Health Product InfoWatch: November 2022
Contents
- Health products mentioned in this issue
- Coronavirus disease (COVID-19)
- Drug and vaccine authorizations and communications for COVID-19
- Nuvaxovid COVID-19 Vaccine
- Spikevax Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran)
- Product monograph update for COVID-19 drugs
- Monthly recap of health product safety information
- New health product safety information
- Product monograph update:
- Adverse reactions to health products – annual report 2021
- Vaccine safety summary:
- Scope
- Reporting adverse reactions
- Helpful links
- Suggestions?
- Copyright
Health products mentioned in this issue
Pharmaceuticals and biologics
Evusheld (tixagevimab and cilgavimab)
Metronidazole Injection, USP (metronidazole)
Nuvaxovid COVID-19 Vaccine
Potassium Chloride in Lactated Ringer's Injection
Spikevax Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran)
Natural and non-prescription health products
Children’s ibuprofen/acetaminophen

Download the alternative format
(PDF format, 1 .57 MB, 11 pages)
Health Products and Food Branch
Marketed Health Products Directorate
Health Product InfoWatch Editorial Team
ISSN:2368-8025
Cat.:H167-1E-PDF
Pub.:210715
Coronavirus disease (COVID-19)
For the most up-to-date information on COVID-19, please visit the Government of Canada Coronavirus disease (COVID-19) website Canada.ca/coronavirus, which includes a dedicated section for healthcare professionals, and for the health product industry.
The COVID-19 vaccines and treatments portal provides information for consumers, healthcare professionals and researchers on vaccines and treatments authorized for COVID-19.
For information about adverse events following immunization that individuals have reported after receiving a COVID-19 vaccine in Canada, new safety signals or other safety updates, please visit the COVID-19 vaccine safety in Canada webpage.
Drug and vaccine authorizations and communications for COVID-19
New information and recent communications related to authorized COVID-19 vaccines and treatments are highlighted in this section.
Nuvaxovid COVID-19 Vaccine
On November 17, 2022, Health Canada authorized Nuvaxovid COVID-19 Vaccine as a booster dose in individuals 18 years of age and older. The booster dose of Nuvaxovid (0.5 mL) may be administered intramuscularly approximately 6 months after completion of the second dose of the primary series.
Authorization with terms and conditions: Nuvaxovid COVID-19 Vaccine
Spikevax Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran)
On November 3, 2022, Health Canada authorized Spikevax Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran) as a booster dose for active immunization against COVID-19 in individuals 18 years of age and older. In order to provide rapid access to the vaccine, Moderna will distribute product vials and cartons labelled in English only with the brand name “Spikevax Bivalent Original / Omicron BA.4/BA.5” for a period of time. Important Canadian-specific information is absent from these labels.
Both Moderna bivalent COVID-19 vaccine presentations, Spikevax Bivalent Original / Omicron BA.4/5 and Spikevax Bivalent (targeting Original / Omicron BA.1), have the same royal blue vial cap as monovalent Spikevax 0.10 mg/mL, 2.5 mL. The royal blue cap represents the 0.10 mg/mL product concentration and should NOT be used alone to identify the product. To avoid medication errors, pay careful attention to the vaccine name and the vial and carton labels.
The vial and carton label of Spikevax Bivalent Original / Omicron BA.4/BA.5 have a grey border and the concentration (0.1 mg/mL) is indicated in a grey band. The booster dose (50 mcg) and dose volume (0.5 mL) do not appear on the English-only vial and /or carton labels.
Health Product Risk Communication: Spikevax Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran)
Authorization with terms and conditions: Spikevax Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran)
Product monograph update for COVID-19 drugs
The following safety labelling update, which was recently made to the Canadian product monograph, has been selected for your awareness. Canadian product monographs for authorized vaccines and treatments for COVID-19 can be accessed through the COVID-19 vaccines and treatments portal or Health Canada's Drug Product Database.
Evusheld (tixagevimab and cilgavimab)
The Dosage and Administration, Adverse Reactions, and Patient Medication Information sections of the Canadian product monograph for Evusheld have been updated with information reflecting the increased initial dose and guidance on repeat dosing when Evusheld is used for pre-exposure prophylaxis of COVID-19.
