Chapter 6-10 - Canadian Biosafety Handbook, Second Edition
As of April 1, 2023, the Canadian Biosafety Standard, Second Edition (CBS2), referenced in this document, is no longer in effect. The Canadian Biosafety Handbook is currently being updated to align with the Canadian Biosafety Standard, Third Edition. We will communicate the publication of this update through the Biosafety and Biosecurity for Pathogens and Toxins News.
[Table of Contents] [Next page]
Chapter 6 - Biosecurity
While the concepts of biosafety and biosecurity are closely related, the distinction between the two is important, particularly when considering facilities where infectious material or toxins are handled and stored. "Biosafety" describes the containment principles, technologies, and operational practices that are implemented to prevent unintentional exposure to pathogens or toxins, or their accidental release. In comparison, "biosecurity" refers to the security measures designed to prevent the loss, theft, misuse, diversion, or intentional release of infectious material or toxins. These concepts are not mutually exclusive and are inherently complementary, as the implementation of good biosafety practices serves to strengthen biosecurity programs and vice versa.
Just as the approach to biosafety is risk- and performance-based, so too is the approach to biosecurity, and facilities may be able to meet the intent of regulatory requirements through a combination of alternative physical measures and operational procedures. The minimum physical containment requirements, operational practice requirements, and performance and verification testing requirements related to biosecurity for regulated facilities in Canada are included in the requirements specified in the Canadian Biosafety Standard (CBS), 2nd Edition.Footnote 1 This chapter aims to outline considerations and approaches to establish a robust biosecurity program.
It is important to note that the term "biosecurity" used here is different from the concept of "agricultural biosecurity", which is outside the scope of the CBH. Agricultural biosecurity is intended to protect livestock and Canada's food supply from disease, and consists of preventive measures to minimize the possibility that a disease will enter an animal or plant population, and to minimize the spread of a pathogen within already infected premises.
6.1 Biosecurity Risk Assessment
The preliminary step in developing a biosecurity program is to conduct a biosecurity risk assessment. There are several resources available to assist with biosecurity risk assessments and developing biosecurity programs.Footnote 2Footnote 3Footnote 4Footnote 5 Similar to other risk assessments described in this volume (see Chapters 4 and 5), the complexity and detail of the biosecurity program is dependent on the level of risk posed by the pathogens, infectious material, or toxins in possession. It is recommended that the biosecurity risk assessment be reviewed on an annual basis and updated as necessary to address any change that affects the level of risk (i.e., introduction of a new pathogen, construction of a new facility). The following are the key elements that are included in a biosecurity risk assessment.
6.1.1 Identify and Prioritize Assets
The first step of the biosecurity risk assessment is to identify all of the relevant assets. In the context of biosecurity, "assets" would include all of the pathogens, infectious material, and toxins in the possession of the facility; however, other materials, equipment, non-infectious material, animals, knowledge and information, and even people may also be identified as assets. At minimum, it is required to maintain an inventory of pathogens, toxins, and other regulated infectious material in long-term storage (i.e., greater than 30 days) in the facility, including their risk group(s) and location. For higher risk material (i.e., security sensitive biological agents [SSBAs], Risk Group 3 [RG3], and Risk Group 4 [RG4]), it is required to have a means to allow for the timely detection of a missing or stolen sample. Good practice would dictate that other factors such as the concentration, quantity, and state of the material, also be included in the inventory. With this information at hand, the potential for the intentional misuse of the pathogens or toxins can be determined and the asset can be prioritized based on the consequences of this misuse. The impact that the loss would have on facility operations should also be considered.
Pathogens and toxins with dual-use potential (i.e., where the inherent qualities of a pathogen or toxin allow for its use in legitimate scientific applications, as well as for intentional and malicious misuse as a biological weapon to cause disease in humans or animals) are of the greatest biosecurity concern. These are the human pathogens and toxins that have been determined to have a potential for misuse and are identified as "prescribed pathogens" and "prescribed toxins" in the Human Pathogens and Toxins Act (HPTA) and Human Pathogens and Toxin Regulations (HPTR), and collectively specified in the CBS and this volume as SSBAs.Footnote 6Footnote 7
Assets are prioritized according to their biosecurity risk based on a number of key factors, including the consequences of malicious use, the ease of use of the material, and the impact of loss of material on the facility.
6.1.2 Identify and Define Threats and Vulnerabilities
Individuals, organizations, or groups that may pose a risk to the security of assets present within the facility (e.g., theft) should be identified and listed. Such individuals or groups are considered adversaries or threats. Adversaries can be categorized as "outsider threats" (i.e., the threat from someone without authorization or access to the assets, containment zone, or facility and who may not have a formal relationship with the facility) and "insider threats" (i.e., an authorized individual who has access to the assets as part of his or her job and has the potential to steal or deliberately misuse the assets). Potential insider threats can include disgruntled current employees, including individuals with malevolent intent who intend to steal, release, or divert pathogens or toxins; or employees with access to pathogens and toxins that are coerced or manipulated into providing access or expertise to unauthorized individuals. Former employees, terrorist groups, organized criminal groups, extremist protest groups, persons suffering from mental illness, and opportunistic criminals are examples of outsider threats.
The biosecurity risk assessment involves an assessment of threats to determine if and how each threat could gain access to, damage, or misuse assets such as pathogens or toxins.Footnote 4 Threat analysis considers the motive, means, and opportunity for each threat. The threat analysis also provides an indication of vulnerabilities. These are the weaknesses in existing security measures that may impact on the "means and opportunity" of the threat to access an asset, and therefore on the likelihood of an event occurring. Identified vulnerabilities can be addressed with mitigation strategies.
6.1.3 Determine Risk Levels and Mitigation Strategies
The biosecurity risk level is determined based on an analysis of the risk associated with each asset (or group of assets with similar characteristics) in combination with each threat. The highest biosecurity risks are those events with the greatest consequences, even if it is fairly unlikely they would occur, followed by events with moderate consequences that are more likely to occur.
Risks can be mitigated using physical security measures, enhanced screening for personnel, a clear accountability framework for pathogens and toxins, effective incident and emergency response protocols, and information security measures. Assets can be managed according to risk level as follows:
- assets that are deemed to be at low risk of unauthorized access need minimal management and control measures;
- assets that are at medium or high risk of unauthorized access will require moderate management and risk mitigation; and
- assets that are at very high risk require extensive management and controls.
Senior management within the organization is responsible for determining the acceptable levels of risk for identified scenarios (i.e., risk tolerance), as well as the resources available to mitigate the risks. Possible mitigation strategies for the identified vulnerabilities should be outlined and include preventive measures that may be implemented to counter the identified risk, and it may be found that identified risks are already controlled through existing biosafety and/or biosecurity measures. Any risks that have not been mitigated or that have been deemed acceptable should be documented along with an explanation of the decision.
Mitigation strategies developed to protect against unacceptable risks can be used to develop a biosecurity plan that will complement the biosafety program.
6.1.4 Develop Risk Statements and Risk Registers
A risk statement provides an accurate picture of a risk and is a key tool used in the risk management process.Footnote 8 Risk statements serve to identify and document biosecurity risks during the biosecurity risk assessment. A risk statement for a threat involves at least two elements: the event and the potential negative impact of such an event should it occur. A risk statement (i.e., the threat) can be structured to read: "If [event] occurs, the consequences could result in [negative impact]".
A risk register is a common project management tool to document the results of qualitative and quantitative risk analysis and risk response planning. It is essentially a list of all of the identified risk statements and the risk level in a format that can be easily reviewed, modified, and updated as necessary.Footnote 9
6.2 Biosecurity Plans
A biosecurity plan is a key biosafety program element for any facility where infectious material or toxins are handled or stored. The biosecurity plan is based on the biosecurity risk assessment and can be simple or complex, depending on the pathogens, infectious material, and toxins possessed by the facility and the structure or complexity of the facility or organization.Footnote 10 The biosecurity plan should address the unacceptable risks posed by both outsider and insider threats. The biosecurity plan should be developed through a collaborative process that involves facility staff members, such as scientific directors, principal investigators, laboratory personnel, administrators, information technologists, occupational health and safety personnel, security personnel, and engineering staff. Involving personnel responsible for the facility's overall security in this process is crucial as certain biosecurity measures may already be in place as part of an existing security program. It may also be appropriate to involve local law enforcement in the development of the biosecurity plan. Regular reviews and updates of the biosecurity plan keep the biosecurity measures, policies, and procedures accurate and effective to maintain biosecurity. Matrix 4.1 of the CBS specifies the minimum requirements for biosecurity plans and biosecurity risk assessments.
6.2.1 Elements of a Biosecurity Plan
Once the biosecurity risk assessment is complete, a biosecurity plan tailored to the facility can be developed, implemented, evaluated, and continually improved as necessary. Integrating the elements of the biosecurity plan within the overall biosafety program will minimize duplication of information and allow for a more efficient biosafety management system. A biosecurity plan addresses the elements that follow. At minimum, a biosecurity plan must document that the risks associated with each element have been assessed and describe the strategies, if any, that are already in place or have been added to mitigate these risks. The PHAC and the CFIA have developed an additional guideline that elaborates on a number of the biosecurity topics introduced in the CBH and serves as a resource for stakeholders seeking additional information and to establish a comprehensive and robust biosecurity plan; please visit the PHAC or CFIA website for further information.
6.2.1.1 Physical Security
Physical security elements of a biosecurity plan aim to reduce the risk of unauthorized access to identified assets and other sensitive materials (i.e., protect against outsider threats). Adequate physical security measures should be in place to minimize opportunities for the unauthorized entry of individuals into containment zones and the unauthorized access to infectious material or toxins in the facility. An evaluation of the physical security measures should include a thorough review of the premises, building, containment zones, and storage areas. Security barriers (i.e., a physical structure designed to prevent entry by unauthorized personnel), such as locked doors and windows, controlled access systems, and secure containers or storage equipment, can be incorporated to increase the security of a containment zone and to restrict access to authorized personnel only. Security barriers can be considered at the property or building perimeter, facility, containment zone, and pathogen or toxin-specific level. Security barriers at the points of access to the containment zone (e.g., lockable doors, manned security stations or checkpoints), access control measures (i.e., limited or restricted), mechanisms to detect unauthorized access and attempts (e.g., security cameras, access control system software records), additional security barriers (e.g., lock boxes or lockable freezers), and maintenance of security barriers are key considerations when determining the appropriate level of physical security. The minimum physical security requirements for regulated containment zones are specified in Matrices 3.1, 3.2, and 3.3 of the CBS; operational practice requirements relating to biosecurity practices are included in Matrices 4.5 and 4.6 of the CBS.
6.2.1.2 Personnel Suitability and Reliability
Hiring managers should screen candidates to establish they have the appropriate credentials, skills, and personal traits to undertake the work, and are the best fit for the position prior to being granted access to pathogens and toxins or other assets.Footnote 11 Academic credentials and prior experience may qualify an individual's scientific ability, but they do not always measure the individual's suitability to handle or access pathogens and toxins. Personnel suitability and reliability policies and procedures should be established to address the risk from a potential insider threat; the training, experience, competency, and other suitability requirements for personnel who handle or have access to pathogens or toxins should be clearly defined and documented. Employee pre-appointment screening is a crucial step in determining personnel suitability. Procedures may also be needed for approving and granting visitor access.
An ongoing reliability assessment program aims to verify that access to pathogens and toxins granted to an individual continues to be justified based on the established criteria for personnel suitability. Moreover, an ongoing assessment program also aims to identify insider threats from personnel who have previously been determined to be suitable for access. Circumstances that may affect an employee's ability to safely and securely perform their duties can also affect that individual's Human Pathogens and Toxins Act Security Clearance (HPTA Security Clearance) status. These may include, for example, participation in criminal activities, immigration or financial concerns, dramatic changes in behaviour, attitudes, demeanor, or actions (e.g., increasingly withdrawn, anger or aggression, unexplained absences, signs of alcohol or drug use), or willful non-compliance with policies and legislation. The availability of programs that identify and offer assistance to employees who are experiencing problems may be considered as a possible method to reduce these risks.
