Health Canada 2017–2018 Departmental Results Report

Download the entire report
(PDF format, 1,237 KB, 77 pages)
Organization: Health Canada
Published: 2018-11-20
The Honourable Ginette Petitpas Taylor, P.C., M.P.
Minister of Health
© Her Majesty the Queen in Right of Canada,
represented by the President of the Treasury Board, 2018
Catalogue No H1-9 / 32E-PDF
ISSN 2560-810X
Pub. 180069
This document is available on the Treasury Board of Canada Secretariat website
This document is available in alternative formats upon request.
Table of contents
- Minister’s message
- Results at a glance
- Raison d’être, mandate and role: who we are and what we do
- Operating context and key risks
- Results: what we achieved
- Program 1.1: Canadian Health System Policy
- Program 1.2: Specialized Health Services
- Program 1.3: Official Language Minority Community Development
- Program 2.1: Health Products
- Program 2.2: Food Safety and Nutrition
- Program 2.3: Environmental Risks to Health
- Program 2.4: Consumer Product and Workplace Hazardous Materials
- Program 2.5: Problematic Substance Use
- Program 2.6: Radiation Protection
- Program 2.7: Pesticides
- Program 3.1 First Nations and Inuit Primary Health Care
- Program 3.2 Supplementary Health Benefits for First Nations and Inuit
- Program 3.3 Health Infrastructure Support for First Nations and Inuit
- Internal Services
- Analysis of trends in spending and human resources
- Supplementary information
- Appendix: definitions
Minister's message
As Minister of Health, I am pleased to present Health Canada’s 2017-18 Departmental Results Report. This report outlines the important work the Department has accomplished over the past year to help protect the health and safety of Canadians.
Without a doubt, this has been a year of exceptional challenges and I am proud of the actions that we have taken to meet them.
Among our greatest challenges has been addressing the opioid crisis. In 2017-18, we introduced many concrete measures to better protect Canadians from opioid harms, reduce stigma associated with drug use, and support those dealing with problematic substance use. For example, we removed regulatory barriers to allow patients to receive prescription heroin and methadone treatment outside a hospital setting. Through recent regulatory amendments, Canadians now have greater access to a comprehensive array of treatment options. Budget 2018 included significant investments over five years to address the opioid crisis, including support for a range of actions to improve treatment, address stigma and gather more data.
As part of the Government’s commitment to legalizing, regulating and restricting access to cannabis, we introduced Bill C-45. With its passage, Canada has adopted a public health approach to cannabis that will better protect our youth, displace the illegal market, and provide adults with a legal source of regulated cannabis. Public education has been, and will continue to be, a cornerstone of our efforts to help ensure that Canadians get the facts on cannabis to make informed choices.
Health Canada also made important progress in its work to reduce tobacco use, which remains the leading preventable cause of disease and premature death in Canada. As of October 2017, Health Canada prohibited the manufacture and sale of cigarettes, blunt wraps and most cigars that contain menthol, and prohibited the promotion of menthol on the packaging of these tobacco products. In addition, we introduced Bill S-5, which proposed amendments to the Tobacco Act to implement a legislative framework for vaping products, representing a major milestone for Canada’s efforts to protect youth and non-smokers. With its passage, Health Canada will be able to implement measures to regulate the appearance, shape and size of tobacco products and their packaging, an important element of Canada's Tobacco Strategy.
Health Canada also made significant progress in its Healthy Eating Strategy, which continues to be a priority for this Government. In September 2017, we took the final step to ban the main source of industrially produced trans fats in all food sold in Canada. In February 2018, the Department consulted with Canadians about mandatory nutrition symbols on the front of packaged foods that are high in sodium, sugars or saturated fat. We are also updating Canada’s Food Guide to make it more relevant and useful for Canadians. These measures will make it easier for Canadians to make healthier and more informed decisions about the food they eat.
We are committed to improving access to medicines for Canadians. We are working with our partners in Canada and abroad to find ways to streamline and speed up our approvals process for new medicines while continuing to ensure that those products are safe and effective. This means that the medicines that the healthcare system needs most will move through the approvals system faster.
In August 2017, the Prime Minister announced plans for the creation of Indigenous Services Canada, a new federal Department with a mandate to improve the delivery of programs and services for Indigenous Peoples and to close socio-economic gaps. Recognizing the important role that health services play in reducing the health inequities between Indigenous Peoples and non-Indigenous populations, the functions delivered by Health Canada’s First Nations and Inuit Health Branch were transferred to the new Department. Health Canada is committed to working to reduce the health inequities between Indigenous Peoples and non-Indigenous Canadians, and continues to engage with Indigenous partners, other federal departments, and provincial and territorial governments to address Indigenous health priorities.
We continue to work with all provinces and territories to improve access to home and community care and mental health and addiction services as part of the Common Statement of Principles on Shared Health Priorities. This stable, predictable and long-term funding represents a major step towards achieving better health care and health outcomes for Canadians across the country.
These are a just a few examples of Health Canada’s work on behalf of Canadians. I invite you to read this report to learn more about how Health Canada is helping the people of Canada to maintain and improve their health.
The Honourable Ginette Petitpas Taylor, P.C., M.P.
Minister of Health
Results at a glance
Resources used to achieve results for Canadians
Health Canada's total actual spending for 2017-18: $3,491,052,712
Health Canada's total actual full time equivalents for 2017-18: 8,218
For more information on Health Canada’s plans, priorities and results achieved, see the “Results: what we achieved” section of this report.
Priority I: Support health system innovation.
Description
A highly functioning health care system is vital to addressing the health needs of Canadians. Although health care delivery is primarily under provincial and territorial jurisdiction, the federal government has an ongoing role in providing financial support through fiscal transfers to the provinces and territories, maintaining the core principles of the Canada Health Act, and supporting health care innovation and collaboration across the country. Health Canada will contribute to improving the quality and sustainability of health care as the system continues to evolve in a context of technological and social changes, demographic shifts and fiscal pressures. The Government is engaging provinces and territories on multi-year health accord agreements as part of its commitment to ensure the health care system continues to evolve and innovate.
Key Results
- In 2017-18, Health Canada negotiated shared priorities for action in home and community care (including palliative care), mental health, pharmaceuticals and innovation with provinces and territories in support of $11B in new federal investments over 10 years to improve home care and mental health services, as well as $544M over five years to federal and pan-Canadian health organizations to support innovation and pharmaceutical initiatives. All provinces and territories have agreed to a Common Statement Of Principles On Shared Health Priorities, and five jurisdictions have finalized and signed bilateral agreements with the federal government.
- The Government published proposed changes to the Patented Medicines Regulations, representing the first major update to the regulations in more than 20 years. These proposed amendments to the regulations are estimated to lower drug prices for Canadians by $12.6B over 10 years.
- Health Canada continued to work towards addressing outstanding and emerging Canada Health Act (CHA) issues. In 2017-18, provinces and territories began participating in discussions with Health Canada on three inter-related CHA initiatives: clarifying the federal position on patient charges for medically necessary diagnostic services; introducing a reimbursement policy; and strengthening reporting requirements. These steps, which are still on going, are to ensure CHA compliance issues are addressed.
Priority II: Strengthen openness and transparency as modernization of health protection legislation, regulation and delivery continues.
Description
Health Canada’s operating environment is constantly evolving. For example, the integrity of the global supply chain is changing; the speed of innovation continues to accelerate; and there is increased demand for greater openness and transparency. To address these challenges in the environment and to help Canadians live healthier lives and protect them from unsafe food, products, and threats, Health Canada will continue its efforts with its partners at home and abroad to modernize regulatory frameworks and service delivery models and to strengthen openness and transparency. The Department will provide credible and timely information to empower Canadians to make informed health decisions and support businesses’ responsibility for the safety of their products.
Key Results
- Health Canada worked with partners and other levels of government to address the opioid crisis on many fronts, as close to 4,000 Canadians died from apparent opioid overdoses in 2017. As part of these efforts, the engagement of people with lived and living experience related to substance use was enhanced, so that their perspectives and expertise were well integrated into the federal response to this significant public health crisis.
- Health Canada worked with Public Safety Canada and the Department of Justice to legalize, strictly regulate and restrict access to cannabis to keep it out of the hands of Canadian children and youth, and to keep profits away from criminals and organized crime. In April 2017, the Minister of Health supported the introduction of effective, evidence-informed legislation on the legalization and strict regulation of cannabis for consideration by Parliament. The Cannabis Act received Royal Assent on June 21, 2018, and came into force on October 17, 2018. In 2017-18, Health Canada continued to administer the Access to Cannabis for Medical Purposes Regulations.
- The Department made significant progress in implementing its Healthy Eating Strategy as part of the Government of Canada’s Vision for a Healthy Canada. Five public consultations related to the Healthy Eating Strategy were held in 2017-18 including extensive consultations to revise Canada's Food Guide and the proposed approach to restricting unhealthy food and beverage marketing to children.
- Significant progress was also made on commitments to address risks and potential benefits of vaping, plain packaging for tobacco products and implementing a ban on menthol cigarettes to protect young Canadians from inducements to tobacco use. As of October 2017, Health Canada prohibited the manufacture and sale of cigarettes, blunt wraps and most cigars that contain menthol, and prohibited the promotion of menthol on the packaging of these tobacco products.
- Health Canada identified 49 planned Regulatory Transparency and Openness Framework (public and non-public) activities to advance transparency and openness, a ministerial priority. Health Canada completed 25 (51%) of the total activities and 9 of the 11 public commitments. With exception of one activity with a revised target of 2019-20, the remaining activities will be completed in 2018-19.
- Health Canada continued to improve risk communication for pesticides to help Canadians make informed decisions about their health and the environment, and to advise industry of current and changing regulations to support compliance. Improved risk communication in 2017-18 included plain language summaries for re-evaluation decisions, to provide Canadians with easier to understand information about potential risks associated with pesticide use as well as measures to mitigate risk.
Priority III: Strengthen First Nations and Inuit health programming.Footnote 1
Description
First Nations and Inuit continue to experience serious health challenges. In an effort to close the Indigenous health gap, Health Canada plays an important role in supporting the delivery of health programs and services for First Nations and Inuit. The Department works with partners on innovative approaches to strengthen access to health services to ensure better integration of those services and to encourage greater control and management of health care delivery by First Nations and Inuit to better respond to their own needs. In addition, Health Canada continues to work with partners to further the implementation of a First Nations and Inuit Health Strategic Plan which provides stronger coherence and direction for the Department's activities in this area, and demonstrates how the Department collectively contributes to improving health outcomes for First Nations and Inuit. While the First Nations and Inuit Health Branch transferred responsibilities to Indigenous Services Canada (ISC), effective November 30, 2017, Health Canada continues to work closely with ISC to ensure that First Nations and Inuit continue to receive the highest standard of programs and services.
Priority IV: Recruit, maintain and foster an engaged, high performing and diverse workforce within a healthy workplace.
Description
Health Canada’s greatest strength is an engaged, empowered and well-equipped workforce with employees that have the competencies, tools and opportunities to succeed in the pursuit of excellence in program and service delivery. Two of the key priorities for the Government of Canada for 2017-18, as referenced in the Clerk’s 23rd Annual Report to the Prime Minister on the Public Service of Canada, are respectful workplaces with a focus on mental health, and recruitment. Health Canada is achieving this by building a healthy, respectful and supportive work environment and by developing an engaged, high-performing and diverse workforce across Canada, which includes recruiting for the future.
Key Results
- The Department continued significant investments in supporting the government’s pay stabilization efforts to support its employees in mitigating Phoenix/pay issues.
- Health Canada continued to implement the Multi-Year Strategy for Mental Health and Wellness in the Workplace promoting a corporate culture that supports workplace well-being, employment equity and healthy working relationships. The Multi-Year Diversity and Employment Equity Plan raised awareness of the importance of promoting a diverse, inclusive and respectful workplace and supported open mental health dialogue.
- Progress in support of enabling a culture of high performance continued in 2017-18 via ongoing initiatives such as the Performance Management Initiative assessment and the Post-Secondary Recruitment program, both of which exceeded their targets for the year, the promotion of the Canada School of Public Service’s new learning model, and the continued utilization of Career Connexions opportunities platform.
- Health Canada organized a live event and webcast titled "Health Talks on Reconciliation with Indigenous Peoples." Participants, including employees from the Health Portfolio and from several other federal departments, heard the perspectives of national Indigenous leaders on the path to putting reconciliation into action. A post-event video, which highlighted key themes of the event, was produced for dissemination to employees and more broadly through the Canada School of Public Service.
- Health Canada continued to support innovative employee engagement and launched a new storytelling series called “HC: Working for Canadians” that features videos, articles and other information describing how Health Canada is tackling serious public health issues to keep Canadians safe and healthy.
Raison d’être, mandate and role: who we are and what we do
Raison d’être
Health Canada regulates specific products and controlled substances, works with partners to support improved health outcomes for First Nations and Inuit, supports innovation and information sharing in Canada’s health system to help Canadians maintain and improve their health, and contributes to strengthening Canada’s record as a country with one of the healthiest populations in the world.
The Minister of Health is responsible for this organization.
Mandate and role
Health Canada is the federal Department responsible for helping Canadians maintain and improve their health, while respecting individual choices and circumstances. The Department plays five core roles in order to deliver its mandate. In fulfilling these roles, Health Canada draws on its strengths as a science-based Department, generating knowledge through the research, analysis and evaluations that it conducts, partners in and supports. The Department also draws on the knowledge that is being generated around the world to inform decision-making.
Core Roles
As a partner, Health Canada administers the Canada Health Act, which embodies the key values and principles of Canada’s publicly funded health care system.
The Department is also a funder, providing grants and contributions to various organizations that reinforce the Department's health objectives. Health Canada also transferred financial resources to First Nations and Inuit organizations and communities to deliver community health services until the transfer of Health Canada’s First Nations and Inuit health programs and services to Indigenous Services Canada (ISC) on November 30, 2017. It also provides policy support for the Canada Health Transfer.
In addition, Health Canada is a regulator, playing a stewardship role that involves both protecting Canadians and facilitating the provision of products vital to the health and well-being of our citizens. The Department regulates and approves the use of thousands of products, including: biologics, consumer goods, foods, medical devices, natural health products, pesticides, pharmaceuticals, and toxic substances. Health Canada also delivers a range of programs and services in environmental health and protection and has responsibilities in the areas of problematic substance use, tobacco policy, workplace health and the safe use of consumer products. As well, Health Canada monitors and tracks diseases and takes action where required.
Until the transfer of Health Canada’s First Nations and Inuit health programs and services to Indigenous Services Canada (ISC) on November 30, 2017, Health Canada was a service provider of supplementary health benefits to more than 849,000 eligible First Nations and Inuit. These supplementary health benefits covered: pharmaceuticals, dental services, vision services, medical transportation, medical supplies and equipment, and mental health counselling. The Department supported the delivery of public health and health promotion services on-reserve and in Inuit communities. Health Canada also provided primary care services on-reserve in remote and isolated areas, where provincial services were not readily available.
Lastly, Health Canada is an information provider. Through performing high quality science and research, we support policy development, regulate increasingly-sophisticated products and provide the services, information and management essential to affordable and world-class health care for Canadians. Through research and surveillance, we provide information that Canadians can use to maintain and improve their health.