Key messages for healthcare professionals:Footnote 1
- The initial Evusheld dose for pre-exposure prophylaxis of COVID-19 has been increased from 300 mg to 600 mg (300 mg of tixagevimab and 300 mg of cilgavimab), administered as two separate 3 mL, sequential, intramuscular injections.
- For individuals who require repeat dosing for ongoing prevention of COVID-19, subsequent doses of 600 mg of Evusheld (300 mg of tixagevimab and 300 mg of cilgavimab) should be given once every 6 months.
- The dose recommendations for prophylaxis are based on the available data including clinical pharmacology, pharmacokinetics, antiviral activity, and clinical trial data. Detailed information can be found in the Canadian product monograph for Evusheld.
Reference
- Footnote 1
-
Evusheld (tixagevimab and cilgavimab) [product monograph]. Mississauga (ON): AstraZeneca Canada Inc., 2022.
Monthly recap of health product safety information
The following is a list of health product advisories, type I recalls and summaries of completed safety reviews published in October 2022 by Health Canada.
For health product advisories related to COVID-19 vaccines and treatments, please see the Drug and vaccine authorizations and communications for COVID-19 section.
Children’s ibuprofen/acetaminophen
There is a current shortage of acetaminophen and ibuprofen products for infants and children across Canada. There has been unprecedented demand for these products, and while supply is increasing, shortages continue. To keep Canadians informed on the situation and the actions that Health Canada is taking, the Department has created a webpage dedicated to the shortage of infant and children's acetaminophen and ibuprofen.
Advisory: Children’s ibuprofen/acetaminophen
Potassium Chloride in Lactated Ringer's Injection, USP
One lot of Potassium Chloride in Lactated Ringer's Injection, USP was recalled as the affected lot is labelled with the incorrect strength and ingredient.
Type 1 drug recall: Potassium Chloride in Lactated Ringer's Injection, USP
Unauthorized health products
Health Canada advised Canadians about various unauthorized health products being sold at retail locations across Canada or online that may pose serious health risks.
Advisory: Unauthorized products may pose serious health risks
New health product safety information
The following topics have been selected to raise awareness and encourage reporting of adverse reactions.
Product monograph update
The following safety labelling update, which was recently made to the Canadian product monograph, has been selected for your awareness. A complete list of safety labelling updates for pharmaceuticals is available on Health Canada's Product monograph brand safety updates page. Canadian product monographs can be accessed through Health Canada's Drug Product Database.
Metronidazole Injection, USP (metronidazole)
The Contraindications, Adverse Reactions (Post-Market Adverse Reactions), and Patient Medication Information sections of the Canadian product monograph for Metronidazole Injection, USP have been updated with the risk of severe irreversible hepatotoxicity/acute liver failure with fatal outcomes in patients with Cockayne syndrome.
Key messages for healthcare professionals:Footnote 1
- Metronidazole Injection, USP is contraindicated in patients with Cockayne syndrome.
- Cases of severe irreversible hepatotoxicity/acute liver failure, including cases with fatal outcomes with very rapid onset after initiation of systemic use of metronidazole, have been reported in patients with Cockayne syndrome (latency from drug start to signs of liver failure as short as 2 days).
Reference
- Footnote 1
-
Metronidazole Injection, USP (metronidazole) [product monograph]. Mississauga (ON): Baxter Corporation, 2022.
Adverse reactions to health products – annual report 2021
Introduction
Post-market reporting systems help in the identification and analysis of new safety information for health products so that appropriate action can be taken to minimize risks to human health. In Canada, adverse reactions (ARs), or medical device incidents (MDIs), suspected of being associated with the use of health products can be reported to the Canada Vigilance Program (CVP). This report summarizes information about domestic AR cases reported for pharmaceuticals, natural health products, biologics, radiopharmaceuticals, disinfectants, and sanitizers with disinfectant claims received by the CVP in 2021. Although foreign AR cases are included in the internal CVP database, these are not included in this report.
