Archived - Detecting E. coli O157 and non-O157

 Download this article as a PDF
Download this article as a PDFPublished by: The Public Health Agency of Canada
Issue: Volume 44-11: Healthcare-associated infections and antimicrobial resistance
Date published: November 1, 2018
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 44-11, November 1, 2018: Healthcare-associated infections and antimicrobial resistance
Guidance
CPHLN recommendations for the laboratory detection of Shiga toxin-producing Escherichia coli (O157 and non-O157)
L Chui1, S Christianson2, DC Alexander3, V Arseneau4, S Bekal5, B Berenger1, Y Chen6, R Davidson7, DJ Farrell8, GJ German4, L Gilbert9, LMN Hoang10, RP Johnson2, A MacKeen11, A Maki6, C Nadon2, E Nickerson12, A Peralta6, SM Radons Arneson11, Y Yu6, K Ziebell2 on behalf of Canadian Public Health Laboratory Network (CPHLN)
Affiliations
1 Provincial Laboratory for Public Health, Alberta Public Laboratories, AB
2 National Microbiology Laboratory, MB & ON
3 Cadham Provincial Laboratory, MB
4 Health Prince Edward Island, PE
5 Laboratoire de Santé Publique du Québec, QC
6 Public Health Ontario, ON
7 Queen Elizabeth II Health Science Centre, NS
8 Roy Romanow Provincial Laboratory, SK
9 Provincial Public Health and Microbiology Laboratory, Eastern Health, NL
10 BC Centre for Disease Control Public Health Laboratory, BC
11 Canadian Public Health Laboratory Network, MB
12 The New Brunswick Enteric Reference Centre, NB
Correspondence
Suggested citation
Chui L, Christianson S, Alexander DC, Arseneau V, Bekal S, Berenger B, Davidson R, Farrell DJ, German GJ, Gilbert L, Hoang LMN, Johnson RP, MacKeen A, Maki A, Nadon C, Nickerson E, Peralta A, Radons Arneson SM, Yu Y, Ziebell K, on behalf of Canadian Public Health Laboratory Network (CPHLN). CPHLN recommendations for the laboratory detection of Shiga toxin-producing Escherichia coli (O157 and non‑O157). Can Commun Dis Rep 2018;44(11):304–7. https://doi.org/10.14745/ccdr.v44i11a06
Keywords: Laboratory testing recommendations, Shiga toxin-producing Escherichia coli, O157 STEC, non‑O157 STEC, culture independent diagnostic tests, nucleic acid amplification tests (NAATs), enzyme immunoassays (EIAs), chromogenic agar culture
Abstract
Shiga toxin-producing Escherichia coli (STEC) are important enteric pathogens responsible for sporadic cases and outbreaks of gastroenteritis. E.coli O157:H7/NM (STEC O157) are the most commonly known STEC serotypes but it is now increasingly apparent that non‑O157 STEC serotypes have been underreported in the past because they were not part of routine screening in many front-line laboratories. The Canadian Public Health Laboratory Network (CPHLN) has identified the need for improved detection and surveillance of non‑O157 STEC and has developed the following recommendations to assist in the decision-making process for clinical and reference microbiology laboratories. These recommendations should be followed to the best of a laboratory’s abilities based on the availability of technology and resources.
The CPHLN recommends that when screening for the agents of bacterial gastroenteritis from a stool sample, front-line laboratories use either a chromogenic agar culture or a culture-independent diagnostic test (CIDT). CIDT options include nucleic acid amplification tests (NAATs) to detect Shiga toxin genes or enzyme immunoassays (EIAs) to detect Shiga toxins. If either CIDT method is positive for possible STEC, laboratories must have a mechanism to culture and isolate STEC in order to support both provincial and national surveillance as well as outbreak investigations and response. These CPHLN recommendations should result in improved detection of STEC in patients presenting with diarrhea, especially when due to the non‑O157 serotypes. These measures should enhance the overall quality of healthcare and food safety, and provide better protection of the public via improved surveillance and outbreak detection and response.
Introduction
Escherichia coli are part of the normal flora of the gut. However, Shiga toxin-producing Escherichia coli (STEC) are intestinal pathogens. Although they typically cause a self-limited episode of diarrhea and abdominal pain, on rare occasions they cause severe—and potentially fatal—sequelae such as hemolytic uremic syndrome Footnote 1.
E. coli O157:H7/NM (STEC O157) are the most common STEC serotypes causing infection in humans, but many non‑O157 STEC serotypes have been associated with serious illness and major outbreaks Footnote 2. In 2011 there was a European outbreak of E. coli O104:H4 Footnote 3. In 2016, there was an outbreak in Canada and the United States of STEC O121 infections associated with flour Footnote 4. These recent outbreaks of non‑O157 serotypes have highlighted the clinical importance of timely and reliable detection and surveillance of these organisms Footnote 5Footnote 6Footnote 7Footnote 8Footnote 9Footnote 10.