For more general information about the Department, see the “Supplementary information” section of this report. For more information on the Department’s organizational mandate letter commitments, see the Minister’s mandate letter on the Prime Minister of Canada’s website.
Text Description
The figure represents a map of Canada entitled “A partner in health for Canadians” with the following four pillars;
- Funds organizations to promote innovation and best practices across Canada.
- Supports the delivery of healthcare to First Nations and InuitFootnote 1.
- Sets and administers national principles so that quality healthcare is available to all Canadians.
- Regulates food, health and consumer products to keep Canadians safe.
Footnotes
- Footnote 1
-
Health Canada’s First Nations and Inuit health programs and services were transferred to Indigenous Services Canada (ISC) on November 30, 2017.
* Health Canada's First Nations and Inuit health programs and services were transferred to Indigenous Services Canada (ISC) on November 30, 2017.
Operating context and key risks
Operating context
Health Canada operates in a complex and dynamic environment, facing several challenges as it works to deliver results for Canadians. Many of these challenges are beyond the sole control of the Department and involve working collaboratively with a range of partners, including stakeholders, the public and various levels of government.
Canada’s public health care systems were designed more than half a century ago. All levels of government are aware of the need to adjust to the changing needs and expectations of Canadians and leverage technological advances in support of improving health outcomes and quality of care. As a partner in the national health care system, the Department works closely with provincial and territorial governments and stakeholders to develop national approaches to health systems issues and to promote the pan-Canadian adoption of best practices.
The increased pace of scientific and technological innovation, globalization, and the complexity of the global supply chain challenges the Department’s ability to effectively regulate new, innovative and complex products, substances, food and emerging product categories. Given the evolving and expanding nature of the global marketplace, a key area of focus is on creating and strengthening relationships with domestic and international partners in order to leverage cooperation and best practices.
Canadians continue to expect their Government to be more open and transparent and to effectively engage them in decision-making. The provision of credible and timely information is critical to helping Canadians make informed health decisions for themselves and their families. However, the Department is one of many sources of health information for Canadians. The varying scientific quality and accuracy of information available to the general public can hinder the Department’s efforts to reach Canadians, but also provides an opportunity for leadership in the provision of high quality, evidence-based health information.
The Department also underwent an internal transformation. The Government of Canada announced the creation of Indigenous Services Canada (ISC) and the transfer of First Nations and Inuit health programs and services from Health Canada to this new Department effective November 30, 2017, as per the Order in Council P.C. 2017-1465. Health Canada ensured the smooth transition of these programs and resources to ISC while minimizing the impact of the transition on employees. The Department also provides internal support services to these programs until all First Nations and Inuit-health-related internal support services are transferred to the new Department.
Innovation and Experimentation: how we further fostered creative thinking, the exchange of ideas and continuous improvement
Innovation and experimentation were critical to Health Canada’s ability to meet its mandate in the face of rapidly evolving science, new trends in the marketplace, and the changing demands and expectations of Canadians. Consistent with the Government of Canada’s commitment to support a more innovative federal public service, Health Canada took concrete steps to further foster innovation and experimentation in the development of new policy; program and service delivery; and in its regulatory enforcement activities.
Specifically, over the past fiscal year, the Department took steps to incent innovation and experimentation in its work. This included measures to more systematically consider innovative approaches to achieving regulatory, program and policy objectives; purposefully seeking out new and different perspectives, including through exposure to leading-edge outside thinkers; and seed funding to support departmental innovations with a view to test new ideas in a responsible way, with the possibility of scaling-up successes.
Budget 2017 announced the creation of Innovative Solutions Canada, a new program with more than $100M dedicated to supporting the scale up and growth of Canada's innovators and entrepreneurs by having the federal government act as a first customer. Health Canada is part of twenty participating federal departments and agencies that have set aside a portion of funding ($1.4M) to support the creation of innovative solutions by Canadian small businesses.
Last fiscal year, Health Canada created a new Medical Device Digital Health Review Division to enhance expertise in the areas of cybersecurity, software as a medical device, 3D printing, and artificial intelligence/machine learning. This work will focus on rapidly changing technologies in digital health which have the potential to make the delivery of health care more accessible, convenient and cost-effective.
Key risks
Health Canada has a well-established integrated risk management process that enables the Department to respond proactively to change and uncertainty by defining and understanding its operating environment and the factors that drive risks.
Key risks are identified through the development of the Corporate Risk Profile (CRP), which is aligned with the Departmental Plan (DP). The CRP sets out the key opportunities and threats that have the potential to affect the Department’s ability to achieve results, and deliver on its mandate and commitments. It outlines in detail the strategies to respond to these risks and benefit from any opportunities. Each risk is monitored to ensure that the associated response strategies are effective in reducing or maximizing their potential impact, and to raise any areas of concern for potential course correction, as required.
The following table outlines the Department’s three key external corporate risks. Health Canada identified four key risks in its 2017-18 DP; however, two pertained to First Nations and Inuit health programming which has since been transferred to the Department of Indigenous Services Canada. Further, an additional key risk was identified for Health Canada following the publication of the 2017-18 DP, which has been included as Risk 1 in the table below.
| Existing Risk Uphold the Canada Health Act |
Link to the Department’s Programs | Link to mandate letter commitments or to government wide and departmental priorities |
|---|---|---|
| Health Canada’s ability to effectively uphold the Canada Health Act (CHA) could be put at risk by challenges in administering the Act. | Program 1.1: Canadian Health System Policy | Mandate Letter Commitment: Promote and defend the CHA. Government Priority: Healthy Canadians. Organizational Priority I: Support Health System Innovation. |
Mitigating strategy and effectiveness
To facilitate consistent and even-handed administration of the CHA, Health Canada’s mitigating strategies over two fiscal years will: implement new policies; work to resolve issues with provinces and territories (PTs) in a consistent manner; normalize the internal administration of the CHA through a delegation matrix; and monitor litigation that may impact the CHA and support legal services as required.
- In 2017-18, PTs began participating in discussions with Health Canada on three inter-related CHA initiatives: clarifying the federal position on patient charges for medically necessary diagnostic services; introducing a reimbursement policy; and strengthening reporting requirements.
- The Department will be in a position to report on the percentage of CHA compliance issues addressed at the 2018-19 reporting period.
| Existing Risk Canadian confidence in the safety of health and consumer products. |
Link to the Department’s Programs | Link to mandate letter commitments or to government wide and departmental priorities |
|---|---|---|
| There is a risk that Canadians will lose confidence in the safety of health and consumer products if Health Canada is not regarded as a trusted regulator and used as a credible source of information. | Program 2.1: Health Products Program 2.2: Food Safety and Nutrition Program 2.3: Environmental Risks to Health Program 2.4: Consumer Product and Workplace Chemical Safety Program 2.5: Problematic Substance Use Program 2.6: Radiation Protection Program 2.7: Pesticides Program |
Government Priority: Open and Transparent Government. Organizational Priority II: Strengthen openness and transparency as modernization of health protection legislation, regulation and service delivery continues. |
Mitigating strategy and effectiveness
Planned mitigation strategies were successfully implemented, and proved to be effective in reducing the risk and leveraging opportunities.
To ensure that Health Canada continued to be seen as a trusted regulator and credible source of information, and to help Canadians make informed health and safety decisions, the following risk responses were executed:
- Health Canada expanded the amount of regulatory health and safety information made available to Canadians in a simple and accessible way through the implementation and communication of Health Canada’s various Regulatory Transparency and Openness Framework (RTOF) activities:
- As of 2017-18, Canadians have access to more information about Health Canada’s stakeholder engagement activities on key policy files, scientific advisory bodies, regulated drugs and medical devices, scientific data and information, policies and procedures for sharing confidential business information related to therapeutic products, and illicit drug analysis statistics and trends.
- As well, a total of 44 proactive communications were completed using social media (e.g. Twitter, Linked-in, Facebook) in support of RTOF initiatives. The majority were related to a new initiative aimed at tweeting certain approvals of new drugs and medical devices.
- Canadians and stakeholders were given more opportunities to provide input for consideration during the regulatory process.
- By March 31, 2018, almost 120,000 individual messages to participate in consultations had been sent to Canadians and stakeholders through the Consultation and Stakeholder Information Management System (CSIMS) – surpassing Health Canada’s established target of 50,000. Topics included nutrition symbols, the Food Guide, patented medicines regulations and cannabis regulations. There were 4,478 stakeholders and individuals registered in CSIMS by year-end, exceeding the Department’s established target of 2,500.
- Health Canada continued to implement a digital-first approach to inform, communicate and engage Canadians on Canada.ca and on Health Canada social media channels.
- Health Canada’s websites were successfully migrated to the Canada.ca publishing platform, which provides Canadians with one single website for health-related information.
- Social media channels were streamlined to provide a health-themed approach. Multiple Facebook and Twitter channels were merged to provide a single Facebook health channel and a single Twitter health channel.
- Health Canada made health-related online information easier to find and use from any device, accessible and compliant with Web 2.0 requirements.
| Existing Risk Protect Canadians from product risks. |
Link to the Department’s Programs | Link to mandate letter commitments or to government wide and departmental priorities |
|---|---|---|
| Health Canada’s ability to protect Canadians from the risks of products may be weakened due to the changing integrity of the global supply chain and the rapid pace of innovation. | Program 2.1: Health Products Program 2.2: Food Safety and Nutrition Program 2.3: Environmental Risks to Health Program 2.4: Consumer Product and Workplace Hazardous Materials Program 2.6: Radiation Protection Program 2.7: Pesticides |
Organizational Priority II: Strengthen openness and transparency as modernization of health protection legislation, regulation and service delivery continues. |
Mitigating strategy and effectiveness
Planned mitigation strategies were successfully implemented, and proved to be effective in reducing risk and leveraging opportunities.
To ensure that Health Canada is able to protect Canadians from the risks of products in an innovative and globalized environment, the following risk responses were executed:
- Health Canada collaborated with international regulatory organizations and aligned where appropriate with foreign regulators. The Department achieved the following:
- Initiated the preparation of a guidance document that would provide interpretation of the Food and Drug Regulations relevant to clinical trials involving human subjects, and the interpretation of international harmonized technical requirements for pharmaceuticals in the Canadian context as related to clinical trial inspections.
- Held two joint United States (U.S.) Food and Drug Administration (FDA)-Health Canada public consultations on International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines to align with the U.S. FDA.
- Published a final notice that reclassifies certain disinfectants and sterilants under the medical device regulatory framework.
- Completed analysis and carried out targeted consultations and engagements to refine the proposal to modernize the regulation of self-care products, including engagement with international regulatory partners.
- Conducted simultaneous regulatory reviews for veterinary drugs with the U.S. FDA Center for Veterinary Medicine including two drug approvals.
- Renewed the Common Electronic Submission Gateway and Interconnect Agreements with the U.S. FDA.
- Continued to collaborate with the U.S. Occupational Safety and Health Administration (OSHA) on the finalization of 2016 Regulatory Cooperation Council (RCC) Work Plan deliverables. Three guidance documents, which promote alignment between both jurisdictions, are in the final stages of review. Health Canada continues to collaborate and share information with the U.S. OSHA to align, to the greatest extent possible, Canadian and U.S. requirements for hazard classification and communication.
- Leveraged international collaborative relationships to make efficiency gains in the risk assessment of existing substances under the Chemical Management Plan. Through the U.S.-Canada RCC Chemicals Management Project, Health Canada, Environment and Climate Change Canada and the U.S. Environmental Protection Agency finalized a collaboration framework and related work plan which will enable enhanced alignment on risk assessment of chemicals, including the identification of risk assessment priorities, information gathering and sharing, risk assessment methodologies and work sharing. The RCC project also allowed for a joint US-Canadian educational primer for industry to understand the similarities and differences between the two jurisdictions related to Significant New Use Rule (SNUR) programs in the US and Significant New Activity (SNAc) compliance in Canada.
- Health Canada developed oversight strategies and tools to strengthen market surveillance and oversight of emerging products. The Department achieved the following:
- Established a scientific advisory panel to provide advice about the regulation of medical device digital health technologies, and created a new Medical Device Digital Health Review Division. This new division will allow Health Canada to keep pace with innovation and advances in the medical device and digital health technology sectors, and aims to benefit Canadian patients through improved access and convenience of these products
- Completed a pilot project between Health Canada and the Canadian Agency for Drugs and Technologies in Health to accelerate access to drugs through parallel and concurrent review (Alignment of the Health Technology Assessment review with the Health Canada review project).
- Posted a Notice of Intent for potential amendments to the Food and Drug Regulations related to improving access to generic drugs which will benefit Canadians through more timely access to affordable drugs and more treatment options.
- Continued ongoing work related to consumer product safety market surveillance including market surveys, media monitoring, the annual surveillance report, and watchlist materials/products.
- Finalized a multi-year implementation plan for the consumer product safety surveillance innovation project, which explored and developed transformative solutions for enhanced surveillance possibilities.
- Took action on consumer products where warranted, based on industry and consumer reported incidents, to mitigate risks to human health and safety, and provided data to inform future activities on known risks to children. This included proposed regulations on small powerful magnets found in toys.
- Conducted environmental scans and collected data to inform compliance and enforcement approaches to emerging products (e.g. drones, devices, treated articles). In addition, Health Canada conducted surveillance activities to ensure those products deemed non-compliant through inspections return to compliance in a timely manner.
- Health Canada increased the use of regulatory and non-regulatory activities which address changing business models in the supply chain, specifically for foreign sites.
- Health Canada completed 100% (40/40) of planned foreign on-site Good Manufacturing Practices inspections in 2017-18.
- The work on joint activities under the North America Consumer Product Safety Summit with the U.S. Consumer Product Safety Commission and Mexico continued and included joint project teams and joint recalls where appropriate. In 2017-18, this work led to 86 consumer product joint recalls, which accounts for almost 40% of the consumer product recalls communicated to Canadians for the year. Also, a trilateral cooperative Memorandum of Understanding (MOU) with the U.S. and Mexico, which formalized the three-way cooperation that has been taking place, was signed at the 2018 North American Summit.
- Preliminary discussions took place with the European Commission on next steps for a MOU under the Canadian-European Union Comprehensive Economic and Trade Agreement.
- Health Canada continued to implement the renewed Action Plan with China's Administration of Quality Supervision, Inspection and Quarantine, that was part of a renewed MOU between Canada and China which also includes an Alert and Response mechanism. Work under the Canada-China Action Plan continues in three working group areas and includes the identification of recalled products made in China for the Alert and Response mechanism.
Results: what we achieved
Programs
Strategic Outcome 1: A health system responsive to the needs of Canadians
Program 1.1: Canadian Health System Policy
Description
The Canadian Health System Policy program provides strategic policy advice, research, and analysis to support decision-making on health care system issues, as well as program support to provinces and territories, partners, and stakeholders on health care system priorities. Mindful of equity, sustainability and affordability, Health Canada collaborates and targets its efforts in order to support improvements to the health care system such as improved access, quality, and integration of health care services. Through the management of grants and contributions agreements with key pan-Canadian health partners, the Canadian Health System Policy program contributes to priority health issues requiring national leadership and strong partnership. The program objective is to support improvement in the health care system to help Canadians maintain and improve their health.
