian market.
We know that it is important for Health Canada to be at the forefront of innovation. We will maintain, and invest in, our core business of reviewing innovative and generic drugs to protect the health and safety of Canadians. Our discussions with our stakeholders will continue as we develop our regulations, to make sure the regulations will respond to new science and technology and to the needs of Canadians.
Focus on … regulatory innovation
"This is a turning point for health product regulation as we work to ensure our regulations and programs adapt to the changing landscape of health product innovation. It is essential that the work we do protects the safety of Canadians and enables innovation to improve their health."

Elizabeth Toller,
Executive Director,
Regulatory Innovation,
Health Canada
Building international partnerships
Health Canada has a long history of working with regulators around the world. This co-operation ranges from ad-hoc meetings about global drug safety issues, to work with international organizations to harmonize our requirements for drugs.
We come together to combine our expertise, develop policies and set standards. Strong relationships with other countries help us all address health risks more effectively. In addition, working together helps us all support timely access to safe new drugs.
Some examples of our collaborations with other regulators in 2019 included:
- International Coalition of Medicines Regulatory Authorities (ICMRA) – This organization focuses on developing strategies to address issues that impact global health. It is led by the heads of the agencies that regulate medicines from every region of the world. Health Canada is a founding member of ICMRA, which involves 29 regulatory authorities. In 2019, as part of ICMRA's Innovation Project, Health Canada led a group that examined new ways to regulate innovation.
- International Council for Harmonisation (ICH) – The mission of ICH is to achieve greater harmonisation worldwide to ensure that medicines are developed and approved efficiently. Health Canada is a member of ICH, and as such adopts and implements all ICH guidelines and standards. In 2019 Health Canada became Vice-Chair of the ICH Assembly, and implemented three ICH guidelines. For example, the "Good Clinical Practice" and "Multi Regional Clinical Trials" guidelines will help improve the quality and efficiency of clinical trials for Canadians.
- International Pharmaceutical Regulators Programme (IPRP) – The IPRP is a forum to exchange information about pharmaceutical products for human use, and enable regulatory cooperation. In 2019 Health Canada acted as Vice-Chair of the IPRP Management Committee. We also co-chaired two IPRP Working Groups: Biosimilars and Nanomedicines.
- Australia-Canada-Singapore-Switzerland (ACSS) Consortium – Through the ACSS Consortium, Health Canada works with partners to review new drugs together, to get them to market quickly and efficiently. In 2019 Health Canada approved two products with Australia: Verzenio and Zejula. For more information, go to "Drugs for human use: Approved in 2019".
- Project Orbis – Project Orbis is an initiative of the United States Food and Drug Administration (FDA) Oncology Center of Excellence. Project Orbis brings together regulators from multiple countries to review cancer drugs at the same time so that patients can receive earlier access to needed treatments. Through the pilot project, companies submit their information to all participating regulatory agencies at the same time and the agencies review the information collaboratively. This pilot can encourage earlier access to new drugs for Canadian patients. In 2019 Health Canada approved the use of Keytruda in combination with Lenvima for endometrial carcinoma, and Calquence for adults with chronic lymphocytic leukemia through Project Orbis.
- Canada-United States Regulatory Cooperation Council (RCC) – The RCC was created to reduce unnecessary differences between the countries' regulatory frameworks. As part of this work, Health Canada and the FDA continue to hold joint consultation meetings on ICH guidance documents. The results of these consultations inform discussions at subsequent ICH meetings. They also help Health Canada better understand, and work to minimize, areas where our requirements differ from those of the FDA.
Focus on … international collaboration
"The EMA and Health Canada will work further together in the future and we will continue to collaborate on key issues. We want to understand how you are operating and how we can benefit from the work you have done. Building collaboration with Canada is important to us and we need to structure our relationship more."

Professor Guido Rasi,
Executive Director,
European Medicines Agency (EMA)
Focus on … international collaboration
"Project Orbis was a unique opportunity to collaborate with our international partners that enabled early access to a new treatment for a disease with an unmet medical need. The aligned review allowed for real-time insights into the FDA review approach, analysis, and interpretation of data common to that submitted to Health Canada. It was certainly a productive learning experience that will help us improve our own processes and facilitate further projects with our international counterparts."

Dr. Jian Wang,
Division Manager,
Health Canada
Dr. Bradley Scott,
Senior Clinical Evaluator,
Health Canada
Dr. Jaigi Mathai,
Senior Clinical Evaluator,
Health Canada
Dr. Maxime Sasseville,
Manager,
Health Canada
Focus on … international collaboration to improve drug safety
In 2017 a Canadian case was reported of a patient who had taken an anti-cancer drug and then developed a tear in an artery wall. Health Canada reviewed the potential risks of these types of products and found there may be a link between their use and structural changes to the walls of arteries. The products are called "vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR TKIs)" and they are approved in Canada for the treatment of various types of cancer. In 2019 Health Canada and the European Medicines Agency shared information and reviews, and both regulators put in place measures to help reduce the risk associated with the products. In Canada, we are working with manufacturers to update the product safety information for all VEGFR TKIs so that patients and healthcare professionals are informed of the risk.
Focus on … Medication Safety Week, November 25-29, 2019
Every year during Medication Safety Week, international regulators work together through ICMRA to promote awareness about adverse drug reactions. To coincide with Medication Safety Week, Health Canada launched a series of social media messages to help encourage reporting of adverse drug reactions.
Publishing clinical information about drugs for human use
"Clinical information" is data about the safety and efficacy of a drug, and includes information about the design and results of clinical trials. In 2019 Health Canada began publishing the clinical information that is provided by companies when they seek approval to sell a drug in Canada. The clinical information is made available after Health Canada has finished its review and has made a decision about approving or rejecting a drug.
Providing public access to this clinical information will benefit Canadians by:
- making it easier for the clinical data to be re-analysed independently,
- encouraging new research questions,
- reducing the duplication of clinical trials, and
- helping patients and healthcare providers make better decisions about their health.
We make clinical information available through our "Clinical Information Portal". In 2019 we published 455,129 pages of clinical information on 21 drugs.
For more information, go to "Clinical information on drugs and health products".
Mandatory reporting of serious adverse drug reactions
Health Canada continues to look for ways to strengthen our knowledge about the safety of drugs on the Canadian market. Reports of adverse drug reactions (ADRs) help us identify emerging safety issues. However, we know that ADRs are under-reported around the world. In addition, the reports that are submitted can be missing important information.
To help address these issues, in 2019 Health Canada published final regulations that aim to increase the quantity and improve the quality of reports of serious ADRs. Hospitals are now required to report to Health Canada all serious ADRs, whether the ADR took place in the hospital or before a patient went to the hospital.
To help hospitals implement this change, Health Canada helped to develop educational materials and online tools to make it easier to submit reports.
The additional, higher quality data will help Health Canada improve the safety of drugs by:
- detecting new safety issues related to drugs,
- assessing the impact of safety issues for Canadians,
- updating the drug labelling, and
- publishing warnings or issuing recalls when needed.
Using real world evidence
During drug development, sponsors (researchers and manufacturers) conduct clinical trials to demonstrate that a drug is safe and effective. However, it may be challenging to conduct clinical trials for certain diseases (such as rare diseases) or patient populations (such as children or pregnant women). In these cases, information about how drugs are being used after they are available for sale can help us understand how to use the drugs safely and effectively. We call this information "real world evidence".
The use of real world evidence already informs our decisions throughout the life cycle of a drug. It is used in the review of new drugs, and to monitor the safety and effectiveness of drugs once they are available for sale in Canada. The availability of real world evidence is increasing steadily worldwide. Health Canada is working with key partners to make sure that the evidence we collect can help assess the safety and effectiveness of a drug throughout its life cycle.
Health Canada will continue to use high quality real world evidence to support our decisions, to help protect the health and safety of Canadians and to increase patient access to treatments.
Addressing the opioid crisis
The opioid crisis continues to be one of the most serious public health issues in Canada's recent history. Increasing access to treatments, as well as harm reduction options, is a key element in addressing the opioid crisis.
In 2019 Health Canada became the first country in the world to approve injectable hydromorphone in adults with severe opioid use disorder. We also added diacetylmorphine (pharmaceutical-grade heroin) to the List of Drugs for an Urgent Public Health Need, at the request of Canada's Chief Public Health Officer. This list enables medical officers of health to import drugs that are not yet available in Canada but have been approved for sale in certain other countries, to meet an urgent public health need. Diacetylmorphine can now be imported for the treatment of opioid use disorder.
This past year, we took additional steps to further restrict the marketing and advertising of prescription opioids. All promotional materials must be limited to only statements that have been approved by Health Canada in the Product Monograph. Statements have to be presented verbatim, and convey the benefits and risks of opioids in a balanced way. These measures build on already-announced initiatives to address the pharmaceutical industry's opioid marketing and advertising practices.
Regulatory review of drugs and devices
Health Canada developed the "Regulatory Review of Drugs and Devices" initiative in 2017 to provide patients with more timely access to drugs and medical devices. Other goals include increasing our work with partners in the healthcare system in Canada and with other countries, and making better use of real-world evidence (data collected outside of clinical trials) across a product's life cycle.
In 2019 Health Canada continued to achieve significant progress in support of drug access. For example, we continued to align our review processes with those of the health technology assessment organizations (Canadian Agency for Drugs and Technologies in Health [CADTH], and Institut national d'excellence en santé et services sociaux [INESSS]). This alignment helps drugs reach patients more quickly. We approved 14 new drugs with this "aligned review" process in 2019. For more information, go to "Drugs for human use: Approved in 2019".
We also launched a pilot project to provide early parallel scientific advice to companies with CADTH. This project will help companies understand the requirements of both Health Canada and its health technology assessment partners, at the same time. This work will help companies create more effective drug development plans.
We consulted on a draft guidance document on accelerated reviews to meet healthcare system needs. This approach will help improve access to those drugs that are needed most by Canadians.
Addressing antimicrobial resistance
Antimicrobials, such as antibiotics and antivirals, are used to treat people and animals. Bacteria, viruses, fungi and parasites can resist antimicrobials. This is known as antimicrobial resistance (AMR). The misuse and over-use of antimicrobials has led to the rapid development and spread of AMR in Canada and around the world.
Antimicrobials are essential in modern health care. When they do not work as well as they should, we are less effective at treating common infectious diseases. This is a major threat to global public health, economic prosperity and security.
Health Canada continues to take important steps to encourage the development of new and innovative therapeutic products. In 2019 we approved a new use for the antibiotic drug Zerbaxa through an expedited review, because it targeted difficult-to-treat pathogens on our Pathogens of Interest List.
As part of our work with international partners, Health Canada led the development of a joint statement on AMR from ICMRA. We also issued a challenge under Innovative Solutions Canada. This challenge will fund the development of a novel tool so that health care providers can detect or diagnose antibiotic resistant bacteria in humans or animals.
To learn about antimicrobial resistance related to animals and veterinary drugs, go to "Drugs for veterinary use: 2019 accomplishments".
Focus on … AMR Awareness Week, November 18-22, 2019
Every year Health Canada, along with international health agencies, supports AMR Awareness Week. This global initiative aims to raise awareness of antimicrobial resistance and highlight best practices to help stop the spread of antibiotic resistance. To coincide with AMR Awareness Week, we launched a series of social media messages to raise awareness of this important issue as well as the joint statement on AMR that Health Canada issued with ICMRA.
Providing more cost-effective treatment options
Generic and biosimilar drugs provide additional options for more cost-effective prevention and treatment.
Generic drugs contain the same medicinal ingredients as the brand name drug, and are considered bioequivalent to the brand name drug.
In 2019 Health Canada proposed regulations and draft guidance documents to improve access to affordable generic drugs. We also continued to work on the Australia-Canada-Singapore-Switzerland (ACSS) Consortium Generic Medicines Work Sharing Trial. In this program, a submission for a generic drug is filed with multiple countries in the ACSS Consortium and the review work is shared between countries.
Biosimilars are biologic drugs that enter the market subsequent to a previously authorized drug in Canada with a demonstrated similarity to the previously authorized biologic drug.
In 2019 Health Canada provided a number of publications to support biosimilars regulation and stakeholder education. We published a Policy Statement on the Naming of Biologic Drugs. Under this policy, biologic drugs including biosimilars can be clearly identified by use of the unique brand name and non-proprietary (medicinal ingredient) name, in addition to other product-specific identifiers such as the Drug Identification Number (DIN). This naming convention supports product-specific identification of biologic drugs, including biosimilars, throughout the medication use process and in adverse drug reaction reporting.
Health Canada also updated our Biosimilar biologic drugs in Canada: Fact Sheet to provide additional information to health professionals and patients about biosimilars and their regulation in Canada. In addition, we contributed to the International Coalition of Medicines Regulatory Authorities (ICMRA) statements on biosimilars for healthcare professionals and patients and the general public. These statements give assurance that regulators have robust processes in place for the authorization and monitoring of biosimilars.
Non-prescription drugs
Health Canada is responsible for the review of non-prescription drugs, also known as "over-the-counter" products, to ensure that they are safe, effective and of high quality. These products include, but are not limited to:
- antiseptics,
- pain relievers,
- cold and cough medicines, and
- sunscreens.
The nature of our review of non-prescription drugs depends on a number of factors. These include the ingredients, the health claims and the evidence that is needed to support the safety, efficacy and quality of the product. Health Canada issues a Drug Identification Number (DIN) to every approved non-prescription drug. Canadians should look for this number on the product label, which indicates that the drug has met our requirements.
In 2019 Health Canada approved four new non-prescription drugs, offering more options to Canadians for treating and managing symptoms of minor ailments.
Improving the labelling of non-prescription drugs
The review of product labels is a very important component in the evaluation of non-prescription drugs. The labels are the main source of information in a self-care environment. As such, it is critical that the label provides simple information in a legible way, so that Canadians can make informed choices for themselves and their families.
In recent years, Health Canada introduced new requirements for labels so that information about non-prescription drugs is easier to read, understand and locate on the package. Our requirements include larger text size, better contrast and a greater emphasis on providing Canadians with the information they need.
In addition, for a consistent look and feel, important information about a non-prescription drug is presented in a tabular format called the Canadian Drug Facts Table.
In 2019 Health Canada approved 516 non-prescription drug submissions that met our new requirements. We expect that non-prescription drugs with these new labels will be available soon.
Focus on … safety
"It's very important that people use non-prescription drugs appropriately. Through the introduction of the Canadian Drug Facts Table, we hope to promote the safe use of medications and reduce preventable medication errors."

Jason DiMuzio,
Label Review Coordinator,
Health Canada
Focus on … our science
In 2019 our regulatory scientists studying biologic drugs published a paper titled "Remarkable Structural Diversity of N-Glycan Sulfation on Influenza Vaccines". The publication describes their analysis of the sugar-structure of the influenza vaccine. Their work will contribute to a better understanding of the influenza vaccine.

Dr. Sean Li,
Research Scientist,
Health Canada
Dr. Terry Cyr,
Research Scientist,
Health Canada
Yi-Min She,
Research Chemist,
Health Canada
Focus on … drug safety
In the summer of 2018, potentially unsafe levels of an impurity were identified in some lots of drugs containing valsartan. These impurities could be cancer causing. This prompted an investigation that resulted in a recall of affected valsartan products. In 2019 Health Canada continued to work closely with regulators worldwide to understand and address this issue. Similar impurities were found in other drugs in the "sartan" class, and later in drugs containing ranitidine. We continued to investigate the root causes and we set strict limits for the impurity. Other areas of Health Canada inspected manufacturing sites, and tested drug products in laboratories. We sent letters to manufacturers to inform them about the factors that can cause these impurities to form, and steps they should take to make sure the impurities are not present in their products.
Focus on … our science
In 2019 our regulatory scientists studying biologic drugs published a paper titled "Chitosan alters inactivated respiratory syncytial virus vaccine elicited immune responses without affecting lung histopathology in mice". Their work is focussed on the evaluation of a future vaccine against the respiratory syncytial virus. This virus infects almost all children under the age of one, and is a top priority of the World Health Organization.

Dr. Terry Cyr,
Research Scientist,
Health Canada
Dr. Simon Sauvé,
Research Scientist,
Health Canada
Dr. Sean Li,
Research Scientist,
Health Canada
Louise Larocque,
Senior Research Technologist,
Health Canada
Caroline Gravel,
Biologist,
Health Canada
Marsha Russell,
Biologist,
Health Canada
Not pictured:
- Abeneya Muralidharan, Graduate Student, Health Canada
- Dr. Michael Rosu-Myles, Director, Health Canada
Healthy clicks – Drugs for human use at a glance
To stay informed about our activities:
- Follow us on Facebook
- Follow us on Twitter
- Follow us on YouTube
- See the latest news from Health Canada on our website
- Find other Health-related information on the Government of Canada website
You can also find specific information about drugs by following the links below.
New drugs approved
Health Canada regularly tweets about new drugs approved.
- Twitter #Drugandmeddevice
The Drug and Health Product Register provides information for consumers about drugs that are currently marketed in Canada.
The Drug Product Database is a listing of all drugs approved for sale in Canada. In the database, many drugs are accompanied by their Product Monographs, which describe the conditions of use of the product.
New Search for data about the tests and trials that were performed on drugs to evaluate their safety and efficacy.
