Health Product InfoWatch: June 2023
Download the alternative format
(606 KB, 9 pages)
Health Products and Food Branch
Marketed Health Products Directorate
Health Product InfoWatch Editorial Team
ISSN: 2368-8025
Cat.: H167-1E-PDF
Pub.: 230000
Contents
- Health products mentioned in this issue
- Announcement
- Drug and vaccine authorizations and communications for COVID-19
- Monthly recap of health product safety information
- New health product safety information
- Scope
- Reporting Adverse Reactions
- Helpful links
- Suggestions?
- Copyright
Health products mentioned in this issue
Pharmaceuticals and biologics
- 0.9% Sodium Chloride Injection USP
- Combination sulfamethoxazole and trimethoprim
- Emerade epinephrine auto-injectors
- Evusheld (tixagevimab and cilgavimab)
- Mekinist (trametinib)
- Mercaptopurine
- Nitroglycerin tablets
- Proglycem (diazoxide)
- Tafinlar (dabrafenib mesylate)
Medical devices
Natural and non-prescription health products
Other
Announcement
Drug and Health Product Portal
In May 2023, Health Canada’s Drug and Health Product Register was replaced by the Drug and Health Product Portal (DHPP) to provide trusted and timely information to Canadians on drugs and health products authorized by Health Canada. The DHPP includes new features, such as an improved search function, improved accessibility, and improved links for health product regulatory information, including Summary Safety Reviews (SSRs).
Drug and vaccine authorizations and communications for COVID-19
New information and recent communications related to authorized COVID-19 vaccines and treatments are highlighted in this section.
Update on the COVID-19 situation in Canada
The World Health Organization (WHO) announced that COVID-19 is now an established and ongoing health issue and no longer constitutes a Public Health Emergency of International Concern. The Government of Canada will continue to work with the WHO, international partners and with Canadian provinces and territories to monitor the COVID-19 situation and to mitigate domestic health and societal impacts of this virus. As of July 2023, the Health Product InfoWatch will no longer include a separate section for COVID-19 communications. Moving forward, COVID-19 related information will be included in the monthly recap or new information sections, as for all health products.
Update on the COVID-19 situation in Canada (May 5, 2023)
Evusheld (tixagevimab and cilgavimab)
Evusheld (tixagevimab and cilgavimab) may not be effective against certain SARS-CoV-2 Omicron subvariants when used as a prophylaxis or treatment for COVID-19. Updated neutralization data has been added to the Canadian product monograph.
Healthcare professionals should routinely review the Antiviral Resistance information
in the product monograph, in conjunction with literature and local guidelines, for details regarding specific variants and resistance. This information will continue to be updated as new evidence emerges.
COVID-19 vaccines and treatments portal: Evusheld (tixagevimab and cilgavimab)
Monthly recap of health product safety information
The following is a list of health product advisories, type I recalls and summaries of completed safety reviews published in May 2023 by Health Canada.
0.9% Sodium Chloride Injection USP
One lot of 0.9% Sodium Chloride Injection USP was recalled as the solution bags may be leaking.
Type 1 drug recall: 0.9% Sodium Chloride Injection USP
Combination sulfamethoxazole and trimethoprim
This safety review evaluated the risk of hemophagocytic lymphohistiocytosis (HLH) associated with combination sulfamethoxazole and trimethoprim-containing products. Health Canada’s review of the available information found a possible link. Health Canada is working with the manufacturers to update the Canadian product monograph for combination sulfamethoxazole and trimethoprim-containing products to include the risk of HLH.
Summary Safety Review: Combination sulfamethoxazole and trimethoprim
Emerade epinephrine auto-injectors
Bausch Health, Canada Inc. recalled all lots of Emerade epinephrine auto-injectors (0.3 mg and 0.5 mg) after testing by the company identified the potential risk that the auto-injector may fail to activate, or it may activate prematurely if dropped. The affected lots of Emerade were distributed in Canada between April 2022 and May 2023.
Advisory: Emerade epinephrine auto-injectors
Mercaptopurine
This safety review evaluated the risk of hypoglycemia associated with mercaptopurine-containing products. Health Canada’s review concluded that there may be a link between the use of mercaptopurine and the risk of hypoglycemia in children (less than 18 years of age). Health Canada is working with the manufacturers to update the Canadian product monograph for mercaptopurine-containing products to include the risk of hypoglycemia in children.