Results
Health Canada managed initiatives and funding agreements that advanced priority health issues and:
- Negotiated shared priorities for action in home and community care (including palliative care), mental health, pharmaceuticals and innovation with provinces and territories in support of $11B in new federal investments over 10 years to improve home care and mental health services, as well as $544M over five years to federal and pan-Canadian health organizations to support innovation and pharmaceutical initiatives. So far, all jurisdictions have agreed to a Common Statement Of Principles On Shared Health Priorities, and five jurisdictions (Newfoundland and Labrador, New Brunswick, Prince Edward Island, the Northwest Territories and Saskatchewan) have finalized and signed bilateral agreements with the federal government.
- Worked closely with the provinces and territories to support the implementation of the federal commitments of medical assistance in dying legislation. The primary focus of activity has been the development of regulations for the collection of information on assisted dying in support of a federal monitoring regime. Draft regulations were published in Canada Gazette Part I (December 2017) for public consultation and final regulations were published in Canada Gazette Part II in August, 2018. Health Canada, in partnership with Statistics Canada, developed and implemented a secure web portal to facilitate the collection of information from practitioners and pharmacists. Independent reviews on circumstances falling outside the scope of the legislation are ongoing.
- Following the passing of Private Members Bill C-277, for the development of a framework on palliative care in Canada, which received Royal Assent in December 2017, worked with key stakeholders, provinces and territories and other government departments to plan the Spring 2018 consultation required by the new legislation.
- Conducted research, analysis and policy work on health care system issues to support departmental priorities, including work on home and community care (including palliative care), mental health, prescription drugs, and Federal Action on Opioids.
Health Canada supported organisations that contribute to health system improvements and:
- Provided $81.7M to the Canadian Institute for Health Information to deliver comparable and actionable data and information to accelerate improvements in health care, health system performance and population health across Canada.
- Provided $47.5M to support the Canadian Partnership Against Cancer which has, through collaboration with key stakeholders including the provinces and territories, accelerated uptake of new knowledge and coordinated approaches to advance cancer control in Canada.
- Provided $29M to Canada Health Infoway to support short-term digital health activities in e-prescribing and tele-homecare (committed in Budget 2016), and provided $18M to further expand e-prescribing and virtual care initiatives, as well as improve access to electronic health records (this additional funding is a Budget 2017 commitment).
- Contributed $27M to support the Canadian Brain Research Foundation, which is managed by the Brain Canada Foundation.
- Provided $17M to the Canadian Foundation for Healthcare Improvement to work with provinces and territories to incubate, identify and support adoption of innovations that improve health care delivery.
- Provided $16.1M for the Canadian Agency for Drugs and Technologies in Health in support of health system effectiveness and sustainability by promoting, through the development of evidence, the cost-effective and optimal use of drugs and other health technologies.
- Provided $14.25M to the Mental Health Commission of Canada to advance work on substance misuse, suicide prevention, engagement with federal partners and other stakeholders, and targeting mental health and wellness initiatives on vulnerable populations.
- Provided $8.23M to the Canadian Centre on Substance Use and Addiction (CCSA) to provide national leadership to address substance use in Canada. CCSA promotes collaboration and knowledge exchange and provides objective, evidence-based information and advice to help reduce the health, social and economic harm from problematic substance use and addictions.
- Provided $7.8M in funding for 11 contribution agreements under the Health Care Policy Contribution Program and advanced health care innovation and health system renewal through collaborative working arrangements with provinces, territories, academic institutions, and non-governmental organizations.
- Provided $7.6M to the Canadian Patient Safety Institute to support efforts to improve the safety of health care across Canada, including the development of a new strategic plan to accelerate the pace of safety improvement in future years.
- Provided $1.8M to Pallium Canada to expand their successful Learning Essential Approaches to Palliative Care program to train more health care providers and others in palliative care so that more Canadians can access palliative care when and where they need it. The project will receive up to $6M over three years (2017-2020).
For more detailed results information on these grants and contributions, please see the Supplementary Information Table “Details on transfer payment programs of $5 million or more.”
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Recipients contribute to improvements in the health care system. | % of recipients demonstrating a contribution to health care system improvements | 100 | March 31, 2018 | 100 | 100 | N/AFootnote 1 |
Footnotes
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 297,012,268 | 297,012,268 | 402,292,934 | 385,167,016 | 88,154,748 |
Note: The variance of $88.2M between actual and planned spending is mainly due to the following:
Additional funding of $105.3M for the Territorial Health Investment Fund, modernizing health care delivery, including building better digital health systems and improving the health information available to support health care decision-making, and statutory funding for payments pursuant to section 103 of the Patent Act as well as Canada Health Infoway.
This is offset by $17.1M mainly resulting from the reprofile of funds for the Canada Brain Research Fund and the Canadian Partnership Against Cancer, as well as the reimbursement of a reduction under the Canada Health Act.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 238 | 176 | -62 |
Note: The variance of 62 in FTE utilization is mainly due to attrition and longer than anticipated staffing processes.
Program 1.2: Specialized Health Services
Description
The Specialized Health Services program supports the Government of Canada's obligation to protect the health and safety of its employees and the health of visiting dignitaries. Health Canada delivers counselling, organizational development and critical incident support services to federal government departments through a network of contracted mental health professionals, and also provides immediate response to employees following traumatic incidents in the workplace. Health Canada delivers occupational health and occupational hygiene consultative services to ensure that public servants meet medical requirements to safely and effectively perform their duties and to prevent work-related illness and injury. Health Canada proactively contributes to reducing the number of work days lost to illness across the federal government through the provision of occupational and psycho-social health services to federal public servants. Health Canada also arranges for the provision of health services for Internationally Protected Persons (IPP) who have come to Canada for international events or for regular meetings or official visits. An IPP is a representative of a State, usually Heads of State and/or Government, members of the Royal Family, or officials of an international organization of an intergovernmental character. The Specialized Health Services Program objective is to ensure continuity of services and the occupational health of federal public servants who can deliver results to Canadians in all circumstances and to arrange access to health services for IPPs.
Results
Through key initiatives, in 2017-18 Health Canada continued to support the Government of Canada's obligation to protect the health of its employees.
- The Employee Assistance Program (EAP) suite of services was expanded to offer the LifeSpeak Digital Wellness Library. The LifeSpeak platform is a bilingual suite of over 300 brief informative videos, podcasts and tip sheets available to employees and their family members. Each video is presented by a leading expert on issues ranging from depression and nutrition to financial health and parenting, and available via smartphone, tablet or computer.
- Initial progress made in 2017-18 on the Public Service Occupational Health Program's Occupational Health Assessment Guide (OHAG) update focussed on the development of the two-year work plan for OHAG renewal, and the initial identification of relevant occupational groups in four key federal departments.
- The Internationally Protected Persons (IPP) Program met its performance target of 95 percent of health plans delivered to client departments at least 24 hours before the visit. Preparations were carried out for the 2018 G7 summit by the IPP Program's special G7 organizing team. These preparations involved negotiating federal-provincial agreements with the Province of Quebec to obtain the human resources and equipment to deliver medical services and food surveillance at the G7 summit. The results of these efforts will be examined and lessons learned will be identified in fiscal year 2018-19.
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Federal employees are able to manage their psycho-social issues during and immediately following, stressful or traumatic events. | % of clients that achieve problem resolution within the Employee Assistance Services short term counselling model. (Baseline TBD) |
75Footnote 1 | March 31, 2018 | 84.3 | 97 | 98 |
| Reduced absenteeism in the workplace for employees who access employee assistance services. | % reduction in absenteeism in the 30 days that follow an employee's last Employee Assistance Program session versus the 30 days prior. | 25Footnote 1 | March 31, 2018 | 26.8 | 50 | 41 |
| Internationally Protected Persons (IPPs) have timely Health Plans available for emergency medical services and appropriate food surveillance services when they are in Canada. | % of Health Plans delivered to client departments at least 24 hours prior to the visit. | 95 | March 31, 2018 | 95 | 96 | 94 |
Footnotes
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 18,326,068 | 18,326,068 | 19,783,625 | 19,688,067 | 1,361,999 |
Note: The variance of $1.4M between actual and planned spending is mainly due to in-year resources received for the 2018 G7 Summit in Charlevoix, Quebec.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 255 | 188 | -67 |
Note: The variance of 67 in FTE utilization is mainly due to the Employee Assistance Services program not using its full revenue authority. FTE utilization is a reflection of workforce requirements based on actual workload.
Program 1.3: Official Language Minority Community Development
Description
The Official Language Minority Community Development program involves the administration of Health Canada's responsibilities under Section 41 of the Official Languages Act (OLA). This Act commits the federal Government to enhance the vitality of official language minority communities and foster the full recognition and use of English and French in Canadian society. This program includes: consulting with Canada's official language minority communities on a regular basis; supporting and enabling the delivery of contribution programs and services for official language minority communities; reporting to Parliament and Canadians on Health Canada's achievements under Section 41; and coordinating Health Canada's activities and awareness in engaging and responding to the health needs of official language minority communities. The program objectives are to improve access to health services in official language minority communities and to increase the use of both official languages in the provision of health care services. This program uses funding from the following transfer payment: Official Languages Health Contribution Program.
Results
In 2017-18, as part of this program, Health Canada successfully completed the following initiatives:
- Health Canada continued to improve access to health services for Official Language Minority Communities, by supporting a range of initiatives through the Official Languages Health Contribution Program, in the following three areas:
- training and retention of health professionals;
- strengthening the capacity of health networks through, among others, knowledge development and dissemination; and,
- projects to improve access to health services.
- The Department conducted in depth consultations with the public, Official Language Minority Communities and targeted recipients under the Program, to inform the renewal of the Official Languages Health Contribution Program for 2018–2023 under the new Federal Action Plan for Official Languages 2018 to 2023. The Action Plan granted Health Canada an additional $16.9M for the next five years, and $3.6M on an annual basis thereafter, in contribution funds to strengthen activities that improve access to health services in Official Language Minority Communities.
Health Canada ensured that its proposals, memoranda and decision documents submitted to the Treasury Board and to other Cabinet committees respected Official Languages Act requirements, such as communicating with or offering services to the public in both official languages as well as consulting official language minority communities, when necessary.
The Federal Health Portfolio Consultative Committee for Canada's Official Language Minority Communities was put in place to facilitate the integration of Official Language Minority Communities' health needs in the Portfolio's various programs and policies. Membership includes Health Canada, the Canadian Institutes of Health Research, the Public Health Agency of Canada as well as targeted recipients under the Official Languages Health Contribution Program, including: Société Santé en Français, Consortium national de formation en santé, and the Community Health and Social Services Network and McGill University.
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Official Language Minority Communities have access to health care services in the official language of their choice. | % of healthcare professionals who successfully complete Health Canada funded training programsFootnote 1. | 70 | March 31, 2018 | 69Footnote 2 | 72 | 73 |
| % of program trained health professionals who are retained. | 86 | March 31, 2018 | N/AFootnote 3 | 71Footnote 4 | 75Footnote 4 | |
Footnotes
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 35,328,730 | 35,328,730 | 35,280,087 | 34,436,737 | -891,993 |
Note: The variance of $0.9M between actual and planned spending is mainly due to attrition and longer than anticipated staffing processes.
| 2017–18 Planned |
Actual | 2017-18 Difference (actual minus planned) |
|---|---|---|
| 10 | 4 | -6 |
Note: The variance of 6 in FTE utilization is mainly due to attrition and longer than anticipated staffing processes.
Strategic Outcome 2: Health risks and benefits associated with food products, substances, and environmental factors are appropriately managed and communicated to Canadians
Program 2.1: Health Products
Description
The Department of Health Act, and the Food and Drugs Act and Regulations provide the authority for Health Canada to develop, maintain, and implement a regulatory framework associated with a broad range of health products that affect the everyday lives of Canadians, including pharmaceutical drugs, biologics and radiopharmaceuticals, medical devices, and natural health products. Health Canada verifies that the regulatory requirements for the safety, quality, and efficacy of health products are met through risk assessments, including monitoring and surveillance, compliance, and enforcement activities. In addition, Health Canada provides evidence-based, authoritative information to Canadians and key stakeholders, including health professionals such as physicians, pharmacists and natural health practitioners, to enable them to make informed decisions. The program objective is to ensure that health products are safe, effective, and of high quality for Canadians.
Results
In 2017-18 Health Canada continued its work to support the Government's commitment to address the opioids crisis.
- In June 2017, Health Canada pre-published proposed amendments to the Food and Drug Regulations that would require mandatory warning stickers and information handouts for patients receiving prescribed opioids. The regulations would also require Pharmaceutical companies to develop and implement Risk Management Plans to identify, monitor, and mitigate risks associated with opioids.
- Health Canada also began the process to update the labelling for all opioids to support better prescribing practices and reduce unnecessary use.
- In July 2017, Health Canada published regulatory amendments to allow the importation and sale of drugs not approved in Canada, but approved in the United States, the European Union, or Switzerland for urgent public health needs. The amendments applied immediately to facilitate access to some drugs used to treat opioid use disorder and other urgent public health needs.
- Health Canada also initiated work to address industry's opioid marketing practices and to increase transparency in the marketing and advertising of opioids. This work supported public consultations launched in June 2018 via a Notice of Intent to restrict the marketing and advertising of opioids.
Health Canada continued to provide Canadians with timely access to safe health products by reviewing the safety, efficacy and quality of pharmaceuticals. The Department approved 61 new drugs, of which 17 were drugs containing new active substances not previously approved in Canada. The Department also approved 304 supplements to new drugs already on the market; 15 of these new drugs and supplements were approved through an expedited pathway, to address unmet medical needs. Health Canada also approved 157 new generic drugs. Generics are drugs that enter the market subsequent to a version previously authorized in Canada, thereby increasing therapeutic options available to Canadians.
Health Canada issued market authorizations for 34 biological drugs, including eight new drugs to treat cancer. Recognizing the needs of Canadians, the Department prioritized the scientific evaluation of six new products based on unmet medical needs. As well, three of the 34 market authorizations issued this past year were for biosimilar drugs, which are drugs that enter the market subsequent to a version previously authorized in Canada, thereby increasing therapeutic options available to Canadians.
In 2017-18, as part of the Government's ongoing efforts to modernize its approach to regulating health products and to meet healthcare system needs, Health Canada carried out several initiatives and activities to support the work related to the Regulatory Review of Drugs and Devices. Health Canada:
- Published draft regulations on the Public Release of Clinical Information on drugs and medical devices. Making available more information about the safety and efficacy of these products can have widespread benefits for patients and the health care system by providing more details about drugs and medical devices to support independent analysis and research. This information could help health professionals make more informed decisions about the appropriate use of these products.
- Solicited submissions for international work-sharing which will help Canadians gain greater access to new products that meet health care system needs.
- Consulted on a Notice of Intent for potential amendments to the Food and Drug Regulations to improve access to generic drugs, which will benefit Canadians through more timely access to affordable drugs and more treatment options.
- Piloted an aligned review process with the Canadian Agency for Drugs and Technologies in Health to reduce the time it takes to make new drugs available to Canadians.