Domestic adverse reaction reports and cases
In 2021, Health Canada received 194,560 domestic post-market AR reports. These reports represented 81,211 AR cases (Table 1). A case consists of all information describing the AR(s) experienced by one patient at one time, which is suspected of being related to the use of one or more health products. A case may include an initial AR report and possibly several follow-up reports that provide additional information. Duplicate cases may exist if an AR report about the same event was received from different reporters (e.g., from a healthcare professional, consumer, hospital, and/or manufacturer).
| Product type | No. (%) of reports |
|---|---|
| Pharmaceuticals | 42,520 (52.4) |
| BiologicsFootnote * | 36,821 (45.3) |
| Radiopharmaceuticals | 502 (0.6) |
| Natural health products | 468 (0.6) |
| OtherFootnote † | 900 (1.1) |
| Total | 81,211 (100) |
| |
In Canada, Market Authorization Holders (MAHs) and hospitals are required to submit AR reports to the CVP in accordance with the requirements of the Food and Drugs Act and its Regulations. For serious ARs that have occurred in Canada, MAHs are required to send a report within 15 days of becoming aware of the incident. In accordance with the Protecting Canadians from Unsafe Drugs Act (Vanessa’s Law), hospitals are required to send, within 30 days of documentation, all reports of serious ARs to therapeutic products that have been documented in their facility. Community members (consumers, patients and non-hospital-based healthcare professionals) can voluntarily submit AR reports at any time.
In 2021, MAHs submitted 87.2% of all domestic AR cases. The remaining cases were mainly submitted by community members (6.3%) and hospitals (6.2%). For most of the domestic AR cases reported to Health Canada directly or via a MAH, the originating reporter was a healthcare professional (Figure 1).
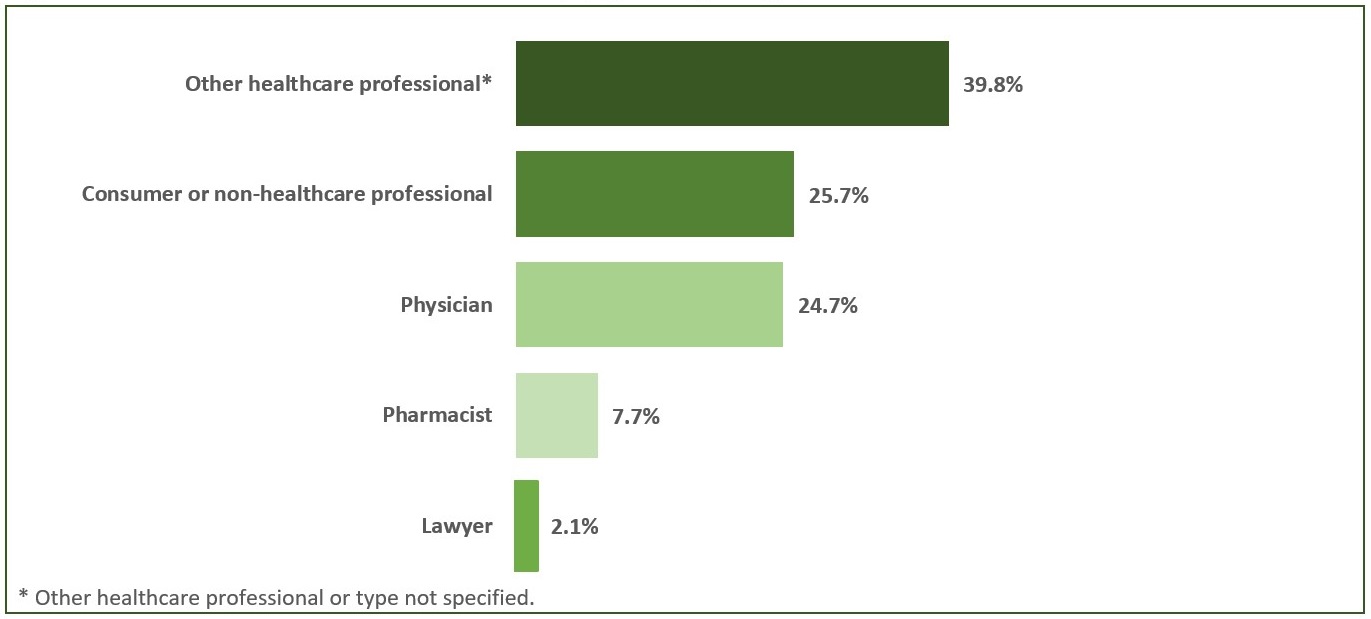
Figure 1 - Text description
The figure shows the percentage of number of domestic AR cases reported in 2021 by type of originating reporter.
