Diphtheria toxoid: Canadian Immunization Guide
For health professionals
Last partial content update (see table of updates): November 2016
Last complete chapter revision (see table of updates): December 2014
On this page
- Key Information
- Epidemiology
- Preparations Authorized for Use in Canada
- Efficacy, Effectiveness and Immunogenicity
- Recommendations for Use
- Vaccine Administration
- Serologic Testing
- Storage Requirements
- Simultaneous Administration with Other Vaccines
- Vaccine and Antitoxin Safety and Adverse Events
- Other Considerations
- Selected References
Key Information (Refer to text for details)
- What
-
- Diphtheria is rare in Canada. It occurs worldwide and is endemic in many developing countries.
- Case-fatality rate is about 5% to 10%; highest death rates occur in the very young and the elderly, and in non-endemic countries because diagnosis is often late.
- Diphtheria toxoid-containing vaccines are only available in combination vaccines.
- Diphtheria toxoid-containing vaccines may be used for diphtheria post-exposure immunization in susceptible persons.
- Diphtheria antitoxin for treatment of diphtheria is available on an emergency basis through local public health officials.
- After a complete primary series (at least 3 doses), more than 97% of vaccinees develop antibody concentrations that are protective against diphtheria.
- Redness, swelling and pain at the injection site are the most common adverse reactions to diphtheria toxoid-containing vaccines.
- Who
-
- Diphtheria toxoid-containing vaccine is recommended for:
- routine immunization of infants and children
- immunization of children who missed diphtheria immunization on the routine schedule
- immunization of previously unvaccinated or incompletely vaccinated adults
- routine booster immunization of adolescents and adults
- Diphtheria toxoid-containing vaccine is recommended for:
- How
-
- Routine diphtheria immunization of infants and children: administer DTaP-IPV-Hib vaccine at 2, 4, 6 and 12 to 23 months of age (generally given at 18 months of age). If infant immunization for hepatitis B is undertaken, DTaP-HB-IPV-Hib vaccine may be used. Subsequently administer a booster dose of either DTaP-IPV or Tdap-IPV vaccine at 4 to 6 years of age (school entry) and a booster dose of Tdap vaccine 10 years later at 14 to 16 years of age.
- Adults previously immunized with diphtheria-toxoid containing vaccine: administer one dose of Tdap vaccine if the patient has not previously received this vaccine in adulthood (18 years of age and older) and give a booster dose of Td vaccine every 10 years.
- Diphtheria toxoid-containing vaccines may be administered concomitantly with routine vaccines at different injection sites, using separate needles and syringes.
- Why
-
- Diphtheria occurs worldwide and is endemic in many developing countries.
- Inadequately immunized or unimmunized travellers to areas with endemic diphtheria are at higher risk of acquiring disease.
- Occasional cases of imported diphtheria are identified in developed countries, like Canada.
- Death occurs in 5% to 10% of diphtheria cases.
Epidemiology
Disease description
Infectious agent
Diphtheria is caused by exotoxin-producing strains of the bacterium Corynebacterium diphtheriae.
Reservoir
Humans
Transmission
Diphtheria is transmitted by person-to-person spread from the respiratory tract or, rarely, by contact with articles soiled with excretions of infected persons. The incubation period is about 2 to 5 days (range, 1 to 10 days). The infectious period in untreated persons is usually 2 weeks or less and, rarely, more than 4 weeks. Chronic carriers are asymptomatically colonized with C. diphtheriae on the skin or in the nasopharynx and may shed organisms for 6 months or more.
Risk factors
Inadequately immunized or unimmunized travellers to areas with endemic diphtheria are at higher risk of acquiring disease. A list of countries where diphtheria is endemic is available from the United States Centers for Disease Control and Prevention (CDC) or the current version of the CDC's Health Information for International Travel Yellow Book.
Seasonal/temporal patterns
Diphtheria occurs most frequently in winter and spring months in temperate climates.
Spectrum of clinical illness
Respiratory diphtheria affects the mucous membrane of the upper respiratory tract. Symptoms include a mild fever, sore throat, difficulty swallowing, malaise and loss of appetite. It can progress to acute respiratory distress, upper airway obstruction and asphyxia in young children. An adherent, asymmetrical, grayish white membrane, visible on the tonsils and oropharynx, typically appears within 2 to 3 days of illness. Dissemination of diphtheria toxin can result in systemic complications such as myocarditis and central nervous system effects. The case-fatality rate is about 5% to 10%; the highest rates occur among the unimmunized who are very young, and unimmunized elderly, and in non-endemic countries, because diagnosis is often late. Localized infection of the skin (cutaneous diphtheria) may occur, but is rarely associated with systemic complications.