- Consulted with industry associations, patient groups and academics on using foreign review decisions to improve access to new drugs for Canadians through a new regulatory pathway that would allow drugs that may not otherwise be filed for market approval authorization in Canada to be approved by a respected international regulator.
- Established a scientific advisory panel under the Building Better Access to Digital Health Technologies. This undertaking will allow Health Canada to keep pace with innovation and advances in the medical device and digital health technology sectors, and should further benefit Canadian patients and the health care system by improving access to these technologies.
In February 2018, Health Canada announced that it is taking a phased approach to updating the way self-care products (cosmetics, natural health products, and non-prescription drugs) are regulated. The Department consulted stakeholders across the country from April to June 2017 to develop a new approach for consistent and aligned regulation of self-care products. Furthermore, consistent with the Government of Canada's commitment to using Gender Based Analysis Plus (GBA+) in the development of policies, programs and legislation, Health Canada is integrating GBA+ in the development of a new framework for self-care products. Additionally, in February 2018, a two year partnership project with McGill University and Health Canada was established to investigate consumer behaviours and perceptions of health product labelling.
In October 2017, Health Canada initiated consultations on revisions to the fees charged to industry to support regulatory activities related to drugs and devices, as current fees are between 10-20 years out of date and no longer reflect the costs of doing business. Revised fees will allow Health Canada to review new drugs and devices within internationally recognized timelines, to best support timely access to these products by Canadians.
In June 2017, a consultation paper was posted on the proposed design of the regulations aimed at requiring certain health care institutions to provide Health Canada with information on serious adverse drug reactions (ADRs) and medical device incidents (MDIs). The feedback received during the subsequent public consultation period was used to develop a “What We Heard” report that was published in December 2017. Reports about serious ADRs and MDIs can be important signals of emerging safety issues. These post-market observations can improve Health Canada's knowledge about product safety, which in turn will help Canadians and their health care providers make better, more informed decisions about treatment.
As well, the Department published a What We Heard report on the feedback from the consultation Toward a Strengthened Assisted Human Reproduction Act in January 2018. The feedback was used to inform the development of regulations for reducing the risks to human health and safety arising from the use of donor sperm and ova; reimbursement of expenses incurred by donors and surrogates; and, the administration and enforcement framework.
In support of the 2015 Federal Action Plan on Antimicrobial Resistance (AMR), Health Canada amended the Food and Drug Regulations in May 2017 to improve the oversight of antimicrobials for veterinary use. The regulatory changes include: restrictions to the own use importation of veterinary drugs; mandatory annual sales reporting of medically important antimicrobials; increased oversight on the importation and quality of veterinary active pharmaceutical ingredients; and a new regulatory pathway to import and sell certain low-risk veterinary drugs, known as veterinary health products.
The Department also:
- Published a number of drug good manufacturing practices guidance documents on its website to address emerging trends using plain language principles.
- Implemented a risk-based inspection strategy for scheduling domestic inspections and site-risk profiles for foreign on-site inspections.
- Conducted ten joint inspections with international regulators including the Food and Drug Administration, Medicines and Healthcare products Regulatory Agency and Therapeutic Goods Administration.
- Carried out activities to keep the public informed of emerging issues and inspection-related information including inspection rating and observations by posting to the Drug and Health Product Inspections Database and the Inspection Tracker database.
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Health products available to Canadians on the Canadian market are safe, effective, and of high quality. | % of regulated parties who are deemed to be in compliance with the Food and Drugs Act and its associated Regulations. (Baseline is 97) |
95Footnote 1 | March 31, 2018 | 97 | 97 | 96 |
Footnotes
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 147,322,313 | 147,322,313 | 185,692,315 | 177,165,339 | 29,843,026 |
Note: The variance of $29.8M between actual and planned spending is mainly due to the following:
In-year resources of $38.4M were received for Maintaining Core Regulatory Operation for Therapeutic Products, as well as Improving the Accessibility, Affordability and Appropriate Use of Prescription Drugs and Medical Devices.
This is offset by $8.5M mainly due to lower than anticipated expenditures for staffing, legal services and replacement of laboratory equipment.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 1,974 | 1,753 | -221 |
Note: The variance of 221 in FTE utilization is mainly due to the Pharmaceutical Drugs and Medical Devices sub-programs not using the full revenue authority. FTE utilization is a reflection of workforce requirements based on actual workload.
Program 2.2: Food Safety and Nutrition
Description
The Department of Health Act and the Food and Drugs Act provide the authority for Health Canada to develop, maintain, and implement a regulatory framework associated with the safety and nutritional quality of food. Food safety standards are enforced by the Canadian Food Inspection Agency. Health Canada develops and promotes evidence-based, national healthy eating policies and standards for Canadians and key stakeholders, including non-governmental organizations, health professionals, and industry associations, to enable all stakeholders to make informed decisions about food and nutrition safety as well as healthy eating. The program objectives are to manage risks to the health and safety of Canadians associated with food and its consumption, and to enable Canadians to make informed decisions about healthy eating.
Results
As part of the Government of Canada's Vision for a Healthy Canada, Health Canada continued to implement the various activities under the Healthy Eating Strategy to improve healthy eating information, improve the nutrition quality of foods and to protect vulnerable populations.
- Health Canada held the second phase of consultations on the Revision of Canada's Food Guide in the summer of 2017. The objective was to determine how Canadians use healthy eating information and to seek feedback on proposed healthy eating recommendations. In support of the Government of Canada's Open Government initiative and Health Canada's Regulatory Transparency and Openness Framework, a summary of the feedback received was reported in the Canada's Food Guide Consultation - Phase 2 What We Heard Report, which was posted in March 2018.
- Additional progress towards the strategy came by way of regulatory work. Specifically, in September 2017, Health Canada published the Notice of Modification: Prohibiting the Use of Partially Hydrogenated Oils (PHOs) in Foods, the main source of industrial produced trans-fat. PHOs have now been added to Part 1 of the List of Contaminants and Other Adulterating Substances in Foods. The prohibition took effect in September 2018.
- In addition, in February 2018, proposed regulations were published in Canada Gazette Part I that would require a nutrition symbol on the front of the package of foods high in sodium, sugars and saturated fat to help consumers make more informed decision on the food they purchase. A 75 day online consultation was held to help inform the design of the final nutrition symbol.
- In January 2018, the report entitled Sodium Reduction in Processed Foods in Canada: An Evaluation of Progress toward Voluntary Targets from 2012 to 2016 was released. The evaluation presents the results of the food industry's efforts to meet 2012 sodium reduction targets established for 94 categories of processed foods.
- At that time, the evaluation of industry's voluntary efforts showed only modest progress, with only 14% of the 94 categories meeting the ultimate targets. This is likely due to technical challenges in reducing sodium in certain foods, consumer acceptance, and lack of a regular monitoring and reporting program to strengthen industry's commitment and accountability. Health Canada has proposed to require a front-of-package nutrition symbol on foods high in sodium to help consumers make healthier food choices and encourage industry to lower sodium in processed foods and will be closely monitoring changes in sodium levels in the food supply.
- The Department engaged the food services sector (restaurants, caterers, institutions, suppliers, distributors and retailers selling home meal replacements, etc.) through the Call for Information on Sodium Reduction Initiatives in the Canadian Food Services Sector between September and November 2017. The objective was to better understand current practices and identify effective strategies to reduce sodium levels in food.
- Health Canada consulted Canadians on a proposed approach to restrict the marketing of unhealthy food and beverages to children between June and August 2017 and posted a consultation report entitled Restricting Marketing of Unhealthy Food and Beverages to Children in Canada, in December 2017. This information will be used to inform the development of regulations. Health Canada also hosted a meeting on the monitoring of unhealthy food and beverage marketing to children with Canadian and international experts in March 2018. The purpose of the meeting was to guide the development of a draft monitoring framework to inform annual reports and the five-year parliamentary review of marketing to children legislation and regulations.
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Policies, standards and guidelines exist that protect Canadians from identified risks in the Canadian food supply. | % of current and emerging high risk food safety issues which generate the development of either a regulatory or a non-regulatory response. (Baseline is 100) |
100 | March 31, 2018 | 100 | 100 | N/AFootnote 1 |
Footnotes
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 67,881,855 | 67,881,855 | 68,223,946 | 68,064,842 | 182,987 |
Note: The variance of $0.2M between actual and planned spending is mainly due to in-year resources received for maintaining critical food safety activities.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 602 | 485 | -117 |
Note: The variance of 117 in FTE utilization is mainly due to attrition and longer than anticipated staffing processes.
Program 2.3: Environmental Risks to Health
Description
The Canadian Environmental Protection Act (CEPA), 1999, and the Department of Health Act provide the authorities for the Environmental Risks to Health program to assess and manage the health risks associated with climate change, air quality, drinking water quality, and new and existing substances. This program activity links closely with Health Canada's Health Products, Food Safety and Nutrition, Consumer Product Safety and Pesticides program activities, as the Food and Drugs Act, the Pest Control Products Act, and the Canada Consumer Product Safety Act provide the authority to manage the health risks associated with substances in products under the purview of these program activities. Key activities include: risk assessment and management, as well as research and bio monitoring of substances; provision of technical support for chemical emergencies that require a coordinated federal response; development of guidelines on indoor and outdoor air quality; development and dissemination of water quality guidelines; and provision of expert support related to environmental assessments and contaminated sites. The program objective is to protect the health of Canadians through the assessment and management of health risks associated with environmental contaminants, particularly substances, and to provide expert advice and guidelines to Canadians and Government partners on the health impacts of environmental factors such as air and water contaminants and a changing climate.
Results
Health Canada met its program objective of protecting the health of Canadians through the assessment and management of health risks associated with chemical substances and by providing expert advice and guidelines to partners on the health impacts of environmental factors such as air and water contaminants and a changing climate.
In 2017-18, Health Canada continued to implement the Chemicals Management Plan (CMP). The CMP's overall objective for existing substances is to assess the potential health and ecological risks associated with 4,363 substances that were prioritized for assessment by March 31, 2021. The third and final phase of the CMP, which began in 2016-17 and runs until 2020-21, is intended to assess the approximately 1,550 substances remaining from this commitment.
- In 2017-18, Health Canada assessed 300 (or 19%) of these approximately 1,550 CMP3 substances at the draft assessment stage, and 101 (or 7%) at the final assessment stage. As of March 31, 2018, Health Canada has published draft screening assessment reports (DSARs) for 3,470 substances (approximately 80% of 4,363) and final screening assessment reports (FSARs) for 2,840 substances (65% of 4,363). In addition, one Science Approach Document was published in 2017-18 covering 14 low concern CMP substances. Conclusions for these substances will be included in screening assessment reports at a later date. With respect to the targets set for Year Two of the Two Year Risk Assessment Rolling Workplan, 309 of 340 (91%) of planned substances were published in DSARs. In addition, five substances planned for publication in DSARs in future years were published early. 96% (198 of 206) of the planned substances were published in FSARs. Health Canada is, therefore, well on its way to meeting its 2021 target for the assessment of existing substances. However, the scientific complexity of some assessments, and the additional time needed to determine risk management approaches, are posing challenges. Steps are being taken to address/mitigate identified risks (e.g., prioritizing regulatory packages and acquiring surge capacity for peak periods).
- In 2017-18, as part of the Chemicals Management Plan, 80% (8 of 10) of planned risk management actions for existing substances were completed by March 31, 2018. Two were behind schedule due to unexpected delays in the finalization/publication of risk management instruments for hydrazine and selenium, but will be completed in 2018-19. Work to improve internal development processes continues to ensure that 100% of planned risk management actions will be taken in a timely manner for substances deemed harmful to human health in the future.
- As well, 100% (395) of new substances for which a notification of their manufacture or import had been received from industry were assessed within targeted timelines in 2017-18, and 100% (14 of 14) of new substances assessed to be harmful to human health also had control measures developed within mandated timeframes. The 14 substances that were risk managed represent 4% of new substances notifications received from industry and assessed in 2017-18, which is consistent with historical levels.
- Health Canada also continued to conduct research and monitoring and surveillance activities in support of the CMP to address existing and emerging chemicals of concern, to inform risk assessment needs and risk management activities and to address outstanding questions and knowledge gaps related to the effects and exposure of chemical substances to humans. Key results from projects funded in the second phase of CMP (2011-12 to 2015-16) were shared in a workshop organized jointly by Environment and Climate Change Canada (ECCC) and Health Canada in October 2017. The workshop enabled dissemination of research findings to a broad audience of 200 participants from research, monitoring and surveillance, risk assessment and risk management groups from both organizations.
- Health Canada also released its Fourth Report on Human Biomonitoring of Environmental Chemicals in Canada as part of the Canadian Health Measures Survey (CHMS), an ongoing national direct health measures survey. Data for cycle 4 of the CHMS were collected between January 2014 and December 2015 from approximately 5,700 Canadians aged 3-79 years at 16 sites across Canada and included 54 environmental chemicals. These latest results add important new knowledge to understanding Canadians' exposure to chemicals.
- Through continued implementation of the Department's outreach strategy in support of improved risk communications, Health Canada reached over 23,000 Canadians at outreach events, providing information on how to reduce the risk of potential health effects of exposure to household chemicals. National public opinion research on household chemicals awareness was undertaken and has informed the development of a new multi-platform communications campaign that will expand messaging to Canadians on the health risks of chemicals.
In 2017-18, the Department continued to provide health science and guidance to support actions to address air quality and improve health, including:
- Continued support for the development of Canadian Ambient Air Quality Standards (CAAQS), in collaboration with ECCC and the provinces and territories. New CAAQS for nitrogen dioxide were endorsed by the Canadian Council of Ministers of the Environment (CCME) in November 2017, and the CAAQS for sulphur dioxide and nitrogen dioxide were also issued as federal objectives under CEPA (1999).
- Analysis of the health and economic benefits from improved air quality were conducted to support the development of regulations led by ECCC, including proposed regulations to limit air and CO2 emissions from the oil and gas sector and to accelerate the phase-out of coal-fired electricity.
- Publication of a new Health Impacts of Air Pollution in Canada report which showed that air pollution from human sources contributes to 14,400 premature deaths a year in Canada; a new State of the Air website was also launched with ECCC and the CCME; and the Air Quality Health Index was also promoted to Canadians through retail engagement and The Weather Network.
- On indoor air, support for the development of a Carbon Monoxide Monitoring and Response Framework in Long-term Care Facilities, led by the British Columbia Centre for Disease Control. Health Canada also provided Canadians with guidance on preventing carbon monoxide and mould in homes and on improving ventilation to improve indoor air quality.
- The conduct and publication of leading research, including studies on the relationship between wood smoke and heart attacks, how exposure to air pollution during pregnancy may affect children's health later in life, time spent and exposure to air pollutants when commuting in Canada and a review of how traffic management strategies could improve air quality. For example, in one study, researchers found that people who lived in areas of Ontario with higher pollution had a greater chance of developing dementia compared to their counterparts in areas of Ontario that experience cleaner air. This new study adds to the body of information linking outdoor air pollution to development of disease.
Health Canada protected the health of Canadians in 2017-18 by finalizing three health-based drinking water guidelines/guidance documents, which were endorsed by provinces/territories, and are used as the basis for drinking water quality requirements across Canada. The Department also supported the implementation of evidence-based heat health measures across five provinces, and launched a National Heat Health Community of Practice.