6.2.1.3 Accountability of Pathogens and Toxins and Inventory
Pathogen and toxin accountability procedures are established in order to track and document pathogens and toxins, which include all regulated infectious material in long-term storage (i.e., greater than 30 days) within the containment zone or organization, so that material can be located when necessary and missing items can be identified more readily. Effective inventory measures for pathogens and toxins can be a successful way to deter a variety of insider threats. The level of detail of the inventory system is determined based on the risk associated with the pathogens, toxins, and other infectious material being handled and stored. For example, where SSBAs, RG3, or RG4 pathogens are in long-term storage, the inventory will require more detail than Risk Group 2 (RG2) pathogens so that the samples of specific pathogens, toxins, and other regulated infectious material can be easily identified, and consequently located or determined to be missing or stolen in a timely manner (CBS Matrix 4.10). Provisions for maintaining accountability during shipping, receiving, monitoring, and storage of packages that contain pathogens, toxins, and other regulated infectious material should also be incorporated into the biosecurity plan. Pathogen and toxin accountability and inventory systems are discussed in detail in Chapter 19.
6.2.1.4 Information Management and Security
Information management and security policies and procedures are created to protect sensitive information from unauthorized access or theft and to ensure the appropriate level of confidentiality. Examples of sensitive information may include facility biosecurity plans, employee information, access codes, passwords, infectious material and toxin inventories, and storage locations. In some cases, scientific information may be considered sensitive information (e.g., cloning procedure to reconstitute an extinct virus).Footnote 12 Information management and security policies should govern the classification and handling of sensitive information and address how the information is collected, documented, transmitted, accessed, and destroyed. Proper access control to sensitive information, containment zones and associated areas is often the first step to mitigating the risk of information misuse by outsider threats.
The protection of information should be consistent with the level of risk posed by the material in question. In certain environments, access to records and documentation pertaining to activities with pathogens and toxins is restricted to authorized personnel only (CBS Matrix 4.10).
Clear policies or protocols for basic information technology security, such as strong user passwords, discouraged or limited use of unsecured wireless connections, and the use of a virtual private network (VPN) to communicate between several offices, are general considerations for information security. The use and control of mobile electronic devices (e.g., tablets, personal data storage devices) and digital cameras should be considered as a vulnerability to information security, as they can be easily hidden from sight and are capable of storing or transferring information on media that can be removed and stored separately.
6.2.1.5 Incident and Emergency Response
The incident and emergency response elements of a biosecurity plan should be integrated into the overall biosafety program for greater efficiency (i.e., a component of the emergency response plan [ERP]). For example, it is recommended that a mechanism is included for the removal of unauthorized individuals. All incidents should be reported. Reporting of biosecurity-related incidents (e.g., missing pathogens or toxins, unauthorized entry or access to sensitive information, loss of keys or passwords) to the biological safety officer (BSO) should be encouraged so that incidents can be appropriately documented, investigated, and reported as necessary. Depending on the incident, the BSO may consider reporting the incident to local law enforcement and may be obligated to report the incident to the Public Health Agency of Canada (PHAC) under the conditions of licence. More details about ERPs and incident investigation are provided in Chapters 17 and 18, respectively.
6.3 Human Pathogen and Toxin Act Security Clearances
The PHAC has determined that an HPTA Security Clearance is an important employee screening procedure to complement suitability assessments in an effort to mitigate the risk from potential insider threats in Canadian facilities authorized to conduct controlled activities with SSBAs. In accordance with the HPTA, an individual must possess a valid HPTA Security Clearance issued by the PHAC to enter a part of a facility in which controlled activities with SSBAs have been authorized under a licence (HPTA 33). The HPTA Security Clearance issued by the PHAC is a comprehensive background check of law enforcement and intelligence databases that also includes a credit check. An individual without an HPTA Security Clearance may only enter the part of a facility in which controlled activities with SSBAs have been authorized if there are no SSBAs in that part of the facility, if any SSBAs that are present are locked up and inaccessible, or if accompanied and supervised by a person who holds a valid HPTA Security Clearance for that part of the facility (HPTA 33). Security screening is a key biosecurity element of the regulatory framework under the HPTA to assess the credibility and suitability of all individuals who will be authorized to access SSBAs. The HPTA Security Clearance cannot be processed without consent; the applicant is screened based on the information provided on the security screening application form. Throughout this section, the relevant sections of the HPTA or HPTR are indicated for ease of reference.
6.3.1 HPTA Security Clearance Process
The HPTA Security Clearance process involves an electronic records check by the Royal Canadian Mounted Police (RCMP), and the Canadian Security Intelligence Service (CSIS), as well as a credit history check. The RCMP determines if an individual has a criminal record in Canada and conducts law enforcement record checks to determine if the individual has engaged in or has been associated with criminal activities that would indicate unacceptable risk. The CSIS reviews domestic and foreign threats to Canadian security posed by both individuals and organized entities, and assesses the applicant's loyalty to Canada as it pertains to threats to national security. In accordance with Section 12 of the HPTR, applicants for an HPTA Security Clearance will be requested to submit additional information to support their application, including but not limited to: a copy of their birth certificate, copies of two valid pieces of government issued photo identification, fingerprints taken at an RCMP accredited facility, and a statement signed by the licence holder certifying that the applicant requires an HPTA Security Clearance for a particular part or parts of a facility. Foreign nationals will also be required to supply a copy of their curriculum vitae, a valid visa if applicable, and the results of a police record check from every jurisdiction they have. At the time of publication, the service standard to review and issue an HPTA Security Clearance had not been established, although it is estimated that the entire security screening process may take up to 80 working days or longer. Longer delays may be encountered due to incomplete applications or additional investigations by the RCMP or CSIS. The decision to issue, suspend, refuse, or revoke an HPTA Security Clearance is made by the Minister of Health, or his or her delegate, in accordance with the HPTR. If an applicant or HPTA Security Clearance holder has had their clearance denied, suspended, or revoked, they will have the option to appeal by requesting a reconsideration of this decision within 30 calendar days after the day on which the notice of denial, suspension, or revocation was received. Further information on the HPTA Security Clearance Application Form and the appeal process can be obtained by visiting the PHAC website (www.publichealth.gc.ca/pathogens).
6.3.2 Exemptions
Personnel in licensed facilities that are authorized to handle and store toxins identified in Schedule 1 of the HPTA in quantities equal to or less than the trigger quantity specified in the HPTR 10(2) do not need an HPTA Security Clearance. Access to an RG3 or an RG4 pathogen that is also an SSBA and that has been modified to the extent that it no longer meets the risk profile described by the definition of the respective risk groups in the HPTA may not require an HPTA Security Clearance (e.g., a vaccine strain of an RG3 pathogen that has been attenuated to a point that it meets the risk profile of an RG2 pathogen).
6.3.3 Validity and Portability
An HPTA Security Clearance issued by the PHAC is valid for a period of up to 5 years and authorizes access to the part(s) of the facility (or facilities) identified on the initial application. The HPTA Security Clearance is issued to an individual and is transferable between licensed facilities. In order to access a facility not indicated on the initial HPTA Security Clearance application without being accompanied by an authorized individual, an HPTA Security Clearance holder will be asked to provide the PHAC with a signed statement by the licence holder of the new licensed facility certifying that they require an HPTA Security Clearance (HPTR 18). In this case, the HPTA Security Clearance holder may not enter the new licensed facility until the signed statement by the licence holder has been provided to the PHAC. A new supporting letter signed by the licence holder will be requested each time an HPTA Security Clearance holder moves to a different licensed location during the tenure of their clearance.
6.3.4 Suspension and Revocation
An HPTA Security Clearance may be suspended by the PHAC in the event that it receives any information that, should it have been obtained in support of the initial application, may have resulted in a refusal to issue an HPTA Security Clearance. The suspension will stand until such time as the analysis of this new information is complete and the suspension is lifted or the HPTA Security Clearance is revoked. An HPTA Security Clearance will be revoked if the PHAC determines that the holder of a clearance is no longer deemed to be suitable.
6.3.5 Notification of Criminal Offences
An individual who has been issued an HPTA Security Clearance is obliged to notify the PHAC if he or she is convicted of a criminal offence (HPTR 19). Depending on the nature of the offence, a review of the individual's HPTA Security Clearance may be initiated. The notification can be submitted electronically to the PHAC by email or through the Notification of Criminal Offence form accessible through the PHAC website (www.publichealth.gc.ca/pathogens). Failure of an individual to report conviction of a criminal offence may result in suspension or revocation of his or her HPTA Security Clearance.
6.3.6 Accompaniment and Supervision
An individual without an HPTA Security Clearance may only enter the part of a facility in which controlled activities with SSBAs have been authorized when accompanied and supervised by a person who holds a valid HPTA Security Clearance for that part of the facility (HPTA 33). In this case, the escort must have an HPTA Security Clearance issued by the PHAC and may only accompany and supervise one person who does not hold a clearance at any one time (i.e., a 1 to 1 ratio). The escort must be in the same room and monitor the activities of the person who does not hold a clearance at all times (HPTR 23); a direct line of sight of the individual without an HPTA Security Clearance should be maintained at all times. Individuals without an HPTA Security Clearance are not considered authorized personnel and are, therefore, not permitted to have access to SSBAs, except under direct supervision as described above. An individual who has been refused an HPTA Security Clearance or whose HPTA Security Clearance has been suspended or revoked may not enter the part of a facility where controlled activities with SSBAs have been authorized at any time, even under accompaniment and supervision (HPTR 24).
6.3.7 Shared Facilities
Shared spaces located inside the part of a facility where controlled activities with SSBAs have been authorized under a licence may present access challenges for all personnel. Facility management, directors, and the licence holder will have to determine how best to address these challenges. There are essentially two options available, each with benefits and challenges, dependent on scheduling and secure storage areas for the SSBAs:
- each individual who works in the shared spaces holds a valid HPTA Security Clearance so that they can freely access the part of the facility when SSBAs are present, regardless of whether or not he or she actually needs access to the SSBAs; or
- access by persons without an HPTA Security Clearance (i.e., those who do not actually need access to the SSBAs) to shared facilities is limited to times when:
- there are no SSBAs present;
- the SSBAs are locked away and inaccessible; or
- they are accompanied and directly supervised by an individual who holds a valid HPTA Security Clearance for that part of the facility (i.e., 1 to 1 ratio).
Option #1 provides more flexibility in terms of what work can take place at what time in the shared space, but increases the reliance on operational controls to manage the number of personnel who would have access to the SSBAs. This option can also weaken a security posture by breeding complacency (i.e., if it is assumed everyone accessing the room possesses an HPTA Security Clearance, and these numbers are significant, staff may be less likely to challenge an unfamiliar individual). In contrast, Option #2 addresses the challenges with the former option, with less reliance on operational controls, but may depend on strict scheduling of activities.
References
- Footnote 1
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 2
- CEN Workshop 31 - Laboratory biosafety and biosecurity. CEN Workshop Agreement (CWA) 15793:2011, Laboratory biorisk management. (2011). Brussels, Belgium: European Committee for Standardization.
- Footnote 3
- CEN Workshop 55 - CEN Workshop Agreement (CWA) 16393:2012, Laboratory biorisk management - Guidelines for the implementation of CWA 15793:2008. (2012). Brussels, Belgium: European Committee for Standardization.
- Footnote 4
- Salerno, R. M., & Gaudioso, J. (2007). Laboratory Biosecurity Handbook. Boca Raton, FL, USA: CRC Press.
- Footnote 5
- United States Centers for Disease Control and Prevention Division of Select Agents and Toxins & United States Animal and Plant Health Inspection Service Agriculture Select Agent Program. (2013). Security Guidance for Select Agent or Toxin Facilities (2nd Revision). Retrieved 11/03, 2015 from http://www.selectagents.gov/resources/Security_Guidance_v3-English.pdf
- Footnote 6
- Human Pathogens and Toxins Act (S.C. 2009, c. 24). (2015).
- Footnote 7
- Human Pathogens and Toxins Regulations (SOR/2015-44) (2015).