*Other health professional or type not specified.
| Originating reporter | Number of cases by percentage |
|---|---|
| Other health professional* | 39.8% |
| Consumer Or Non-Health Professional | 25.7% |
| Physician | 24.7% |
| Pharmacist | 7.7% |
| Lawyer | 2.1% |
Sex and age distribution
The distribution for the 81,211 cases by sex was 56.9% female, 38.9% male and 4.2% unknown or unreported, which reflects the sex distribution of AR cases seen globally.Footnote 1 The distribution by age group was 3.4% pediatric (< 19 years), 52.3% adult (19-64 years), 26.6% elderly (≥ 65 years) and 17.6% age unknown or unreported.
Suspect products and adverse reactions
The top 10 groups of suspect products most commonly identified in domestic AR cases are listed in Table 2. The groups are classified according to the World Health Organization's Anatomical Therapeutic Chemical (ATC) classification system.
| Health product (ATC group) | No. (%) of times reportedFootnote ‡ |
|---|---|
| Immunosuppressants (L04) | 33,586 (41.4) |
| Antineoplastic agents (L01) | 12,148 (15.0) |
| Vaccines (J07) | 6,384 (7.9) |
| Analgesics (N02) | 5,948 (7.3) |
| PsycholepticsFootnote § (N05) | 3,551 (4.4) |
| Drugs for obstructive airway diseases (R03) | 2,942 (3.6) |
| Corticosteroids for systemic use (H02) | 2,515 (3.1) |
| Antiepileptics (N03) | 2,408 (3.0) |
| Antibacterials for systemic use (J01) | 2,126 (2.6) |
| Antidiarrheals, intestinal antiinflammatory/antiinfective agents (A07) | 1,831 (2.3) |
| |
Table 3 displays the top 10 domestic ARs reported to the CVP based on System Organ Class codes. The ARs are coded using Medical Dictionary for Regulatory Activities (MedDRA) terminology. The most commonly reported ARs were general disorders and administration site conditions, which include disorders that affect several body systems or sites (e.g., drug ineffective, fatigue, fever, edema, pain, reactions at the administration site), followed by injury, poisoning and procedural complications.
| System Organ Class | No. (%) of times reportedFootnote ‡ |
|---|---|
| General disorders and administration site conditions | 44,767 (55.2) |
| Injury, poisoning and procedural complications | 21,666 (26.7) |
| Gastrointestinal disorders | 18,116 (22.4) |
| Nervous system disorders | 15,196 (18.8) |
| Investigations | 14,626 (18.0) |
| Infections and infestations | 14,620 (18.0) |
| Musculoskeletal and connective tissue disorders | 13,381 (16.5) |
| Skin and subcutaneous tissue disorders | 10,270 (12.7) |
| Respiratory, thoracic and mediastinal disorders | 9,992 (12.3) |
| Psychiatric disorders | 9,552 (11.8) |
|
|
Reason for seriousness
Of the 81,211 AR cases, 74.3% were considered serious.Footnote * A case can have more than one reported reason for seriousness. In 2021, 22.6% of all AR cases indicated that hospitalization was required, 2.7% indicated a life-threatening condition, and 7.6% indicated a death had occurred.
Investigation of reported adverse reactions
As part of routine surveillance monitoring activities, an AR report submitted to the CVP is assessed for potential safety issues and signal detection through progressing levels of escalation. Results of concern are presented to the evaluation bureau responsible for the product for signal confirmation, prioritization, and assessment.