Through its Environmental Assessment Program, Health Canada continued to provide its expert human health advice in the areas of noise, air/water quality, radiation and country/traditional foods and on reducing the potential risks from proposed major energy development projects (such as pipelines, mines, windfarms, electrical generation, etc.). Additionally, Health Canada finalized four guidance documents on noise, air quality, recreational and drinking water, and radiation, which are now publically available to all Canadians.
In its role as an Expert Support Department under the Government of Canada's Federal Contaminated Sites Action Plan, Health Canada's Contaminated Sites Program also continued to provide its expert scientific risk assessment/mitigation advice, technical training and other tools to assist federal custodian departments in assessing human health risks and risk managing approximately 120 contaminated sites in Canada
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Canadians and Government partners have the guidance they need to respond to potential and actual environmental health risks. | % of planned guidance materials made available. (Baseline is 93) |
100 | March 31, 2018 | 100 | 100 | 83 |
| Substances deemed to be harmful to human health are risk managed according to the Canadian Environmental Protection Act (CEPA) (1999) and other “Best Placed Acts”.Footnote * | % of planned risk management actions taken under CEPA (1999) for new substances. (Baseline is 96) |
100 | March 31, 2018 | 100 | 100 | 100 |
| % of planned risk management actions taken under CEPA (1999) or another Act for existing substances. (Baseline is 96) |
100 | March 31, 2018 | 80Footnote 1 | 100 | 100 | |
Footnotes
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 96,356,868 | 96,356,868 | 93,471,137 | 89,496,610 | - 6,860,258 |
Note: The variance of $6.9M between actual and planned spending is mainly due to attrition and longer than anticipated staffing processes.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 720 | 532 | -188 |
Note: The variance of 188 in FTE utilization is mainly due to attrition and longer than anticipated staffing processes.
Program 2.4: Consumer Product and Workplace Hazardous Materials
Description
The Consumer Product Safety and Workplace Hazardous Materials programs support efforts to protect Canadians from unsafe products and chemicals. The Consumer Product Safety program supports industry's responsibility for the safety of their products under the authorities of the Canada Consumer Product Safety Act and the Food and Drugs Act and its Cosmetic Regulations. In addition, the program supports consumers' responsibility to make informed decisions about product purchase and use. Health Canada's efforts are focussed in three areas: active prevention; targeted oversight; and rapid response. The Hazardous Products Act and the Hazardous Materials Information Review Act provide the authorities for the Workplace Hazardous Materials program to maintain a national hazard communication standard of cautionary labelling and safety data sheets for hazardous chemicals supplied for use in Canadian workplaces and to protect related confidential business information. The objectives of the programs are to identify, assess, manage and communicate health or safety risks to Canadians associated with consumer products and cosmetics, as well as to communicate the hazards of workplace chemicals.
Results
In 2017-18, Health Canada continued to protect Canadians from unsafe products and chemicals by:
- Supporting early detection of potentially unsafe consumer products and cosmetics. Health Canada triaged 3,222 incident reports (51% from industry, 49% from consumers) and assessed 715 incident reports of consumer products related to electrical hazards (such as USB adapters), mechanical/physical hazards (such as BBQ brushes), and chemical hazards (including cosmetic ingredients such as methylisothiazolinone (MI)).
- Taking prompt action when risks posed by dangerous consumer products and cosmetics are identified. Health Canada continued its risk management activities. When these risks are identified through Health Canada's Cyclical Enforcement Plan, or through incident reporting, Health Canada responded with the appropriate risk management action within the established service standard 92% of the time.
- Working with industry to communicate recalls of products when it was the appropriate risk management action to take. Health Canada worked with industry to communicate 238 consumer product recalls to Canadians, of which approximately 40% were released in coordination with regulatory partners such as the United States Consumer Product Safety Commission. In response to the Commissioner of the Environment and Sustainable Development's Audit on Chemicals in Consumer Products and Cosmetics, Health Canada updated its recall policy to provide more information for industry on how to complete an effective voluntary recall of their products. Proposed new or amended regulations for corded window coverings, toys with small powerful magnets, and playpens were also published for public consultation in order to allow Health Canada to take appropriate action.
- Supporting consumers in the safe selection and use of consumer products. Health Canada continued to provide timely and credible consumer product information, often through social media, including product awareness campaigns, alerts and recall communications, such as the awareness campaign on how to baby proof your home, and the alert on the risks of buying prohibited, non-compliant and unsafe consumer products online.
- Ensuring critical health and safety information was available to workers while providing protection to industry for confidential business information in accordance with the requirements of the Hazardous Materials Information Review Act (HMIRA). To further protect Canadian workers from potential incidents related to workplace hazardous chemicals, Health Canada launched a compliance and enforcement program delivered with Federal, Provincial and Territorial partners under the authorities of the Hazardous Products Act (HPA).
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Risks associated with consumer products and cosmetics in the Canadian marketplace are managed. | % of non-compliant products identified through the Cyclical Enforcement Plan and incident reporting, for which risk management actions are completed within service standards. (Baseline is 97) |
85Footnote 1 | March 31, 2018 | 92 | 92 | 85 |
| Suppliers are compliant with Canadian WHMIS 2015 requirements. | % of Safety Data Sheets (SDS) which are compliant as reviewed by Health Canada. (Baseline Year 2017-18) |
Baseline year 2017-18Footnote 2 |
March 31, 2018 | 0Footnote 3 | N/AFootnote 4 | N/AFootnote 4 |
Footnotes
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 38,015,185 | 38,015,185 | 38,648,378 | 34,846,833 | - 3,168,352 |
Note: The variance of $3.2M between actual and planned spending is mainly due to the reallocation of resources within the Department to meet program needs and priorities, attrition, and longer than anticipated staffing processes, as well as delays in the procurement of laboratory equipment.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 305 | 268 | -37 |
Note: The variance of 37 in FTE utilization is mainly due to the reallocation of resources within the Department based on operational requirements, as well as attrition and longer than anticipated staffing processes.
Program 2.5: Problematic Substance UseFootnote 2
Description
Under the authority of several Acts, the Problematic Substance Use program regulates tobacco products and controlled substances. Through the Tobacco ActFootnote 3 and its regulations the program regulates the manufacture, sale, labelling and promotion of tobacco products. The program leads the Federal Tobacco Control StrategyFootnote 4, the goal of which is to further reduce the prevalence of smoking through regulatory, programming, educational and enforcement activities. Through the Controlled Drugs and Substances Act and its regulations, the program regulates access to controlled substances and precursor chemicals to support their legitimate use and minimize the risk of diversion for illegal use. As the lead for the Canadian Drugs and Substances Strategy (CDSS)Footnote 5, the program supports prevention, health promotion, treatment initiatives, harm reduction and enforcement with the goal of reducing problematic substance use, including problematic prescription drug use. In addition, the program provides timely, evidence-based information to key stakeholders including, but not limited to, health professionals, law enforcement agencies, provincial and territorial governments and Canadians. The program objective is to minimize risks to the health of Canadians associated with the use of tobacco products, and the illegal use of controlled substances and precursor chemicals.
Results
In 2017-18, Health Canada made addressing the opioid crisis a top priority and continued its ongoing efforts to address the national opioid crisis through a targeted public health and evidence-based response. This included working with provincial/territorial/municipal partners, health experts and practitioners, law enforcement officials, and people with lived experience who were directly affected by the crisis to understand how best to implement actions.
- To support increased access to treatment for opioid use disorder, Health Canada developed regulatory amendments to make it easier for health practitioners to access methadone and prescription heroin for their patients with opioid use disorder; and implemented regulations to allow the importation of drugs not approved in Canada for urgent public health needs.
- To reduce harms caused by problematic opioid use, Health Canada streamlined the supervised consumption site application process and authorized more than 25 supervised consumption sites in four provinces (this interactive map shows the locations of operating supervised consumption sites across the country); supported the development of a national guideline to provide recommendations on best practices related to the use of opioids for the management of chronic, non-cancer pain; authorized drug checking services for the supervised consumption sites that wanted them; worked with provinces, as requested, to temporarily authorize overdose prevention sites; developed information for the safe handling of opioid substances for front-line workers; and supported the passage of the Good Samaritan Drug Overdose Act, which can protect those who experience an overdose and the people who try to help them from some drug possession charges.
- Health Canada dedicated funding to support opioid-related prevention and harm reduction projects through the Substance Use and Addictions Program and developed awareness and communications materials to better inform Canadians about the potential risks of opioid use (such as an Opioids web toolkit, videos and wallet cards).
- In 2017-18, Health Canada also started posting online quarterly reports on the analysis of seized drugs and hosted four ministerial roundtables to talk to people at the front-lines of the crisis. These activities contributed to the efforts of partners in building the evidence needed to effectively respond to the opioid crisis.
- Health Canada also conducted ongoing surveillance and monitoring through the Canadian Tobacco Alcohol and Drug Survey (CTADS) and the Canadian Student Tobacco Alcohol and Drug Survey (CSTADS). For school students (Grades 7-12), the rate of use of at least one of six illegal drugs (cannabis, amphetamines, MDMA (ecstasy), hallucinogens including salvia, heroin and cocaine) in the past 12 months remained unchanged from 17.6% in 2014-15 to 17.5% in 2016-17, and was more prevalent among males than females (18.5% versus 16.3% in 2016-17).
Health Canada also made progress in protecting Canadians, particularly young people, from the health consequences of nicotine addiction, tobacco use and vaping.
- Canada continued its efforts to reduce tobacco use, which remains the leading preventable cause of disease and premature death in Canada. In the most recently available results from the Canadian Tobacco, Alcohol and Drugs Survey (CTADS, 2015), 15% (4.6 million) of Canadians aged 15 years and older reported using at least one tobacco product in the past 30 days, down from 23% (5.8 million) in 2003. Prevalence of past-30-day use of at least one tobacco product in 2015 continued to be higher among males (20% or 2.8 million) than females (12% or 1.7 million). In 2017, Health Canada consulted the public on the future of tobacco control in Canada. The responses helped the Department modernize the Federal Tobacco Control Strategy, a comprehensive, integrated and sustained tobacco control program aimed at reducing tobacco-related disease and death. The modernized approach sets the path to reduce Canada's rate of tobacco use to less than 5% by 2035, which means there would be fewer than 1.8 million Canadians using tobacco. Budget 2018 announced $80.5M in new funding for the modernized strategy over five years, starting in 2018-19.
- Canada took another important step to protect young Canadians from inducements to tobacco use by banning menthol cigarettes. As of October 2017, the manufacture and sale of cigarettes, blunt wraps and most cigars that contain menthol are prohibited across Canada. As well, the promotion of menthol on the packaging of these tobacco products is now prohibited.
- A major milestone for Canada's legislative and regulatory efforts to protect youth and non-smokers was the introduction of new legislation (Bill S-5 “An Act to amend the Tobacco Act and the Non-smokers' Health Act and to make consequential amendments to other Acts”), to implement a legislative framework for vaping products and provide the authority for action on plain packaging. Bill S-5, which was passed by the Senate and introduced in the House of Commons in June 2017 received Royal Assent on May 23, 2018 and amended the Tobacco Act, changing its title to the "Tobacco and Vaping Products Act" (TVPA).
- The TVPA provides authorities to regulate the manufacture, sale, labelling and promotion of vaping products as a set of products separate from tobacco products, addressing recommendations outlined in the Evaluation of the Federal Tobacco Control Strategy (January 2017). Canada is internationally recognized for its leadership and expertise in regulatory action to address smoking and tobacco use. With the enactment of Bill S-5, Health Canada can implement plain packaging measures to regulate the appearance, shape and size of tobacco products and their packaging. In 2017-18, a draft regulatory package was completed for plain and standardized packaging based on responses gathered from a 2016 public consultation.
- From August to October 2017, the Department also consulted the public on proposals for the regulation of vaping products. Comments on the proposed regulations were generally supportive, with some clear but expected differences of opinion and specific concerns identified by some groups. The comments received have been taken into consideration in the development of the new vaping regime (promotion, labelling, and reporting regulations for vaping products).
As part of the Government's commitment to legalize, regulate and restrict access to cannabis to keep it out of the hands of Canadian children and youth and to keep profits out of the hands of criminals, the Minister of Health supported the introduction of effective, evidence-informed legislation to legalize and strictly regulate cannabis for consideration by Parliament.
- The legislation was introduced in April 2017, and was informed by the report of the Task Force on Cannabis Legalization and Regulation, which reflected extensive consultations with a wide variety of experts in relevant fields. (The Cannabis Act received Royal Assent on June 21, 2018 and came into force on October 17, 2018.)
- In November 2017, Health Canada undertook public consultations on a comprehensive set of cannabis-related regulatory proposals, and in March 2018, published a summary of the feedback.
- Final regulations were published in the Canada Gazette Part II on July 11, 2018 and came into force with the Act on October 17, 2018.
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Decrease in current tobacco prevalence. | % of Canadians (aged 15+) who have used tobacco products (including cigarettes) in the past 30 days. (Baseline 15) |
<15Footnote 1 | March 31, 2018 | 15.5Footnote 2 | 15.5Footnote 2 | 17.4Footnote 3 |
| % of Canadians students (grades 7-12) who have used any tobacco products (including cigarettes) in the past 30 days. (Baseline 14) |
<12 | March 31, 2018 | 10Footnote 4 | 12Footnote 5 | 12Footnote 5 | |
| Decrease in illicit drug use among Canadians. | % of Canadians (aged 15+) who report using at least one of 6 illicit drugs (cannabis, cocaine or crack, speed, ecstasy, hallucinogens or heroin). (Baseline 11) |
<11 | March 31, 2018 | 12.6Footnote 2 | 12.6Footnote 2 | 10.9Footnote 3 |
| % of Canadians (grades 7-12) who report using at least one of 6 illicit drugs (cannabis, cocaine or crack, speed, ecstasy, hallucinogens or heroin). (Baseline 21) |
<21 | March 31, 2018 | 17.5Footnote 4 | 17.6Footnote 5 | 17.5Footnote 5 | |
FootnotesNote: Unless otherwise specified, the sources of data are the Canadian Student Tobacco, Alcohol and Drugs Survey (CSTADS) and the Canadian Tobacco, Alcohol and Drugs Survey (CTADS), which are conducted biennially.
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 88,941,061 | 88,941,061 | 128,572,801 | 103,843,391 | 14,902,330 |
Note: The variance of $14.9M between actual and planned spending is mainly due to the following:
In-year resources of $39.6M were received to implement the Government's priorities to Legalize and Strictly Regulate Cannabis and the Canadian Drug Substance Strategy.
This is offset by $24.8M mainly resulting from the reprofile of funds for the Substance Use and Addictions Program, as well as shifting of some elements to implement the Legalization and Strict Regulation of Cannabis into the 2018-19 fiscal year.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 415 | 696 | 281 |
Note: The variance of 281 in FTE utilization is mainly due to in-year resources received to implement the Government's priority to Legalize and Strictly Regulate Cannabis.