Disease distribution
Incidence/prevalence
Global
Diphtheria occurs worldwide and is endemic in many developing countries as well as in Albania, Russia and other countries of the former Soviet Union. In other countries, occasional cases of imported diphtheria are identified. Resurgence of diphtheria has been reported in countries with low vaccine coverage. A total of 4,187 cases of diphtheria were reported to the World Health Organization (WHO) in 2010.
National
Routine infant and childhood diphtheria immunization has resulted in a dramatic decline in reported cases of diphtheria (refer to Figure 1). A small number of toxigenic strains of diphtheria bacilli are detected each year (0 to 5 isolates), although classic diphtheria is rare. Serosurveys of healthy adult populations in Canada indicate that approximately 20% (higher in some age groups) do not have protective concentrations of antibody to diphtheria; adult booster doses are required.
Figure 1: Diphtheria - reported cases and incidence, Canada, 1924-2008
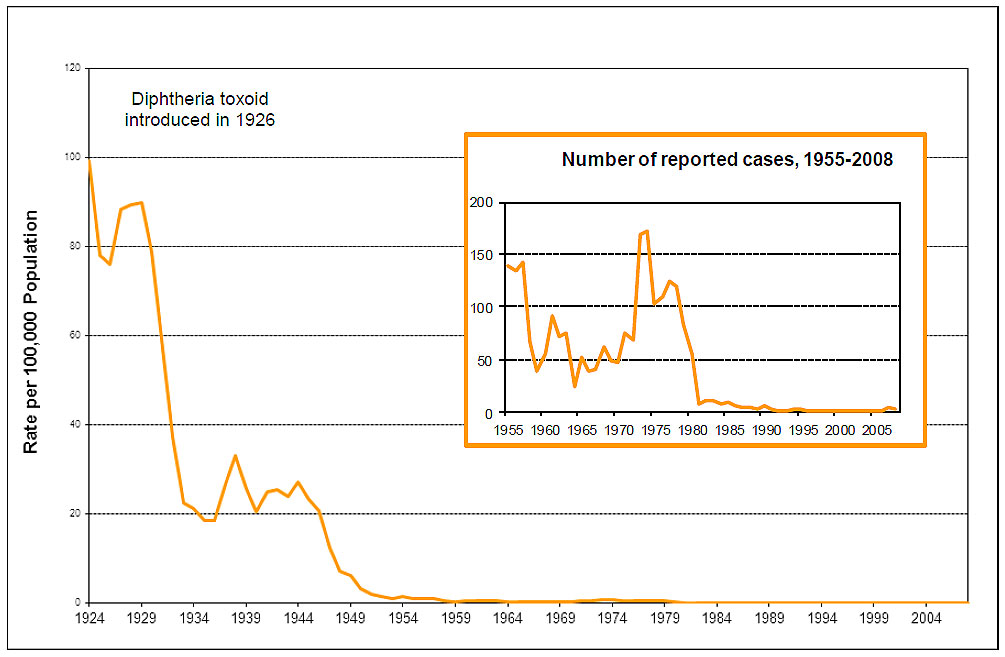
Figure 1 Footnote
Population data sources: Statistics Canada, Population by Sex and Age, 1921-1971, revised annual estimates of population, Canada and the provinces, (Catalogue 91-512)
Statistics Canada, Population estimates 0-90+ July Canada - Provinces 1971-2008.xls
Figure 1: Diphtheria - reported cases and incidence, Canada, 1924-2008 - Text Equivalent
This image includes two graphs showing the reported cases and incidence of diphtheria in Canada. The bigger graph shows the trends in diphtheria incidence over the years, with x axis representing time in years between 1924 and 2004. The y axis represents rate per 100,000 population starting with 0 at the bottom and ending in 120 at the top. The graph showing the incidence rates has an orange line inside representing the trends since 1924, when the rate was close to 98 per 100,000. Although it declined to about 75 by 1926, it quickly climbed to a peak of 90 by 1927. It is apparent that the introduction of diphtheria toxoid in 1926 resulted in a drastic decline in incidence starting from around 1929. The rate fell to less than 20 by 1936. Another peak of about 32 occurred around 1938, which fell to 20 by 1941. Although the rate continued to fluctuate around 25 until 1944, the rate of incidence started to decline thereafter, reaching 10 per 100,000 by 1947 and close to 0 by 1969. The rate of incidence continues to remain around 0 until 1984. No reports of incidence rates are available since 1984.