When a reported AR is known and included in the product monograph, it is not considered to be a new signal, unless there is a change in the frequency or severity of the AR. Post-market ARs may be attributed to a variety of factors, including previously unrecognized pharmacological effects of the product, idiosyncratic effects, drug interactions (e.g., drug-drug, drug-disease, drug-natural health product interactions), individual patient factors (e.g., pharmacogenomic factors), medication incidents, or other factors that may have been too infrequent to be identified in clinical trials.
It is difficult to compare the risk of health products based solely on submitted reports. Several factors may influence AR reporting patterns, such as the known risks associated with a product, the length of time a product has been on the market, volume of use, publicity about an AR, regulatory actions taken to minimize risks, and/or method of data collection. For example, rare and serious ARs may be reported more frequently in organized data collection systemsFootnote † compared to voluntary reporting, which may affect the pattern of reporting. In general, there is underreporting of adverse events to spontaneous reporting programs like the CVP.
AR reports are an important part of Health Canada’s monitoring of health products. AR reports, along with other sources of information from domestic and international sources, help in the identification and analysis of new safety information and support Health Canada's decisions to take action. For example, a causal association between the product and the AR may prompt an action from Health Canada. The same applies if new risks are determined from a cluster of similarly reported ARs, or from AR reports suggesting labelling gaps or product quality issues. Drug-related information received and assessed by Health Canada may lead to actions that include informing Canadians and healthcare professionals about new safety information, recommending label changes, or removing a drug product from the market.
In 2021, drug-related information received and assessed by Health Canada resulted in over 1,180 actions: 26 safety signals, 22 regulatory actions, 33 health product risk communications, and addressing over 1,100 false and misleading advertisements. The important new safety information for healthcare professionals and Canadians is communicated via the Recalls and Safety Alerts Database on the Healthy Canadians website. The new safety information is also distributed through the MedEffect™ e-Notice email notification system. In addition, Health Canada published 24 summaries of its safety reviews, which describe Health Canada’s findings and risk management actions related to potential safety issues.
Conclusion
Each year, the CVP receives thousands of reports that contribute to a better understanding of the safety associated with marketed health products. Health Canada would like to thank all who have contributed information and encourage the continued support of post-market surveillance through AR and MDI reporting. Any ARs or MDIs suspected of being associated with the use of health products should be reported to the CVP. Every report counts, and together, they tell a story.
References
- Footnote 1
-
Watson, S., Caster, O., Rochon, P.A. & den Ruijter, H. (2019). Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine, 17, 100188, ISSN 2589-5370. https://doi.org/10.1016/j.eclinm.2019.10.001
Vaccine safety summary
Health Canada and the Public Health Agency of Canada (PHAC) share the responsibility of monitoring the safety of vaccines in Canada.
Market authorization holders are required to report serious adverse events following immunization (AEFIs) to the Canada Vigilance Program in Health Canada. The Canada Vigilance Program also receives voluntary reports from healthcare professionals and consumers.
Provincial and territorial public health authorities report AEFIs from publicly funded vaccine programs to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) in PHAC.
Summary for July 1, 2020 to December 31, 2020
Key messages:
- From July 1, 2020 to December 31, 2020, the Canada Vigilance Program received 533 reports of adverse events following immunization for which vaccines were a suspected cause.
- No new safety signals (potential safety issues) were identified during this period.
This vaccine safety summary includes reports of adverse events following immunization (AEFIs) received by the Canada Vigilance Program between July 1, 2020 and December 31, 2020 for vaccines not including COVID-19 vaccines. To access summaries published by CAEFISS, please visit the CAEFISS Web site. For information about AEFIs that individuals have reported after receiving a COVID-19 vaccine in Canada, please visit the Reported side effects following COVID-19 vaccination in Canada webpage.
- From July 1, 2020 to December 31, 2020, the Canada Vigilance Program received 533 reportsFootnote ‡ of adverse events following immunization for which vaccines were a suspected cause.
- The majority of the reports were received from healthcare professionals (Figure 1) through spontaneous reporting.
