Tetanus toxoid: Canadian Immunization Guide
For health professionals
Updated: October 2014
On this page
- Key Information
- Epidemiology
- Preparations Authorized for Use in Canada
- Efficacy, Effectiveness and Immunogenicity
- Recommendations for Use
- Vaccine Administration
- Serologic Testing
- Storage Requirements
- Simultaneous Administration with Other Vaccines
- Vaccine and Immunoglobulin Safety and Adverse Events
- Other Considerations
- Selected References
Key Information (Refer to text for details)
- What
-
- Tetanus (lockjaw) occurs worldwide but is rare in Canada because of immunization against this infection.
- Tetanus toxoid is only available in combination vaccines.
- A primary series and boosters, including post-exposure boosters, are recommended to develop and maintain high circulating concentrations of tetanus antibody in the event of exposure to Clostridium tetani spores and subsequent toxin production. In unvaccinated or inadequately vaccinated individuals, tetanus immunoglobulin is recommended post-exposure in certain situations.
- After a complete primary series (at least 3 doses), more than 99% of vaccinees develop antibody concentrations that are protective against tetanus, but there is declining immunity over time.
- Redness, swelling and pain at the injection site are the most common adverse reactions to tetanus toxoid-containing vaccines.
- Who
-
- Tetanus toxoid-containing vaccine is recommended for:
- routine immunization of infants and children
- immunization of children who missed tetanus immunization on the routine schedule
- immunization of previously unvaccinated or incompletely vaccinated adults
- routine booster immunization of adolescents and adults
- post-exposure prophylaxis in some wound management situations
- Tetanus toxoid-containing vaccine is recommended for:
- How
-
- Routine tetanus immunization of infants and children: administer DTaP-IPV-Hib vaccine at 2, 4, 6 and 12 to 23 months of age (generally given at 18 months of age). If infant immunization for hepatitis B is undertaken, DTaP-HB-IPV-Hib vaccine may be used. Subsequently, administer a booster dose of either DTaP-IPV or Tdap-IPV vaccine at 4 to 6 years of age (school entry) and a booster dose of Tdap vaccine 10 years later at 14 to 16 years of age.
- Adults previously immunized with tetanus toxoid-containing vaccine: administer one dose of Tdap vaccine if not previously received in adulthood (18 years of age and older) and give a booster dose of Td vaccine every 10 years.
- Post exposure/wound management: the need for tetanus toxoid-containing vaccine in wound management, with or without tetanus immunoglobulin depends on both the nature of the wound and the vaccination history
- Tetanus toxoid-containing vaccines may be administered concomitantly with routine vaccines at different injection sites using separate needles and syringes.
- Why
-
- Tetanus occurs worldwide.
- Many Canadians, especially those who are older or born outside of Canada, do not have protective concentrations of tetanus antitoxin.
- The case fatality rate in the unvaccinated varies from 10% to over 80% and is highest in infants and the elderly.
Epidemiology
Disease description
Infectious agent
Tetanus (lockjaw) is caused by a neurotoxin produced by the bacterium Clostridium tetani.
Reservoir
C. tetani spores are widely distributed in soil worldwide and have also been detected in the intestines of animals and humans.
Transmission
C. tetani spores are usually introduced into the body through a wound that is contaminated with soil, animal/human feces or dust. C. tetani spores will germinate into bacilli in an anaerobic environment, such as necrotic tissue. The bacilli release a potent neurotoxin. The incubation period is generally 3 to 21 days (range, 1 day to several months). Since tetanus is caused by the neurotoxin, it is not transmitted person-to-person.
Risk factors
Cases of tetanus related to lacerations (most frequent), injection drug use, and animal bites have been reported, as well as rare cases occurring after bowel surgery or aspiration of soil and feces. Cases may occur following small, insignificant wounds, especially when there is necrotic tissue present. It is often associated with blunt trauma or deep puncture wounds. Tetanus rarely occurs in fully vaccinated people and, if it does, it is usually mild.
Spectrum of clinical illness
Tetanus is characterized by muscle spasms, usually in a descending pattern, beginning in the jaw muscles. As the disease progresses, prolonged frequent spasms may occur, contributing to serious complications and death, unless treatment is provided. Globally, tetanus is common in neonates who are delivered without adequate sterile procedures. The case fatality rate in the unvaccinated varies from 10% to over 80%, and is highest in infants and the elderly.
Disease distribution
Incidence/prevalence
Global
Tetanus occurs worldwide but occurs most frequently in agricultural regions and densely populated regions. A total of 9,683 cases of tetanus were reported to the World Health Organization (WHO) in 2010. Tetanus is relatively uncommon in most developed countries because of long-standing immunization programs. Although neonatal tetanus has been eliminated in North America, it remains an important global issue.