The Submissions Under Review Lists show the drugs that are currently being reviewed by Health Canada.
The Generic Submissions Under Review List shows the generic drugs that are currently being reviewed by Health Canada.
The Notice of Compliance database lists the approvals (Notices of Compliance or NOCs) issued for new drugs.
Regulatory Decision Summaries include the purpose of a drug submission and the reasons for Health Canada's decision to approve or reject it.
Summary Basis of Decision documents give the detailed regulatory, safety, effectiveness and quality considerations that factored into Health Canada's decision to approve certain drug submissions.
Drug shortages
The Drug Shortages in Canada website gives information on actual and anticipated drug shortages.
Clinical trials for drugs
The Clinical Trials Database lists the clinical trial applications that have been approved for drugs in Canada.
Surveillance of drugs
You can report adverse drug reactions to your medical professional, to a hospital or to the company that made the product. You can also report them to Health Canada through the Canada Vigilance Program or by phone at 1-866-234-2345.
The Recalls and Safety Alerts Database includes the recalls, advisories, safety alerts and other publications issued by Health Canada.
Summary Safety Reviews summarize our completed reviews of potential safety issues for drugs.
New Safety and Effectiveness Reviews are tables listing reviews that are currently ongoing in Health Canada.
Health Product InfoWatch is a monthly publication intended primarily for healthcare professionals. The Health Product InfoWatch provides clinically relevant information about health products and their safety.
The summary tables of advertising complaints list the complaints about health product advertising that have been filed with Health Canada, and the action we have taken.
The Canada Vigilance Adverse Reaction Online Database includes information about suspected adverse reactions to health products and about medical device incidents. These reports have been submitted by consumers and health professionals as well as drug manufacturers and distributors.
Medical devices
Medical devices: 2019 in brief
Safe, effective and high quality medical devices can help Canadians maintain and improve their health. Medical devices are used in the treatment, diagnosis or prevention of diseases or abnormal physical conditions.
In Canada, medical devices are categorized into four groups based on the level of risk associated with their use. These groups are called "Classes" and range from I to IV. Class I devices are considered low-risk devices – for example, a wheelchair. Class IV devices present the greatest potential risk – for example, a defibrillator.
Health Canada provides timely access to new and innovative technology, while overseeing the safety and effectiveness of the medical devices on the Canadian market.
The Medical Devices Action Plan continued to be one of our key priorities this year. The objective of the Action Plan is to strengthen the regulation of medical devices in Canada by:
- improving how devices get on the market,
- strengthening, monitoring and follow-up of devices once they are being used by Canadians, and
- providing more information to Canadians about the medical devices they use.
In 2019 we made significant progress in meeting these goals. We expanded our scientific expertise, strengthened our monitoring of medical devices and provided more information to Canadians about the medical devices they use.
For example, we created a Scientific Advisory Committee on Health Products for Women. This committee gives voice to patient advocates, physicians and researchers on current and emerging issues relating to women's health, and the regulation of the medical devices and drugs that they use.
The "Medical devices: 2019 accomplishments" section describes our progress this year in these and other areas.
Making safe and effective medical devices available to Canadians in a timely manner is the core of our work. In the "Medical devices: Approved in 2019" section you will learn about the new high-risk medical devices we approved, and how we addressed safety issues that arose.
New medical devices approved
In 2019 we approved 360 new medical devices in the highest risk categories (Classes III and IV). These devices provide patients and healthcare professionals with new and innovative options for the treatment, prevention and diagnosis of various health conditions.
For a list and description of the 56 new Class IV (highest-risk) medical devices we approved in 2019, go to "Medical devices: Approved in 2019".
Investigational testing and Special Access Programme
We review applications to allow companies to conduct investigational testing on medical devices in Canada. New trials mean Canadians may have access to more innovative choices. In 2019, 144 new investigational testing applications for medical devices were approved.
Through our Special Access Programme, we give access to medical devices that have not been approved for sale in Canada. We grant access to doctors for emergency use or when alternatives are unsuitable or unavailable. In 2019, 2829 requests for special access to medical devices were authorized.
Surveillance
After we approve a medical device for sale in Canada, we continue to monitor and evaluate reports of suspected incidents involving that medical device.
In 2019 we received 43,832 reports of medical device incidents. These come from domestic and international sources. We undertook 36 actions related to medical devices. These actions can include informing the public and healthcare professionals of new safety information or recommending labelling changes. In the most serious situations, we may remove a medical device from the market.
This "Medical Devices" chapter gives you more information about our work in 2019. For up-to-date information about our activities see the "Healthy clicks – Medical devices at a glance" section, and follow us on social media.

John Patrick Stewart,
Director General,
Therapeutic Products,
Health Canada

Marc Mes,
Director General,
Marketed Health Products,
Health Canada
Medical devices: What's new in 2019
In 2019 Health Canada approved 56 new Class IV (highest-risk) medical devices. More detail is available in the section "Medical devices: Approved in 2019".
Anaesthesia and respiratory devices
Body tissue manipulation and reparation devices
- da Vinci X Surgical System
- DSM Biomedical Calcium Phosphate Cement
- DSM Biomedical Calcium Phosphate Cement with Microspheres
- Endoform Antimicrobial Dermal Template
- Filasilk Sterilised Surgical Needled Suture
- Filasilk Sterilised Surgical Suture
- Gentrix Surgical Matrix
- Mericron XL Sterilised Surgical Needled Suture
- Optium DBM Gel
Cardiovascular devices
- Acticor
- Amplatzer Valvular Plug III
- Arctic Front Advance Pro
- Edwards Sapien 3 Ultra Transcatheter Heart Valve Systems
- Figulla Flex II PFO
- Flow Re-Direction Endoluminal Device (FRED) System
- Gore Molding & Occlusion Balloon Catheter
- Gore Viabahn VBX Balloon Expandable Endoprosthesis
- Ilivia Neo 7 VR-T, VR-T DX, DR-T, HF-T, HF-T QP
- MitraClip G4 System
- Novasight Hybrid System
- Optowire III Pressure Guidewire
- Plexa and Plexa (ProMRI) ICD Leads
- Promus ELITE Monorail Everoliums-Eluting Platinum Chromium Coronary Stent System
- PuraStat
- Reprocessed ViewFlex Xtra ICE Catheter
- Resolute Onyx Zotarolimus-Eluting Coronary Stent System
- RotaPro Rotational Atherectomy System
- SoundBite Crossing System
- Squid Liquid Embolic Agent
- Stellarex 0.035 inch OTW Drug-Coated Angioplasty Balloon
- True Dilatation Balloon Valvuloplasty Catheter
- Valiant Navion Thoracic Stent Graft System
- VersaCross RF Wire
- Watchman FLX Left Atrial Appendage Closure Device with Delivery System
- Xience Sierra Everolimus Eluting Coronary Stent System
Dental devices
Disability-assistive products
In vitro diagnostic medical devices
- Alinity s System
- Alinity s Anti-HBc Assay (Donor Screening & Cadaveric Testing)
- Alinity s Anti-HCV (Donor Screening & Cadaveric Testing)
- Alinity s Chagas Assay (Donor Screening & Cadaveric Testing)
- Alinity s HBsAg Assay (Donor Screening/Cadaveric)
- Alinity s HBsAg Confirmatory Reagent Kit
- Alinity s HTLV I/II Assay (Donor Screening & Cadaveric Testing)
- Beckman Coulter PK7400 Automated Microplate System
- Elecys Chagas (Donor Screening)
- NEO Iris
- Procleix Zika Virus Assay (Donor Screening/Cadaveric Testing)
- Virotrol Plus-R
Neurological devices
Plastic surgery and cosmetic devices
Medical devices: 2019 accomplishments
Regulating innovative devices
Scientific and technological advances have led to new ways to diagnose, treat and monitor patients. For example, technologies like 3D printing and artificial intelligence mean that products can be personalized to an individual patient.
In 2019 Health Canada announced it would develop new regulations that are agile and that will support innovative new therapies. Health Canada met with key stakeholders across the country and around the world to better understand the changes we are seeing. We also discussed the challenges that medical device manufacturers and other stakeholders face in bringing innovative new products to market. Health Canada published a "What we heard" document that describes the key findings from these discussions.
As part of our ongoing Regulatory Innovation Initiative, we are focussing on how we oversee investigational testing and approve complex new therapies.
Changing how we regulate investigational testing
Investigational testing is usually the first step in developing new medical devices. Health Canada is enhancing patient protection by strengthening the way clinical trials involving medical devices are conducted in Canada.
As products evolve, so do the trials that are conducted. We need regulations that are more flexible so that new medical devices can be developed and tested.
These changes will improve the safety of participants in trials and align our approach with other regulators. It will also encourage research in Canada.
Creating a flexible approach for approving complex new medical devices
Some therapeutic products are so novel, complex and distinct that they do not fit within our current regulations. We need a way to approve these types of products that is flexible and based on their potential risk.
Health Canada is proposing a new pathway for these "Advanced Therapeutic Products". Using this pathway, we will collaborate with stakeholders to create regulatory requirements that can be tailored to the specific product. It will allow us to manage these products throughout their life cycle, and still maintain our high standards for patient safety.
We know that it is important for Health Canada to be at the forefront of innovation. We will maintain, and invest in, our core business of reviewing innovative new medical devices to protect the health and safety of Canadians. Our discussions with stakeholders will continue as we develop our regulations, to make sure the regulations will respond to new science and technology and to the needs of Canadians.
Focus on … regulatory innovation
"This is a turning point for health product regulation as we work to ensure our regulations and programs adapt to the changing landscape of health product innovation. It is essential that the work we do protects the safety of Canadians and enables innovation to improve their health." - Elizabeth Toller, Executive Director, Regulatory Innovation, Health Canada
Building international partnerships
Health Canada has a long history of working with regulators around the world. This co-operation ranges from meetings about global medical device safety issues, to working with international organizations to harmonize our requirements for medical devices.
We come together to combine our expertise, develop policies and set standards. Strong relationships with other countries help us all address health risks more efficiently and effectively. In addition, working together helps us all support timely access to safe new medical devices.
International Medical Devices Regulators Forum
In 2019 Health Canada continued its work with the International Medical Devices Regulators Forum (IMDRF). This group of medical device regulators works to harmonize the regulation of medical devices. This cooperation can make innovative medical devices available to patients more quickly around the world.
This year we implemented an IMDRF guidance document that describes a common structure that companies should use when they apply for approval of a high-risk medical device.
Health Canada leads an IMDRF working group on electronic formats for medical device applications and co-chairs a working group on cybersecurity. We also participate in the IMDRF adverse events working group, which is developing a harmonized terminology for reporting incidents related to medical devices.
Canada-United States Regulatory Cooperation Council
The Canada-United States Regulatory Cooperation Council (RCC) was created to reduce unnecessary differences between the countries' regulatory frameworks. As part of this work, Health Canada and the United States Food and Drug Administration (FDA) will continue to work together on regulatory topics, particularly through the IMDRF. We are working with the FDA towards the development of the Medical Device Single Review Program (MDSRP). The MDSRP aims to improve patient access to medical devices, support innovation and strengthen the development of standards.
International Cooperation
The high quality of a medical device helps ensure that it functions as it is intended. This is particularly important for devices with long lifetimes and those that are implanted in the body. In 2019 Health Canada completed its transition to an audit system called the Medical Device Single Audit Program (MDSAP). The MDSAP was designed and developed so that one single audit is needed, that can then meet the requirements of multiple regulatory agencies around the world.
This program aligns with our partners in other countries, and continues to ensure the quality of the medical devices sold in Canada. To date more than 99% of active medical device licences have complied with this requirement.
Focus on … Medical Device Single Audit Program (MDSAP)
International partners that are participating in the MDSAP include:
MDSAP Members:
- Health Canada
- Therapeutic Goods Administration of Australia
- Brazil's Agência Nacional de Vigilância Sanitária
- Japan's Ministry of Health, Labour and Welfare, and the Japanese Pharmaceuticals and Medical Devices Agency
- United States Food and Drug Administration
MDSAP Official Observers:
- The World Health Organization (WHO) Prequalification of In Vitro Diagnostics Programme
- European Union
MDSAP Affiliate Members:
- Argentina's National Administration of Drugs, Foods and Medical Devices
- Republic of Korea's Ministry of Food and Drug Safety
Medical Devices Action Plan
Health Canada's Medical Devices Action Plan is a strategy to strengthen the regulatory system for medical devices. Through the Action Plan, we aim to continuously improve the safety, effectiveness and quality of devices in Canada.
In 2019 we continued to focus on the three components of the Medical Devices Action Plan.
Improving how medical devices get on the Canadian market
We expanded our use of outside experts and improved our internal expertise. For example, Health Canada created a new Scientific Advisory Committee on Health Products for Women (SAC-HPW), which held its first two meetings in 2019. We also held meetings of the Scientific Advisory Committees on Digital Health Technologies and Medical Devices Used in the Cardiovascular System. Through these committees, we improved our internal expertise through advice from the scientific, medical and patient communities about current and emerging issues.
Focus on … expert advice
In 2019 as part of the Medical Devices Action Plan, Health Canada formed a new Scientific Advisory Committee on Health Products for Women (SAC-HPW). This committee includes members representing the patient and healthcare professional communities. This committee will provide Health Canada with advice on current and emerging issues regarding women's health. Members will examine issues across the life cycle of medical devices and drugs.
"Optimal health relies on sex and gender sensitive evidence and analysis, especially in regulating drugs and devices used primarily by women and girls. This important opportunity to advise on actions for improving the safety and monitoring of women's health products is welcome and timely, in light of increasing scrutiny on health products designed for women in particular."

Dr. Lorraine Greaves,
Chair,
Scientific Advisory Committee on Health Products for Women
Strengthening monitoring and follow-up of medical devices
Health Canada put forward two initiatives in 2019 as part of our work on the Medical Devices Action Plan. The first implemented mandatory reporting of medical device incidents by hospitals. For more information, go to "Medical devices: 2019 Accomplishments – Mandatory reporting of medical device incidents". The second proposed draft regulations for surveillance of medical devices currently available in Canada. Together, these initiatives will help us improve how we monitor and address potential safety issues.
Providing more information to Canadians
In 2019 Health Canada began publicly releasing clinical information that was submitted to Health Canada in medical device applications. For more information, go to "Medical devices: 2019 Accomplishments – Publishing clinical information about medical devices".
We also began publishing summaries of our decisions for more new medical devices and for amendments to existing higher-risk products. With this change, Health Canada will now publish over 1,000 regulatory decision summaries per year. Canadians can consult these summaries to learn more about our decisions and the medical devices they use.
In addition, we are working to launch a medical device incident database that contains reports in a user-friendly, searchable, online format.
Publishing clinical information about medical devices
"Clinical information" is data about the safety and efficacy of medical devices, and includes information about the design and results of investigational testing. In 2019 Health Canada began publishing the clinical information that is provided by companies when they seek approval to sell a medical device in Canada. The clinical information is made available after Health Canada has finished its review and has made a decision about approving or rejecting a medical device.
Providing public access to this clinical information will benefit Canadians by:
- making it easier for the clinical data to be re-analysed independently,
- encouraging new research questions,
- reducing the duplication of clinical trials, and
- helping patients and healthcare providers make better decisions about their health.
We make clinical information available through our "Clinical Information Portal". In 2019 we published 465 pages of clinical information on 8 medical devices.
For more information, go to "Clinical information on drugs and health products".
Mandatory reporting of medical device incidents
Health Canada continues to look for ways to strengthen our knowledge about the safety of medical devices on the Canadian market. Reports of medical device incidents (MDIs) help us identify emerging safety issues. However, we know that MDIs are under-reported around the world. In addition, the reports that are submitted can be missing important information.
To help address these issues, in 2019 Health Canada published final regulations that aim to increase the quantity and improve the quality of MDI reports. Hospitals are now required to report to Health Canada all MDIs for medical devices documented within the hospital.
To help hospitals implement this change, Health Canada helped to develop educational materials and online tools to make it easier for hospitals to submit reports.
The additional, higher quality data will help Health Canada improve the safety of medical devices by:
- detecting new safety issues related to medical devices,
- assessing the impact of safety issues for Canadians,
- updating the instructions for use, and
- publishing warnings or issuing recalls when needed.
Regulatory review of drugs and devices
Health Canada developed the "Regulatory Review of Drugs and Devices" initiative in 2017 to provide patients with more timely access to drugs and medical devices. Other goals include increasing our work with partners in the healthcare system in Canada and with other countries, and making better use of real-world evidence (data collected outside of clinical trials) across a product's life cycle.
As part of this initiative, in 2019 we launched an e-Learning tool, "Understanding How Medical Devices are Regulated in Canada – Premarket Regulation". This tool is designed to provide an overview of Health Canada's premarket regulatory requirements for medical devices. It is expected to help companies improve the completeness and quality of their applications. This in turn will result in more positive, and more timely, regulatory decisions from Health Canada.
Digital health technologies
Digital health technologies are evolving quickly and can benefit Canadians. For example, artificial intelligence is helping to diagnose certain diseases and develop treatment options that are more accurate and efficient.
These innovations can make healthcare delivery more accessible, convenient and cost-effective. However, advances in medical devices pose unique challenges. Health Canada must keep pace with innovation, and also make sure these products are safe and effective.