Program 2.6: Radiation Protection
Description
The Department of Health Act, the Radiation Emitting Devices Act, and the Comprehensive Nuclear Test-Ban Treaty Implementation Act provide the authority for the Radiation Protection program to monitor, regulate, advise, and report on exposure to radiation that occurs both naturally and from non-natural sources. In addition, the program is licensed under the Nuclear Safety and Control Act to deliver the National Dosimetry Service, which provides occupational radiation monitoring services. The key components of the program are environmental and occupational radiation monitoring; management of inter-organizational plans, procedures, capabilities and committees for a nuclear emergency that requires a coordinated federal response; delivering a national radon outreach program; and regulation of radiation emitting devices. The program objective is to inform and advise other Canadian government departments, collaborate with international partners, and inform Canadians about the health risks associated with radiation and strategies to manage associated risks.
Results
In 2017-18, Health Canada undertook the following key initiatives:
- Health Canada is the lead federal department responsible for coordinating the response to a nuclear emergency under the Federal Nuclear Emergency Plan (FNEP). As part of a series of exercises to test the revised FNEP (FNEP, 5th edition), Health Canada participated in Exercise Staunch Maple in April 2017 to test the response to the use of an Improvised Nuclear Device and participated in Exercise Unified Control in December 2017 to test the response to a nuclear emergency at the Pickering Nuclear Generating Station in Ontario. In addition, Health Canada also conducted a number of drills to verify operational readiness, and identify any gaps in response plans and operational arrangements so that issues may be resolved prior to an emergency. Health Canada also participated in five exercises led by the International Atomic Energy Agency. Planning was also initiated for an international peer review of national nuclear emergency preparedness arrangements in 2019. Health Canada maintained and ensured all Comprehensive Nuclear-Test-Ban Treaty monitoring stations and laboratory capabilities were operational, and maintained and operated national radionuclear monitoring stations according to agreed maintenance schedules, and reported results.
- In reference to the 2015-16 departmental evaluation of Health Canada's Radiation Protection Activities (September 2016), Health Canada clarified roles and responsibilities for nuclear emergency preparedness and response with the Canadian Nuclear Safety Commission, and enhanced public communications and access to available data by leveraging online tools through the Regulatory Transparency and Openness Framework and Government of Canada Web Renewal Initiative.
- The Department also continued to increase awareness on the risks, health impacts and mitigation strategies related to radon gas - the leading cause of lung cancer for non-smokers. Health Canada supported and participated in the 5th annual National Radon Action Month in November 2017 led by the Canadian Lung Association. Health Canada also participated in over 100 outreach events, responded to over 1,100 public inquiries and distributed over 1 million radon outreach materials across Canada. The aim is to encourage all Canadians to test the levels of radon gas in their homes, and to reduce the radon levels, if necessary. Health Canada published a summary of the Residential Radon Mitigation Actions Follow-Up Study. The study surveyed Canadians whose homes tested near or above the radon guideline from the two previous cross-Canada surveys (undertaken between 2009 and 2013) to determine the proportion of Canadians that took action to reduce radon levels in their homes; obtain statistics on the types of radon mitigation actions taken; and gather insights into public risk perception versus costs of mitigation that could guide future policies. Health Canada also published a revised edition of the Guide for Radon Measurements in Residential Dwellings (Homes). This publication provides guidance on how to measure radon properly in order to accurately estimate indoor air concentrations and manage radon exposure in homes.
- Environmental radioactivity surveillance data was posted to the Health Canada website (4680 new data points), Open Data Canada website (4128 new data points) and internationally. This included the real-time posting of Fixed Point Surveillance station dose data (over 2.5 million data points) to the European Radiological Data Exchange Platform, which allows Canadians and the international community to view various environmental radioactivity level data from across Canada, improving access to and understanding of their radioactivity exposure from natural and non-natural sources.
- The Department also developed a regulatory proposal to amend the Dental X-ray Equipment standard of the Radiation Emitting Devices Regulations (Schedule II, Part II) to align the Canadian standard with the radiation safety requirements of new international standards and to reflect the current state of dental x-ray equipment design and technology. The proposed amendments were published in Canada Gazette Part II in November 2017 and came into force on May 15, 2018.
- Health Canada published Guidelines for Tanning Equipment Owners, Operators and Users on February 14, 2018. Health Canada also updated its information regarding UV radiation, including tanning equipment and sun safety/sun awareness material, in September 2017 based on the latest national recommendations for sun safety messages in Canada.
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Canadians, Institutions and Government partners have the guidance they need to respond to potential and actual radiation risk. | % of targeted guidance documents accessed by Canadians, Institutions and Government partners. (Baseline is 100) |
100 | March 31, 2018 | 100 | 100 | N/AFootnote 1 |
Footnotes
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 18,294,915 | 18,294,915 | 20,458,193 | 20,381,720 | 2,086,805 |
Note: The variance of $2.1M between actual and planned spending is mainly due to in-year resources received for the 2018 G7 Summit in Charlevoix, Quebec.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 202 | 171 | -31 |
Note: The variance of 31 in FTE utilization is mainly due to attrition and longer than anticipated staffing processes.
Program 2.7: Pesticides
Description
The Pest Control Products Act provides Health Canada with the authority to regulate and register pesticides under the Pesticides program. In the delivery of this program, Health Canada conducts activities that span the lifecycle of a pesticide, including: product assessment for health and environmental risks and product value; risk management; post-market surveillance, compliance and enforcement; changes in use, cancellation, or phase-out of products that do not meet current standards; and consultations and public awareness building. Health Canada is also an active partner in international efforts (e.g., North American Free Trade Agreement; Organisation for Economic Co-operation and Development, Regulatory Cooperation Council) to align regulatory approaches. These engagements provide access to the best available science to support regulatory decisions and promote consistency in the assessment of pesticides. The program objective is to protect the health and safety of Canadians relating to the use of pesticides.
Results
Outreach and engagement are core activities of Health Canada's Pesticides Program, as a means of communicating and raising awareness of the regulatory requirements under the Pest Control Products Act (PCPA). The primary objective of the PCPA is to mitigate risks to people and the environment from the use of pesticides. Engaged and informed Canadians and stakeholders will have greater confidence that science supporting the registration of pesticides is sound and will understand that pesticides can be used safely. Health Canada's Pesticides Compliance Program optimizes opportunities to engage the agricultural and non-agricultural pesticide sectors across the country which responds to recommendations outlined in the departmental evaluation of the Pesticide Program (December 2015).
- In January 2018, Health Canada's Pesticide Program conducted a stakeholder webinar, to provide an overview of proposed pollinator re-evaluation decisions regarding clothianidin and thiamethoxam, which was attended by organizations and individuals of various interests (e.g., pesticide manufacturers, environmental groups, users, etc.). Health Canada published plain language re-evaluation summaries and decisions that emphasize what has changed as a result of a decision, who is affected by it, and how Canadians can protect themselves and the environment. In addition, Health Canada has actively engaged on pollinator issues, reaching out to agriculture, industry and beekeepers to keep them informed. To raise awareness of how it considers such issues in its work, Health Canada developed, and published online in December 2017, a fact sheet called “Sex- and Gender-based Considerations in the Scientific Risk Assessment of Pesticides in Canada.”
- A total of 163 outreach activities were conducted during fiscal year 2017-18, including presentations, meetings and exhibit booths at trade shows attended by users of pesticides. Communication materials such as posters, fact sheets, pest notes, power point presentations and YouTube videos were distributed and included topics such as Personal Protective Equipment, Incident Reporting, Restricted Entry Intervals (REI) and Pre-Harvest Intervals (PHI).
- To keep up with changes in technology and its operating environment, Health Canada continued to modernize the Pest Control Products Regulations to: enhance the protection of Canadians' health and their environment; update the Regulations to reflect Health Canada policy; and support the implementation of an international treaty. Health Canada made several amendments to the Pest Control Products Regulations in 2017-18, and communicated them through the Canada Gazette; these included amendments to: address concerns of the Standing Joint Committee for the Scrutiny of Regulations (e.g., regarding imposing third party liability); align the regulations with Health Canada policy to no longer issue conditional registrations; and align the regulations with changes to the Pest Control Products Act that were made to support the implementation of the Canada-European Union Comprehensive Economic and Trade Agreement.
- Additionally, Health Canada updated the “personal use import exemption” to reduce the likelihood of pest control products with unacceptable risks being imported illegally. Health Canada continues to make progress on modernizing other aspects of the Pest Control Products Regulations.
- Health Canada is dedicated to modernizing its technology tools to ensure efficiencies and effective case management. In 2017-18, Health Canada implemented a number of improvements to the Electronic Pesticide Regulatory System (ePRS), PMRA's principal e-tool for pesticide case management, including adaptation of ePRS for the implementation of a new cost recovery regime.
| Expected Results | Performance Indicators | Target | Date to achieve target | Actual results | ||
|---|---|---|---|---|---|---|
| 2017-18 | 2016-17 | 2015-16 | ||||
| Industry meets the Canadian regulatory requirements for new pesticides. | % of submissions that meet regulatory requirements. (Baseline is 90) |
80Footnote 1 | March 31, 2018 | 95 | 94 | 92 |
| Pesticides in the marketplace continue to meet modern scientific standards. | % of re-evaluations initiated for registered pesticides according to the Re-evaluation Work PlanFootnote 2. (Baseline is 90) |
80Footnote 1 | March 31, 2018 | 100 | 100 | 100 |
| International collaboration is leveraged to maximize access to global science for the risk assessment of pesticides. | % of new pesticides reviewed in collaboration with international partners. (Baseline is 90) |
80Footnote 1 | March 31, 2018 | 100 | 100 | 100 |
Footnotes
|
||||||
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 39,983,502 | 39,983,502 | 41,772,731 | 41,702,395 | 1,718,893 |
Note: The variance of $1.7M between actual and planned spending is mainly due to the reallocation of resources within the Department for replacing aging laboratory equipment.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 489 | 406 | -83 |
Note: The variance of 83 in FTE utilization is mainly due to attrition and longer than anticipated staffing processes.
Strategic Outcome 3: First Nations and Inuit communities and individuals receive health services and benefits that are responsive to their needs so as to improve their health status
Program 3.1 First Nations and Inuit Primary Health CareFootnote 6
Description
The Department of Health Act and the Indian Health Policy (1979) provide the authority for the delivery of the First Nations and Inuit Primary Health Care program to First Nations and Inuit in Canada. Primary health care includes health promotion and disease prevention, public health protection (including surveillance), and primary care (where individuals are provided diagnostic, curative, rehabilitative, supportive, palliative/end of life care, and referral services). The Department administers contribution agreements and direct departmental spending related to child development, mental wellness and healthy living, communicable disease control and management, environmental health, clinical and client care, as well as home and community care. The program objective is to improve the health and safety of First Nations and Inuit individuals, families, and communities.
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 1,099,570,276 | 1,099,570,276 | 845,731,488 | 762,970,471 | -336,599,805 |
Note: The variance of $336.6M between actual and planned spending is due to the transfer of the First Nations and Inuit Health Branch to the Department of Indigenous Services Canada, effective November 30, 2017, as per the Order in Council P.C. 2017-1465.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 1,436 | 829 | -607 |
Note: The 1,436 FTEs correspond to the planned FTEs for a full year, whereas the 829 FTE utilization represents the 8 months during which the First Nations and Inuit Health Branch (FNIHB) was still part of Health Canada.
The variance of 607 in FTE utilization is mainly due to the transfer of the FNIHB to the Department of Indigenous Services Canada, effective November 30, 2017, as per the Order in Council P.C. 2017-1465.
Program 3.2 Supplementary Health Benefits for First Nations and InuitFootnote 7
Description
Under the Supplementary Health Benefits for First Nations and Inuit program, the Non-Insured Health Benefits (NIHB) Program provides registered First Nations and recognized Inuit residents in Canada with a specified range of medically necessary health-related goods and services, which are not otherwise provided to eligible clients through other private or provincial/territorial programs. NIHB include: pharmaceuticals; medical supplies and equipment; dental care; vision care; short term crisis intervention; mental health counselling; and medical transportation to access medically required health services not available on reserve or in the community of residence. The NIHB Program also pays health premiums on behalf of eligible clients in British Columbia (BC) (as of July 2013, NIHB no longer pays premiums for First Nations residents of BC, who became clients of the First Nations Health Authority in accordance with the BC Tripartite Health Agreement and sub agreements). Benefits are delivered through registered, private sector health benefits providers (e.g., pharmacists and dentists) and funded through NIHB's electronic claims processing system or through regional offices. Some benefits are also delivered via contribution agreements with First Nations and Inuit organizations and the territorial Governments in Nunavut and Northwest Territories. The program objective is to provide benefits in a manner that contributes to the improved health status of First Nations and Inuit. This program uses funding from the following transfer payment: First Nations and Inuit Supplementary Health Benefits.
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 1,238,036,465 | 1,238,036,465 | 871,988,424 | 795,718,923 | -442,317,542 |
Note: The variance of $442.3M between actual and planned spending is due to the transfer of the First Nations and Inuit Health Branch to the Department of Indigenous Services Canada, effective November 30, 2017, as per the Order in Council P.C. 2017-1465.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 460 | 294 | -166 |
Note: The 460 FTEs correspond to the planned FTEs for a full year whereas the 294 FTE utilization represents the 8 months during which the First Nations and Inuit Health Branch (FNIHB) was still part of Health Canada.
The variance of 166 in FTE utilization is mainly due to the transfer of the FNIHB to the Department of Indigenous Services Canada, effective November 30, 2017, as per the Order in Council P.C. 2017-1465.
Program 3.3 Health Infrastructure Support for First Nations and InuitFootnote 8
Description
The Department of Health Act and the Indian Health Policy (1979) provide the authority for the Health Infrastructure Support for First Nations and Inuit program to administer contribution agreements and direct departmental spending to support the delivery of health programs and services. The program promotes First Nation and Inuit capacity to design, manage, deliver, and evaluate health programs and services. To better meet the unique health needs of First Nations and Inuit individuals, families, and communities, this program also supports: innovation in health program and service delivery; health governance partnerships between Health Canada, the provinces, and First Nation and provincial health services; and improved integration of First Nation and provincial health services. The program objective is to help improve the health status of First Nations and Inuit People, to become comparable to that of the Canadian population. The program objective is to help improve First Nations and Inuit capacity to influence and/or control the delivery of health programs and services to First Nations and Inuit individuals, families and communities.
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 796,373,302 | 796,373,302 | 589,200,730 | 588,407,867 | -207,965,435 |
Note: The variance of $208.0M between actual and planned spending is due to the transfer of the First Nations and Inuit Health Branch to the Department of Indigenous Services Canada, effective November 30, 2017, as per the Order in Council P.C. 2017-1465.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 187 | 100 | -87 |
Note: The 187 FTEs correspond to the planned FTEs for a full year whereas the 100 FTE utilization represents the 8 months during which the First Nations and Inuit Health Branch (FNIHB) was still part of Health Canada.
The variance of 87 in FTE utilization is mainly due to the transfer of the FNIHB to the Department of Indigenous Services Canada, effective November 30, 2017, as per the Order in Council P.C. 2017-1465.
Internal Services
Description
Internal Services are those groups of related activities and resources that the federal government considers to be services in support of programs to meet corporate obligations of an organization. Internal Services refers to the activities and resources of the 10 distinct service categories that support Program delivery in the organization, regardless of the Internal Services delivery model in a department. The 10 service categories are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Services; Materiel Services; and Acquisition Services.