The smaller graph illustrating the reported cases of diphtheria has a dark orange line showing the trends since 1955 until 2008. The x axis of this graph represents time in years between 1955 and 2008. The y axis of this graph represents the number of reported cases starting from 0 at the bottom to 200 at the top. Starting at around 137 in 1955, the initial peak appears to have occurred around 1957, followed by a steep decline to about 37 by the year 1960. The number of cases increased to almost 90 by 1962. Another transient decline can be noticed at around 1965 when the number of cases fell to 25. The cases steadily increased over the next decade, reaching an all-time high peak of almost 175 by 1974. Diphtheria cases then declined to 100 by 1975. Except for another increase to 125 cases by 1977, the number declined sharply to single digits by 1982. The number of diphtheria cases continued its downward trend reaching 0 by 1990 and close to 0 ever since.
The notes at the bottom acknowledge the population data sources including "Statistics Canada, Population by Sex and Age, 1921-1971, revised annual estimates of population, Canada and the provinces, (Catalogue 91-512); and Statistics Canada, Population estimates 0-90+ July Canada - Provinces 1971-2008.xls
Recent outbreaks
The potential for re-emergence of diphtheria if immunization levels decline was demonstrated during the 1990s in the Commonwealth of Independent States (former Soviet Union) when over 140,000 cases and 4,000 deaths were reported.
Preparations Authorized for Use in Canada
Diphtheria toxoid-containing vaccines
- ADACEL® (adsorbed vaccine containing tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis vaccine), Sanofi Pasteur Ltd. (Tdap).
- ADACEL®-POLIO (adsorbed vaccine containing tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis vaccine combined with inactivated poliomyelitis vaccine), Sanofi Pasteur Ltd. (Tdap-IPV).
- BOOSTRIX® (adsorbed vaccine containing tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis vaccine), GlaxoSmithKline Inc. (Tdap).
- BOOSTRIX®-POLIO (adsorbed vaccine containing tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis vaccine combined with inactivated poliomyelitis vaccine), GlaxoSmithKline Inc. (Tdap-IPV).
- INFANRIX®-IPV/Hib (adsorbed vaccine containing diphtheria and tetanus toxoids, acellular pertussis, inactivated poliomyelitis and conjugated Haemophilus influenzae type b vaccine), GlaxoSmithKline Inc. (DTaP-IPV-Hib).
- INFANRIX hexa™ (adsorbed vaccine containing diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis and conjugated Haemophilus influenzae type b vaccine), GlaxoSmithKline Inc. (DTaP-HB-IPV-Hib).
- PEDIACEL® (adsorbed vaccine containing diphtheria and tetanus toxoids and acellular pertussis vaccine combined with inactivated poliomyelitis vaccine and Haemophilus influenzae type b conjugate vaccine), Sanofi Pasteur Ltd. (DTaP-IPV-Hib).
- QUADRACEL® (adsorbed vaccine containing diphtheria and tetanus toxoids and acellular pertussis vaccine combined with inactivated poliomyelitis vaccine), Sanofi Pasteur Ltd. (DTaP-IPV).
- Td ADSORBED (adsorbed vaccine containing tetanus and reduced diphtheria toxoids), Sanofi Pasteur Ltd. (Td).
Diphtheria toxoid is only available in combination vaccines. The amount of diphtheria toxoid present varies by product. Preparations containing higher concentrations of diphtheria toxoid (designated as "D") are administered for primary immunization of infants and young children less than 7 years of age (pediatric formulation). Preparations containing a lower concentration (designated as "d" and referred to as "reduced") may be administered as a booster dose to children 4 years to less than 7 years of age and are the recommended product for older children, adolescents and adults (adolescent/adult formulation).
Diphtheria antitoxin
- ANTIDIPHTHERIA SERUM: purified immunoglobulins obtained from the plasma of horses hyper-immunized with diphtheria toxoid, Instituto Butantan, (DAtx)
DAtx is a specific immunoglobulin preparation for IM or IV administration that is available on an emergency basis through local public health officials.
For complete prescribing information, consult the product leaflet or information contained within Health Canada's authorized product monographs available through the Drug Product Database. Refer to Table 1 and Table 2 in Contents of Immunizing Agents Authorized for Use in Canada in Part 1 for lists of all vaccines and passive immunizing agents authorized for use in Canada and their contents.
Efficacy, Effectiveness and Immunogenicity
Diphtheria toxoid protects against the systemic effects of diphtheria toxin but does not directly protect against infection. Carriage of C. diphtheriae can occur in immunized individuals, but the rate of carriage is lower in immunized populations. After a complete primary series, more than 97% of vaccinees develop antibody concentrations that are protective against diphtheria toxin. In studies assessing booster response, 100% of vaccinees had a protective antibody titre one month after the booster dose. Antitoxin is believed to persist at protective concentrations for 10 years or more.