Summary Safety Review: Mercaptopurine
Nature’s Bounty Kids Multivitamin Gummies
Nestlé Health Science recalled 12 lots of Nature’s Bounty Kids Multivitamin Gummies
because the product’s label does not indicate that the gummies should not be given to children under 4 years of age. Giving these products to children in this age group could create a choking hazard. The affected products were distributed to multiple retailers across Canada between January and May 2023.
Advisory: Nature’s Bounty Kids Multivitamin Gummies
Nitroglycerin tablets
Canada is experiencing a shortage of nitroglycerin tablets due to an increase in demand caused by the nitroglycerin spray shortage. Together with its partners, Health Canada is looking at ways to conserve existing supply, expedite resupplies to hospitals and pharmacies, and access foreign-authorized supply or alternatives, where possible.
Advisory: Nitroglycerin tablets
Proglycem (diazoxide)
This safety review evaluated the risk of necrotizing enterocolitis in newborns and infants associated with Proglycem (diazoxide). Health Canada’s review of the available information found a possible link. Health Canada is working with the manufacturer to update the Canadian product monograph for Proglycem to include the risk of necrotizing enterocolitis.
Summary Safety Review: Proglycem (diazoxide)
Unauthorized health products
Health Canada advised Canadians about various unauthorized health products being sold at retail locations across Canada or online that may pose serious health risks.
- Advisory: Unauthorized amoxicillin capsules
- Advisory: Unauthorized drugs for abortion and emergency contraception
- Advisory: Unauthorized Kobayashi eye washes and Sante FX eye drops
- Advisory: Unauthorized sexual enhancement products
- Advisory: Unauthorized skin lightening products
- Advisory: Unauthorized workout supplements
New health product safety information
The following topics have been selected to raise awareness and encourage reporting of adverse reactions.
Product monograph and medical device instructions for use updates
The following safety labelling updates, which were recently made to the Canadian product monograph and medical device instructions for use, have been selected for your awareness. A complete list of safety labelling updates for pharmaceuticals is available on Health Canada's Product monograph brand safety updates page. Canadian product monographs can be accessed through Health Canada's Drug Product Database.
Tafinlar (dabrafenib mesylate) and Mekinist (trametinib)
The Warnings and Precautions and the Patient Medication Information sections of the Canadian product monographs for Tafinlar and Mekinist have been updated with the risk of haemophagocytic lymphohistiocytosis.
Key messages for healthcare professionals: Footnote 1, Footnote 2
- In post-marketing experience, haemophagocytic lymphohistiocytosis (HLH) has been observed with combination dabrafenib and trametinib treatment. Patients should be closely monitored.
- If HLH is suspected, treatment should be interrupted. If HLH is confirmed, treatment should be discontinued and appropriate management of HLH should be initiated.
References
- Footnote 1
-
Tafinlar (dabrafenib mesylate) [product monograph]. Montreal (QC): Novartis Pharmaceuticals Canada Inc, 2023.
- Footnote 2
-
Mekinist (trametinib) [product monograph]. Montreal (QC): Novartis Pharmaceuticals Canada Inc, 2023.
SpaceOAR System
SpaceOAR System is an absorbable polyethylene glycol (PEG) based perirectal spacer that temporarily positions the anterior rectal wall away from the prostate during radiotherapy for prostate cancer, with the intent to reduce the radiation dose delivered to the anterior rectum. The Adverse Events, Warnings and Device Description sections of the instructions for use (IFU) for SpaceOAR System have been updated to include new risks and to elaborate upon previously labelled risks. The updated IFU (version 51607315) will be in distribution by February 2024.
Key messages for healthcare professionals:
To determine the appropriateness of SpaceOAR System for each individual patient, healthcare professionals are advised to carefully review the IFU, which has been updated since initial authorization in Canada. The following information has been added or elaborated upon:
Adverse Events
Potential complications that may be associated with the use of SpaceOAR System include, but are not limited to:
- infection (including abscess)
- urgency (e.g., urinary and rectal)
- constipation (acute, chronic, or secondary to outlet obstruction)
- rectal tenesmus/muscle spasm
- fistula
- allergic reactions (includes localized or more severe such as anaphylaxis)
- syncope
Warnings
The SpaceOAR product contains PEG. The use of this product in patients with documented PEG sensitivities or allergies has not been studied. The use of SpaceOAR in patients with an allergy to PEG could potentially lead to an allergic reaction (including anaphylaxis).