- Footnote 8
- Treasury Board of Canada Secretariat. (2014). Guide to Risk Statements. Retrieved 11/03, 2015 from http://www.tbs-sct.gc.ca/tbs-sct/rm-gr/guides/rmg-gertb-eng.asp
- Footnote 9
- Public Works and Government Services Canada. (2014). Risk Register - Introduction. Retrieved 11/03, 2015 from http://www.tpsgc-pwgsc.gc.ca/biens-property/sngp-npms/ti-it/rgtenjx-rsklg-eng.html
- Footnote 10
- United States Department of Health and Human Services, United States Centers for Disease Control and Prevention Division of Select Agents and Toxins and United States Department of Agriculture Animal and Plant Health Inspection Service Agriculture Select Agent. (2007). Select Agents and Toxins Security Plan Template. Retrieved 11/03, 2015 from http://www.selectagents.gov/resources/Security_Plan_Template_Final_APHIS-CDC-English.pdf
- Footnote 11
- United States Centers for Disease Control and Prevention Division of Select Agents and Toxins & United States Animal and Plant Health Inspection Service Agriculture Select Agent Program. (2013). Guidance for Suitability Assessments (2nd Revision). Retrieved 11/03, 2015 from http://www.selectagents.gov/resources/Tier_1_Suitability_Guidance_v3-English.pdf
- Footnote 12
- Tumpey T, Basler C, Aguilar P, Zeng H, Solórzano A, Swayne D, Cox N, Katz J, Taubenberger J, Palese P, García-Sastre A. (2005). Characterization of the Reconstructed 1918 Spanish Influenza Pandemic Virus. Science. 310(5745):77-80.
Chapter 7 - Medical Surveillance Program
The basic purpose of a medical surveillance program is to help prevent and detect illnesses related to the exposure of personnel to pathogens or toxins. The focus of this program is primarily preventive, although it also provides a response mechanism through which a potential infection can be identified and treated before serious injury, disease, or secondary transmissions occur. The medical surveillance of personnel handling pathogens and toxins can often be integrated into an existing workplace medical surveillance program (e.g., occupational health and safety programs for chemical or radiological hazards).
The requirements for a medical surveillance program are specified in Matrix 4.2 of the Canadian Biosafety Standard (CBS), 2nd Edition.Footnote 1 The medical surveillance program is developed and based on an overarching risk assessment and local risk assessments (LRAs) in order to identify the pathogens and toxins handled, stored, or encountered in the containment zone or throughout the organization, and to identify the associated risks. A description of the medical surveillance program is included in the containment zone's Biosafety Manual so that it is available for reference to all personnel. It is important to update the medical surveillance program accordingly whenever changes are made to a laboratory program (e.g., when different pathogens or toxins will be introduced or new procedures or activities will be carried out). It may be appropriate to involve an occupational health professional or a local health care provider (e.g., physician, nurse, local hospital), as well as emergency responders (e.g., local paramedic, fire, and police department personnel), in the process of developing the medical surveillance program, especially with programs involving higher risk pathogens.
This chapter presents a number of aspects to be considered in developing a medical surveillance program. The level of detail and the complexity of the program will depend on the nature (i.e., size, structure, complexity) of the organization, the activities carried out involving pathogens and toxins, and the safety-related provisions of applicable legislation. Some components that may be considered when developing a medical surveillance program include a pre-placement medical examination of personnel; serum screening, testing or storage; immunizations; and other tests, as determined by an LRA.
The medical surveillance program complements medical emergency procedures, which form part of a facility's emergency response plan (ERP). ERPs and incident investigation and reporting are described further in Chapter 17 and 18, respectively.
7.1 Laboratory Exposures and Laboratory Acquired Infections/Intoxications
Individuals who work in areas where infectious material or toxins are handled or stored are at risk of exposure to these pathogens and toxins and the adverse consequences of an exposure event (i.e., infection or intoxication). The term that is commonly used to describe diseases associated with workplace exposures to infectious material or toxins in a laboratory setting is laboratory acquired infections/intoxications (LAIs); however, the term exposure more accurately includes both infections and intoxications (i.e., resulting from exposure to toxins), whether symptomatic or asymptomatic in nature, as well as those that can be linked to a containment zone but that occur outside of a laboratory environment (e.g., infection of an office worker in a licensed facility by a pathogen handled or stored in that facility).
In addition to the immediate risk to individuals handling infectious materials, exposed persons can pose a risk to the community via transmission of infections within or outside the laboratory setting. Although it may be difficult to determine the root cause(s) in all cases, exposures resulting in LAIs are not uncommon. The most recent comprehensive epidemiological review found 5,527 cases and 204 deaths reported worldwide from 1930 to 2004.Footnote 2 While LAIs do still occur and are documented, the incidence of LAIs appears to have declined over the years; this may be attributable to enhanced biosafety practices, improved design of containment facilities and equipment, or simply due to under-reporting of incidents.Footnote 3Footnote 4Footnote 5 Despite this apparent decline, exposures and LAIs continue to occur, and data on these incidents may be used by biosafety professionals to better understand and quantify the risk associated with a given pathogen or a specific laboratory activity. Likewise, the information can be used to improve biosafety and biocontainment standards, guidelines, training, equipment and systems, and best practices, as well as medical surveillance programs (e.g., immunization, post-exposure prophylaxis, or treatment recommendations). The Public Health Agency of Canada (PHAC) is currently collecting incident reports documenting LAIs or exposures to human pathogens and toxins to analyze this information and help shape current and future biocontainment and biosafety practices in Canada.
Exposure to an infectious material or toxin is not always immediately followed by symptoms or overt disease. In addition, LAIs themselves can be symptomatic or asymptomatic in nature. Some facilities may employ medical surveillance practices that could identify a seroconversion, which may provide an additional source of information for recognition or confirmation of recent or previous infection or disease. Seroconversion can occur following initial infection and clearance of the pathogen, and may indicate a post-infection latency period prior to onset of the disease associated with certain pathogens (e.g., human immunodeficiency virus [HIV], Mycobacterium tuberculosis, hepatitis C virus, and prions). Sound judgement is needed in evaluating historical LAI data, as the accuracy of statistics may be impacted due to the likelihood of under-reporting of incidents. Under-reporting of exposures and LAIs may be attributed to a variety of factors, including:
- a lack of mechanisms for the reporting and tracking of exposures and LAIs;
- recognition and reporting of only symptomatic or laboratory-confirmed cases of disease;
- limited publication of LAI cases in scientific or medical journals due to factors such as space limitations;
- uncertainty as to whether an illness is due to an exposure that occurred in the laboratory setting or the community;
- a lack of interest or motivation to report common incidents or incidents involving a commonly used pathogen; and
- fear of reproach or reprisal.
The Human Pathogens and Toxins Act (HPTA), Section 13, requires that any exposure to human pathogens or toxins that may cause disease or any disease that may have been caused by an exposure to a human pathogen or toxin in the facility be reported to the PHAC without delay.Footnote 6 This reporting allows the PHAC to assess the severity of the exposure incident and assist the facility in their response, if requested or necessary. The PHAC can also provide expertise and assistance to the facility in developing corrective actions to address the cause of the incident and prevent a recurrence. Information provided in an exposure notification report following an exposure incident will allow the PHAC to monitor developing trends, prompt the issuance of biosafety advisories, and amend or update best practices in biosafety practices and training, and at the same time analyze this data at the national level to inform current and future biocontainment and biosafety directions. Local investigation, documentation, and reporting for all types of incidents are intended to capture near misses and LAIs for which no clear exposure event can be identified. Incident reporting and investigation are further discussed in Chapter 18.
7.2 Pre-Placement Medical Evaluation
In some circumstances, it may be beneficial to conduct pre-placement medical evaluations for all personnel. This section describes options when considering the implementation of pre-placement medical evaluations. A pre-placement medical evaluation may be conducted for new personnel or when personnel are given new responsibilities, prior to commencing activities with human pathogens, toxins, or zoonotic pathogens. The primary purpose of such an evaluation is to assess the initial health status of the individual and identify if there are any underlying medical conditions that may increase the risk of harm associated with the anticipated job activities. This evaluation may include an interview with the institutional occupational health care provider or completion of a personal medical history questionnaire to document the individual's previous and current medical problems; current medications; known allergies to medications, animals, or environmental allergens; and prior immunizations. Personnel who are immunocompromised or immunosuppressed (e.g., through medical therapy, pregnancy, diabetes, or other conditions) may be particularly susceptible to infection or intoxication, unable to take post-exposure treatment, or experience more severe illness if they develop disease following exposure to a pathogen or toxin. A complete physical examination is rarely necessary as part of this process but may be appropriate.
Before commencing any controlled activities, the individual should be informed of the hazards associated with, and the signs and symptoms of disease(s) caused by, the pathogens and toxins to be manipulated, and of all preventive measures available against the pathogens or toxins, such as vaccinations or other treatments, along with the risks and benefits of these vaccinations and treatments. They should also be informed of the steps to follow in the event of potential exposure, including appropriate first aid measures, incident reporting, timely post-exposure prophylaxis and medical treatments. In addition, the early signs and symptoms of a possible infection or intoxication with the pathogen(s) or toxin(s) being handled should be described to personnel, and they should be told what immediate steps to take if they develop these symptoms. In a clinical diagnostic setting, it may not always be possible or practical to advise personnel of all potential pathogens that they may encounter; rather, it may be more reasonable to inform personnel of symptoms of key concern in situations when illnesses caused by unusual pathogens have been diagnosed in the laboratory.
Personnel with a considerable risk of exposure to pathogens may be encouraged to provide a blood sample for serum testing and storage prior to the initiation of work with the pathogen(s). Such samples can be stored long-term and later used to determine pre-existing immunity from prior vaccination or infection, and to establish a baseline seroreactivity for comparison with supplementary blood samples collected following a potential exposure.
7.3 Vaccinations
Vaccines are highly regulated and complex biological products designed to induce a protective immune response both effectively and safely. The availability of vaccines or other prophylaxis should be evaluated, and these should be offered to personnel as required prior to commencing work with a pathogen. Periodic testing of antibody titres should be conducted post-vaccination to determine if the required level of protective immunity has been achieved and is being maintained, or if a booster vaccination is necessary. Should an individual decline or not respond immunologically to a vaccination that is deemed a prerequisite for working in a containment zone, a re-evaluation of placement, the implementation of additional environmental controls, or the use of additional personal protective equipment (PPE) should be considered.
Further recommendations on vaccines can be obtained from health care professionals specializing in this area or from the National Advisory Committee on Immunization (NACI). The NACI is a national advisory committee of medical and health sciences experts that makes recommendations to the PHAC on the use of vaccines in Canada, including the identification of groups at risk (e.g., occupational groups, age groups) for vaccine-preventable diseases. All NACI recommendations are published in the Canadian Immunization Guide with additional statements and updates published in the Canada Communicable Disease Report (CCDR).Footnote 7Footnote 8
7.4 Ongoing Medical Surveillance
Ongoing medical surveillance for personnel who are at risk of exposure to pathogens or toxins may provide an indication of occupational exposure. Personnel should be encouraged by the supervisor, without fear of reprisal, to disclose any changes in their health status that could increase their risk of exposure or disease susceptibility. This could include developing an immunodeficiency or a temporary condition, such as the need to take prescribed antibiotics, impaired vision, or even stress. Routine or periodic medical evaluations are generally not necessary; however, such evaluations may be appropriate in the case of personnel with a substantial risk of exposure to pathogens or toxins since they may permit earlier recognition of an infection that may be due to a laboratory exposure. Any clinical tests (e.g., serum testing) requested by a medical advisor or practitioner should be limited to approved, commercially available tests with adequate sensitivity to identify an infection or previous infection (i.e., seroconversion). Serum samples collected during the pre-placement evaluation can be used to establish a baseline or "pre-exposure" reference for any tests to be conducted as part of the medical surveillance program. While medical test results are only reported to the patient, individuals who discover a positive infection or seroconversion that may be associated with a laboratory-related exposure have an obligation to inform their supervisor or internal organizational authority (i.e., biological safety officer [BSO], licence holder), who, in turn, is legally required to notify the PHAC of the exposure (HPTA 13).
7.5 Post-Exposure Response Plan
Post-exposure response plans outline the specific procedures to follow and actions to be taken in the event of a known, suspected, or potential exposure to a pathogen or toxin (e.g., reporting, medical testing, and treatment) and could be a component of an overall ERP. For containment zones where pathogens or toxins are handled or stored, a post-exposure response plan may be created in consultation with the occupational health care provider or practitioner, the institutional biosafety committee, the BSO, and the occupational health and safety advisor. Incident reporting and investigation are discussed in further detail in Chapter 18.