In 2019 Health Canada continued to focus on providing access to digital health technologies. For example, we are receiving an increasing number of applications for medical devices that use artificial intelligence, such as applications that are used in the analysis of images from MRIs and CT scans.
Our new Digital Health Division continues to work with other regulators and with scientific experts in Canada and around the world. We have collaborated on topics such as machine learning and artificial intelligence, as well as cybersecurity.
Health Canada has also finalized guidance documents for industry on 3D printing and cybersecurity, and we published a guidance on software as a medical device. These documents will help clarify Health Canada's standards in these important areas.
Focus on … our science
"Medical devices that are manufactured by 3D printing can offer a personalized solution: a device designed based on the needs of an individual patient. The guidance will help our decision-making in the face of rapid advances in technology. It will also inform manufacturers of our expectations with respect to the evidence we require ensuring 3D-printed medical devices are safe and effective."

Andrea Katynski,
Scientific Evaluator,
Health Canada
Addressing safety issues
In 2019 Health Canada focussed on possible risks related to several types of medical devices. We met with patients who had received pelvic meshes for transvaginal repairs, and those with breast implants.
Working with mesh manufacturers, Health Canada addressed concerns related to pelvic meshes by limiting their use through labelling revisions. Meshes that did not meet our safety and effectiveness requirements were removed from the Canadian market by the manufacturers.
Health Canada found that "macro-textured" breast implants had an increased risk of a type of non-Hodgkin lymphoma, a cancer that affects the immune system. Health Canada suspended the licences of the "macro-textured" implants that were available in Canada. We updated our published information about breast implants. We also published information about the safety review and the licence suspension. Health Canada continues to monitor and review all available scientific and clinical information regarding the safety of textured breast implants.
Healthy clicks – Medical devices at a glance
To stay informed about our activities:
- Follow us on Facebook
- Follow us on Twitter
- Follow us on YouTube
- See the latest news from Health Canada on our website
- Find other Health-related information on the Government of Canada website
You can also find specific information about medical devices by following the links below.
New medical devices approved
Health Canada regularly tweets about new medical devices approved.
- Twitter #Drugandmeddevice
The Medical Devices Active Licences Listing (MDALL) is a listing of all approvals (licences) issued for medical devices.
New Search for data about the tests and trials that were performed on medical devices to evaluate their safety and efficacy.
Regulatory Decision Summaries include the purpose of an application for a medical device licence and the reasons for Health Canada's decision to approve or reject it.
Summary Basis of Decision documents give the detailed regulatory, safety, effectiveness and quality considerations that factored into Health Canada's decision to approve certain medical devices.
Surveillance of medical devices
You can report medical device incidents to your medical professional, to a hospital or to the company that made the product. You can also report them to Health Canada through the Canada Vigilance Program or by phone at 1-866-234-2345.
The Recalls and Safety Alerts Database includes the recalls, advisories, safety alerts and other publications issued by Health Canada.
Summary Safety Reviews summarize our completed reviews of potential safety issues for medical devices.
New Safety and Effectiveness Reviews are tables listing reviews that are currently ongoing in Health Canada.
Health Product InfoWatch is a monthly publication intended primarily for healthcare professionals. The Health Product InfoWatch provides clinically relevant information about health products and their safety.
The summary tables of advertising complaints list the complaints about health product advertising that have been filed with Health Canada, and the action we have taken.
The Medical Devices Incident database includes suspected medical device incidents reported to Health Canada. These reports have been submitted by consumers and health professionals as well as medical device manufacturers.
Drugs for veterinary use
Drugs for veterinary use: 2019 in brief
Safe, effective and high quality drugs for veterinary use play an important role in protecting human and animal health. Health Canada evaluates and monitors the safety, quality and effectiveness of veterinary drugs. In doing so, we work to protect Canada's food supply.
This year one of our key priorities remained taking action to address antimicrobial resistance. We continued our work to improve the responsible use of antimicrobials in animals.
The world in which we work is changing rapidly and so are the drugs we regulate. We have focussed on developing flexible new regulatory tools. We have also continued our collaborations with our international partners. Together, these initiatives will help bring new drugs for veterinary use to the Canadian market.
The "Drugs for veterinary use: 2019 accomplishments" section describes our progress this year in this and other areas.
New health products approved
In 2019 we approved seven new drugs for companion and food-producing animals. This enabled access to innovative new products and therapies to help maintain and improve the health of animals.
We also approved seven new generic drugs to provide additional options for more cost-effective prevention and treatment.
For a list and description of the new drugs we approved in 2019, go to "Drugs for veterinary use: Approved in 2019".
This year we also helped make 723 veterinary health products available. These offer more low-risk options to maintain or promote health and wellness in animals.
Clinical trials and Emergency Drug Release Programme
We review applications to allow companies and researchers to conduct studies on veterinary drugs in Canada. New veterinary drug trials (called investigational drugs and experimental studies) mean Canadians may have access to new products in the future. In 2019, 87 Experimental Studies Certificates that support clinical trials or research activities were authorized.
Through our Emergency Drug Release Programme, we give access to drugs for veterinary use that are not available in Canada. We may grant access to veterinarians in the case of a medical emergency. In 2019, 671 requests under the Emergency Drug Release program were authorized.
Surveillance
After we approve a drug for sale in Canada, we continue to monitor and evaluate reports of suspected adverse veterinary drug reactions.
This "Drugs for Veterinary Use" chapter gives you more information about our work in 2019. For up-to-date information about our activities see the "Healthy clicks – Drugs for veterinary use at a glance" section, and follow us on social media.

Mary Jane Ireland,
Director General,
Veterinary Drugs,
Health Canada
Drugs for veterinary use: What's new in 2019
In 2019 Health Canada approved seven new drugs for veterinary use. More detail is available in the section "Drugs for veterinary use: Approved in 2019".
New drugs for veterinary use
Drugs for veterinary use: 2019 accomplishments
Addressing antimicrobial resistance related to animals
Using antimicrobials in animals can affect the way that humans and animals respond to treatment.
Antimicrobials, such as antibiotics and antivirals, are used to treat people and animals. Bacteria, viruses, fungi and parasites can resist antimicrobials. This is known as antimicrobial resistance (AMR). The impact of AMR on human and animal health is a concern in Canada and around the world.
In animals, antimicrobial use contributes to the development and spread of resistant bacteria. These bacteria can be transferred to humans through direct contact with animals, and also through the animal products we eat.
Taking action to address antimicrobial resistance
A large portion of the antimicrobials sold in Canada are used in food-producing animals. In 2019 we continued to improve the oversight and responsible use of antimicrobials in animals.
Involving a licensed veterinarian in treatment decisions is an important part of overseeing antimicrobial use. Beginning in December 2018, many antimicrobials used in animals that were once available without a prescription ("over-the-counter") now require a prescription.
To help our stakeholders adapt to this change, we worked with our partners to develop a variety of tools such as prescription templates. For more information, go to Responsible use of medically important antimicrobials in animals.
In 2019 Health Canada launched the Veterinary Antimicrobial Sales Reporting System. This is an electronic tool that helps manufacturers, importers and compounders submit reports directly to Health Canada about the volume of certain antimicrobials they have sold. We will use this information to give us a better understanding of how antimicrobials are used in animals. It will also help us identify patterns of antimicrobial resistance. For more information, go to Veterinary antimicrobial sales reporting.
Our work to promote effective stewardship of veterinary antimicrobials is part of the Federal Action Plan on Antimicrobial Resistance and Use in Canada and the Pan-Canadian Framework for Action on Tackling Antimicrobial Resistance and Antimicrobial Use.
To learn about antimicrobial resistance related to humans, go to "Drugs for human use: 2019 accomplishments".
Focus on … AMR Awareness Week, November 18-22, 2019
Every year Health Canada, along with international health agencies, supports AMR Awareness Week. This global initiative aims to raise awareness of antimicrobial resistance and highlight best practices to help stop the spread of antibiotic resistance. To coincide with AMR Awareness Week, we launched a series of social media messages to raise awareness of this important issue as well as the joint statement on AMR that Health Canada issued with ICMRA.
Regulating innovative products
Scientific and technological advances have led to new ways to diagnose, treat and monitor animals. In 2019 Health Canada announced it would develop new regulations that are agile and that will support innovative new therapies.
As part of our ongoing Regulatory Innovation initiative, we are focusing on how we oversee clinical trials, approve complex new therapies and use foreign reviews and decisions.
Changing how we regulate experimental studies
Experimental studies are usually the first step in developing new veterinary drugs. As products evolve, so do experimental studies. We need regulations that are more flexible so that new veterinary drugs can be developed and tested. The level of oversight should be based on the potential risk of the study.
Health Canada will propose regulations that would streamline and clarify the processes for experimental studies for veterinary drugs. This approach will improve our alignment with regulators around the world. It will also encourage more sponsors to conduct studies in Canada.
Creating a flexible approach for approving complex new therapeutic products
Some therapeutic products are so novel, complex and distinct that they do not fit within our current regulations. We need a way to approve these products for veterinary use that is flexible and based on their potential risk.
Health Canada is proposing a new pathway for these "Advanced Therapeutic Products". Using this pathway, we will collaborate with stakeholders to create regulatory requirements that can be tailored to a specific product. It will allow us to manage these products throughout their life cycle, and still maintain our high standards for safety.
Improving access to products for minor uses and minor species
Health Canada is aiming to facilitate access to veterinary drugs for use in minor species (for example, farmed fish and sheep) and to treat rare and life-threatening conditions in major species (for example, cattle and chickens). Health Canada is exploring using foreign decisions to approve certain veterinary drugs that have been authorized by specified foreign regulators, have an adequate amount of post-market experience and meet internationally established food safety standards.
We know that it is important for Health Canada to be at the forefront of innovation. We will maintain, and invest in, our core business of reviewing drugs for veterinary use to protect the health and safety of Canadians and to protect Canada's food supply. Our discussions with stakeholders will continue as we develop our regulations, to make sure the regulations will respond to new science and technology and to the needs of Canadians.
Focus on … regulatory innovation
"This is a turning point for health product regulation as we work to ensure our regulations and programs adapt to the changing landscape of health product innovation. It is essential that the work we do protects the safety of Canadians and enables innovation to improve their health." - Elizabeth Toller, Executive Director, Regulatory Innovation, Health Canada
Providing more choices to improve animal health and wellness
By keeping animals healthy, we can reduce the need to use drugs, including antimicrobials. Veterinary health products are low-risk products that can help maintain or promote the health and wellness of animals. They include ingredients such as vitamins, minerals and traditional medicines.
More than 100 additional ingredients are now permitted in low-risk products. In 2019 we also helped make 723 veterinary health products available. Together, these will expand the range of choices available to animal owners and veterinarians. To learn more about products available, go to the List of Notified Products.
Collaborating internationally
Health Canada has a long history of working with regulators around the world. This co-operation ranges from ad-hoc meetings about global drug safety issues, to work with international organizations to harmonize our requirements for drugs.
We come together to combine our expertise, develop policies and set standards. Strong relationships with other countries:
- improve simultaneous access to safe and effective veterinary drugs,
- contribute to important public health objectives,
- promote harmonization of our regulations, and
- encourage innovation.
Some examples of our collaborations with other international regulators in 2019 included:
- Canada-United States Regulatory Cooperation Council (RCC) – The RCC was created to reduce unnecessary differences between the countries' regulatory frameworks. As a result, Health Canada and the United States Food and Drug Administration Center for Veterinary Medicine are conducting simultaneous reviews of veterinary drugs. This partnership expanded in 2019 to include the simultaneous review of generic drugs. This will increase the number of veterinary drugs available at the same time in both countries, so that Canadian animal owners will have more treatment options. In 2019, 1 drug was approved in Canada through this pathway, with 1 more accepted for review.
- Regulatory Cooperation with Australia and New Zealand – Health Canada continues to review veterinary drug submissions with the Australian Pesticides and Veterinary Medicines Authority Australia and the New Zealand Ministry of Primary Industries. These joint reviews enable simultaneous access to a new animal drug in three major markets, leading to improved animal health and food safety.
- International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH) – The mission of VICH is to harmonize requirements for the approval of veterinary drugs. Health Canada is an observer of VICH. Through the VICH, scientific experts from around the world work together to develop guidelines that help regulators ensure that veterinary drugs are safe, effective and of high quality. In 2019 we implemented one VICH guideline.
- Codex Alimentarius – The Codex Alimentarius is a collection of standards, guidelines and codes of practice. Health Canada helps set international standards for veterinary drug residues in foods under the Codex Alimentarius via the Codex Committee on Veterinary Drugs Residues in Foods. We are also involved in the Adhoc Intergovernmental Task Force on AMR to develop guidance on the management of AMR in the food chain.
For more information, go to Veterinary drugs – International collaboration.
Focus on … international collaboration
"Reviewing submissions under regulatory cooperation continues to strengthen our international relationships. Evaluators enjoy the opportunity to discuss submissions and challenges, finding that we often share the same concerns. Simultaneous reviews continue being a rewarding experience for all reviewers involved."

Dr. Elise Tatone,
Acting Senior Science Advisor,
Health Canada
Focus on … international collaboration
"Our collective work with Health Canada, Veterinary Drugs Directorate, has allowed us to build a collaborative environment where scientists from both countries are able to work together and share their respective expertise. The animal drug approvals that arise out of this partnership are incredibly beneficial to our public and animal health mission on a broader scale by increasing animal drug availability across our two countries. We look forward to continuing this work as we evaluate future animal drug applications."

Dr. Matthew Lucia,
Director,
Office of New Animal Drug Evaluation,
Center for Veterinary Medicine,
United States Food and Drug Administration
Focus on … product safety
In 2019 Health Canada implemented new software that allows veterinary drug manufacturers to report suspected adverse drug reactions electronically. This new system will enhance surveillance of veterinary drugs currently on the market to ensure their benefits continue to outweigh their risks.
Healthy clicks – Drugs for veterinary use at a glance
To stay informed about our activities:
- Follow us on Facebook
- Follow us on Twitter
- Follow us on YouTube
- See the latest news from Health Canada on our website
- Find other Health-related information on the Government of Canada website
You can also find specific information about veterinary drugs by following the links below.
New drugs approved
The Drug Product Database is a listing of all drugs approved for sale in Canada. In the database, many drugs are accompanied by their veterinary labelling, which describe the conditions of use of the product.
The Notice of Compliance database lists the approvals (Notices of Compliance or NOCs) issued for new drugs.
Surveillance of veterinary drugs
Drugs for human use: Life cycle
As part of Health Canada's mission to help Canadians maintain and improve their health, we evaluate drugs before they reach the Canadian market and continue to monitor real world evidence while they are on the market. Health Canada is involved throughout the life cycle of a drug for human use, from clinical trials to after the drug is being sold in Canada.
Clinical trials
Clinical trials are conducted by sponsors (manufacturers or researchers) to gather information on a drug's safety and effectiveness in humans. Sponsors of clinical trials submit their applications to conduct a clinical trial with a drug in Canada. Health Canada reviews these applications before the trial is conducted in Canada.
Special Access Programme
Drugs that are not approved in Canada may be available through our Special Access Programme. In this program, access is given to an individual healthcare practitioner who is treating a specific patient. Access may be granted for emergency use, or to patients with serious or life-threatening conditions when conventional therapies have failed, are unavailable or are unsuitable.
Drug submission and review
When a company decides that it would like to market a drug in Canada, it files a submission with Health Canada. A new drug submission contains detailed scientific information about the drug's safety, quality and efficacy.
Our scientists and medical officers perform a thorough review of the information submitted. Sometimes we also consult with advisory committees or external consultants. Reviewers evaluate the safety, effectiveness and quality data to assess the benefits and potential risks of the drugs. They also review the information that will be provided to healthcare practitioners and consumers about the drug.
Expedited review pathways
We have several review processes that can provide an expedited path for certain drugs, including those that target specific healthcare needs. That is, there are several review pathways that have shorter review targets for certain drugs. Products approved through expedited review pathways can be available to patients sooner.
Priority review: Drugs for serious, life-threatening, or severely debilitating diseases or conditions can be given a priority review status. Drug submissions that are granted priority review status are subject to an expedited review process.
Notice of Compliance with conditions: When a new drug is approved it is issued a Notice of Compliance (NOC). An NOC may be issued with conditions (NOC/c) to a drug that showed promising clinical benefit, for serious, life-threatening, or severely debilitating diseases or conditions. The manufacturer must still demonstrate that the drug has an acceptable safety profile and is of high quality, and also commits to undertake additional studies to verify the clinical benefit of the drug. Submissions that are reviewed under this pathway are subject to an expedited review process.
Approval of drugs
After its review of a drug submission, Health Canada may conclude that the benefits of the product outweigh the potential risks and approve the drug for sale in Canada. When a new drug is approved, it is issued a Notice of Compliance (NOC) and a Drug Identification Number (DIN). This does not mean the drug will immediately be available to patients, as many other factors can influence that timeline.
Surveillance
It is not possible to know or predict all of the possible adverse reactions to a drug through clinical studies. After a product is approved and available for sale in Canada, we continue to monitor its use in the real world, that is, in the broader Canadian population that may be taking other medications. We evaluate potential safety and effectiveness issues, and take action when there are identified problems.