Recommendations for Use
Infants and children (2 months to 17 years of age)
Diphtheria toxoid-containing vaccine is recommended for routine infant immunization beginning at 2 months of age. DTaP-IPV (with or without Hib) vaccine is authorized for use in children less than 7 years of age. DTaP-HB-IPV-Hib vaccine is authorized for use in children 6 weeks to 23 months of age and may be given to children aged 24 months to less than 7 years, if necessary. DTaP-IPV or Tdap-IPV vaccine should be used as the booster dose for children at 4 to 6 years of age. Children 7 years of age and older should receive the adolescent/adult formulation of diphtheria-tetanus-pertussis-containing vaccine, with or without polio (Tdap or Tdap-IPV), as it contains less diphtheria toxoid than preparations given to younger children and is less likely to cause reactions in older children. Tdap vaccine should be administered to adolescents at 14 to 16 years of age as the first 10-year booster dose; Tdap-IPV vaccine should be used if IPV vaccine is also indicated.
Adults (18 years of age and older)
Adults who have not previously received a primary series (at least 3 doses) of diphtheria toxoid-containing vaccine should complete their schedule with a Td-containing vaccine, including one dose of Tdap-IPV vaccine. There is new evidence that a booster dose of Td vaccine may not be required every 10 years. Until a full review of the literature has been completed, a booster dose of Td vaccine is recommended every 10 years.
Refer to Schedule and Booster doses and re-immunization. Refer to Tetanus Toxoid, Pertussis Vaccine, Poliomyelitis Vaccine, Haemophilus influenzae type b Vaccine and Hepatitis B Vaccine in Part 4 for additional information.
Persons with inadequate immunization records
Children and adults lacking adequate documentation of immunization should be considered unimmunized and started on an immunization schedule appropriate for their age and risk factors. When available, serologic testing for diphtheria and tetanus antitoxin concentrations may guide the need for continued immunization. Refer to Immunization of Persons with Inadequate Immunization Records in Part 3 for additional general information.
Pregnancy and breastfeeding
Immunization with Tdap in pregnancy has been shown to be safe and effective in preventing neonatal and infant pertussis and tetanus infection. For the use of Tdap vaccine during pregnancy, refer to Pertussis Vaccine in Part 4. Refer to Immunization in Pregnancy and Breastfeeding in Part 3 for additional information.
Infants born prematurely
Premature infants in stable clinical condition should be immunized with a diphtheria toxoid-containing vaccine at the same chronological age and according to the same schedule as full-term infants. Infants born prematurely, especially those weighing less than 1,500 grams at birth, are at higher risk of apnea and bradycardia following vaccination. Hospitalized premature infants should have continuous cardiac and respiratory monitoring for 48 hours after their first immunization. Refer to Immunization of Infants Born Prematurely in Part 3 for additional general information.
Persons/residents in health care institutions
Residents of long-term care facilities should receive all routine immunizations appropriate for their age and risk factors, including diphtheria toxoid-containing vaccine. Refer to Immunization of Patients in Health Care Institutions in Part 3 for additional general information.
Immunocompromised persons
Diphtheria-tetanus-pertussis-polio-Hib-containing vaccines may be administered to immunocompromised persons. When considering immunization of an immunocompromised person, consultation with the individual's attending physician may be of assistance. For complex cases, referral to a physician with expertise in immunization or immunodeficiency is advised. Refer to Immunization of Immunocompromised Persons in Part 3 for additional information.
Persons with chronic diseases
Neurologic disorders
People with neurological disorders with onset preceding immunization should receive all routinely recommended immunizations. Refer to Tetanus Toxoid and Pertussis Vaccine in Part 4 for information regarding other components in diphtheria toxoid-containing combination vaccines. Refer to Immunization of Persons with Chronic Diseases in Part 3 for additional general information.
Travellers
Unimmunized or incompletely immunized travellers should receive diphtheria-tetanus-pertussis-polio-Hib-containing vaccine as appropriate for age. For infants embarking on travel, the first dose of DTaP-IPV-Hib or DTaP-HB-IPV-Hib vaccine can be given at 6 weeks of age (refer to Schedule). Previously immunized adult travellers should receive a booster dose of a tetanus-diphtheria toxoid-containing preparation every 10 years. For adults who have not previously received a dose of acellular pertussis vaccine in adulthood, it is recommended that the Td vaccine booster dose be replaced by Tdap vaccine. Some travellers may also need a polio booster. Refer to Poliomyelitis Vaccine in Part 4 for additional information. Refer to Immunization of Travellers in Part 3 for additional general information.
Persons new to Canada
Health care providers who see people newly arrived in Canada should review the immunization status and update immunization for these individuals. Refer to Immunization of Persons New to Canada in Part 3 for additional general information.