National
Tetanus is rare in Canada (refer to Figure 1). Between 1990 and 2010, the number of cases reported annually ranged from 1 to 10, with an average of 4 per year. During this period, persons 60 years of age and older accounted for 48% of the cases, of which 59% were males. No cases were reported among neonates. The immunization status of the reported cases was not known. Only eight deaths due to tetanus have been reported since 1990; the last two were reported in 2009. There is limited evidence on the protective concentrations of tetanus antitoxin in the Canadian population. A serosurvey of adult blood donors in Toronto found that 17.5% of donors did not have protective levels of tetanus antitoxin. Factors associated with lack of immunity to tetanus include increasing age, birth outside Canada, and absence of immunization records.
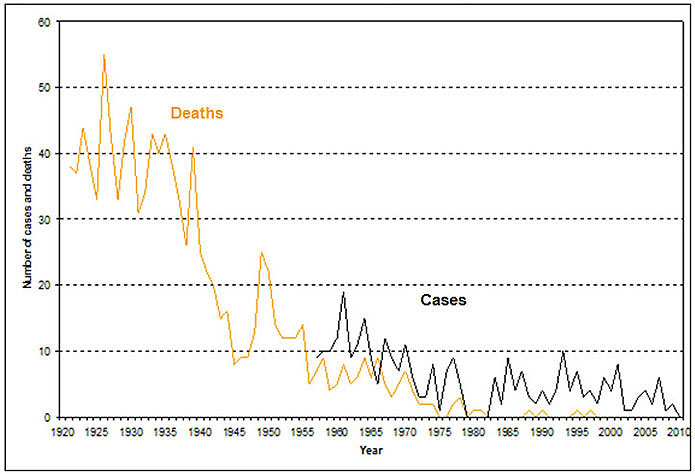
Figure 1: Tetanus - number of cases and deaths, Canada, 1921-2010: Text description
This image is a graph illustrating the numbers of cases and deaths in Canada attributed to tetanus between the years 1921 and 2010. The x axis represents the time in years between 1920 and 2010. The y axis represents the number of cases and deaths starting from 0 at the bottom and 60 at the top. The orange line within the graph represents deaths due to tetanus while the black line portrays the trends in tetanus cases over the years. The pattern suggests that the number of deaths increased from around 37 in 1921 to 43 by 1923. The deaths declined to 33 by 1926 only to hit another peak by 1927 declining sharply to 33 by 1929. Another peak followed in 1930 at 47 cases. Another sharp decline to 31 cases occurred in 1932, followed by a rise in the number of deaths between the years 1934 and 1936. By 1938 the deaths declined to about 26, followed by another peak at 41 in the year 1939. A sharp decline thereafter resulted in less than 9 deaths by 1945. After yet another increase in tetanus deaths around 1950 to a peak of 25, the downward spiral led to fewer and fewer deaths, with the number falling to 5 by 1956 and has been in single digits since then. There were zero deaths by the year 1975, and between 1982 and 1987, 1991 and 1993, and none whatsoever since 1998 until the end of the reporting period.
The black line within the graph representing the number of cases shows declining peaks following an initial report of less than 10 cases around 1957. The first peak of about 19 cases occurred at around 1961. After another brief decline to 9 cases by 1963, the next peak occurs at around 1964. After a few fluctuations, the number of cases declined to 3 by 1973 and further to a couple of cases by 1975. After rising to about 9 in 1978, for the first time the reported cases declined to zero in 1980. No reports are available for the years 1981 and 1982. However, by 1983, a transient upward trend is noticed, close to 9 by 1985. In 1994, 10 cases were reported. Between 1990 and 2010, the number of cases reported annually ranged from 1 to 10, with an average of 4 per year.
Preparations Authorized for Use in Canada
Tetanus toxoid is only available in combination vaccines.