7.6 Additional Considerations for High Containment
Any potential occupational exposure that occurs in a high containment zone (i.e., containment level 3 [CL3], which includes CL3 large animal containment zones [LA zone; CL3-Ag], or containment level 4 [CL4]) should be promptly evaluated, as infection with a higher risk pathogen may lead to severe illness or death. The pathogens manipulated in CL4 zones are typically exotic and an LAI would represent a serious health concern for the community. Ensuring adherence to all medical surveillance protocols and procedures by all containment zone personnel, including facilities and support personnel, is particularly important in high containment zones. It is strongly recommended that an infectious disease specialist be involved in the development of the medical surveillance program, including risk assessment, pre-placement evaluations, and development of a post-exposure response plan. Additionally, it is required (CBS Matrix 4.2) for CL4 zones and strongly recommended for CL3 and CL3 LA zones (CL3-Ag), that the post-exposure response plan be prepared in consultation with local health care facilities, to keep health care providers informed of the pathogens being handled and that the appropriate procedures and treatments are in place. Specific quarantine procedures for potentially infected personnel may need to be established prior to an exposure incident. In CL4 zones, it is also required that the supervisor contact any containment zone personnel with unexpected work absences to determine if the absence is due to an illness that could be related to activities with the pathogens in use (CBS Matrix 4.2).
7.7 Emergency Medical Contact Card
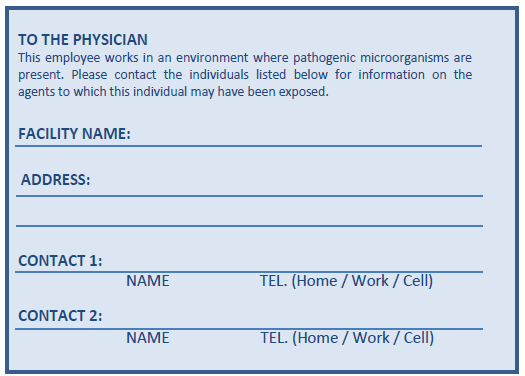
Emergency medical contact cards are issued by the employer to personnel working with non-human primates (NHPs), personnel working with pathogens or toxins that cause diseases unlikely to be recognized by a physician, and all personnel working in CL4 zones to provide a means to facilitate communication with health care providers and other individuals, particularly during emergency situations. The card should summarize important information regarding the pathogen(s) or toxin(s) that are handled by the individual, such as routes of infection or intoxication, transmission, symptoms, and preventive and therapeutic treatments. This measure is also recommended for personnel working in CL3 and CL3 LA zones (i.e., CL3-Ag). In the event of an unexplained illness, this card can be presented to hospital or heath care facility staff, or emergency responders. The containment zone supervisor should provide guidance as to when the card should be carried by the personnel (e.g., at all times on the premises except inside the containment zone, or at all times during a period that an active study involving the particular pathogen is being conducted). It is the responsibility of the facility to determine when the emergency medical contact card is to be carried by personnel. An example of an emergency medical contact card can be found in Figure 7-1.
Figure 7-1: Example of an Emergency Medical Contact Card
FRONT

BACK
Text Equivalent - Figure 7-1
This figure depicts the front and back of an example of an emergency medical contact card. The front of the card contains the card holder's name, the date of issue, a summary of the infectious materials or toxins that are handled by the individual and whether the card holder is working with non-human primates. In addition, a statement reads "This card is to be kept in the possession of the laboratory employee and presented to a physician if an illness occurs that may be associated with a pathogen used within the laboratory (see reverse)". The back of the card contains the name and address of the facility where the individual works as well as the name, and work and home telephone numbers for two emergency contacts. In addition, a short statement reads "To the physician – this employee works in an environment where pathogenic microorganisms are present. Please contact the individuals listed below for information on the agents to which this individual may have been exposed".
References
- Footnote 1
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 2
- Harding, A. L., & Brandt Byers, K. (2006). Epidemiology of Laboratory-Associated Infections. In Fleming, D. O., & Hunt, D. L. (Eds.), Biological Safety: Principles and Practices (4th ed., pp. 53-77). Washington, DC, USA: ASM Press.
- Footnote 3
- Singh K. (2011). It’s time for a centralized registry of laboratory acquired infections. Nature Medicine. 17(8):919.
- Footnote 4
- Singh K. (2009). Laboratory-Acquired Infections. Clinical Infectious Diseases. 49:142-147.
- Footnote 5
- Willermarck N., Van Vaerenbergh B., Descamps E., Brosius B., Dai Do Thi C., Leunda A., Baldo A., Herman P. (2015). Laboratory-Acquired Infections in Belgium (2007-2012). Retrieved 11/03, 2015 from http://www.biosafety.be/CU/PDF/2015_Willemarck_LAI%20report%20Belgium_2007_2012_Final.pdf
- Footnote 6
- Human Pathogens and Toxins Act (S.C. 2009, c. 24). (2015).
- Footnote 7
- Public Health Agency of Canada. (2014). Canadian Immunization Guide (Evergreen Edition). Retrieved 11/03, 2015 from http://www.phac-aspc.gc.ca/publicat/cig-gci/index-eng.php
- Footnote 8
- Public Health Agency of Canada. (2014). Canada Communicable Disease Report. Retrieved 11/03, 2015 from http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/
Chapter 8 - Training Program
A training program is essential to the success of a biosafety program. It is critical that personnel be knowledgeable about the hazards present in the work environment and the practices and tools that can protect them from these hazards. The training program encompasses both education and training. Education relates to the provision of general information or theoretical knowledge. Personnel can be educated on work-related hazards through various means, including classroom courses, videos, e-learning, on-the-job training, and printed materials, such as manuals, Pathogen Safety Data Sheets (PSDSs), and posters. Training refers to more practical and hands-on job-specific instruction, including the demonstration of practices and procedures. These two concepts are complementary and necessary to create a robust training program. A supervisor or facility director is generally responsible for ensuring that containment zone personnel receive proper training. The biological safety officer (BSO) or biosafety representative plays a role to promote and monitor that training related to biosafety and biosecurity policies, standards, and practices is arranged and documented in regulated facilities where human and animal pathogens and toxins are handled and stored in accordance with the Human Pathogens and Toxins Regulations (HPTR) (HPTR 9[1][c][i]) and the Canadian Biosafety Standard (CBS), 2nd Edition.Footnote 1Footnote 2 The required elements of a biosafety training program are specified in Matrix 4.3 of the CBS.
8.1 Training Needs and Objectives
The specific content of the training program will vary between organizations and even between containment zones within the same facility. A training needs assessment is the first step in developing an effective training program. This assessment is performed to identify the current and future training needs of the facility, and gaps in the existing training program. The results of a training needs assessment should be used as the foundation for determining instructional objectives, the selection and design of instructional programs, the implementation of the programs, the retraining cycle, and the evaluation of the training provided. The needs assessment should take into consideration the risks identified through the pathogen and biosecurity risk assessments, and the specific issues that can be mitigated through training.
The Biosafety Manual describes the core elements of the biosafety program, including training program objectives and goals. The training objectives should be measurable and clearly identify the desired behaviour or skill to be learned in the training.
8.2 Training Program Content
All training programs share certain components and requirements. Combining biosafety and biosecurity training with other workplace training requirements could be beneficial, as well as an efficient use of resources. Training on the Biosafety Manual and standard operating procedures (SOPs) requires personnel to be familiar with the contents of the Biosafety Manual, such as the biosecurity and emergency response plans (ERP). Personnel demonstrating knowledge and proficiency in the SOPs on which they were trained will be able to safely handle the pathogens and toxins they will encounter in the workplace and respond accordingly in an emergency situation.
Training related to the potential hazards associated with the work carried out is particularly important and may include the following elements:
- information on the nature of infectious material and toxins used in the workplace and how to identify them;
- signs and symptoms of disease caused by exposure to the pathogens that will be handled. In facilities where a wide variety of pathogens could potentially be handled (e.g., diagnostic facilities), a broader approach may be considered (i.e., training on general signs and symptoms of concern rather than the symptoms for each pathogen);
- safe work practices and physical control measures, including handling and disposal of infectious material or toxins (i.e., decontamination and waste management), and the correct choice, use, and maintenance of personal protective equipment (PPE);
- instruction on relevant safety information (e.g., PSDSs) and on how to find and use these materials; and
- information on the legislative and regulatory requirements related to activities involving the infectious material or toxins concerned.
There are many teaching and training resources available to assist in the development of a training program. The Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA) provide numerous resources, including biosafety training materials (such as the "Principles of Laboratory Biosafety" e-learning course), templates, toolkits, posters, instructional videos, and more, available through an e-learning portal (www.publichealth.gc.ca/training).
8.2.1 Biosecurity Training
Biosecurity training for all personnel is essential to establish a culture of responsibility and security awareness and may include the following elements:
- awareness of insider threats and outsider threats;
- behaviours of concern;
- identifying and removing a suspicious person;
- policies concerning access to security sensitive biological agents (SSBAs);
- escort procedures;
- self and peer reporting procedures;
- corrective actions, procedures and policies;
- information security;
- security policies, including:
- entry and access procedures and prevention of "tailgating";
- preventing the sharing of unique means of access;
- reporting the loss or compromise of passwords;
- how to identify and report suspicious persons or activities; and
- inventory documentation and records management;
- responding to an alarm; and
- responding to a security breach.
8.2.2 Training on Containment Systems and Equipment
Training programs are to include information on the physical design and operation of the containment zone and containment systems that are relevant to the individual being trained (CBS Matrix 4.3). This should include a review of the types of primary containment devices and containment systems associated with the containment zone, a basic overview of how each functions, their correct use and operation, and how to determine if the equipment or systems are functioning properly or not, to protect against a release or exposure to a pathogen or toxin. This training should also include a review of secondary containment systems (e.g., backup containment systems) where these have been incorporated into the containment zone.
Personnel are to demonstrate knowledge and proficiency in the SOPs on which they were trained, including SOPs for the correct operation of containment systems, devices, and other equipment. Examples of primary containment devices and containment systems that should be reviewed during biosafety training include, but not limited to:
- biological safety cabinets (BSCs);
- heating, ventilation, and air conditioning (HVAC) and control systems;
- decontamination technologies (e.g., autoclaves, effluent decontamination systems, dunk tanks);
- primary containment caging systems used in small animal containment zones (SA zones);
- centrifuges; and
- laboratory equipment and apparatus used for activities with pathogens and toxins.
8.3 Identification of Trainees
Identification of the intended audience is a key component of designing a training program, since it permits the identification of specific training needs and the type of training suitable for the learning styles of the target audience.
8.3.1 New Personnel
Implementation of an orientation program for new personnel gives them the requisite instruction prior to exposure to work-related hazards. Training of new personnel should include all training elements identified in Section 8.2 of this chapter, and any other relevant topics (e.g., a review of the organization's history, safety program, policies, personnel rights and responsibilities, general Workplace Hazardous Materials Information System (WHMIS) information). Hands-on training and extra guidance and supervision should be provided during the initial period of employment for a new employee. In addition to formal training, on-the-job experience is also important. Trainees may carry out activities with infectious material and toxins within the containment zone, provided that they are supervised by authorized personnel.
8.3.2 Existing Laboratory Personnel
Training is an ongoing process and is therefore not limited to new personnel. Existing personnel may require training or education with respect to new procedures, work in new environments, or work with new infectious material or toxins. Refresher training, provided at a frequency determined by a training needs assessment review or when warranted by a change in the biosafety program, helps keep personnel knowledgeable about the hazards, risks, resources, and control measures in their workplace. Annual refresher training on emergency response procedures is required (CBS Matrix 4.3). Refresher training also provides an opportunity to educate personnel with respect to any new information about the infectious material or toxins being used, changes in recommended practices, or changes in regulatory requirements.
8.3.3 Other Personnel
A training program needs to consider all personnel who will access the containment zone; not just those handling the infectious material and toxins. Visitors, contractors, janitorial staff, security staff, and maintenance staff require training on the hazards, risks, and control measures, in accordance with anticipated activities and supervision by authorized personnel while conducting activities in a containment zone (CBS Matrix 4.3).