Device Description
The materials in the SpaceOAR System include PEG SG (Polyethylene Glycol Succinimidyl Glutarate, 15K Da MW), Sodium Phosphate Dibasic, Trilysine Acetate, Sodium Borate and Sodium Phosphate Monobasic.
Vaccine safety summary
Health Canada and the Public Health Agency of Canada (PHAC) share the responsibility of monitoring the safety of vaccines in Canada.
Market authorization holders are required to report serious adverse events following immunization (AEFIs) to the Canada Vigilance Program in Health Canada. The Canada Vigilance Program also receives voluntary reports from healthcare professionals and consumers.
While hospitals must report serious adverse drug reactions that were documented within their facility, they do not have to report an adverse reaction to a vaccine if they have submitted an AEFI report on that case to their local public health unit. These reports are submitted by provincial and territorial public health authorities to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) in PHAC.
This vaccine safety summary includes reports of AEFIs received by the Canada Vigilance Program between January 1, 2021, and December 31, 2021, for vaccines. The summary excludes reports where a COVID-19 vaccine was the sole vaccine suspected to be linked to the AEFI. For information about adverse events that individuals have reported after receiving a COVID-19 vaccine in Canada, please visit the Reported side effects following COVID-19 vaccination in Canada webpage.
Summary for January 1, 2021 to December 31, 2021
Key messages:
- From January 1, 2021 to December 31, 2021, the Canada Vigilance Program received 466 reports of adverse events following immunization (AEFI) for which vaccines were the suspected cause.
- There were no significant changes in the characteristics (sex and age) of the population who experienced the reported AEFIs, nor in the types of vaccines most frequently reported compared to previous years. Notably, 2021 was the first year since 2013 for which the majority of reports were reported by consumers.
- No new safety signals (potential safety issues) for non-COVID vaccines were identified during this period.
- In 2021, the Canada Vigilance Program received 466 reports of AEFI for which vaccines were the suspected cause. The majority of the reports were received from consumers (Figure 1) through spontaneous reporting, marking the first year since 2013 where consumers were the primary source of AEFI reporting to the Canada Vigilance program.
Figure 1: Total number of reports received by reporter type 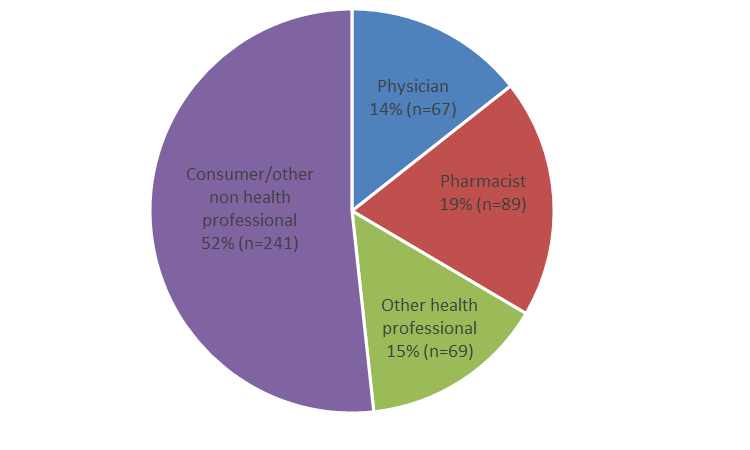
Figure 1 - Text description
The figure shows the total number of reports received by reporter type. Reporter type Percentage (%) Number of reports (n) Consumer/other non health professional 52 241 Other health professional 15 69 Pharmacist 19 89 Physician 14 67
- Most of the reports involved adults over 18 years of age (219 out of 466: 47%) (Figure 2), which is similar to what has been observed since 2013 (when Health Canada began publishing periodic reports of AEFIs submitted to the Canada Vigilance Program).
- The distribution for the 466 reports by sex was 69% female, 21% male and 10% unknown. This trend is similar to what has been observed previously.