Tetanus toxoid-containing vaccines
- ADACEL® (adsorbed vaccine containing tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis vaccine), Sanofi Pasteur Ltd. (Tdap)
- ADACEL®-POLIO (adsorbed vaccine containing tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis vaccine combined with inactivated poliomyelitis vaccine), Sanofi Pasteur Ltd. (Tdap-IPV)
- BOOSTRIX® adsorbed vaccine containing tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis vaccine), GlaxoSmithKline Inc. (Tdap)
- BOOSTRIX®-POLIO (adsorbed vaccine containing tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis vaccine combined with inactivated poliomyelitis vaccine), GlaxoSmithKline Inc. (Tdap-IPV)
- INFANRIX®-IPV/Hib (adsorbed vaccine containing diphtheria and tetanus toxoids, acellular pertussis, inactivated poliomyelitis and conjugated Haemophilus influenzae type b vaccine), GlaxoSmithKline Inc. (DTaP-IPV-Hib)
- INFANRIX hexa™ (adsorbed vaccine containing diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis and conjugated Haemophilus influenzae type b vaccine, GlaxoSmithKline Inc. (DTaP-HB-IPV-Hib)
- PEDIACEL®(adsorbed vaccine containing diphtheria and tetanus toxoids and acellular pertussis vaccine combined with inactivated poliomyelitis vaccine and Haemophilus influenzae type b conjugate vaccine), Sanofi Pasteur Ltd. (DTaP-IPV-Hib)
- QUADRACEL®(adsorbed vaccine containing diphtheria and tetanus toxoids and acellular pertussis vaccine combined with inactivated poliomyelitis vaccine), Sanofi Pasteur Ltd. (DTaP-IPV)
- Td ADSORBED (adsorbed vaccine containing tetanus and reduced diphtheria toxoids). Sanofi Pasteur Ltd. (Td)
Tetanus immunoglobulin
- HYPERTET®S/D (tetanus immunoglobulin (human) solvent/detergent treated), Grifols Therapeutics Inc. (TIg)
- HyperTET®(tetanus immunoglobulin (human)), Grifols Therapeutics LLC. (TIg)*
* NACI has not yet deliberated on the use of HyperTET®. NACI will review this immunizing agent and update the chapter in due course. For information regarding the use of these immunizing agents in the interim, please refer to the product monograph available through Health Canada's Drug Product Database.
TIg is a solution of tetanus Ig for IM administration prepared from pooled human plasma of screened donors immunized with tetanus toxoid.
For complete prescribing information, consult the product leaflet or information contained within Health Canada's authorized product monographs available through the Drug Product Database. Refer to Contents of Immunizing Agents Authorized for Use in Canada in Part 1 for lists of all vaccines and passive immunizing agents authorized for use in Canada and their contents.
Efficacy, Effectiveness and Immunogenicity
Efficacy and effectiveness
Protective antitoxin concentrations occur in virtually all healthy infants and children who receive primary tetanus immunization. Efficacy in standard pre-exposure and post-wound booster immunization regimens in adults has not been assessed in randomized trials but has been demonstrated in observational studies. Cases of tetanus occurring in fully immunized persons whose last dose was within the last 10 years are extremely rare.
Immunogenicity
It has been consistently demonstrated in clinical trials that one month after completion of a three dose primary series at least 99% of vaccinees have protective antibody titer.
Recommendations for Use
Infants and children (2 months to 17 years of age)
Tetanus toxoid-containing vaccine is recommended for routine infant immunization, beginning at 2 months of age. DTaP-IPV, with or without Hib vaccine, is authorized for use in children less than 7 years of age. DTaP-HB-IPV-Hib vaccine is authorized for use in children 6 weeks to 23 months of age and may be given to children aged 24 months to less than 7 years, if necessary. DTaP-IPV or Tdap-IPV vaccine should be used as the booster dose for children at 4 to 6 years of age. Children 7 years of age and older should receive the adolescent/adult formulation of diphtheria-tetanus-pertussis-containing vaccine, with or without polio, (Tdap or Tdap-IPV) as it contains less diphtheria toxoid than preparations given to younger children and is less likely to cause reactions in older children. Tdap vaccine should be administered to adolescents at 14 to16 years of age as the first 10-year booster dose; Tdap-IPV vaccine should be used if IPV vaccine is also indicated.
Adults (18 years of age and older)
Adults who have not previously received a primary series (at least 3 doses) of tetanus toxoid-containing vaccine should complete their schedule with a Td-containing vaccine, including one dose of Tdap-IPV vaccine. There is new evidence that a booster dose of Td vaccine may not be required every 10 years. Until a full review of the literature has been completed, a booster dose of Td vaccine is recommended every 10 years.
Refer to Schedule and Booster doses and re-immunization. Refer to Diphtheria Toxoid, Pertussis Vaccine, Poliomyelitis Vaccine, Haemophilus influenzae type b Vaccine and Hepatitis B Vaccine in Part 4 for additional information.
Persons with inadequate immunization records
Children and adults lacking adequate documentation of immunization should be considered unimmunized and started on an immunization schedule appropriate for their age and risk factors. When available, serologic testing for diphtheria and tetanus antitoxin concentrations may guide the need for continued immunization. Refer to Immunization of Persons with Inadequate Immunization Records in Part 3 for additional general information.