Workers
All health care workers should be immune to diphtheria and receive a booster dose of Td vaccine every 10 years as recommended for all adults. All health care and child care workers, regardless of age, should receive a single dose of Tdap vaccine for pertussis protection if not previously administered in adulthood, even if they are not due for a tetanus and diphtheria booster. Refer to Immunization of Workers in Part 3 for additional general information.
Post-exposure immunization
Diphtheria toxoid-containing vaccine
Close contacts (e.g., household, classroom) of a diphtheria case should receive a dose of a diphtheria toxoid-containing vaccine as appropriate for age unless the contact is known to have been fully immunized and the last dose of diphtheria toxoid-containing vaccine was given within 10 years. The diphtheria toxoid-containing vaccine series should be completed for previously unimmunized or incompletely immunized contacts.
Diphtheria antitoxin (equine)
Prophylaxis of diphtheria
Diphtheria antitoxin is not recommended for prophylaxis of immunized or unimmunized close contacts of diphtheria cases, given the substantial risk of allergic reaction to equine serum and lack of evidence of additional benefit of antitoxin for contacts who have received antimicrobial prophylaxis.
Treatment of diphtheria
Diphtheria antitoxin for treatment of diphtheria disease is available on an emergency basis through local public health officials. Antitoxin should be administered when there is clinical suspicion of diphtheria, before bacteriologic confirmation. The method of testing for sensitivity to equine serum, as well as the dose and route of administration, are provided in the manufacturer's product leaflet. If sensitivity tests are positive, desensitization must be undertaken according to the manufacturer's recommendations. Intramuscular administration usually suffices, but intravenous administration may be necessary in some cases.
Persons who have recovered from diphtheria should receive diphtheria toxoid-containing vaccine as recommended for people who have not had the disease. Because symptoms of diphtheria are largely mediated through toxins produced by the diphtheria bacterium and not through the bacterium itself, recovery from diphtheria disease does not necessarily confer immunity.
Refer to Vaccine and Antitoxin Safety and Adverse Events for safety information.
For complete prescribing information, consult the product leaflet or information contained within Health Canada's authorized product monographs available through the Drug Product Database.
Vaccine Administration
Dose, route of administration, and schedule
Dose
Each dose of diphtheria toxoid-containing vaccine is 0.5 mL.
Route of administration
Diphtheria toxoid-containing vaccines must be administered intramuscularly. Refer to Vaccine Administration Practices in Part 1 for additional information.
Schedule
Infants and children (2 months to 6 years of age)
Routine diphtheria immunization of infants: DTaP-IPV-Hib vaccine should be given at 2, 4, 6 and 12 to 23 months of age (the last generally is given at 18 months of age).
If infant immunization for hepatitis B is undertaken, DTaP-HB-IPV-Hib vaccine may be used as an alternative to separately administered hepatitis B and DTaP-IPV-Hib vaccines. DTaP-HB-IPV-Hib vaccine is authorized for use in children 6 weeks to 23 months of age and may be given to children aged 24 months to less than 7 years, if necessary. DTaP-HB-IPV-Hib vaccine may be given at 2, 4, 6 and 12 to 23 months of age but the fourth dose is unlikely to provide significant additional hepatitis B protection and will increase cost. Alternative schedules may be used as follow:
- DTaP-HB-IPV-Hib vaccine (2, 4 and 6 months of age) with DTaP-IPV-Hib vaccine at 12 to 23 months of age
- DTaP-HB-IPV-Hib vaccine (2, 4 and 12 to 23 months of age) with DTaP-IPV-Hib vaccine at 6 months of age.
If rapid protection is required for an infant, the first dose of DTaP-IPV-Hib or DTaP-HB-IPV-Hib vaccine can be given at 6 weeks of age. The first three doses may be administered at intervals of 4 weeks and, optimally, the fourth dose given 12 months after the third dose. The fourth dose may be given at a minimum interval of 6 months after the third dose in certain situations (e.g., travel) but must be administered at or after 12 months of age for sustained immunity.
Children less than 7 years of age not immunized in infancy: should receive three doses of DTaP-IPV (with or without Hib depending on the age of the child) vaccine with an interval of 8 weeks between doses, followed by a dose of DTaP-IPV vaccine 6 to 12 months after the third dose. A booster dose of either DTaP-IPV or Tdap-IPV vaccine should be administered at 4 to 6 years of age (school entry). The booster dose at 4 to 6 years of age is not required if the fourth dose of tetanus-toxoid containing vaccine was administered after the fourth birthday.
If rapid protection is required for a child less than 7 years of age not immunized in infancy, the first three doses of vaccine may be administered at intervals of 4 weeks and, optimally, the fourth dose given 12 months after the third dose. The fourth dose may be given at a minimum interval of 6 months after the third dose in certain situations (e.g., travel).