In 2016, 642 cases of STEC infections were reported to the Canadian National Enteric Surveillance Program (NESP); approximately 35% were caused by non‑O157 STEC Footnote 11. Current data for Canada on non‑O157 STEC infections are likely an underestimate as they are not part of routine screening in many laboratories Footnote 11Footnote 12. There are several reasons for this gap. STEC O157 is readily identified using differential and selective media such as Sorbitol-MacConkey agar and chromogenic O157 agar. Unlike O157 STEC, non‑O157 STEC lack the phenotypic characteristics that readily distinguish them from generic E. coli, making culture-based isolation challenging.
Detection of non-O157 STEC subtypes
Improvements to laboratories' ability to identify non‑O157 STEC are based on the use of chromogenic and/or selective agars and culture-independent diagnostic tests (CIDT). The two common CIDTs include nucleic acid amplification tests (NAATs) for detection of Shiga toxin genes, and enzyme immunoassays (EIAs) that detect Shiga toxins. Less common CIDTs include Shiga toxin detection by cell culture cytotoxicity assays or reverse passive latex agglutination test, and isolation of selected serogroups by O-antigen immunomagnetic bead-capture methods. However, these methods are not practical in most front-line microbiology laboratories. Not only is it important to identify the STEC subtype, it is also essential to have isolates for further characterization such as serotyping, molecular genotyping and whole genome sequencing.
Each detection method has its advantages and disadvantages. Chromogenic agar has a sensitivity of greater than 85% Footnote 13. It is less costly than other methods and can be easily substituted into a laboratory’s workflow by replacing the current O157-specific plates. However, chromogenic agar can be inhibitory to some STEC serotypes Footnote 14. As for the detection of Shiga toxins by EIA, both microwell and lateral flow formats are available. The sensitivity of EIAs is lower for direct stool testing; however, overnight enrichment in MacConkey broth Footnote 15 or other suitable broth may provide sensitivity approaching that of NAATs Footnote 13Footnote 16. Both in-house and commercial NAATs have equivalent sensitivities and are the most sensitive methods available Footnote 15Footnote 17. Many NAATs can be performed directly on stool samples Footnote 18, improving the turn-around time compared to culture and EIA. Multiplex NAATs have the added advantage of detecting multiple pathogens concurrently. Both EIAs and NAATs will detect Shiga toxins and Shiga toxin genes respectively from any serotype of STEC. However, EIAs and NAATs are more expensive than agar for screening. NAATs also require laboratories to purchase additional equipment, designated space for molecular set-up and specialized training of personnel, which may be impractical for many frontline laboratories.
Public health implications
Isolation and further subtyping enables the comparison of STEC strains in order to identify outbreaks and potential sources of infection. Once the suspected source of the outbreak is identified, tracing and outbreak management activities by public health officials can prevent further transmission and promote public awareness.
The objectives of the following recommendations are to identify laboratory choices in a testing workflow for the detection, confirmation of STEC in stool specimens, and recovery of positive isolates for further characterization.
Recommendations
The Canadian Public Health Laboratory Network (CPHLN) recommends that when screening for the agents of bacterial gastroenteritis, front-line laboratories use one or more of three options for the detection of STEC: NAAT, culture on selective agar, or broth enrichment plus an EIA (Figure 1). Stool specimens submitted for STEC detection should follow local guidelines for submission and testing. Laboratories using chromogenic agar as their primary screening method may consider using an EIA method with broth enrichment or a NAAT in selected cases where there is a high suspicion of STEC infection and chromogenic agar results are negative.
If CIDT is implemented for STEC testing, culture is still recommended for the recovery of isolates for further characterization when Shiga toxins or Shiga toxin genes are identified. CPHLN culture recommendations following CIDT can be found in Berenger et al. Footnote 19. It is imperative that front-line laboratories communicate with their referral public health laboratories to determine the required work-up for culture, isolation or characterization of isolates before submitting any samples.
Figure 1: Recommendations for the detection of Shiga toxin-producing Escherichia coli in stool specimens
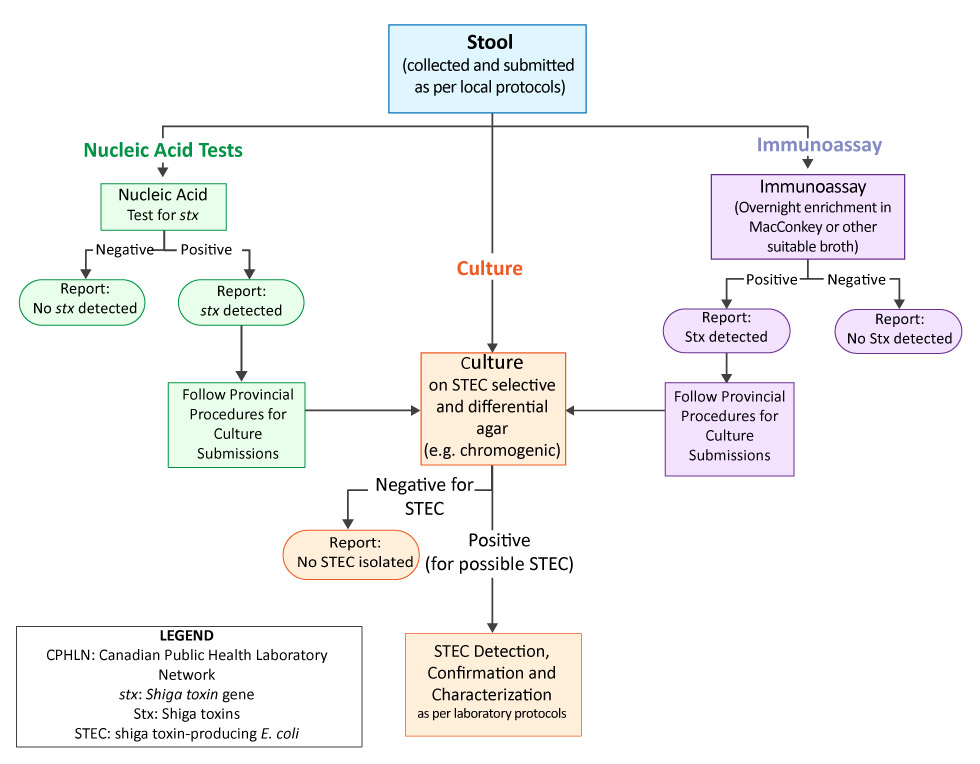
Text description: Figure 1
Figure 1: Recommendations for the detection of Shiga toxin-producing Escherichia coli in stool specimen.
This is a flow chart describing recommended paths for the laboratory detection of Shiga toxin-producing Escherichia coli in stool specimen. Three main paths are illustrated: Nucleic acid testing, immunoassay and stool culture.
The Nucleic acid testing path for Shiga toxin gene shows two possible results. For nucleic acid tests that are Shiga toxin gene negative, a report should be completed and no further laboratory action is required. For positive nucleic acid tests, the report should indicate that no Shiga toxin gene was found and the next step is to follow provincial procedures for culture submissions. The “culture” path is discussed later in this text description.
The immunoassay path for Shiga toxin detection follows. The immunoassay is performed using overnight enrichment in MacConkey or other suitable broth. When immunoassay results are negative for Shiga toxin, a report should be completed and no further laboratory action is required. For positive immunoassays, the report should indicate that Shiga toxin was found and the next step is to follow provincial procedures for culture submissions.
The culture path can be followed either as a first step in the detection process, or as a result of negative nucleic acid tests or positive immunoassays. If the culture is negative for Shiga toxin-producing Escherichia coli (STEC), a report should be completed and no further laboratory action is required. If the culture is positive for possible STEC, laboratory protocols should be followed for STEC detection, confirmation and characterization.
Discussion
To accurately diagnose all STEC-related cases of gastroenteritis that may lead to outbreaks and have public health implications, it is important that both O157 and non‑O157STEC serotypes are identified. To facilitate this, screening should be done using either culture or CIDT by a NAAT or an EIA. If the CIDT is positive for possible STEC, then culture is needed for STEC confirmation and characterization.
Laboratories should follow the CPHLN recommendations to the best of their abilities based on the availability of technology and resources. It is important to emphasize that when STEC are detected, culturing the organism is of paramount importance for further characterization (typing and subtyping), as well as to facilitate the outbreak response and support surveillance programs, including PulseNet Canada and the National Enteric Surveillance Program.
The role of the provincial or federal laboratories is to support front-line laboratories by performing confirmatory testing as necessary, as well as serotyping and other reference laboratory services for STEC isolates. These laboratories are also available to assist with facilitating the implementation of these new testing algorithms for non‑O157 STEC. The public health laboratory system varies among provinces; so any changes in public health laboratory protocols that may impact the capacity of front-line laboratories to follow these recommendations must be discussed with the front-line laboratories and other stakeholders prior to implementation.
Conclusion
Following these CPHLN recommendations should result in improved detection of STEC in patients presenting with diarrhea, especially the non‑O157 serotypes. These measures will enhance the overall quality of health care and food safety, and provide better protection of the public via improved surveillance, and outbreak detection and response.
Authors’ statement
All authors are members of the Canadian Public Health Laboratory Network (CPHLN) Shiga toxin-producing Escherichia coli (STEC) Working Group. This group was chaired by L Chui (Provincial Co-Chair) and S Christianson (Federal Co-Chair).
Conflict of interest
None.
Acknowledgements
The authors would like to acknowledge members of the Canadian Public Health Laboratory Network, Laboratory Directors Council and Secretariat support for their advice and guidance in the development of this recommendation.
Funding
Funding for the Canadian Public Health Laboratory Network Secretariat is provided by the National Microbiology Laboratory of the Public Health Agency of Canada.