Pregnancy and breastfeeding
Immunization with Tdap in pregnancy has been shown to be safe and effective in preventing neonatal and infant pertussis and tetanus infection. Neonatal tetanus may occur in infants born to unimmunized mothers under unhygienic conditions. For the use of Tdap vaccine during pregnancy refer to Pertussis Vaccine in Part 4. Refer to Immunization in Pregnancy and Breastfeeding in Part 3 for additional general information.
Infants born prematurely
Premature infants in stable clinical condition should be immunized with a tetanus toxoid-containing preparation at the same chronological age and according to the same schedule as full-term infants. Infants born prematurely (especially those weighing less than 1,500 grams at birth) are at higher risk of apnea and bradycardia following vaccination. Hospitalized premature infants should have continuous cardiac and respiratory monitoring for 48 hours after their first immunization. Refer to Immunization of Infants Born Prematurely in Part 3 for additional general information.
Persons/residents in health care institutions
Residents of long-term care facilities should receive all routine immunizations appropriate for their age and risk factors, including tetanus toxoid-containing vaccine. Refer to Immunization of Persons in Health Care Institutions in Part 3 for additional general information.
Immunocompromised persons
Diphtheria-tetanus-pertussis-polio-Hib-containing preparations may be administered to immunocompromised persons. When considering immunization of an immunocompromised person, consultation with the individual's attending physician may be of assistance, in addition to the guidance provided in Immunocompromised persons in Diphtheria Toxoid and Haemophilus influenzae type b Vaccine in Part 4. For complex cases, referral to a physician with expertise in immunization or immunodeficiency is advised.
Refer to Immunization of Immunocompromised Persons in Part 3 for additional information.
Persons with chronic diseases
Neurologic disorders
People with neurological disorders with onset preceding immunization should receive all routinely recommended immunizations, including tetanus toxoid-containing preparations. Cases of Guillain Barré Syndrome (GBS) have been reported only very rarely following administration of a tetanus toxoid-containing vaccine, and the benefit of the vaccine normally outweighs this very small risk. Refer to Contraindications and precautions for additional information. Refer to Immunization of Persons with Chronic Diseases in Part 3 for additional general information.
Travellers
Unimmunized or incompletely immunized travellers should receive diphtheria-tetanus-pertussis-polio-Hib-containing vaccine as appropriate for age. Refer to Diphtheria Toxoid and Poliomyelitis Vaccine in Part 4 for information regarding other components in tetanus toxoid-containing combination vaccines. Refer to Immunization of Travellers in Part 3 for additional general information.
Persons new to Canada
Health care providers who see people newly arrived in Canada should review the immunization status and update immunization for these individuals. Refer to Immunization of Persons New to Canada in Part 3 for additional general information.
Workers
All health care workers should be immune to tetanus and receive a booster dose of Td vaccine every 10 years as recommended for all adults. All health care and child care workers, regardless of age, should receive a single dose of Tdap vaccine for pertussis protection if they have not been immunized previously with this vaccine in adulthood, even if they are not due for a tetanus and diphtheria booster. Refer to Immunization of Workers in Part 3 for additional general information.
Post-exposure prophylaxis
The most important goals of post-exposure prophylaxis are removing the source of toxin production and neutralizing any toxin which may have been released. The first goal is best achieved by timely, thorough wound cleaning. The second goal is achieved by providing or inducing high circulating concentrations of tetanus antibody, which inactivate the toxin. Effective neutralizing antibody concentrations at the time of the injury can only be achieved by prior completion of the tetanus toxoid-containing vaccine series or immediate administration of tetanus immunoglobulin (TIg).
It is important to ascertain the number of doses of tetanus toxoid-containing vaccine that an injured person previously has received, any severe reaction that they have experienced with any previous dose, and the interval since the last dose. Post-exposure prophylaxis of individuals who are unimmunized or incompletely immunized (unknown or fewer than 3 doses) and who sustain more than a minor, clean wound, should consist of both TIg and tetanus toxoid-containing vaccine (as appropriate for age and immunization history), given at different injection sites and using separate needles and syringes. TIg provides immediate passive protection until the exposed person mounts an immune response to the tetanus toxoid-containing vaccine. The vaccine series should be completed subsequently unless there is a contraindication. Refer to Table 1 for additional information.
Previously immunized persons (3 or more doses) may require a booster dose of a tetanus toxoid-containing vaccine, depending on the interval since the last booster and the type of wound. A booster dose of tetanus toxoid-containing vaccine is recommended if ten or more years have elapsed for those with clean, minor wounds and if five or more years have elapsed, for all other wounds.
People who have a tetanus-prone injury and have experienced a severe injection site reaction following a tetanus toxoid-containing vaccine usually have very high serum antitoxin levels and should not receive routine or emergency booster doses of tetanus toxoid-containing vaccine for 10 years following the last dose. A tetanus-prone injury can be defined as an injury significantly contaminated with material likely to contain tetanus spores (i.e. soil, animal or human feces) or an injury with the presence of necrotic tissue.
Some individuals with humoral immune deficiency (e.g., HIV, agammaglobulinemia or hypogammaglobulinemia) may not respond adequately to tetanus toxoid-containing vaccine. Individuals with humoral immune deficiency who have wounds that are not minor and clean should receive both TIg and tetanus toxoid-containing vaccine, regardless of the time elapsed since the last booster. Table 1 summarizes the recommended use of immunizing agents in wound management.
| History of tetanus immunization | Clean, minor wounds | All other wounds | ||
|---|---|---|---|---|
| Tetanus toxoid-containing vaccineTable 1 - Footnote * | TIg | Tetanus toxoid-containing vaccineTable 1 - Footnote * | TIgTable 1 - Footnote ** | |
| Unknown or less than 3 doses in a vaccine seriesTable 1 - Footnote † | Yes | No | Yes | Yes |
| 3 or more doses in a vaccine series and less than 5 years since last booster dose | No | No | No | NoTable 1 - Footnote ¶ |
| 3 or more doses in a vaccine series and more than 5 years but less than 10 years since last booster dose | No | No | Yes | NoTable 1 - Footnote ¶ |
| 3 or more doses in a vaccine series and more than 10 years since last booster dose | Yes | No | Yes | NoTable 1 - Footnote ¶ |
Persons who have recovered from tetanus disease should receive tetanus toxoid-containing vaccine as recommended for people who not had the disease. Because tetanus is caused by the toxins produced by the tetanus bacterium and not by the bacterium itself, recovery from tetanus disease does not confer immunity.
Tetanus immunoglobulin (TIg) for prophylaxis
TIg provides immediate passive protection until the exposed person mounts an immune response to the tetanus toxoid. The recommended dose for adults and children 7 years of age and older is 250 units by deep intramuscular injection. In small children less than 7 years old, the routine prophylactic dose of 4 units/kg. However, it may be advisable to administer the entire contents of the vial or syringe (250 units) regardless of the child's size, since theoretically the same amount of toxin will be produced in the child's body by the infecting tetanus organism as it will in an adult's body.
Tetanus immunoglobulin (TIg) for treatment
When used in the treatment of tetanus, TIg should be administered intramuscularly in an effort to neutralize tetanus toxin in body fluids. It has no effect on toxin already fixed to nerve tissue. The optimal therapeutic dose has not been established.
Intramuscular injections are preferably administered in the deltoid muscle of the upper arm or lateral thigh muscle. The gluteal muscle should not be used as an injection site because of the risk of injury to the sciatic nerve.
Refer to Adverse Events Following Immunization for safety information.
For complete prescribing information, consult the product leaflet or information contained within Health Canada's authorized product monographs available through the Drug Product Database.
Vaccine Administration
Dose, route of administration, and schedule
Dose
Each dose of tetanus toxoid-containing vaccine is 0.5 mL
Route of administration
Tetanus toxoid-containing vaccines must be administered intramuscularly. Refer to Vaccine Administration Practices in Part 1 for additional information.
Schedule
Infants and children (2 months to 6 years of age)
Routine tetanus immunization of infants: DTaP-IPV-Hib vaccine should be given at 2, 4, 6 and 12 to 23 months of age (generally given at 18 months of age).
If infant immunization for hepatitis B is undertaken, DTaP-HB-IPV-Hib vaccine may be used as an alternative to separately administered hepatitis B and DTaP-IPV-Hib vaccines. DTaP-HB-IPV-Hib vaccine is authorized for use in children 6 weeks to 23 months of age and may be given to children aged 24 months to less than 7 years, if necessary. DTaP-HB-IPV-Hib vaccine may be given at 2, 4, 6 and 12 to 23 months of age but the fourth dose is unlikely to provide significant additional hepatitis B protection and will increase cost. Alternative schedules may be used as follow:
- DTaP-HB-IPV-Hib vaccine (2, 4 and 6 months of age) with DTaP-IPV-Hib vaccine at 12 to 23 months of age
- DTaP-HB-IPV-Hib vaccine (2, 4 and 12 to 23 months of age) with DTaP-IPV-Hib vaccine at 6 months of age.
If rapid protection is required for an infant, the first dose of DTaP-IPV-Hib or DTaP-HB-IPV-Hib vaccine can be given at 6 weeks of age. The first three doses may be administered at intervals of 4 weeks and, optimally, the fourth dose given 12 months after the third dose. The fourth dose may be given at a minimum interval of 6 months after the third dose in certain situations (e.g., travel) but must be administered at or after 12 months of age for sustained immunity.
Children less than 7 years of age not immunized in infancy: should receive three doses of DTaP-IPV (with or without Hib) vaccine with an interval of 8 weeks between doses, followed by a dose of DTaP-IPV vaccine 6 to 12 months after the third dose. A booster dose of either DTaP-IPV or Tdap-IPV vaccine should be administered at 4 to 6 years of age (school entry). The booster dose at 4 to 6 years of age is not required if the fourth dose of tetanus-toxoid containing vaccine was administered after the fourth birthday.
If rapid protection is required for a child less than 7 years of age and not immunized in infancy, the first three doses of vaccine may be administered at intervals of 4 weeks and, optimally, the fourth dose given 12 months after the third dose. The fourth dose may be given at a minimum interval of 6 months after the third dose in certain situations (e.g., travel).
Children who received a primary series) of tetanus toxoid-containing vaccine and a booster dose 6-12 months later as outlined above should receive a booster dose of either DTaP-IPV or Tdap-IPV vaccine at 4 to 6 years of age (school entry); and, 10 years later, a booster dose of Tdap vaccine at 14 to 16 years of age. The booster dose at 4 to 6 years of age is not required if the fourth dose of tetanus-toxoid containing vaccine was administered after the fourth birthday.
Children and adolescents (7 years to 17 years of age)
Children 7 years of age and older, not previously immunized, should receive three doses of Tdap-IPV vaccine with an interval of 8 weeks between the first two doses followed by a third dose administered 6 to 12 months after the second dose. A booster dose of Tdap vaccine should be administered 10 years after the last dose.
Adults (18 years of age and older)
Adults who have not previously received a primary series (at least 3 doses) of tetanus toxoid-containing vaccine should complete their schedule with a Td-containing vaccine, including one dose of Tdap-IPV vaccine.
Booster doses and re-immunization
The preschool booster dose of either DTaP-IPV or Tdap-IPV vaccine should be administered at 4 to 6 years of age. Adolescents should routinely receive a booster dose of Tdap vaccine at 14 to 16 years of age. Currently, booster doses of Td vaccine are recommended every 10 years. Adults who have not received an adult dose of pertussis-containing vaccine should receive one dose of Tdap vaccine, which can be administered regardless of the interval since the last dose of tetanus and diphtheria toxoid-containing vaccine. Refer to Schedule.
Serologic Testing
Serologic testing is not recommended before or after receiving tetanus toxoid-containing vaccine.
Storage Requirements
Tetanus toxoid-containing preparations should be stored in a refrigerator at +2°C to +8°C and must not be frozen. Refer to Storage and Handling of Immunizing Agents in Part 1 for additional general information.
Simultaneous Administration with Other Vaccines
Tetanus toxoid-containing vaccines may be administered concomitantly with routine vaccines at different injection sites using separate needles and syringes. Refer to Timing of Vaccine Administration in Part 1 for additional general information.
Vaccine and Immunoglobulin Safety and Adverse Events
Refer to Adverse Events Following Immunization in Part 2 for additional general information. Refer to Diphtheria Toxoid, Pertussis Vaccine, Poliomyelitis Vaccine, Haemophilus influenzae type b Vaccine and Hepatitis B Vaccine in Part 4 for additional information regarding other components in tetanus toxoid-containing combination vaccines.
Common and local adverse events
Tetanus-toxoid containing vaccines
Redness, swelling and pain at the injection site are the most common adverse reactions to childhood tetanus toxoid-containing combination vaccines. A nodule may be palpable at the injection site and persist for several weeks. Abscess at the injection site has been reported. These events are most often related to administering the vaccine subcutaneously in error.
In clinical trials, injection site adverse reactions, including tenderness, erythema, swelling, or any combination, were reported in 10% to 40% of children after each of the first 3 doses of tetanus toxoid-containing vaccine. Mild systemic reactions such as fever, irritability fussiness or any combination were commonly reported (8% to 29%), as well as drowsiness (40% to 52%).
In two clinical studies, swelling (greater than 5 cm) and erythema were reported in 15 % to 20% of vaccinees after the fourth or fifth doses of DTaP vaccines. Extensive limb swelling (greater than 10 cm in diameter) possibly involving the entire proximal limb may occur in 2% to 6% of children. While these injection site reactions produce significant swelling, pain is generally limited. There is some evidence that children with extensive limb swelling following the fourth dose of a DTaP vaccine are at increased risk of such an event following the fifth dose. The presence of a large injection site reaction to a previous dose is not a contraindication to continuing the recommended schedule.