Collecting information
Health Canada collects safety information about a product after it is approved from a variety of sources. One source of information is suspected adverse reactions that are reported after products are approved for sale. Adverse reactions are undesirable effects potentially caused by drugs.
Another source of information are documents called Risk Management Plans (RMPs) that are submitted by manufacturers as part of their drug submissions. An RMP includes information on a drug's safety profile and how its risks will be prevented or minimized. It also contains plans for studies and other activities to learn more about the safety and effectiveness of the drug.
Evaluating safety signals
Health Canada evaluates the data we collect to detect new safety signals, which we then investigate more closely. A "safety signal" can be defined as information on a new or known adverse event that may be associated with a drug. These investigations are called signal assessments and they may result in recommendations for actions to be taken by the company, by Health Canada, or both. These actions can include informing the public and healthcare professionals of new safety information or recommending labelling changes. In the most serious situations, we may remove a drug from the market.
Advertising complaints
Health Canada also regulates the advertising of drugs in Canada to ensure that companies are not making false claims about their products. We review advertising complaints to determine if a company is complying with our requirements, and we take appropriate action when non-compliance is identified. This may include requesting a company to stop disseminating non-compliant advertising and taking steps towards avoiding any future issues.
Drugs for human use: Approved in 2019
This section outlines the new drugs, generic drugs and biosimilars approved for sale in Canada in 2019, and the safety updates issued.
You can report adverse drug reactions to your medical professional, to a hospital or to the company that made the product. You can also report them to Health Canada through the Canada Vigilance Program or by phone at 1-866-234-2345.
Health categories
The drugs listed have been divided into categories according to the Anatomical Therapeutic Chemical Classification (ATC) System, a system of codes developed by the World Health Organization. ATC codes are often assigned according to the mechanism of action (that is, how the drug works) rather than the disease or condition to be treated.
We have included the indication of each new drug to give you some additional information. In addition, each new drug has a hyperlink to the Decision Summary (when available). These documents provide a brief overview of the rationale for our decision to approve the drug.
The categories are:
- Alimentary tract and metabolism – for example, drugs for the gastrointestinal tract and drugs for diabetes.
- Antiinfectives for systemic use – for example, antibacterials, antivirals and vaccines.
- Antineoplastic and immunomodulating agents – for example, drugs for the treatment of cancer and drugs that stimulate or suppress the immune system.
- Antiparasitic products, insecticides and repellents – for example, drugs to treat infestations of parasites.
- Blood and blood forming organs – for example, drugs such as antihemorrhagics.
- Cardiovascular system – for example, drugs for high blood pressure and cholesterol reducers.
- Dermatologicals – for example, drugs for the skin such as drugs to treat acne.
- Genito urinary system and sex hormones – for example, hormonal contraception, sex hormones and drugs for the urinary tract system.
- Musculo-skeletal system – for example, drugs affecting the muscles, bones and joints, including anti-inflammatories and muscle relaxants.
- Nervous system – for example, drugs that affect the brain and the nervous system, including analgesics, antidepressants and anti-seizure drugs.
- Respiratory system – for example, drugs that affect the respiratory system, including antihistamines.
- Sensory organs – for example, drugs for the eyes.
- Systemic hormonal preparations, excluding sex hormones and insulins – for example, drugs that affect the endocrine system, including thyroid hormones.
- Various – for example, drugs unable to be classified into the other categories, such as diagnostic agents and drugs to treat high levels of potassium in the blood.
Important definitions
Aligned review
An aligned review is one where the drug company allowed information to be shared between Health Canada and heath technology assessment organizations.
Biologic drug
Biologic drugs are biologically-derived products such as vaccines, blood-derived products and products produced through biotechnology.
Biosimilar
A biosimilar is a biologic drug that enters the market subsequent to a previously authorized biologic drug in Canada with a demonstrated similarity to the previously authorized biologic drug.
Generic drug
A generic drug is a copy of a brand name product. Generic drugs contain the same medicinal ingredients as the brand name drug and are considered bioequivalent to the brand name drug. There may be many generic versions of one brand name drug. Generic drugs cost less, so approving generic drugs can mean considerable savings to the healthcare system.
New drug
New drugs give new and innovative options for treatment, prevention and diagnosis of various health conditions.
New active substance
A new drug that contains a medicinal ingredient not previously approved in a drug in Canada and that is not a variation of a previously approved medicinal ingredient.
Non-prescription ("over-the-counter") drug
Non-prescription drugs, also called over-the-counter (OTC) drugs, are products that can be bought without a doctor's prescription.
Notice of Compliance with conditions
A Notice of Compliance may be issued with Conditions (NOC/c) to a drug with promising clinical benefit, for a serious, life-threatening, or severely debilitating disease or condition. The manufacturer must still demonstrate that the product has an acceptable safety profile based on a benefit/risk assessment, and is of high quality, and also commits to undertake additional studies to verify the clinical benefit of the drug. Submissions that are reviewed under this pathway are subject to shorter review targets.
Orphan drug
Orphan drugs are used to treat rare diseases, and have received orphan designation in either the United States or the European Union.
Pediatric indication
This indicates that the drug has been approved for use in children less than 18 years old.
Priority review
Priority review status may be granted to a drug submission for a product for a serious, life-threatening, or severely debilitating disease or condition. Submissions that are granted priority review status are subject to shorter review targets.
Review with international partners
A review with international partners is one where Health Canada worked with certain regulators to share the work of drug reviews. For more information, go to "Drugs for human use: 2019 accomplishments".
Safety updates
Safety updates are designed to communicate information about potential health risks, so that patients and healthcare professionals can make informed decisions about their health.
For more information about the types of risk communications that can be found on the Government of Canada's website, go to "Healthy clicks – Drugs for human use at a glance".
Figure 1: New drugs, new generic drugs and new biosimilars approved in 2019

Text description
Number of new drugs, generic drugs, and biosimilars approved in 2019, by ATC Category (the Anatomical Therapeutic Chemical Classification (ATC) System, a system of codes developed by the World Health Organization).
| ATC Category | Number of new drugs approved | Number of generic drugs approved | Number of biosimilars approved |
|---|---|---|---|
| Alimentary tract and metabolism | 2 | 10 | 0 |
| Antiinfectives for systemic use | 5 | 24 | 0 |
| Antineoplastic and immunomodulating agents | 21 | 27 | 5 |
| Antiparasitic products, insecticides and repellents | 0 | 2 | 0 |
| Blood and blood forming organs | 2 | 0 | 0 |
| Cardiovascular system | 3 | 14 | 0 |
| Dermatologicals | 1 | 3 | 0 |
| Genito-urinary system and sex hormones | 2 | 7 | 0 |
| Musculo-skeletal system | 1 | 7 | 0 |
| Nervous system | 5 | 22 | 0 |
| Respiratory system | 1 | 2 | 0 |
| Sensory organs | 3 | 4 | 0 |
| Systemic hormonal preparations, excluding sex hormones and insulins | 1 | 4 | 0 |
| Various | 6 | 3 | 0 |
| Total new drugs approved in 2018 | 53 | 129 | 5 |
Alimentary tract and metabolism
For example, drugs for the gastrointestinal tract and drugs for diabetes.
2 New drugs were approved in this category
Jorveza
- Orphan drug
- Priority review
- Medicinal Ingredient
- Budesonide
- Indication
- For the treatment of eosinophilic esophagitis.
Trulance
- New active substance
- Medicinal Ingredient
- Plecanatide
- Indication
- Trulance is used in adults to treat a condition called irritable bowel syndrome with constipation (IBS-C).
10 New generic drugs were approved in this category
- 2 products containing acarbose
- 1 product containing calcitriol
- 1 product containing levocarnitine
- 1 product containing metformin hydrochloride
- 3 products containing odansetron
- 2 products containing prucalopride
Safety updates published in this category
- Metformin: Information Update: Health Canada evaluating NDMA in metformin drugs
- Polyethylene glycol (PEG) 3350: Health Product InfoWatch: Interaction between polyethylene glycol laxative and starch-based thickener
- Ranitidine:
- Information Update: Health Canada assessing NDMA in ranitidine
- Information Update: Health Canada requests that companies stop distributing ranitidine drugs in Canada while it assesses NDMA; some products being recalled
- Information Update: Health Canada requests that companies stop distributing ranitidine drugs in Canada while it assesses NDMA; additional products being recalled
- Information Update: Additional ranitidine products recalled, including Zantac; request to stop distribution remains in place while Health Canada continues to assess NDMA
- Information Update: Additional ranitidine products recalled as a precautionary measure; request to stop distribution remains in place while Health Canada continues to assess NDMA
- Information Update: Ranbaxy Pharmaceuticals Canada Inc. recalls prescription ranitidine products as a precaution; request to stop distribution remains in place while Health Canada continues to assess NDMA
Antiinfectives for systemic use
For example, antibacterials, antivirals and vaccines.
5 New drugs were approved in this category
Dovato
- Aligned review
- Pediatric indication
- Medicinal Ingredients
- Dolutegravir, lamivudine
- Indication
- Dovato is used to treat HIV (human immunodeficiency virus) infection in adults and adolescents over the age of 12 years and weighing at least 40 kg.
Flucelvax Quad
- Biologic drug
- Pediatric indication
Flucelvax Quad Decision Summary
- Medicinal Ingredients
- Haemagglutinin-strain A(H1N1), haemagglutinin-strain A(H3N2), haemagglutinin-strain B (Victoria), haemagglutinin-strain B (Yamagata)
- Indication
- Flucelvax Quad is used in adults and children 9 years and older to prevent influenza, often called "the flu." Influenza is caused by infection with specific influenza viruses. New types of influenza viruses can appear each year. Flucelvax Quad vaccine contains fragments of four different types of influenza virus. Each year the World Health Organization decides which four types of viruses are most suitable to include in the vaccine. For this season (2019 – 2020) the viruses are A/[Official strain] (H1N1) – like strain, A/[Official Strain] (H3N2) – like strain, B/[Official Strain] (Victoria) – like strain and B/[Official strain ] (Yamagata) – like strain. You cannot catch influenza from the vaccine, as the virus in the vaccine has been killed and split into small non-infectious particles. The National Advisory Committee on Immunization (NACI) encourages annual influenza vaccination for all Canadians who are able to have the vaccine. Vaccination against influenza is recommended every year, for anyone wanting to lower their chance of catching influenza. Flucelvax Quad has been used by many people to lower their risk of catching the flu.
Influvac Tetra
- Biologic drug
Influvac Tetra Decision Summary
- Medicinal Ingredients
- Haemagglutinin-strain A(H1N1), haemagglutinin-strain A(H3N2), haemagglutinin-strain B (Victoria), haemagglutinin-strain B (Yamagata)
- Indication
- Influvac Tetra is a vaccine used to prevent adults of 18 years of age and older from developing influenza (the flu).
Ivozfo
- Pediatric indication
- Priority review
- Medicinal Ingredient
- Fosfomycin
- Indication
- Ivozfo is used in adults and children to treat infections of the:
- lung
- bones
- kidney and bladder
- brain (called meningitis)
- blood that are caused by any of the infections listed above
- Ivozfo is used when other antibiotics cannot be used or have not worked.
- This medicine is usually given in combination with other antibiotics.
- Ivozfo is used in adults and children to treat infections of the:
Xembify
- Biologic drug
- Pediatric indication
- Medicinal Ingredient
- Immunoglobulin (human)
- Indication
- Xembify can help prevent infections in patients who have poorly functioning immune systems.
24 New generic drugs were approved in this category
- 1 product containing acyclovir
- 2 products containing amikacin
- 1 product containing caspofungin
- 1 product containing cephalexin
- 1 product containing clindamycin
- 1 product containing daptomycin
- 1 product containing efavirenz
- 2 products containing efavirenz, emtricitabine, tenofovir disoproxil
- 3 products containing emtricitabine, tenofovir disoproxil fumarate
- 2 products containing entecavir
- 2 products containing ertapenem
- 1 product containing levofloxacin
- 1 product containing linezolid
- 1 product containing meropenem
- 1 product containing tigecycline
- 1 product containing vancomycin
- 2 products containing voriconazole
Safety updates published in this category
- Biaxin BID, Biaxin SL, and Biaxin (clarithromycin): Health Product InfoWatch: Concomitant administration of clarithromycin with domperidone is contraindicated
- Sulfamethoxazole containing products: Summary Safety Review: Assessing the potential risk of drug reaction with eosinophilia and systemic symptoms (DRESS)
- Systemic fluoroquinolones: Summary Safety Review: Assessing the potential risk of aneurysm (a balloon-like bulge) and dissection (a separation or tear in the layers of the wall) of the aorta (a major blood vessel)
Antineoplastic and immunomodulating agents
For example, drugs for the treatment of cancer and drugs that stimulate or suppress the immune system.
21 New drugs were approved in this category
Balversa
- Aligned review
- New active substance
- Notice of compliance with conditions
- Medicinal Ingredient
- Erdafitinib
- Indication
- Balversa is used to treat a type of bladder cancer called urothelial carcinoma (cancer in the bladder and urinary tract organs). It is used in adults:
- whose cancer has spread to other parts of the body or cannot be removed by surgery; and
- whose cancer was previously treated with chemotherapy, which did not work or is no longer working; and
- whose cancer has changes in certain genes called FGFR (known as fibroblast growth factor receptors).
- Balversa is used to treat a type of bladder cancer called urothelial carcinoma (cancer in the bladder and urinary tract organs). It is used in adults:
Calquence
- Aligned review
- New active substance
- Notice of compliance with conditions
- Orphan drug
- Medicinal Ingredient
- Acalabrutinib
- Indication
- Calquence is used to treat patients with a kind of cancer called mantle cell lymphoma (MCL). It is only used in patients who received at least one other MCL therapy before using Calquence.
Dacogen
- New active substance
- Orphan drug
- Medicinal Ingredient
- Decitabine
- Indication
- Dacogen is used to treat adults with Myelodysplastic Syndromes (MDS). These are blood disorders. It is used when MDS:
- was untreated or previously treated with chemotherapy
- cannot be treated with stem cell transplant
- Dacogen is used to treat adults with Myelodysplastic Syndromes (MDS). These are blood disorders. It is used when MDS:
Demylocan
- New active substance
- Orphan drug
- Medicinal Ingredient
- Decitabine
- Indication
- Demylocan is used to treat adults with myelodysplastic syndromes (MDS). In MDS, the bone marrow does not make enough healthy mature blood cells. Instead, immature blood cells, called blasts, build up in the bone marrow and the blood. These blasts do not work properly, which results in fewer healthy red blood cells, white blood cells and platelets.
Envarsus PA
- Orphan drug
- Medicinal Ingredient
- Tacrolimus
- Indication
- Envarsus PA is used to help prevent organ rejection. It is used in adults who have had a kidney or a liver transplant. It is used along with other immunosuppressants.
Idhifa
- New active substance
- Notice of compliance with conditions
- Orphan drug
- Priority review
- Medicinal Ingredient
- Enasidenib
- Indication
- Idhifa is used to treat Acute Myeloid Leukemia (AML) in adults with a particular change (mutation) in the enzyme "IDH2". AML is a form of cancer which affects your bone marrow and can cause problems with producing normal blood cells. Idhifa is used when your AML has come back (relapsed) or, has not improved with another treatment (refractory).
Libtayo
- Biologic drug
- New active substance
- Notice of compliance with conditions
- Medicinal Ingredient
- Cemiplimab
- Indication
- Libtayo is a medicine used to treat a rare type of skin cancer which may or may not have spread called advanced cutaneous squamous cell carcinoma.
Lorbrena
- New active substance
- Notice of compliance with conditions
- Orphan drug
- Medicinal Ingredient
- Lorlatinib
- Indication
- Lorbrena is used to treat adult patients with a type of lung cancer called non-small cell lung cancer (NSCLC). It is used in a special type of NSCLC that is anaplastic lymphoma kinase (ALK)-positive. Lorbrena is used if your cancer has spread to other parts of your body and:
- your cancer has gotten worse after taking crizotinib and at least one other ALK tyrosine kinase inhibitor (TKI) medicine or
- your cancer has gotten worse on ceritinib or alectinib.
- Lorbrena is used to treat adult patients with a type of lung cancer called non-small cell lung cancer (NSCLC). It is used in a special type of NSCLC that is anaplastic lymphoma kinase (ALK)-positive. Lorbrena is used if your cancer has spread to other parts of your body and:
Mylotarg
- Aligned review
- Biologic drug
- New active substance
- Orphan drug
- Medicinal Ingredient
- Gemtuzumab ozogamicin
- Indication
- Mylotarg is used to treat a certain type of cancer called acute myeloid leukaemia (AML) in which the bone marrow makes abnormal white blood cells. Mylotarg is intended for the treatment of AML in adult patients who have not tried other treatments. Mylotarg is not for use in patients with a type of cancer called acute promyelocytic leukaemia (APL).
Nerlynx
- New active substance
- Medicinal Ingredient
- Neratinib
- Indication
- Nerlynx is used to treat women who have early-stage breast cancer, when:
- the cancer cells produce a larger amount of HER2 proteins; and
- the cancer cells are sensitive to female hormones.
- To receive Nerlynx you will have had previous treatment with the medicine trastuzumab within the last 12 months. Tests are used to find out if your cancer cells produce large amounts of HER2 proteins and are hormone-sensitive.