Figure 2: Total number of reports received by age group 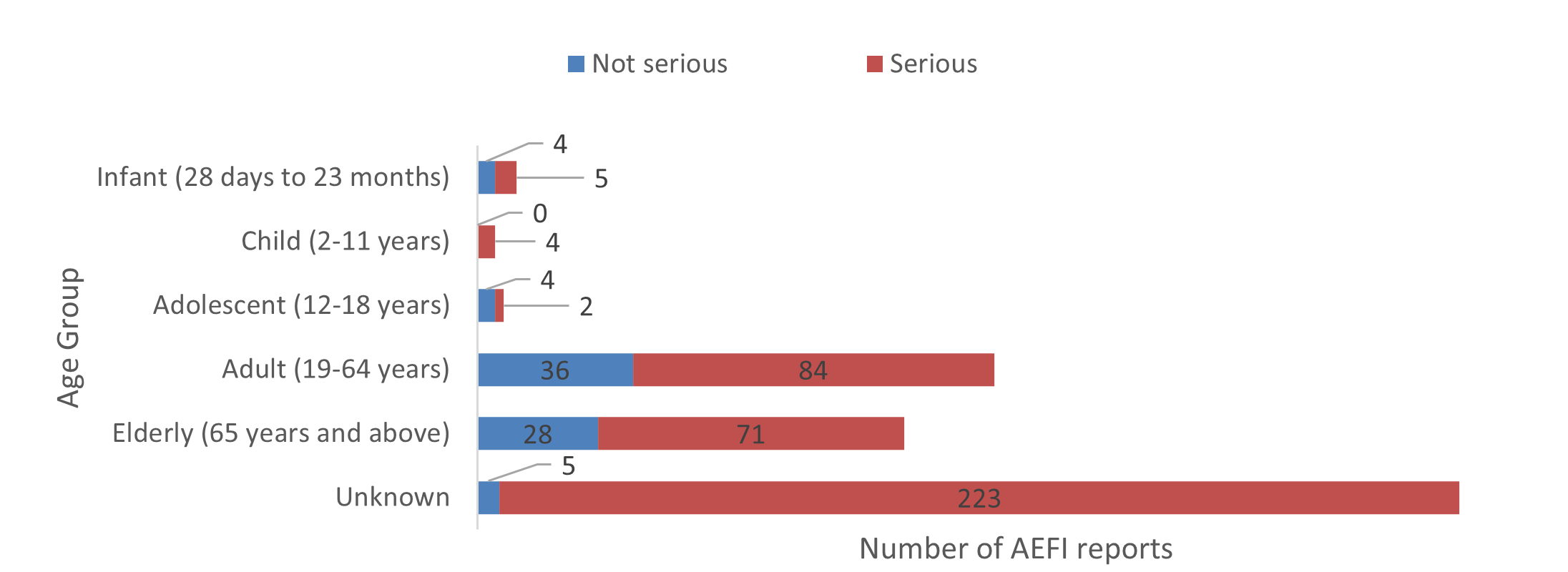
Figure 2 - Text description
The figure shows the total number of reports received by age group. Age group Number of non-serious reports Number of serious reports Infant (28 days to 23 months)
4
5
Child (2-11 years)
0
4
Adolescent (12-18 years)
4
2
Adult (19-64 years)
36
84
Elderly (65 years and above)
28
71
Unknown
5
223
- The highest number of reports (serious and non-serious) involved herpes zoster vaccines (283 reports) followed by influenza vaccines (86 reports), and pneumococcal vaccines (44 reports) (Figure 3). This trend is similar to what has been observed previously.