8.3.4 Learning Conditions
Incorporation of adult learning principles into the design of biosafety education and training programs will promote program success. This may include focusing on motivation, reinforcement, retention, and transference of existing skills and knowledge. Since people differ in their learning styles, a variety of teaching methods and tools are recommended in order to reach a broader audience. Training is most effective when a variety of education tools are used, such as a presentation combined with visual aids, videos, self-directed tutorials, and problem solving activities. Acting out scenarios or rehearsing emergency drills will reinforce knowledge and skills acquired through other teaching methods. Trainers should also consider accessibility issues, such as language barriers and hearing impaired participants, and adjust their approach accordingly.
8.4 Training Evaluation
A variety of methods can be used to evaluate the uptake of knowledge and skills conveyed in a training program. The evaluation method that is selected (e.g., written test, hands-on evaluation) should effectively measure the trainee's skill development and knowledge acquisition. Pre- and post-training tests or quizzes are helpful tools for measuring whether the learning objectives were achieved. The evaluation of workplace practices and behaviour through facility audits, inspections, or regular monitoring by supervisors can also provide a useful indication of how well the training was understood, and whether retraining or review of the training program is warranted. Training evaluation forms may be handed out at the end of a training course or session to solicit feedback from trainees. This will provide valuable feedback on the effectiveness and efficiency of the course content, instructor(s), and teaching practices, and can assist in improving the training program.
An annual (or periodic) performance review of individuals with access to human or animal pathogens or toxins provides an opportunity to gauge their adherence to, and understanding of, biosafety and biosecurity procedures. The annual review also provides an opportunity for the supervisor to review and reinforce the importance of biosafety and biosecurity, to discuss the requirements of the Human Pathogens and Toxins Act (HPTA), HPTR, Health of Animals Act (HAA), Health of Animals Regulations (HAR), and the CBS, and address any potential problems that have affected work performance in the past or may impact future performance.Footnote 3Footnote 4Footnote 5
8.5 Training Records
Training and retraining records document the participation and successful completion of training. These records may include attendance sheets, orientation checklists, examinations, certificates, or other types of records (e.g., a description of the training with signatures of the attendee, trainer, and supervisor), as deemed appropriate by the organization. Training and retraining records provide documentation of the date the course or training was provided, the names of individual participants/trainees, and the type or name of the course/training to facilitate tracking of retraining requirements. Biosafety training and retraining records may be combined with other occupational health and safety program training records, where applicable. All training and retraining records should be kept up to date and the most recent version should be kept on file (i.e., if training is repeated, updated, or a refresher course given, only the most recent record of training needs to be retained for a given individual). Minimum retention periods for training records can be found in Matrix 4.10 of the CBS. Such records will be used to determine refresher training needs.
8.6 Training Program Review
Regular evaluation of the content of the training program will help identify areas that need to be updated to ensure that the information is accurate and relevant. It is recommended that the program be reviewed and updated annually at a minimum, or whenever changes occur in working conditions, procedures, hazards, or hazard information. Training and retraining records should also be included in the review of the biosafety and biosecurity programs as a measure of training program performance (e.g., frequency of training sessions, number of attendees, variety of topics/programs). This will provide an opportunity to adjust resources so as to optimize the training program.
References
- Footnote 1
- Human Pathogens and Toxins Regulations (SOR/2015-44). (2015).
- Footnote 2
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 3
- Human Pathogens and Toxins Act (S.C. 2009, c. 24. (2015).
- Footnote 4
- Health of Animals Act (S.C. 1990, c. 21). (2015).
- Footnote 5
- Health of Animals Regulations (C.R.C., c. 296). (2015).
Chapter 9 - Personal Protective Equipment
Personal protective equipment (PPE) refers to devices and clothing designed to minimize the risk of exposure to various hazards. PPE is the last line of defence to protect personnel and to minimize the risk of transmitting pathogens and toxins to the public and the animal population. PPE helps to prevent the release of pathogens and toxins on contaminated individuals (or their clothes) by providing a barrier between the individual and the infectious material and toxins being handled or stored. PPE should be the last form of control considered as it provides an additional barrier to protect against exposure to hazardous materials in the event of failure in the administrative or engineering controls. PPE requirements are described in Matrix 4.4 of the Canadian Biosafety Standard (CBS), 2nd Edition.Footnote 1
In Canada, occupational health and safety is regulated provincially, territorially, and federally, and requirements pertaining to PPE have been incorporated into the relevant occupational health and safety legislation. In general, the employer is responsible for ensuring that appropriate PPE is available, properly maintained, used, and that personnel are appropriately trained on how to use it. The PPE requirements specified in the CBS are specific to biosafety and not intended to supersede any provincial or territorial legislation, nor the regulatory requirements of local occupational health and safety legislation. This chapter provides guidance on the types and general use of the PPE commonly used in containment zones where infectious material and toxins are handled or stored.
9.1 Types and Selection of Personal Protective Equipment
PPE can include respirators, hand and foot protection, head and eye protection, and full body protection. Selection of PPE is based on a local risk assessment (LRA) of the containment zone and is specific to the work to be performed.
9.1.1 Hand Protection
Gloves protect hands from contamination and reduce the risks associated with ingestion (e.g., hand-to-mouth transfer) or absorption through the skin. Gloves provide a protective barrier when handling infectious material, toxins, infected animals, or material potentially contaminated with a pathogen or toxin (e.g., tissues, cultures, blood, and body fluids). Gloves can be made from many different materials, and the type of glove selected will depend on the specific activity and hazard concerned; they should be clean, disposable, and fluid-resistant for handling infectious material or toxins.
Fluid resistance of gloves is affected by conditions of use including type and concentration of chemicals used, duration of use, temperature, physical stress, and material thickness.Footnote 2 Table 9-1 provides general recommendations for glove materials commonly used for work involving pathogens, infectious materials, toxins, and chemical disinfectants based only on chemical compatibility data. Glove material compatibility with a chemical can be assessed by measuring the amount of time elapsed between exposing the outer surface of a glove to a chemical and detecting the chemical on the inside of the glove (i.e., breakthrough time); measuring the flow of the chemical through the glove material (i.e., permeation rate); and assessing the degradation in the physical properties (e.g., brittleness, softening, swelling) of the glove material as a result of exposure to the chemical.Footnote 3
| Disinfectant Type | Chemical Disinfectant | Natural Rubber Latex | Synthetic Rubber | Plastic Polymer | |
|---|---|---|---|---|---|
| Neoprene | Nitrile | Polyvinyl Chloride (PVC) | |||
| Oxidizing Agents | Sodium Hypochlorite (<15%) | G | G | F | VG |
| Iodine | G | G | G | G | |
| Hydrogen peroxide (30%) | VG | VG | VG | VG | |
| Sodium Hydroxide (50%) | VG | VG | VG | VG | |
| Ammonium hydroxide | G | G | G | VG | |
| Phenolics | Hexachlorophene | G | G | G | -- |
| Quaternary ammonium compounds | N, N Didecyl Dimethyl Ammonium Chloride | G | G | VG | -- |
| Aldehydes | Glutaraldehyde (25%)1 | VG | VG | VG | VG |
| Formaldehyde (37% in 1/3 methanol/water) | F | G | VG | VG | |
| Alcohols | Ethanol (92%) | F | VG | VG | G |
| Isopropyl alcohol | G | VG | VG | VG | |
| Bisbiguanides | Chlorhexidine digluconate (4%) | F | F | VG | -- |
|
|||||
In addition to chemical compatibility, the degree that the glove material impedes dexterity or punctures should also be considered when selecting gloves. Static-free gloves may be needed when handling dry toxins. In general, hand exposure to infectious material or toxins can be managed effectively through the use of latex, nitrile, or vinyl gloves. Cut- or puncture-resistant gloves provide suitable hand protection for activities where there is a risk of accidental cuts, bites, punctures, or abrasions.
9.1.1.1 Double-Gloving
While typical glove materials create a suitable barrier against infectious material and toxins, they will not always remain impermeable. Gloves are susceptible to wear, punctures, rips, and tears, causing the glove barrier to fail with use. Wearing two pairs of disposable fluid-resistant gloves significantly reduces breaches to the innermost glove barrier.Footnote 8 Double-gloving may be recommended in certain situations, and indicated in higher containment levels, based on an LRA.
9.1.1.2 Protection from Physical Hazards
It is important to verify that the glove material selected provides an effective protective barrier against the hazards that will be encountered prior to handling the hazardous materials (i.e., compatible with disinfectants or solvents in use). For example, latex, nitrile, and vinyl gloves offer little to no protection from physical hazards such as temperature extremes (e.g., heat from an autoclave, cold from liquid nitrogen) or from sharp objects (e.g., needles, scalpels, animal teeth). In these situations, alternative or additional gloves would be more suitable. Insulated glove materials include terry cloth or wool to protect against high temperatures, and jersey- or cotton-lined nylon gloves to protect against low temperatures. When protection against sharps, cuts, or bites is needed, para-aramid fiber or stainless steel mesh gloves provide an effective barrier. For some activities, para-aramid fiber or stainless steel mesh gloves may need to be paired with fluid-resistant gloves for appropriate protection.
9.1.2 Foot Protection
Protective footwear is meant to provide an effective barrier against the pathogens and toxins encountered in the containment zone. This may include protection from spills of infectious material or toxins, or slipping, tripping, crushing, or puncture injuries, all of which have the potential of leading to or increasing the risk of an exposure to pathogens and toxins, and thereby to a risk of subsequent transmission to the public or the animal population. Completely enclosed footwear will protect the entire foot from exposure to hazardous liquids in the event of a spill. The risk of an accidental trip or fall can be reduced by wearing footwear with no heels or low heels, or footwear with a non-slip sole in areas where the walking surface is often wet or slippery. Steel-toe footwear can be worn to protect against crushing injuries when working with heavy objects or large-sized animals. For activities involving the use of sharp objects, footwear with puncture-resistant soles provides protection against puncture injuries. Footwear made of non-absorbent materials will enable easy cleaning and disinfection. The use of dedicated footwear limits the movement of infectious or potentially infectious material out of the containment zone on contaminated footwear.
Disposable, fluid-resistant shoe covers provide an added layer of protection from liquid contamination. Reusable boot covers can be used, provided that appropriate decontamination procedures are in place. Alternatively, rubber boots may be used in conjunction with disinfectant footbaths to protect personnel whenever large volumes of water will be used (e.g., cubicle decontamination and cage washing). Additional protective footwear for work in animal rooms, animal cubicles, and post mortem rooms (PM rooms), may include boot covers in animal containment zones housing small-sized animals, and rubber boots or other safety footwear in animal cubicles and PM rooms housing large-sized animals.
In addition to the requirements specified in Matrix 4.4 of the CBS, foot protection should comply with Canadian Standards Association (CSA) standard CSA-Z195, Protective Footwear, where applicable.Footnote 9
9.1.3 Head Protection
Some activities involving infectious material and toxins pose a risk of exposure due to sprays, splashes, or airborne exposure. This can be mitigated through the use of head protectors or covers (e.g., liquid-repellent bouffant caps) to shield the hair and scalp from contamination. Where applicable, head protection should comply with standard CSA Z94.1, Industrial protective headwear - Performance, selection, care, and use.Footnote 10
9.1.4 Eye and Face Protection
There are many different types of eye and face protection that can be used to shield the eyes, nose, or mouth from flying objects or splashes from infectious liquids or toxins. The type of eye and face protection selected will depend on the degree of coverage needed for the specific task at hand. Safety glasses protect the eyes from injuries associated with larger objects, including chips, fragments, sand, and dirt, as well as minor splashes. Safety goggles provide a higher level of protection due to the snug fit over and around the eyes, which creates a barrier to liquid hazards. Face shields provide coverage of the nose, mouth, and skin, in addition to the eyes. Depending on the type of protective eye and face equipment selected, prescription eye glasses may be worn underneath; safety glasses can also have prescription lenses. Some activities may pose additional risks for users of contact lenses in the event of an exposure or injury affecting the eye. A means of identifying contact lens wearers will help facilitate a timely and appropriate response in the event of an eye-related exposure or injury. Eye and face protection may be reused, provided that it is appropriately decontaminated after coming in contact with infectious material or toxins. Used disposable eye and face protection is considered contaminated waste.
Where applicable, eye and face protection should comply with standard CSA Z94.3, Eye and Face Protectors and CSA Z94.3.1, Selection, Use, and Care of Protective Eyewear. According to CSA Z94.3, face shields are considered secondary protectors only and provide adequate eye protection only when worn with safety glasses or goggles.Footnote 11Footnote 12
9.1.5 Body Protection
A lab coat is the most common type of PPE used to protect an individual's body and personal clothes against contamination with infectious material. Lab coats that are approximately knee-length and cover the arms to the wrists protect the skin and personal clothing from exposure to hazardous materials. Lab coats that fit closely to the body and have cuffed sleeves help prevent dragging and catching of clothing during laboratory work. Snap closures are preferred over buttons to allow quick removal of the lab coat in the event of an emergency. Lab coats are commercially available in single-use (i.e., disposable) or reusable materials. Fire-resistant and fluid-resistant varieties are also available; these provide increased protection against flammable or liquid hazards. Restricting storage and use of lab coats and other protective clothing to designated areas within the containment zone helps prevent contamination of clean areas.
Wearing appropriate personal clothing also contributes to body protection. Wearing clothes that cover the legs down to the ankles will offer protection. Shorts, skirts, and other clothing that leaves the legs exposed below the lab coat should not be worn in the containment zone.
9.1.5.1 Additional Layer of Protective Clothing
An additional layer of dedicated PPE provides enhanced protection for work with infectious material, toxins, or animals infected with zoonotic pathogens. This additional layer may include a second pair of gloves; head covers; solid-front gowns with tight-fitting wrists worn over dedicated laboratory clothing; full-body suits and coveralls; or, disposable sleeve covers or waterproof aprons worn over a solid-front gown or lab coat. A solid-front, rear-closing gown, typically worn over dedicated clothing (e.g., scrubs) in place of a lab coat, provides protection to the torso and can be worn when working with open vessels of infectious material or toxins. Surgical scrubs can be worn under the outer layer of protective clothing to avoid potential contamination of personal attire in the event the outer layer of protection is breached. Surgical scrubs are commonly part of the dedicated PPE for high containment zones or animal rooms and cubicles as they can be sterilized and laundered for reuse. Surgical gowns designed for use in operating rooms are reinforced with impermeable fabric to provide a full fluid-resistant layer, and have back tape ties and an overlapping back for improved coverage. Aprons are commonly used in PM rooms and necropsy suites; they are worn over lab coats or gowns and offer additional protection against spills or splashes of infectious material or toxins. Full-body suits and coveralls provide further protection and are available in disposable or reusable materials. Individuals working with large-sized animals commonly wear coveralls to provide protection from organic material. Coverall materials such as flashspun high-density polyethylene fibres, rubberized cloth, polyvinyl chloride (PVC), and neoprene provide a good barrier as they are hard to tear or puncture and will prevent penetration by biological, chemical, or particulate contaminants.
9.1.5.2 Positive-Pressure Suits
Positive-pressure suits provide the maximum full-body coverage (i.e., head-to-toe) to protect from the containment zone environment, and include integral boots, gloves, and headpiece. Breathable air is provided through a supplied air hose connected to the suit, which creates a positive pressurization within the suit. Integrity testing is conducted to demonstrate that suits are gas tight (i.e., no tears or leaks) and able to maintain a fixed positive pressure when inflated.
9.1.6 Masks and Respiratory Protection
Safe operational practices and the use of primary containment devices can limit the creation of, and exposure to, infectious aerosols or aerosolized toxins. Surgical masks and many types of dust masks offer little protection from airborne pathogens, infectious aerosols, or aerosolized toxin, but will protect mucous membranes of the nose and mouth from spills and splashes. Masks are not intended to be used more than once. Respirators are used when there is a risk of exposure to aerosolized toxins or infectious aerosols that can be transmitted through the inhalation route. Respirators are divided into two classes: air purifying respirators and atmosphere-supplying respirators. The type of respirator selected will depend on the hazard associated with the particular activity being carried out. Personnel education on airborne hazards and training on respirator selection, fit, inspection, and maintenance are some examples of elements of a workplace respiratory protection program, which is required for any workplace where respirators are used. Where applicable, respiratory protection should conform to standard CSA Z94.4, Selection, Use and Care of Respirators.Footnote 13
9.1.6.1 Respirator Fit
All respirators need to fit properly in order to function as intended. Some types of respirators require a seal between the apparatus and the wearer's face in order to provide adequate protection. Using the wrong respirator or misusing one can be as dangerous as not wearing one at all. The respirator should be individually selected and fitted to the operator's face, and fit tested for its seal. Facial hair, imperfections of the skin, cosmetics, and changes in a person's weight can affect respirator fit. Most jurisdictions within Canada currently require qualitative or quantitative fit-testing to be conducted to demonstrate proper fit for the selected respirator(s) before an individual carries out any activities that require respiratory protection. In addition, standard CSA Z94.4, Selection, Use, and Care of Respirators, requires that an employer take reasonable precautions to verify that an individual is medically cleared to wear a respirator. Proper use and care of respiratory protection equipment is a core component of the training program in workplaces where respirators are used.
9.1.6.2 Air Purifying Respirators
Air purifying respirators help reduce the concentration of microorganisms and particulates in the air inhaled by the user to an acceptable exposure level by passing the air through a particulate filter or chemical cartridge. Half-mask air purifying respirators cover the nose and mouth but not the eyes, while full-face air purifying respirators cover the entire face. Disposable half-mask air purifying respirators, including the N95 and N100 type respirators, are designed for single use. Non-powered half-mask and full-face respirators can also use disposable filter cartridges to provide a similar level of protection. Non-powered respirators work through the creation of negative-pressure inside the respirator during inhalation. There are nine classifications of particulate filters used with non-powered respirators approved by the United States National Institute of Occupational Safety and Health (NIOSH).Footnote 14 These are the N-Series (N95, N99, N100; not resistant to oil), R-Series (R95, R99, R100; oil-resistant), and P-Series (P95, P99, P100; oil-proof). The associated numbers identify the efficiency in removing contaminants. Respirators rated at N95 or higher are adequate to protect personnel carrying out most activities with microorganisms.
9.1.6.3 Powered Air Purifying Respirators
Powered air purifying respirators (PAPRs) create a positive-pressure around the wearer's head. PAPRs are designed to be decontaminated and reused, and the disposable filter cartridges are replaced on a regular basis, as determined by an LRA. Particulate filters for PAPR units are all high efficiency (HE), which are certified to be 99.97% efficient at filtering the most penetrating particle size (0.3 µm). Due to the effects of impaction, diffusion, and interception, high efficiency particulate air (HEPA) filters are even more efficient for particles that are either smaller or larger than 0.3 µm.Footnote 15 Most PAPR filters are suitable for use against oil-based aerosols; however, this is not always the case and users should check the manufacturer instructions before use in oil environments.
9.1.6.4 Atmosphere-Supplying Respirators
Atmosphere-supplying respirators deliver clean, breathable air from a source such as a compressed air cylinder or tank. These are generally supplied-air respirators, but could be a self-contained breathing apparatus (SCBA). Supplied-air respirators deliver air through a small hose connected to an air compressor or a cylinder of compressed air, whereas SCBAs supply breathable air from a portable cylinder worn on the back.
9.2 Key Considerations for the Selection of Personal Protective Equipment
No single glove or respirator type can be expected to provide protection against all the different types of hazards in a work environment. Poorly chosen PPE can impair personnel performance (e.g., stiff, bulky or inappropriately sized gloves may reduce dexterity and control), creating the potential for accidents that can lead to the exposure to hazards. The first step to be taken prior to handling infectious material or toxins is to perform an LRA to develop safe work practices and select appropriate PPE. The selection of PPE will depend on the containment level, the amount and nature of the infectious material or toxins in use, and the activities being performed. This type of evaluation should be conducted by the biological safety officer (BSO) or other appropriate personnel (i.e., infection control or industrial hygiene specialist) and the employee(s) concerned, possibly in consultation with the employer, the institutional biosafety committee (IBC), and the health and safety committee. Once the need for PPE has been identified, the correct PPE is chosen based on the degree of protection required and the suitability of the equipment for the situation (e.g., gloves that provide appropriate dexterity, clothing and footwear that provide adequate protection). For example, in large animal containment zones (LA zones), where the animal cubicle provides primary containment and PPE is the worker's primary protection against exposure to pathogens and toxins, it is essential that personnel select their PPE accordingly. It is important to involve employees in the selection of the PPE to verify fit and comfort, and to encourage use. Once selected, employees should be adequately trained in the proper use of the PPE, including when it must be worn, the appropriate methods of donning and doffing PPE, limitations, proper care and maintenance, and decontamination and disposal of PPE.
Many factors related to the hazard must be taken into consideration when selecting proper PPE, but it is also important to take allergies and ergonomics into account. Allergies to certain materials (e.g., latex in gloves) can sometimes pose more of a health risk than the hazards themselves. Comfort and fit are key factors in addressing potential ergonomic issues; personnel may be inclined to remove PPE if it does not fit correctly or is uncomfortable. When selecting PPE that will be used when handling large-sized animals, consideration should be given to selecting PPE that is lightweight, non-encumbering, cool, and that is not prone to becoming snagged or entangled by animals or equipment.
9.3 Use of Personal Protective Equipment
9.3.1 Donning
Procedures to don, or put on, PPE describe the specific order to don each article. Standard operating procedures (SOPs) to don PPE prior to entering laboratory work areas, animal rooms, animal cubicles, PM rooms, and containment zones are developed based on an LRA and vary in complexity depending on the types of PPE used. It is important that these SOPs are understood and adhered to by all personnel. Storage of PPE at all points of routine entry enables easy access to PPE when preparing to enter the containment zone. Individuals should carefully inspect the articles for damage or breaches prior to donning PPE. For containment zones where only a lab coat and gloves are worn, the following donning procedure is recommended:
- Single Gloves and Lab Coat
- Donning Order
- Lab coat (properly fastened)
- Gloves (fitted over cuffs of lab coat)
- Doffing Order
- Gloves
- Lab coat
- Donning Order
- Double Gloves and Lab Coat
- Donning Order
- Inner gloves
- Lab coat (properly fastened)
- Outer gloves (fitted over cuffs of lab coat)
- Doffing Order
- Outer gloves
- Lab coat
- Inner gloves
- Donning Order
In containment level 2 (CL2) large animal containment zones (LA zones) (i.e., CL2-Ag zones) and high containment zones, clothing change areas are used to separate personal clothing and dedicated containment clothing, or to separate the dedicated protective clothing worn in the different areas of containment (i.e., animal containment zone versus animal cubicle). An example of a donning procedure when multiple layers of PPE are involved is shown below. Personal clothing and accessories, including jewelry and identification (ID) cards, are removed and stored in a dedicated area before donning PPE.
- Multiple Layers of PPE
- Donning Order
- Dedicated containment clothing, such as scrubs, dedicated footwear, shoe covers, and head covers
- Inner gloves
- Back-closing gown or equivalent protective layer
- Mask or respirator
- Eye protection, including safety glasses, goggles, or face shield
- Outer gloves, fitted over gown cuffs
- Doffing Order
- Outer gloves
- Eye protection, including safety glasses, goggles, or face shield
- Mask or respirator
- Back-closing gown or equivalent protective layer
- Inner gloves
- Dedicated containment clothing, such as scrubs, dedicated footwear, shoe covers, and head covers
- Donning Order
Depending on the nature of the activities carried out in the containment zone, there may be special requirements posted at the point(s) of entry for personnel to follow prior to entering, including the use of specific PPE. The PPE and donning order could be different in other scenarios (e.g., working in a containment level 3 [CL3] LA zone [i.e., CL3-Ag] or PM room where a PAPR is worn based on the LRA).
9.3.2 Doffing
It is important to doff, or remove, PPE with care to minimize the risk of contamination of the skin and hair. The SOPs for doffing PPE when exiting laboratory work areas, animal rooms, animal cubicles, PM rooms, and containment zones describe the removal order and any specific instructions related to each article of PPE being removed. Doffing is generally done in the reverse order to the donning procedure, as outlined in Section 9.3.1 of this chapter. It is important to remember that the front and sleeves of the lab coat may be contaminated.
