Health Product InfoWatch: October 2023
Download the alternative format
(325 KB, 4 pages)
Health Products and Food Branch
Marketed Health Products Directorate
Health Product InfoWatch Editorial Team
Published: 2023-10-25
Pub.: 230000
Cat.: H167-1E-PDF
ISSN: 2368-8025
Contents
- Health products mentioned in this issue
- Monthly recap of health product safety information
- New health product safety information
- Scope
- Reporting Adverse Reactions
- Helpful links
- Suggestions?
Health products mentioned in this issue
Pharmaceuticals and biologics
- Allergenic Extract Non Pollens
- Atropine Sulfate Injection USP
- Fosamax (alendronate sodium)
- Fosavance (alendronate sodium and cholecalciferol)
- Levothyroxine sodium products
Medical devices
Omnipod Insulin Management System
Natural and non-prescription health products
Other
Monthly recap of health product safety information
The following is a list of health product advisories, type I drug recalls and summaries of completed safety reviews published in September 2023 by Health Canada.
Allergenic Extract Non Pollens
Certain lots of Allergenic Extract Non Pollens (Pecan Nut) were recalled as the affected lots may result in a false negative for skin test (pecan nut allergy).
- Type 1 drug recall: Allergenic Extract Non Pollens, 2023-09-07
- Type 1 drug recall: Allergenic Extract Non Pollens, 2023-09-29
Atropine Sulfate Injection USP
One lot of Atropine Sulfate Injection USP was recalled as the affected lot may contain the presence of glass particulate.
Unauthorized Health Products
Health Canada advised Canadians about various unauthorized health products being sold at retail locations across Canada or online that may pose serious health risks.
- Advisory: Unauthorized health products seized from a Tokyo Beauty and Healthcare store in Richmond, B.C.
- Advisory: Unauthorized sexual enhancement products
- Advisory: Unauthorized skin lightening and skin treatment products
New health product safety information
The following topics have been selected to raise awareness and encourage reporting of adverse reactions.
Safety briefs
Use of probiotics in preterm infants
In Canada, probiotic natural health products (NHPs) are authorized under the Natural Health Products Regulations. These products contain live microorganisms (single and/or multi-strain bacteria and/or yeast) and are indicated to help support intestinal/gastrointestinal health and to promote a favorable gut flora in authorized populations.Footnote 1 Probiotic NHPs are contraindicated in certain vulnerable populations, such as immunocompromised individuals, due to the risk of infection (e.g., bacteremia, fungemia and sepsis).Footnote 1,Footnote 2,Footnote 3
Market Authorization Holders (MAHs) of probiotic NHPs must meet specific quality standards (as outlined in Health Canada's Probiotics monograph) at the time of authorization. These quality standards are expected to be maintained thereafter by the MAHs or the product manufacturers. These standards are related to strain identity, microbial impurities, chemical contaminants, antibiotic resistance, virulence factor production, and toxigenic activity.Footnote 1Footnote 4 The quality standards put in place by Health Canada with respect to probiotic NHPs are specifically tailored for authorized indications and populations.
As of October 2023, Health Canada has received 5 serious (including 3 deaths) domestic adverse reaction reports involving preterm infants who were administered a probiotic NHP in hospitals for the prevention of necrotizing enterocolitis (NEC). This practice is considered off-label as probiotic NHPs are not authorized for use in preterm infants. Sepsis and bacteremia were reported in the 3 fatal cases. Upon evaluation by Health Canada, a causal association between probiotic exposure and the adverse events could not be established for the 5 reported cases.
While the off-label use of health products, including probiotic NHPs, falls within the scope of the practice of medicine, Health Canada is reminding healthcare professionals that there are currently no probiotic NHPs authorized by Health Canada for preterm infants nor for the prevention of NEC. The safety and efficacy of probiotics for this subpopulation and indication have not been substantiated through evidence submitted to and reviewed by Health Canada. The Department is providing this information to help healthcare professionals weigh the risks and benefits when deciding on appropriate treatments for their patients.
Healthcare professionals are encouraged to report to the Canada Vigilance Program any adverse reactions suspected of being associated with the use of probiotics including, but not limited to, adverse reactions associated with off-label use in preterm infants.
Health Canada will continue to monitor the safety of probiotics, as it does for all health products on the Canadian market, to identify and assess potential harms. Health Canada will take appropriate and timely action should new health risks be identified.
Omnipod Insulin Management Systems and the risks of Pod detachment and needle deformation
The Omnipod and Omnipod Dash Insulin Management Systems (Omnipod) are intended for subcutaneous delivery of insulin at set and variable rates to control blood glucose levels in people with diabetes mellitus who require insulin. They are licensed in Canada as Class III medical devices.
The Omnipod contains 2 parts:
- a Pod, which is a single-use disposable device, filled with insulin and placed directly on the body for up to 72 hours (3 days) to deliver a maximum of 200 units of insulin; and
- a Personal Diabetes Manager (PDM), which is a remote controller that manages insulin delivery from the Pod.
Since Health Canada's 2018 review of the Omnipod system, the Department has continued to monitor reports of adverse events associated with the system. Through this ongoing monitoring, Health Canada has investigated new complaints related to Pod detachment or deformation of the Pod's needle during insertion into the skin.
Health Canada's investigation found that these risks are often associated with Pod misuse, such as wearing the Pod for longer than recommended or inserting the Pod in a non-recommended infusion site. Although the device labelling provides detailed instructions on Pod placement, insertion, replacement and care, including warnings and precautions to help mitigate risks, this information may be overlooked by patients. A lack of understanding of the safe and proper use of the device may result in an interruption of insulin delivery to the patient, potentially leading to adverse events, which can include hyperglycemia, diabetic ketoacidosis or diabetic coma.
Recognizing the important role that healthcare providers have in onboarding patients to new diabetes management systems, Health Canada would like to highlight certain aspects of the manufacturer's labelling to facilitate patient counselling.
The manufacturer recommends that patients:
- Select an appropriate placement site for the Pod on their body. Ideal sites for Pod placement are body areas that have a layer of fatty tissue and offer easy access and viewing, such as the abdomen, hip, back of the upper arm, upper thigh, or lower back. Patients should avoid areas where belts, waistbands, or tight clothing may rub against, disturb or dislodge the Pod.
- Change the site of Pod placement each time a new Pod is applied. The new site should be at least 2.5 cm (one inch) away from the last site.
- Follow the manufacturer's step-by-step instructions when applying and verifying the placement of the Pod.
- Check often to make sure the Pod and soft cannula are securely attached and in place. A loose or dislodged cannula may interrupt insulin delivery.
- Replace the Pod at least once every 48 to 72 hours (2 to 3 days) or after 200 units of insulin have been delivered, or more often if recommended by their healthcare provider.
Health Canada will continue to monitor the safety of the Omnipod system and will take appropriate action should new health risks be identified. Healthcare providers are encouraged to report to Health Canada any adverse events suspected of being associated with the Omnipod.
Product monograph updates
The following safety labelling updates, which were recently made to the Canadian product monographs, have been selected for your awareness. A complete list of safety labelling updates for pharmaceuticals is available on Health Canada's Product monograph brand safety updates page. Canadian product monographs can be accessed through Health Canada's Drug Product Database.
Fosamax (alendronate sodium) and Fosavance (alendronate sodium and cholecalciferol)
The Warnings and Precautions, Adverse Reactions (Post-Market Adverse Reactions) and Patient Medication Information sections of the Canadian product monographs for Fosamax and Fosavance have been updated with information about the risk of low-energy fractures observed in bones other than the femur. While atypical femoral fractures were previously reported in the product monographs, there is emerging post-market data showing fractures with atypical presentation occurring in bones other than the femur.
Key messages for healthcare professionals:Footnote 5,Footnote 6
- Low-energy fractures of the subtrochanteric and proximal femoral shaft and other bones have been reported in some long-term (time to onset in the majority of reports ranged from 18 months to 10 years) alendronate-treated patients.
- Some were stress fractures (some of which were reported as insufficiency fractures) occurring in the absence of apparent trauma or induced by mild external force.
Levothyroxine sodium products
The Warnings and Precautions, Drug Interactions, and Patient Medication Information sections of the Canadian product monographs for levothyroxine sodium productsFootnote * have been or will be updated with the risk of biotin interfering with streptavidin-based thyroid function immunoassays.
Key messages for healthcare professionals:Footnote 7,Footnote 8,Footnote 9
- Serum biotin may interfere with thyroid function immunoassays that are based on a biotin/streptavidin interaction, leading to either falsely decreased or falsely increased test results. The risk of interference increases with higher doses of biotin.
- When possible, it is recommended that patients abstain from taking biotin supplements for at least 2 days prior to specimen collection.
- When interpreting results of laboratory tests, possible biotin interference should be taken into consideration, especially if a lack of coherence with the clinical presentation is observed.
Vaccine safety summary
Health Canada and the Public Health Agency of Canada (PHAC) share the responsibility of monitoring the safety of vaccines in Canada.
Market authorization holders are required to report serious adverse events following immunization (AEFIs) to the Canada Vigilance Program in Health Canada. The Canada Vigilance Program also receives voluntary reports from healthcare professionals and consumers.
While hospitals must report serious adverse drug reactions that are documented within their facility, they do not have to report an adverse reaction to a vaccine if they have submitted an AEFI report on that case to their local public health unit. These reports are submitted by provincial and territorial public health authorities to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) in PHAC.
This vaccine safety summary includes reports of AEFIs received by the Canada Vigilance Program between January 1, 2022, and December 31, 2022. The summary excludes reports where a COVID-19 vaccine was the sole vaccine suspected to be linked to the AEFI. For information about adverse events that individuals have reported after receiving a COVID-19 vaccine in Canada, please visit the Reported side effects following COVID-19 vaccination in Canada webpage.
Summary for January 1, 2022 to December 31, 2022
Key messages:
- From January 1, 2022 to December 31, 2022, the Canada Vigilance Program received 402 reports of adverse events following immunization (AEFI) for which vaccines were the suspected cause.
- There were no significant changes in the characteristics (sex and age) of the population who experienced the reported AEFIs, nor in the types of vaccines most frequently reported compared to previous years.
- No new safety signals (potential safety issues) were identified for non-COVID vaccines during this period.
- In 2022, the Canada Vigilance Program received 402 reports of AEFI for which vaccines were the suspected cause. The majority of the reports were received from consumers through spontaneous reporting (Figure 1).
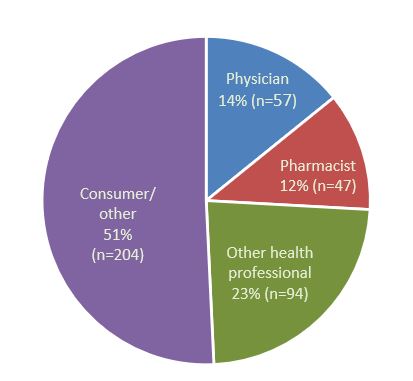
Figure 1 - Text description
| Reporter type | Percentage (%) | Number of reports (n) |
|---|---|---|
| Consumer/other non health professional | 51 | 204 |
| Other health professional | 23 | 94 |
| Pharmacist | 12 | 47 |
| Physician | 14 | 57 |
- Most of the reports involved adults over 18 years of age (189 out of 402: 47%) (Figure 2), which is similar to what has been observed since 2013 when Health Canada began publishing periodic reports of AEFIs submitted to the Canada Vigilance Program.
- The distribution by sex for the 402 reports was 59% female, 25% male and 16% unknown. This trend is similar to what has been observed previously.

Figure 2 - Text description
| Age group | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Infant (28 days to 23 months) | 0 | 13 |
| Child (2-11 years) | 1 | 8 |
| Adolescent (12-18 years) | 0 | 2 |
| Adult (19-64 years) | 24 | 72 |
| Elderly (65 years and above) | 11 | 82 |
| Unknown | 5 | 184 |
- The highest number of reports (serious and non-serious) involved herpes zoster vaccines (239 reports) followed by influenza vaccines (93 reports), and pneumococcal vaccines (41 reports) (Figure 3). This trend is similar to what has been observed previously.

Figure 3 - Text description
| Vaccine type | Number of non-serious reports | Number of serious reports | |
|---|---|---|---|
| Herpes Zoster | HZ | 20 | 219 |
| Influenza | INF | 13 | 80 |
| Pneumococcal | PNEU | 5 | 36 |
| COVID-19 | COVID | 3 | 34 |
| Meningococcal | MEN | 0 | 12 |
| Varicella | VAR | 1 | 11 |
| Human papillomavirus | HPV | 1 | 9 |
| Measles, mumps, rubella | MMR | 0 | 10 |
| Hepatitis B | HB | 2 | 7 |
| Diphtheria, tetanus, acellular pertussis, and inactivated poliomyelitis | DTaP-IPV | 0 | 7 |
| Hepatitis A | HA | 0 | 4 |
| Rotavirus | ROT | 0 | 4 |
| Typhoid | Typh | 1 | 3 |
| Diphtheria, tetanus, acellular pertussis | DTaP | 0 | 3 |
| Tetanus | T | 0 | 3 |
| Hepatitis A and Hepatitis B | HAHB | 0 | 2 |
| Tetanus, diphtheria (reduced), acellular pertussis | TdaP | 1 | 1 |
| Hepatitis (type of hepatitis vaccine was not reported) | Hep | 0 | 2 |
| Tetanus, diphtheria (reduced) | Td | 0 | 2 |
| Measles, mumps, rubella, varicella | MMRV | 0 | 2 |
| Tetanus, diphtheria (reduced), acellular pertussis and inactivated poliomyelitis | TdaP-IPV | 0 | 1 |
| Smallpox | Sma | 0 | 1 |
| Cholera and Enterotoxigenic Escherichia Coli Travellers' Diarrhea | Chol Ecol | 0 | 1 |
| Inactivated poliomyelitis | IPV | 0 | 1 |
| Yellow fever | YF | 1 | 0 |
- Of the 402 reports, 361 (90%) were serious reports, and among them,
- 219 serious reports were associated with herpes zoster vaccines. The 5 most frequently reported serious adverse events following immunization with herpes zoster vaccines included herpes zoster, vaccination failure, pain, ophthalmic herpes zoster, and pyrexia. Most of the events of herpes zoster and ophthalmic herpes zoster were co-reported with vaccination failure.
- 80 serious reports were associated with influenza vaccines. The 5 most frequently reported serious adverse events following immunization with influenza vaccines included cough, pyrexia, pneumonia, fatigue, and headache.
- Overall, the majority of serious reports were not assessable for relatedness to the vaccine given the lack of medical information regarding the recipients, vaccination status, and the event. In reports where medical information was provided, it was found that the reported adverse events were unlikely to be related to vaccination given the patients had underlying medical conditions and/or were taking concomitant medications that could have contributed to the event. In some reports, the events described were included in the product monographs of the suspected vaccines.
- In total, there were 16 reports with an outcome of death; 3 reports involved males, 7 involved females and 6 did not provide information on sex. Four cases occurred in individuals who were 65 years of age or older, 1 was reported in an individual 18 to 64 years old, 1 was reported in an infant, while 10 cases did not report age. The reported vaccines were herpes zoster vaccine (11), influenza vaccine (2), influenza vaccine co-reported with a COVID-19 vaccine (2) and measles, mumps and rubella vaccine co-reported with herpes zoster vaccine and varicella vaccine (1). The percentage of fatal cases reported in 2022 (16 out of 402: 4%) is higher than what has been reported in 2021 (2%). Although these deaths occurred after vaccination with the suspected vaccines, a causal association between the vaccine and the reported event could not be established, due to the lack of detail provided in the reports.
Conclusion
- No new safety signals (potential safety issues) were identified for vaccines during this period.
- The benefits of vaccines authorized in Canada continue to outweigh the risks.
- Health Canada, in collaboration with PHAC, will continue to closely monitor the safety of vaccines authorized in Canada.
For additional information, contact the Marketed Health Products Directorate.
Note that because of updated information received by the Canada Vigilance Program, there may be differences in the number of reports and adverse events retrieved at different dates.
Scope
This monthly publication is intended primarily for healthcare professionals and includes information on pharmaceuticals, biologics, medical devices and natural health products. It provides a summary of key health product safety information published in the previous month by Health Canada, as well as a selection of new health product safety information meant to raise awareness. New information contained in this issue is not comprehensive but rather represents a selection of clinically relevant items warranting enhanced dissemination.
Reporting Adverse Reactions
Canada Vigilance Program
Telephone: 1-866-234-2345
Fax or mail: Form available on MedEffect Canada
For more information on how to report an adverse reaction, visit the Adverse Reaction and Medical Device Problem Reporting page.
Helpful links
- MedEffectTM Canada
- Recalls and Safety Alerts Database
- New Safety and Effectiveness Reviews
- Canada Vigilance Adverse Reaction Online Database
- Drug Product Database
- Medical Devices Active Licence Listing
- Licensed Natural Health Products Database
- The Drug and Health Product Portal
- Drug Shortages Canada
- Medical device shortages: List of shortages and discontinuations
- Stop Illegal Marketing of Drugs and Devices
- List of drugs for exceptional importation and sale
- Coronavirus disease (COVID-19)
- Drug and vaccine authorizations for COVID-19: List of authorized drugs, vaccines and expanded indications
- COVID-19 vaccines and treatments portal
- Reported side effects following COVID-19 vaccination in Canada
Suggestions?
Your comments are important to us. Let us know what you think by reaching us at:
infowatch-infovigilance@hc-sc.gc.ca
Health Product InfoWatch Editorial Team
Marketed Health Products Directorate
Health Canada
Address Locator 1906C
Ottawa ON K1A 0K9
Telephone: 613-954-6522
Teletypewriter: 1-800-465-7735 (Service Canada)
Footnotes
- Footnote 1
-
Probiotics [monograph]. Ottawa (ON): Health Canada; 2023 January 27 (accessed October 12, 2023).
- Footnote 2
-
Costa RL, Moreira J, Lorenzo A, et al. Infectious complications following probiotic ingestion: a potentially underestimated problem? A systematic review of reports and case series.(url: https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-018-2394-3) BMC Complement Altern Med 2018;18:329.
- Footnote 3
-
Hojsak I, Fabiano V, Pop TL, et al. Guidance on the use of probiotics in clinical practice in children with selected clinical conditions and in specific vulnerable groups. (url: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5969308) Acta Paediatr. 2018;107(6):927–37.
- Footnote 4
-
Health Canada. Quality of Natural Health Products Guide. Version 3.1. Published May 1, 2015 (accessed October 12, 2023).
- Footnote 5
-
Fosamax (alendronate sodium) [product monograph]. Kirkland (QC): Organon Canada Inc.; 2023.
- Footnote 6
-
Fosavance (alendronate sodium and cholecalciferol) [product monograph]. Kirkland (QC): Organon Canada Inc.; 2023.
- Footnote 7
-
Eltroxin (levothyroxine sodium)[product monograph]. Oakville (ON): Aspen Pharmacare Canada Inc.; 2023.
- Footnote 8
-
Euthyrox (levothyroxine sodium) [product monograph]. Mississauga (ON): EMD Serono, A Division of EMD Inc.; 2023.
- Footnote 9
-
Synthroid (levothyroxine sodium) [product monograph]. Etobicoke (ON): BGP Pharma ULC; 2023.