Figure 3: Total number of reports received by vaccine (some reports include multiple vaccines)Footnote † 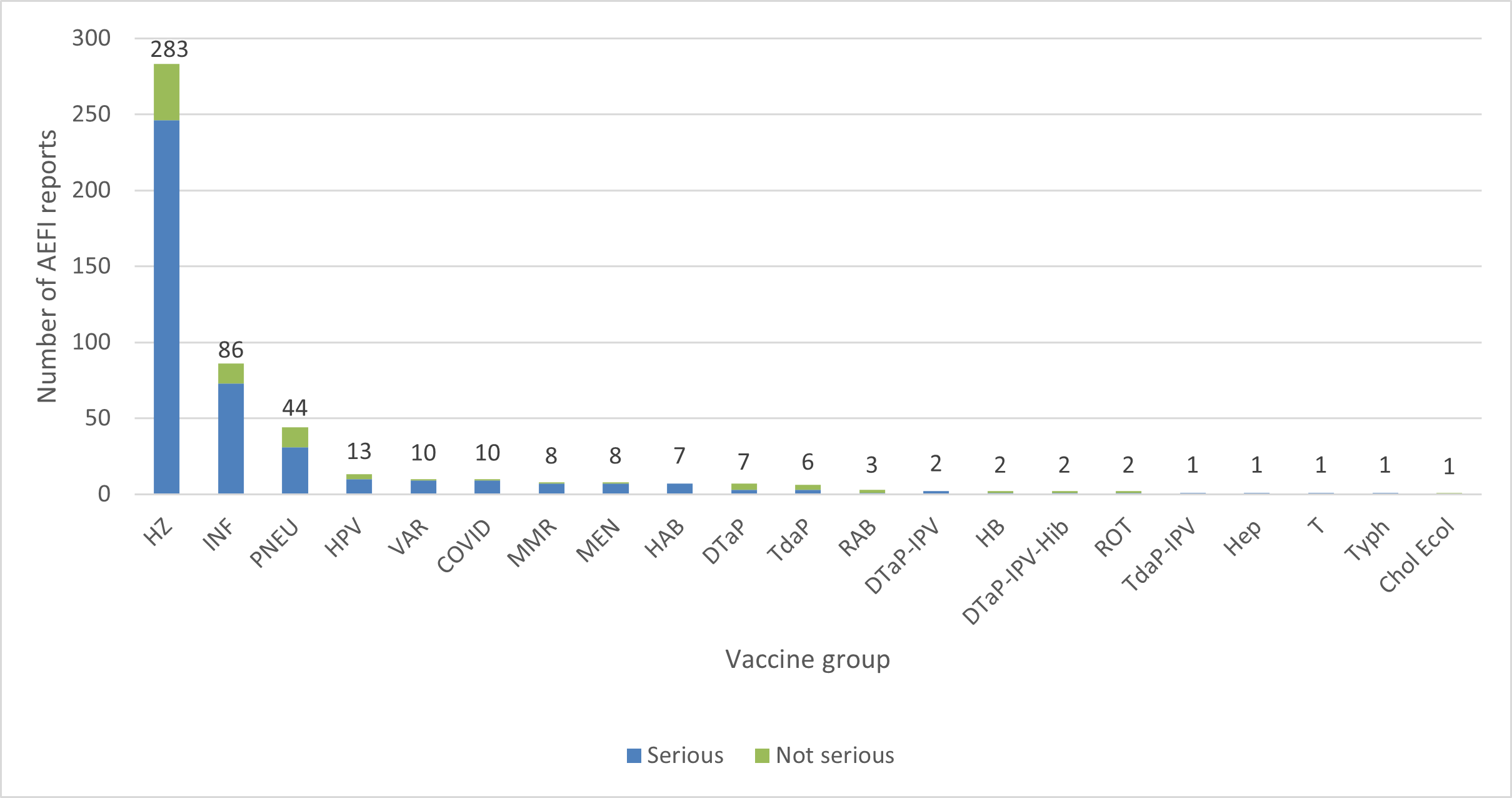
Figure 3 - Text description
The figure shows the total number of reports received by vaccine (some reports include multiple vaccines). Vaccine type Number of non-serious reports Number of serious reports Herpes Zoster HZ 37 246 Influenza INF 13 73 Pneumococcal PNEU 13 31 Human papillomavirus HPV 3 10 Varicella VAR 1 9 COVID-19 vaccines COVID 1 9 Measles, mumps, rubella MMR 1 7 Meningococcal MEN 1 7 Hepatitis A, B HAB 0 7 Diphtheria, tetanus, acellular pertussis DTaP 4 3 Tetanus, diphtheria (reduced), acellular pertussis TdaP 3 3 Rabies RAB 2 1 Diphtheria, tetanus, acellular pertussis, and inactivated poliomyelitis DTaP-IPV 0 2 Hepatitis B HB 1 1 Diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis and Haemophilus influenzae type b DTaP-IPV-Hib 1 1 Rotavirus ROT 1 1 Tetanus, diphtheria (reduced), acellular pertussis and inactivated poliomyelitis TdaP-IPV 0 1 Hepatitis
* type of hepatitis vaccine was not reportedHep 0 1 Tetanus T 0 1 Typhoid Typh 0 1 Cholera and Enterotoxigenic Escherichia Coli Travellers' Diarrhea Chol Ecol 1 0
- Of the 466 reports, 389 (83%) were serious reports, and among them,
- 246 serious reports were associated with herpes zoster vaccines. The 5 most frequently reported serious adverse events following immunization with herpes zoster vaccines included herpes zoster, vaccination failure, pain, rash and pyrexia. Most of the herpes zoster and vaccination failure events were co-reported.