- Nerlynx is used to treat women who have early-stage breast cancer, when:
Rinvoq
- New active substance
- Medicinal Ingredient
- Upadacitinib
- Indication
- Rinvoq is used to treat adults with rheumatoid arthritis when treatment with methotrexate has not worked well or was not tolerated well. Rinvoq may be taken alone or in combination with other medicines. Rinvoq is not recommended for use in children and adolescents under 18 years of age.
Skyrizi
- Biologic drug
- New active substance
- Medicinal Ingredient
- Risankizumab
- Indication
- Skyrizi is a prescription medicine used to treat adults with moderate to severe plaque psoriasis, an inflammatory condition affecting the skin and nails. Plaque psoriasis can cause raised, thick, red and scaly patches ("psoriatic lesions") that can appear anywhere on your body.
Talzenna
- New active substance
- Medicinal Ingredient
- Talazoparib
- Indication
- Talzenna is taken by itself to treat a specific type of breast cancer (known as human epidermal growth factor receptor 2 [HER2]-negative) in adults:
- who have mutations (changes) in certain genes called BRCA (known as the breast cancer gene),
- who have had previous chemotherapy for your breast cancer, and
- whose cancer has spread beyond the original tumor or to other parts or organs of the body.
- Talzenna is taken by itself to treat a specific type of breast cancer (known as human epidermal growth factor receptor 2 [HER2]-negative) in adults:
Tolak
- Medicinal Ingredient
- Fluorouracil
- Indication
- Tolak is used in adults to treat actinic keratosis (AK) on the face, ears, or scalp. AK is a rough, crusty or scaly patch on the top layer of skin that is made up of fast growing precancerous cells.
Ultomiris
- Biologic drug
- New active substance
- Orphan drug
- Medicinal Ingredient
- Ravulizumab
- Indication
- Ultomiris is used to treat adult patients with a certain type of disease affecting the blood system called Paroxysmal Nocturnal Haemoglobinuria (PNH). In patients with PNH, their red blood cells can be destroyed which can lead to low blood counts (anemia), tiredness, difficulty in functioning, pain, dark urine, shortness of breath, and blood clots.
Verzenio
- Aligned review
- New active substance
- Reviewed with international partners (Australia)
- Medicinal Ingredient
- Abemaciclib
- Indication
- Verzenio is used to treat breast cancer in adult women, only when it has spread to other parts of the body. The breast cancer must be hormone receptor positive. It is taken:
- With another drug for breast cancer called an aromatase inhibitor. You must have already gone through menopause to take Verzenio this way. This is an initial therapy. Or,
- With another drug for breast cancer called fulvestrant. This is used when the cancer gets worse after initial therapy. If you have not yet gone through menopause, you must take a medicine that will stop your ovaries from making estrogen. Or,
- By itself. You must have breast cancer that came back after having had hormone therapy and at least two kinds of prior chemotherapy treatment.
- Verzenio is used to treat breast cancer in adult women, only when it has spread to other parts of the body. The breast cancer must be hormone receptor positive. It is taken:
Vitrakvi
- Aligned review
- New active substance
- Notice of compliance with conditions
- Orphan drug
- Pediatric indication
- Medicinal Ingredient
- Larotrectinib
- Indication
- Vitrakvi is indicated for children and adults. It can treat solid tumours that have a Neurotropic Tyrosine Receptor Kinase (NTRK) gene fusion. The NTRK gene fusion should not have a known resistance mutation. It can treat cancers that have spread to different parts of the body. Or, it can treat cancers where removal is likely to cause serious problems. Vitrakvi is for patients without other treatment choices. To benefit from Vitrakvi the patient must have a tumour that has an NTRK gene fusion. This can be checked by a test that is done before you start Vitrakvi.
Vizimpro
- Aligned review
- New active substance
- Orphan drug
- Medicinal Ingredient
- Dacomitinib
- Indication
- Vizimpro is a prescription medicine used in adults to treat a type of lung cancer called non-small cell lung cancer (NSCLC). Vizimpro is typically used as your first treatment when your cancer:
- cannot be removed with surgery or has spread to other parts of the body
- has certain changes in the genes that produce a protein on the surface of the cells called epidermal growth factor receptor (EGFR)
- Vizimpro is a prescription medicine used in adults to treat a type of lung cancer called non-small cell lung cancer (NSCLC). Vizimpro is typically used as your first treatment when your cancer:
Xospata
- Aligned review
- New active substance
- Orphan drug
- Priority review
- Medicinal Ingredient
- Gilteritinib
- Indication
- Xospata works by blocking certain enzymes of the cells that are not normal. It prevents cancer cells from growing and dividing. Xospata may also slow down or stop the cancer from growing. It also kills cancer cells.
Yescarta
- Aligned review
- Biologic drug
- New active substance
- Orphan drug
- Priority review
- Medicinal Ingredient
- Axicabtagene ciloleucel
- Indication
- Yescarta is a treatment for your large B-cell lymphoma – a form of white blood cell cancer. It is used when you have failed at least two other kinds of treatment.
Zejula
- New active substance
- Orphan drug
- Reviewed with international partners (Australia)
- Medicinal Ingredient
- Niraparib
- Indication
- Zejula is used to treat the following types of cancer in adult women whose cancer has come back:
- ovarian cancer,
- fallopian tube cancer, and
- primary peritoneal cancer (the membrane that lines the inside of the abdomen).
- Zejula is used after the cancer has responded to chemotherapy treatment with a platinum medicine. It helps to keep that response.
- Zejula is used to treat the following types of cancer in adult women whose cancer has come back:
5 New biosimilars were approved in this category
Herzuma
- Biologic drug
- Medicinal Ingredient
- Trastuzumab
- Indication
- Herzuma is a cancer medicine that must be prescribed by a doctor.
- Herzuma is used to slow down the growth of specific breast cancer cells that produce large amounts of HER2 protein. It is used only for patients whose tumours are growing more rapidly than normal because of a genetic problem in the cells. This occurs in about 25 to 30% of breast cancer tumours.
- Herzuma is also approved for the treatment of gastric cancer that has spread to other parts or organs of the body to slow down the growth of specific gastric cancer cells that produce large amounts of HER2 protein. Herzuma is used in combination with chemotherapy (capecitabine or intravenous 5-fluorouracil and in combination with cisplatin) for the treatment of gastric cancer that has spread to other parts or organs of the body.
Ogivri
- Biologic drug
- Medicinal Ingredient
- Trastuzumab
- Indication
- Ogivri is a cancer medicine that must be prescribed by a doctor.
- Ogivri is used to slow down the growth of specific breast cancer cells that produce large amounts of HER2 protein. It is used only for patients whose tumours are growing more rapidly than normal because of a genetic problem in the cells. This occurs in about 25 to 30% of breast cancer tumours.
- Ogivri is used for certain patients with gastric cancer that has spread to other parts or organs of the body to slow down the growth of specific gastric cancer cells that produce large amounts of HER2 protein. Ogivri is used in combination with chemotherapy (capecitabine or intravenous 5-fluorouracil and in combination with cisplatin) for the treatment of gastric cancer that has spread to other parts or organs of the body.
Trazimera
- Biologic drug
- Medicinal Ingredient
- Trastuzumab
- Indication
- Trazimera is a cancer medicine that must be prescribed by a doctor.
- Trazimera is used to slow down the growth of specific breast cancer cells that produce large amounts of HER2 protein. It is used only for patients whose tumours are growing more rapidly than normal because of a genetic problem in the cells. This occurs in about 25 to 30% of breast cancer tumours.
- Trazimera is used for certain patients with gastric cancer that has spread to other parts or organs of the body to slow down the growth of specific gastric cancer cells that produce large amounts of HER2 protein. Trazimera is used in combination with chemotherapy (capecitabine or intravenous 5-fluorouracil and in combination with cisplatin) for the treatment of gastric cancer that has spread to other parts or organs of the body.
Truxima
- Biologic drug
- Medicinal Ingredient
- Rituximab
- Indication
- Truxima (also known as rituximab for injection) is a cancer medicine that is used to stop cancer cell growth and ideally cause the death of cancer cells. It is a cancer medicine that must be prescribed by a doctor. It is used to treat patients with certain types of non-Hodgkin's lymphoma and chronic lymphocytic leukemia.
- Truxima is also used to reduce signs and symptoms of rheumatoid arthritis (in combination with methotrexate).
Zirabev
- Biologic drug
- Medicinal Ingredient
- Bevacizumab
- Indication
- Metastatic Colorectal Cancer: Zirabev is used in combination with a specific type of chemotherapy (intravenous 5-fluorouracil [5-FU]-based chemotherapy) for treatment of people diagnosed with metastatic colorectal cancer for the first time. Metastatic colorectal cancer is cancer of the colon or rectum that has spread to other organs in the body.
- Metastatic Lung Cancer: Zirabev is used in combination with a specific type of chemotherapy (carboplatin and paclitaxel) for the treatment of people diagnosed with metastatic non-small cell lung cancer. Metastatic non-small cell lung cancer is cancer of the lungs that has spread to other organs in the body.
- Recurrent Platinum-Resistant Ovarian Cancer: Zirabev is used in combination with a specific type of chemotherapy (paclitaxel, topotecan, or pegylated liposomal doxorubicin) for the treatment of people diagnosed with recurrent, platinum-resistant, epithelial ovarian, fallopian tube, or primary peritoneal cancer who received no more than two prior chemotherapy regimens. Recurrent platinum-resistant ovarian cancer is the type of cancer that progresses within 6 months after the last time the patient responded to a chemotherapy regimen containing a platinum agent.
- Recurrent Glioblastoma: Zirabev is used in combination with lomustine (a specific type of chemotherapy) for the treatment of patients with a particular type of brain cancer called glioblastoma in which the cancer reoccurred after a previous treatment.
27 New generic drugs were approved in this category
- 2 products containing arsenic trioxide
- 2 products containing bortezomib
- 1 product containing cabazitaxel
- 1 product containing cladribine
- 1 product containing dasatinib
- 1 product containing doxorubicin hydrochloride
- 1 product containing everolimus
- 8 products containing fingolimod
- 5 products containing fulvestrant
- 2 products containing gefitinib
- 2 products containing imatinib
- 1 product containing methotrexate
Safety updates published in this category
- Actemra (tocilizumab): Dear Healthcare Professional Letter: Risk of hepatotoxicity
- Benlysta (belimumab): Dear Healthcare Professional Letter: Increased risk of serious depression, suicidal ideation or behaviour, or self-injury
- Darzalex (daratumumab): Dear Healthcare Professional Letter: Hepatitis B virus reactivation
- Gilenya (fingolimod): Summary Safety Review: Assessing the potential risk of worsening multiple sclerosis symptoms after product withdrawal (rebound effect) and Dear Healthcare Professional Letter: Risk of congenital malformations
- Istodax (romidepsin): Health Product InfoWatch: Risk of romidepsin use in patients with severe hepatic impairment
- Lartruvo (olaratumab): Dear Healthcare Professional Letter: New clinical trial information important to prescribing decisions
- Lemtrada (alemtuzumab): Dear Healthcare Professional Letter: Risk of autoimmune hepatitis, hemophagocytic lymphohistiocytosis, and associated serious cardiovascular reactions
- Opdivo (nivolumab) and Yervoy (ipilimumab): Summary Safety Review: Assessing the potential risk of hemophagocytic lymphohistiocytosis (HLH)
- pms-Methotrexate (methotrexate): Health Product InfoWatch: Contraindication in patients with severe renal impairment and risk of pulmonary alveolar haemorrhage
- Revlimid (lenalidomide): Health Product InfoWatch: Risk of progressive multifocal leukoencephalopathy
- Tacrolimus: Dear Healthcare Professional Letter: Risk of graft rejection due to medication errors: inadvertent switching between different oral formulations
- Tamoxifen: Information Update: Health Canada update on the tamoxifen shortage
- Tecentriq (atezolizumab): Dear Healthcare Professional Letter: Risk of immune-related myositis
- Trisenox (arsenic trioxide): Health Product InfoWatch: Risk of encephalopathy
- Xeljanz and Xeljanz XR (tofacitinib): Information Update: Clinical trial finds an increased risk of blood clots in the lungs and of death in rheumatoid arthritis patients taking high dose of tofacitinib and Dear Healthcare Professional Letter: Risk of thrombosis
Antiparasitic products, insecticides and repellents
For example, drugs to treat infestations of parasites.
2 New generic drugs were approved in this category
- 1 product containing hydroxychloroquine sulfate
- 1 product containing metronidazole
Blood and blood forming organs
For example, drugs such as antihemorrhagics.
2 New drugs were approved in this category
Esperoct
- Aligned review
- Biologic drug
- New active substance
- Pediatric indication
- Medicinal Ingredient
- Antihemophilic factor VIII (recombinant, B-domain truncated), pegylated
- Indication
- Esperoct is a long-acting recombinant coagulation Factor VIII product. Factor VIII is a protein found in the blood that helps to prevent and stop bleeding.
- Esperoct is used to treat and prevent bleeding in people with hemophilia A.
Vonvendi
- Biologic drug
- New active substance
- Medicinal Ingredient
- Von Willebrand factor (recombinant)
- Indication
- To treat and control bleeding in patients (age ≥18) with von Willebrand disease.
- To prevent and treat bleeding during and after surgery in patients (age ≥18) with von Willebrand disease.
Safety updates published in this category
- Brilinta (ticagrelor): Health Product InfoWatch: Risk of thrombotic thrombocytopenic purpura (TTP)
- Eliquis (apixaban): Health Product InfoWatch: Risk of hemorrhage with the concomitant use of selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs), and dosing information according to renal function
- Extraneal peritoneal dialysis solution: Advisory: Baxter recalls two lots of Extraneal peritoneal dialysis solution because of high levels of sodium hydroxide, which may pose serious health risks and Advisory: Updated information on dates of distribution of previously recalled lot
- PregVit and PregVit folic 5 prenatal and postpartum vitamin-mineral supplements: Advisory: Duchesnay Inc. recalls certain lots of PregVit and PregVit folic 5 prenatal and postpartum vitamin-mineral supplements because of a packaging error
- Xarelto (rivaroxaban): Summary Safety Review: Assessing the potential risk of liver injury
Cardiovascular system
For example, drugs for high blood pressure and anticholesterol agents.
3 New drugs were approved in this category
pdp-amlodipine
- Pediatric indication
pdp-amlodipine Decision Summary
- Medicinal Ingredient
- Amlodipine
- Indication
- pdp-Amlodipine is used by itself or with other medicines to treat:
- Mild to moderate high blood pressure (hypertension).
- A type of chest pain called angina for patients who still have angina symptoms after receiving certain other medications.
- pdp-Amlodipine is approved for use in patients aged 6 and above.
- pdp-Amlodipine is used by itself or with other medicines to treat:
Sandoz Bisoprolol Tablets
- Medicinal Ingredient
- Bisoprolol fumarate
- Indication
- Sandoz Bisoprolol Tablets is used to treat hypertension (high blood pressure).
Vascepa
- Aligned review
- New active substance
- Priority review
- Medicinal Ingredient
- Icosapent ethyl
- Indication
- Vascepa is used to reduce cardiovascular events, such as heart attacks or strokes, in high risk patients with elevated triglycerides.
14 New generic drugs were approved in this category
- 1 product containing amlodipine, perindopril arginine
- 1 product containing colesevelam hydrochloride
- 1 product containing diltiazem hydrochloride
- 1 product containing doxazosin
- 1 product containing flecainide acetate
- 1 product containing furosemide
- 1 product containing hydralazine hydrochloride
- 2 products containing hydrochlorothiazide, quinapril
- 1 product containing labetalol hydrochloride
- 1 product containing propranolol hydrochloride
- 1 product containing rosuvastatin
- 1 product containing spironolactone
- 1 product containing trandolapril
Safety updates published in this category
- Auro-Irbesartan HCT: Information Update: Auro Pharma Inc. voluntarily recalls one lot of Auro-Irbesartan HCT tablets because of nitrosamine impurity
- EpiPen (0.3 mg) auto-injectors: Information Update: Pfizer Canada is reporting a shortage of EpiPen (0.3 mg) auto-injectors and taking precautions to minimize impact on Canadians
- EpiPen and EpiPen Jr auto-injectors: Advisory: Some EpiPen and EpiPen Jr auto-injectors may be difficult to remove from their carrier tubes
- Hydrochlorothiazide: Summary Safety Review: Assessing the potential risk of non-melanoma skin cancer
- Irbesartan: Information Update: Pro Doc Limitée voluntarily recalls two lots of irbesartan drugs because of nitrosamine impurity
- Losartan-containing drugs: Information Update: Multiple losartan-containing drugs voluntarily recalled because of potential for nitrosamine impurity
- Opsumit (macitentan): Summary Safety Review: Assessing the potential risk of liver injury
Dermatologicals
For example, drugs for the skin such as drugs to treat acne.
1 New drug was approved in this category
Aklief
- New active substance
- Pediatric indication
- Medicinal Ingredient
- Trifarotene
- Indication
- Aklief is used to treat acne vulgaris on the face and trunk (upper, middle and lower back, shoulders and chest) for patients 12 years and older.