Children who received a primary series of a diphtheria toxoid-containing vaccine and a booster dose 6-12 months later as outlined above should receive a booster dose of either DTaP-IPV or Tdap-IPV vaccine at 4 to 6 years of age (school entry); and, 10 years later, a booster dose of Tdap vaccine at 14 to 16 years of age. The booster dose at 4 to 6 years of age is not required if the fourth dose of diphtheria toxoid-containing vaccine was administered after the fourth birthday.
Children and adolescents (7 years to 17 years of age)
Children 7 years of age and older not previously immunized should receive three doses of Tdap-IPV vaccine with an interval of 8 weeks between the first two doses followed by a third dose administered 6 to 12 months after the second dose. A booster dose of Tdap vaccine should be administered 10 years after the last dose.
Adults (18 years of age and older)
Adults who have not previously received a primary series (at least 3 doses) of diphtheria toxoid-containing vaccine should complete their schedule with a Td-containing vaccine, including one dose of Tdap-IPV vaccine.
Booster doses and re-immunization
The preschool booster dose of either DTaP-IPV or Tdap-IPV vaccine should be administered at 4 to 6 years of age. Adolescents should routinely receive a booster dose of Tdap vaccine at 14 to 16 years of age.
There is new evidence that booster doses of Td vaccine may not be required every 10 years. Until a full review of the literature has been completed , booster doses of Td vaccine continue to be recommended every 10 years. Adults who have not received an adult dose of pertussis-containing vaccine should receive one dose of Tdap vaccine, which can be administered regardless of the interval since the last dose of tetanus and diphtheria toxoid-containing vaccine. Refer to Schedule.
Serologic Testing
Serologic testing is not recommended before or after receiving diphtheria toxoid-containing vaccine.
Storage Requirements
Diphtheria toxoid-containing preparations should be stored in a refrigerator at +2°C to +8°C and must not be frozen. Refer to Storage and Handling of Immunizing Agents in Part 1 for additional general information.
Simultaneous Administration with Other Vaccines
Diphtheria toxoid-containing vaccines may be administered concomitantly with routine vaccines at different injection sites using separate needles and syringes. Refer to Timing of Vaccine Administration in Part 1 for additional general information.
Vaccine and Antitoxin Safety and Adverse Events
Refer to Adverse Events Following Immunization in Part 2 for additional general information. Refer to Tetanus Toxoid, Pertussis Vaccine, Poliomyelitis Vaccine, Haemophilus influenzae type b Vaccine and Hepatitis B Vaccine in Part 4 for additional information regarding other components in diphtheria toxoid-containing combination vaccines.
Common and local adverse events
Diphtheria-toxoid containing vaccines
Redness, swelling and pain at the injection site are the most common adverse reactions to childhood diphtheria toxoid-containing combination vaccines. A nodule may be palpable at the injection site and persist for several weeks. Abscess at the injection site has been reported.
In clinical trials, injection site adverse reactions, including tenderness, erythema, swelling or any combination of these findings, were reported in 10% to 40% of children after each of the first 3 doses of diphtheria-toxoid containing vaccine. Mild systemic reactions such as fever, irritability fussiness or any combination of these findings were commonly reported (8% to 29%), as well as drowsiness (40% to 52%).
In two clinical studies, swelling (greater than 5 cm) and erythema were reported in 15% to 20% of vaccinees after the fourth or fifth doses of DTaP vaccines. Extensive limb swelling (greater than 10 cm in diameter) possibly involving the entire proximal limb may occur in 2% to 6% of children. While these injection site reactions produce significant swelling, pain is generally limited. There is some evidence that children with extensive limb swelling following the fourth dose of a DTaP vaccine are at increased risk of such an event following the fifth dose. The presence of a large injection site reaction to a previous dose, however, is not a contraindication to continuing the recommended schedule.
Among adults given a booster dose of Tdap vaccine, common reactions include pain, redness and swelling at the injection site, headache, and fatigue. Fever and chills also are common reactions. Adverse reactions following Td vaccine are similar. Overall, adverse reactions are less common in adults than adolescents. The interval between the childhood DTaP vaccine series or a dose of Td vaccine, and a dose of Tdap vaccine does not affect the rate of injection site or systemic adverse events.
DAtx
Diphtheria antitoxin may trigger allergic reactions of varying severity including: skin pruritus, pain, swelling or redness; urticaria; dry cough; hoarseness; nausea; vomiting; and asthma-like crisis. The frequency varies and the reactions occur within the first 24 hours after administration of DAtx. Persons previously treated with serum of equine origin may have a higher risk of reaction.