- 73 serious reports were associated with influenza vaccines. The 5 most frequently reported serious adverse events following immunization with influenza vaccines included headache, cough, fatigue, diarrhoea, and nasopharyngitis.
- Overall, the majority of serious reports were not assessable for relatedness to the vaccine given the lack of medical information regarding the recipients, vaccination status, and the event. Several reports involved patients with underlying medical conditions and/or concomitant medications, and the serious adverse events were unlikely to be related to vaccination. In other reports, the events were captured in the product monographs for the suspected vaccines.
- In total, there were 9 reports with an outcome of death; 2 reports involved males, 5 involved females and 2 did not provide information on sex. Two cases occurred in individuals who were 65 years of age or older, while 7 cases did not report age. The reported vaccines were herpes zoster vaccine (6), influenza vaccine (2), and influenza vaccine co-reported with a COVID-19 vaccine (1). The information provided in these reports was not sufficiently detailed to assess the causal association between the reported event and the vaccine. The percentage of fatal cases observed in 2021 (9 out of 466 reports: 2%) is consistent with what has been reported in previous years.
Conclusion
- No new safety signals (potential safety issues) were identified for vaccines during this period.
- The benefits of vaccines authorized in Canada continue to outweigh the risks.
- Health Canada, in collaboration with PHAC, will continue to closely monitor the safety of vaccines authorized in Canada.
For additional information, contact the Marketed Health Products Directorate.
Note: that because of updated information received by the Canada Vigilance Program, there may be differences in the number of reports and adverse events retrieved at different dates.
Scope
This monthly publication is intended primarily for healthcare professionals and includes information on pharmaceuticals, biologics, medical devices and natural health products. It provides a summary of key health product safety information published in the previous month by Health Canada, as well as a selection of new health product safety information meant to raise awareness. New information contained in this issue is not comprehensive but rather represents a selection of clinically relevant items warranting enhanced dissemination.
Reporting Adverse Reactions
Canada Vigilance Program
Telephone: 1-866-234-2345
Fax or mail: Form available on MedEffect Canada
For more information on how to report an adverse reaction, visit the Adverse Reaction and Medical Device Problem Reporting page.
Helpful links
- MedEffectTM Canada
- Recalls and Safety Alerts Database
- New Safety and Effectiveness Reviews
- Canada Vigilance Adverse Reaction Online Database
- Drug Product Database
- Medical Devices Active Licence Listing
- Licensed Natural Health Products Database
- The Drug and Health Product Portal
- Drug Shortages Canada
- Medical device shortages: List of shortages and discontinuations
- Stop Illegal Marketing of Drugs and Devices
- List of drugs for exceptional importation and sale
- Coronavirus disease (COVID-19)
- Drug and vaccine authorizations for COVID-19: List of authorized drugs, vaccines and expanded indications
- COVID-19 vaccines and treatments portal
- Reported side effects following COVID-19 vaccination in Canada
Suggestions?
Your comments are important to us. Let us know what you think by reaching us at:
infowatch-infovigilance@hc-sc.gc.ca
Health Product InfoWatch Editorial Team
Marketed Health Products Directorate
Health Canada
Address Locator 1906C
Ottawa ON K1A 0K9
Telephone: 613-954-6522
Teletypewriter: 1-800-465-7735 (Service Canada)
Copyright
© 2023 His Majesty the King in Right of Canada. This publication may be reproduced without permission provided the source is fully acknowledged. The use of this publication for advertising purposes is prohibited. Health Canada does not assume liability for the accuracy or authenticity of the information submitted in case reports.
Adverse reactions (ARs) to health products are considered to be suspicions, as a definite causal association often cannot be determined. Spontaneous reports of ARs cannot be used to estimate the incidence of ARs because ARs remain underreported and patient exposure is unknown.
Due to time constraints relating to the production of this publication, information published may not reflect the most current information.
Footnotes
This summary does not include cases reported for COVID vaccines unless they were co-administered with another vaccine, and both suspected to be linked to the AEFI.