3 New generic drugs were approved in this category
- 1 product containing adapalene, benzoyl peroxide
- 2 products containing clobetasol propionate
Safety updates published in this category
- Atoma-brand Diphenhydramine Hydrochloride 2% Anti-Itch Cream: Advisory: One lot of Atoma-brand Diphenhydramine Hydrochloride 2% Anti-Itch Cream recalled because of a labelling error regarding use in children under two years of age
- Gentian violet liquid topical and medical devices: Summary Safety Review: Assessing the potential risk of cancer
Genito urinary system and sex hormones
For example, hormonal contraception, sex hormones and drugs for the urinary tract system.
2 New drugs were approved in this category
Intrarosa
- New active substance
- Medicinal Ingredient
- Prasterone
- Indication
- Intrarosa is used to treat postmenopausal women with vulvovaginal atrophy. At menopause, there can be a lack of sex hormones. This may cause the tissues of the vulva and vagina to become thin and dry. Below are the possible symptoms:
- vaginal dryness
- pain during sexual activity
- irritation
- itching
- Intrarosa is used to treat postmenopausal women with vulvovaginal atrophy. At menopause, there can be a lack of sex hormones. This may cause the tissues of the vulva and vagina to become thin and dry. Below are the possible symptoms:
Tibella
- New active substance
- Medicinal Ingredient
- Tibolone
- Indication
- Tibella is a hormone replacement therapy (HRT). It is used to treat some symptoms that occur when the level of estrogen produced by a woman's body drops after menopause. These symptoms can include hot flashes, flushing and night sweats.
- Tibella is used over a short term in women when more than 12 months have passed since their last period (called postmenopausal). Only women with an intact uterus should take Tibella.
7 New generic drugs were approved in this category
- 1 product containing cyproterone acetate, ethinyl estradiol
- 1 product containing ethinyl estradiol, norgestimate
- 1 product containing progesterone
- 1 product containing solifenacin succinate
- 2 products containing trospium chloride
- 1 product containing vardenafil
Safety updates published in this category
- Elmiron (pentosan polysulfate sodium): Health Product InfoWatch: Risk of pigmentary maculopathy
- Estradiol: Health Product InfoWatch: Risk of testicular cancer
- Fibristal (ulipristal acetate): Information Update: Health Canada safety review finds possible link between Fibristal and risk of liver injury
- Hormonal Birth Control Products (including oral contraceptive pills, transdermal patch, vaginal ring, intrauterine contraceptive device [IUD], and injectable contraception): Summary Safety Review: Assessing the potential risk of suicidal thoughts and behaviours (suicidality)
- Linessa 28 (desogestrel, ethinyl estradiol): Advisory: Labelling error may lead to patients receiving Linessa 28 birth control pills instead of Linessa 21
- Mifegymiso (mifepristone and misoprostol): Information Update: Health Canada approves updates to Mifegymiso prescribing information: Ultrasound no longer mandatory
- Propecia and Proscar (finasteride): Summary Safety Review: Assessing the potential risk of suicidal thoughts and/or behaviour (suicidal ideation)
Musculo-skeletal system
For example, drugs affecting the muscles, bones and joints, including anti-inflammatories and muscle relaxants.
1 New drug was approved in this category
Evenity
- Biologic drug
- New active substance
- Medicinal Ingredient
- Romosozumab
- Indication
- Evenity contains romosozumab, a medicine that helps build bone, which makes bone stronger and less likely to break. Evenity is used to treat osteoporosis in women after menopause who are at high risk of fracture (broken bone). Osteoporosis is a disease that makes your bones thin and fragile. Osteoporosis is most common in women following menopause. Many patients with osteoporosis have no symptoms, but they are still at risk of breaking bones because osteoporosis has made their bones weak.
7 New generic drugs were approved in this category
- 1 product containing baclofen
- 1 product containing cyclobenzaprine hydrochloride
- 1 product containing diphenhydramine hydrochloride, ibuprofen (non-prescription drug)
- 3 products containing febuxostat
- 1 product containing ibuprofen (non-prescription drug)
Safety update published in this category
- Uloric (febuxostat): Dear Healthcare Professional Letter: Increased risk of cardiovascular fatal outcomes
Nervous system
For example, drugs that affect the brain and the nervous system, including analgesics, antidepressants and anti-seizure medications.
5 New drugs were approved in this category
Combogesic
- Non-prescription ("over-the-counter") drug
- Medicinal Ingredients
- Acetaminophen, ibuprofen
- Indication
- Combogesic (Acetaminophen/Ibuprofen) is indicated for the:
- short term management of mild to moderate acute pain
- reduction of fever
- Combogesic (Acetaminophen/Ibuprofen) is indicated for the:
Emgality
- Biologic drug
- New active substance
- Medicinal Ingredient
- Galcanezumab
- Indication
- Emgality is a medicine used to prevent migraine in adults who have at least 4 migraine days per month.
Onpattro
- New active substance
- Orphan drug
- Priority review
- Medicinal Ingredient
- Patisiran
- Indication
- Onpattro is a medicine that treats the nervous system problems due to an illness which runs in families called hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis).
Onstryv
- New active substance
- Medicinal Ingredient
- Safinamide
- Indication
- Onstryv is used along with levodopa alone or with levodopa in combination with other medicines for Parkinson's disease, to treat the signs and symptoms of Parkinson's disease in adults having an "off" episode.
pdp-levetiracetam
- Pediatric indication
- Medicinal Ingredient
- Levetiracetam
- Indication
- pdp-levetiracetam is used to help reduce the number of your seizures, or your child's seizures when taken together with other seizure medications.
22 New generic drugs were approved in this category
- 1 product containing bupivacaine hydrochloride
- 5 products containing dexmedetomidine
- 1 product containing duloxetine
- 1 product containing eletriptan
- 1 product containing haloperidol
- 3 products containing hydromorphone hydrochloride
- 5 products containing lacosamide
- 1 product containing pregabalin
- 2 products containing rasagiline
- 1 product containing tramadol hydrochloride
- 1 product containing zolpidem tartrate
Safety updates published in this category
- Alertec (modafinil): Dear Healthcare Professional Letter: Alertec (modafinil) and the risk of congenital anomalies
- Cymbalta (duloxetine hydrochloride): Health Product InfoWatch: Risk of postpartum hemorrhage
- Fentora (fentanyl citrate): Health Product InfoWatch: Risks of respiratory depression, coma and death
- Gabapentin or pregabalin with opioids: Information Update: Health Canada advises Canadians to exercise caution when taking gabapentin or pregabalin with opioids
- Lamictal (lamotrigine): Health Product InfoWatch: Risk of Brugada-type ECG
- Propofol-containing products: Summary Safety Review: Assessing the potential risk of prolonged erection of the penis (priapism)
- Spinraza (nusinersen): Health Product InfoWatch: Risk of hydrocephalus
Respiratory system
For example, drugs that affect the respiratory system, including antihistamines.
1 New drug was approved in this category
Loratadine Soft Gelatin Capsules
- Non-prescription ("over-the-counter") drug
- Pediatric indication
- Medicinal Ingredient
- Loratadine
- Indication
- Loratadine Soft Gelatin Capsules provide:
- fast relief from symptoms of allergies, including sneezing, runny nose and itchy nose, as well as itchy watery red eyes, caused by exposure to seasonal allergens (trees, grass, and ragweed pollen) and perennial (year round) allergens (dust mites, animal dander and molds)
- fast relief of allergic skin conditions, such as skin itch and hives
- Loratadine Soft Gelatin Capsules provide:
2 New generic drugs were approved in this category
- 1 product containing budesonide
- 1 product containing cetirizine hydrochloride
Safety update published in this category
- Opioid-containing cough and cold products: Summary Safety Review: Assessing the potential risk of opioid use disorder and related harms in children and adolescents
Sensory organs
For example, drugs for the eyes.
3 New drugs were approved in this category
Cystadrops
- Orphan drug
- Pediatric indication
- Priority review
- Medicinal Ingredient
- Cysteamine
- Indication
- Cystadrops is used to reduce cysteine crystals in the surface of the eye (cornea) in adults and children from 2 years of age with cystinosis.
Netildex (With Preservative), Netildex (Without Preservative)
- Medicinal Ingredient
- Netilmicin
- Indication
- Netildex is used to reduce inflammation of the eye following cataract surgery. It is also used to reduce the risk of bacterial infection.
Oxervate
- Biologic drug
- New active substance
- Orphan drug
- Priority review
- Medicinal Ingredient
- Cenegermin
- Indication
- Oxervate is used to treat adults with moderate or severe "neurotrophic keratitis". This disease affects the cornea (the transparent layer in the front part of the eye) and causes defects. These defects will not heal on their own. They may get worse and turn into corneal ulcers. Oxervate helps the cornea heal.
4 New generic drugs were approved in this category
- 1 product containing gatifloxacin
- 1 product containing ketotifen
- 1 product containing moxifloxacin
- 1 product containing timolol, travoprost
Systemic hormonal preparations, excluding sex hormones and insulins
For example, drugs that affect the endocrine system, including thyroid hormones.
1 New drug was approved in this category
Baqsimi
- Aligned review
- Orphan drug
- Pediatric indication
- Medicinal Ingredient
- Glucagon
- Indication
- Baqsimi is a medicine used to treat severe low blood sugar. This is called severe hypoglycemia. It is used in adults and children who are older than 4 that take insulin for diabetes. It is used when the person is unable to swallow sugar.
4 New generic drugs were approved in this category
- 1 product containing carbetocin
- 1 product containing liothyronine
- 1 product containing methimazole
- 1 product containing teriparatide
Safety update published in this category
- Tapazole (methimazole) and generics: Summary Safety Review: Assessing the potential risk of inflammation of the pancreas (acute pancreatitis)
Various
For example, drugs unable to be classified into the other categories, such as diagnostic agents and drugs to treat high levels of potassium in the blood.
6 New drugs were approved in this category
Aridol
- Pediatric indication
- Medicinal Ingredient
- Mannitol
- Indication
- Aridol is a bronchial challenge test. It is used:
- in patients 6 years of age and older
- to check how your airways (bronchi) respond when you do not have typical asthma symptoms
- Aridol is used as part of the doctor's overall assessment for asthma.
- Aridol is a bronchial challenge test. It is used:
Galli Eo
- Medicinal Ingredient
- Gallium 68 Ga chloride
- Indication
- The gallium (68Ga) chloride eluate from the Galli Eo generator is not intended for direct administration to patients. The gallium (68Ga) chloride eluate is indicated for in vitro radiolabelling of radiopharmaceutical ligands for diagnostic procedures using positron emission tomography (PET).
Lokelma
- Aligned review
- New active substance
- Medicinal Ingredient
- Sodium zirconium cyclosilicate
- Indication
- To treat hyperkalemia in adults. Hyperkalemia means that there is a high level of potassium in the blood.
Lutathera
- Aligned review
- New active substance
- Orphan drug
- Priority review
- Medicinal Ingredient
- Lutetium (177Lu) oxodotreotide
- Indication
- Lutathera is a radiopharmaceutical medicine used for the treatment of certain tumors (gastroenteropancreatic neuroendocrine tumors) that have somatostatin receptors, which cannot be completely removed from your body by surgery, have spread in your body (metastatic) and no longer responds to your current treatment.
Netspot
- New active substance
- Orphan drug
- Medicinal Ingredient
- Dotatate
- Indication
- Netspot is a kit used to prepare the radiopharmaceutical (radioactive) product gallium (68Ga) dotatate injection, which is used to find and diagnose certain cancer tumors called somatostatin receptor positive neuroendocrine tumors (NETs).
SSP+
- Medicinal Ingredients
- Disodium phosphate, magnesium chloride, potassium chloride, sodium acetate, sodium chloride, sodium citrate, sodium phosphate monobasic
- Indication
- SSP+ is a solution designed to:
- Be used by a healthcare professional to partially replace plasma in the preparation and storage of platelets concentrates obtained either by buffy coat pooling or by apheresis device.
- Enable platelets to be stored in a mix of SSP+ and plasma at 20°C – 24°C, under gentle agitation, for up to 7 days following collection and according to local regulations.
- SSP+ is a solution designed to:
3 New generic drugs were approved in this category
- 1 product containing deferasirox
- 1 product containing leucovorin
- 1 product containing sincalide
Safety updates published in this category
- Ferriprox (deferiprone): Summary Safety Review: Assessing the potential risk of brain and nervous system (neurological) disorders in children
- Sartans, ranitidine, and other potential drugs: Information Update: Health Canada updates Canadians on its ongoing assessment of nitrosamine impurities in certain drugs
Medical devices: Life cycle
As part of Health Canada's mission to help Canadians maintain and improve their health, we evaluate medical devices before and after they reach the Canadian market. Health Canada is involved throughout the life cycle of a medical device, from investigational testing to after the device is being sold in Canada.
Clinical trials (investigational testing)
Clinical trials are conducted by sponsors (manufacturers or importers) to gather information on a medical device's safety and effectiveness in humans. Sponsors of investigational tests submit their applications to conduct investigational testing with a medical device in Canada. Health Canada reviews these applications before the testing is conducted in Canada.
Special Access Programme
Medical devices that are not approved in Canada may be available through our Special Access Programme. In this program, access is given to an individual healthcare practitioner who is treating a specific patient. Access may be granted for emergency use, or when conventional therapies have failed, are unavailable or are unsuitable.
Medical device application and review
In Canada, medical devices are categorized in four groups based on their risk of use. These groups are called "Classes" and range from I to IV. Class I devices are considered low-risk devices – for example, a wheelchair. Class IV devices present the greatest potential risk – for example, a defibrillator.
When a company decides that it would like to market a Class II, III or IV medical device in Canada, it files an application to Health Canada for a new medical device licence. The application contains scientific information about the medical device's safety, effectiveness and quality. Class I devices do not require a medical device licence, but are monitored through establishment licences.
Applications for higher-risk medical devices are reviewed by our scientists and engineers. Sometimes we also consult with advisory committees or external consultants. Reviewers evaluate the safety, effectiveness and quality data submitted to assess the potential benefits and risks of the medical devices. They also review the information that will be provided to healthcare practitioners and consumers about the medical device.
Expedited review pathway: Priority review
The priority review pathway provides an expedited path to a final decision for certain medical devices, including those that target specific healthcare needs. Medical devices for serious, life-threatening, or severely debilitating diseases or conditions can be given a priority review status. Products approved through expedited review pathways can be available to patients sooner because they have shorter review targets.
Approval of medical devices
After its review of a medical device application, Health Canada may conclude that the benefits of the product outweigh the potential risks and approve the device for sale in Canada. When a new medical device is approved, it is issued a medical device licence. This does not mean the medical device will immediately be available to patients, as many other factors can influence that timeline.
Surveillance
It is not possible to know or predict all of the possible problems related to a medical device through clinical studies. After a product is approved and available for sale in Canada, we continue to monitor its use in the real world, that is, in the broader Canadian population. We evaluate potential safety and effectiveness issues and take action when there are identified problems.
Collecting information
Health Canada collects safety information about a product after it is approved from a variety of sources. One source of information is suspected medical device problems that are reported after products are approved for sale. These are undesirable effects potentially caused by medical devices.
Evaluating safety signals
Health Canada evaluates the data we collect to detect new safety signals, which we then investigate more closely. A "safety signal" can be defined as information on a new or known adverse event that may be associated with a medical device. These investigations are called signal assessments and they may result in recommendations for actions to be taken by the company, by Health Canada, or both. These actions can include informing the public and healthcare professionals of new safety information or recommending labelling changes. In the most serious situations, we may remove a medical device from the market.
Advertising complaints
Health Canada also regulates the advertising of medical devices in Canada to ensure that companies are not making false claims about their products. We review advertising complaints to determine if a company is complying with our requirements, and we take appropriate action when non-compliance is identified.
Medical devices: Approved in 2019
There are different classes of medical devices, ranging from Class I to IV. Class I devices are considered low-risk devices, for example, a tongue depressor. Class IV devices present the greatest potential risk, for example, a pacemaker.
This section outlines the new Class IV medical devices approved for sale in Canada in 2019, and the safety updates issued.
You can report medical device incidents to your medical professional, to a hospital or to the company that made the product. You can also report them to Health Canada through the Canada Vigilance Program or by phone at 1-866-234-2345.
Health categories
The medical devices listed have been divided into categories according to the Global Medical Device Nomenclature (GMDN) system for naming and grouping medical devices.
We have included the indication of each new medical device to give you some additional information. In addition, each new device has a hyperlink to the Decision Summary (when available). These documents provide a brief overview of the rationale for our decision to approve the medical device.
The categories are
- Anaesthesia and respiratory devices – for example, inhalation therapy devices.
- Blood fluid and tissue management devices – for example, photopheresis systems.
- Body tissue manipulation and reparation devices – for example, bone matrix implants, sutures and robotic surgical systems.
- Cardiovascular devices – for example, cardiovascular catheters, vascular grafts and haemostatic agents.
- Dental devices – for example, dental bone matrix implants.
- Disability-assistive products – for example, hearing implant systems.
- Gastro-urological devices – for example, intragastric balloons.
- General hospital devices – for example, infusion sets and insulin pumps.
- In vitro diagnostic medical devices – for example, instrument/analyser and viral infection disease in vitro devices.
- Neurological devices – for example, neurological monitors/monitoring systems and surgical procedure kits.
- Obstetrical/gynaecological devices – for example, tampons and fetal monitors.