Considerations when doffing gloves are as follows:
- Gloves should be carefully removed by grasping the outside of the glove near the wrist with the opposite gloved hand and carefully peeling the glove off, turning it inside out.
- The removed glove should be held in the opposite gloved hand. A finger from the ungloved hand should slide under the wrist of the remaining glove to peel it off from the inside, creating a bag for both gloves that is carefully discarded in a designated biohazardous waste container. If two pairs of gloves are worn, these steps are repeated for the second pair.
- Hands are then to be washed, in accordance with exit procedures, before leaving the containment zone, animal room, animal cubicle, or PM room. It is recommended that hands be washed with soap under clean running water; the hands should be rubbed together to make a lather and thoroughly scrubbed, including the backs of the hands, between fingers, and under the fingernails, for a minimum of 15-30 seconds before rinsing.Footnote 16 Alcohol-based hand sanitizers are not as effective as handwashing with soap and water and cannot eliminate all types of pathogens.Footnote 17 A hand sanitizer that has been demonstrated to be effective against the pathogen(s) or toxin(s) in use in the containment zone may be an alternative where handwashing sinks are not easily accessible to avoid the spread of contamination. See Appendix B for proper handwashing technique.
Considerations for the doffing of other items and the recommended doffing sequence for all PPE are reflected in the following example:
- Gloves should be removed prior to removing hands from a biological safety cabinet (BSC) after handling infectious material or toxins, and they should be discarded as biohazardous waste within the BSC. This will help prevent the inadvertent spread of contamination outside the BSC. If two pairs of gloves are worn, it is the outermost layer of gloves that is removed prior to exiting the BSC. The inner layer of gloves will protect the user's hands from exposure to any residual infectious aerosols or aerosolized toxins prior to removal from the BSC. In order to prevent the spread of contamination outside of the BSC, gloved hands should be disinfected or decontaminated immediately after removal from the BSC; bare hands should be washed immediately after removal from the BSC.
- Gowns should next be removed, keeping in mind that the gown front and sleeves may be contaminated. The gown should be removed by unfastening the ties and peeling the gown away from the neck and shoulders, keeping the contaminated side away from the body and folding or rolling it into a bundle before discarding it in the designated waste container for decontamination.
- Face shields and protective eyewear should then be removed, keeping in mind that the outside of the eyepiece may be contaminated. These should be handled by the head band or ear pieces and pulled away from the face, then placed in a designated receptacle for decontamination.
- Masks and respirators can then be removed, keeping in mind that the front of the mask may be contaminated. Masks are removed as per the manufacturer directions and precautions should be taken to avoid transfer of contamination from the outside of the mask. The mask is then discarded.
- Hair covers and protective headgear can be removed and discarded or decontaminated.
- Protective footwear and shoe covers should be removed next and stored or discarded.
- Finally, the inner pair of gloves (when two pairs of gloves are worn), can be removed and discarded. Hands are immediately washed thoroughly with soap and water to remove and decontaminate any potential pathogens that may have penetrated the layers of PPE.
Doffing of PPE should always be immediately followed by handwashing at a sink dedicated for the purpose, after which personnel can change out of surgical scrubs and back into their personal clothes. Hand sanitizers may only be used as an alternative to handwashing where sinks are not easily accessible, provided the hand sanitizer has been demonstrated to be effective against the pathogen(s) or toxin(s) in use in the containment zone. This example does not represent the procedure when a walk-through body shower is required upon exit, but it does indicate the order in which PPE should be removed to minimize the risk of contamination. Personnel working in high containment zones are required to remove the additional layer of full-body coverage clothing when exiting across the containment barrier. Personnel exiting animal rooms, animal cubicles and PM rooms at any containment level, are to remove dedicated clothing (including footwear) or remove the additional layer of PPE and footwear (when worn), unless exiting to the dirty corridor. Where a clothing change is not required for exiting animal cubicles and PM rooms, it is good practice for personnel to use a disinfectant footbath appropriate for the pathogen in use to effectively decontaminate footwear when exiting the animal work area.Footnote 18
9.3.3 General Use Tips
The following sections provide general information for the use of different types of PPE.
9.3.3.1 Gloves
- Nitrile or vinyl gloves can be worn instead of latex to provide fluid-resistance (e.g., if allergic to latex).
- Verify that gloves are intact; inspect for rips, tears and flaws before use.
- Change gloves often if wearing for long periods of time.
- Never reuse disposable gloves. Dispose of used gloves in an appropriate contaminated waste receptacle.
- Remove gloves and wash hands prior to exit from the containment zone, animal room, animal cubicle, or PM room.
9.3.3.2 Footwear
- Wear shoes that cover the entire foot with no heels or low heels.
- Footwear should protect from hazardous liquids and be easily cleaned and disinfected. Verify that disposable shoe covers are intact; inspect for rips and tears before use.
- Never reuse disposable shoe covers. Dispose of used shoe covers in an appropriate contaminated waste receptacle.
- Never wear dedicated footwear outside the containment zone.
- Wear waterproof boots in wet environments.
9.3.3.3 Head Protection
- Remove head protection prior to exiting from the containment zone.
- Decontaminate reusable head protection after use; collect disposable head covers and decontaminate prior to removal from the containment zone for disposal.
9.3.3.4 Eye and Face Protection
- Wear safety eyewear in environments where there is a chance of eye exposure.
- Wear safety goggles to protect eyes against splashes and spills.
- Wear a face shield to protect nose, mouth, and skin against splashes and spills.
- Decontaminate reusable eye and face protection after every use, even if stored in the containment zone.
- Decontaminate prescription eyeglasses at the containment barrier prior to exiting high containment zones, unless protected by additional PPE (e.g., PAPR or other full head cover, as determined by an LRA).
- Never wear dedicated eye or face protection outside the containment zone.
9.3.3.5 Body Protection
- Wear completely fastened body protection with sleeves covering arms.
- Remove, decontaminate, and launder protective clothing after it has become contaminated. In high containment zones, remove protective layer prior to exit from the containment barrier; all PPE (reusable or disposable) is decontaminated before removal from the containment zone. Reusable protective clothing is decontaminated before being sent to laundry; laundering facilities located inside the containment zone may be suitable for decontamination provided they have been demonstrated to be effective for decontamination of the pathogen(s) in use (i.e., validated).
- Never wear dedicated body protection outside the containment zone (e.g., in offices, cafeteria).
9.3.3.6 Masks and Respiratory Protection
- Complete respirator training and verify proper fit through qualitative or quantitative fit-testing prior to commencing any activities requiring respirator.
- Perform a seal check every time the respirator is donned.
- After every use, clean and sanitize or decontaminate the respirator according to the manufacturer's instructions or SOPs developed in consultation with the manufacturer, even if it is stored in the containment zone.
- Care must be taken to prevent the filters or cartridge media from becoming wet during decontamination. Replace cartridges that are near the end of their service life.
- Never reuse disposable respirators or masks; decontaminate used respirators and masks prior to disposal.
- Inspect the respirator after use; discard or repair any defective parts.
- Remove respiratory protection at the point at which a risk assessment deems it safe to do so upon exit from the containment zone.
- Reusable respirators should be stored in a manner that will protect them against any potential hazard that can have a detrimental effect (e.g., dust, sunlight, heat, extreme cold).
References
- Footnote 1
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 2
- Klingner, T. D., & Boeniger, M. F. (2010). A critique of assumptions about selecting chemical-resistant gloves: A case for workplace evaluation of glove efficacy. Applied Occupational and Environmental Hygiene. 17(5): 360-367.
- Footnote 3
- ASTM F739-12. Standard Test for Permeation of Liquids and Gases through Protective Clothing Materials under Conditions of Continuous Contact. (2012). West Conshohocken, PA, USA: American Society for Testing and Materials.
- Footnote 4
- Ansell Occupational Healthcare. (2008). Chemical Resistance Guide: Permeation & Degradation Data (8th Edition). Retrieved 11/03, 2015 from http://www.ansellpro.com/download/Ansell_8thEditionChemicalResistanceGuide.pdf
- Footnote 5
- Ansell Occupational Healthcare. A Guide to Safe Handling of Hazardous Materials. Retrieved 11/03, 2015 from http://www.ansellhealthcare.com/pdf/guide_hazardous_materials.pdf
- Footnote 6
- All Safety Products. Glove Selection Chart - Chemical Breakthrough Ratings. Retrieved 11/03, 2015 from http://www.allsafetyproducts.com/glove-selection-chart-chemical-breakthrough-ratings.html
- Footnote 7
- National Research Council. (1981). Prudent Practices for Handling Hazardous Chemicals in Laboratories. Washington, DC, USA: The National Academy Press, pp. 159-160.
- Footnote 8
- Albin, M. S., Bunegin, L., Duke, E. S., Ritter, R. R., & Page, C. P. (1992). Anatomy of a defective barrier: sequential glove leak detection in a surgical and dental environment. Critical Care Medicine. 20(2):170-184.
- Footnote 9
- CSA Z195-14, Protective Footwear. (2014). Mississauga, ON, Canada: Canadian Standards Association.
- Footnote 10
- CSA Z94.1-15, Industrial protective headwear - Performance, selection, care, and use. (2015). Mississauga, ON, Canada: Canadian Standards Association.
- Footnote 11
- CSA Z94.3-07 (R2014), Eye and Face Protectors. (2007). Mississauga, ON, Canada: Canadian Standards Association.
- Footnote 12
- CSA Z94.3.1-09, Selection, Use, and Care of Protective Eyewear. (2009). Mississauga, ON, Canada: Canadian Standards Association.
- Footnote 13
- CSA Z94.4-11, Selection, Use, and Care of Respirators. (2011). Mississauga, ON, Canada: Canadian Standards Association.
- Footnote 14
- United States National Institute for Occupational Safety and Health (1996). NIOSH Guide to the Selection and Use of Particulate Respirators. DHHS (NIOSH) Publication Number 96-101. Retrieved on 11/30, 2015 from http://www.cdc.gov/niosh/docs/96-101/
- Footnote 15
- Richardson, A. W., Eshbaugh, J. P., Hofacre, K. C., & the Edgewood Chemical Biological Center United States Army Research, Development and Engineering Command. (2006). ECBC-CR-085: Respirator Filter Efficiency Testing Against Particulate and Biological Aerosols Under Moderate to High Flow Rates. Columbus, OH, USA: Battelle Memorial Institute.
- Footnote 16
- Public Health Agency of Canada. (2012). Hand Hygiene Practices in Healthcare Settings. Ottawa, ON, Canada: Public Health Agency of Canada.
- Footnote 17
- United States Centers for Disease Control and Prevention. Show Me the Science - When to Use Hand Sanitizers. Retrieved on 11/03, 2015 from http://www.cdc.gov/handwashing/show-me-the-science-hand-sanitizer.html
- Footnote 18
- Morley, P. S., Morris, S. N., Hyatt, D. R., & Van Metre, D. C. (2005). Evaluation of the efficacy of disinfectant footbaths as used in veterinary hospitals. Journal of the American Veterinary Medical Association. 226(12):2053-2058.
Chapter 10 - Air Handling
The heating, ventilation, and air conditioning (HVAC) system provides fresh air and maintains good indoor air quality. It provides general cleaning and filtration of air in the indoor environment and controls temperature, humidity, and odours from animals, as well as providing ventilation (e.g., for chemical use during decontamination). Guidelines on ventilation for laboratory environments are provided in several standards, including the American National Standards Institute (ANSI)/American Industrial Hygiene Association (AIHA) Z9.5, ANSI/American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) 62.1 and National Standard of Canada (CAN)/Canadian Standards Association (CSA)-Z317.2, although local regulations and building and fire codes should also be consulted.Footnote 1Footnote 2Footnote 3 The Canadian Council on Animal Care (CCAC) Guidelines on: Laboratory Animal Facilities provides further guidance on HVAC systems for activities involving animals.Footnote 4 The requirements for air handling in containment zones regulated by the Public Health Agency of Canada (PHAC) and the Canadian Food Inspection Agency (CFIA) are specified in Matrix 3.5 of the Canadian Biosafety Standard (CBS), 2nd Edition.Footnote 5
10.1 Inward Directional Airflow
HVAC systems can be designed to maintain a negative differential air pressure within the containment zone so that air flows into the containment zone, across the containment barrier, from areas of lower containment to areas of higher containment. Inward directional airflow (IDA) helps to establish a buffer zone of air at the containment barrier that serves to reduce the potential for aerosolized infectious materials or toxins to be released from the work area. Where inward directional airflow (IDA) is provided, anterooms and door interlocks (or standard operating procedures [SOPs]) are often included to accommodate the entry of personnel, animals, and equipment through the containment barrier. HVAC systems that provide inward directional airflow (IDA) are critical containment systems. Further guidance and considerations regarding anterooms can be found in Chapter 3. The requirements for anterooms are specified in Matrix 3.3 of the CBS; the requirements for inward directional airflow (IDA) are specified in Matrix 3.5 of the CBS.