Results
Human Resources Management Services
Health Canada continued to implement the Multi-Year Strategy for Mental Health and Wellness in the Workplace and promote a corporate culture that supports workplace well-being and healthy working relationships through a number of initiatives such as:
- The establishment of the Respect in the Workplace Joint Partnership Committee comprised of Health Canada senior management and union representatives.
- The continued delivery of Mental Health training sessions. In 2017-18, 1,310 employees (approximately 20%) completed Mental Health First Aid, for a cumulative total of 3,780 employees (approximately 57%), and 1,565 employees (approximately 23%) completed Building Blocks of Respect in the Workplace, for a cumulative total of 4,855 employees (approximately 73%), both mandatory workplace wellness training courses.
- The continued implementation of the National Standard for Psychological Health and Safety in the Workplace action plans.
- Continued implementation of initiatives such as the Multi-Year Diversity and Employment Equity Plan, which supported Health Canada in meeting the statutory requirements under the provisions of the Employment Equity Act. Actions were taken to recruit, develop, and retain a diverse workforce and build an inclusive, respectful and healthy workplace. As a result, Health Canada continues to meet representation of Women, Persons with Disabilities, Aboriginal Peoples, and Visible Minorities with respect to labour market availability.
Health Canada continued to enable a culture of high performance through initiatives such as the Performance Management Initiative (PMI) and the Post-Secondary Recruitment (PSR) program:
- The PMI completion rate for Health Canada year-end assessments was 89.7% (adjusted for employees on leave), which is higher than last year's completion rate and above the core public service average of 75.7%. In addition, 7.8% of employees have Talent Management Plans, which demonstrates a strong commitment to supporting employee development.
- Health Canada hired 191 new, indeterminate, entry-level recruits in FY 2017-18. Of the 191 recruits that were hired, 76 were hired through student bridging. This represents an 11% increase from last year's 172 hires and exceeds the initial 2017-18 target of 178. Health Canada also offered 864 student placements through the Federal Student Work Experience Program, the Research Affiliate Program and CO-OP.
The Canada School of Public Service's (CSPS) new learning model continues to be promoted, with 12,731 learning activities completed by Health Canada staff at the CSPS in 2017-18.
The department continued to utilize Career Connexions and Career Marketplace (the new name for the Career Connexions Opportunities Platform), with participation in over 159 opportunities in 2017-18. Commonly explored opportunities included micro missions, mentoring, secondment, acting, assignment and deployment opportunities.
Health Canada continued to support employees and managers in resolving pay issues and concerns by providing advice and guidance, and liaising with Public Services and Procurement Canada. Notable progress for 2017-18 included:
- The launch of an in-house pay pilot team of Compensation Advisors who processed nearly 4000 pay transactions and continued to grow in capacity throughout the year.
- The continuation of compensation escalation services to assist employees in troubleshooting and liaising with the Pay Centre on their behalf to follow up on their outstanding pay cases.
- A dedicated Virtual Nursing Unit to support primarily First Nations and Inuit Health Branch (FNIHB) nurses and a seamless transition of compensation services following FNIHB's transfer to Indigenous Services Canada.
- Continued offering of training sessions on Pay Action Request (PAR) completion and pay stubs, as well a PAR video and updated guides for managers, employees and Trusted Sources.
Management and Oversight
The Departmental Results Framework, approved by Treasury Board in 2017, is now the basis upon which Health Canada communicates the changes it is seeking to influence or achieve for Canadians. Performance Information Profiles, management tools used by programs to plan for and guide the generation of performance information, were also developed and are being refined, with attention turning to capturing data to track progress on planned results.
Health Canada has also strengthened its risk-based data monitoring by employing analytics on contracts and all acquisition card transactions. The Department continues to make progress on its initiative to standardize and streamline business processes, and to develop a system that integrates operational and financial planning and reporting.
Communication Services
Throughout the past year, Health Canada continued its work to ensure that Canadians had access to the information they needed to take action on their health and safety. The Department undertook numerous initiatives to inform and engage Canadians and support the Minister in delivering on her mandate by communicating with Canadians.
Health Canada adopted an enhanced digital-first approach to ensure Canadians received timely, relevant and accessible information about cannabis, opioids, healthy eating, tobacco cessation, consumer product safety, and other subjects, through online and social media channels. Overall, social media posts communicated through the Department's social media channels were viewed more than 60 million times. The Department also advanced its use of social media analytics to evaluate its activities and outreach capabilities.
Health Canada, through strengthened integration and collaboration between communications and program functions within the Department, also worked to improve how it engages with and communicates to Canadians about their health and safety. As an example, strong internal collaboration in the context of the cannabis file enabled improved coordination of public communications and stakeholder engagement, and greater consistency in communications to target audiences. It further resulted in the production and release of advertising to support public education about cannabis, successful creation and distribution of the Drug Free Kids Canada Toolkit, and numerous consultative activities that have engaged Canadians.
The department also launched a new opioids-focussed landing page on Canada.ca to deliver more comprehensive information to better address the information needs of Canadians.
| 2017–18 Main Estimates |
2017–18 Planned spending |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2017–18 Difference (actual minus planned) |
|---|---|---|---|---|
| 286,918,200 | 286,918,200 | 384,517,278 | 369,162,501 | 82,244,301 |
Note: The variance of $82.2M between actual and planned spending is mainly due to additional funding of $97.6M for the operating budget carry forward, and internal services resources received from various Treasury Board approved initiatives.
This is offset by $15.3M due to changes in the timing of investment plan projects.
| 2017–18 Planned |
2017-18 Actual |
2017-18 Difference (actual minus planned) |
|---|---|---|
| 1,968 | 2,316 | 348 |
Note: The variance of 348 in FTE utilization is mainly due to a transfer of FTEs to Health Canada from Public Health Agency of Canada which is associated with the health portfolio Shared Services Partnership model, and additional resources received in-year for the internal support services from various Treasury Board approved initiatives.
Analysis of trends in spending and human resources
Actual expenditures
Departmental Spending Trend
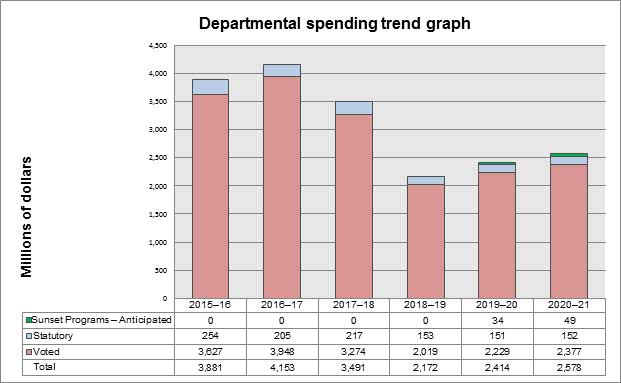
Text Description - Departmental spending trend graph
The figure illustrates Health Canada's spending trend from fiscal year 2015-16 to fiscal year 2020-21 where spending, in millions of dollars, is shown on the vertical axis and time period, in fiscal years, is shown on the horizontal axis.
Health Canada's actual spending for fiscal year 2015-16: $3,881 million (Voted: $3,627 million, Statutory: $254 million); 2016-17: $4,153 million (Voted: $3,948 million, Statutory: $205 million); 2017-18: $3,491 million (Voted: $3,274 million, Statutory: $217 million).
Health Canada's planned spending for fiscal year 2018-19: $2,172 million (Voted: $2,019 million, Statutory: $153 million).
Health Canada's 2019-20 and 2020-21 planned spending includes the assumed renewal of certain sunsetting programs; 2019-20: $2,414 million (Voted: $2,229 million, Statutory: $151 million, Sunset Programs – Anticipated: $34 million); 2020-21: $2,578 million (Voted: $2,377 million, Statutory: $152 million, Sunset Programs – Anticipated: $49 million).
Note: The variance in planned spending between fiscal year 2017-18 through 2020-21 is mainly due to the transfer of the First Nations and Inuit Health Branch to the Department of Indigenous Services Canada, effective November 30, 2017, as per the Order in Council P.C. 2017-1465.
Note: The variance in planned spending between fiscal year 2017-18 through 2020-21 is mainly due to the transfer of the First Nations and Inuit Health Branch to the Department of Indigenous Services Canada, effective November 30, 2017, as per the Order in Council P.C. 2017-1465.
| Programs and Internal Services | 2017–18 Main Estimates |
2017–18 Planned spending |
2018–19 Planned spendingFootnote * |
2019–20 Planned spendingFootnote * |
2017–18 Total authorities available for use |
2017–18 Actual spending (authorities used) |
2016–17 Actual spending (authorities used) |
2015–16 Actual spending (authorities used) |
|---|---|---|---|---|---|---|---|---|
| Strategic Outcome 1: A health system responsive to the needs of Canadians | ||||||||
| 1.1 Canadian Health System Policy | 297,012,268 | 297,012,268 | N/A | N/A | 402,292,934 | 385,167,016 | 329,454,933 | 329,580,184 |
| 1.2 Specialized Health Services | 18,326,068 | 18,326,068 | N/A | N/A | 19,783,625 | 19,688,067 | 13,588,652 | 15,260,199 |
| 1.3 Official Language Minority Community Development | 35,328,730 | 35,328,730 | N/A | N/A | 35,280,087 | 34,436,737 | 37,435,684 | 37,221,431 |
| Strategic Outcome 2: Health risks and benefits associated with food, products, substances, and environmental factors are appropriately managed and communicated to Canadians | ||||||||
| 2.1 Health Products | 147,322,313 | 147,322,313 | N/A | N/A | 185,692,315 | 177,165,339 | 149,469,788 | 145,641,623 |
| 2.2 Food Safety and Nutrition | 67,881,855 | 67,881,855 | N/A | N/A | 68,223,946 | 68,064,842 | 69,079,818 | 63,941,395 |
| 2.3 Environ-mental Risks to Health | 96,356,868 | 96,356,868 | N/A | N/A | 93,471,137 | 89,496,610 | 84,862,213 | 87,559,410 |
| 2.4 Consumer Product and Workplace Hazardous Materials | 38,015,185 | 38,015,185 | N/A | N/A | 38,648,378 | 34,846,833 | 34,148,234 | 34,513,091 |
| 2.5 Problematic Substance Use.Footnote 9 | 88,941,061 | 88,941,061 | N/A | N/A | 128,572,801 | 103,843,391 | 94,866,751 | 84,450,294 |
| 2.6 Radiation Protection | 18,294,915 | 18,294,915 | N/A | N/A | 20,458,193 | 20,381,720 | 19,866,574 | 20,871,026 |
| 2.7 Pesticides | 39,983,502 | 39,983,502 | N/A | N/A | 41,772,731 | 41,702,395 | 42,621,685 | 41,360,034 |
| Strategic Outcome 3: First Nations and Inuit communities and individuals receive health services and benefits that are responsive to their needs so as to improve their health status | ||||||||
| 3.1 First Nations and Inuit Primary Health Care | 1,099,570,276 | 1,099,570,276 | N/A | N/A | 845,731,488 | 762,970,471 | 940,569,090 | 888,041,558 |
| 3.2 Supplementary Health Benefits for First Nations and Inuit | 1,238,036,465 | 1,238,036,465 | N/A | N/A | 871,988,424 | 795,718,923 | 1,251,632,266 | 1,138,729,982 |
| 3.3 Health Infrastructure Support for First Nations and Inuit | 796,373,302 | 796,373,302 | N/A | N/A | 589,200,730 | 588,407,867 | 781,886,051 | 672,276,324 |
| Subtotal | 3,981,442,808 | 3,981,442,808 | - | - | 3,341,116,789 | 3,121,890,211 | 3,849,481,739 | 3,559,446,551 |
| Internal Services | 286,918,200 | 286,918,200 | N/A | N/A | 384,517,278 | 369,162,501 | 303,735,385 | 321,685,601 |
| Total | 4,268,361,008 | 4,268,361,008 | 2,171,515,042 | 2,380,296,471 | 3,725,634,067 | 3,491,052,712 | 4,153,217,124 | 3,881,132,152 |
Footnotes
|
||||||||
Actual human resources
| Programs and Internal Services | 2015–16 Actual |
2016–17 Actual |
2017–18 Planned |
2017–18 Actual |
2018–19 PlannedFootnote * |
2019–20 PlannedFootnote * |
|---|---|---|---|---|---|---|
| Strategic Outcome 1: A health system responsive to the needs of Canadians | ||||||
| 1.1 Canadian Health System Policy | 175 | 177 | 238 | 176 | N/A | N/A |
| 1.2 Specialized Health Services | 179 | 178 | 255 | 188 | N/A | N/A |
| 1.3 Official Language Minority Community Development | 7 | 8 | 10 | 4 | N/A | N/A |
| Strategic Outcome 2: Health risks and benefits associated with food, products, substances, and environmental factors are appropriately managed and communicated to Canadians | ||||||
| 2.1 Health Products | 1,763 | 1,733 | 1,974 | 1,753 | N/A | N/A |
| 2.2 Food Safety and Nutrition | 500 | 500 | 602 | 485 | N/A | N/A |
| 2.3 Environmental Risks to Health | 561 | 553 | 720 | 532 | N/A | N/A |
| 2.4 Consumer Product and Workplace Hazardous Materials | 290 | 289 | 305 | 268 | N/A | N/A |
| 2.5 Problematic Substance Use.Footnote 10 | 476 | 522 | 415 | 696 | N/A | N/A |
| 2.6 Radiation Protection | 192 | 180 | 202 | 171 | N/A | N/A |
| 2.7 Pesticides | 428 | 434 | 489 | 406 | N/A | N/A |
| Strategic Outcome 3: First Nations and Inuit communities and individuals receive health services and benefits that are responsive to their needs so as to improve their health status | ||||||
| 3.1 First Nations and Inuit Primary Health Care | 1,337 | 1,363 | 1,436 | 829 | N/A | N/A |
| 3.2 Supplementary Health Benefits for First Nations and Inuit | 473 | 489 | 460 | 294 | N/A | N/A |
| 3.3 Health Infrastructure Support for First Nations and Inuit | 188 | 183 | 187 | 100 | N/A | N/A |
| Subtotal | 6,569 | 6,609 | 7,293 | 5,902 | - | - |
| Internal Services | 2,171 | 2,243 | 1,968 | 2,316 | N/A | N/A |
| Total | 8,740 | 8,852 | 9,261 | 8,218 | 7,554 | 7,591 |
Footnotes
|
||||||
Expenditures by vote
For information on Health Canada’s organizational voted and statutory expenditures, consult the Public Accounts of Canada 2018.
Government of Canada spending and activities
Information on the alignment of Health Canada’s spending with the Government of Canada’s spending and activities is available in the GC InfoBase.
Financial statements and financial statements highlights
Financial statements
Health Canada’s financial statements [unaudited] for the year ended March 31, 2018, are available on Health Canada’s web site.