Among adults given a booster dose of Tdap vaccine, very common reactions include pain, redness and swelling at the injection site, headache, and fatigue. Fever and chills are common reactions. Adverse reactions following Td vaccine are similar. Overall, adverse reactions are less common in adults than adolescents. The interval between the childhood DTaP vaccine series or a dose of Td vaccine, and a dose of Tdap vaccine does not affect the rate of injection site or systemic adverse events.
TIg
Mild soreness at the injection site and slight temperature elevation may occur following TIg injection.
Less common and serious or severe adverse events
Tetanus-toxoid containing vaccines
Serious adverse events are rare following immunization with tetanus toxoid-containing vaccines and, in most cases, data are insufficient to determine a causal association. Severe systemic reactions such as generalized urticaria, anaphylaxis, or neurologic complications have been reported rarely.
Serum sickness, brachial plexus neuropathy, encephalomyelitis and transverse myelitis have rarely been reported in association with tetanus vaccination.
Severe arthus-type injection site reactions are occasionally reported following receipt of diphtheria toxoid or tetanus toxoid-containing vaccines. There may be extensive painful swelling around the injection site, often involving the arm from shoulder to elbow and generally beginning 2 to 8 hours after injection. Such reactions are most often reported in adults, particularly those who have received frequent doses of diphtheria or tetanus toxoid-containing vaccines or both. Persons experiencing severe injection site reactions usually have very high serum antitoxin concentrations and should not receive further routine doses of Td vaccine for at least 10 years.
TIg
Angioneurotic edema, nephrotic syndrome, and anaphylaxis after injection have been reported rarely.
Other reported adverse events and conditions
Trismus (inability to normally open the mouth) associated with tetanus toxoid immunization has rarely been reported. The pathogenesis is unexplained and it may be attributable to a reporting bias. Outcomes have been favourable.
Cases of Guillain-Barré Syndrome (GBS) or polyneuritis have been reported following administration of tetanus toxoid-containing vaccine and there has been one case report of relapsing GBS following each of three doses of vaccine. However, population studies have not supported a causal association.
Guidance on reporting Adverse Events Following Immunization (AEFI)
Vaccine providers are asked to report, through local public health officials, any serious or unexpected adverse event felt to be temporally related to vaccination. An unexpected AEFI is an event that is not listed in available product information but may be due to the immunization or a change in the frequency of a known AEFI. Refer to Reporting Adverse Events Following Immunization (AEFI) in Canada and Adverse Events Following Immunization Part 2 for additional information about AEFI reporting.
Contraindications and precautions
Tetanus toxoid-containing vaccines are contraindicated in persons with a history of anaphylaxis after previous administration of the vaccine and in persons with proven immediate or anaphylactic hypersensitivity to any component of the vaccine or its container. Refer to Contents of Immunizing Agents Authorized for use in Canada in Part 1 for lists of all vaccines and passive immunizing agents authorized for use in Canada and their contents.
If indicated, TIg should be given with caution to persons with a history of prior systemic allergic reactions following the administration of human Ig preparations.
Hypersensitivity to yeast is very rare and a personal history of yeast allergy is not generally reliable. In situations of suspected hypersensitivity or non-anaphylactic allergy to vaccine components, investigation is indicated, which may involve immunization in a controlled setting. Consultation with an allergist is advised.
Administration of tetanus toxoid-containing vaccine should be postponed in persons with severe acute illness. Persons with minor acute illness, with or without fever, may be vaccinated.
It is prudent to not administer further doses of tetanus toxoid-containing vaccine to persons who develop GBS within 6 weeks of receiving such vaccine. Those who develop GBS outside the 6 week interval may receive subsequent doses of tetanus toxoid-containing vaccine. If there is a history of both Campylobacter infection (which has been associated with GBS) and receipt of a tetanus and diphtheria toxoid-containing vaccine within the 6 weeks before the onset of GBS, consultation with an infectious disease specialist is advised.
People who experience a severe injection site reaction following a dose of tetanus toxoid-containing vaccine should not be given another dose for at least 10 years.
Refer to Contraindications and Precautions in Part 2 for additional information.
Other Considerations
Interchangeability of vaccines
The primary series of three doses tetanus toxoid-containing vaccine should be completed with an appropriate vaccine from the same manufacturer whenever possible. However, if the original vaccine is unknown or unavailable, an alternative combination vaccine from a different manufacturer may be used to complete the primary series. On the basis of expert opinion, an appropriate product from any manufacturer can be used for all booster doses. Refer to Principles of Vaccine Interchangeability in Part 1 for additional general information.