High containment zones are designed so that air pressure decreases when progressing deeper into the containment zone (e.g., through a sequence of air pressure differentials between the clean and dirty sides of anterooms and showers). In high containment zones, HVAC systems can be supported by emergency power, and at containment level 4 (CL4), building automation systems are supported by uninterruptible power supply (UPS) to maintain operation in case of a power failure. Interlocks, visual and audible alarms, and protocols can be used to prevent the opening of the critical door on the containment barrier simultaneously with the door leading into the anteroom from outside of the containment zone, and the door(s) leading from the anteroom into the laboratory work area, animal room, animal cubicle, or post mortem room (PM room), which could disrupt the inward directional airflow (IDA) and the integrity of the containment barrier.
Sealing openings in the barrier (e.g., windows, doors, ductwork, conduits), and the correct use of appropriately designed equipment located on the containment barrier (e.g., barrier autoclaves, pass-through chambers, and dunk tanks), will help to maintain inward directional airflow (IDA) and the integrity of the containment barrier (Matrices 3.2 and 4.8 of the CBS). Monitoring devices such as ball-in-tube monitors above doors that visually demonstrate inward directional airflow (IDA), or a differential pressure gauge to measure the pressure differential between rooms, are provided to allow containment zone personnel to verify that the inward directional airflow (IDA) is being maintained prior to entry (Matrices 3.5 and 4.5 of the CBS).
High efficiency particulate air (HEPA) filtration of exhaust air reduces the risk of releasing infectious material or toxins from high containment zones, while small in-line HEPA filters are used to protect the lines to pressure differential monitoring devices that penetrate the containment barrier. Supply air may also be HEPA filtered, depending on the containment level (CBS Matrix 3.5).
The following list highlights some requirements and recommendations for the installation of HVAC systems:
- Air should be exhausted from high containment zones to avoid re-introduction into the building, in accordance with applicable standards such as ANSI/ASHRAE 62.1.Footnote 2
- Controls such as interlocked supply and exhaust air systems will prevent sustained pressurization of the laboratory during fan failures, and audible and visual alarms will notify personnel of such failures (CBS Matrix 3.5).
- Transfer air devices designed to provide controlled leakage into the containment zones should be designed to ensure directional airflow is maintained and that backdraft protection is provided. These are devices that can be mounted into walls or doors to allow air transfer to limit pressure differentials between rooms.
- The use of auxiliary localized humidifiers may contribute to personnel and animal well-being by providing additional moisture.
- Mechanical support services for HVAC systems should be located as close as possible to the containment barrier. Locating HEPA filter housings as close to the containment barrier as possible will reduce the length of potentially contaminated ductwork. Installing valves to isolate sections of the ductwork will facilitate decontamination.
10.1.1 Verifying Inward Directional Airflow and Containment Barrier Integrity
Visual demonstration of inward directional airflow (IDA) at all critical doors of the containment barrier will confirm that air flows toward areas of higher containment, according to design, and never the reverse. Pressurization across adjacent areas can be visually tested under normal HVAC system operations by holding a smoke pencil at each door with the door in its normal state (i.e. typically closed). Testing with a smoke pencil or other visual aid should be conducted under normal operating conditions, as well as under simulated failure scenarios.
Smoke testing is also used to detect leaks in the surfaces within a containment zone that form part of the containment barrier. All joints, corners, sealed penetrations (e.g., conduits, plumbing, wiring), as well as seals around doors, windows, autoclaves, and dunk tanks should be surveyed for leaks. Visual inspections of floors, walls and ceilings, as well as floor/wall and wall/ceiling joints can identify cracks, chips, or wear that need repair.
10.1.1.1 Pressure Decay Testing
In containment zones with airtight doors or sealable doors, pressure decay testing of the containment zone (whole room) provides an indication of the integrity of the room perimeter (i.e., the ability of gases and liquids to move through the perimeter membrane and service penetrations). The basic procedure for pressure decay testing under negative pressure is as follows:Footnote 6
- Isolate the area by closing and securing all doors, valves, and isolation dampers at the containment barrier. Avoid temporary sealing measures in doors, windows, and services that would cover permanent seals and not permit their testing for leakage. Plug all pressure sensor lines (e.g., differential pressure gauges).
- Install a calibrated manometer across the containment barrier such that it is not affected by air distribution. The manometer should have a minimum accuracy of 10 Pa (i.e., 0.05 inches water gauge [in. w.g.]) and be capable of reading pressure up to 750 Pa (i.e., 3 in. w.g.).
- Install a ball valve in the piping between the vacuum source (i.e., pump or fan) and the room to allow the room to be sealed once the test pressure has been attained.
- Connect a vacuum source to the room and create a 500 Pa (i.e., 2 in. w.g.) negative pressure differential. Allow the room pressure to stabilize and close the valve between the vacuum source and the room to seal the room at 500 Pa (i.e., 2 in. w.g.).
- Dynamically trend pressure loss starting at 500 Pa (i.e., 2 in. w.g.) negative pressure differential; record the differential pressure at 1 minute intervals for 20 minutes.
- If repeat testing is required, allow a 20 minute wait period or longer if necessary for equilibration of the HVAC system.
- Disconnect the vacuum source and open the ball valve slowly to allow room pressure to return to normal.
- If the leak rate exceeds the acceptance value:
- pressurize the room to a pressure adequate to locate leaks;
- with the room under continuous pressure, apply bubble solution to areas to be tested (e.g., joints, corners, sealed penetrations); or if using audible leak location method, locate audible leaks (i.e., using electronic sound detection equipment);
- identify places where bubbles are found; and
- after repair of leak, retest as required.
10.2 High Efficiency Particulate Air Filters
HEPA filters are capable of filtering greater than 99.97% of airborne particles 0.3 µm in diameter, the most penetrating particle size. Due to the effects of impaction, diffusion, and interception, HEPA filters are even more efficient for particles that are either smaller or larger than 0.3 µm in diameter.Footnote 7 Although HEPA filters are factory rated at 99.97% efficient, they will typically achieve a much higher efficiency. HEPA filter performance of a minimum efficiency of 99.99% should be used for containment facilities. Consumers need to make the installed filtration requirements clear to the HEPA filter suppliers prior to purchase.Footnote 8
Typical HEPA filters are fabricated from a single pleated sheet of fibres. The pleats are divided by separators (e.g., corrugated aluminum) to prevent the pleats from collapsing in the air stream (see Figure 10-1 inset). The filter medium is glued into a wood, metal, or plastic frame, which can be easily damaged or distorted if handled incorrectly. For this reason, it is important that filter integrity and performance be verified after installation or relocation, and regularly thereafter.
HEPA filters are typically installed in filter housings (Figure 10-1) by means of a gasket (e.g., neoprene) or fluid (e.g., gel) seal. A common problem with gaskets is that they can be compressed, torn, or may be incompatible with gaseous decontaminants. For example, some types of neoprene (e.g., open-celled black neoprene gaskets) are degraded by hydrogen peroxide (H2O2). Dense gasket materials can be more resistant to frequent decontamination than open-celled gaskets made of similar material. Gel seals establish an airtight seal between the filter and housing by means of a channel filled with gel that surrounds the filter perimeter. The housing knife-edge flange seals into this channel. Gel seals are not prone to the compression and compatibility problems associated with gasket seals. The integrity of HEPA filters is performance tested to confirm that there are no leaks in the filter media, the gaskets, or the seal to the filter housing; this filter housing test is performed by challenging with a known particulate concentration and scanning for percentage of penetration downstream of the filter (i.e., "scan" testing).
Filters that are loaded should be replaced when airflow cannot be maintained within target range, or as per the manufacturer's instructions. Consideration should be given, especially in animal containment zones, to the installation of pre-filters to protect HEPA filters from dust and debris (e.g., hair, fur). The standard ASHRAE 52.2, Gravimetric and Dust-Spot Procedures for Testing Air-Cleaning Devices Used in General Ventilation for Removing Particle Matter can be consulted for more information on pre-filters.Footnote 9
HEPA filters that can be decontaminated through in situ fumigation with a gas (e.g., formaldehyde or vaporized hydrogen peroxide [VHP], chlorine dioxide [ClO2]) allows for them to be decontaminated prior to their removal. In containment zones where available decontamination technologies are not effective against the pathogens and toxins handled (e.g., prions are not completely inactivated by most common decontamination methods), an alternative mechanism for the safe removal of HEPA filters is required. Examples of suitable alternatives include using HEPA filters with a bag-in/bag-out capability or using procedures to contain the HEPA filter for removal followed by its subsequent decontamination.
Figure 10-1: Representative Diagram of a High Efficiency Particulate Air (HEPA) Filter Housing with Cut Away Showing HEPA Filters within the Housing
The inset shows the filter media: a pleated sheet of fibres divided by separators.

Text Equivalent - Figure 10-1
The figure shows air ducts leading into and out of a housing that contains a HEPA filter. There are dampers on the ducts to either side of the housing to allow for the decontamination of the filter. A door on the housing allows access for changing the filters and the cutaway shows the location of the filters. An inset shows the filter media: a pleated sheet of fibres divided by separators that provide rigidity.
Figure 11-1a: Illustration of a Class one Biological Safety Cabinet (BSC)
In this figure, a Class one BSC is hard-ducted and functions using the building's HVAC system. Room air is drawn through the front opening of the cabinet and moves across the negatively-pressurized workspace. It is then drawn through an air grille situated at the rear of the cabinet, flows up a plenum and through a HEPA filter before being discharged to the outside environment.
References
- Footnote 1
- ANSI/AIHA/ASSE Z9.5-2012, Laboratory Ventilation. (2012). Fairfax, VA, USA: American National Standards Institute / American Industrial Hygiene Association.
- Footnote 2
- ANSI/ASHRAE 62.1-2013, Ventilation for Acceptable Indoor Air Quality. (2013). Atlanta, GA, USA: American National Standards Institute / American Society of Heating, Refrigerating and Air-Conditioning Engineers.
- Footnote 3
- CAN/CSA Z317.2-10, Special requirements for heating, ventilation, and air-conditioning (HVAC) systems in health care facilities. (2010). Mississauga, ON, Canada: Canadian Standards Association.
- Footnote 4
- Canadian Council on Animal Care. (2003). CCAC Guidelines on: Laboratory Animal Facilities - Characteristics, Design and Development. Ottawa, ON, Canada: Canadian Council on Animal Care.
- Footnote 5
- Government of Canada. (2015). Canadian Biosafety Standard (2nd ed.). Ottawa, ON, Canada: Government of Canada.
- Footnote 6
- United States Department of Agriculture Research, Education, and Economics Division. (2012). Agriculture Research Service (ARS) Facilities Design Standards, ARS-242.1. Washington, DC, USA: United States Government Printing Office.
- Footnote 7
- Richardson, A. W., Eshbaugh, J. P., Hofacre, K. C., & the Edgewood Chemical Biological Center United States Army Research, Development and Engineering Command. (2006). ECBC-CR-085: Respirator Filter Efficiency Testing Against Particulate and Biological Aerosols Under Moderate to High Flow Rates. Columbus, OH, USA: Battelle Memorial Institute.
- Footnote 8
- IEST RP-CC001.5, HEPA and ULPA Filters. (2010). Rolling Meadows, IL, USA: Institute of Environmental Sciences and Technology.
- Footnote 9
- ANSI/ASHRAE 52.2-2012, Method of Testing General Ventilation Air-Cleaning Devices for Removal Efficiency by Particle Size. (2012). Atlanta, GA, USA: American National Standards Institute / American Society of Heating, Refrigerating and Air-Conditioning Engineers.