Financial Statements Highlights
| Financial information | 2017–18 Planned results |
2017–18 Actual results |
2016–17 Actual results |
Difference (2017–18 actual results minus 2017–18 planned results) | Difference (2017–18 actual results minus 2016–17 actual results) |
|---|---|---|---|---|---|
| Total expenses | 1,320,845,000 | 1,543,017,000 | 1,394,262,000 | 222,172,000 | 148,755,000 |
| Total revenues | 202,327,000 | 184,298,000 | 176,138,000 | (18,029,000) | 8,160,000 |
| Transferred operations | 3,142,559,000 | 2,159,998,000 | 2,977,814,000 | (982,561,000) | (817,816,000) |
| Net cost of operations before government funding and transfers | 4,261,077,000 | 3,518,717,000 | 4,195,938,000 | (742,360,000) | (677,221,000) |
Pursuant to Order-in-Council P.C. 2017-1465, Health Canada transferred the control and supervision of the First Nations and Inuit Health Branch (FNIHB) to the Department of Indigenous Services Canada (ISC) effective November 30, 2017.
The Department’s total expenses from continuing operations were $1.5B in 2017-18.
There was an increase of $222.2M when comparing actual expenditures to planned results for 2017-18. This is primarily a result of an increase in funding for: implementing and administering a federal framework to legalize and strictly regulate cannabis; maintaining core regulatory operations for therapeutic products; renewal of the Territorial Health Investment Fund; Canada Health Infoway Inc.; and payments in connection with section 103 of the Patent Act.
There was an increase of $148.8M when comparing year-over-year actual expenditures. The significant changes were:
- an increase of $123.2M in salaries and employee benefits resulting from the signing of collective agreements, the creation of an Opioid Response Team and the creation of the Cannabis Legalization and Regulation Branch;
- an increase of $39.9M in transfer payments due primarily to an increase in transfer payments made to Canada Health Infoway, as well as a statutory payment made pursuant to section 103 of the Patent Act; and,
- a decrease of $37.1M in other expenses primarily as a result of a provision for contingent liabilities recorded in the prior year.
The Department’s total revenues were $184.3M in 2017-18 representing a decrease of $18.0M from planned results and an increase of $8.2M over the prior year actual revenues. This year-over-year variance is primarily a result of increased revenue authority granted to the Pest Management Regulatory Agency and increases in demand for other regulatory and non-regulatory services provided by the Department.
Transferred operations for the 2017-18 fiscal year of $2,160.0M represent those expenses and revenues incurred by FNIHB up to November 29, 2017. In comparing these results to those planned for 2017-18 and to the 2016-17 results, the variance is primarily due to the fact that the comparative figures are for the entire fiscal year.
Pursuant to paragraph 2(a) of the Public Service Rearrangement and Transfer of Duties Act and in accordance with Order-in-Council P.C. 2018-0381, the control and supervision of the internal services that support FNIHB, including the stewardship responsibility for the assets and liabilities related to those services, were transferred to the ISC, effective April 1, 2018. The estimated net cost of operations for this unit is $33.9 million for 2017-18, and has been recorded as part of the net cost from continuing operations in financial statements.
| Financial Information | 2017-18 | 2016-17 | Difference (2017-18 minus 2016-17) |
|---|---|---|---|
| Total net liabilities | 368,954,000 | 497,243,000 | (128,289,000) |
| Total net financial assets | 201,796,000 | 305,331,000 | (103,535,000) |
| Departmental net debt | 167,158,000 | 191,912,000 | (24,754,000) |
| Total non-financial assets | 142,602,000 | 141,057,000 | 1,545,000 |
| Departmental net financial position | (24,556,000) | (50,855,000) | 26,299,000 |
Total net liabilities were $369.0M at the end of 2017-18, representing a decrease of $128.3M from the previous year. This variance is mainly due to decreased liabilities following the transfer of control and supervision of FNIHB to ISC and $26.0M reduction in the liability to Canada Health Infoway Inc. originating from the 2007 and 2009 Budgets. These decreases were partially offset by an increase in contingent liabilities and an increase in accrued liabilities for salaries as a result of challenges with the government payroll system.
The year-over-year decrease in total net financial assets of $103.5M is primarily a result of decreased assets following the transfer of control and supervision of FNIHB to ISC and offset by an increase in accounts receivable and employee advances arising from challenges with the government pay system.
Total non-financial assets increased $1.5M resulting from capital asset acquisitions net of amortization, and is offset by the transfer of FNIHB capital assets to ISC.
Supplementary information
Corporate information
Organizational profile
Appropriate Minister: The Honourable Ginette Petitpas Taylor, P.C., M.P.
Institutional Head: Simon Kennedy
Ministerial Portfolio: Health
Enabling Instrument(s): Canada Health Act, Canada Consumer Product Safety Act, Controlled Drugs and Substances Act, Food and Drugs Act, Tobacco Act, Hazardous Products Act, Hazardous Materials Information Review Act, Department of Health Act, Radiation Emitting Devices Act, Pest Control Products Act.
Year of Incorporation / Commencement: 1913
Other: Canadian Food Inspection Agency joined the Health Portfolio in October 2013.
Reporting framework
Health Canada’s Strategic Outcomes and Program Alignment Architecture of record for 2017–18 are shown below.
- 1 Strategic Outcome: A health system responsive to the needs of Canadians
- 1.1 Program: Canadian Health System Policy
- 1.1.1 Sub-Program: Health System Priorities
- 1.1.2 Sub-Program: Canada Health Act Administration
- 1.2 Program: Specialized Health Services
- 1.3 Program: Official Language Minority Community Development
- 1.1 Program: Canadian Health System Policy
- 2 Strategic Outcome: Health risks and benefits associated with food, products, substances, and environmental factors are appropriately managed and communicated to Canadians
- 2.1 Program: Health Products
- 2.1.1 Sub-Program: Pharmaceutical Drugs
- 2.1.2 Sub-Program: Biologics and Radiopharmaceuticals
- 2.1.3 Sub-Program: Medical Devices
- 2.1.4 Sub-Program: Natural Health Products
- 2.2 Program: Food Safety and Nutrition
- 2.2.1 Sub-Program: Food Safety
- 2.2.2 Sub-Program: Nutrition Policy and Promotion
- 2.3 Program: Environmental Risks to Health
- 2.3.1 Sub-Program: Air Quality
- 2.3.2 Sub-Program: Water Quality
- 2.3.3 Sub-Program: Health Impacts of Chemicals
- 2.4 Program: Consumer Product and Workplace Hazardous Materials
- 2.4.1 Sub-Program: Consumer Product Safety
- 2.4.2 Sub-Program: Workplace Hazardous Materials
- 2.5 Program: Problematic Substance UseFootnote 11
- 2.5.1 Sub-Program: Tobacco Control
- 2.5.2 Sub-Program: Controlled Substances
- 2.6 Program: Radiation Protection
- 2.6.1 Sub-Program: Environmental Radiation Monitoring and Protection
- 2.6.2 Sub-Program: Radiation Emitting Devices
- 2.6.3 Sub-Program: Dosimetry Services
- 2.7 Program: Pesticides
- 2.1 Program: Health Products
- 3 Strategic OutcomeFootnote 12 : First Nations and Inuit communities and individuals receive health services and benefits that are responsive to their needs so as to improve their health status
- 3.1 Program: First Nations and Inuit Primary Health Care
- 3.1.1 Sub-Program: First Nations and Inuit Health Promotion and Disease Prevention
- 3.1.1.1 Sub-Sub-Program: Healthy Child Development
- 3.1.1.2 Sub-Sub-Program: Mental Wellness
- 3.1.1.3 Sub-Sub-Program: Healthy Living
- 3.1.2 Sub-Program: First Nations and Inuit Public Health Protection
- 3.1.2.1 Sub-Sub-Program: Communicable Disease Control and Management
- 3.1.2.2 Sub-Sub-Program: Environmental Public Health
- 3.1.3 Sub-Program: First Nations and Inuit Primary Care
- 3.1.3.1 Sub-Sub-Program: Clinical and Client Care
- 3.1.3.2 Sub-Sub-Program: Home and Community Care
- 3.1.3.3 Sub-Sub-Program: Jordan's Principle – A Child First Initiative
- 3.1.1 Sub-Program: First Nations and Inuit Health Promotion and Disease Prevention
- 3.2 Program: Supplementary Health Benefits for First Nations and Inuit
- 3.3 Program: Health Infrastructure Support for First Nations and Inuit
- 3.3.1 Sub-Program: First Nations and Inuit Health System Capacity
- 3.3.1.1 Sub-Sub-Program: Health Planning and Quality Management
- 3.3.1.2 Sub-Sub-Program: Health Human Resources
- 3.3.1.3 Sub-Sub-Program: Health Facilities
- 3.3.2 Sub-Program: First Nations and Inuit Health System Transformation
- 3.3.2.1 Sub-Sub-Program: Health Systems Integration
- 3.3.2.2 Sub-Sub-Program: e-Health Infostructure
- 3.3.3 Sub-Program: Tripartite Health Governance
- 3.3.1 Sub-Program: First Nations and Inuit Health System Capacity
- 3.1 Program: First Nations and Inuit Primary Health Care
Internal Services
- IS 1: Management and Oversight Services
- IS 2: Communications Services
- IS 3: Legal Services
- IS 4: Human resources Management Services
- IS 5: Financial Management Services
- IS 6: Information Management Services
- IS 7: Information Technology Services
- IS 8: Real Property Services
- IS 9: Materiel Services
- IS 10: Acquisition Services
Supporting information on lower-level programs
Supporting information on lower-level programs is available on the GC InfoBase.
Supplementary information tables
The following supplementary information tables are available on the Health Canada website.
- Departmental Sustainable Development Strategy
- Details on transfer payment programs of $5 million or more
- Evaluations
- Fees
- Horizontal initiatives
- Internal audits
- Response to parliamentary committees and external audits
- Up-front multi-year funding
Federal tax expenditures
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance Canada publishes cost estimates and projections for these measures each year in the Report on Federal Tax Expenditures . This report also provides detailed background information on tax expenditures, including descriptions, objectives, historical information and references to related federal spending programs. The tax measures presented in this report are the responsibility of the Minister of Finance.
Organizational contact information
Marc Desjardins
Director General
Health Canada
DIRECTOR GENERAL’S OFFICE
200 Eglantine Driveway, Tunney’s Pasture
Ottawa, Ontario K1A 0K9
Telephone: 613-948-6357
Fax: 613-946-0807
marc.desjardins@canada.ca
Appendix: definitions
appropriation
Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
audit
An independent, objective assurance and consulting activity designed to add value and improve an organization's operations. It helps an organization accomplish its objectives by bringing a systematic, disciplined approach to evaluate and improve the effectiveness of risk management, control, and governance processes.
budgetary expenditures
Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
Core Responsibility
An enduring function or role performed by a department. The intentions of the department with respect to a Core Responsibility are reflected in one or more related Departmental Results that the department seeks to contribute to or influence.
Departmental Plan
A report on the plans and expected performance of an appropriated department over a three‑year period. Departmental Plans are tabled in Parliament each spring.
Departmental Result
A Departmental Result represents the change or changes that the department seeks to influence. A Departmental Result is often outside departments’ immediate control, but it should be influenced by program-level outcomes.
Departmental Result Indicator
A factor or variable that provides a valid and reliable means to measure or describe progress on a Departmental Result.
Departmental Results Framework
Consists of the department’s Core Responsibilities, Departmental Results and Departmental Result Indicators.
Departmental Results Report
A report on an appropriated department’s actual accomplishments against the plans, priorities and expected results set out in the corresponding Departmental Plan.
evaluation
In the Government of Canada, the systematic and neutral collection and analysis of evidence to judge merit, worth or value. Evaluation informs decision making, improvements, innovation and accountability. Evaluations typically focus on programs, policies and priorities and examine questions related to relevance, effectiveness and efficiency. Depending on user needs, however,
evaluations can also examine other units, themes and issues, including alternatives to existing interventions. Evaluations generally employ social science research methods.
experimentation
Activities that seek to explore, test and compare the effects and impacts of policies, interventions and approaches, to inform evidence-based decision-making, by learning what works and what does not.
full‑time equivalent
A measure of the extent to which an employee represents a full person‑year charge against a departmental budget. Full‑time equivalents are calculated as a ratio of assigned hours of work to scheduled hours of work. Scheduled hours of work are set out in collective agreements.
gender-based analysis plus (GBA+)
An analytical approach used to assess how diverse groups of women, men and gender-diverse people may experience policies, programs and initiatives. The “plus” in GBA+ acknowledges that the gender-based analysis goes beyond biological (sex) and socio-cultural (gender) differences. We all have multiple identity factors that intersect to make us who we are; GBA+ considers many other identity factors, such as race, ethnicity, religion, age, and mental or physical disability. Examples of GBA+ processes include using data disaggregated by sex, gender and other intersecting identity factors in performance analysis, and identifying any impacts of the program on diverse groups of people, with a view to adjusting these initiatives to make them more inclusive.
government-wide priorities
For the purpose of the 2017–18 Departmental Results Report, those high-level themes outlining the government’s agenda in the 2015 Speech from the Throne, namely: Growth for the Middle Class; Open and Transparent Government; A Clean Environment and a Strong Economy; Diversity is Canada’s Strength; and Security and Opportunity.
horizontal initiative
An initiative where two or more departments are given funding to pursue a shared outcome, often linked to a government priority.
Management, Resources and Results Structure
A comprehensive framework that consists of an organization’s inventory of programs, resources, results, performance indicators and governance information. Programs and results are depicted in their hierarchical relationship to each other and to the Strategic Outcome(s) to which they contribute. The Management, Resources and Results Structure is developed from the Program Alignment Architecture.
non‑budgetary expenditures
Net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
performance
What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
performance indicator
A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of an organization, program, policy or initiative respecting expected results.
performance reporting
The process of communicating evidence‑based performance information. Performance reporting supports decision making, accountability and transparency.
plan
The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead up to the expected result.
planned spending
For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts that receive Treasury Board approval by February 1. Therefore, planned spending may include amounts incremental to planned expenditures presented in the Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports.
priority
A plan or project that an organization has chosen to focus and report on during the planning period. Priorities represent the things that are most important or what must be done first to support the achievement of the desired Strategic Outcome(s) or Departmental Results.
program (applies to departments reporting using the Program Alignment Architecture)
A group of related resource inputs and activities that are managed to meet specific needs and to achieve intended results and that are treated as a budgetary unit.
Program (applies to departments reporting using the Departmental Results Framework)
Individual or groups of services, activities or combinations thereof that are managed together within the department and focus on a specific set of outputs, outcomes or service levels.
Program Alignment Architecture
A structured inventory of an organization’s programs depicting the hierarchical relationship between programs and the Strategic Outcome(s) to which they contribute.
Program Inventory
Identifies all of the department’s programs and describes how resources are organized to contribute to the department’s Core Responsibilities and Results.
result
An external consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead they are within the area of the organization’s influence.
statutory expenditures
Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
Strategic Outcome
A long‑term and enduring benefit to Canadians that is linked to the organization’s mandate, vision and core functions.
sunset program
A time‑limited program that does not have an ongoing funding and policy authority. When the program is set to expire, a decision must be made whether to continue the program. In the case of a renewal, the decision specifies the scope, funding level and duration.
target
A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
voted expenditures
Expenditures that Parliament approves annually through an Appropriation Act. The Vote wording becomes the governing conditions under which these expenditures may be made.
Page details
- Date modified:
