Front-of-package nutrition symbol labelling guide for industry
Version 2
On this page
- 1. Introduction
- 2. Overview of the front-of-package (FOP) nutrition symbol labelling rules
- 3. Foods prohibited from displaying the front-of-package (FOP) nutrition symbol
- 4. Nutrient thresholds for the front-of-package (FOP) nutrition symbol
- 4.1 What are the FOP nutrition symbol thresholds based on?
- 4.2 What % DV triggers the FOP nutrition symbol?
- 4.3 What quantity of a food must be used as the basis for determining whether the amount of saturated fat, sugars and/or sodium meets or exceeds the symbol threshold?
- 4.4 What % DV threshold applies to prepackaged products with a reference amount ≤ 30 g or 30 mL whose serving size is larger than the reference amount?
- 5. Foods exempt from the front-of-package (FOP) nutrition symbol requirements
- 6. Presentation of the front-of-package (FOP) nutrition symbol
- 6.1 Where on the labels of prepackaged products does the symbol have to be displayed?
- 6.2 How does the symbol have to be displayed on labels of prepackaged products?
- 6.3 What is the minimum buffer surrounding the symbol and how is it determined?
- 6.4 What is the symbol size based on?
- 6.5 How many bars have to appear in the symbol?
- 6.6 In what order do the nutrient bars have to appear in the symbol?
- 6.7 How are the different symbol formats identified in the regulations?
- 6.8 How can I obtain high resolution graphic files of all variations of the symbol?
- 7. Front-of-package (FOP) nutrition labelling of prepackaged products that contain assortments of foods, and prepackaged products that contain either ingredients that are intended to be combined together or foods that are intended to be consumed together
- 8. Nutrient and health-related claims and the front-of-package (FOP) nutrition symbol
- 9. Definitions
- Appendix 1: Steps for determining whether the amount of saturated fat, sugars and/or sodium in a prepackaged product meets or exceeds the FOP nutrition symbol thresholds
- Example A: the serving of stated size (serving size) is greater than the reference amount (food contains trans fat)
- Example B: the serving size is greater than the reference amount (food does not contain trans fat)
- Example C: the reference amount is greater than the serving size
- Example D: the food requires preparation and has a reference amount for its unprepared form
- Example E: the serving size is greater than the reference amount and the product is intended solely for children one year or older but less than four years of age
- Example F: the serving size and reference amount are equal and the food has a reference amount ≤ (less than or equal to) 30 g or 30 mL
- Appendix 2: Steps for choosing a FOP nutrition symbol format
1. Introduction
1.1 Background
On July 20, 2022, Health Canada published the Regulations Amending the Food and Drug Regulations (Nutrition Symbols, Other Labelling Provisions, Vitamin D and Hydrogenated Fats or Oils) in the Canada Gazette, Part II (CGII). These regulations amend the Food and Drug Regulations (FDR) to add a new requirement for front-of-package (FOP) nutrition symbol labelling (FOP labelling) for most prepackaged products containing nutrients of public health concern (saturated fat, sugars and/or sodium) at or above specified thresholds. The FOP nutrition symbol (the symbol) will help Canadians to more easily identify foods high in these nutrients. Avoiding excess consumption of these nutrients can help reduce associated health risks.
1.2 About this document
This guide is intended for stakeholders in the Canadian food industry. This includes Canadian manufacturers, retailers and importers of foods for sale in Canada and foreign companies who export food to Canada. The guide provides the Government of Canada's interpretation of the new regulations related to FOP labelling that came into force on July 20, 2022. It is intended to help regulated parties become familiar with core elements of the regulations. The user is encouraged to consult the regulations including Schedule K.1, the Directory of Nutrition Symbol Specifications, the Compendium of Nutrition Symbol Formats, the Table of Daily Values, the Table of Reference Amounts, and the Table of Permitted Nutrient Content Statements and Claims while using this guide.
While this guide provides interpretation of core elements of the symbol requirements, at the end of the transition period, the Canadian Food Inspection Agency's Industry Labelling Tool (ILT) will be updated with guidance on other components of the Regulations Amending the Food and Drug Regulations (Nutrition Symbols, Other Labelling Provisions, Vitamin D and Hydrogenated Fats or Oils) not covered in this guide, such as the addition of vitamin D to milks and margarine and the labelling requirements for foods containing high-intensity sweeteners.
1.3 Disclaimer
This is not a legal document. It is not intended to be used as legal advice about the requirements for FOP labelling as set out in the FDR. The user is encouraged to consult the official version of the applicable legislation and regulations in the Food and Drugs Act and FDR for the purposes of interpreting and applying the law.
The words "prepackaged product", "product" and "food" are used interchangeably throughout this document as are "principal display panel" and "label".
The images used in this guide are not to scale and are for illustrative purposes only. The nutrition symbols are not proportional to the principal display surface (PDS) of the mock-ups.
1.4 Send us your feedback
Health Canada is committed to providing all stakeholders with timely, accurate and reliable information. This includes providing information needed to comply with the requirements for FOP labelling as set out in the FDR. We would appreciate receiving your feedback on whether this guide was useful, and we welcome your suggestions for improvement. Email your feedback to nut.labelling-etiquetage@hc-sc.gc.ca and indicate in the subject line Feedback on the FOP nutrition symbol labelling guide.
2. Overview of the front-of-package (FOP) nutrition symbol labelling rules
The rules for FOP nutrition symbol labelling (FOP labelling) consist of four major parts, which are explained in this guide:
- Prohibitions from carrying the FOP nutrition symbol (the symbol)
- Nutrient thresholds for the symbol
- Exemptions from carrying the symbol
- Presentation of the symbol
2.1 When do the regulations come into force?
These regulations came into force on the date they were published in the Canada Gazette, Part II on July 20, 2022. The amendments related to FOP labelling, nutrient content claims, vitamin D fortification and high-intensity sweetener labelling are subject to a transition period that ends December 31, 2025. The transitional provisions for the different components of these amendments are independent of one another. This means that implementation of any requirement within a component (for example, applying the new high-intensity sweetener labelling requirements to prepackaged cookies that contain aspartame) during the transition period will trigger implementation of all requirements within that component but will not trigger implementation of requirements in other components (for example, FOP labelling). Regulated parties must comply with the requirements for all components of these amendments as of January 1, 2026. As of January 1, 2026, information presented in accordance with the former requirements will no longer be compliant. However, products imported, manufactured in Canada or packaged at retail before January 1, 2026 can remain in the warehouse and continue to be sold on store shelves.
2.2 Which foods are subject to the new FOP labelling rules?
The Food and Drug Regulations (FDR) state that the symbol is mandatory for most prepackaged foods sold in Canada, including those manufactured in Canada or imported for sale in Canada. When the symbol is required on a food label, it must be presented in the manner described in the FDR. These requirements are outlined in the Presentation section.
Certain foods or types of foods hold either prohibitions or exemptions from displaying the symbol. The symbol may be voluntarily displayed on labels of foods that have an exemption; however, when it is displayed, the symbol must be presented in the manner described in the FDR.
2.3 How will the regulations be enforced?
While it is Health Canada that develops regulations pertaining to FOP labelling, it is the Canadian Food Inspection Agency (CFIA) that is responsible for the enforcement of the regulations. Health Canada and the CFIA have developed an implementation plan for the transition period. This plan outlines CFIA's compliance and enforcement approach during the transition period.
2.4 Where can I submit my questions?
Health Canada and the CFIA share the responsibility for answering enquiries on the new regulations. Questions on the new requirements and their intent can be submitted to Health Canada at nut.labelling-etiquetage@hc-sc.gc.ca. For questions dealing with compliance and enforcement activities, please refer to the implementation plan on the CFIA website.
3. Foods prohibited from displaying the front-of-package (FOP) nutrition symbol
3.1 Are any foods prohibited from displaying the FOP nutrition symbol?
Yes. The following foods are prohibited from carrying the symbol on their label:
- Products intended solely for infants six months of age or older but less than one year of age
- Human milk fortifiers
- Human milk substitutes (infant formula)
- Foods represented as containing a human milk substitute
- Formulated liquid diets as defined in section B.24.001 of the Food and Drug Regulations (FDR)
- Meal replacements
- Nutritional supplements
- Foods represented for protein-restricted diets
- Foods represented for low (naming the amino acid) diets
- Foods represented for use in a very low energy diet as defined in section B.24.001 of the FDR
Reference: subsection B.01.350(15), FDR
4. Nutrient thresholds for the front-of-package (FOP) nutrition symbol
The Food and Drug Regulations (FDR) require that a nutrition symbol appear on the label (specifically, on the principal display panel (PDP)) of a prepackaged product when the amount of saturated fat, sugars and/or sodium is equal to or higher than the specified nutrient thresholds. The regulations specify the thresholds and the approach for determining whether the amount of one or more of these nutrients meets or exceeds them.
Reference: subsections B.01.350(1) to (4), FDR
4.1 What are the FOP nutrition symbol thresholds based on?
The thresholds for the FOP nutrition symbol (the symbol) are based on Daily Values (DVs). For some nutrients, such as fibre, potassium and calcium, the DV is the recommended amount that people in a specific age group should try to consume each day. For other nutrients, such as saturated fat, sugars and sodium, the DV is the amount that people should try not to exceed. The thresholds for the symbol are expressed as percentages of the DV (% DV).
The DVs are found in the Table of Daily Values, which is incorporated by reference into the FDR and available on the Government of Canada website.
4.2 What % DV triggers the FOP nutrition symbol?
For most prepackaged products, a symbol must appear on the label when the amount of saturated fat, sugars and/or sodium is ≥ (equal to or greater than) 15% of the applicable DV set out in column 2 or column 3 of Part 1 of the Table of Daily Values. The DVs in column 2 are the basis of the thresholds for foods intended solely for children one year of age or older but less than four years of age (children one to four years only). The DVs in column 3 are the basis of the thresholds for foods intended for children one to four years of age, or for children four years of age or older and adults (children and/or adults). This is the default requirement.
However, there are two exceptions:
- For prepackaged products with a reference amount ≤ (equal to or less than) 30 g or 30 mL, the symbol must appear when the amount of saturated fat, sugars and/or sodium is ≥ 10% of the applicable DV
- For prepackaged main dishes with a reference amount ≥ 170 g (when intended solely for children one to four years) or ≥ 200 g (when intended for children and/or adults), the symbol must appear when the amount of saturated fat, sugars and/or sodium is ≥ 30% of the applicable DV.
Reference: table to section B.01.350, FDR
| Saturated fat | Sugars | Sodium | |
| Prepackaged foods with a reference amount > 30 g or 30 mL that are not main dishes | ≥ 15% DV Footnote 1 | ||
| Prepackaged foods with a reference amount ≤ 30 g or 30 mL | ≥ 10% DV | ||
| Prepackaged main dishes with a reference amount ≥ 200 g or 170 g Footnote 2 | ≥ 30% DV | ||
|
|||
The Table of Daily Values includes a DV for the sum of saturated fatty acids and trans fatty acids. There is no DV for saturated fat only. The % DV threshold for the "high in sat fat" symbol is based on the DV for the sum of saturated fatty acids and trans fatty acids and the % DV must be calculated using only the saturated fat content of the food. This differs from how to calculate the % DV for saturated fat and trans fat declared in the Nutrition Facts table (NFt). The % DV declared in the NFt must be calculated using the saturated fat and trans fat content combined.
- % DV calculated for FOP labelling purposes: (saturated fat content per serving size or reference amount, whichever is greater / DV for the sum of saturated fatty acids and trans fatty acids) x 100
- % DV calculated for NFt labelling purposes: (saturated fat and trans fat content combined per serving size / DV for the sum of saturated fatty acids and trans fatty acids) x 100
4.3 What quantity of a food must be used as the basis for determining whether the amount of saturated fat, sugars and/or sodium meets or exceeds the symbol threshold?
In order to determine whether a symbol is required, the amount of saturated fat, sugars and/or sodium in a specific quantity of a food must be assessed against the applicable % DV threshold. That quantity of food is either the serving of stated size (serving size) or the reference amount for the product, whichever quantity is greater.
Reference: subsection B.01.350(2), FDR
An interim policy statement published on March 6, 2025, conveys Health Canada’s decision that it would be appropriate for the symbol assessment for certain dense, ready-to-eat breakfast cereals (like granola) to be based on the reference amount of 55 grams, the amount typically consumed in one eating occasion, rather than on the greater of the reference amount or serving size. This policy applies to ready-to-eat breakfast cereals (weighing 43 g or more per 250 mL) in item C.4 of the Table of Reference Amounts for Food that are required to declare a 1 cup serving size in the Nutrition Facts table.
There are no prescribed rounding rules for determining whether the nutrient content meets or exceeds the applicable thresholds. However, regulated parties should apply rounding rules that exist for calculating the % DV declared in the NFt.
4.4 What % DV threshold applies to prepackaged products with a reference amount ≤ 30 g or 30 mL whose serving size is larger than the reference amount?
The 10% DV threshold always applies to a product with a small reference amount (more specifically, ≤ 30 g or 30 mL), even when the serving size is larger than 30 g or 30 mL and must be used as the basis when assessing the amount of saturated fat, sugars and/or sodium to determine if a symbol is required. For example, consider a dried meat, such as beef jerky, with a reference amount of 30 g and a serving size of 45 g. Given that the reference amount is 30 g, the applicable threshold is 10% DV. Given that the serving size of 45 g is greater than the reference amount, it must be used as the basis when assessing the amount of saturated fat, sugars and/or sodium against the 10% DV threshold.
5. Foods exempt from the front-of-package (FOP) nutrition symbol requirements
5.1 Are any foods exempt?
Yes, some foods are exempt from the FOP nutrition symbol requirements. An exemption means that a symbol is not required even if the product's saturated fat, sugars and/or sodium content meets or exceeds the applicable threshold, as described in the Thresholds section.
There are two types of exemptions: full exemptions and conditional exemptions.
- Full exemption
- Conditional exemption
- Associated with the Nutrition Facts table (NFt)
- Nutrient-specific and associated with ingredients
5.2 Which foods have a full exemption?
The products in the following list are always exempt from the FOP requirement. In other words, they are always exempt from the need to assess the saturated fat, sugars and sodium content against the appropriate threshold and therefore they are never required to carry the symbol even if the nutrient content meets or exceeds the threshold. This is known as a full exemption.
- Shipping containers, if the container and its contents are not sold as a single unit to a consumer at retail
- Examples: a 4 kg box of frozen chicken strips to be served in a cafeteria; a large bag of mixed nuts to be repackaged from bulk by the retailer into smaller amounts; deli meat chubs (whole, not sliced) sold to retailers for the purpose of resale directly by a clerk at the time of sale, from bulk into consumer-sized portions; a shipping container that is destined for retailers who will remove units of fully-labelled consumer prepackaged foods from the container to display them for sale; and a box of six 1.8 kg packages of alfredo sauce to be served in a restaurant.
- However, if a shipping container and its contents are sold as a single unit to a consumer at retail the shipping container is subject to the FOP labelling requirements. This includes examples such as a box of 6 x 948 mL tetra packs of chicken broth and 24 x 300 mL bottles of apple juice in shrink wrap that are sold as single units at the retail level.
- Products with an available display surface < (less than) 15 cm2
- Examples: one-bite confections such as wrapped hard candies, caramels, mints, individual sticks of gum or balls of bubble gum
- Individual portions of food that are intended solely to be served by a restaurant or other commercial enterprise with meals or snacks
- Examples: individually portioned crackers served with soup and creamers served with a cup of coffee
- Ready-to-serve multiple-serving products intended only to be served in a commercial or industrial enterprise or an institution
- Examples: frozen, pre-cooked lasagna; gravy; cooked seasoned fish fillets; fresh pasta; pasta sauce; fruit pies; bagels; breakfast cereals; jam; sliced processed meats; and condiments and salad dressings
- Products intended only to be used as ingredients in other prepackaged products intended to be sold to consumers at retail or as ingredients in the preparation of food by a commercial or industrial enterprise or an institution
- Examples: unbaked lasagna noodles; raw seasoned fish fillets; dried pasta noodles; frozen fries; unbaked fruit pies; canned pie filling; instant potato flakes; dried soup mix; and corn starch
- Whole, partly skimmed and skimmed cow or goat's milk sold in refillable glass containers, flavoured whole, partly skimmed and skimmed cow's milk sold in refillable containers as well as cream sold in refillable glass containers
- Sweetening agents, including those listed in Division 18 of the Food and Drug Regulations (FDR) and sold as such
- Examples: sugar; agave syrup; corn syrup; maple syrup, table syrup; honey; and molasses
- Salt and seasoning salt that includes "salt" in its common name and sold as such
- Examples: table salt; celery salt; garlic salt; and onion salt
- Fats and oils referred to in Division 9 of the FDR, fish and other marine fats and oils, butter, ghee, margarine and other similar substitutes for butter and sold as such
- Examples: brick of butter; tub of margarine; bottle of coconut oil; and bottle of canola oil
- Individual rations intended for use by military personnel engaged in operations or exercises
Reference: subsection B.01.350(5), FDR
An interim policy statement published on March 6, 2025, conveys Health Canada’s determination that prepackaged fresh single ingredient coconut should not be required to carry a nutrition symbol.
5.3 Which foods have a conditional exemption?
Some products have a conditional exemption from the nutrition symbol requirements. In other words, specific conditions trigger the need to assess the saturated fat, sugars and/or sodium content of the product against the appropriate threshold to determine whether a symbol is required. Losing a conditional exemption does not necessarily mean a product will display the nutrition symbol. The symbol is required on the label only if the content of one or more of these nutrients meets or exceeds the threshold.
For some products, the conditional exemption is based on their conditional exemption from the NFt requirement and for other products it is nutrient-specific based on their ingredients.
5.3.1 Conditional exemption associated with the NFt
The following prepackaged products are conditionally exempt from the symbol requirements when they are also conditionally exempt from carrying an NFt:
- Beverages with an alcohol content > (greater than) 0.5%
- Raw single ingredient meat, meat by-products, poultry meat or poultry meat by-products that are not ground
- Raw single ingredient meat, meat by-products, poultry meat or poultry meat by-products that are ground are always required to carry the NFt, however, they are conditionally exempt from the FOP requirement
- Raw single ingredient fish or seafood products
- Products sold only in the retail establishment where they are prepared and processed from their ingredients, including from a pre-mix to which an ingredient other than water is added during preparation or processing
- Products sold only at road-side stands, craft shows, flea markets, fairs, farmers' markets or sugar bushes by the individual who prepared or processed the products
- Individual servings of products sold for immediate consumption and that have not been subjected to a process to extend their durable life, including special packaging
- Products sold only in the retail establishment where they are packaged, if they are labelled with a sticker and have an available display surface < 200 cm2
- Products that have an available display surface < 100 cm2
Reference: subsection B.01.350(13), FDR
Loss of conditional exemption: As stated above, except for ground meat, meat by-products, poultry meat or poultry meat by-products, the products lose their exemption from the symbol requirements if they lose their exemption from the NFt requirement. The products lose their exemption from carrying an NFt if any of the triggers listed in subsection B.01.401(3) or section B.01.467 of the FDR are present. Ground meat, meat by-products, poultry meat or poultry meat by-products lose their exemption from the FOP requirement if any of the triggers listed in paragraphs B.01.401(3)(a), (b) or (e) are present. For more information, refer to Foods usually exempt from carrying a Nutrition Facts table on the Canadian Food Inspection Agency's (CFIA) Industry Labelling tool.
However, if regulated parties choose to voluntarily display an NFt on one of these otherwise exempt products, the product still maintains its conditional exemption from the symbol requirements.
Reference: subsection B.01.350(13.01), FDR
5.3.2 Nutrient-specific conditional exemption associated with ingredients
For the types of prepackaged products exempt under subsections B.01.350(6) to (12), the conditional nature of the nutrient-specific exemption means that naturally occurring saturated fat, sugars and/or sodium does not trigger the need to assess whether the level of these nutrients meets or exceeds the threshold for the symbol.
5.3.2.1 Foods with health protection benefits
The types of prepackaged products in the following list are conditionally exempt from the symbol requirements, whether standardized or unstandardized, unless otherwise stated:
- Whole or cut fresh, frozen, canned or dried fruits or vegetables
- Examples of eligible products: chopped, diced, grated, riced and shredded forms of fruits and vegetables
- Examples of products not eligible: juices, purées, pastes and powdered forms of fruits and vegetables and all forms of coconut
- Milk from any animal, in liquid or powdered form
- Examples of eligible products: cow, sheep and goat milk
- Example of products not eligible: plant-based beverages
- Whole eggs, fresh or in liquid, frozen, or dried form, or whole egg mixes
- o Examples of eligible products: hard boiled eggs, quail, turkey or duck eggs, dried whole egg mix
- Examples of products not eligible: liquid egg whites, plant-based egg, dried yolk mix
- Nuts, seeds or their butters that contain less than 30% (<30%) of their total fat content as saturated fat
- Examples of eligible products: almonds, cashews, sunflower seed butter
- Examples of products not eligible: hazelnut spread, coconut
- Vegetable or marine oils that contain < 30% of their total fat content as saturated fat
- Examples of eligible products: olive oil, canola oil, sunflower oil
- Examples of products not eligible: animal fats and oils, coconut oil, palm oil
- Marine or fresh water animal products referred to in Division 21 of the FDR that contain < 30% of their total fat content as saturated fat
- Examples of eligible products: salmon, trout, sardines, shellfish
- Examples of products not eligible: none identified
- Any combination of products above
- Examples of eligible products: sardines packed in olive oil, trail mix of unsalted roasted nuts and unsweetened dried fruit, mixed cut fruit
This conditional exemption applies to these foods when they are packaged and sold as such and may also apply when these foods are included in prepackaged products that contain an assortment of foods, or prepackaged products that contain either ingredients that are intended to be combined together or foods that are intended to be consumed together. Section 7 provides more information on the application of this exemption to such products.
Reference: subsections B.01.350(6) and (10), FDR
Loss of conditional exemption: The prepackaged products lose their conditional exemption when they contain an ingredient that has saturated fat, sugars or sodium other than ingredients set out in subsections B.01.350(7) and (8) and shown in the lists below.
Ingredients that will not trigger the loss of the exemption in foods with health protection benefits
In relation to saturated fat and sodium, the following ingredients will not trigger a loss of the exemption when no saturated fat or sodium has been added to them:
- whole or cut fresh, frozen, canned or dried vegetables and fruits (other than coconut)
- milk from any animal, in liquid or powdered form, whether standardized or unstandardized
- whole eggs, fresh or in liquid, frozen, or dried form, or whole egg mixes, whether standardized or unstandardized
- nuts, seeds or their butters that contain < 30% of their total fat content as saturated fat
- vegetable or marine oils that contain < 30% of their total fat content as saturated fat
- marine or fresh water animal products referred to in Division 21 of the FDR that contain < 30% of their total fat content as saturated fat
Reference: subsections B.01.350(7) and (10), FDR
In relation to sugars, the following ingredients will not trigger a loss of the exemption when no sugars have been added to them:
- whole or cut fresh, frozen, canned or dried vegetables and fruits (other than coconut)
- milk from any animal, in liquid or powdered form, whether standardized or unstandardized
- dairy products such as cheese and yogurt
- nuts, seeds or their butters that contain < 30% of their total fat content as saturated fat
- grains such as barley, oats and quinoa
- legumes such as lentils and soybeans
Reference: subsection B.01.350(8), FDR
When the exemption is lost the total amount of the nutrient of concern in the products (from all ingredients) must be assessed to determine whether it meets or exceeds the threshold for that nutrient.
This conditional exemption is nutrient-specific. This means that a product can lose the exemption for any or all nutrients of concern.
The nutrient-specific aspect of the exemption works as follows:
Consider a can of artichoke hearts with the following list of ingredients:
Ingredients: Artichoke hearts, Water, Salt, Citric acid, Ascorbic acid.
Artichoke hearts are eligible for the conditional exemption under paragraph B.01.350(6)(a) whole or cut fruits or vegetables, including frozen, canned or dried fruits or vegetables.
The presence of a sodium-containing ingredient that is not provided for in subsection B.01.350(7), such as salt in this example, triggers the loss of the exemption for sodium. Therefore, the product's total sodium content needs to be assessed against the threshold. Total sodium content includes the sodium from all ingredients including any naturally occurring sodium in the artichoke hearts.
If the total sodium content meets or exceeds the threshold, the product must carry a symbol indicating that it is "high in sodium".
However, the presence of such a sodium-containing ingredient does not trigger the need to assess the total saturated fat or total sugars content against the applicable thresholds.
5.3.2.2 Foods that are important sources of calcium, a shortfall nutrient that is not readily available in other foods
The prepackaged products in this list are conditionally exempt from the symbol requirements for saturated fat and sugars:
- Cheese that is made from dairy products, whether standardized or unstandardized
- Yogurt, including drinkable yogurt, that is made from dairy products
- Kefir
- Buttermilk
This conditional exemption applies to these foods when they are packaged and sold as such and may apply when these foods are included in prepackaged products that contain an assortment of foods, or prepackaged products that contain either ingredients that are intended to be combined together or foods that are intended to be consumed together. Section 7 provides more information on the application of this exemption to such products.
To benefit from the exemption, these products must contain: ≥ 10% DV calcium per serving or reference amount, whichever is greater, for products with a reference amount of 30 g or 30 mL or less and ≥ 15% DV calcium per serving size or reference amount, whichever is greater, for products with a larger reference amount.
Reference: subsections B.01.350(9) and (12), FDR
A marketing authorization published on June 5, 2024, extends the eligibility for this exemption to products that contain ≥ 5% DV calcium per serving or reference amount, whichever is greater, regardless of the product’s reference amount.
Loss of conditional exemption: The prepackaged products lose their conditional exemption when they contain an ingredient that has saturated fat or sugars other than ingredients set out in subsection B.01.350(9), shown in the lists below.
Ingredients that will not trigger the loss of the exemption in cheese and yogurt, including drinkable yogurt, made from dairy products, kefir and buttermilk
In the case of saturated fat, the following ingredients will not trigger a loss of the exemption:
- milk ingredients
- modified milk ingredients
- nuts or seeds that contain < 30% of their total fat content as saturated fat
- vegetable or marine oils that contain < 30% of their total fat content as saturated fat
- marine or fresh water animal products referred to in Division 21 of the FDR that contain < 30% of their total fat content as saturated fat
Reference: paragraph B.01.350(9)(a), FDR
In the case of sugars, the following ingredients will not trigger a loss of the exemption when no sugars have been added to them:
- whole or cut fresh, frozen, canned or dried vegetables and fruits
- dairy products such as milk and cream
- grains such as barley, oats and quinoa
- legumes such as lentils and soybeans
- nuts or seeds
Reference: paragraph B.01.350(9)(b), FDR
When the exemption is lost the total amount of the nutrient of concern in the products (from all ingredients) must be assessed to determine whether it meets or exceeds the threshold for that nutrient.
As with foods that have a health protection benefit (see Section 5.3.2.1), this conditional exemption is nutrient specific. This means that a product can lose the exemption for saturated fat or sugars or both.
There is no conditional exemption from sodium for yogurt, kefir and buttermilk. Cheese that meets the calcium threshold, however, is always exempt from the requirement to assess sodium content against the threshold and will not have to display a "high in sodium" symbol.
Reference: subsection B.01.350(11), FDR
The nutrient-specific aspect of the exemption works as follows:
Consider a yogurt with the following list of ingredients:
Ingredients: Yogurt (skim milk, cream, bacterial culture) ● Milk chocolate chips (sugar, cocoa butter, unsweetened chocolate, milk ingredients, soy lecithin, vanilla extract) ● Shredded coconut preparation (sugar, water, shredded coconut, rice starch, natural flavour, pectin, citric acid).
Contains: Milk, Soy
The presence of one or more ingredients that contain sugars that is not provided for in paragraph B.01.350(9)(b), such as the milk chocolate chips and the shredded coconut preparation, triggers the loss of the exemption for sugars. Therefore, the total sugars content of the product needs to be assessed against the threshold. The presence of one or more ingredients that contain saturated fat that is not provided for in paragraph B.01.350(9)(a), such as the milk chocolate chips and shredded coconut preparation, triggers the loss of the exemption for saturated fat and therefore, the total saturated fat content of the product needs to be assessed against the threshold. Total nutrient content includes the amount in all ingredients including any naturally occurring sugars and saturated fat in the yogurt.
If the total sugars content meets or exceeds the threshold, the product must carry a symbol indicating that it is "high in sugars".
Similarly, if the total saturated fat content meets or exceeds the threshold, the product must carry a symbol indicating that it is "high in sat fat".
5.4 What exemption applies to fats and oils?
A full exemption applies to fats and oils referred to in Division 9 of the FDR, fish and other marine fats and oils, butter, ghee and margarine and other similar substitutes for butter when they are packaged and sold as such. Examples of products eligible for this exemption include bricks of butter, tubs of margarine and bottles of coconut oil. The full exemption applies to animal, fish, marine and vegetable sources of fats and oils regardless of their saturated fat content (or fatty acid profile).
A conditional exemption applies to vegetable and marine fats and oils that contain < 30% of their total fat content as saturated fat when they are packaged and sold as such and when they are ingredients in foods identified in subsection B.01.350(6).
Product-specific full exemptions and conditional exemptions may interact. For example, coconut oil is exempt from carrying a nutrition symbol when packaged and sold as such [B.01.350(5)(i), FDR]. Coconut oil has more than 30% of its total fat content as saturated fat. As a result, it does not benefit from the conditional exemption set out in B.01.350(6)(e) for vegetable oils that contain less than 30% of their total fat content as saturated fat. However, the FDR still fully exempts coconut oil, when it is sold as such, from the requirement to carry a nutrition symbol, because the provision for a full exemption takes precedence over that for the conditional exemption [B.01.350(14), FDR].
6. Presentation of the front-of-package (FOP) nutrition symbol
The Food and Drug Regulations (FDR) prescribe where and how the FOP nutrition symbol (the symbol) must be displayed on the label of a prepackaged product that meets or exceeds established thresholds for saturated fat, sugars and/or sodium.
a.

b.
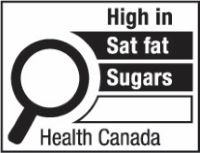
c.

d.
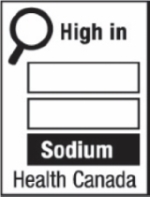
Figure 6.1 - Text description
This figure shows four front-of-package (FOP) symbol designs side by side labelled a, b, c and d.
The first symbol labelled 'a' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'b' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat and sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is white, is outlined by a thin black line and contains no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'c' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top left of the box is a black magnifying glass. To the right of the magnifying glass is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is one horizontal bar. There is a small amount of white space between the right side of the bar and the thin black line that outlines the box. The bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'd' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in sodium. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top left of the box is a black magnifying glass. To the right of the magnifying glass is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading are three bars that are stacked. There is a small amount of white space between each bar, as well as between both ends of the bars and the thin black line that outlines the box. The first and second bars are white, are outlined by a thin black line and contain no words. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
Figure 6.1 illustrates four possible symbol formats. As shown, formats vary based on the nutrient(s) declared, number of bars, language and orientation. Schedule K.1 in the FDR contains images of the 78 unique symbol formats. However, all formats include the following mandatory design elements:
- The symbol is in black and white and consists of a solid white rectangular box with a thin black line border
- There is a black magnifying glass inside the box and it is left-justified
- The heading "High in" appears in bold black letters inside the box at the top
- There is at least one bar inside the box that identifies the product as "high in sat fat", "high in sugars" and/or "high in sodium", as applicable
- The symbol is attributed to Health Canada inside the box at the bottom
- The elements that make up the symbol design do not touch each other
- The symbol is surrounded by a minimum buffer that is free of text and other graphic material
6.1 Where on the labels of prepackaged products does the symbol have to be displayed?
The symbol must be displayed on the principal display panel (PDP) as follows:
- When the height of the PDP is equal to or greater than the width of the PDP, the symbol must be displayed within the upper half (50%) of the PDP as shown in Figure 6.2. The PDP of the package is shaded in the figure.
Reference: paragraph B.01.355(1)(b), FDR
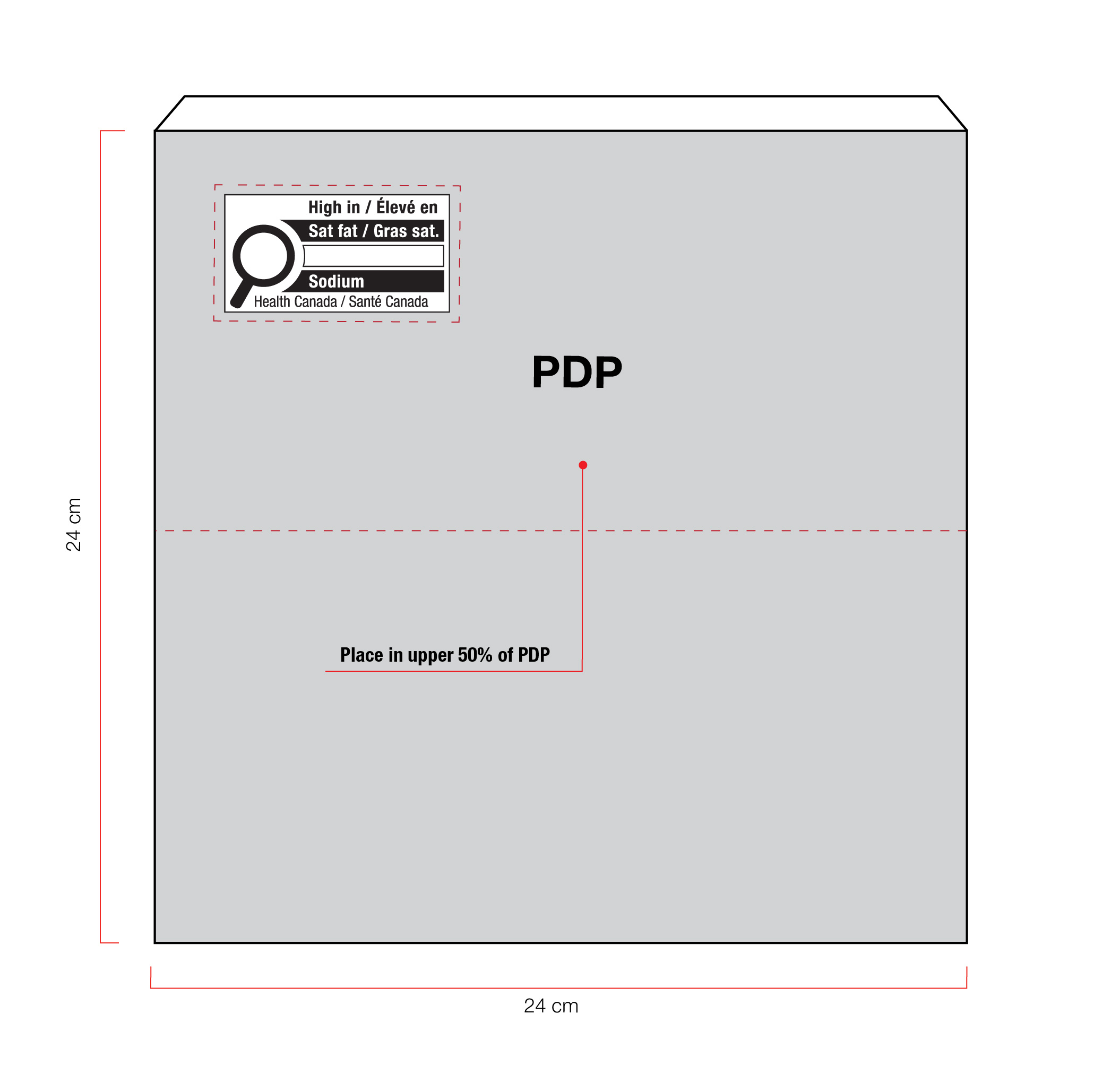
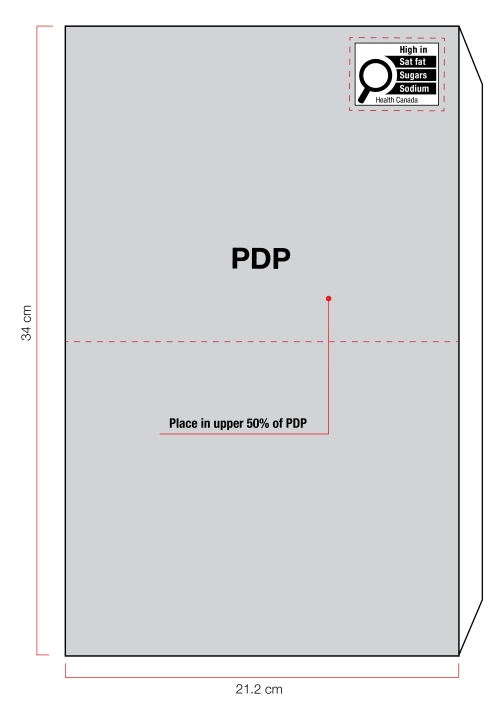
Figure 6.2 - Text description
This figure shows two boxes.
On the left of the figure is a square box. The front panel is shaded grey to identify it as the Principal Display Panel or PDP and measures 24 cm wide and 24 cm high. The PDP is divided in half horizontally by a red dashed line. The top half is identified with the statement "Place in upper 50% of PDP".
There is a horizontal nutrition symbol in the top left corner of the PDP that identifies the food as high in saturated fat and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is white, is outlined by a thin black line and contains no words. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a red dashed rectangular box around the nutrition symbol at a slight distance from it.
On the right of the figure is a rectangular box. The front panel is shaded grey to identify it as the Principal Display Panel or PDP and measures 21.2 cm wide and 34 cm high. The PDP is divided in half horizontally by a red dashed line. The top half is identified with the statement "Place in upper 50% of PDP".
There is a horizontal nutrition symbol in the top right corner of the PDP that identifies the food as high in saturated fat, sugars and sodium. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a red dashed rectangular box around the nutrition symbol at a slight distance from it.
- When the height of the PDP is less than the width of the PDP, the symbol must be displayed within the right half (50%) of the PDP as shown in Figure 6.3. The PDP of the package is shaded in the figure.
Reference: paragraph B.01.355(1)(a), FDR
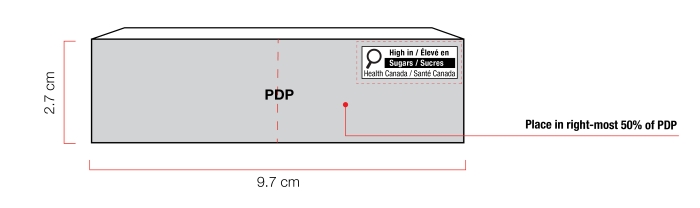
Figure 6.3 - Text description
This figure shows a rectangular box. The front panel is shaded grey to identify it as the Principal Display Panel or PDP and measures 9.7 cm wide and 2.7 cm high. The PDP is divided in half vertically by a red dashed line. The right half is identified with the statement "Place in right-most 50% of PDP".
There is a horizontal nutrition symbol in the top right corner of the PDP that identifies the food as high in sugars. This figure shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in sugars. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top left of the box is a black magnifying glass. To the right of the magnifying glass is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is one horizontal bar. There is a small amount of white space between the right side of the bar and the thin black line that outlines the box. The bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a red dashed rectangular box around the nutrition symbol at a slight distance from it.
A buffer must surround the nutrition symbol (see Section 6.3).
Reference: subsection B.01.355(2), FDR
6.1.1 Cylindrical packages
On cylindrical packages, the outer edge of the buffer surrounding the symbol must be a minimum distance of 10% of the width of the principal display surface (PDS) from the left or right edge of that surface.
Reference: subsection B.01.355(3), FDR
For example, a can has a PDS with a width of 6 cm. Ten percent (10%) of the width of the PDS is equal to 0.6 cm. Therefore, the outer edge of the buffer must be at least 0.6 cm from either the left or right edge of the PDS and the symbol must be within the upper half (50%) of the PDP (Figure 6.4).
This requirement also applies to cylindrical packages on which the symbol must be displayed in the right half (50%) of the PDP. In this case, if it is not possible to display the symbol entirely in the right half of the PDP due to the requirement to be at a minimum distance from the edge of the PDS, the symbol can appear in the left half but only to the extent necessary. This means the outer edge of the buffer is as close as possible to the 10% line as shown in Figure 6.5 so that there is minimal crossing over of the symbol into the left half of the PDP.
Reference: subsection B.01.355(4), FDR
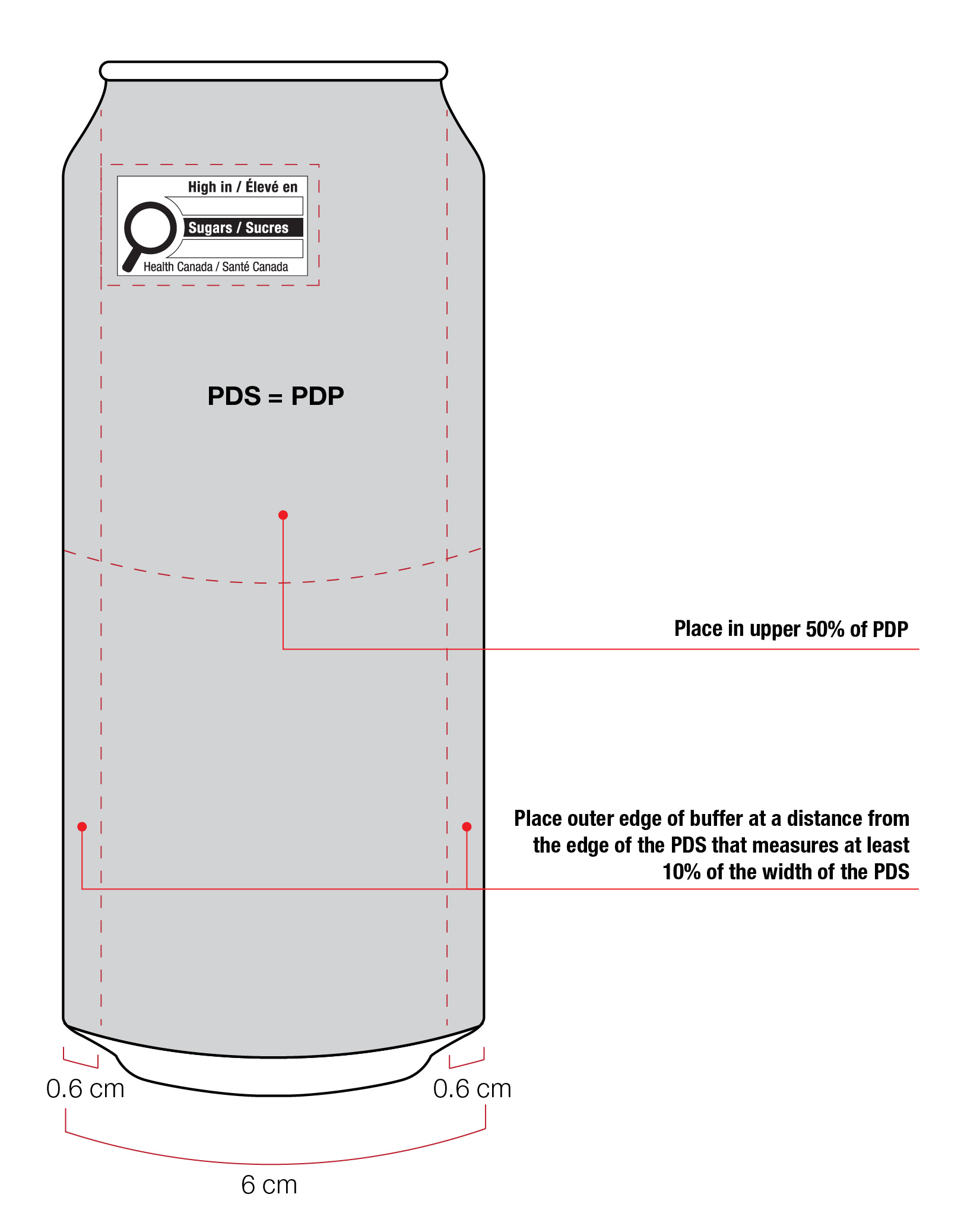
Figure 6.4 - Text description
This figure shows a can. The front surface of the can is shaded grey to identify it as the Principal Display Panel or PDP and measures 6 cm wide. The Principal Display Surface or PDS and the PDP are of equal dimensions. The PDP is divided in half horizontally by a red dashed line. The top half is identified with the statement "Place in upper 50% of PDP".
There is a horizontal nutrition symbol in the top left corner of the PDP that identifies the food as high in sugars. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a red dashed rectangular box around the nutrition symbol at a slight distance from it.
A vertical red dashed line appears on the PDP at 0.6 cm from the edge of the can, on each side. These areas are identified with the statement "Place outer edge of buffer at a distance from the edge of the PDS that measures at least 10% of the width of the PDS".
The left edge of the red dashed rectangular box around the nutrition symbol overlaps the vertical dashed line.
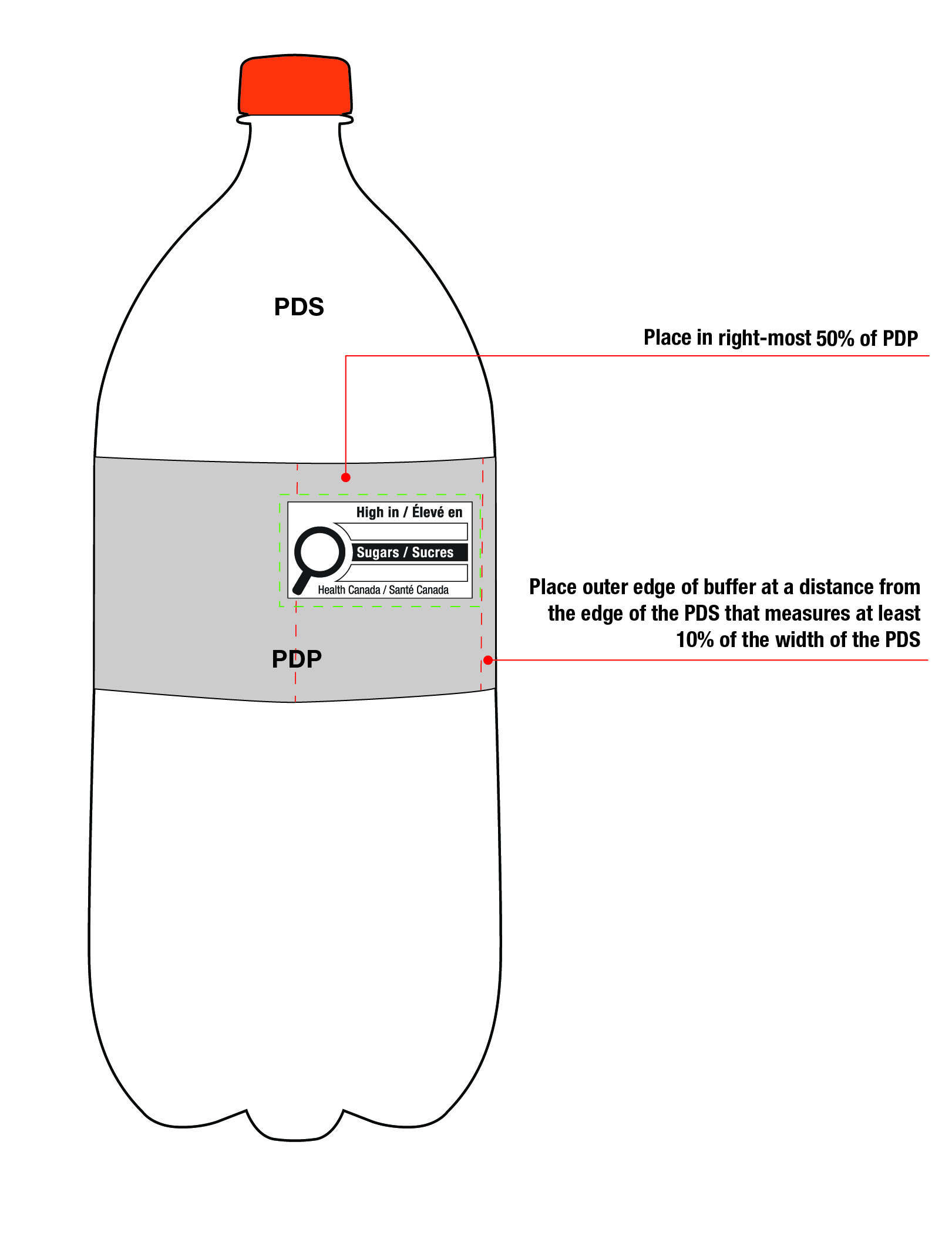
Figure 6.5 - Text description
This figure shows a white bottle with an orange cap. Towards the top of the bottle, below the cap, are the letters PDS to identify the Principal Display Surface. Towards the middle of the bottle is a label that spans the width of the bottle. The label is shaded grey to identify it as the Principal Display Panel or PDP. The PDP is divided in half vertically by a red dashed line. The right half is identified with the statement "Place in right-most 50% of PDP".
There is a horizontal nutrition symbol in the right half of the PDP, which slightly exceeds into the left half, that identifies the food as high in sugars. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a green dashed rectangular box around the nutrition symbol at a slight distance from it.
A vertical red dashed line appears on the PDP at a slight distance from the right edge of the bottle. That area is identified with the statement "Place outer edge of buffer at a distance from the edge of the PDS that measures at least 10% of the width of the PDS".
6.1.2 Irregular shapes
The upper half and right half of the PDP each refer to a proportion of the surface area of the panel. The line delineating these proportions may not correspond to the halfway point of the height measurement or width measurement of the panel, respectively. This is notably the case for products with an irregular shape PDP, as shown in Figure 6.6. The Canadian Food Inspection Agency's Industry Labelling Tool provides information on mathematical calculations for determining the area of various geometric shapes.
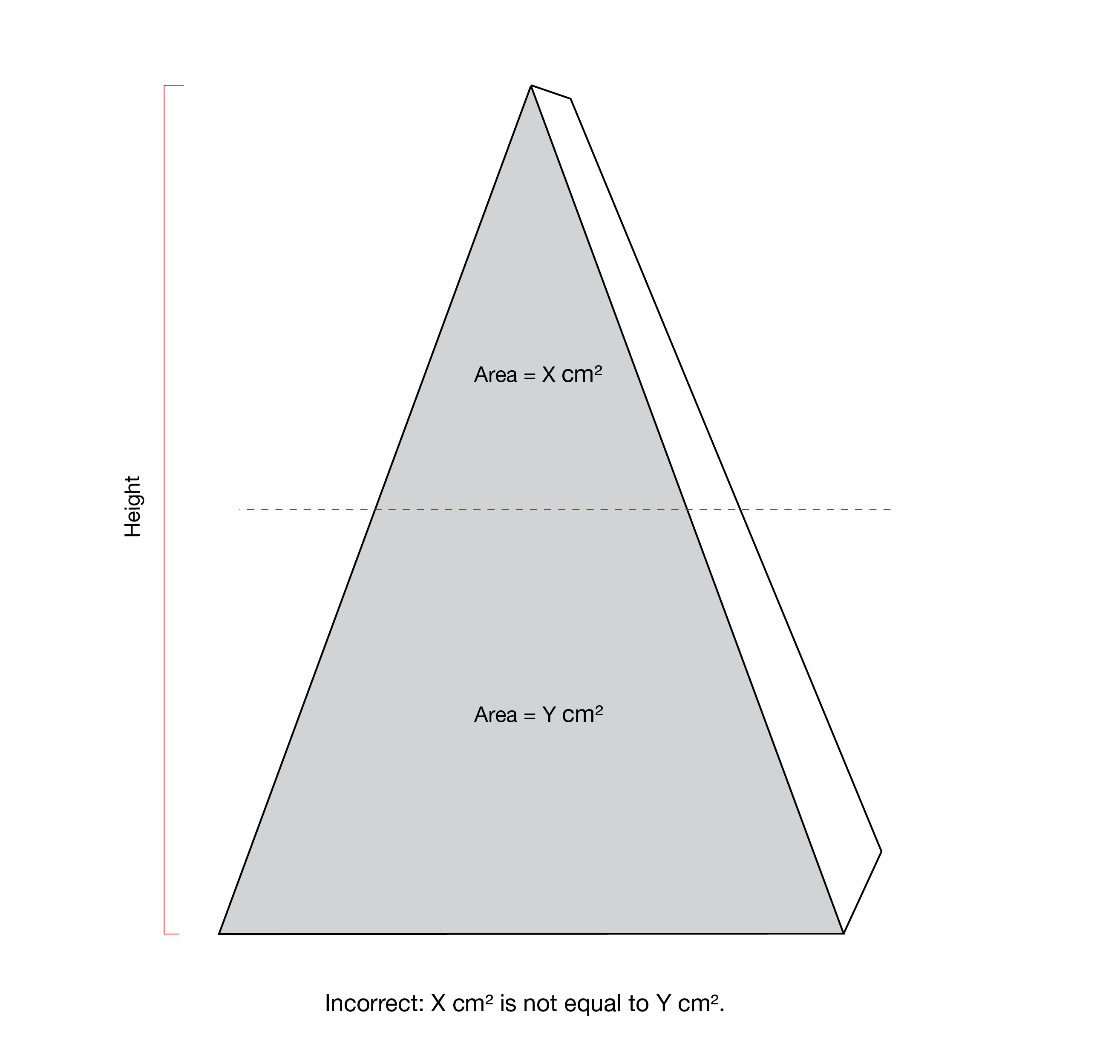
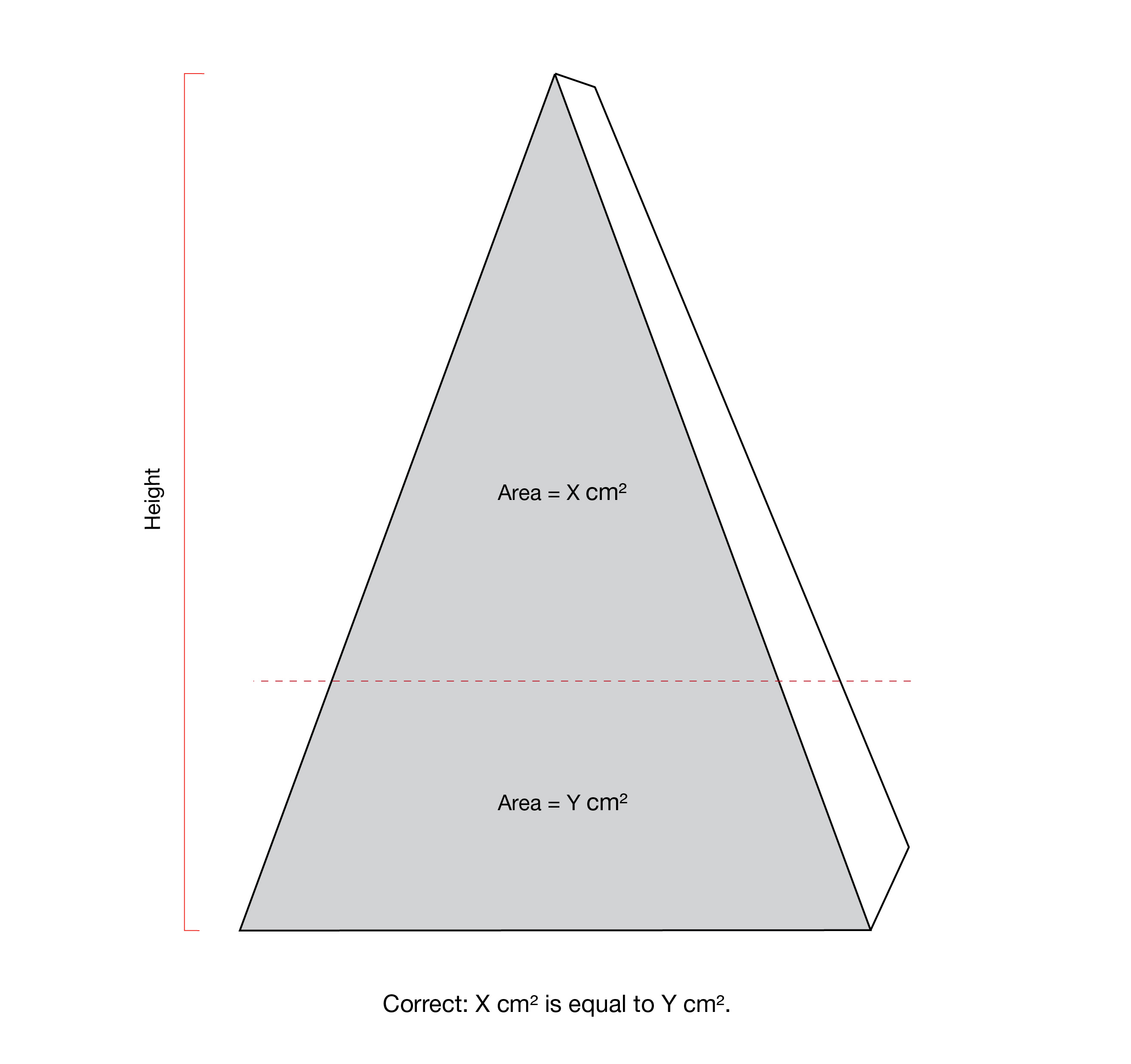
Figure 6.6 - Text description
This figure shows two triangular products.
The front panel of the triangular product on the left is shaded grey and is divided horizontally by a red dashed line. The top portion contains the statement "Area = X cm2". The bottom portion contains the statement "Area = Y cm2". To the left of the triangular product is a red vertical line indicating the height of the product. Below the triangular product is the statement "Incorrect: X cm2 is not equal to Y cm2."
The front panel of the triangular product on the right is shaded grey and is divided in half horizontally by a red dashed line. The top half contains the statement "Area = X cm2". The bottom half contains the statement "Area = Y cm2". To the left of the triangular product is a red vertical line indicating the height of the product. Below the triangular product is the statement "Correct: X cm2 is equal to Y cm2."
6.1.3 Products displayed for sale in different ways
Regulated parties are responsible to ensure that the FOP nutrition symbol appears on the PDP, which is generally on the side or surface of the container that is displayed under customary conditions of sale.
As prepackaged products may differ in the way they are displayed at the time of sale, manufacturers and importers share joint responsibility with retailers to ensure that all information is displayed on the appropriate surface at the time of sale. Mandatory information may be repeated on multiple surfaces.
6.1.4 Products with a scale label
Location of the nutrition symbol
Products with a scale label can display the symbol as a separate sticker or printed directly on the scale label. In both cases, regulated parties must first try to display the symbol in a manner that meets the requirements for symbol location as set out in B.01.355(1), FDR and described in subsection 6.1.
If it is not possible to meet the regulatory requirements for symbol location, products with a scale label may display the nutrition symbol anywhere on the scale label (Figure 6.7) or elsewhere on the PDS (Figure 6.8). This applies regardless of the establishment where they are packaged and labelled. The symbol must be visible under customary conditions of sale for the consumer. It must not cover any other mandatory information and must not be on the bottom of the container.
If placed elsewhere on the PDS, regulated parties are encouraged to display the symbol within the upper half or right half of the PDS (Figure 6.8), similar to the general location requirements described in subsection 6.1.
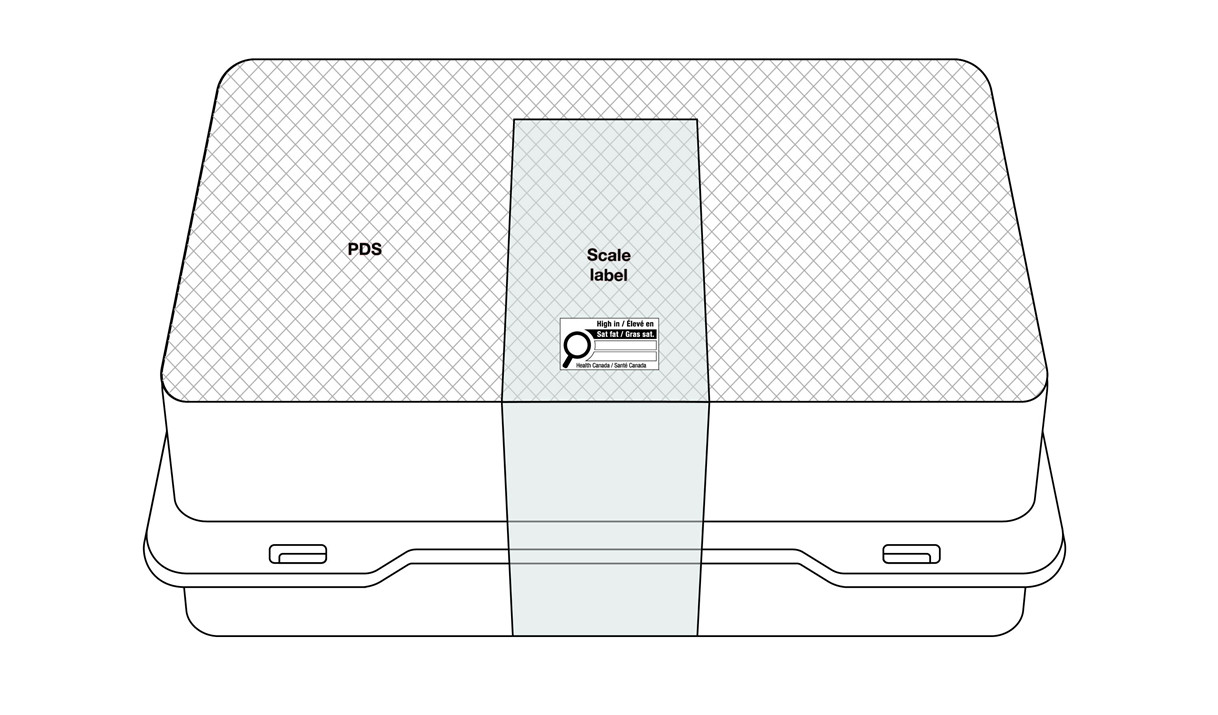
Figure 6.7 - Text description
This figure shows a rectangular clamshell container. The top of the lid is crosshatched with grey lines to identify it as the Principal Display Surface or PDS. There is a sticker scale label wrapped vertically down the middle of the container. It is shaded grey to identify it as the Scale label.
There is a horizontal nutrition symbol along the bottom of the section of the scale label that overlaps with the top of the lid. It identifies the product as high in saturated fat. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second and third bars are white, are outlined by a thin black line and contain no words. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
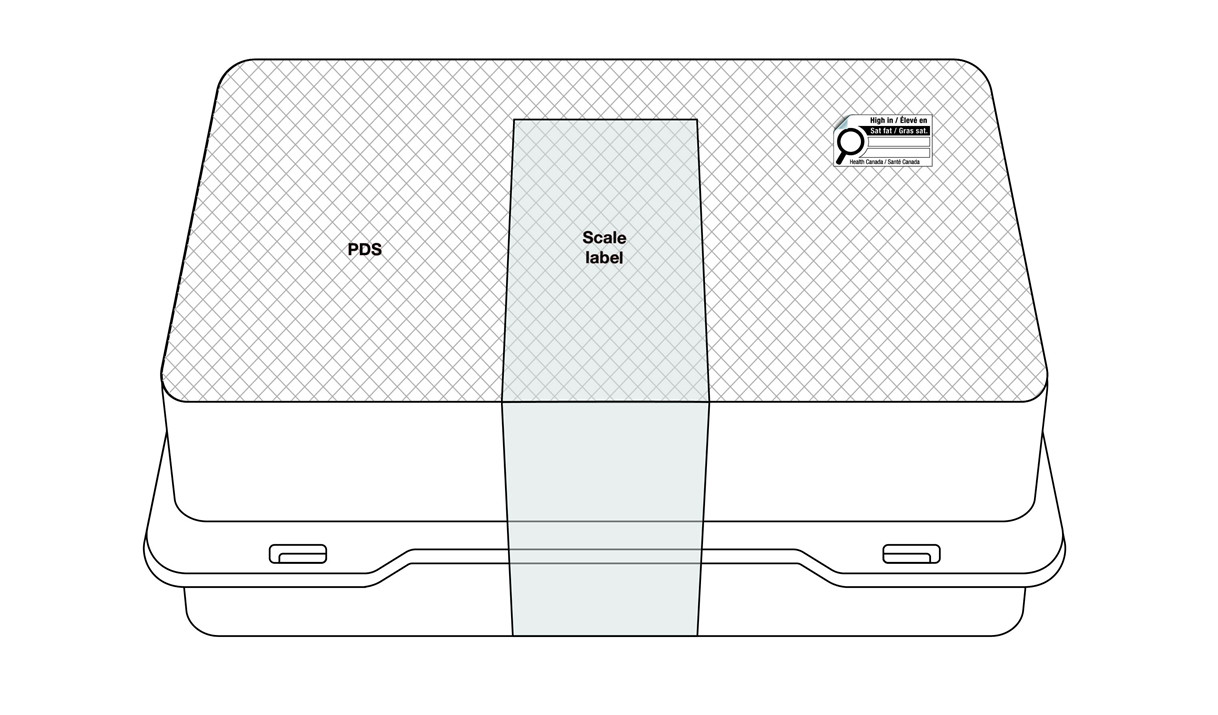
Figure 6.8 - Text description
This figure shows a rectangular clamshell container. The top of the lid is crosshatched with grey lines to identify it as the Principal Display Surface or PDS. There is a horizontal nutrition symbol in sticker format in the top right corner of the PDS that identifies the product as high in saturated fat. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second and third bars are white, are outlined by a thin black line and contain no words. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case. The top left corner of the symbol is lifted to indicate it is a sticker.
There is a sticker scale label wrapped vertically down the middle of the container. It is shaded grey to identify it as the scale label.
Symbol size
See Section 6.4 for information regarding the symbol size for products with a PDS > 250 cm2 that are sold only in the retail establishment where they are packaged and labelled by means of a sticker. This does not apply to establishments other than a retailer.
6.1.5 Ornamental and decorative containers
Ornamental container means a container that, except on the bottom, does not have any promotional or advertising material thereon, other than a trade mark or common name and that, because of any design appearing on its surface or because of its shape or texture, appears to be a decorative ornament and is sold as a decorative ornament in addition to being sold as the container of a product.
Reference: subsection B.01.001(1), FDR
Ornamental containers have the potential for an extended life, as they are reusable. Ornamental containers must be substantial enough to be sold on their own merit (without the food). They are usually made of metal (for example, cookie tins), plastic or glass (for example, candy-filled figurines).
To align with the FDR, which allow mandatory labelling information to be shown on a label that is applied to the bottom of an ornamental container, the FOP nutrition symbol can be displayed on a label that is applied to the bottom of an ornamental container. The symbol can also be displayed on a tag if one is attached to an ornamental container. No information should appear on the top of the ornamental container other than a trade mark or common name.
The container on the left in Figure 6.9 is an ornamental container. The container on the right is no longer considered an ornamental container as it has a label on the top of the container. The FOP nutrition symbol cannot be displayed on the bottom of the container on the right.


Figure 6.9 - Text description
This figure shows two blue rectangular tin containers.
The tin container on the left has a glittery lid with a gold swirl frame design and gold stars scattered throughout.
This tin container on the right has a glittery lid with gold stars scattered throughout. There is a light blue rectangular sleeve wrapped vertically around the middle of the container. On the top left of the sleeve area covering the lid is the brand name "Middleton Bakes" in brown cursive letters. On the top right is a nutrition symbol that indicates that the product is high in saturated fat. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second and third bars are white, are outlined by a thin black line and contain no words. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
Below this, towards the centre of the sleeve, are two lines of text in blue cursive letters. On the first line are the words "Butter Cookies" and on the second line are the words "Biscuits au beurre". Below this is an image of an assortment of butter cookies. Overlayed above the cookies are the words "No artificial flavours, colours or preservatives" and "Sans arômes, colorants ni agents de conservation artificiels" in white, bold, upper and lower case letters, on two blue cloud-shaped backgrounds. On the bottom of the sleeve is the net quantity of "1 kg", in blue, bold, upper and lower case letters, followed by the common name "Butter cookies", forward slash, "Biscuits au beurre" in blue upper and lower case letters.
The sleeve continues down the side of the container. Centred at the top is the brand name "Middleton Bakes" in brown cursive letters. Below this, toward the centre of the sleeve, are two lines of text in blue cursive letters. On the first line are the words "Butter Cookies" and on the second line are the words "Biscuits au beurre". Below this is an image of an assortment of stacked butter cookies.
Decorative containers, although aesthetically pleasing, are usually not reusable (as opposed to ornamental containers) because they are not sturdy enough and often get torn or damaged upon opening. Fabric-covered or embossed cardboard boxes for chocolates (for example, for Valentine's Day) are normally considered decorative containers. The symbol cannot be displayed on the bottom of a decorative container.

Figure 6.10 - Text description
This figure shows a heart-shaped box of assorted chocolates. The lid is open and placed over the left half of the box of chocolates.
The lid is beige with patterned red hearts and keys scattered throughout. There is a gold ribbon spanning diagonally from the middle left to the top right of the lid, with a gold bow in the middle. Attached to the bow of the ribbon with a white string is a white tag.
Towards the top right of the tag is a nutrition symbol that indicates the product is high in sugars and saturated fat. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box are the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, upper and lower case letters. Under this heading is a left-justified black magnifying glass with three bars horizontally stacked to its right. The left side of the three bars forms a concave curve. There is a small amount of white space between each bar, as well as between the bars and the side of the white rectangular box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, upper and lower case letters. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, upper and lower case letters. The third bar is white, outlined by a thin black line and contains no words. At the bottom of the white rectangular box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black upper and lower case letters.
In the middle of the tag are various curled pieces of chocolate shavings. Below these are the words "ASSORTED CHOCOLATES" and "CHOCOLATS ASSORTIS" on two lines in red, bold, upper case letters. At the bottom of the tag is the net quantity of "375 g" in black, lower case letters.
The base of the box is beige with a red lining. There is also a gold insert with individual sections for each piece of chocolate. Only the right half of the box of chocolates is visible. At the top is a piece of fan shaped chocolate. Below in the next row are a round piece of chocolate with white chocolate drizzle and a diamond shaped piece of chocolate. Below these in the next row are a seashell shaped piece of chocolate wrapped in gold foil and a geometric round piece of chocolate. Below these is a rounded triangular piece of chocolate with white chocolate drizzle. Below it is a piece of chocolate made up of half white and half milk chocolate. At the bottom is a domed shaped piece of chocolate.
6.2 How does the symbol have to be displayed on labels of prepackaged products?
There are requirements related to the symbol's visibility, language, orientation and graphic specifications. The nutrition symbol must be displayed in accordance with the applicable symbol set out in Schedule K.1, FDR. Regulated parties are encouraged to display the nutrition symbol in the applicable part of the PDP in such a manner that it will remain intact when the package is opened.
The nutrition symbol can be printed directly on a label or applied to it as a sticker. In the latter case, the sticker must be able to withstand any of the normal conditions that the product may be exposed to until such time the product reaches the consumer.
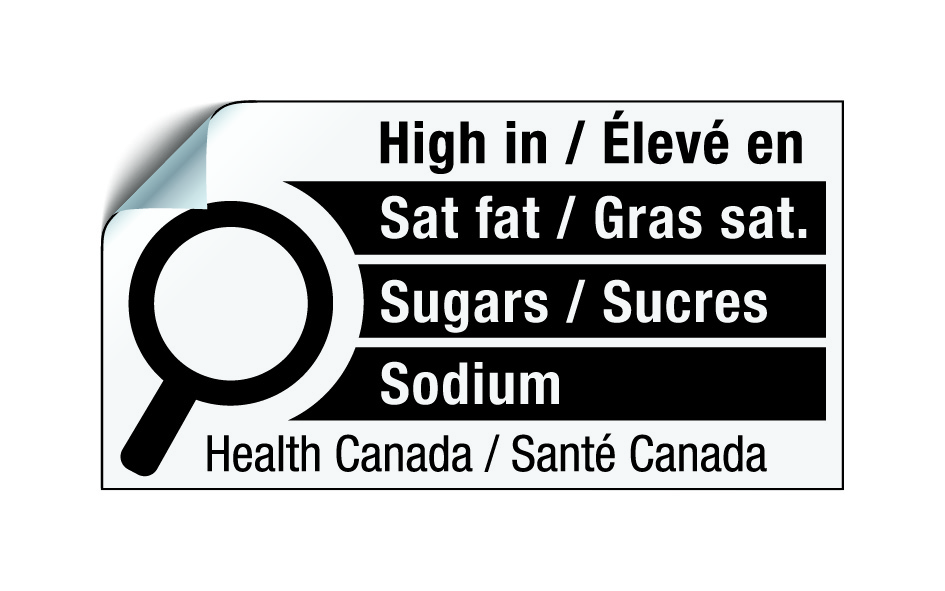
Figure 6.11 - Text description
This figure shows the nutrition symbol in sticker format for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case. The top left corner of the symbol is lifted to indicate it is a sticker.
Section 7 provides additional information about how to display the nutrition symbols on prepackaged products containing an assortment of foods when one or more of the foods requires a nutrition symbol.
Visibility
The symbol must be clearly visible and distinguishable from all other information appearing on the PDP of the product.
Reference: section A.01.016, FDR
Language
The symbol must be presented in both official languages (English and French) unless otherwise exempt from bilingual labelling. For more information, refer to Bilingual food labelling requirements – Exemptions on the Canadian Food Inspection Agency's (CFIA) Industry Labelling tool.
Product labels can carry two separate unilingual symbols, one in French and one in English, or bilingual symbol(s). In a bilingual symbol, the order of languages may be reversed from the order shown in the applicable format (in other words, English before French or French before English).
Reference: subsections B.01.351(2) to (5), FDR


Figure 6.12 - Text description
This figure shows two front-of-package symbols side by side.
The image on the left is a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The image on the right shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is bilingual, with the French text shown first, followed by the English text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "Élevé en" followed by a forward slash and the words "High in" in black, bold, lower case letters, except that the first letter of the words "Élevé" and "High" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Gras sat." followed by a forward slash and the words "Sat fat" in white, bold, lower case letters, except that the first letter of the words "Gras" and "Sat" are in upper case. The second bar is black and contains the word "Sucres" followed by a forward slash and the word "Sugars" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Santé Canada" followed by a forward slash and the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The FDR state that where there are one or more surfaces on the label of a food that are of at least the same size and prominence as the PDP, the information required by the regulations to be shown on the PDP may be shown in one official language if such information is shown in the other official language on one of those other surfaces. This means that a unilingual symbol in English can be shown on a unilingual English PDP and a unilingual symbol in French can be shown on another surface that is of at least the same size and prominence as the PDP, as illustrated in Figure 6.13
Reference: subsection B.01.012(8), FDR


Figure 6.13 - Text description
This figure shows two orange and yellow rectangular boxes of cereal with a unilingual front panel, one in English and one in French.
The English panel appears on the left of the figure. On the top right of the panel is a nutrition symbol that indicates that the product is high in sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
On the top left corner of the panel is the brand name "Mr. Timpleton" in white, upper case letters. Below this, toward the centre, is the word "Honey" with the word "Swirls" underneath, both in white, cursive text. Centred below this is an image of a bowl cereal. To the left of the bowl are two lines of text. On the first line are the words "Enlarged to show texture" and on the second line are the words "Suggested serving" in black, upper and lower case letters. On the bottom right of the panel is the net quantity of "465g" in bold, followed by the common name "Cereal" in black upper case letters.
The French panel appears on the right of the figure. On the top right of the panel is a nutrition symbol that indicates that the product is high in sugars. This symbol is in French only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading " Élevé en" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sucres" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words " Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
On the top left corner of the panel is the brand name "Mr. Timpleton" in white upper case letters. Below this, toward the centre, is the word "Tourbillons" with the words "au miel" underneath, both in white, cursive text. Centred below this is an image of a bowl cereal. To the left of the bowl are two lines of text in black, upper and lower case letters. On the first line are the words " Agrandie pour illustrer la texture" and on the second line are the words " Présentation suggérée" in black upper and lower case letters. On the bottom right of the panel is the net quantity of "465g" in bold, followed by the common name "Céréales" in black upper case letters.
Orientation
The symbol must be oriented in the same manner as most of the other information that appears on the principal display panel. However, when the panel is displayed in the vertical plane and most of the other information is not displayed parallel with the base of the package, the symbol must be oriented in such a manner that the words appearing in it are parallel with the base of the package.
Orientation also refers to either a horizontal symbol format (where its width is greater than its height) or a vertical symbol format (where its height is greater than its width).
The horizontal format is the default orientation, therefore the majority of products that need to carry the symbol will display a horizontal format.
The use of the vertical format is required when:
- the product has a PDS ≤ 450 cm2, and
- the horizontal format that would otherwise need to be used is wider than the PDP.
Reference: subsections B.01.351(2) and (3) and section B.01.356, FDR
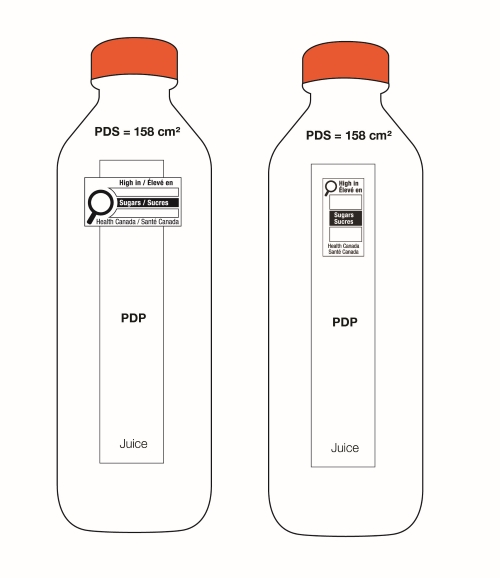
Figure 6.14 - Text description
This figure shows two white juice bottles side by side.
On the left is a white juice bottle with an orange cap. The Principal Display Surface or PDS is identified at the top centre of the bottle and represents a total surface area of 158 cm2. Centred on the bottle is a thin black line that creates the outline of a white rectangular label. The white rectangular label is identified as the Principal Display Panel or PDP.
There is a horizontal nutrition symbol at the top of the PDP that identifies the juice is high in sugars. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol exceeds the width of the PDP on both sides.
To the right is a white juice bottle with an orange cap. The Principal Display Surface or PDS is identified at the top centre of the bottle and represents a total surface area of 158 cm2. Centred on the bottle is a thin black line that creates the outline of a white rectangular label. The white rectangular label is identified as the Principal Display Panel or PDP.
There is a vertical nutrition symbol at the top of the PDP that identifies the juice is high in sugars. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top left of the box is a black magnifying glass. To the right of the magnifying glass is a heading composed of the words "High in" above the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading are three bars that are stacked. There is a small amount of white space between each bar, as well as between both ends of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" above the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred at the bottom of the box are the words "Health Canada" above the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol is more narrow than the PDP.
Specifications
The term "specifications" refers to the symbol size (height and width), the minimum buffer surrounding the symbol, the height of letters in the symbol, the height of nutrient bars in the symbol and the diameter of the lens of the magnifying glass in the symbol. The specifications for each format are set out in the Directory of Nutrition Symbol Specifications (the Directory), which is incorporated by reference into the FDR and available on the Government of Canada website.
Section 7 provides additional information about how to display the nutrition symbols on prepackaged products containing an assortment of foods when one or more of the foods requires a nutrition symbol.
6.3 What is the minimum buffer surrounding the symbol and how is it determined?
The buffer zone is the space surrounding the symbol in which no other text or graphic material can appear. The regulations set out the minimum dimension of this zone for each symbol format. It is equal to the size of the "x" height of text in the symbol (except for the words Health Canada / Santé Canada). The "x" height is the height of the lowercase "x" in the OpenType version of the font Helvetica Neue LT STD (Figure 6.15).
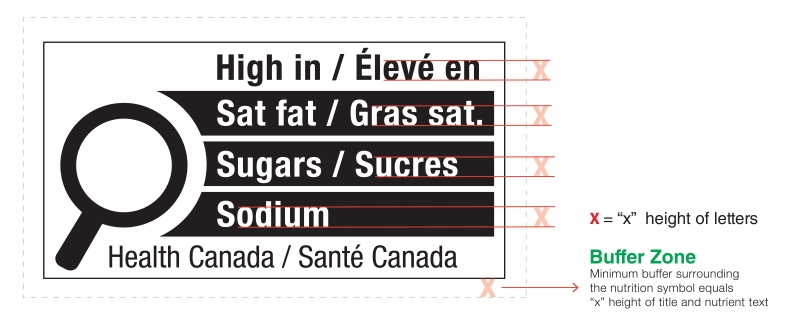
Figure 6.15 - Text description
This figure shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a green dotted rectangular box around the nutrition symbol at a slight distance from it representing the buffer zone. Additional specifications are shown around the symbol with lines pointing to the areas addressed by the specification:
- x height of lowercase letters in the title Élevé en
- x height of lowercase letters in the nutrient text Gras sat.
- x height of lowercase letters in the nutrient text Sucres
- x height of lowercase letters in the nutrient text Sodium
- x height of the buffer zone
There is an arrow pointing from the x height of the buffer zone to the text Buffer Zone and directly below is the statement "Minimum buffer surrounding the nutrition symbol equals x height of title and nutrient text".
6.4 What is the symbol size based on?
The size of the symbol is proportional to the PDS of the package. In other words, the height and width of the symbol decreases as the PDS decreases.
For each format, the symbol specifications shown in Figure 6.16 are set out in the Directory according to a hierarchy based on the PDS. This is a similar approach to the existing hierarchies based on the available display surface for the Nutrition Facts table formats.
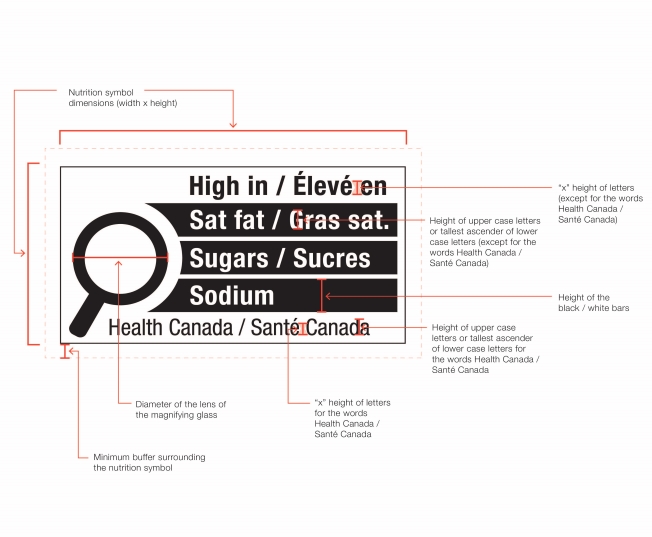
Figure 6.16(a) - Text description
This figure shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a red dotted rectangular box around the nutrition symbol at a slight distance from it.
There is text shown around the outside of the nutrition symbol with red arrows pointing to the symbol specifications. This is described below.
Starting at the top left of the nutrition symbol, there are two sets of arrows pointing to square brackets representing the width and height of the nutrition symbol followed by the words "Nutrition symbol dimensions" open bracket "width x height" closed bracket.
At the bottom left of the nutrition symbol, there is an arrow pointing to the space between the nutrition symbol and the red dotted rectangular box followed by the words "Minimum buffer surrounding the symbol".
Next to this, there is another arrow pointing to the magnifying glass followed by the words "Diameter of the lens of the magnifying glass".
Towards the right, at the bottom of the nutrition symbol, there is an arrow pointing to the letter x between the word "Santé" and the word "Canada" followed by open quotation mark x closed quotation mark "height of letters for the words Health Canada" forward slash "Santé Canada".
At the bottom right of the nutrition symbol, there is an arrow pointing to the letter d in the word "Canada" followed by the words "Height of upper case letters or tallest ascender of lower case letters for the words Health Canada" forward slash "Santé Canada".
Continuing upward on the right side of the nutrition symbol, there is an arrow pointing to a black bar followed by the words "Height of the black" forward slash "white bars".
Above this, there is an arrow pointing to the letter G in the word "Gras" followed by the words "Height of upper case letters or tallest ascender of lower case letters" open bracket "except for the words Health Canada" forward slash "Santé Canada" closed bracket.
At the top right of the nutrition symbol, there is an arrow pointing to the letter x between the word "Élevé" and the word "en" followed by open quotation mark "x" closed quotation mark "height of letters" open bracket "except for the words Health Canada" forward slash "Santé Canada" closed bracket.
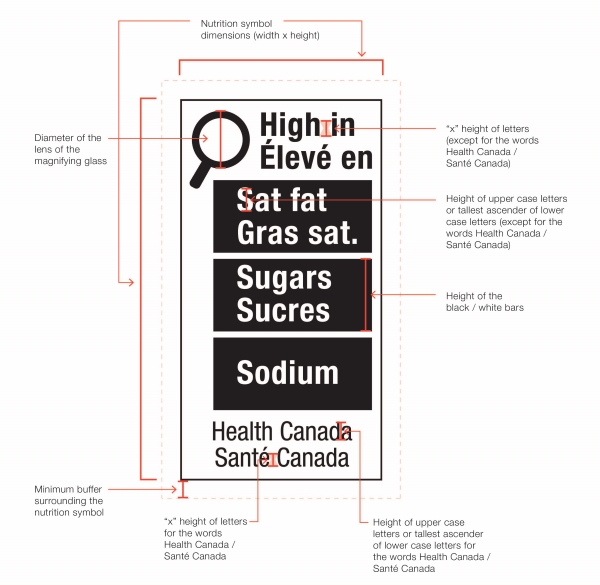
Figure 6.16(b) - Text description
This figure shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top left of the box is a black magnifying glass. To the right of the magnifying glass is a heading composed of the words "High in" above the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading are three bars that are stacked. There is a small amount of white space between each bar, as well as between both ends of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" above the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is black and contains the word "Sugars" above the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" above the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a red dotted rectangular box around the nutrition symbol at a slight distance from it.
There is text shown around the outside of the nutrition symbol with red arrows pointing to the symbol specifications. This is described below.
Starting at the top of the nutrition symbol, there are two sets of arrows pointing to square brackets representing the width and height of the nutrition symbol followed by the words "Nutrition symbol dimensions" open bracket "width x height" closed bracket.
Moving on to the top left side of the nutrition symbol, towards the center, there is an arrow pointing to the magnifying glass followed by the words "Diameter of the lens of the magnifying glass".
At the bottom left of the nutrition symbol, there is an arrow pointing to the space between the nutrition symbol and the red dotted rectangular box followed by the words "Minimum buffer surrounding the symbol".
Towards the right, at the bottom of the nutrition symbol, there is an arrow pointing to the letter x between the word "Santé" and the word "Canada" followed by open quotation mark x closed quotation mark "height of letters for the words Health Canada" forward slash "Santé Canada".
At the bottom right of the nutrition symbol, there is an arrow pointing to the letter d in the word "Canada" followed by the words "Height of upper case letters or tallest ascender of lower case letters for the words Health Canada" forward slash "Santé Canada".
Continuing upward, towards the centre, on the right side of the nutrition symbol there is an arrow pointing to a black bar followed by the words "Height of the black" forward slash "white bars".
Towards the centre, on the right side of the nutrition symbol there is an arrow pointing to the letter S in the word "Sat" followed by the words "Height of upper case letters or tallest ascender of lower case letters" open bracket "except for the words Health Canada" forward slash "Santé Canada" closed bracket.
At the top right of the nutrition symbol, there is an arrow pointing to the letter x between the word "High" and the word "in" followed by open quotation mark "x" closed quotation mark "height of letters" open bracket "except for the words Health Canada" forward slash "Santé Canada" closed bracket.
The hierarchy of symbol specifications is based on the following PDS ranges:
- > 600 cm2 (applies only to horizontal formats)
- > 450 cm2 to ≤ 600 cm2 (applies only to horizontal formats)
- > 250 cm2 to ≤ 450 cm2
- > 100 cm2 to ≤ 250 cm2
- > 30 cm2 to ≤ 100 cm2
- ≤ 30 cm2
The hierarchy of specifications (proportional to the PDS) applies in the majority of cases.
However, there is an exception for products with a PDS > 250 cm2 that are sold only in the retail establishment where they are packaged and labelled by means of a sticker. These products can carry a smaller symbol than would otherwise be required. They can carry a horizontal symbol with the specifications set out for a PDS in the range of > 100 cm2 to ≤ 250 cm2. Section 6.1.4 provides information on where the symbol can be displayed on products that have scale labels.
The symbol may be displayed with larger dimensions than those set out in Column 3 of the applicable table in the Directory if it is scaled in a proportional manner vertically and horizontally.
Reference: Directory of Nutrition Symbol Specifications and section B.01.352, FDR
6.5 How many bars have to appear in the symbol?
The number of bars that have to appear in the symbol depends on the PDS and the number of nutrients that must be declared.
PDS > 30 cm2
Three bars must always appear in the symbol on packages with a PDS > 30 cm2. This includes the bars required to identify the product as "high in sat fat", "high in sugars" and/or "high in sodium" (nutrient bars), as applicable, and blank bars. This ensures the nutrients are always in the same order.
The following are examples of formats for use on packages with a PDS > 30 cm2. Each has three bars in total; however, the number of nutrient bars and blank bars varies.
a.

b.

c.
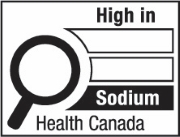
d.
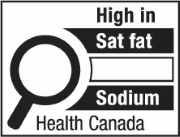
Figure 6.17 - Text description
This figure shows four front-of-package (FOP) nutrition symbols side by side labelled a, b, c and d.
The first symbol labelled 'a' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'b' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is white, is outlined by a thin black line and contains no words. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'c' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in sodium. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and second bars are white, are outlined by a thin black line and contain no words. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'd' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat and sodium. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is white, is outlined by a thin black line and contains no words. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
PDS ≤ 30 cm2
The only bars that must appear in the symbol on packages with a PDS of ≤ 30 cm2 are the bars required to identify the product as "high in sat fat", "high in sugars" and/or "high in sodium", as applicable. Blank bars are not required to be shown. Therefore, some small packages may display symbols with fewer than three bars.
The following are examples of formats for use on packages with a PDS ≤ 30 cm2.
a.

b.

c.

d.
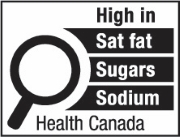
e.

Figure 6.18 - Text description
This figure shows five front-of-package (FOP) nutrition symbols side by side labelled a, b, c, d, and e.
The first symbol labelled 'a' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'b' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with two bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the two bars. The left side of the two bars forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The second bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'c' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top left of the box is a black magnifying glass. To the right of the magnifying glass is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is one horizontal bar. There is a small amount of white space between the right side of the bar and the thin black line that outlines the box. The bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'd' shows a nutrition symbol for the principal display panel that indicates that prepackaged product is high in saturated fat, sugars and sodium. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The symbol labelled 'e' shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat and sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top left of the box is a black magnifying glass. To the right of the magnifying glass is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading are two bars that are stacked. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
6.6 In what order do the nutrient bars have to appear in the symbol?
The order of the nutrient bars in the symbol is always:
- Sat fat
- Sugars
- Sodium
In symbol formats with three bars, the top bar is always and only for saturated fat, the middle bar is always and only for sugars and the bottom bar is always and only for sodium. The order doesn't change because of blank bars.
6.7 How are the different symbol formats identified in the regulations?
Each symbol format is identified by the same unique number and letter combination in the table to B.01.352, Schedule K.1 and the Directory. Numbers distinguish formats based on nutrient combination and letters indicate language and symbol orientation. The diagram in Figure 6.19 further describes this naming convention.
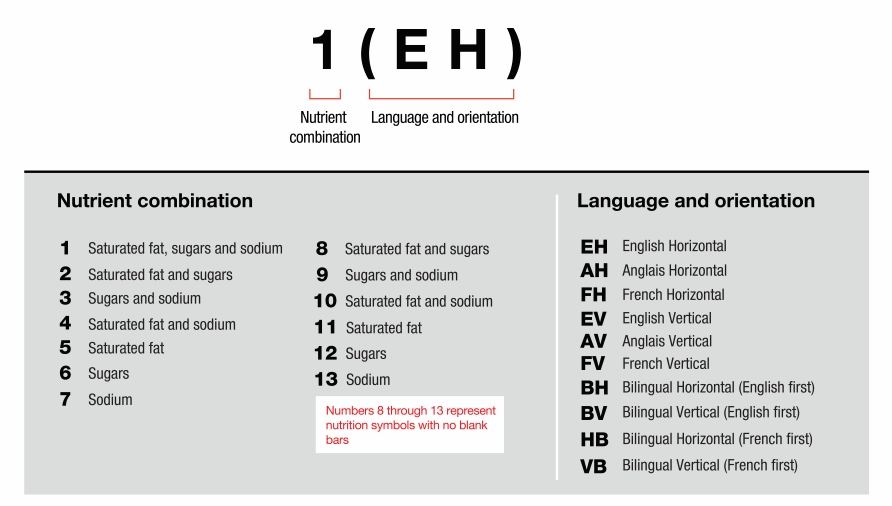
Figure 6.19 - Text description
The notation 1(EH) is centered at the top of the image in black, bold letters. A red bracket points to the number 1 indicating nutrient combination and another bracket points to the letters EH indicating language and orientation. Below this notation is a grey table of three columns. The first two columns carry the heading in black, bold letters Nutrient combination followed by a list of numbers with their respective nutrient combination: 1 Saturated fat, sugars and sodium, 2 Saturated fat and sugars, 3 Sugars and sodium, 4 Saturated fat and sodium, 5 Saturated fat, 6 Sugars, 7 Sodium. This is end of the first column. The second column continues with the list of numbers and their respective nutrient combination: 8 Saturated fat and sugars, 9 Sugars and sodium, 10 Saturated fat and sodium, 11 Saturated fat, 12 Sugars, 13 Sodium. A white box appears below 13 sodium with the following text in red: Numbers 8 through 13 represent nutrition symbols with no blank bars. The third column carries the heading in black, bold letters Language and orientation followed by a list of abbreviations and their meanings: EH English Horizontal, AH Anglais Horizontal, FH French Horizontal, EV English Vertical, AV Anglais Vertical, FV French Vertical, BH Bilingual Horizontal (English first), BV Bilingual Vertical (English first), HB Bilingual Horizontal (French first), VB Bilingual Vertical (French first). All numbers and abbreviations are in black, bold letters. All nutrient combinations, and abbreviation meanings are in black without any bolding.
6.8 How can I obtain high resolution graphic files of all variations of the symbol?
A Compendium of Nutrition Symbol Formats (the Compendium) is available in PDF as a reference document for label designers and the food and packaging industry to comply with the format specifications set out in the FDR and in the Directory. Ready-to-use high resolution graphic files of all variations of the symbol are available in .EPS format. To obtain a copy of the Compendium and the graphic files, please send an e-mail to smiu-ugdi@hc-sc.gc.ca with the subject line "HPFB BNS Compendium of Nutrition Symbol Formats".
In addition to the number and letter combination used in the naming convention in the regulations, the Compendium includes a number to distinguish the figures based on the PDS (Figure 6.20). For example, consider a product with a PDS > 600 cm2, which requires symbol format 6(BH) as per the naming convention used in the regulations. In the Compendium, this is the symbol format equivalent to Figure 1.6(BH). Appendix A in the Compendium shows how these different conventions align.
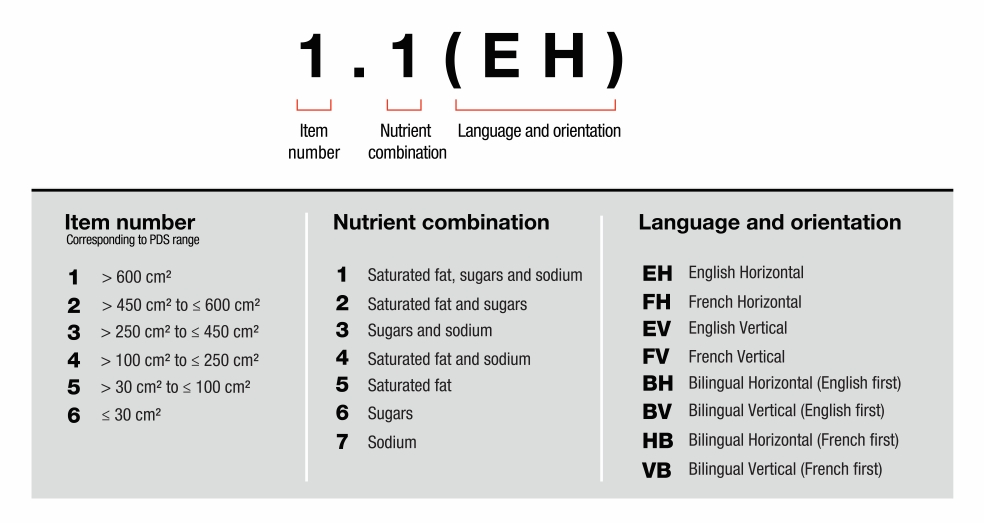
Figure 6.20 - Text description
The notation 1.1(EH) is centered at the top of the image in black, bold letters. A red bracket points to the first number 1 indicating Item number, another bracket points to the second number 1 indicating Nutrient combination and a third bracket points to the letters EH indicating Language and orientation. Below this notation is a grey table of three columns. The first column carries the heading Item number in black, bold letters and the words corresponding to PDS range directly below it. A list of numbers with their respective PDS range follows: 1 followed by > 600 cm2, 2 followed by > 450 cm2 to ≤ 600 cm2, 3 followed by > 250 cm2 to ≤ 450 cm2, 4 followed by > 100 cm2 to ≤ 250 cm2, 5 followed by > 30 cm2 to ≤ 100 cm2, 6 followed by ≤ 30 cm2. The second column carries the heading Nutrient combination followed by a list of numbers with their respective nutrient combination: 1 Saturated fat, sugars and sodium, 2 Saturated fat and sugars, 3 Sugars and sodium, 4 Saturated fat and sodium, 5 Saturated fat, 6 Sugars, 7 Sodium. The third column carries the heading Language and orientation in black, bold letters followed by a list of abbreviations and their meanings: EH English Horizontal, FH French Horizontal, EV English Vertical, FV French Vertical, BH Bilingual Horizontal (English first), BV Bilingual Vertical (English first), HB Bilingual Horizontal (French first), VB Bilingual Vertical (French first). All numbers and abbreviations are in black, bold letters. All PDS ranges, nutrient combinations, and abbreviation meanings are in black without any bolding.
7. Front-of-package (FOP) nutrition labelling of prepackaged products that contain assortments of foods, and prepackaged products that contain either ingredients that are intended to be combined together or foods that are intended to be consumed together
This section provides information on the following three topics as they relate to prepackaged products that contain an assortment of foods and prepackaged products that contain either ingredients that are intended to be combined or foods that are intended to be consumed together:
- assessment of saturated fat, sugars and sodium content against the % daily value (DV) thresholds
- conditional exemptions set out in subsections B.01.350(6) to (12)
- symbol presentation where one or more of the foods in these types of products require a nutrition symbol.
7.1 How does FOP nutrition labelling apply to prepackaged products that contain an assortment of foods?
Assessment of saturated fat, sugars and sodium content against thresholds
For prepackaged products that contain an assortment of foods, the nutrient content assessment may align with the format of the Nutrition Facts table (NFt): standard or aggregate. This flexibility means that:
- For assortments with an aggregate NFt, such as a multi-pack of granola bars, the nutrient content of each food in the assortment is assessed against the threshold individually.
- For assortments with a standard NFt with composite values, such as a box of assorted chocolates, the nutrient content of the composite can be assessed against the threshold.
- For assortments of similar types of foods, such as a multi-pack of frozen popsicles, where a serving consists of only one of the foods and where information for serving size, energy and core nutrients is the same for all individual items and must be set out in a standard NFt, the nutrient content of any one item is assessed against the threshold.
Section 4 provides information on the % DV thresholds and the approach for determining whether the amount of saturated fat, sugars and/or sodium in a prepackaged product meets or exceeds them.
Conditional exemptions
The conditional exemptions set out in subsections B.01.350(6) to (12), FDR, can be applied to prepackaged products that contain an assortment of foods.
- When assortments carry an aggregate NFt, the nutrient content of each food in the assortment is assessed against the threshold individually. As a result, each food maintains the exemption that would apply to it when packaged and sold as such.
- When assortments contain only foods set out in subsection B.01.350(6) or in subsection B.01.350(9) and are labelled with composite values in a standard NFt, the exemption applies to the product as a whole.
Section 5.3.2 provides information on the exemptions set out in subsections B.01.350(6) to (12).
Symbol presentation where one or more of the foods require a nutrition symbol
If a prepackaged product contains an assortment of foods and one or more of the foods require a nutrition symbol, the nutrition symbols must be displayed in a manner that clearly indicates the applicable nutrients that are contained in each food.
Reference: subsection B.01.353(1), FDR
Examples of acceptable symbol presentation for assortments where one or more foods requires a symbol
It is acceptable to place the name of the food, variety or flavour in the assortment that requires a symbol immediately adjacent to the buffer zone of the applicable symbol as shown in Figure 7.1. The use of an image that clearly identifies the food, variety or flavour is also acceptable (Figure 7.2). Note that text or other graphic material cannot appear in the buffer zone (B.01.355(2)(b), FDR).

Figure 7.1 - Text description
This figure shows a grey box of oat bars. The front and top panels of the box are visible.
Most of the information on the top panel is blurry given the angle of the image. The top left of this panel has 3 bowls corresponding to the 3 bar varieties. The first bowl has almonds, the second bowl has peanut butter and the third bowl has chocolate. Below the bowls is a bilingual aggregate nutrition facts table. In the centre of this panel is the list of ingredients in English and French. In the top right of this panel is a green logo with the word "WELL" in light green, upper case letters, and the word "NESS" under it in white, upper case letters. Below this is text, a barcode, and additional text.
The top left of the front panel has a bowl of almonds and sketches of 3 almonds in the background. In the bottom left corner is the net quantity of "1.68 kg" and "48 Bars" in black lower case letters, expect that the first letter of the word "Bars" is in uppercase.
In the middle of the front panel is a large green circle with wavy lines around it. Within the circle, at the top, is the brand name "arya eating" in white, lower case letters. In the middle of the circle is the word "WELL" in light green, upper case letters, and the word "NESS" under it in white, upper case letters. Below this are the words "OAT BARS" in white upper case letters, with a horizontal white line on either side. Extending from the left side of the green circle is a white rectangle with the words "Made with whole grain oats" in orange, lower case letters, except that the first letter of the first word is in upper case. Below the green circle is a bowl of chocolate. To the right of the green circle, at the top, is a bowl of peanut butter. Under it are 3 different horizontally stacked oat bars. On the right of the front panel is the name of each oat bar variety along with the applicable nutrition symbol. At the top are the words "Roasted Almond" in black, lower case letters, except that the first letter of each word is in upper case. Under it is the first nutrition symbol that indicates that this bar is high in saturated fat and sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is white, is outlined by a thin black line and contains no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
Under this symbol are the words "Peanut Butter" in black, lower case letters, except that the first letter of each word is in upper case. This is followed by the second nutrition symbol that indicates that this bar is high in saturated fat. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second and third bar are white, are outlined by a thin black line and contain no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
Under this symbol are the words "Dark Chocolate Nut" in black, lower case letters, except that the first letter of each word is in upper case. This is followed by the third nutrition symbol that indicates that this bar is high in sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a callout box to the right of the oat bar box showing a zoomed-in view of the 3 nutrition symbols and bar varieties indicated on the front panel.

Figure 7.2 - Text description
This figure shows a grey box of oat bars. The front and top panels of the box are visible.
Most of the information on the top panel is blurry given the angle of the image. The top left of this panel has 3 bowls corresponding to the 3 oat bar varieties. The first bowl has almonds, the second bowl has peanut butter and the third bowl has chocolate. Below the bowls is a bilingual aggregate nutrition facts table. In the centre of this panel is the list of ingredients in English and French. In the top right of this panel is a green logo with the word "WELL" in light green, upper case letters, and the word "NESS" under it in white, upper case letters. Below this is text, a barcode, and additional text.
The top left of the front panel has a bowl of almonds and sketches of 3 almonds in the background. On the bottom left corner of the panel is the net quantity of "1.68 kg" and "48 Bars" in black lower case letters, except that the first letter of the word bars is in upper case, expect that the first letter of the word bars is in uppercase.
In the middle of the front panel is a large green circle with wavy lines around it. Within the circle is, at the top, the brand name "arya eating" in white, lower case letters. In the middle of the circle is the word "WELL" in light green, upper case letters, and the word "NESS" under it in white, upper case letters. Below this are the words "OAT BARS" in white upper case letters, with a horizontal white line on either side. Extending from the left side of the green circle is a white rectangle with the words "Made with whole grain oats" in orange, lower case letters, except that the first letter of the first word is in upper case. Below the green circle is a bowl of chocolate. To the right of the green circle, at the top, is a bowl of peanut butter. Under it are 3 different horizontally stacked oat bars. At the bottom of the first bar are the words "ROASTED ALMOND" in black, bold, upper case letters, on a white rectangular background. At the bottom of the second bar are the words "PEANUT BUTTER" in black, bold, upper case letters, on a white rectangular background. At the bottom of the third bar are the words "DARK CHOCOLATE NUT" in black, bold, upper case letters, on a white rectangular background.
On the right of the front panel are three nutrition symbols horizontally stacked. The first nutrition symbol is beside the first bar and indicates that this variety is high in saturated fat and sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is white, is outlined by a thin black line and contains no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The second nutrition symbol is beside the second bar and indicates that this variety is high in saturated fat. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second and third bar are white, are outlined by a thin black line and contain no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The third nutrition symbol is beside the third bar and indicates that this variety is high in sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
When more than one food, variety or flavour in an assortment require the same nutrition symbol, regulated parties do not need to duplicate the symbol on the PDP. The symbol can be displayed only once if the food, variety or flavour it applies to is clearly indicated (Figure 7.3).

Figure 7.3 - Text description
This figure shows a grey box of oat bars. The front and top panels of the box are visible.
Most of the information on the top panel is blurry given the angle of the image. The top left of this panel has 3 bowls corresponding to the 3 oat bar varieties. The first bowl has almonds, the second bowl has peanut butter and the third bowl has chocolate. Below the bowls is a bilingual aggregate nutrition facts table. In the centre of this panel is the list of ingredients in English and French. In the top right of this panel is a green logo with the word "WELL" in light green, upper case letters, and the word "NESS" under it in white, upper case letters. Below this is text, a barcode, and additional text.
The top left of the front panel has a bowl of almonds and sketches of 3 almonds in the background. On the bottom left corner of the panel is the net quantity of "1.68 kg" and "48 Bars" in black lower case letters, expect that the first letter of the word bars is in uppercase.
In the middle of the front panel is a large green circle with wavy lines around it. Within the circle, at the top, is the brand name "arya eating" in white, lower case letters. In the middle of the circle is the word "WELL" in light green, upper case letters, and the word "NESS" under it in white, upper case letters. Below this are the words "OAT BARS" in white upper case letters, with a horizontal white line on either side. Below the green circle is a bowl of chocolate. Extending from the left side of the green circle is a white rectangle with the words "Made with whole grain oats" in orange, lower case letters, except that the first letter of the first word is in upper case. To the right of the green circle, at the top, is a bowl of peanut butter. Under it are 3 different horizontally stacked oat bars.
On the top right of the front panel are two nutrition symbols horizontally stacked. The first nutrition symbol indicates that two bar varieties are high in saturated fat and sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is white, is outlined by a thin black line and contains no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case. Below this symbol are two lines of text corresponding to the bar varieties associated with the symbol. On the first line are the words "Roasted Almond" and on the second line are the words "Chocolate Coconut", in black, lower case letters, except that the first letter of each word is in upper case.
Under this text is the second nutrition symbol which indicates that the third bar variety is high in saturated fat. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second and third bar are white, are outlined by a thin black line and contain no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case. Below this symbol are the words "Peanut Butter" in black, lower case letters, except that the first letter of each word is in upper case.
There is a callout box to the right of the oat bar box showing a zoomed-in view of the 2 nutrition symbols and bar varieties indicated on the front panel.
Furthermore, when all foods, varieties or flavours in an assortment require the same symbol, regulated parties must clearly indicate that the symbol applies to all items in the assortment.
Consider a multi-pack of frozen popsicles in three flavours. All three flavours have the same nutrition information and the product must carry the standard format NFt. The nutrient content of one flavour is assessed. It is determined that the sugars content exceeds the % DV threshold. The "high in sugars" symbol must be displayed in a manner that clearly indicates it applies to the three flavours (Figure 7.4). The symbol does not need to be shown three times.

Figure 7.4 - Text description
This figure shows an orange and yellow box of ice pops. On the top left corner of the panel is the brand name "ChillinPops" in white, upper and lower case, bubble letters. Bordering this text are layers of red, orange, and purple backgrounds.
In the top right corner of the front panel is a nutrition symbol that indicates that the product is high in sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case. Above this symbol are the words "All varieties" in black, lower case letters, except that the first letter of the first word is in upper case.
On the middle left of the panel are the flavours of the ice pops. There is a red rectangle with the word "BERRY" in white, upper case letters. Below this is an orange rectangle with the word "ORANGE" in white, upper case letters. Below this is a purple rectangle with the word "GRAPE" in white, upper case letters. Below this are the words "Natural and artificial flavours" in black, lower case letters, except that the first letter of the first word is in upper case.
In the middle of the panel are three stacked ice pops in red, orange and purple.
On the bottom left of the panel is the net quantity of "12 x 75 mL" in bold black, followed by the common name "Ice pops" in black lower case letters, except that the first letter of the first word is in upper case.
Any text used to meet the requirement in subsection B.01.353(1) must meet bilingual requirements, if applicable. The text must be clearly and prominently displayed on the label and readily discernible to the purchaser or consumer under the customary conditions of purchase.
References: section A.01.016 and subsection B.01.012(2), FDR
When more than one food in an assortment requires a symbol, the buffer zones can overlap in the space between the symbols as shown in Figure 7.5.
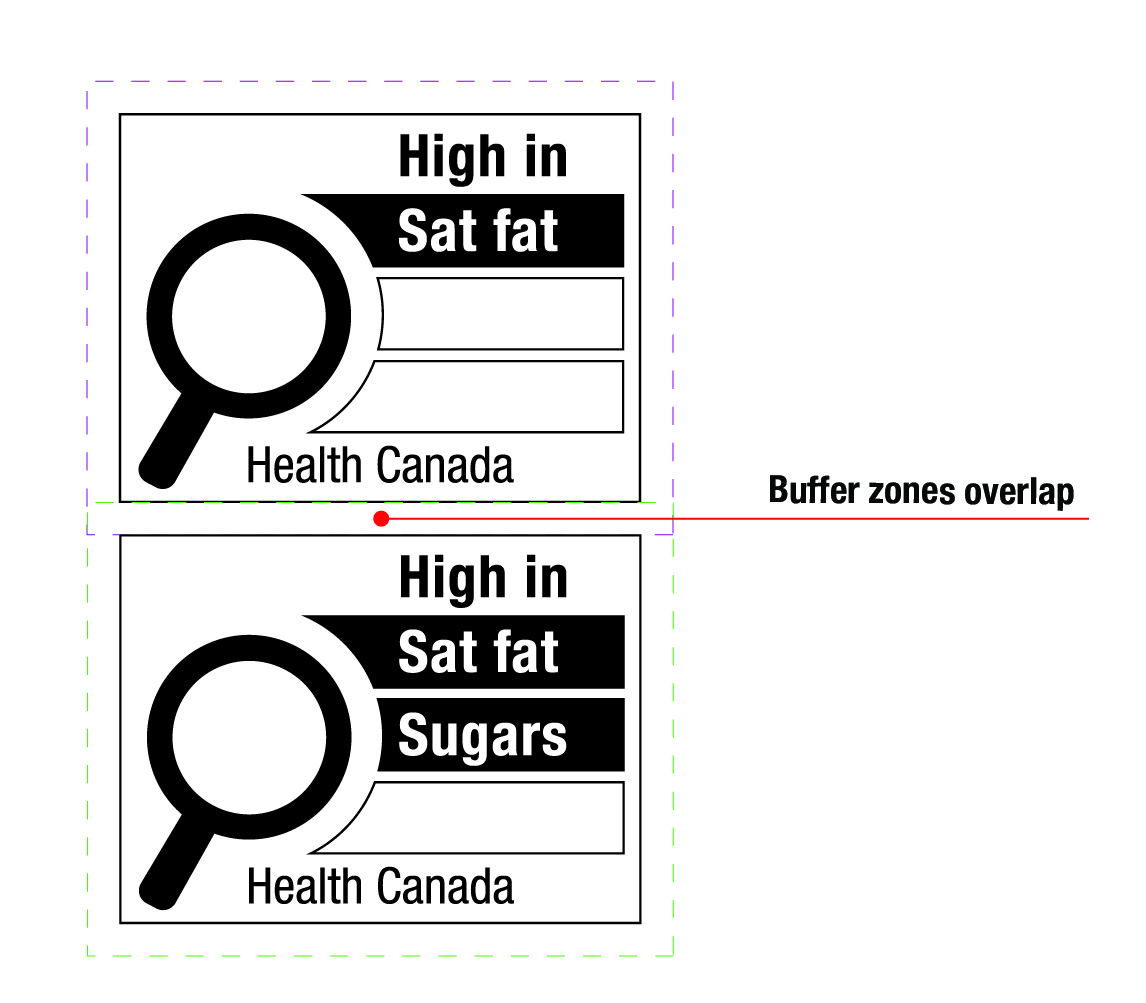
Figure 7.5 - Text description
This figure shows two nutrition symbols stacked vertically.
The first nutrition symbol for the principal display panel indicates that a prepackaged product is high in saturated fat. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second and third bar are white, are outlined by a thin black line and contain no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case. There is a purple dashed rectangular box around the nutrition symbol at a slight distance from it to indicate the buffer zone.
The second nutrition symbol for the principal display panel indicates that a prepackaged product is high in saturated fat and sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is white, is outlined by a thin black line and contains no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case. There is a green dashed rectangular box around the nutrition symbol at a slight distance from it to indicate the buffer zone.
The bottom buffer zone of the top symbol overlaps with the top buffer zone of the second symbol. This overlap area is identified with the statement "Buffer zones overlap".
Examples of unacceptable presentation for assortments where one or more foods require a nutrition symbol
- Using a legend to indicate which nutrient in a nutrition symbol applies to which food, variety or flavour (Figure 7.6)
- Associating one food, variety or flavour to more than one nutrition symbol (Figure 7.7)
- Displaying only the nutrition symbol for the food, variety or flavour with the most "high in" nutrients and referring the consumer to the NFt for information on the other foods (Figure 7.8)
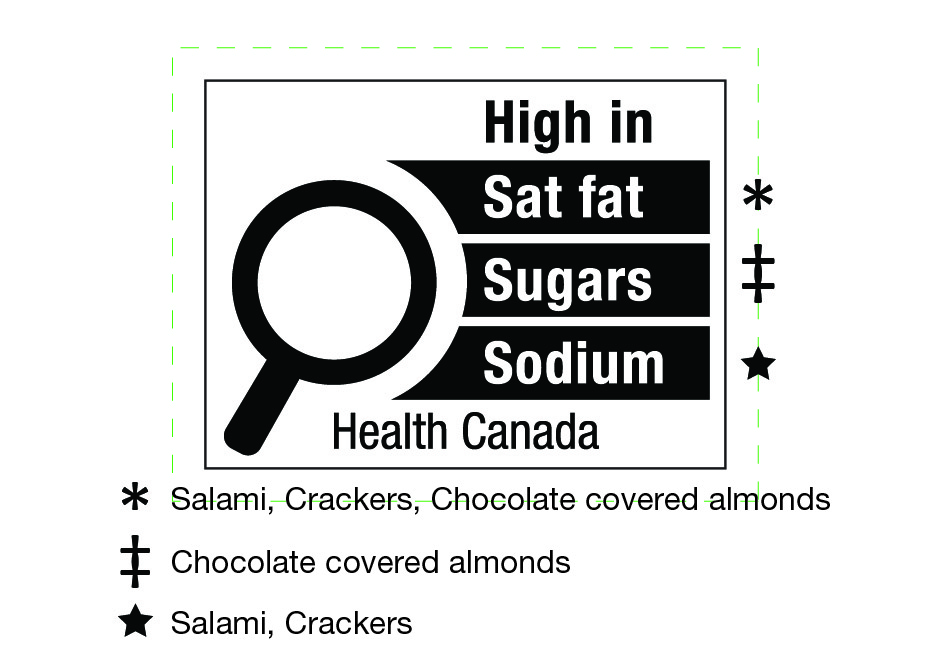
Figure 7.6 - Text description
This figure shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat, sugars and sodium. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a green dashed rectangular box around the nutrition symbol at a slight distance from it to indicate the buffer zone.
To the right of the nutrition symbol are 3 icons overlapping with the buffer zone. The first icon is an asterisk and is aligned with the saturated fat bar. The second icon is a dagger and is aligned with the sugars bar. The third icon is a star and is aligned with the sodium bar.
Below the nutrition symbol is a legend. The first line of the legend starts with the asterisk symbol, followed by the words "Salami, Crackers, Chocolate covered almonds" in black, upper and lower case letters. The second line of the legend starts with the dagger symbol, followed by the words "Chocolate covered almonds" in black, lower case letters, except that the first letter of the first word is in upper case. The third line of the legend starts with the star symbol, followed by the words "Salami, Crackers" in black, lower case letters, except that the first letter of each word is in upper case.
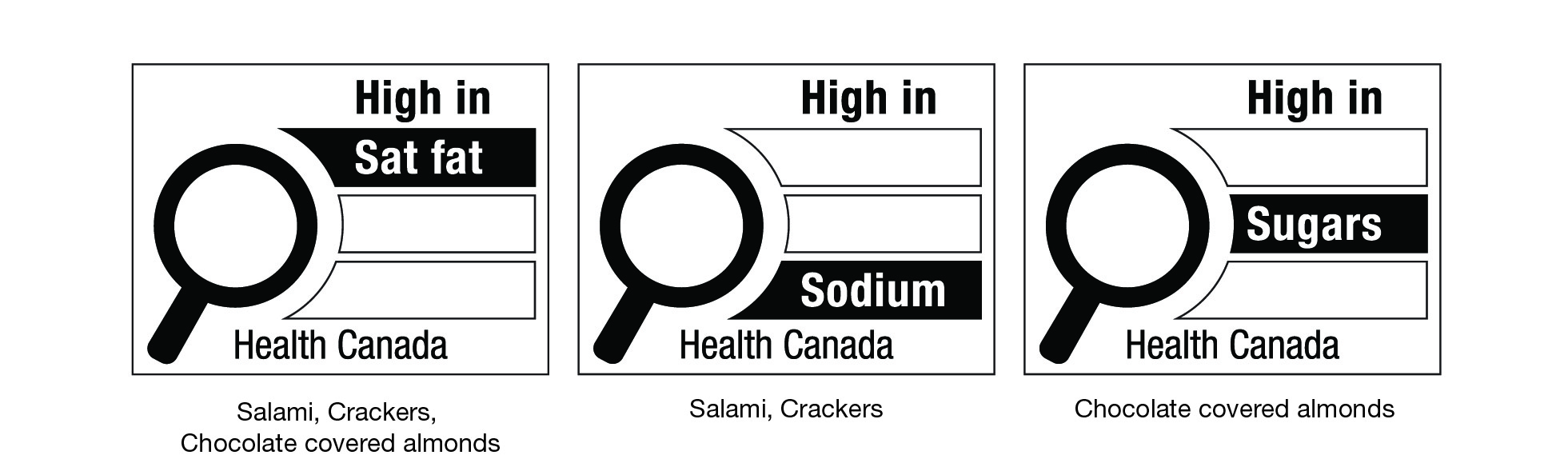
Figure 7.7 - Text description
This figure shows three nutrition symbols side by side.
The first nutrition symbol for the principal display panel indicates that a prepackaged product is high in saturated fat. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second and third bar are white, are outlined by a thin black line and contain no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case. Below this symbol are the words "Salami, Crackers, Chocolate covered almonds" on two lines, in black, upper and lower case letters.
The second nutrition symbol for the principal display panel indicates that a prepackaged product is high in sodium. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and second bars are white, are outlined by a thin black line and contain no words. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case. Below this symbol are the words "Salami, Crackers" in black, lower case letters, except that the first letter of each word is in upper case.
The third nutrition symbol for the principal display panel indicates that a prepackaged product is high in sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case. Below this symbol are the words "Chocolate covered almonds" in black, lower case letters, except that the first letter of the first word is in upper case.

Figure 7.8 - Text description
This figure shows a nutrition symbol for the principal display panel that indicates that a prepackaged product is high in saturated fat and sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is white, is outlined by a thin black line and contains no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
There is a green dashed rectangular box around the nutrition symbol at a slight distance from it to indicate the buffer zone. Above the nutrition symbol, and overlapping with the buffer zone, are the words "Chocolate covered almonds" in black, lower case letters, except that the first letter of the first word is in upper case. Below the symbol, and overlapping with the buffer zone, are the words "Consult the Nutrition Facts table for details about other foods" in black, lower case letters, except that the first letter of the first word is in upper case.
| Description of the prepackaged product | Examples | NFt format | Nutrient content assessment (saturated fat, sugars, sodium) and application of conditional exemptions set out in B.01.350(6) to (12) | Nutrition symbol presentation where one or more foods require a symbol |
|---|---|---|---|---|
Assortment of foods of the same type where:
|
Multi-pack of instant oatmeal Multi-pack of granola bars |
These products must use the aggregate NFt with information declared for each food in the assortment (B.01.406(3)(a), FDR) |
Each food in the assortment is assessed against the threshold Conditional exemptions apply to eligible foods individually |
More than one symbol could be required Symbols must be displayed in a manner that clearly indicates to which food they apply (B.01.353(1), FDR) |
Assortment of foods of the same type where:
|
Multi-pack of popsicles Multi-pack of yogurt |
These products must use the standard NFt with information declared for one of the foods (B.01.406(3)(b), FDR) | Any food in the assortment is assessed against the threshold as the nutrition information is the same for all foods in the package Conditional exemptions apply to eligible foods |
One symbol could be required Symbol must be displayed in a manner that clearly indicates it applies to all foods in the assortment (B.01.353(1), FDR) |
Assortment of foods of the same type where:
|
Tray of mixed nuts Box of assorted chocolate Tin of assorted cookies |
These products can use the aggregate NFt with information declared for each food in the assortment or the standard NFt with composite values (B.01.406(4), FDR) |
When the product carries the aggregate NFt: Conditional exemptions apply to eligible foods individually |
More than one symbol could be required Symbols must be displayed in a manner that clearly indicates to which food they apply (B.01.353(1), FDR) |
When the product carries the standard NFt: Conditional exemptions apply to product as a whole |
One symbol could be required No requirement to indicate that the symbol applies to the composite or each food |
|||
Assortment of foods of different types intended to be consumed together |
Charcuterie tray Assorted appetizers and sauces |
These products can use the aggregate NFt with information declared for each food (B.01.406(2), FDR) | Each food in the assortment is assessed against the threshold Conditional exemptions apply to eligible foods individually |
More than one symbol could be required Symbols must be displayed in a manner that clearly indicates to which food they apply (B.01.353(1), FDR) |
Consider an assortment that contains plain roasted almonds, cheddar cheese, ham and cookies. The product carries an aggregate NFt with information declared for each food in the assortment. The almonds are eligible for the conditional exemption set out in paragraph B.01.350(6)(d) and the cheddar cheese is eligible for the conditional exemption set out in subsections B.0.350(9) and (11). Assuming the almonds are roasted with an oil with a healthy fat profile and the cheddar cheese meets the calcium threshold set out in subsection B.01.350(12), regulated parties are not required to assess the nutrient content of these two foods against the % DV thresholds. (Section 5.3.2 provides more information about these conditional exemptions.) It is determined that the sodium content of the ham exceeds the threshold as does the sugars content of the cookies. It must be clearly indicated that the "high in sodium" symbol applies to the ham and the "high in sugars" symbol applies to the cookies.
Consider an assortment that contains two varieties of plain nuts and two varieties of seasoned nuts. The product carries the standard format NFt with information declared as a single composite value. The product as a whole is eligible for the conditional exemption set out in paragraph B.01.350(6)(d). The product loses the conditional exemption for sodium because of the salt used in the seasoned nuts. (Section 5.3.2 provides more information about the conditional exemption.) The total sodium content of the product as a whole is assessed against the % DV threshold; total sodium content includes the sodium in the seasoned nuts and the naturally occurring sodium in the plain nuts. It is determined that the sodium content meets the threshold. The product maintains the conditional exemption from the requirement to assess saturated fat and sugars content. The label must display the "high in sodium" symbol. There is no requirement to indicate that the symbol applies to the product as a whole.
7.2 How does FOP nutrition labelling apply to prepackaged products that contain either ingredients intended to be combined together or foods intended to be consumed together?
Assessment of saturated fat, sugars and sodium content against thresholds
For prepackaged products that contain either ingredients intended to be combined together or foods intended to be consumed together, the nutrient content assessment may align with the format of the Nutrition Facts table (NFt): standard or aggregate. This flexibility means that:
- When these products carry a standard or aggregate NFt, such as hors d'oeuvres with sauce, the nutrient content of the product as a whole (ingredients or foods combined) is assessed against the threshold. A reference amount for the product as a whole is required.
- Alternatively, when these products carry an aggregate NFt, the nutrient content of each ingredient or food can be assessed against the threshold individually.
Section 4 provides information on the % DV thresholds and the approach for determining whether the amount of saturated fat, sugars and/or sodium in a prepackaged product meets or exceeds them.
Conditional exemptions
The conditional exemptions set out in subsections B.01.350(6) to (12), FDR, can be applied to prepackaged products that contain either ingredients intended to be combined together or foods intended to be consumed together.
- When these products carry an aggregate NFt and the nutrient content of each food or ingredient is assessed against the threshold individually, the exemptions apply to each food or ingredient. As a result, each food can maintain the exemption that would apply to it when packaged and sold as such.
- When these products carry a standard or aggregate NFt and the nutrient content of the product as a whole is assessed against the threshold, the exemptions apply to the product as a whole. As a result, the product can maintain the exemption set out in subsection B.01.350(6) if the product contains only foods in that subsection.
Section 5.3.2 provides information on the exemptions set out in subsections B.01.350(6) to (12).
Symbol presentation where one or more of the foods require a nutrition symbol
Subject to subsection B.01.353(2) of the FDR, if a prepackaged product contains either ingredients that are intended to be combined together or foods that are intended to be consumed together, the nutrition symbol must display the nutrients that are contained in the product as a whole. No further indication near the FOP symbol would be required.
However, if a prepackaged product contains either ingredients that are intended to be combined together or foods that are intended to be consumed together, carries an aggregate NFt and one or more of the ingredients or foods require a nutrition symbol, regulated parties are encouraged to display the symbols in a manner that clearly indicates the applicable nutrients that are contained in each ingredient or food as described in Section 7.1.
| Description of the prepackaged product | Examples | NFt format | Nutrient content assessment (saturated fat, sugars, sodium) and application of conditional exemptions set out in B.01.350(6) to (12) | Nutrition symbol presentation where one or more foods or ingredients require a symbol |
|---|---|---|---|---|
| Prepackaged products that contain either ingredients that are intended to be combined together or foods that are intended to be consumed together | Sushi rolls and sauce Chicken wings and sauce Taco kit Salad kit Cheese and cracker snack pack Yogurt with granola topping |
These products can use the standard NFt with values for the product as a whole or the aggregate NFt with information declared for each ingredient or food (B.01.406(2), FDR) | When the product carries the standard or aggregate NFt: The nutrient content of the product as a whole is assessed against the threshold. A reference amount for the product as a whole is required Conditional exemption set out in subsection B.01.350(6) applies to product as a whole if it contains only foods set out in paragraphs (a) to (g) |
One symbol could be required The symbol must display the nutrients that are contained in the product as a whole (B.01.353(2), FDR). No requirement to indicate that the symbol applies to the entire product |
When the product carries the aggregate NFt: Each ingredient or food can also be assessed against the threshold Conditional exemptions apply to eligible ingredients or foods individually |
More than one symbol could be required Regulated parties are encouraged to display the symbols in a manner that clearly indicates to which food they apply |
Consider a prepackaged product that contains chicken wings and 2 dipping sauces. The foods are intended to be consumed together and the product carries an aggregate NFt with information declared for each food. Each food is assessed against the % DV threshold to determine whether one or more of the foods require a symbol. If one or more foods require a symbol, regulated parties are encouraged to display the symbols in a manner that clearly indicates to which food they apply.
Consider a salad kit that contains mixed greens, apples, dried unsweetened cranberries, plain almonds, bacon and creamy dressing. The ingredients are intended to be combined together and the product carries a standard NFt with information declared for the product as a whole. The conditional exemption set out in subsection B.01.350(6) does not apply because the product contains foods other than those referred to in paragraphs (a) to (f). The saturated fat, sugars and sodium content of the product as a whole is assessed against the % DV threshold to determine whether the product requires a nutrition symbol.
8. Nutrient and health-related claims and the front-of-package (FOP) nutrition symbol
For the purposes of FOP nutrition labelling, in subsection B.01.357(3), the regulations define "health-related representation" as:
| Health-related representation means | Examples |
|---|---|
| (a) a declaration referred to in subsection B.01.301(1) or (2) | Quantitative declarations outside of the Nutrition Facts table "360 mg of calcium per bar (40 g)" |
| (b) a statement or claim referred to in subsection B.01.311(2) or (3) | Nutrient function claims "Protein helps build strong muscles" |
| (c) a representation referred to in any of sections B.01.503 to B.01.513 | Nutrient content claims "Reduced in sugar" |
| (d) a statement or claim referred to in subsection B.01.601(1) | Health claims "A healthy diet rich in a variety of vegetables and fruit may help reduce the risk of heart disease." |
| (e) any other health-related statement, logo, symbol, seal of approval or mark | The use of a name, logo, symbol, seal of approval or other certification mark of a third party-organization Using a check mark to draw attention to good or healthy choices. |
Nutrition and health-related statements and claims may be made on the label or in advertisements for foods on a voluntary basis. However, when they are made, they must comply with subsection 5(1) of the Food and Drugs Act, the food provisions of the FDR, and subsection 6(1) of the Safe Food for Canadians Act and should follow any applicable guidance.
8.1 Are health-related representations permitted on the principal display panel (PDP) when a food also carries the FOP nutrition symbol (the symbol)?
Yes. Most health-related representations are permitted on the PDP when it displays the symbol. This includes representations not related to saturated fat, sugars or sodium (for example, protein, fibre or energy) and representations that are related to these nutrients of concern as long as there is no "high in" symbol for that nutrient on the PDP. For example, a "low sodium" claim is permitted on the PDP when the product carries a "high in sat fat" symbol. Statements or claims described in sections D.01.004 to D.01.007 and D.02.002 to D.02.005 are also permitted on the PDP when it carries a symbol. However, the "unsweetened" claim is prohibited on the PDP when it carries a symbol for "high in sugars" and the representation that a food is for use in a sodium-restricted diet is prohibited when the PDP carries a symbol for "high in sodium".
Furthermore, all nutrient content claims related to saturated fat, sugars or sodium, as set out in the Table of Permitted Nutrient Content Statements and Claims (the Table) incorporated by reference into the FDR, are prohibited on the PDP when it carries a symbol to identify the food as "high in" that particular nutrient, except for "reduced in" claims.
For instance, the claims related to saturated fat set out in item 18, 19 and 21 of the Table are prohibited on the PDP when it carries a symbol for "high in sat fat". The "reduced in saturated fatty acids" claim (item 20) is the only claim about saturated fat in the Table that is permitted on the PDP when it carries a symbol for "high in sat fat". The nutrient content claims "lean" and "extra lean" set out in item 46 and 47 of the Table are considered related to the total fat content of a meat or poultry that has not been ground, a marine or fresh water animal or a product of any of these. These claims are not considered related to saturated fat and are not prohibited on the PDP when it carries a symbol for "high in sat fat".
The same applies to claims about sugars and sodium set out in column 4 of the Table:
- The claims related to sugars set out in item 37, 37.1, 39 and 40 of the Table are prohibited on the PDP when it carries a symbol for "high in sugars". The "reduced in sugar" claim (item 38) is the only claim about sugars in the Table that is permitted on the PDP when it carries a symbol for "high in sugars".
- The claims related to sodium set out in item 31, 32, 34 and 35 of the Table are prohibited on the PDP when it carries a symbol for "high in sodium". The "reduced in sodium or salt" claim (item 33) is the only claim about sodium in the Table that is permitted on the PDP when it carries a symbol for "high in sodium".
Using a representation such as a word, phrase, illustration, sign, mark, symbol or design that could be mistaken for the symbol is also prohibited regardless of whether the food carries the symbol. Note that a representation does not include a supplemented food caution identifier.
Reference: subsection B.01.357(3), section B.01.358, subsection B.01.503(1.1), B.01.508(2) and B.01.509(2), FDR
8.2 Do restrictions apply to the size of health-related representations displayed on the PDP when a food also carries the symbol?
Yes. There are limits on the size (in other words, the height of letters) of any health-related representation defined in subsection B.01.357(3) and any statement or claim described in sections D.01.004 to D.01.007 and D.02.002 to D.02.005 displayed on a PDP that carries the symbol. The maximum permitted height of the letters in the claims, statements or representations depends on whether the nutrient they feature is declared in the symbol.
However, these size limits do not apply to the brand name or product name that appear on the PDP, such as those that include the name of a nutrient or nutritive substance, for example "fibre" or "probiotics".
When the nutrient featured in a health-related representation displayed on the PDP is also declared in the symbol:
- the height of the upper case letters in the claim, statement or representation must not exceed the prescribed height of the upper case letters (excluding any accents) in the applicable symbol format, other than in the words "Health Canada" and "Santé Canada"; and
- the height of the tallest ascender of the lower case letters in the claim, statement or representation must not exceed the prescribed height of the tallest ascender of the lower case letters in the applicable symbol, other than in the words "Health Canada" and "Santé Canada".

Figure 8.1 - Text description
This figure shows the words "High in" forward slash "Élevé en" in black, bold lower case letters except that the first letters of the words "High" and "Élevé" are in upper case. A double-ended red arrow appears to the left of the word High that spans the height of the uppercase letter H. To the left of that arrow is the statement "Height of upper case letters forward slash tallest ascender of lower case letters". A red line appears both above and below this text. The lines span the width of the figure.
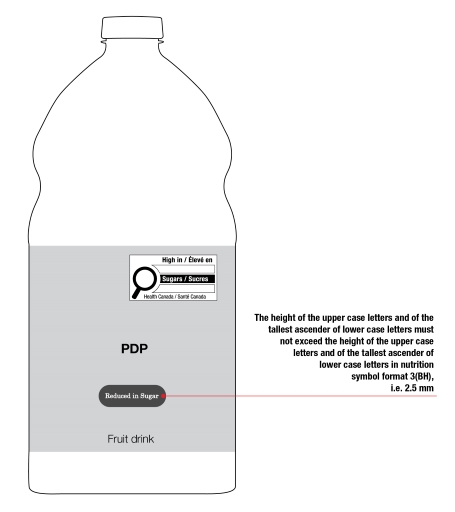
Figure 8.2 - Text description
This figure shows a white bottle of fruit drink. A label spans the width of the bottom half of the bottle. The label is shaded grey to identify it as the Principal Display Panel or PDP.
There is a horizontal nutrition symbol in the top right corner of the PDP that identifies the food as high in sugars. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The PDP carries a "Reduced in Sugar" claim centred and at the bottom of the panel. This claim is linked to the statement "The height of the upper case letters and of the tallest ascender of lower case letters must not exceed the height of the upper case letters and of the tallest ascender of lower case letters in nutrition symbol format 3(BH), i.e. 2.5 mm".
When the nutrient featured in a health-related representation or a statement or claim referred to in any of sections D.01.004 to D.01.007 and D.02.002 to D.02.005 displayed on the PDP is not declared in the symbol:
- the height of the upper case letters in the claim, statement or representation must not exceed two times the prescribed height of the upper case letters (excluding any accents) in the applicable symbol format, other than in the words "Health Canada" and "Santé Canada"; and
- the height of the tallest ascender of the lower case letters in the claim, statement or representation must not exceed two times the prescribed height of the tallest ascender of the lower case letters in the applicable symbol format, other than in the words "Health Canada" and "Santé Canada".

Figure 8.3 - Text description
This figure shows a granola cereal box. The front panel is shaded grey to identify it as the Principal Display Panel or PDP.
There is a horizontal nutrition symbol in the top right corner of the PDP that identifies the food as high in saturated fat and sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is white, is outlined by a thin black line and contains no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The PDP carries a "Source of Fibre" and a "Low in Sodium" claim to the left and near the bottom of the panel. These two claims are linked to the statement "The height of the upper case letters and of the tallest ascender of lower case letters must not exceed 2 times the height of the upper case letters and of the tallest ascender of lower case letters in nutrition symbol format 1(EH), i.e. 3.5 mm".
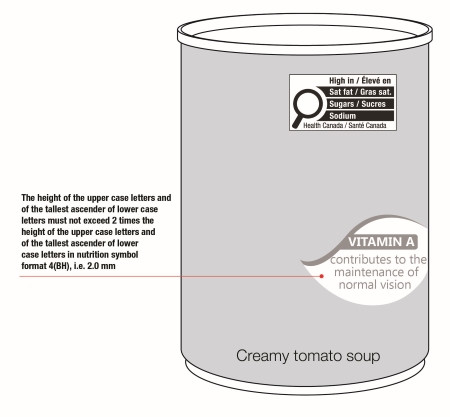
Figure 8.4 - Text description
This figure shows a creamy tomato soup can. The front surface is shaded grey to identify it as the Principal Display Panel or PDP.
There is a horizontal nutrition symbol in the top right corner of the PDP that identifies the soup as high in saturated fat, sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
The PDP carries the claim "Vitamin A contributes to the maintenance of normal vision" to the right and near the bottom of the panel. This claim is linked to the statement "The height of the upper case letters and of the tallest ascender of lower case letters must not exceed 2 times the height of the upper case letters and of the tallest ascender of lower case letters in nutrition symbol format 4(BH), i.e. 2.0 mm".
The Directory of Nutrition Symbol Specifications (the Directory) sets out the height of upper case letters and tallest ascender of lower case letters that must be used in each symbol format. The information is in column 5 of each table in the Directory. See the Presentation section for more information on how to identify which symbol format must appear on the PDP.
Reference: section B.01.357 and D.01.001.2, FDR
8.3 Do restrictions apply to health-related representations displayed elsewhere than with the symbol on the PDP ?
No. Restrictions on use and size only apply to health-related representations when they are displayed with the FOP nutrition symbol on the PDP. No restrictions apply to health-related representations that appear elsewhere than on the PDP with the symbol, for example, on the back of the package. Note that these representations remain subject to existing legibility requirements.
9. Definitions
The Food and Drug Regulations (FDR) should be referred to for definitions. The definitions in this section are provided for ease of reference only.
Daily value: As defined in subsection B.01.001(1) of the FDR, means, in respect of a nutrient, the quantity applicable to the nutrient according to subsection B.01.001.1(2).
Main dish: As defined in subsection B.01.001(1) of the FDR, means a combination dish, as set out in the Table of Reference Amounts, that does not require the addition of ingredients, other than water, for its preparation and that contains food from at least two of the following categories:
- dairy products and their alternatives, except butter, cream, sour cream, ice cream, ice milk, sherbet and alternatives for those foods;
- meat products, poultry products, marine and fresh water animal products referred to in Division 21, and their alternatives such as eggs, tofu, legumes, nuts, seeds, nut or seed butters and spreads made from legumes;
- fruits and vegetables except pickles, relishes, olives and garnishes; and
- breads, breakfast cereals, rice and other grains, and alimentary pastes.
Milk ingredients: As defined in item 7 of Table 2 in the Common Names for Ingredients and Components Document, which is incorporated by reference into the FDR, means any of the following in liquid, concentrated, dry, frozen or reconstituted form, namely, butter, buttermilk, butter oil, milk fat, cream, milk, partly skimmed milk, skim milk and any other component of milk the chemical composition of which has not been altered and that exists in the food in the same chemical state in which it is found in milk.
Modified milk ingredients: As defined in item 7.1 of Table 2 in the Common Names for Ingredients and Components Document, which is incorporated by reference into the FDR, means any of the following in liquid, concentrated, dry, frozen or reconstituted form, namely, calcium-reduced skim milk (obtained by the ion-exchange process), casein, caseinates, cultured milk products, milk serum proteins, ultrafiltered milk, whey, whey butter, whey cream and any other component of milk the chemical state of which has been altered from that in which it is found in milk. As defined in item 7.2 of the Common Names for Ingredients and Components Document, which is incorporated by reference into the FDR, it also means one or more ingredients or components set out in item 7 combined with any one or more ingredients or components set out in item 7.1.
Nutrition symbol: As defined in subsection B.01.001(1) of the FDR, means a symbol set out in Schedule K.1 that is carried on the principal display panel (PDP) of a prepackaged product.
Principal display panel (PDP): As defined subsection B.01.001(1) of the FDR, means, despite the meaning assigned to that term in section A.01.010:
- in the case of a label that is applied to a consumer prepackaged food within the meaning of section 1 of the Safe Food for Canadians Regulations, the principal display panel as described in paragraphs (a) to (c) of the definition of that term in that section;
- in the case of a label that is applied to a prepackaged product other than a consumer prepackaged food subject to the Safe Food for Canadians Regulations, the part of the label that is applied to all or part of any side or surface of the container that is displayed or visible under normal or customary conditions of sale or use and, if the container does not have such a side or surface, the part of the label that is applied to any part of the container except on the bottom; or
- in the case of a label that is applied to a food that is not a prepackaged product, the part of the label that is applied to all or part of the side or surface of the food that is displayed or visible under normal or customary conditions of sale or use.
Principal display surface (PDS): As defined in subsection B.01.001(1) of the FDR and in respect of a prepackaged product, means:
- if the package has a surface that is displayed or visible under customary conditions of sale or use, the total area of that surface, excluding any surface that is the top of the package;
Figure 9.1. PDS of cereal box 
Figure 9.1 - Text description
This figure shows a box. The front panel is crosshatched with grey lines to identify it as the Principal Display Surface or PDS. It is also shaded grey.
For example, the front panel of this cereal box, is usually visible when the product is displayed for sale. Therefore, the total area of that panel is considered the PDS, which is the area shown here with crosshatching. The grey shaded area is considered the PDP.
- if the package has a lid that is the part of the package that is displayed or visible under customary conditions of sale or use, the total area of the top surface of the lid;
Figure 9.2. PDS of hummus container 
Figure 9.2 - Text description
This figure shows a round container with a lid. The outer edge of the lid is orange. The top total area of the lid is crosshatched with grey lines to identify it as the Principal Display Surface or PDS. Covering half of the PDS is a label shaded in grey.
For example, the lid of this hummus container is usually visible when displayed for sale. Therefore, the total area of the lid is considered the PDS, which is the area shown here with crosshatching. The grey shaded area is considered the PDP.
- if the package does not have a particular surface that is displayed or visible under customary conditions of sale or use, 40% of the total surface area of the package, excluding any surface area that is its top and bottom, if it is possible for that proportion of the total surface area to be displayed or visible under customary conditions of sale or use;
Figure 9.3. PDS of soup can 
Figure 9.3 - Text description
This figure shows a can. The front surface is crosshatched with grey lines to identify it as the Principal Display Surface or PDS. It is also shaded grey.
For example, cylindrical shaped packages, such as this soup can, don't have a particular surface that is usually visible when displayed for sale. Therefore, 40% of the package's total surface area, excluding the top and bottom is considered the PDS. It is the area shown here with crosshatching. The grey shaded area is considered the PDP.
Figure 9.4. PDS of jar of toffee peanuts 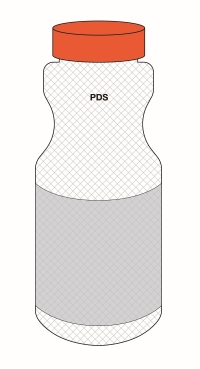
Figure 9.4 - Text description
This figure shows a cylindrical jar with an orange lid. The front surface is crosshatched with grey lines to identify it as the Principal Display Surface or PDS. Covering approximately half of the PDS is a label shaded in grey.
This definition also applies to cylindrical containers that do not have a constant diameter such as this jar of toffee peanuts, in which the PDS is shown here with crosshatching. The grey shaded area is considered the PDP.
- if the package is a bag with surfaces of equal dimensions, the total area of one of the surfaces;
Figure 9.5. PDS of bag of popcorn 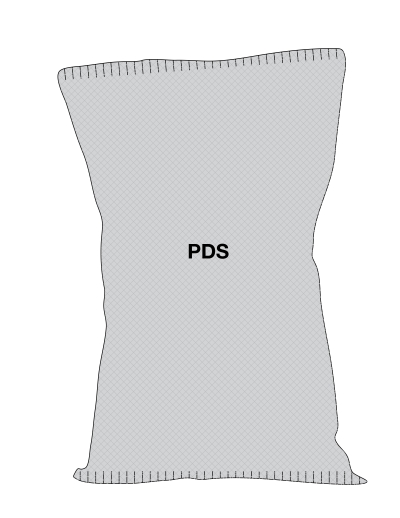
Figure 9.5 - Text description
This figure shows a bag. The front surface is crosshatched with grey lines to identify it as the Principal Display Surface or PDS. It is also shaded grey.
For example, the surfaces of this bag of popcorn are the same size. Therefore, the total area of one surface is considered the PDS. It is the area shown here with crosshatching. The grey shaded area is considered the PDP.
- if the package is a bag with surfaces of different dimensions, the total area of one of the largest surfaces;
Figure 9.6. PDS of bag of mini brownies 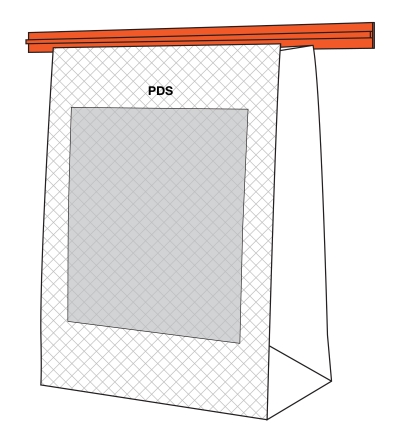
Figure 9.6 - Text description
This figure shows a bag with an orange tin tie closure. The front surface is crosshatched with grey lines to identify it as the Principal Display Surface or PDS. Covering the majority of the PDS is a label shaded in grey.
For example, the surfaces of this bag of mini brownies are different sizes. Therefore, the total area of the largest surface is considered the PDS. It is the area shown here with crosshatching. The grey shaded area is considered the PDP.
- despite paragraphs (a) to (e), if the package does not have a surface that is displayed or visible under customary conditions of sale or use to which a label can be applied, the total area of one side of a tag that is attached to the package;
Figure 9.7. PDS of mesh bag with individually-wrapped chocolates 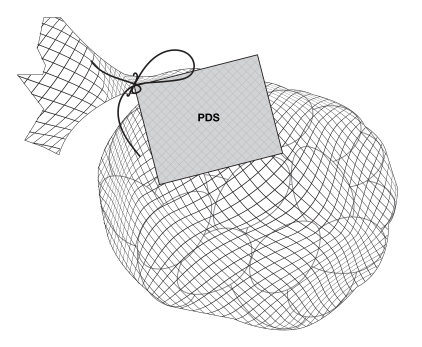
Figure 9.7 - Text description
This figure shows a mesh bag held together by a string. A tag is hanging from the string. One side of the tag is crosshatched with grey lines to identify it as the Principal Display Surface or PDS. It is also shaded grey.
For example, given that a mesh bag such as this one containing individually wrapped chocolates doesn't have a surface on which a label can be applied, the total area of one side of the tag attached to the bag is considered the PDS. It is the area shown here with crosshatching. The grey shaded area is considered the PDP.
- despite paragraphs (a) to (e), if the package contains wine that is exposed for sale, any part of the surface of the package, excluding its top and bottom, that can be seen without having to turn the package; and
- if the package is a wrapper or confining band that is so narrow in relation to the size of the food it contains that it cannot reasonably be considered to have any surface that is displayed or visible under customary conditions of sale or use, the total area of one side of a tag that is attached to the package.
Reference amount: As defined in subsection B.01.001(1) of the FDR, means, in respect of a food set out in column 1 of the Table of Reference Amounts, the amount of that food set out in column 2.
Serving of stated size: The serving size is a specific amount of food upon which the nutrient information presented in the nutrition facts table is based. The serving size is a quantity of food that can be reasonably consumed at a single eating occasion.
Sweetening agent: As defined in subsection B.01.001(1) of the FDR, includes any food for which a standard is provided in Division 18, but does not include those food additives listed in the tables to Division 16.
Appendix 1: Steps for determining whether the amount of saturated fat, sugars and/or sodium in a prepackaged product meets or exceeds the FOP nutrition symbol thresholds
These instructions describe the steps to determine whether the amount of saturated fat, sugars and/or sodium in a food meets or exceeds the applicable thresholds and triggers the need to display the FOP nutrition symbol (the symbol) on the label. Although most prepackaged products are subject to the FOP nutrition symbol labelling requirements, some foods are exempted from carrying the symbol, and in some cases, the symbol is prohibited on the label of certain foods. See the Exemptions and Prohibitions sections for more information.
Examples A to F illustrate the following scenarios:
- Example A : the serving of stated size (serving size) is greater than the reference amount (food contains trans fat)
- Example B : the serving size is greater than the reference amount (food does not contain trans fat)
- Example C : the reference amount is greater than the serving size
- Example D : the food requires preparation and has a reference amount for its unprepared form
- Example E : the serving size is greater than the reference amount and the product is intended solely for children one year or older but less than four years of age
- Example F : the serving size and reference amount are equal and the food has a reference amount ≤ (less than or equal to) 30 g or 30 mL
Example A –the serving of stated size (serving size) is greater than the reference amount (food contains trans fat)
This example illustrates a scenario in which the serving of stated size (serving size) declared in the Nutrition Facts table (NFt) is larger than the reference amount set out for the food in the Table of Reference Amounts.
Consider a frozen, uncooked lamb burger. The serving size declared in the NFt is 1 burger (142 g). These instructions describe the steps to follow to determine whether the amount of saturated fat, sugars and/or sodium meets or exceeds the applicable threshold and therefore, triggers the need to carry the front-of-package (FOP) nutrition symbol (the symbol) on the label.

Figure A1.1 - Text description
This figure shows a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1 burger open parenthesis 142 g close parenthesis. The next line is pour 1 burger open parenthesis 142 g close parenthesis. There is a thin rule below pour 1 burger open parenthesis 142 g close parenthesis that spans the width of the table. The next line is Calories in bold followed by 290, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 290; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 21 and a lowercase g. Right justified on the same line is 28 followed by a percent symbol. Indented on the next line is Saturated, forward slash, saturés followed by 10 and a lowercase g. Indented on the next line is a plus symbol followed by Trans, forward slash, trans followed by 1 and a lowercase g. Right justified and vertically centered against the saturated and trans information on the left is 55 followed by a percent symbol. There is a thin rule below the trans information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 3 and a lowercase g. Indented on the next line is Fibre, forward slash, Fibres, followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. Indented on the next line is Sugars, forward slash, Sucres, followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. There is a thin rule under the sugars information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 23 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Cholesterol, forward slash, Cholestérol in bold followed by 95 and mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
The next line is Sodium in bold followed by 390 and mg in lowercase. Right justified on the same line is 17 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table.
The next line is Potassium followed by 400 and mg in lowercase. Right justified on the same line is 12 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table. The next line is Calcium followed by 30 and mg in lowercase. Right justified on the same line is 2 followed by a percent symbol. There is a thin rule below the calcium information that spans the width of the table.
The next line is Iron, forward slash, Fer followed by 2 and mg in lowercase. Right justified on the same line is 11 followed by a percent symbol. There is a thick rule under the iron information that spans the width of the Nutrition Facts table.
The next two lines is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, 15 percent symbol or more is a lot and on the second line is an asterisk followed by the statement: 5 percent symbol ou moins c'est peu, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu', and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
-
Identify for whom the product is intended.
Determine which of the following 2 categories applies to the food:
- A food intended solely for children one year of age or older, but less than four years of age (children one to four years only).
- A food intended for children one year of age or older, but less than four years of age or for children four years of age or older and adults (children and/or adults).
- In this example, the product is intended for children and/or adults. Therefore, the thresholds in columns 2 to 4 of the table to section B.01.350 (Thresholds Requiring a Nutrition Symbol) apply.
- Identify the quantity of food that must be used to assess the amount of saturated fat, sugars and/or sodium against the symbol threshold.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- The reference amount for raw patties is 100 g; it is set out in Item L.7, Column 2, of the Table of Reference Amounts.
- Compare the product's reference amount and the serving size declared in the NFt. The larger quantity must be used as the base quantity for the nutrient content assessment.
- In this example, the serving size (142 g) is greater than the reference amount. Therefore, the serving size is the base quantity that must be used for the assessment.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- Identify the applicable % Daily Value (% DV) threshold.
Determine in which of the following classes the product belongs:
- Prepackaged products with a reference amount ≤ (less than or equal to) 30 g or 30 mL: threshold of ≥ (greater than or equal to) 10% DV
- Prepackaged main dishes with a reference amount ≥ 170 g (when intended solely for children one to four years) or ≥ 200 g (when intended for children and/or adults): threshold of ≥ 30% DV
- Prepackaged products with a reference amount > 30 g or 30 mL other than main dishes: threshold of ≥ 15% DV
- In this example, the applicable threshold is ≥ 15% DV given that raw patties have a reference amount > (greater than) 30 g or 30 mL and are not a main dish.
- Determine whether the amount of saturated fat, sugars and/or sodium in the base quantity meets or exceeds the threshold.
When the serving size is the base quantity for the nutrient content assessment the % DV for sugars and sodium declared in the NFt can be compared to the % DV thresholds identified in Step 3. It is important to note that the NFt presents the % DV for saturated fat and trans fat content combined and that for FOP labelling purposes, only saturated fat content must be considered when calculating the % DV to determine whether a "high in sat fat" symbol is required. If a food contains trans fat, the % DV for saturated and trans fat content declared in the NFt should not be compared to the % DV threshold for the symbol. If a food contains no trans fat, the % DV for saturated and trans fat content declared in the NFt can be compared to the threshold.
If the % DV for one or more of the nutrients is equal to or greater than the % DV threshold, a symbol for the nutrient or nutrients is required.
- In this example, the product contains trans fat. For FOP labelling purposes, the % DV must only reflect saturated fat content. The % DV for saturated fat and trans fat declared in the NFt should not be compared to the % DV threshold for saturated fat.
- The % DV should be calculated as follows: (saturated fat content declared in the NFt / DV for the sum of saturated fatty acids and trans fatty acids) x 100 = (10 g / 20 g) x 100 = 50%
- The % DV for sugars (0%) and sodium (17%) declared in the NFt were compared to the 15% DV threshold.
- In this example, the % DV calculated for saturated fat and the % DV for sodium declared in the NFt are equal to or greater than the 15% DV threshold. Therefore, the label must carry a symbol for "high in sat fat and sodium ".
- See the Presentation section for more information on the symbol location and presentation requirements.

Figure A1.2 - Text description
This figure shows a rectangular box of lamb burgers. The front panel is shaded grey. There is a horizontal nutrition symbol in the top right corner of the panel that identifies the food as high in saturated fat and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is white, is outlined by a thin black line and contains no words. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
To the right of the rectangular box of lamb burgers is a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1 burger open parenthesis 142 g close parenthesis. The next line is pour 1 burger open parenthesis 142 g close parenthesis. A red box surrounds these two lines of text. There is a thin rule below pour 1 burger open parenthesis 142 g close parenthesis that spans the width of the table. The next line is Calories in bold followed by 290, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 290; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 21 and a lowercase g. Right justified on the same line is 28 followed by a percent symbol. Indented on the next line is Saturated, forward slash, saturés followed by 10 and a lowercase g. Indented on the next line is a plus symbol followed by Trans, forward slash, trans followed by 1 and a lowercase g. Right justified and vertically centered against the saturated and trans information on the left is 55 followed by a percent symbol. There is a thin rule below the trans information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 3 and a lowercase g. Indented on the next line is Fibre, forward slash, Fibres, followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. Indented on the next line is Sugars, forward slash, Sucres, followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. There is a thin rule under the sugars information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 23 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Cholesterol, forward slash, Cholestérol in bold followed by 95 and mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
The next line is Sodium in bold followed by 390 and mg in lowercase. Right justified on the same line in a red box is 17 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table.
The next line is Potassium followed by 400 and mg in lowercase. Right justified on the same line is 12 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table. The next line is Calcium followed by 30 and mg in lowercase. Right justified on the same line is 2 followed by a percent symbol. There is a thin rule below the calcium information that spans the width of the table.
The next line is Iron, forward slash, Fer followed by 2 and mg in lowercase. Right justified on the same line is 11 followed by a percent symbol. There is a thick rule under the iron information that spans the width of the Nutrition Facts table.
The next two lines is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, 15 percent symbol or more is a lot and on the second line is an asterisk followed by the statement: 5 percent symbol ou moins c'est peu, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu', and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
Example B – the serving size is greater than the reference amount (food does not contain trans fat)
This example illustrates a scenario in which the serving of stated size (serving size) declared in the Nutrition Facts table (NFt) is larger than the reference amount set out for the food in the Table of Reference Amounts
Consider frozen, uncooked plant-based burgers. The serving size declared in the NFt is 1 burger (113 g). These instructions describe the steps to follow to determine whether the amount of saturated fat, sugars and/or sodium meets or exceeds the applicable threshold and therefore, triggers the need to carry the front-of-package (FOP) nutrition symbol (the symbol) on the label.
Figure A1.3 - Text description
This figure shows a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1 burger open parenthesis 113 g close parenthesis. The next line is pour 1 burger open parenthesis 113 g close parenthesis. There is a thin rule below pour 1 burger open parenthesis 113 g close parenthesis that spans the width of the table. The next line is Calories in bold followed by 260, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 260; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 15 and a lowercase g. Right justified on the same line is 20 followed by a percent symbol. Indented on the next line is Saturated, forward slash, saturés followed by 7 and a lowercase g. Indented on the next line is a plus symbol followed by Trans, forward slash, trans followed by 0 and a lowercase g. Right justified and vertically centered against the saturated and trans information on the left is 35 followed by a percent symbol. There is a thin rule below the trans information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 10 and a lowercase g. Indented on the next line is Fibre, forward slash, Fibres, followed by 3 and a lowercase g. Right justified on the same line is 11 followed by a percent symbol. Indented on the next line is Sugars, forward slash, Sucres, followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. There is a thin rule under the sugars information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 20 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Cholesterol, forward slash, Cholestérol in bold followed by 0 and mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
The next line is Sodium in bold followed by 400 and mg in lowercase. Right justified on the same line is 17 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table.
The next line is Potassium followed by 450 and mg in lowercase. Right justified on the same line is 13 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
The next line is Calcium followed by 50 and mg in lowercase. Right justified on the same line is 4 followed by a percent symbol. There is a thin rule below the calcium information that spans the width of the table.
The next line is Iron, forward slash, Fer followed by 7 and mg in lowercase. Right justified on the same line is 39 followed by a percent symbol. There is a thin rule under the iron information that spans the width of the table.
The next line is Thiamine followed by 0.75 and mg in lowercase. Right justified on the same line is 63 followed by a percent symbol. There is a thin rule under the thiamine information that spans the width of the table.
The next line is Riboflavin, forward slash, Riboflavine followed by 0.3 and mg in lowercase. Right justified on the same line is 23 followed by a percent symbol. There is a thin rule under the riboflavin information that spans the width of the table.
The next line is Niacin, forward slash, Niacine followed by 10 and mg in lowercase. Right justified on the same line is 63 followed by a percent symbol. There is a thin rule under the niacin information that spans the width of the table.
The next line is Vitamin B subscript 6, forward slash, Vitamine B subscript 6 followed by 0.5 and mg in lowercase. Right justified on the same line is 29 followed by a percent symbol. There is a thin rule under the vitamin B subscript 6 information that spans the width of the table.
The next line is Folate followed by 50, the symbol µ, a lowercase g and the letters DFE, forward slash, and the letters ÉFA. Right justified on the same line is 13 followed by a percent symbol. There is a thin rule under the folate information that spans the width of the table.
The next line is Vitamin B subscript 12, forward slash, Vitamine B subscript 12 followed by 2, the symbol µ, and a lowercase g. Right justified on the same line is 83 followed by a percent symbol. There is a thin rule under the vitamin B subscript 12 information that spans the width of the table.
The next line is Pantothenate, forward slash, Pantothénate followed by 1 and mg in lowercase. Right justified on the same line is 20 followed by a percent symbol. There is a thin rule under the pantothenate information that spans the width of the table.
The next line is Magnesium, forward slash, Magnésium followed by 25 and mg in lowercase. Right justified on the same line is 6 followed by a percent symbol. There is a thin rule under the magnesium information that spans the width of the table.
The next line is Zinc followed by 6 and mg in lowercase. Right justified on the same line is 55 followed by a percent symbol. There is a thin rule under the zinc information that spans the width of the table.
The next line is Copper, forward slash, Cuivre followed by 0.1 and mg in lowercase. Right justified on the same line is 11 followed by a percent symbol. There is a thick rule under the copper information that spans the width of the table.
The next line is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, forward slash, asterisk 5 percent symbol ou moins c'est peu and stacked below is, 15 percent symbol or more is a lot, forward slash, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu' and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
-
Identify for whom the product is intended.
Determine which of the following 2 categories applies to the food:
- A food intended solely for children one year of age or older, but less than four years of age (children one to four years only).
- A food intended for children one year of age or older, but less than four years of age or for children four years of age or older and adults (children and/or adults).
- In this example, the product is intended for children and/or adults. Therefore, the thresholds in columns 2 to 4 of the table to section B.01.350 (Thresholds Requiring a Nutrition Symbol) apply.
- Identify the quantity of food that must be used to assess the amount of saturated fat, sugars and/or sodium against the symbol threshold.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- The reference amount for raw burger patties is 100 g; it is set out in Item L.7, Column 2, of the Table of Reference Amounts.
- Compare the product's reference amount and the serving size declared in the NFt. The larger quantity must be used as the base quantity for the nutrient content assessment.
- In this example, the serving size (113 g) is greater than the reference amount. Therefore, the serving size is the base quantity that must be used for the assessment.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- Identify the applicable % Daily Value (% DV) threshold.
Determine in which of the following classes the product belongs:
- Prepackaged products with a reference amount ≤ (less than or equal to) 30 g or 30 mL: threshold of ≥ (greater than or equal to) 10% DV
- Prepackaged main dishes with a reference amount ≥ 170 g (when intended solely for children one to four years) or ≥ 200 g (when intended for children and/or adults): threshold of ≥ 30% DV
- Prepackaged products with a reference amount > 30 g or 30 mL other than main dishes: threshold of ≥ 15% DV
- In this example, the applicable threshold is ≥ 15% DV given that burger patties have a reference amount > 30 g and are not a main dish.
- Determine whether the amount of saturated fat, sugars and/or sodium in the base quantity meets or exceeds the threshold
When the serving size is the base quantity for the nutrient content assessment the % DV for sugars and sodium declared in the NFt can be compared to the % DV thresholds for the symbol identified in Step 3. It is important to note that the NFt presents the % DV for saturated fat and trans fat content combined and that for FOP labelling purposes, only saturated fat content must be considered when calculating the % DV to determine whether a "high in sat fat" symbol is required. If a food contains trans fat, the % DV for saturated and trans fat content declared in the NFt should not be compared to the % DV threshold for the symbol. If a food contains no trans fat, the % DV for saturated and trans fat content declared in the NFt can be compared to the threshold.
If the % DV for one or more of the nutrients is equal to or greater than the % DV threshold, a symbol for the nutrient or nutrients is required.
- In this example, the % DV declared for saturated fat and trans fat in the NFt (35%) can be compared to the % DV thresholds because the food contains no trans fat.
- The % DV for sugars (0%) and sodium (17%) declared in the NFt were also compared to the 15% DV threshold.
- In this example, the % DV for saturated fat and sodium meet or exceed the threshold. The label must carry a symbol for "high in sat fat and sodium".
- See Presentation section for more information on the symbol location and presentation requirements.

Figure A1.4 - Text description
This figure shows a rectangular box of plant-based burgers. The front panel is shaded grey. There is a horizontal nutrition symbol in the bottom right corner of the panel that identifies the food as high in saturated fat and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is white, is outlined by a thin black line and contains no words. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
To the right of the rectangular box of plant-based burgers is a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1 burger open parenthesis 113 g close parenthesis. The next line is pour 1 burger open parenthesis 113 g close parenthesis. A red box surrounds these two lines of text. There is a thin rule below pour 1 burger open parenthesis 113 g close parenthesis that spans the width of the table. The next line is Calories in bold followed by 260, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 260; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 15 and a lowercase g. Right justified on the same line is 20 followed by a percent symbol. Indented on the next line is Saturated, forward slash, saturés followed by 7 and a lowercase g. Indented on the next line is a plus symbol followed by Trans, forward slash, trans followed by 0 and a lowercase g. Right justified and vertically centered against the saturated and trans information on the left is 35 followed by a percent symbol. A red box surrounds this percent daily value. There is a thin rule below the trans information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 10 and a lowercase g. Indented on the next line is Fibre, forward slash, Fibres, followed by 3 and a lowercase g. Right justified on the same line is 11 followed by a percent symbol. Indented on the next line is Sugars, forward slash, Sucres, followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. There is a thin rule under the sugars information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 20 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Cholesterol, forward slash, Cholestérol in bold followed by 0 and mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
The next line is Sodium in bold followed by 400 and mg in lowercase. Right justified on the same line in a red box is 17 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table.
The next line is Potassium followed by 450 and mg in lowercase. Right justified on the same line is 13 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
The next line is Calcium followed by 50 and mg in lowercase. Right justified on the same line is 4 followed by a percent symbol. There is a thin rule below the calcium information that spans the width of the table.
The next line is Iron, forward slash, Fer followed by 7 and mg in lowercase. Right justified on the same line is 39 followed by a percent symbol. There is a thin rule under the iron information that spans the width of the table.
The next line is Thiamine followed by 0.75 and mg in lowercase. Right justified on the same line is 63 followed by a percent symbol. There is a thin rule under the thiamine information that spans the width of the table.
The next line is Riboflavin, forward slash, Riboflavine followed by 0.3 and mg in lowercase. Right justified on the same line is 23 followed by a percent symbol. There is a thin rule under the riboflavin information that spans the width of the table.
The next line is Niacin, forward slash, Niacine followed by 10 and mg in lowercase. Right justified on the same line is 63 followed by a percent symbol. There is a thin rule under the niacin information that spans the width of the table.
The next line is Vitamin B subscript 6, forward slash, Vitamine B subscript 6 followed by 0.5 and mg in lowercase. Right justified on the same line is 29 followed by a percent symbol. There is a thin rule under the vitamin B subscript 6 information that spans the width of the table.
The next line is Folate followed by 50, the symbol µ, a lowercase g and the letters DFE, forward slash, and the letters ÉFA. Right justified on the same line is 13 followed by a percent symbol. There is a thin rule under the folate information that spans the width of the table.
The next line is Vitamin B subscript 12, forward slash, Vitamine B subscript 12 followed by 2, the symbol µ, and a lowercase g. Right justified on the same line is 83 followed by a percent symbol. There is a thin rule under the vitamin B subscript 12 information that spans the width of the table.
The next line is Pantothenate, forward slash, Pantothénate followed by 1 and mg in lowercase. Right justified on the same line is 20 followed by a percent symbol. There is a thin rule under the pantothenate information that spans the width of the table.
The next line is Magnesium, forward slash, Magnésium followed by 25 and mg in lowercase. Right justified on the same line is 6 followed by a percent symbol. There is a thin rule under the magnesium information that spans the width of the table.
The next line is Zinc followed by 6 and mg in lowercase. Right justified on the same line is 55 followed by a percent symbol. There is a thin rule under the zinc information that spans the width of the table.
The next line is Copper, forward slash, Cuivre followed by 0.1 and mg in lowercase. Right justified on the same line is 11 followed by a percent symbol. There is a thick rule under the copper information that spans the width of the table.
The next line is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, forward slash, asterisk 5 percent symbol ou moins c'est peu and stacked below is, 15 percent symbol or more is a lot, forward slash, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu' and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
Example C – the reference amount is greater than the serving size
This example illustrates a scenario in which the reference amount set out for the food in the Table of Reference Amounts is larger than the serving of stated size (serving size) declared in the Nutrition Facts table (NFt).
Consider a lasagna sold in a 227 g tray. The serving size declared in the NFt is 1 package (227 g). These instructions describe the steps to follow to determine whether the amount of saturated fat, sugars and/or sodium meets or exceeds the applicable threshold and therefore, triggers the need to display the front-of-package (FOP) nutrition symbol (the symbol) on the label.

Figure A1.5 - Text description
This figure shows a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1 package open parenthesis 227 g close parenthesis. The next line is pour 1 emballage open parenthesis 227 g close parenthesis. There is a thin rule below pour 1 emballage open parenthesis 227 g close parenthesis that spans the width of the table. The next line is Calories in bold followed by 290, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 290; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 8 and a lowercase g. Right justified on the same line is 11 followed by a percent symbol. Indented on the next line is Saturated, forward slash, saturés followed by 5 and a lowercase g. Indented on the next line is a plus symbol followed by Trans, forward slash, trans followed by 0 and a lowercase g. Right justified and vertically centered against the saturated and trans information on the left is 25 followed by a percent symbol. There is a thin rule below the trans information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 34 and a lowercase g. Indented on the next line is Fibre, forward slash, Fibres, followed by 3 and a lowercase g. Right justified on the same line is 11 followed by a percent symbol. Indented on the next line is Sugars, forward slash, Sucres, followed by 1 and a lowercase g. Right justified on the same line is 1 followed by a percent symbol. There is a thin rule under the sugars information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 20 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Cholesterol, forward slash, Cholestérol in bold followed by 35 and mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
The next line is Sodium in bold followed by 580 and mg in lowercase. Right justified on the same line is 25 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table.
The next line is Potassium followed by 75 and mg in lowercase. Right justified on the same line is 2 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
The next line is Calcium followed by 225 and mg in lowercase. Right justified on the same line is 17 followed by a percent symbol. There is a thin rule below the calcium information that spans the width of the table.
The next line is Iron, forward slash, Fer followed by 3.5 and mg in lowercase. Right justified on the same line is 19 followed by a percent symbol. There is a thick rule under the iron information that spans the width of the Nutrition Facts table.
The next two lines is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, 15 percent symbol or more is a lot and on the second line is an asterisk followed by the statement: 5 percent symbol ou moins c'est peu, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu', and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
- Identify for whom the product is intended.
Determine which of the following 2 categories applies to the food:
- A food intended solely for children one year of age or older, but less than four years of age (children one to four years only).
- A food intended for children one year of age or older, but less than four years of age or for children four years of age or older and adults (children and/or adults).
- In this example, the product is intended for children and/or adults. Therefore, the thresholds in columns 2 to 4 of the table to section B.01.350 (Thresholds Requiring a Nutrition Symbol) apply.
- Identify the quantity of food that must be used to assess the amount of saturated fat, sugars and/or sodium against the symbol threshold.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- The reference amount for lasagna is 300 g; it is set out in Item N.1, Column 2, of the Table of Reference Amounts.
- Compare the product's reference amount and the serving size declared in the NFt. The larger quantity must be used as the base quantity for the nutrient content assessment.
- In this example, the reference amount is greater than the serving size (227 g). Therefore, the reference amount is the base quantity that must be used for the assessment.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- Identify the applicable % Daily Value (% DV) threshold.
Determine in which of the following classes the product belongs:
- Prepackaged products with a reference amount ≤ 30 g or 30 mL: threshold of ≥ 10% DV
- Prepackaged main dishes with a reference amount ≥ 170 g (when intended solely for children one to four years) or ≥ 200 g (when intended for children and/or adults): threshold of ≥ 30% DV
- Prepackaged products with a reference amount > 30 g or 30 mL other than main dishes: threshold of ≥ 15% DV
- In this example, the applicable % DV threshold is ≥ 30% DV given that lasagna is a main dish with a reference amount ≥ 200 g.
- Determine the amount of saturated fat, sugars and sodium in the base quantity of the food.
Convert the saturated fat, sugars and sodium content declared per serving size in the NFt into content per reference amount. The following formula could be used: (Nutrient content per serving size / Serving size) x Reference amount = Nutrient content per reference amount.
- The nutrient content per reference amount of lasagna is shown below:
Table A1.1. Nutrient content per reference amount of lasagna Nutrient Content per serving size declared in NFt (227 g) Content per reference amount (300 g) Saturated fat 5 g 6.61 g Sugars 1 g 1.32 g Sodium 580 mg 766.52 mg
- The nutrient content per reference amount of lasagna is shown below:
- Identify the applicable Daily Value for saturated fat, sugars and sodium.
Consult Part 1 of the Table of Daily Values and determine in which column the applicable DVs are specified based on the intended consumers identified in Step 1:
- Column 2: DVs used for thresholds applicable to prepackaged products intended solely for children one to four years or
- Column 3: DVs used for thresholds applicable to prepackaged products intended for children and/or adults
- In this example, the applicable DVs are in column 3. They are 20 g for saturated fat and trans fat, 100 g for sugars and 2300 mg for sodium.
- Determine whether the amount of saturated fat, sugars and/or sodium in the base quantity of the food meets or exceeds the threshold.
- Calculate the % DV for the saturated fat, sugars and sodium content per reference amount using values calculated in Step 4 and the DV identified in Step 5.
- In this example, the % DVs for the nutrient content per reference amount are shown below:
Table A1.2. % DVs for the nutrient content per reference amount Nutrient Content per reference amount (300 g) Daily Value % DV calculationFootnote 1 [(Nutrient content per reference amount/DV for the nutrient) x 100] % DV threshold Saturated fat 6.61 g 20 g 33.05% [(6.61 g ∕ 20 g)*100] ≥ 30% Sugars 1.32 g 100 g 1.32% [(1.32 g ∕ 100 g)*100] ≥ 30% Sodium 766.52 mg 2300 mg 33.47% [(770 mg ∕ 2300 mg)*100] ≥ 30% - 1
-
There are no prescribed rounding rules for determining whether the nutrient content per reference amount meets or exceeds the applicable thresholds. However, regulated parties should apply similar rounding rules that exist for calculating the % DV declared in the NFt. In this example, the unrounded content of saturated fat and of sugars per reference amount were used for the calculation, however rounded content is also permitted. The rounded content of sodium was used in accordance with the NFt rounding rules.
- Compare the % DV calculated for each nutrient and the % DV threshold. If the calculated % DV is equal to or greater than the % DV threshold for one or more of the three nutrients, a symbol for the nutrient or nutrients is required.
- In this example, the % DV for the saturated fat and sodium content per reference amount of lasagna meet or exceed the 30% DV threshold. Therefore, the label must carry a symbol for "high in sat fat and sodium".
- See the Presentation section for more information on the symbol location and presentation requirements.
Figure A1.6 - Text description
This figure shows a rectangular box of lasagna. The front panel is shaded grey. There is a horizontal nutrition symbol in the top right corner of the panel that identifies the food as high in saturated fat and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" followed by a forward slash and the words "Gras sat." in white, bold, lower case letters, except that the first letter of the words "Sat" and "Gras" are in upper case. The second bar is white, is outlined by a thin black line and contains no words. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
Example D – the food requires preparation and has a reference amount for its unprepared form
This example illustrates a scenario in which the prepackaged product is sold in its unprepared form. In other words, it either needs to be reconstituted with water (or other liquid) or combined with another ingredient and it has a reference amount for its unprepared form in the Table of Reference Amounts.
Consider chocolate cake mix. In this example, the reference amount is the amount of unprepared mix required to make 80 g of the final product because it is a medium weight cake. The serving of stated size (serving size) declared in the Nutrition Facts Table (NFt) is 1/10 of package (43 g). These instructions describe the steps to follow to determine whether the amount of saturated fat, sugars and/or sodium meets or exceeds the applicable threshold and therefore, triggers the need to display the front-of-package (FOP) nutrition symbol (the symbol) on the label.
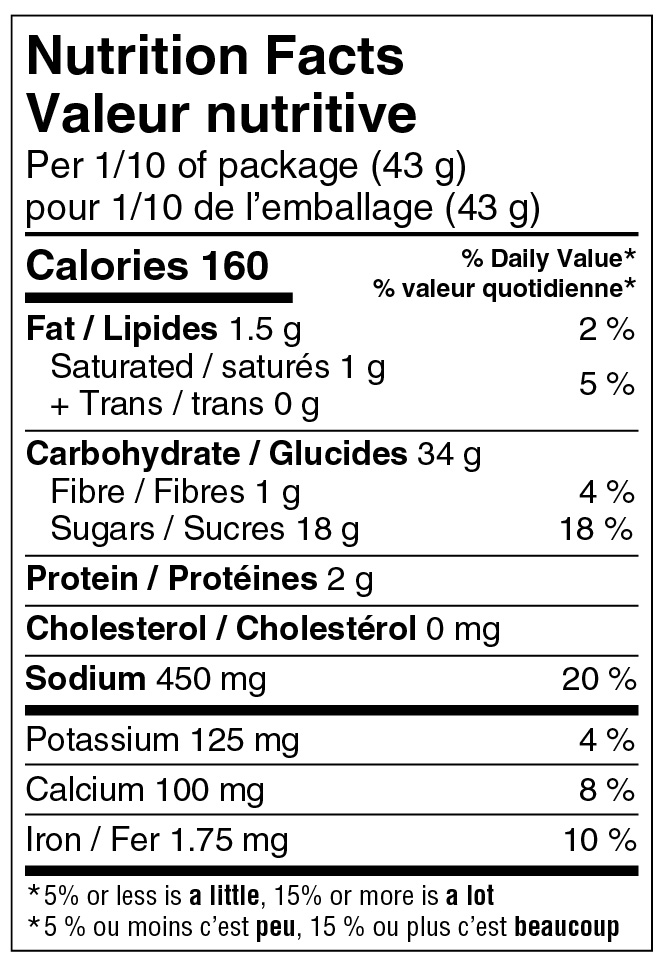
Figure A1.7 - Text description
This figure shows a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1, forward slash, 10 of package open parenthesis 43 g close parenthesis. The next line is pour 1, forward slash, 10 de l'emballage open parenthesis 43 g close parenthesis. There is a thin rule below pour 1, forward slash, 10 de l'emballage open parenthesis 43 g close parenthesis that spans the width of the table. The next line is Calories in bold followed by 160, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 160; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 1.5 and a lowercase g. Right justified on the same line is 2 followed by a percent symbol. Indented on the next line is Saturated, forward slash, saturés followed by 1 and a lowercase g. Indented on the next line is a plus symbol followed by Trans, forward slash, trans followed by 0 and a lowercase g. Right justified and vertically centered against the saturated and trans information on the left is 5 followed by a percent symbol. There is a thin rule below the trans information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 34 and a lowercase g. Indented on the next line is Fibre, forward slash, Fibres, followed by 1 and a lowercase g. Right justified on the same line is 4 followed by a percent symbol. Indented on the next line is Sugars, forward slash, Sucres, followed by 18 and a lowercase g. Right justified on the same line is 18 followed by a percent symbol. There is a thin rule under the sugars information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 2 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Cholesterol, forward slash, Cholestérol in bold followed by 0 and mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
The next line is Sodium in bold followed by 450 and mg in lowercase. Right justified on the same line is 20 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table.
The next line is Potassium followed by 125 and mg in lowercase. Right justified on the same line is 4 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
The next line is Calcium followed by 100 and mg in lowercase. Right justified on the same line is 8 followed by a percent symbol. There is a thin rule below the calcium information that spans the width of the table.
The next line is Iron, forward slash, Fer followed by 1.75 and mg in lowercase. Right justified on the same line is 10 followed by a percent symbol. There is a thick rule under the iron information that spans the width of the Nutrition Facts table.
The next two lines are the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, 15 percent symbol or more is a lot and on the second line is an asterisk followed by the statement: 5 percent symbol ou moins c'est peu, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu', and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
- Identify for whom the product is intended.
Determine which of the following 2 categories applies to the food:
- A food intended solely for children one year of age or older, but less than four years of age (children one to four years only).
- A food intended for children one year of age or older, but less than four years of age or for children four years of age or older and adults (children and/or adults).
- In this example, the product is intended for children and/or adults. Therefore, the thresholds in columns 2 to 4 of the table to section B.01.350 (Thresholds Requiring a Nutrition Symbol) apply.
- Identify the quantity of food that must be used to assess the amount of saturated fat, sugars and/or sodium content against the symbol threshold.
- Consult the Table of Reference Amounts to identify the amount applicable to the product
- The reference amount for medium weight cake mix is the amount of unprepared mix required to make 80 g prepared; it is set out in Item A.6, Column 2, of the Table of Reference Amounts.
- Compare the product's reference amount and the serving size declared in the NFt. The larger quantity must be used as the base quantity for the nutrient content assessment
- In this example, the serving size and reference amount are equal. Therefore, the serving size of 1/10 of package (43 g) of the unprepared product can be used as the base quantity for the assessment.
- Consult the Table of Reference Amounts to identify the amount applicable to the product
- Identify the applicable % Daily Value (% DV) threshold.
Determine in which of the following classes the product belongs:
- Prepackaged products with a reference amount ≤ 30 g or 30 mL: threshold of ≥ 10% DV
- Prepackaged products with a reference amount > 30 g or 30 mL other than main dishes: threshold of ≥ 15% DV
- Prepackaged main dishes with a reference amount ≥ 170 g (when intended solely for children one to four years) or ≥ 200 g (when intended for children and/or adults): threshold of ≥ 30% DV
- In this example, the applicable % DV threshold is ≥ 15% DV given that the reference amount for the unprepared cake mix is > 30 g or 30 mL.
- Determine whether the amount of saturated fat, sugars or sodium in the base quantity of the food meets or exceeds the threshold.
When the serving size is the base quantity for the nutrient content assessment the % DV for sugars and sodium declared in the NFt can be compared to the % DV thresholds for the symbol identified in Step 3. It is important to note that the NFt presents the % DV for saturated fat and trans fat content combined and that for FOP labelling purposes, only saturated fat content must be considered when calculating the % DV to determine whether a "high in sat fat" symbol is required. If a food contains trans fat, the % DV for saturated and trans fat content declared in the NFt should not be compared to the % DV threshold for the symbol. If a food contains no trans fat, the % DV for saturated and trans fat content declared in the NFt can be compared to the threshold.
If the % DV for one or more of the nutrients is equal to or greater than the % DV threshold, a symbol for the nutrient or nutrients is required.
- In this example, the % DV declared for saturated fat and trans fat in the NFt (5%) can be compared to the % DV thresholds because the food contains no trans fat
- The % DV for sugars (18%) and sodium (20%) declared in the NFt were also compared to the 15% DV threshold.
- In this example, the % DV for sugars and sodium meet or exceed the threshold. The label must carry a symbol for "high in sugars and sodium".
- See the Presentation section for more information on the symbol location and presentation requirements.
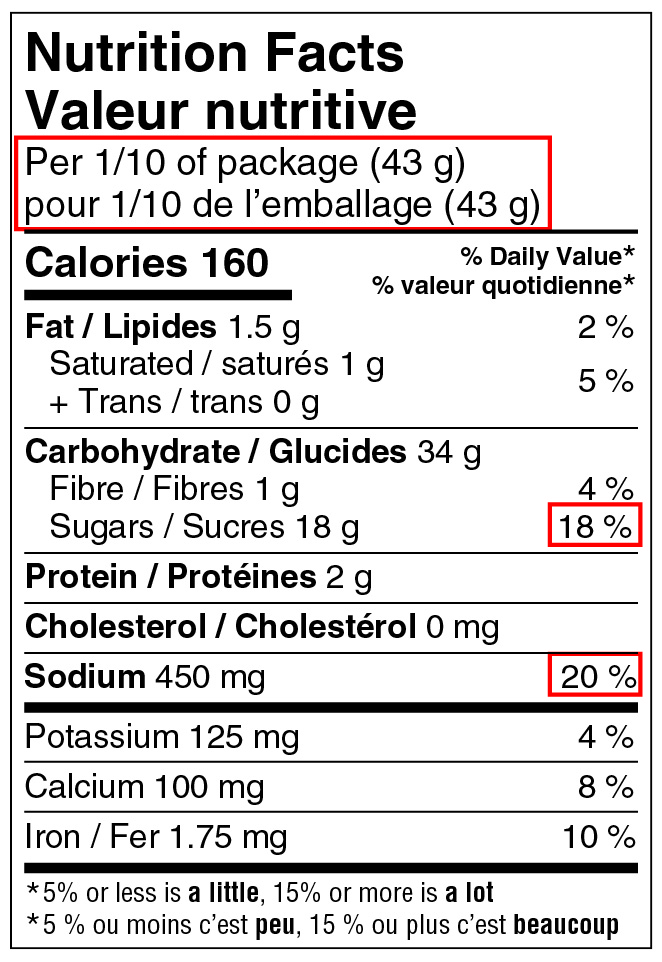
Figure A1.8 - Text description
This figure shows a rectangular box of cake mix. The front panel is shaded grey. There is a horizontal nutrition symbol in the top right corner of the panel that identifies the prepackaged product as high in sugars and sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box are the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, upper and lower case letters. Under this heading is a left-justified black magnifying glass with three bars horizontally stacked to its right. The left side of the three bars forms a concave curve. There is a small amount of white space between each bar, as well as between the bars and the side of the white rectangular box. The first bar is white, outlined by a thin black line and contains no words. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, upper and lower case letters. The third bar is black and contains the word "Sodium" in white, bold, upper and lower case letters. At the bottom of the white rectangular box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black upper and lower case letters.
To the right of the rectangular box of cake mix is a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1, forward slash, 10 of package open parenthesis 43 g close parenthesis. The next line is pour 1, forward slash, 10 de l'emballage open parenthesis 43 g close parenthesis. A red box surrounds these two lines of text. There is a thin rule below pour 1, forward slash, 10 de l'emballage open parenthesis 43 g close parenthesis that spans the width of the table. The next line is Calories in bold followed by 160, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 160; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 1.5 and a lowercase g. Right justified on the same line is 2 followed by a percent symbol. Indented on the next line is Saturated, forward slash, saturés followed by 1 and a lowercase g. Indented on the next line is a plus symbol followed by Trans, forward slash, trans followed by 0 and a lowercase g. Right justified and vertically centered against the saturated and trans information on the left is 5 followed by a percent symbol. There is a thin rule below the trans information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 34 and a lowercase g. Indented on the next line is Fibre, forward slash, Fibres, followed by 1 and a lowercase g. Right justified on the same line is 4 followed by a percent symbol. Indented on the next line is Sugars, forward slash, Sucres, followed by 18 and a lowercase g. Right justified on the same line is 18 followed by a percent symbol. A red box surrounds this percent daily value. There is a thin rule under the sugars information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 2 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Cholesterol, forward slash, Cholestérol in bold followed by 0 and mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
The next line is Sodium in bold followed by 450 and mg in lowercase. Right justified on the same line is 20 followed by a percent symbol. A red box surrounds this percent daily value. There is a thick rule under the sodium information that spans the width of the table.
The next line is Potassium followed by 125 and mg in lowercase. Right justified on the same line is 4 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
The next line is Calcium followed by 100 and mg in lowercase. Right justified on the same line is 8 followed by a percent symbol. There is a thin rule below the calcium information that spans the width of the table.
The next line is Iron, forward slash, Fer followed by 1.75 and mg in lowercase. Right justified on the same line is 10 followed by a percent symbol. There is a thick rule under the iron information that spans the width of the Nutrition Facts table.
The next two lines are the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, 15 percent symbol or more is a lot and on the second line is an asterisk followed by the statement: 5 percent symbol ou moins c'est peu, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu', and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
Example E – the serving size is greater than the reference amount and the product is intended solely for children one to four years of age
This example illustrates a scenario in which the serving of stated size (serving size) declared in the Nutrition Facts table (NFt) is larger than the reference amount set out for the food in the Table of Reference Amounts and the food is intended to be consumed by young children only.
Consider toddler biscuits sold in a box. The serving size declared in the NFt is 1 biscuit (10 g). These instructions describe the steps to follow to determine whether the amount of saturated fat, sugars and/or sodium meets or exceeds the applicable threshold and therefore, triggers the need to display the front-of-package (FOP) nutrition symbol (the symbol) on the label.

Figure A1.9 - Text description
This figure shows a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1 biscuit open parenthesis 10 g close parenthesis. The next line is pour 1 biscuit open parenthesis 10 g close parenthesis. There is a thin rule below pour 1 biscuit open parenthesis 10 g close parenthesis that spans the width of the table. The next line is Calories in bold followed by 40, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 40; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 1.5 and a lowercase g. Right justified on the same line is 3 followed by a percent symbol. Indented on the next line is Saturated, forward slash, saturés followed by 0 and a lowercase g. Indented on the next line is a plus symbol followed by Trans, forward slash, trans followed by 0 and a lowercase g. Right justified and vertically centered against the saturated and trans information on the left is 0 followed by a percent symbol. There is a thin rule below the trans information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 7 and a lowercase g. Indented on the next line is Fibre, forward slash, Fibres, followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. Indented on the next line is Sugars, forward slash, Sucres, followed by 3 and a lowercase g. Right justified on the same line is 6 followed by a percent symbol. There is a thin rule under the sugars information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 0 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Cholesterol, forward slash, Cholestérol in bold followed by 0 and mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
The next line is Sodium in bold followed by 35 and mg in lowercase. Right justified on the same line is 2 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table. The next line is Potassium followed by 20 and mg in lowercase. Right justified on the same line is 1 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
The next line is Calcium followed by 75 and mg in lowercase. Right justified on the same line is 11 followed by a percent symbol. There is a thin rule below the calcium information that spans the width of the table. The next line is Iron, forward slash, Fer followed by 1 and mg in lowercase. Right justified on the same line is 14 followed by a percent symbol. There is a thick rule under the iron information that spans the width of the Nutrition Facts table.
The next two lines is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, 15 percent symbol or more is a lot and on the second line is an asterisk followed by the statement: 5 percent symbol ou moins c'est peu, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu', and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
- Identify for whom the product is intended.
Determine which of the following 2 categories applies to the food:
- A food intended solely for children one year of age or older, but less than four years of age (children one to four years only).
- A food intended for children one year of age or older, but less than four years of age or for children four years of age or older and adults (children and/or adults).
- In this example, the product is intended for children one to four years only. Therefore, the thresholds in columns 5 to 7 of the table to section B.01.350 (Thresholds Requiring a Nutrition Symbol) apply.
- Identify the quantity of food that must be used to assess the amount of saturated fat, sugars and/or sodium against the symbol threshold.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- The reference amount for biscuits for children under four years of age is 7 g; it is set out in Item W.3, Column 2, of the Table of Reference Amounts.
- Compare the product's reference amount and the serving size declared in the NFt. The larger quantity must be used as the base quantity for the nutrient content assessment.
- In this example, the serving size (10 g) is greater than the reference amount. Therefore, the serving size is the base quantity that must be used for the assessment.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- Identify the applicable % Daily Value (% DV) threshold.
Determine in which of the following classes the product belongs:
- Prepackaged products with a reference amount ≤ 30 g or 30 mL: threshold of ≥ 10% DV
- Prepackaged products with a reference amount > 30 g or 30 mL other than main dishes: threshold of ≥ 15% DV
- Prepackaged main dishes with a reference amount ≥ 170 g (when intended solely for children one to four years) or ≥ 200 g (when intended for children and/or adults): threshold of ≥ 30% DV
- In this example, the applicable threshold is ≥ 10% DV given that the biscuits have a reference amount ≤ 30 g or 30 mL.
- Determine whether the amount of saturated fat, sugars and/or sodium in the base quantity of the food meets or exceeds the threshold.
When the serving size is the base quantity for the nutrient content assessment the % DV for sugars and sodium declared in the NFt can be compared to the % DV thresholds identified in Step 3. It is important to note that the NFt presents the % DV for saturated fat and trans fat content combined and that for FOP labelling purposes, only saturated fat content must be considered when calculating the % DV to determine whether a "high in sat fat" symbol is required. If a food contains trans fat, the % DV for saturated and trans fat content declared in the NFt should not be compared to the % DV threshold for the symbol. If a food contains no trans fat, the % DV for saturated and trans fat content declared in the NFt can be compared to the threshold.
If the % DV for one or more of the nutrients is equal to or greater than the % DV threshold, a symbol for the nutrient or nutrients is required.
- In this example, the % DV declared for saturated and trans fat in the NFt (0%) can be compared to the % DV thresholds because the food contains no trans fat
- The % DV for sugars (6%) and sodium (2%) declared in the NFt were also compared to the 10% DV threshold.
- In this example, the % DV for all three nutrients are below the threshold. Therefore, no symbol is required on the label.
Example F – the serving size and reference amount are equal and the food has a reference amount ≤ 30 g or 30 mL
This example illustrates a scenario in which the serving of stated size (serving size) declared in the Nutrition Facts table (NFt) is equal to the reference amount for the food in the Table of Reference Amounts and the food has a reference amount of ≤ 30 mL.
Consider soy sauce sold in a bottle. The serving size declared in the NFt is 1 tbsp. (15 mL). These instructions describe the steps to follow to determine whether the amount of saturated fat, sugars and/or sodium meets or exceeds the applicable threshold and therefore, triggers the need to display the front-of-package (FOP) nutrition symbol (the symbol) on the label.

Figure A1.10 - Text description
This figure shows a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1 tbsp open parenthesis 15 mL close parenthesis. The next line is pour 1 c. à soupe open parenthesis 15 mL close parenthesis. There is a thin rule below pour 1 c. à soupe open parenthesis 15 mL close parenthesis that spans the width of the table. The next line is Calories in bold followed by 10, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 10; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. There is a thin rule under the fat information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 1 and a lowercase g. There is a thin rule under the carbohydrate information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 1 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Sodium in bold followed by 1160 and mg in lowercase. Right justified on the same line is 50 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table. The next line is Potassium followed by 75 and mg in lowercase. Right justified on the same line is 2 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
The next two lines are the English footnote: Not a significant source of saturated fat, trans fat, fibre, sugars, cholesterol, calcium and iron. There is a thin rule below this footnote. The next two lines are the French footnote: Source négligeable de lipides saturés, lipides trans, fibres, sucres, cholestérol, calcium et fer. There is a thin rule below this footnote.
The next two lines is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, 15 percent symbol or more is a lot and on the second line is an asterisk followed by the statement: 5 percent symbol ou moins c'est peu, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu', and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
- Identify for whom the product is intended.
Determine which of the following 2 categories applies to the food:
- A food intended solely for children one year of age or older, but less than four years of age (children one to four years only).
- A food intended for children one year of age or older, but less than four years of age or for children four years of age or older and adults (children and/or adults).
- In this example, the product is intended for children and/or adults. Therefore, the thresholds in columns 2 to 4 of the table to section B.01.350 (Thresholds Requiring a Nutrition Symbol) apply.
- Identify the quantity of food that must be used to assess the amount of saturated fat, sugars and/or sodium against the symbol threshold.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- The reference amount for soy sauce is 15 mL; it is set out in Item R.5, Column 2, of the Table of Reference Amounts.
- Compare the product's reference amount and the serving size declared in the NFt. The larger quantity must be used as the base quantity for the nutrient content assessment.
- In this example, the serving size and reference amount are equal. Therefore, the serving size can be used as the base quantity for the assessment.
- Consult the Table of Reference Amounts to identify the amount applicable to the product.
- Identify the applicable % Daily Value (% DV) threshold.
Determine in which of the following classes the product belongs:
- Prepackaged products with a reference amount ≤ 30 g or 30 mL: threshold of ≥ 10% DV
- Prepackaged products with a reference amount > 30 g or 30 mL other than main dishes: threshold of ≥ 15% DV
- Prepackaged main dishes with a reference amount ≥ 170 g (when intended solely for children one to four years) or ≥ 200 g (when intended for children and/or adults): threshold of ≥ 30% DV
- In this example, the applicable threshold is ≥ 10% DV given that soy sauce has a reference amount ≤ 30 g or 30 mL.
- Determine whether the amount of saturated fat, sugars and/or sodium in the base quantity meets or exceeds the threshold.
When the serving size is the base quantity for the nutrient content assessment the % DV for sugars and sodium declared in the NFt can be compared to the % DV thresholds identified in Step 3. It is important to note that the NFt presents the % DV for saturated fat and trans fat content combined and that for FOP labelling purposes, only saturated fat content must be considered when calculating the % DV to determine whether a "high in sat fat" symbol is required. If a food contains trans fat, the % DV for saturated and trans fat content declared in the NFt should not be compared to the % DV threshold for the symbol. If a food contains no trans fat, the % DV for saturated and trans fat content declared in the NFt can be compared to the threshold.
If the % DV for one or more of the nutrients is equal to or greater than the % DV threshold, a symbol for the nutrient or nutrients is required.
- In this example, only the % DV for sodium declared in the NFt (50%) is equal to or greater than the 10% DV threshold. Therefore, the label must carry a symbol for "high in sodium".
- See the Presentation section for more information on the symbol location and presentation requirements.

Figure A1.11 - Text description
This figure shows a white cylindrical bottle of soy sauce with an orange wrapper around the lid. Covering the bottom third of the front surface is a label shaded in grey. There is a horizontal nutrition symbol in the top right corner of the label that identifies the product as high in sodium. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and second bars are white, are outlined by a thin black line and contain no words. The third bar is black and contains the word "Sodium" in white, bold, lower case letters, except that the first letter is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
To the right of the bottle of soy sauce is a Nutrition Facts table. Left justified at the top of the table is the heading Nutrition Facts and stacked below it is the heading Valeur nutritive. Both are in bold. The next line is Per 1 tbsp open parenthesis 15 mL close parenthesis. The next line is pour 1 c. à soupe open parenthesis 15 mL close parenthesis. A red box surrounds these two lines of text. There is a thin rule below pour 1 c. à soupe open parenthesis 15 mL close parenthesis that spans the width of the table. The next line is Calories in bold followed by 10, also in bold. Right justified on the same line is the subheading percent symbol Daily Value in bold. Stacked under this is percent symbol valeur quotidienne. Both Percent Daily Value and percent valeur quotidienne are followed by an asterisk that refers to a footnote at the bottom of the Nutrition Facts table. There is a thick rule under Calories that ends after the 10; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. There is a thin rule under the fat information that spans the width of the table.
The next line is Carbohydrate, forward slash, Glucides in bold followed by 1 and a lowercase g. There is a thin rule under the carbohydrate information that spans the width of the table.
The next line is Protein, forward slash, Protéines in bold followed by 1 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
The next line is Sodium in bold followed by 1160 and mg in lowercase. Right justified on the same line in a red box is 50 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table.
The next line is Potassium followed by 75 and mg in lowercase. Right justified on the same line is 2 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
The next two lines are the English footnote: Not a significant source of saturated fat, trans fat, fibre, sugars, cholesterol, calcium and iron. There is a thin rule below this footnote. The next two lines are the French footnote: Source négligeable de lipides saturés, lipides trans, fibres, sucres, cholestérol, calcium et fer. There is a thin rule below this footnote.
The next two lines is the percent Daily Value footnote that was referred to at the beginning of the table description. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little, 15 percent symbol or more is a lot and on the second line is an asterisk followed by the statement: 5 percent symbol ou moins c'est peu, 15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu', and 'beaucoup' are in bold. This is the end of the Nutrition Facts table.
Appendix 2: Steps for choosing a FOP nutrition symbol format
These instructions describe steps to follow to identify the FOP nutrition symbol (the symbol) format required on the label of different prepackaged products.
Example A: general rules of application for symbol presentation
Example B: required use of a vertical symbol
Example A – general rules of application for symbol presentation
This example illustrates a typical scenario in which the general rules of application apply.
Consider a box of granola-type breakfast cereal, for which it has been determined that the saturated fat and sugars content exceeds the thresholds (see the Thresholds section for more information). Therefore, a symbol must appear on the label to indicate that the cereal is "high in" these two nutrients.
These instructions describe the steps to follow to identify the symbol format required on the label.
- Identify the product's principal display surface (PDS).
Refer to the PDS definition to determine which surface of the package is considered the PDS.
- The front panel of the box of cereal is displayed under normal conditions of sale. Therefore, it is considered the PDS. In this example, the package is labelled in English on one of the two large panels. It is labelled in French on the other large panel. Either one of the larger panels could be considered the PDS. Both are included in this example.
- Calculate the PDS (size).
- The PDS is equal to 721 cm2. The PDS is crosshatched on both large panels of the cereal box in Figure A2.1.
Figure A2.1. Unilingual English and unilingual French PDS of the box of cereal 
Figure A2.1 - Text description
This figure shows two rectangular boxes of cereal with a unilingual front panel, one in English and one in French. The front panels are crosshatched to identify it as the Principal Display Surface or PDS. The PDS has a total surface area of 721 cm2.
Did you know? The size of the symbol is proportional to the PDS. In most cases, the larger the PDS the larger the symbol on the label.
- The PDS is equal to 721 cm2. The PDS is crosshatched on both large panels of the cereal box in Figure A2.1.
- Identify the product's principal display panel (PDP).
Refer to the PDP definition to determine which part of the label is considered the PDP. The dimensions of the PDP determine where to place the symbol and validate which symbol orientation must be used.
- In this example, the PDP of the box of cereal is the same part of the label as the PDS. The PDP is shaded on both large panels of the cereal box in Figure A2.2. The shading overlaps with the crosshatching used to identify the PDS because the areas are the same part of the label.
Figure A2.2. Unilingual English and unilingual French PDS and PDP of the box of cereal 
Figure A2.2 - Text description
This figure shows two rectangular boxes of cereal with a unilingual front panel, one in English and one in French. The front panels are crosshatched to identify them as the Principal Display Surface or PDS. They are also shaded grey to identify them as the Principal Display Panel or PDP. This means the PDS and PDP both have a total surface area of 721 cm2.
Did you know? The symbol must appear on the PDP.
- In this example, the PDP of the box of cereal is the same part of the label as the PDS. The PDP is shaded on both large panels of the cereal box in Figure A2.2. The shading overlaps with the crosshatching used to identify the PDS because the areas are the same part of the label.
- Consult the table to section B.01.352 of the Food and Drug Regulations (FDR) (Nutrition Symbols and Formats table) to identify the symbol format that must appear on the label.
- Refer to Column 1 (Range of principal display surface) to determine whether the PDS calculated in Step 2 corresponds to Item 1 (> 30 cm2) or Item 2 (≤ 30 cm2).
- The PDS of 721 cm2corresponds toItem 1 (> 30 cm2).
- Within that item, refer to Column 2 (Nutrients that meet or exceed threshold […]) to find the "high in" nutrient combination that applies to the product.
- The cereal is "high in sat fat" and "high in sugars". Therefore, the applicable combination is Saturated fat (Sat fat) and sugars.
- Choose a language option (unilingual or bilingual) to determine which column sets out the applicable format: Column 3, 4, 5 or 6.
- In this example, unilingual symbols are the language option used. This narrows down the relevant columns to Column 3 (Nutrition symbol in unilingual horizontal format) or 4 (Nutrition symbol in unilingual vertical format).
- Validate the symbol orientation (horizontal or vertical).
The regulations set out the horizontal format as the default orientation. The vertical format is required only if the product has a PDS ≤ 450 cm2 and the horizontal format that would otherwise need to be used on the label is wider than the PDP (see the Presentation section for more information).
- In this example, the PDS is larger than 450 cm2, therefore vertical symbols are not permitted. Horizontal symbols must be used. This eliminates Column 4 and means that the applicable symbol format is set out in Column 3 (Nutrition symbol in unilingual horizontal format).
- Identify the symbol format that must appear on the label.
Given that:
- the PDS falls within Item 1 of the table to B.01.352 of the FDR
- the cereal is "high in sat fat" and "high in sugars"
- the language option chosen is unilingual and
- the default horizontal orientation applies
symbol formats 2(EH) and 2(FH) set out in Column 3 must be displayed on the English label and French label, respectively. Refer to Schedule K.1 of the FDR to see an image of these two formats.
Table A2.1. Excerpt from the table to section B.01.352 of the FDR Item Column 1 Range of principal display surface Column 2 Nutrients that meet or exceed threshold in subsection B.01.350(1) Column 3 Nutrition symbol in unilingual horizontal format Column 4 Nutrition symbol in unilingual vertical format Column 5 Nutrition symbol in bilingual horizontal format Column 6 Nutrition symbol in bilingual vertical format 1 > 30 cm2 Saturated fat (Sat fat), sugars and sodium 1(EH) and 1(FH) 1(EV) and 1(FV) 1(BH) 1(BV) Saturated fat (Sat fat) and sugars 2(EH) and 2(FH) 2(EV) and 2(FV) 2(BH) 2(BV)
- Refer to Column 1 (Range of principal display surface) to determine whether the PDS calculated in Step 2 corresponds to Item 1 (> 30 cm2) or Item 2 (≤ 30 cm2).
- Consult the Compendium of Nutrition Symbol Formats and associated graphic files to find the symbol that must appear on the label (refer to section 6.8 for how to obtain these documents).
- Refer to the Table of Contents to identify the section with the language and symbol orientation that matches the symbol formats identified in Step 4.
- In this example, the applicable section is Unilingual Horizontal Format.
- Within that section, find the relevant PDS range.
- In this example, the relevant range is > 600 cm2.
- Within that PDS range, find the symbol with the nutrient combination that applies to the product.
- In this example, the relevant symbols are Figures 1.2(EH) and 1.2(FH).
- Find the appropriate .EPS graphic files in the package.
Did you know? Different naming conventions are used to identify a given symbol format in the regulations and in the Compendium of Nutrition Symbol Formats. Appendix A in the Compendium shows how these different conventions align.
- Refer to the Table of Contents to identify the section with the language and symbol orientation that matches the symbol formats identified in Step 4.
- Determine where to place the symbol on the label.
Compare the height and width of the PDP to determine whether the symbol must appear in the upper half (50%) or in the right half (50%) of the PDP.
- In this example, the height of the PDP is greater than the width. Therefore, the symbol must be placed in the upper half (50%) of the PDP (see the Presentation section for more information).
- Consult the Directory of Nutrition Symbol Specifications to determine the dimension of the buffer surrounding the symbols.
There must be a buffer zone around the symbol without any text or other graphic material.
- Refer to the Table of Contents to identify the table with the language and symbol orientation that matches the symbol formats identified in Step 4.
- In this example, the relevant table is Table 1 - Unilingual Horizontal Format.
- Within that table, refer to Column 1 to identify the relevant PDS range.
- In this example, the relevant PDS range is > 600 cm2.
- Within that PDS range, refer to Column 4 to identify the minimum buffer dimension required.
- In this example, the minimum buffer dimension is 2.7 mm.
- Refer to the Table of Contents to identify the table with the language and symbol orientation that matches the symbol formats identified in Step 4.
Table A2.2. Excerpt of Table 1 from the Directory of Nutrition Symbol Specifications of the FDR
| Item | Column 1 Range of principal display surface | Column 2 Nutrition symbol in Schedule K.1 of the Food and Drug Regulations | Column 3 Nutrition symbol dimensions (width x height) | Column 4 Minimum buffer surrounding the nutrition symbol | Column 5 Height of upper case letters or tallest ascender of lower case letters (except for the words Health Canada / Santé Canada) | Column 6 Height of upper case letters or tallest ascender of lower case letters for the words Health Canada / Santé Canada | Column 7 "x" height of letters (except for the words Health Canada / Santé Canada) | Column 8 "x" height of letters for the words Health Canada / Santé Canada | Column 9 Height of the black / white bars | Column 10 Diameter of the lens of the magnifying glass |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | > 600 cm2 | 1(EH) and 1(FH) 2(EH) and 2(FH) 3(EH) and 3(FH) 4(EH) and 4(FH) 5(EH) and 5(FH) 6(EH) and 6(FH) 7(EH) and 7(FH) |
4.42 cm x 3.30 cm | 2.7 mm | 3.5 mm | 3.0 mm | 2.7 mm | 2.3 mm | 6.2 mm | 17.3 mm |
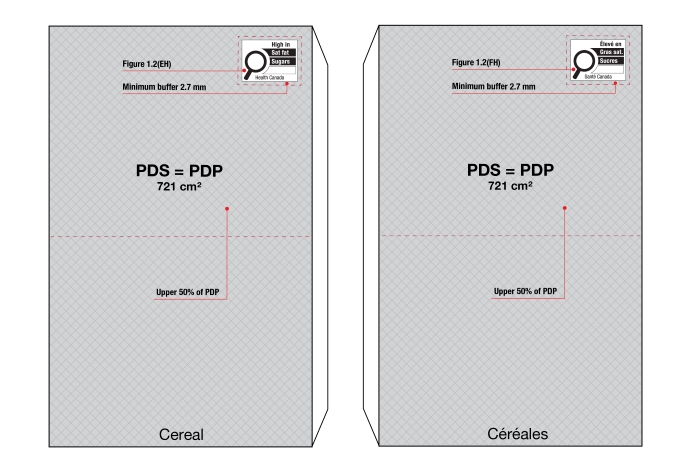
Figure A2.3 - Text description
This figure shows two rectangular boxes of cereal with a unilingual front panel, one in English and one in French. The front panels are crosshatched to identify them as the Principal Display Surface or PDS. They are also shaded grey to identify them as the Principal Display Panel or PDP. This means the PDS and PDP both have a total surface area of 721 cm2. The PDP is divided in half horizontally by a red dashed line. The top half is identified with the statement "Upper 50% of PDP".
On the English front panel, there is a horizontal nutrition symbol in the top right corner of the PDP that identifies the food as high in saturated fat and sugars. This symbol is in English only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "High in" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Sat fat" in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sugars" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is white, is outlined by a thin black line and contains no words. Centred at the bottom of the box are the words "Health Canada" in black, lower case letters, except that the first letter of each word is in upper case.
This symbol is identified as Figure 1.2(EH). There is a red dashed rectangular box around the nutrition symbol at a slight distance from it. That area is identified as Minimum buffer 2.7 mm.
On the French front panel, there is a horizontal nutrition symbol in the top right corner of the PDP that identifies the food as high in saturated fat and sugars. This symbol is in French only. There is a white rectangular box outlined by a thin black line. At the top of the box is the heading "Élevé en" in black, bold, lower case letters, except that the first letter of the first word is in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first bar is black and contains the words "Gras sat." in white, bold, lower case letters, except that the first letter of the first word is in upper case. The second bar is black and contains the word "Sucres" in white, bold, lower case letters, except that the first letter is in upper case. The third bar is white, is outlined by a thin black line and contains no words. Centred at the bottom of the box are the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
This symbol is identified as Figure 1.2(FH). There is a red dashed rectangular box around the nutrition symbol at a slight distance from it. That area is identified as Minimum buffer 2.7 mm.
Example B – required use of a vertical symbol
This example illustrates a scenario where the use of a vertical symbol is assessed and determined to be required.
Consider a bottle of juice for which it has been determined that the sugars content exceeds the threshold (see the Thresholds section for more information). Therefore, a symbol must appear on the label to indicate that the juice is "high in sugars".
These instructions describe the steps to follow to identify the symbol format required on the label.
- Identify the product's principal display surface (PDS).
Refer to the PDS definition to determine which surface of the package is considered the PDS.- The entire front of the bottle is displayed under normal conditions of sale. Therefore, it is considered the PDS.
- Calculate the PDS (size).
- The PDS is equal to 158 cm2. The PDS is crosshatched on the juice bottle in Figure A2.4.
Figure A2.4. PDS of the bottle of juice 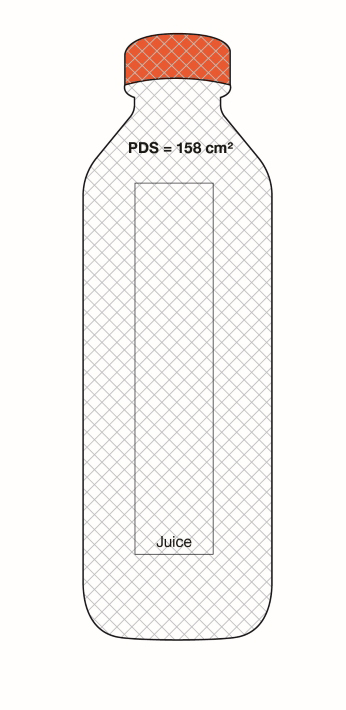
Figure A2.4 - Text description
This figure shows a white juice bottle with an orange cap. The front surface, including the cap, is crosshatched to identify it as the Principal Display Surface or PDS. The PDS has a total surface area of 158 cm2. Centered on the bottle is a thin black line that creates the outline of a rectangular label.
- The PDS is equal to 158 cm2. The PDS is crosshatched on the juice bottle in Figure A2.4.
- Identify the product's principal display panel (PDP).
Refer to the PDP definition to determine which part of the label is considered the PDP. The dimensions of the PDP determine where to place the symbol and validate which symbol orientation must be used.
- In this example, the PDP of the bottle is the part of the label that is applied to the PDS. The PDP is shaded on the bottle of juice in Figure A2.5. The shading overlaps with the crosshatching used to identify the PDS because the PDP covers a portion of the PDS.
Figure A2.5. PDS and PDP of the bottle of juice 
Figure A2.5 - Text description
This figure shows a white juice bottle with an orange cap. The front surface, including the cap, is crosshatched to identify it as the Principal Display Surface or PDS. The PDS has a total surface area of 158 cm2. Centered on the PDS is a rectangular label that is crosshatched and shaded grey. This identifies it as the Principal Display Panel or PDP.
- In this example, the PDP of the bottle is the part of the label that is applied to the PDS. The PDP is shaded on the bottle of juice in Figure A2.5. The shading overlaps with the crosshatching used to identify the PDS because the PDP covers a portion of the PDS.
- Consult the table to section B.01.352 of the Food and Drug Regulations (FDR) (Nutrition Symbols and Formats table) to identify the applicable nutrition symbol format.
- Refer to Column 1 (Range of principal display surface) to determine whether the PDS calculated in Step 2 corresponds to Item 1 (> 30 cm2) or Item 2 (≤ 30 cm2).
- The PDS of 158 cm2 corresponds to Item 1 (> 30 cm2).
- Within that item, refer to Column 2 (Nutrients that meet or exceed threshold […]) to find the "high in" nutrient combination that applies to the product.
- The juice is "high in sugars". Therefore, the applicable combination in Column 2 is Sugars.
- Choose a language option (unilingual or bilingual) to determine which column sets out the applicable format: Column 3, 4, 5 or 6.
- In this example, a bilingual symbol (with English text shown first) is used as the language option. This narrows down the relevant columns to Column 5 (Nutrition symbol in bilingual horizontal format) or 6 (Nutrition symbol in bilingual vertical format).
- Validate the symbol orientation (horizontal or vertical).
The regulations set out the horizontal format as the default orientation. The vertical format is required only if the product has a PDS ≤ 450 cm2 and the horizontal format that would otherwise need to be used on the label is wider than the PDP (see the Presentation section for more information).
- The PDS of this product is < 450 cm2, therefore it is relevant to compare the width of the horizontal symbol and the width of the PDP to determine whether a vertical symbol must be used.
- First, identify the symbol format in the default horizontal orientation.
Given that:
- the PDS falls within Item 1 of the table to B.01.352 of the FDR
- the juice is "high in sugars" and
- the language option chosen is bilingual
symbol format 6(BH) set out in Column 5 is the format in the default horizontal orientation.
Table A2.3. Excerpt from the table to section B.01.352 of the FDR Item Column 1 Range of principal display surface Column 2 Nutrients that meet or exceed threshold in subsection B.01.350(1) Column 3 Nutrition symbol in unilingual horizontal format Column 4 Nutrition symbol in unilingual vertical format Column 5 Nutrition symbol in bilingual horizontal format Column 6 Nutrition symbol in bilingual vertical format 1 > 30 cm2 Saturated fat (Sat fat), sugars and sodium 1(EH) and 1(FH) 1(EV) and 1(FV) 1(BH) 1(BV) Sugars 6(EH) and 6(FH) 6(EV) and 6(FV) 6(BH) 6(BV)
- Next, consult the Directory of Nutrition Symbol Specifications to find the width of the horizontal symbol. Refer to the Table of Contents to identify the table with the applicable language option in the horizontal orientation.
- In this example, the language option is bilingual. Therefore, the relevant table is Table 3 - Bilingual Horizontal Format.
- Within that table, refer to Column 1 to identify the relevant PDS range.
- In this example, the relevant PDS range is > 100 cm2 to ≤ 250 cm2.
- Within that PDS range, refer to Column 3 to find the width of the symbol in the default horizontal orientation.
- In this example, the width of the horizontal symbol (that is, format 6(BH)) is 3.60 cm.
Table A2.4. Excerpt of Table 3 from the Directory of Nutrition Symbol Specifications of the FDR
Table 3 Bilingual Horizontal Format Item Column 1 Range of principal display surface Column 2 Nutrition symbol in Schedule K.1 of the Food and Drug Regulations Column 3 Nutrition symbol dimensions (width x height) Column 4 Minimum buffer surrounding the nutrition symbol Column 5 Height of upper case letters or tallest ascender of lower case letters (except for the words Health Canada / Santé Canada) Column 6 Height of upper case letters or tallest ascender of lower case letters for the words Health Canada / Santé Canada Column 7 "x" height of letters (except for the words Health Canada / Santé Canada) Column 8 "x" height of letters for the words Health Canada / Santé Canada Column 9 Height of the black / white bars Column 10 Diameter of the lens of the magnifying glass 4 > 100 cm2 to ≤ 250 cm2 1(BH)
2(BH)
3(BH)
4(BH)
5(BH)
6(BH)
7(BH)3.60 cm x 1.89 cm 1.5 mm 2.0 mm 1.8 mm 1.5 mm 1.3 mm 3.5 mm 9.9 mm
- In this example, the width of the horizontal symbol (that is, format 6(BH)) is 3.60 cm.
- Refer to Column 1 (Range of principal display surface) to determine whether the PDS calculated in Step 2 corresponds to Item 1 (> 30 cm2) or Item 2 (≤ 30 cm2).
- Compare the width of the PDP and the width of the symbol in the default horizontal orientation to determine whether the vertical orientation must be used.
- In this example, the width of symbol 6(BH) is greater than the width of the PDP. Therefore, the vertical orientation must be used on the label.
- This eliminates Column 5 and means that the vertical symbol format set out in Column 6 (Nutrition symbol in bilingual vertical format) of the table to B.01.352 of the FDR must be used on the label. In this example, the applicable symbol in the vertical orientation is format 6(BV). Refer to Schedule K.1 of the FDR to see an image of this format.
Figure A2.6. Example where the vertical FOP nutrition symbol is required on the label: PDS is < 450 cm2 and the width of horizontal symbol exceeds the width of the PDP 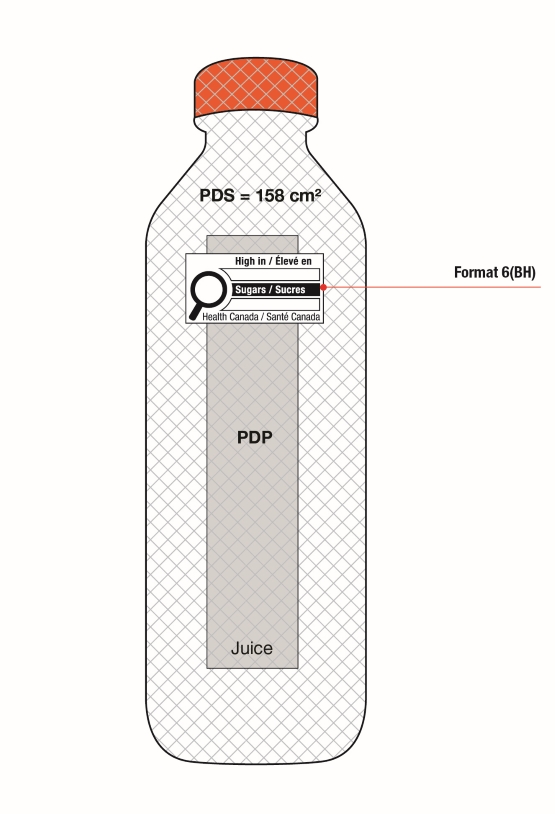
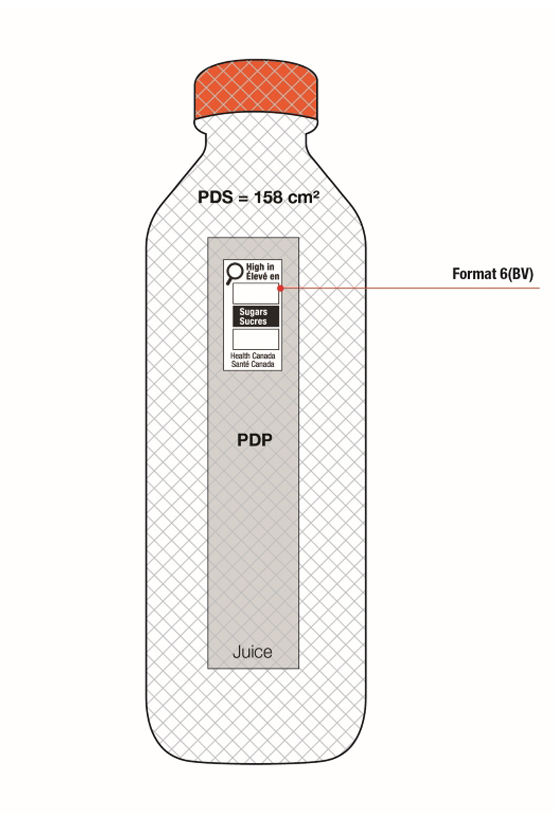
Figure A2.6 - Text description
This figure shows two white juice bottles side by side.
On the left is a white juice bottle with an orange cap. The front surface, including the cap, is crosshatched to identify it as the Principal Display Surface or PDS. The PDS has a total surface area of 158 cm2. Centred on the PDS is a rectangular label that is crosshatched and shaded grey. This identifies it as the Principal Display Panel or PDP.
There is a horizontal nutrition symbol at the top of the PDP that identifies the juice is high in sugars. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top of the box is a heading composed of the words "High in" followed by a forward slash and the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading is a left-justified black magnifying glass with three bars stacked to its right. There is a small amount of white space between the magnifying glass and the left side of the three bars. This left side forms a concave curve that follows the curvature of the magnifying glass. There is a small amount of white space between each bar, as well as between the right side of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" followed by a forward slash and the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred at the bottom of the box are the words "Health Canada" followed by a forward slash and the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
This symbol is identified as Format 6(BH). The symbol exceeds the width of the PDP on both sides.
To the right is a white juice bottle with an orange cap. The front surface, including the cap, is crosshatched to identify it as the Principal Display Surface or PDS. The PDS has a total surface area of 158 cm2. Centred on the PDS is a rectangular label that is crosshatched and shaded grey. This identifies it as the Principal Display Panel or PDP.
There is a vertical nutrition symbol at the top of the PDP that identifies the juice is high in sugars. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top left of the box is a black magnifying glass. To the right of the magnifying glass is a heading composed of the words "High in" above the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading are three bars that are stacked. There is a small amount of white space between each bar, as well as between both ends of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" above the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred at the bottom of the box are the words "Health Canada" above the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
This symbol is identified as Format 6(BV). The symbol is more narrow than the PDP.
- Consult the Compendium of Nutrition Symbol Formats and associated graphic files to find the symbol that must appear on the label (refer to section 6.8 for how to obtain these documents).
- Refer to the Table of Contents to identify the section with the language and symbol orientation that matches the symbol format identified in Step 4.
- In this example, the applicable section is Bilingual Vertical Format.
- Within that section, find the relevant PDS range.
- In this example, the relevant range is > 100 cm2 to ≤ 250 cm2.
- Within that PDS range, find the symbol with the applicable nutrient combination.
- In this example, the relevant symbol is Figure 4.6(BV).
- Find the appropriateness graphic file in the package.
- Refer to the Table of Contents to identify the section with the language and symbol orientation that matches the symbol format identified in Step 4.
- Determine where to place the symbol on the label.
Compare the height and width of the PDP to determine whether the symbol must appear in the upper half (50%) or in the right half (50%) of the PDP.
- In this example, the height of the PDP is greater than the width. Therefore, the symbol must be placed in the upper half (50%) of the PDP (see the Presentation section for more information).
- Consult the Directory of Nutrition Symbol Specifications to determine the dimension of the buffer surrounding the symbol.
There must be a buffer zone around the symbol without any text.
- Refer to the Table of Contents to identify the table with the language and symbol orientation that matches the symbol format identified in Step 4.
- In this example, the relevant table is Table 4 - Bilingual Vertical Format.
- Within that table, refer to Column 1 to find the relevant PDS range.
- In this example, the relevant range is > 100 cm2 to ≤ 250 cm2.
- Within that PDS range, refer to Column 4 to identify the minimum buffer dimension required.
- In this example, the minimum buffer dimension required is 1.5 mm.
Table A2.5. Excerpt of Table 4 from the Directory of Nutrition Symbol Specifications of the Food and Drug Regulations
Table 4 Bilingual Vertical Format Item Column 1 Range of principal display surface Column 2 Nutrition symbol in Schedule K.1 of the Food and Drug Regulations Column 3 Nutrition symbol dimensions (width x height) Column 4 Minimum buffer surrounding the nutrition symbol Column 5 Height of upper case letters or tallest ascender of lower case letters (except for the words Health Canada / Santé Canada) Column 6 Height of upper case letters or tallest ascender of lower case letters for the words Health Canada / Santé Canada Column 7 "x" height of letters (except for the words Health Canada / Santé Canada) Column 8 "x" height of letters for the words Health Canada / Santé Canada Column 9 Height of the black / white bars Column 10 Diameter of the lens of the magnifying glass 4 > 100 cm2 to ≤ 250 cm2 1(BV)
2(BV)
3(BV)
4(BV)
5(BV)
6(BV)
7(BV)1.75 cm x 3.31 cm 1.5 mm 2.0 mm 1.8 mm 1.5 mm 1.3 mm 6.3 mm 5.0 mm
- In this example, the minimum buffer dimension required is 1.5 mm.
- Refer to the Table of Contents to identify the table with the language and symbol orientation that matches the symbol format identified in Step 4.
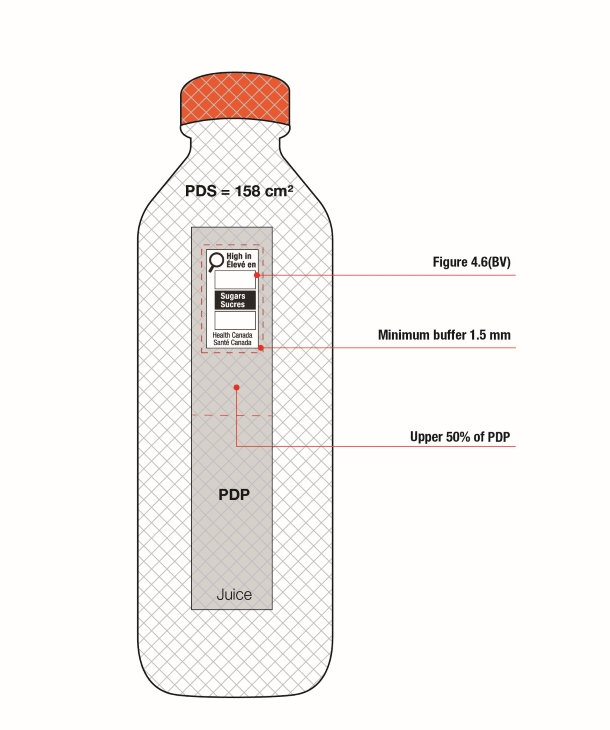
Figure A2.7 - Text description
This figure shows a white juice bottle with an orange cap. The front surface, including the cap, is crosshatched to identify it as the Principal Display Surface or PDS. The PDS has a total surface area of 158 cm2. Centered on the PDS is a rectangular label that is crosshatched and shaded grey. This identifies it as the Principal Display Panel or PDP.
The PDP is divided in half horizontally by a red dashed line. The top half is identified by the statement "Upper 50% of PDP".
There is a vertical nutrition symbol in the top half of the PDP that identifies the beverage as high in sugars. This symbol is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black line. At the top left of the box is a black magnifying glass. To the right of the magnifying glass is a heading composed of the words "High in" above the words "Élevé en" in black, bold, lower case letters, except that the first letter of the words "High" and "Élevé" are in upper case. Under the heading are three bars that are stacked. There is a small amount of white space between each bar, as well as between both ends of the bars and the thin black line that outlines the box. The first and third bars are white, are outlined by a thin black line and contain no words. The second bar is black and contains the word "Sugars" above the word "Sucres" in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred at the bottom of the box are the words "Health Canada" above the words "Santé Canada" in black, lower case letters, except that the first letter of each word is in upper case.
This symbol is identified as Figure 4.6(BV). There is a red dashed rectangular box around the nutrition symbol at a slight distance from it. That area is identified as Minimum buffer 1.5 mm.