Selected References
- Centers for Disease Control and Prevention. ACIP Provisional Recommendations for Health Care Personnel on use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine (Tdap) and use of Postexposure Antimicrobial Prophylaxis.
- Centers for Disease Control and Prevention. Prevention of Pertussis, Tetanus, and Diphtheria Among Pregnant and Postpartum Women and Their Infants. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2008;57(RR-4):1-49.
- Centers for Disease Control and Prevention. The Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases. Updated 11th ed.; May 2009.
- Centers for Disease Control and Prevention. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) Vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
- Centers for Disease Control and Prevention. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis (Tdap) Vaccine in Adults Aged 65 Years and Older — Advisory Committee on Immunization Practices (ACIP), 2012.
- David S, Hemsley C, Pasquali P et al. Enhanced surveillance for vaccine-associated adverse events: dTap catch-up of high school students in Yukon. Can Commun Dis Rep 2005;31(11):117-26.
- Fiorillo L, Robinson JL. Localized tetanus in a child. Ann Emerg Med 1999;33(4):460-63.
- Gautret P, Wilder-Smith A. Vaccination against tetanus, diphtheria, pertussis
and poliomyelitis in adult travellers. Travel Med Infect Dis 2010;8:155-60. - GlaxoSmithKline Inc. Product Monograph - BOOSTRIX®, October 2009.
- GlaxoSmithKline Inc. Product Monograph - BOOSTRIX®-POLIO, June 2008.
- GlaxoSmithKline Inc. Product Monograph - INFANRIX hexa™. July 2008.
- Grifols Therapeuctics LLC. Product Monograph - HyperTET®. September 2021.
- Jacobs R, Lowe R, Lanier B. Adverse reactions to tetanus toxoid. JAMA 1982;247(1):40-2.
- Katz K, Walmsley S. Postoperative tetanus: a case report. CMAJ 2000;163(5):571-73.
- Martin-Munoz M, Pereira M, Posadas S et al. Anaphylactic reaction to diphtheria-tetanus vaccine in a child: specific IgE/IgG determinations and cross-reactivity studies. Vaccine 2002;20(27-28):3409-12.
- Mayaud C, Loupi E, Charara O et al. Trismus et vaccination antitétanique. Arch Pediatr 1999;6(7):752-54.
- McQuillan G, Kruszon-Moran D, Deforest A et al. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med 2002;136(9):660-66.
- National Advisory Committee on Immunization. Interval between administration of vaccines against diphtheria, tetanus, and pertussis. Can Comm Dis Rep 2005;31(ACS-9):17-22.
- National Advisory Committee on Immunization. Statement on the recommended use of pentavalent and hexavalent vaccines. Can Comm Dis Rep. 2007;33(ACS-1):1-15.
- Okaïs C, Gay C, Seon F et al. Disease-specific adverse events following nonlive vaccines: A paradoxical placebo effect or a nocebo phenomenon? Vaccine 2011;May 31. [Epub ahead of print]
- Pascual F, McGinley E, Zanardi L et al. Tetanus surveillance - United States, 1998-2000. MMWR Surveill Summ 2003;52(3):1-8.
- Pichichero ME, DeTora LM, Johnson DR. An adolescent and adult formulation combined tetanus, diphtheria and five-component pertussis vaccine. Expert Rev Vaccines 2006 Apr;5(2):175-87.
- Pollard JD, Selby G: Relapsing neuropathy due to tetanus toxoid: report of a case. J Neurol Sci 1978;37:113-125.
- Sanofi Pasteur Ltd. Product Monograph - ADACEL®, August 2009.
- Sanofi Pasteur Ltd. Product Monograph - ADACEL®-POLIO, October 2010.
- Sanofi Pasteur Ltd. Product Monograph - PEDIACEL®, January 2009.
- Sanofi Pasteur Ltd. Product Monograph - QUADRACEL®, July 2008.
- Sanofi Pasteur Ltd. Product Monograph - Td ADSORBED, July 2010.
- Shimoni Z, Dobrousin A, Cohen J et al. Tetanus in an immunised patient. BMJ 1999;319(7216):1049.
- Shin D, Park J, Jung PJ et al. A case of maternal tetanus in Korea. J Korean Med Sci 2002;17(2):260-62.
- Talecris Biotherapeutics Inc. Product Monograph - HYPERTET™ S/D. June 2005.
- Wassilak S, Roper M, Murphy T et al. Tetanus toxoid. In: Plotkin SA, Orenstein WA, eds. Vaccines. 4th edition. Philadelphia: W.B. Saunders 2004;745-81.
- Yuan L, Lau W, Thipphawong J et al. Diphtheria and tetanus immunity among blood donors in Toronto. CMAJ 1997;156(7):985-90.