Less common and serious or severe adverse events
Diphtheria-toxoid containing vaccines
Serious adverse events are rare following immunization with diphtheria toxoid-containing vaccines and, in most cases, data are insufficient to determine that the vaccine has caused the reactions. Severe systemic reactions such as generalized urticaria, anaphylaxis, or neurologic complications have been reported rarely.
Severe (Arthus-type) injection site reactions are occasionally reported following receipt of diphtheria toxoid or tetanus toxoid-containing vaccines. There may be extensive, painful swelling around the injection site, often involving the arm from shoulder to elbow and generally beginning 2 to 8 hours after injection. Such reactions are most often reported in adults, particularly those who have received frequent doses of diphtheria or tetanus toxoid or both. Persons experiencing severe injection site reactions usually have very high serum antitoxin concentrations and should not receive further routine booster doses of Td vaccine for at least 10 years. A pregnant person with a history of Arthus-type injection site reaction within the past 10 years should be referred to a specialist prior to re-vaccination with Tdap.
DAtx
Severe reactions are uncommon. Fatal anaphylactic shock has been reported in 1:50,000 persons receiving DAtx. Serum sickness (fever, urticaria, arthralgia, adenomegaly and, more rarely, neurological or renal compromise) may occur between 5 and 24 days after the administration DAtx in approximately 8% of recipients.
Other reported adverse events and conditions
Cases of Guillain-Barre Syndrome (GBS) or polyneuritis have been reported following receipt of diphtheria toxoid-containing vaccine. While the evidence favours a causal relationship between tetanus toxoid and GBS, there is little evidence to support an independent association between receipt of diphtheria toxoid and GBS.
Guidance on reporting Adverse Events Following Immunization (AEFI)
Vaccine providers are asked to report, through local public health officials, any serious or unexpected adverse event felt to be temporally related to vaccination, so that a full investigation can be undertaken. An unexpected AEFI is an event that is not listed in available product information, but may be due to the immunization or to an increase in the frequency of a known AEFI. Refer to Reporting Adverse Events Following Immunization (AEFI) in Canada and Adverse Events Following Immunization Part 2 for additional information about AEFI reporting.
Contraindications and precautions
Diphtheria toxoid-containing vaccines are contraindicated in persons with a history of anaphylaxis after previous administration of the vaccine and in persons with proven immediate or anaphylactic hypersensitivity to any component of the vaccine or its container. Refer to Table 1 and Table 2 in Contents of Immunizing Agents Authorized for Use in Canada in Part 1 for lists of all vaccines and passive immunizing agents authorized for use in Canada and their contents.
Hypersensitivity to yeast is very rare and a personal history of yeast allergy is not generally reliable. In situations of suspected hypersensitivity or non-anaphylactic allergy to vaccine components, investigation is indicated that may involve immunization in a controlled setting. Consultation with an allergist is advised.
Administration of diphtheria toxoid-containing vaccine should be postponed in persons with moderate or severe acute illness. Persons with minor acute illness (with or without fever) may be vaccinated.
It is prudent to not administer further doses of tetanus-toxoid containing vaccine to persons who develop GBS within 6 weeks of receiving such vaccine. Those who develop GBS outside the 6 week interval may receive subsequent doses of tetanus toxoid-containing vaccine. If there is a history of both Campylobacter infection (which has been associated with GBS) and receipt of a tetanus and diphtheria toxoid-containing vaccine within the 6 weeks before the onset of GBS, consultation with an infectious disease specialist is advised.
People who experience a severe injection site reaction following a dose of tetanus toxoid-containing vaccine should not be given another dose for at least 10 years.
Refer to Contraindications and Precautions in Part 2 for additional general information.
Other Considerations
Interchangeability of vaccines
The primary series of three doses of diphtheria toxoid-containing vaccine should be completed with an appropriate vaccine from the same manufacturer whenever possible. However, if the original vaccine is unknown or unavailable, an alternative combination vaccine from a different manufacturer may be used to complete the primary series. On the basis of expert opinion, an appropriate product from any manufacturer can be used for all booster doses. Refer to Principles of Vaccine Interchangeability in Part 1 for additional general information.
Selected References
- Centers for Disease Control and Prevention. ACIP Provisional Recommendations for Health Care Personnel on use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine (Tdap) and use of Postexposure Antimicrobial Prophylaxis.
- Centers for Disease Control and Prevention. Notice to Readers: FDA Approval of Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed, (INFANRIX®) for Fifth Consecutive DTaP Vaccine Dose. MMWR Morb Mortal Wkly Rep. 2003 Sep 26;52(38):921.
- Centers for Disease Control and Prevention. Prevention of Pertussis, Tetanus, and Diphtheria Among Pregnant and Postpartum Women and Their Infants. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2008;57(RR-4):1-49.
- Centers for Disease Control and Prevention. The Pink Book: Epidemiology and Prevention of Vaccine Preventable Diseases. Updated 11th ed.; May 2009.
- Centers for Disease Control and Prevention. Health Information for International Travel 2012. The Yellow Book.
- Centers for Disease Control and Prevention. Update: Guillain-Barré syndrome among recipients of Menactra® meningococcal conjugate vaccine-United States, June 2005-September 2006. MMWR 2006; 55:1120-24.
- Centers for Disease Control and Prevention. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis (Tdap) Vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep 2011;60(1):13-15.
- Centers for Disease Control and Prevention. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis (Tdap) Vaccine in Adults Aged 65 Years and Older — Advisory Committee on Immunization Practices (ACIP), 2012.
- David S, Hemsley C, Pasquali P et al. Enhanced surveillance for vaccine-associated adverse events: dTap catch-up of high school students in Yukon. Can Commun Dis Rep 2005;31(11):117-26.
- Decker MD, Edwards KM, Steinhoff MC et al. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics 1995; 96(l):557-66.
- Dittmann S, Wharton M, Vitek C et al. Successful control of epidemic diphtheria in the states of the former Union of Soviet Socialist Republics: lessons learned. J Infect Dis 2000;181(Suppl 1):S10-22.
- Galazka A, Robertson S. Immunization against diphtheria with special emphasis on immunization of adults. Vaccine 1996;14(9):845-57.
- Gautret P, Wilder-Smith A. Vaccination against tetanus, diphtheria, pertussis and poliomyelitis in adult travellers. Travel Med Infect Dis 2010;8:155-60.
- GlaxoSmithKline Inc. Product Monograph - BOOSTRIX®, October 2009.
- GlaxoSmithKline Inc. Product Monograph - BOOSTRIX®-POLIO, June 2008.
- GlaxoSmithKline Inc. Product Monograph - INFANRIX hexa™. July 2008.
- Halperin S, Sweet L, Baxendale D et al. How soon after a prior tetanus-diphtheria vaccination can one give adult formulation tetanus-diphtheria-acellular pertussis vaccine? Pediatr Infect Dis J 2006;25(3):195-200.
- Instituto Butantan. Product Leaflet - ANTIDIPHTHERIA SERUM, undated.
- Institute of Medicine. Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality, Washington, DC, National Academy Press, 1994.
- Kretsinger K, Broder KR, Cortese MM et al. Centers for Disease Control and Prevention; Advisory Committee on Immunization Practices; Healthcare Infection Control Practices Advisory Committee. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006 Dec 15;55(RR-17):1-37.
- Langley JM, Predy G, Guasparina R, et al. An adolescent-adult formulation tetanus and diphtheria toxoids adsorbed combined with acellular pertussis vaccine has comparable immunogenicity but less reactogenicity in children 4-6 years of age than a pediatric formulation acellular pertussis vaccine and diphtheria and tetanus toxoids adsorbed combined with inactivated poliomyelitis vaccine. Vaccine 2007; 25:1121-25.
- National Advisory Committee on Immunization. Interval between administration of vaccines against diphtheria, tetanus, and pertussis. Can Commun Dis Rep 2005;31(ACS- 9).
- National Advisory Committee on Immunization. Statement on the recommended use of pentavalent and hexavalent vaccines. Can Comm Dis Rep 2007;33(ACS-1):1-15.
- Plotkin SA, Orenstein WA. Vaccines, 4th edition. Philadelphia: W.B Saunders Company, 2003: 211-228.
- Rennels MB. Extensive swelling reactions occurring after booster doses of diphtheria-tetanus-acellular pertussis vaccines. Semin Pediatr Infect Dis. 2003 Jul;14(3):196-8.
- Rennels MB, Black S, Woo EJ et al. Safety of a fifth dose of diphtheria and tetanus toxoid and acellular pertussis vaccine in children experiencing extensive, local reactions to the fourth dose. Pediatr Infect Dis J. 2008 May;27(5):464-5.
- Sanofi Pasteur Ltd. Product Monograph - ADACEL®, August 2009.
- Sanofi Pasteur Ltd. Product Monograph - ADACEL®-POLIO, October 2010.
- Sanofi Pasteur Ltd. Product Monograph - PEDIACEL®, January 2009.
- Sanofi Pasteur Ltd. Product Monograph - QUADRACEL®, July 2008.
- Sanofi Pasteur Ltd. Product Monograph - Td ADSORBED, July 2010.
- Varughese P. Diphtheria in Canada - surveillance summary. Can Dis Wkly Rep 1978;4:65-8.