- Plastic surgery and cosmetic devices – for example, tissue expanders.
- Various – applicable to medical devices generally.
Important definitions
Medical device
Medical devices are products that are used for diagnostic and/or therapeutic purposes. Newly approved medical devices provide a broader range of products used to treat, manage, diagnose or prevent a disease or a physical condition.
Licence with conditions
A medical device licence may be issued with conditions set out by Health Canada. For example, the manufacturer may be required to submit additional information on an on-going basis for the medical device to demonstrate that it continues to meet our regulatory requirements.
Safety updates
Safety updates are designed to communicate information about potential health risks, so that patients and healthcare professionals can make informed decisions about their health.
For more information about the types of risk communications that can be found on the Government of Canada's website, go to "Healthy clicks – Medical devices at a glance".
Figure 2: New class IV medical devices approved in 2019
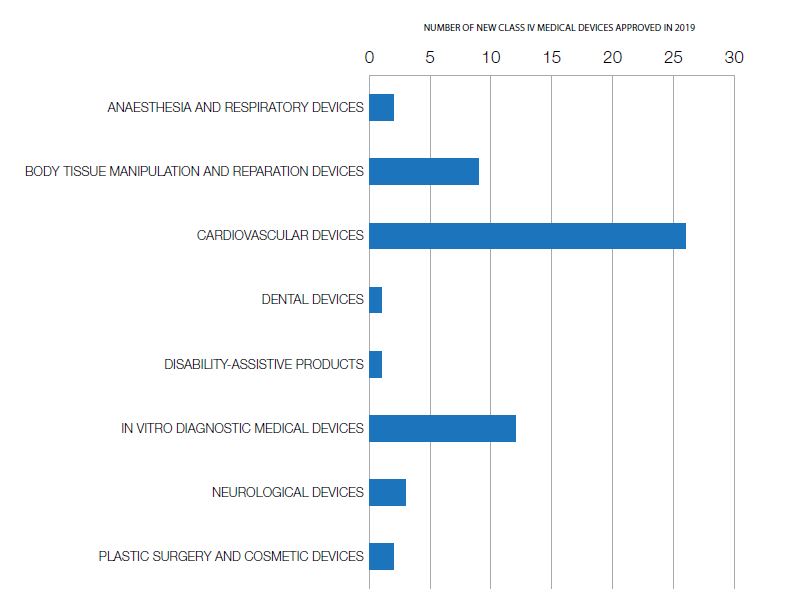
Text description
Number of new Class IV medical devices approved in 2019, according to the Global Medical Device Nomenclature (GMDN) system for naming and grouping medical devices.
| Health Category | Number of Class IV devices approved |
|---|---|
| Anaesthesia and respiratory devices | 2 |
| Body tissue manipulation and reparation devices | 9 |
| Cardiovascular devices | 26 |
| Dental devices | 1 |
| Disability-assistive products | 1 |
| In vitro diagnostic medical devices | 12 |
| Neurological devices | 3 |
| Plastic surgery and cosmetic devices | 2 |
| Total new Class IV medical devices approved in 2018 | 56 |
Anaesthesia and respiratory devices
For example, inhalation therapy devices.
2 New medical devices were approved in this category
FreeO2 Automated Oxygen Therapy Device
FreeO2 Automated Oxygen Therapy Device Decision Summary
- Indication
- The FreeO2 Automated Oxygen Therapy Device (FreeO2), which provides oxygen therapy on demand, based on continuous, non-invasive monitoring of oxygen saturation, is indicated for use under the direction of a physician in a clinical or hospital environment, on spontaneously breathing neonatal, pediatric, and adult patients who are prescribed supplemental oxygen via nasal cannula or face mask.
Intellivent-ASV Software
Intellivent-ASV Software Decision Summary
- Indication
- The Intellivent-ASV feature is a software module that creates a closed-loop system. It controls the automatic adjustment of the minute volume (% MinVol), positive end expiratory pressure (PEEP), and fractional inspired oxygen (FiO2). This closed-loop ventilation mode automatically adjusts the ventilation and oxygenation in both passive and active patients according to the pre-set settings entered by the clinician.
Blood fluid and tissue management devices
For example, photopheresis systems.
Safety update published in this category
- Cellex Photopheresis System: Summary Safety Review: Venous thromboembolism and pulmonary embolism
Body tissue manipulation and reparation devices
For example, bone matrix implants, sutures and robotic surgical systems.
9 New medical devices were approved in this category
da Vinci X Surgical System
da Vinci X Surgical System Decision Summary
- Indication
- The Intuitive Surgical Endoscopic Instrument Control System (da Vinci Surgical System) is intended to assist in the accurate control of Intuitive Surgical Endoscopic Instruments during urologic surgical procedures, general laparoscopic surgical procedures, gynecologic laparoscopic surgical procedures, general thoracoscopic surgical procedures, thoracoscopically-assisted cardiotomy procedures and transoral otolaryngology surgical procedures, restricted to benign tumors and malignant tumors classified as T1 and T2, and for benign base of tongue resection procedures. The system can also be employed with adjunctive mediastinotomy to perform coronary anastomosis during cardiac revascularization.
DSM Biomedical Calcium Phosphate Cement
DSM Biomedical Calcium Phosphate Cement Decision Summary
- Indication
- DSM Biomedical Calcium Phosphate Cement is indicated to fill bony voids or gaps of the skeletal system (i.e. extremities and pelvis). These defects may be surgically created or osseous defects created from traumatic injury to the bone.
DSM Biomedical Calcium Phosphate Cement With Microspheres
DSM Biomedical Calcium Phosphate Cement With Microspheres Decision Summary
- Indication
- DSM Biomedical Calcium Phosphate Cement with Microspheres is indicated to fill bony voids or gaps of the skeletal system (i.e. extremities and pelvis). These defects may be surgically created or osseous defects created from traumatic injury to the bone.
Endoform Antimicrobial Dermal Template
Endoform Antimicrobial Dermal Template Decision Summary
- Indication
- Endoform Antimicrobial Dermal Template is indicated for the management of wounds including:
- Partial and full thickness wounds
- Pressure ulcers
- Venous ulcers
- Diabetic ulcers
- Chronic vascular ulcers
- Tunneled/ undermined wounds
- Surgical wounds (donor sites, grafts, post Mohs surgery, post laser surgery, podiatric, wound dehiscence)
- Traumatic wounds (abrasions, lacerations, first and second degree burns, and skin tears)
- Draining wounds
- Endoform Antimicrobial Dermal Template is indicated for the management of wounds including:
Filasilk Sterilised Surgical Needled Suture
Filasilk Sterilised Surgical Needled Suture Decision Summary
- Indication
- Filasilk Sutures is intended for use as non-absorbable sutures in general soft tissue approximation and/or ligation, including use in cardiac, vascular, ophthalmic, and skin tissue closure.
Filasilk Sterilised Surgical Suture
Filasilk Sterilised Surgical Suture Decision Summary
- Indication
- Filasilk Sutures is intended for use as non-absorbable sutures in general soft tissue approximation and/or ligation, including use in cardiac, vascular, ophthalmic, and skin tissue closure.
Gentrix Surgical Matrix
Gentrix Surgical Matrix Decision Summary
- Indication
- Gentrix Surgical Matrix Plus is intended for implantation to reinforce soft tissue where weakness exists in patients requiring gastroenterological or plastic and reconstructive surgery. Reinforcement of soft tissue within gastroenterological and plastic and reconstructive surgery includes, but is not limited to, the following procedures: hernia and body wall repair, colon and rectal prolapse repair, tissue repair, and esophageal repair.
Mericron XL Sterilised Surgical Needled Suture
Mericron XL Sterilised Surgical Needled Suture Decision Summary
- Indication
- Mericron XL suture is intended for use in general soft tissue approximation and/or ligation including cardiovascular surgery, neurosurgery and ophthalmic procedure.
Optium DBM Gel
Optium DBM Gel Decision Summary
- Indication
- Optium DBM Gel is intended for filling bony voids or gaps that are not intrinsic to the stability of the bony structure. It may be placed into the bony voids or gaps of the skeletal system (for example, the extremities, spine and pelvis). These defects may be surgically created osseous defects or osseous defects created from traumatic injury to the bone.
Safety update published in this category
- Surgical mesh products: Summary Safety Review: Assessing the potential risk of complications associated with transvaginal implantation of non-absorbable synthetic surgical mesh for the treatment of pelvic organ prolapse (POP)
Cardiovascular devices
For example, cardiovascular catheters, vascular grafts and haemostatic agents.
26 New medical devices were approved in this category
Acticor
- Indication
- Acticor can treat life-threatening ventricular arrhythmias with antitachycardia pacing and defibrillation.
Amplatzer Valvular Plug III
- Licence with conditions
Amplatzer Valvular Plug III Decision Summary
- Indication
- The Amplatzer Valvular Plug III (AVP III) is intended for percutaneous, transcatheter closure of a paravalvular leak that has developed after an aortic or mitral surgical valve implant procedure. The Amplatzer Valvular Plug III is intended for use in patients with a clinically significant paravalvular leak (showing signs of heart failure and/or paravalvular leak-associated hemolysis necessitating recurring blood transfusions).
Arctic Front Advance Pro
Arctic Front Advance Pro Decision Summary
- Indication
- The Arctic Front Advance Pro Cardiac Cryoablation Catheter is indicated for the treatment of patients with atrial fibrillation.
Edwards Sapien 3 Ultra Transcatheter Heart Valve Systems
- Licence with conditions
Edwards Sapien 3 Ultra Transcatheter Heart Valve Systems Decision Summary
- Indication
- The Edwards Sapien 3 Ultra Transcatheter Heart Valve System is a heart valve delivered by catheter for replacement of the native aortic or failing aortic or mitral bioprosthetic valve.
Figulla Flex II PFO
- Licence with conditions
Figulla Flex II PFO Decision Summary
- Indication
- The Figulla Flex II PFO is indicated for percutaneous transcatheter closure of a patent foramen ovale (PFO) to reduce the risk of recurrent ischemic stroke in patients who have had a cryptogenic stroke due to a presumed paradoxical embolism, as determined by a neurologist and cardiologist, following an evaluation to exclude known causes of ischemic stroke.
Flow Re-Direction Endoluminal Device (Fred) System
Flow Re-Direction Endoluminal Device (Fred) System Decision Summary
- Indication
- The Flow Re-Direction Endoluminal Device (FRED) System is indicated for use with or without embolic coils for the treatment of intracranial aneurysms that are not amenable to treatment with surgical clipping with parent vessels that are ≥2.0 mm and ≤ 5.0 mm in diameter.
Gore Molding & Occlusion Balloon Catheter
Gore Molding & Occlusion Balloon Catheter Decision Summary
- Indication
- The Gore Molding and Occlusion Balloon Catheter (MOB) is intended for temporary occlusion of large diameter vessels or to assist the expansion of self-expanding endovascular prostheses (stent grafts).
Gore Viabahn VBX Balloon Expandable Endoprosthesis
Gore Viabahn VBX Balloon Expandable Endoprosthesis Decision Summary
- Indication
- The Gore Viabahn VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm - 13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation.
Ilivia Neo 7 VR-T, VR-T DX, DR-T, HF-T, HF-T QP
Ilivia Neo 7 VR-T, VR-T DX, DR-T, HF-T, HF-T QP Decision Summary
- Indication
- Ilivia Neo can treat life-threatening ventricular arrhythmias with antitachycardia pacing and defibrillation.
Mitraclip G4 System
- Licence with conditions
Mitraclip G4 System Decision Summary
- Indication
- The MitraClip G4 Clip Delivery System is indicated for the percutaneous reduction of significant symptomatic degenerative mitral regurgitation (MR ≥ 3+) in patients who have been determined to be too high risk for mitral valve surgery by a heart team, including a cardiac surgeon, and in whom existing co-morbidities would not preclude the expected benefit from correction of the mitral regurgitation.
Novasight Hybrid System
Novasight Hybrid System Decision Summary
- Indication
- The Novasight Hybrid System is intended for intravascular imaging of coronary arteries and is indicated in patients who are candidates for transluminal interventional procedures.
Optowire III Pressure Guidewire
Optowire III Pressure Guidewire Decision Summary
- Indication
- The OptoWire III pressure guidewire is indicated for use to measure pressure in blood vessels including both coronary and peripheral vessels, during diagnostic angiography and/or any interventional procedures.
Plexa and Plexa (ProMri) ICD Leads
Plexa and Plexa (ProMri) ICD Leads Decision Summary
- Indication
- In combination with a compatible ICD, the ICD leads are intended for the following:
- Permanent pacing and sensing in the right ventricle
- Delivery of defibrillation/cardioversion therapies
- With its active fixation screw, this lead is especially suitable for patients with degenerated trabeculae in the ventricle for whom passive fixation with silicone or polyurethane tines is not possible.
- In addition to the aforementioned indications, Plexa (ProMRI) DF-1 S DX also has the following indication:
- Permanent sensing the right atrium
- ICD leads with two dipoles for sensing in both chambers are especially indicated for patients who, in addition to the usual ICD indications, have documented paroxysmal atrial fibrillation.
- In combination with a compatible ICD, the ICD leads are intended for the following:
Promus ELITE Monorail Everolimus-Eluting Platinum Chromium Coronary Stent System
Promus ELITE Monorail Everolimus-Eluting Platinum Chromium Coronary Stent System Decision Summary
- Indication
- The Promus ELITE Everolimus-Eluting Platinum Chromium Coronary Stent System is indicated for improving coronary luminal diameter in patients with symptomatic ischemic heart disease, due to discrete de novo native coronary artery lesions.
PuraStat
- Licence with conditions
- Indication
- PuraStat is indicated for hemostatsis in the following situations encountered during surgery, when hemostatsis by ligation or standard means is insufficient or impractical:
- Bleeding from small blood vessels and oozing from capillaries of parenchyma of solid organs
- Oozing from vascular anastomoses
- Bleeding from small vessels and oozing from capillaries of the GI tract
- PuraStat is indicated for hemostatsis in the following situations encountered during surgery, when hemostatsis by ligation or standard means is insufficient or impractical:
Reprocessed ViewFlex Xtra Ice Catheter
Reprocessed ViewFlex Xtra Ice Catheter Decision Summary
- Indication
- The Reprocessed ViewFlex Xtra ICE Catheter is indicated for use in adult and adolescent pediatric patients to visualize cardiac structures, blood flow and other devices within the heart.
Resolute Onyx Zotarolimus-Eluting Coronary Stent System
- Licence with conditions
Resolute Onyx Zotarolimus-Eluting Coronary Stent System Decision Summary
- Indication
- The Resolute Onyx Zotarolimus-Eluting Coronary Stent System is indicated for improving coronary luminal diameter in one or two vessels and reducing restenosis in patients with symptomatic ischemic heart disease in de novo coronary artery lesions in native coronary arteries with a reference vessel diameter of 2.0 mm to 5.0 mm and a lesion length of ≤35 mm.
RotaPro Rotational Atherectomy System
- Indication
- Percutaneous rotational coronary atherectomy with the RotaPro Rotational Atherectomy System, as a sole therapy or with adjunctive percutaneous coronary intervention (PCI) is indicated in patients with calcific coronary artery disease who meet one of the following selection criteria:
- Single vessel atherosclerotic coronary artery disease with a stenosis that can be passed with a guidewire;
- Multiple vessel coronary artery disease that in the physician's judgment does not pose undue risk to the patient;
- Patients who have had prior PCI, and who have native coronary artery post-balloon angioplasty restenosis; or,
- Native vessel atherosclerotic coronary artery disease that is less than 25 mm in length.
- Percutaneous rotational coronary atherectomy with the RotaPro Rotational Atherectomy System, as a sole therapy or with adjunctive percutaneous coronary intervention (PCI) is indicated in patients with calcific coronary artery disease who meet one of the following selection criteria:
Soundbite Crossing System – Peripheral
- Licence with conditions
- Indication
- The SoundBite Crossing System – Peripheral is indicated to facilitate the intra-luminal placement of conventional guidewires or treatment devices beyond peripheral artery chronic total occlusions via atherectomy.
Squid Liquid Embolic Agent
Squid Liquid Embolic Agent Decision Summary
- Indication
- The device is sold under two names: Squid (18 and 34) and Squidperi (18 and 34) but the products are the same; they only differ in the labelled indications. Squid is indicated for the embolisation of lesions in the peripheral and neurovasculature, including arteriovenous malformations and hypervascular tumours. Squidperi is indicated for the embolisation of lesions in the peripheral vasculature, including arteriovenous malformations and hypervascular tumours.
Stellarex 0.035 inch OTW Drug-Coated Angioplasty Balloon
- Licence with conditions
Stellarex 0.035 inch OTW Drug-Coated Angioplasty Balloon Decision Summary
- Indication
- The Stellarex 0.035 Over-The-Wire (OTW) Drug-coated Angioplasty Balloon is a drug-coated balloon catheter designed for percutaneous treatment of peripheral vascular artery lesions to improve blood flow.
True Dilatation Balloon Valvuloplasty Catheter
True Dilatation Balloon Valvuloplasty Catheter Decision Summary
- Indication
- The True Dilatation Balloon Valvuloplasty Catheter is indicated for balloon aortic valvuloplasty.
Valiant Navion Thoracic Stent Graft System
Valiant Navion Thoracic Stent Graft System Decision Summary
- Indication
- The Valiant Navion thoracic stent graft system is indicated for the endovascular repair of all lesions of the descending thoracic aorta (DTA) in patients having the appropriate anatomy.
Versacross RF Wire
Versacross RF Wire Decision Summary
- Indication
- The VersaCross RF Wire is indicated for creation of an atrial septal defect in the heart.
Watchman FLX Left Atrial Appendage Closure Device with Delivery System
- Licence with conditions
Watchman FLX Left Atrial Appendage Closure Device with Delivery System Decision Summary
- Indication
- The Watchman FLX Left Atrial Appendage Closure Device With Delivery System is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with non-valvular atrial fibrillation who:
- are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc1 scores and are recommended for anticoagulation therapy;
- are deemed by their physicians to be suitable for anticoagulation therapy; and
- have an appropriate rationale to seek a non-pharmacologic alternative to anticoagulation therapy, taking into account the safety and effectiveness of the device compared to anticoagulation therapy.
- The Watchman FLX Left Atrial Appendage Closure Device With Delivery System is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with non-valvular atrial fibrillation who:
Xience Sierra Everolimus Eluting Coronary Stent System
Xience Sierra Everolimus Eluting Coronary Stent System Decision Summary
- Indication
- The Xience Sierra stent system is indicated for improving coronary artery luminal diameter in patients, including those with diabetes mellitus, with symptomatic heart disease due to de novo native coronary artery lesions (length ≤ 32 mm) with reference vessel diameters of ≥ 2.25 mm to ≤ 4.25 mm. In addition, the Xience Sierra stent system is indicated for treating de novo chronic total coronary occlusions.
Safety update published in this category
- Paclitaxel‐Coated Balloons (PCB) and Paclitaxel-Eluting Stents (PES) for the treatment of peripheral arterial disease (PAD): Dear Healthcare Professional Letter: Potential risk of long-term all-cause mortality
Dental devices
For example, dental bone matrix implants.
1 New medical device was approved in this category
Synthetic Mineral Collagen Composite Dental Bone Graft Matrix
Synthetic Mineral Collagen Composite Dental Bone Graft Matrix Decision Summary
- Indication
- SynOss Putty and RegenerOss Synthetic are recommended for:
- Augmentation or reconstructive treatment of the alveolar ridge.
- Filling of periodontal defects.
- Filling of endodontic or open defects after pulp removal, root resection or apicoectomy to enhance stabilization of the tooth.
- Filling of defects after cystectomy.
- Filling of extraction sockets to enhance preservation of the alveolar ridge.
- Elevation of the maxillary sinus floor.
- Filling of periodontal defects in conjunction with products intended for Guided Tissue Regeneration (GTR) and Guided Bone Regeneration (GBR).
- Filling of peri-implant defects in conjunction with products intended for Guided Bone Regeneration (GBR).
- SynOss Putty and RegenerOss Synthetic are recommended for:
Disability-assistive products
For example, hearing implant systems.
1 New medical device was approved in this category
MI1200 Synchrony Auditory Brainstem Implant
- Indication
- The Synchrony Auditory Brainstem Implant (ABI) is used for electrical stimulation of the cochlear nucleus (CN) via an implanted stimulator and a specially designed electrode array to evoke auditory sensations in patients with non-functional cochlear nerves. The Synchrony ABI is indicated for patients 15 years of age or older who have been diagnosed with neurofibromatosis type 2 (NF2) when both cochlear nerves are non‑functional, or are anticipated to be rendered non‑functional by presence or removal of tumour. The surgical procedure for the implantation of the device shall be concurrent with tumour removal surgery.
Gastro-urological devices
For example, intragastric balloons.
Safety update published in this category
- Intragastric balloons: Summary Safety Review: Assessing the potential risk of inflamed pancreas, developing a hole (perforation) in the esophagus and stomach, and unexpected balloon enlargement (overinflation)
General hospital devices
For example, infusion sets and insulin pumps.
Safety updates published in this category
- Alaris Infusion Sets and Alaris 8100 Pump Module: Dear Healthcare Professional Letter: Risk of over-infusion and Dear Healthcare Professional Letter: Risk of over-infusion; expanded list of affected products
- Medtronic MiniMed insulin pumps: Advisory: Certain older Medtronic MiniMed insulin pumps may be vulnerable to cybersecurity risks
In vitro diagnostic medical devices
For example, instrument/analyser and viral infection disease in vitro devices.
12 New medical devices were approved in this category
Alinity s System
Alinity s System Decision Summary
- Indication
- The Alinity s System is a high-throughput, fully-automated immunoassay analyzer designed to determine the presence of specific antigens and antibodies by using chemiluminescent immunoassay technology.
Alinity s Anti-HBc Assay (Donor Screening & Cadaveric Testing)
Alinity s Anti-HBc Assay (Donor Screening & Cadaveric Testing) Decision Summary
- Indication
- The Alinity s Anti-HBc Assay is a chemiluminescent microparticle immunoassay (CMIA), performed on the fully automated licensed Alinity s System, for the qualitative detection of Anti-HBc in human serum and plasma for blood donor screening and cadaveric testing.
Alinity s Anti-HCV (Donor Screening & Cadaveric Testing)
- Indication
- The Alinity s Anti-HCV Assay, a two-step immunoassay for the qualitative detection of anti-HCV in human serum and plasma using chemiluminescent microparticle immunoassay (CMIA) technology, is intended to be run on the Alinity s analyzer (licence # 103483) as is intended for blood donor screening and screening of cadaveric donors.
Alinity s Chagas Assay (Donor Screening & Cadaveric Testing)
- Indication
- The Alinity s Chagas assay is a chemiluminescent microparticle immunoassay (CMIA) used for the qualitative detection of antibodies to Trypanosoma cruzi (the causative agent of Chagas disease) in human serum and plasma specimens on the Alinity s System. The Alinity s Chagas assay is intended for blood donor screening and screening of cadaveric donors.
Alinity s HBsAg Assay (Donor Screening/Cadaveric)
Alinity s HBsAg Assay (Donor Screening/Cadaveric) Decision Summary
- Indication
- The Alinity s HBsAg assay is a chemiluminescent microparticle immunoassay (CMIA), performed on the fully automated licensed Alinity s System, for the qualitative detection of HBsAg human serum and plasma for blood donor screening and cadaveric testing.
Alinity s HBsAg Confirmatory Reagent Kit
Alinity s HBsAg Confirmatory Reagent Kit Decision Summary
- Indication
- The Alinity HBsAg Confirmatory assay is used to confirm the presence of hepatitis B surface antigen (HBsAg) in human serum and plasma, using chemiluminescent microparticle immunoassay (CMIA) technology.
Alinity s HTLV I/II Assay (Donor Screening & Cadaveric Testing)
- Indication
- The Alinity s HTLV I/II assay is a chemiluminescent microparticle immunoassay (CMIA), performed on the fully automated licensed Alinity s System, for the qualitative detection of antibodies to HTLV I/II in human serum and plasma for blood donor screening and cadaveric testing.
Beckman Coulter PK7400 Automated Microplate System
Beckman Coulter PK7400 Automated Microplate System Decision Summary
- Indication
- The Beckman Coulter PK7400 Automated Microplate System is a high throughput, fully automated, batch test system that uses agglutination technology with Beckman Coulter terraced microplates. The PK7400 performs donor ABO blood grouping, Rh typing, including weak D testing, red blood cell antigen screening, and syphilis qualitative screening. This system is for in vitro diagnostic use only.
Elecsys Chagas (Donor Screening)
- Indication
- The Elecsys Chagas Assay is an in vitro diagnostic test for the qualitative determination of antibodies to Trypanosoma cruzi (T. cruzi, the causative agent of the Chagas disease) in human serum and plasma.
NEO Iris
- Indication
- The NEO Iris is designed to automate standard immunohematology assays using a microplate-based platform. Assays include ABO grouping and Rh (D) typing, detection/identification of IgG red blood cell antibodies, compatibility testing, red blood cell phenotyping, antigen screening and infectious disease screening, such as cytomegalovirus (CMV).
Procleix Zika Virus Assay (Donor Screening/Cadaveric Testing)
- Licence with conditions
Procleix Zika Virus Assay (Donor Screening/Cadaveric Testing) Decision Summary
- Indication
- The Procleix Zika Virus Assay is a qualitative in vitro nucleic acid test for the detection of Zika virus (ZIKV) RNA in plasma and serum specimens from individual human donors, including volunteer donors of whole blood and blood components for transfusion. It is also intended for use in testing plasma or serum specimens to screen other living (heart-beating) donors of organs and Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps), and in testing blood specimens to screen cadaveric (non-heart-beating) donors.
Virotrol Plus-R
Virotrol Plus-R Decision Summary
- Indication
- Virotrol Plus-R is intended for use as an unassayed reactive quality control with in vitro assay procedures for determination of antibodies to Human Immunodeficiency Virus Type ½ (HIV-1/2), antibodies to Human T-Lymphotropic Virus Type I/II (HTLV-I/II), antibodies to Hepatitis C Virus (HCV), Hepatitis B Surface Antigen (HbsAg), antibodies to Hepatitis B Core Antigen (HBc), antibodies to Cytomegalovirus (CMV) and total antibodies to Treponema pallidum.
Neurological devices
For example, neurological monitors/monitoring systems and surgical procedure kits.
3 New medical devices were approved in this category
Codman CereLink ICP Monitoring System
Codman CereLink ICP Monitoring System Decision Summary
- Indication
- The Codman CereLink ICP Monitoring System consisting of CereLink ICP Sensors and a CereLink ICP Monitor with associated cables and kits are used for direct intracranial (intraparenchymal, subdural, and intraventricular) pressure monitoring and ventricular drainage.
Neurology Pack
Neurology Pack Decision Summary
- Indication
- The intended use of this pack is to aid in the neurological medical/surgical procedure of Neurosurgery of the back and cervical disc performed by a Neurosurgeon.
Spectra Wavewriter Spinal Cord Stimulator System
- Indication
- The Spectra Wavewriter Spinal Cord Stimulator System is indicated as an aid in the management of chronic intractable pain.
Obstetrical/gynaecological devices
For example, tampons and fetal monitors.
Safety updates published in this category
- Avalon Fetal Monitor: Summary Safety Review: Assessing the potential risk of inaccurate heart rate tracking of unborn babies (fetuses)
- U by Kotex Sleek and U by Kotex Click Tampons: Summary Safety Review: Assessing the risk of tampons coming apart during removal and potential adverse reactions (side effects)
Plastic surgery and cosmetic devices
For example, tissue expanders.
2 New medical devices were approved in this category
CPX4 Breast Tissue Expander Smooth
- Licence with conditions
CPX4 Breast Tissue Expander Smooth Decision Summary
- Indication
- The CPX4 Breast Tissue Expander Smooth is intended for breast reconstruction after mastectomy, correction of an underdeveloped breast, scar revision and tissue defect procedures.
Natrelle 133S Tissue Expander
Natrelle 133S Tissue Expander Decision Summary
- Indication
- The Natrelle 133S Tissue Expander is intended for temporary use following mastectomy or to correct an underdeveloped breast, scar revision, and tissue defect procedures.
Safety updates published in this category
- Biocell breast implants: Information Update: Health Canada advises Allergan of its intent to suspend its licences for Biocell breast implants as a precautionary measure and Summary Safety Review: Assessing the potential risk of cancer (Breast implant associated anaplastic large cell lymphoma)
- Breast implants: Information Update: Health Canada will be updating its safety review of breast implants
Various
Applicable to medical devices generally.
Safety update published in this category
- Medical devices: Information Update: Health Canada encourages Canadians to report incidents involving medical devices to help strengthen patient safety
Drugs for veterinary use: Life cycle
Every year, we approve new veterinary drugs in Canada, giving more choices to help maintain and improve the health and well-being of animals. We work to protect human and animal health and the safety of Canada's food supply.
As part of our work to protect human and animal health and the safety of Canada's food supply, we evaluate veterinary drugs throughout the life cycle of a drug for veterinary use, from clinical trials to after the drug is being sold in Canada.
Clinical trials
Experimental studies certificates and investigational new drug applications support the development of potential new veterinary drugs or new uses of already approved products. Sponsors of clinical trials (including manufacturers and researchers) submit their applications to conduct a trial with a veterinary drug in Canada. Health Canada reviews these applications before the trial is conducted in Canada.
Emergency Drug Release Program
Drugs that are not approved in Canada may be available through our Emergency Drug Release program. This program gives access to veterinary drugs for the purpose of diagnosing or treating a medical emergency. Animals receiving drugs through this program must be under the care of a veterinarian.
Drug submission and review
When a company decides that it would like to market a veterinary drug in Canada, it files a submission with Health Canada. A new drug submission contains detailed scientific information about the drug's safety, effectiveness and quality.
Submissions for drugs are reviewed by our scientists to assess the potential benefits and risks to human and animal health. They also help to ensure veterinary drug labels have clear directions for use and warning statements.
Many veterinary drugs are intended for use in food-producing animals such as cattle, chickens and pigs. We work to ensure the safety of food that comes from animals treated with veterinary drugs. We do this by setting standards such as Maximum Residue Limits for veterinary drugs in foods, and establishing withdrawal periods.
Innovative reviews (international regulatory cooperation)
Through international regulatory cooperation, we conduct reviews of submissions for new and generic veterinary drugs with certain partners. These include the United States Food and Drug Administration Center for Veterinary Medicines, the Australian Pesticides and Veterinary Medicines Authority, and the New Zealand Ministry of Primary Industries.
These collaborative reviews bring veterinary drugs to market in Canada at the same time as in other countries, which would otherwise not be possible. As a result, companion animal owners, veterinarians and producers have faster access to safe, effective and quality veterinary drugs.
Approval of drugs
After its review of a drug submission, Health Canada may conclude that the benefits of the product outweigh the potential risks and approve the veterinary drug for sale in Canada. When a new veterinary drug is approved, it is issued a Notice of Compliance (NOC) and a Drug Identification Number (DIN). This does not mean the drug will immediately be available in Canada, as many other factors can influence that timeline.
Surveillance
After Health Canada approves a veterinary drug, we continue to monitor its use in the real world. We evaluate potential safety and effectiveness issues, and take action when there are identified problems.
Collecting information
Health Canada collects information about a product after it is approved from a variety of sources.
One source of information is suspected adverse events that are reported after products are approved for sale. Adverse events are unwanted or harmful events that occur after administration of a veterinary drug. Included are:
- adverse reactions or events in animals,
- adverse reactions or events in humans who administered a veterinary drug to an animal, and
- events that result from a suspected lack of effect.
Evaluating safety signals
Health Canada evaluates adverse event reports submitted by manufacturers and the public (including animal owners and veterinarians), to find out if they are related to the administered drug(s). We work with manufacturers and veterinarians to ensure that any important safety information is communicated.
Drugs for veterinary use: Approved in 2019
You can report a veterinary drug reaction to Health Canada.
Important definitions
Generic drug
A generic drug is a copy of a brand name product. Generic drugs contain the same medicinal ingredients as the brand name drug and are considered bioequivalent to the brand name drug. There may be many generic versions of one brand name drug.
New drug
New drugs give new and innovative options for treatment, prevention and diagnosis of various health conditions.
New active substance
A new drug that contains a medicinal ingredient not previously approved in a drug in Canada and that is not a variation of a previously approved medicinal ingredient for veterinary use.
7 New drugs were approved for veterinary use
Adaxio Shampoo
- Medicinal Ingredients
- Chlorhexidine gluconate, miconazole nitrate
- Indication
- For use with dogs for the treatment and control of seborrheic dermatitis associated with Malassezia pachydermatis and/or Staphylococcus pseudintermedius.
Forceris
- Medicinal Ingredients
- Iron, toltrazuril
- Indication
- Indicated for use in piglets for the prevention of iron deficiency anaemia and for the prevention of the clinical signs of coccidiosis.
Galliprant
- New active substance
- Medicinal Ingredient
- Grapiprant
- Indication
- For the treatment and control of pain and inflammation associated with osteoarthritis in dogs.
Simparica Trio
- Medicinal Ingredients
- Moxidectin, pyrantel, sarolaner
- Indication
- For use in dogs and puppies for the prevention of heartworm disease; treatment and control of ticks, treatment and prevention of flea infestations, and treatment and control of intestinal worms.
Synovex LA-F
- Medicinal Ingredients
- Estradiol benzoate, trenbolone acetate
- Indication
- For use in feedlot steers and heifers to increase rate of weight gain and improve feed efficiency.
Synovex LA-G
- Medicinal Ingredients
- Estradiol benzoate, trenbolone acetate
- Indication
- For use in steers and heifers maintained on pasture to increase rate of weight gain.
Vitrecto
- Medicinal Ingredients
- Fluralaner, moxidectin
- Indication
- Indicated for use in cats and kittens to treat and prevent flea infestations, for the treatment and control of ticks, prevention of heartworm disease, and treatment of intestinal worms.
7 New generic drugs were approved for veterinary use
- 2 products containing atipamezole hydrochloride
- 1 product containing detomidine hydrochloride
- 1 product containing dexmedetomidine hydrochloride
- 2 products containing meloxicam
- 1 product containing selamectin
Footnotes
- Footnote 1
-
New biosimilar