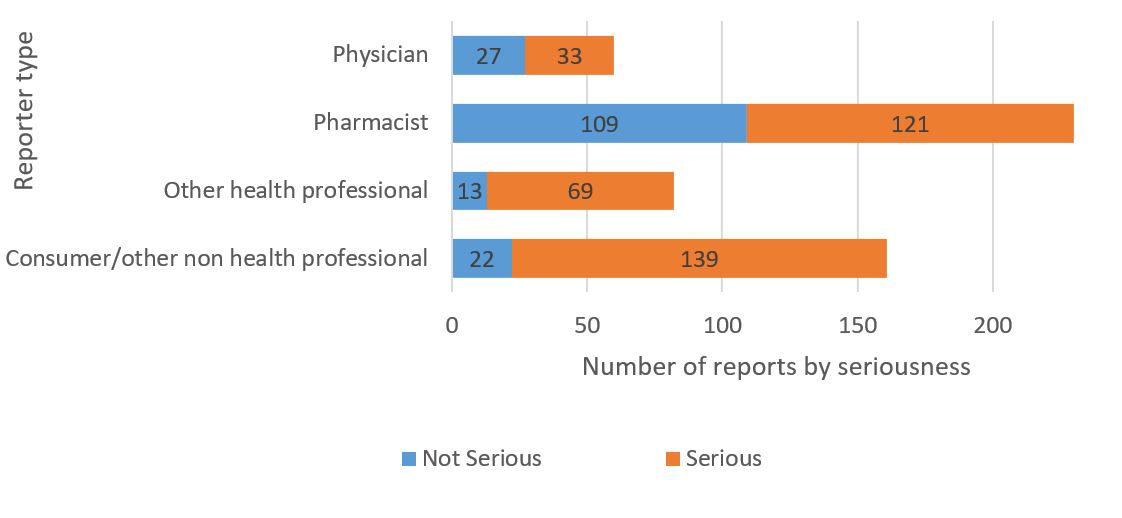
Figure 1 - Text description
| Reporter type | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Physician | 27 | 33 |
| Pharmacist | 109 | 121 |
| Other health professional | 13 | 69 |
| Consumer or other non health professional | 22 | 139 |
- Most of the reports involved adults between 19 and 64 years of age (183 out of 533: 34%) (Figure 2).
- The distribution for the 533 reports by sex was 56% female, 34% male and 10% unknown.
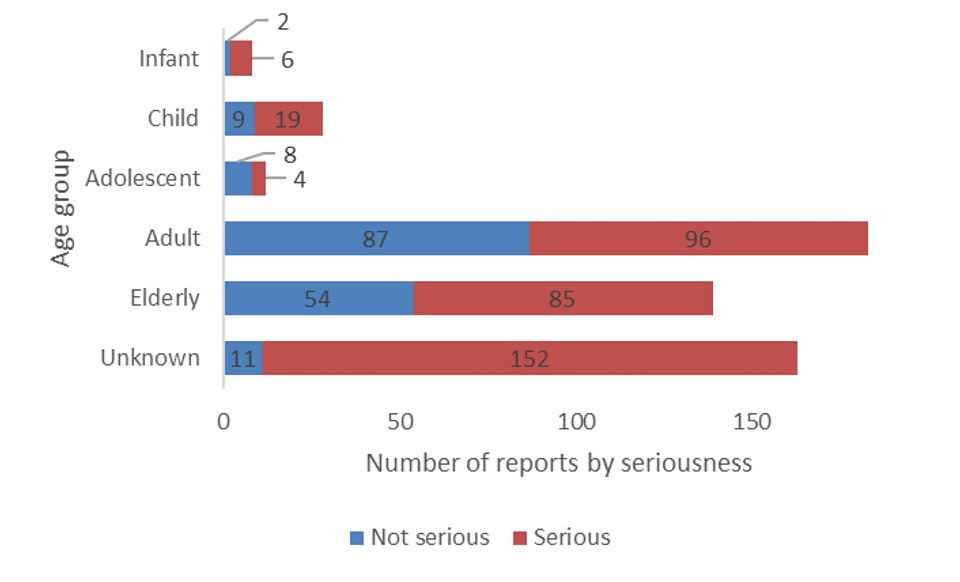
Figure 2 - Text description
| Age group | Number of non-serious reports | Number of serious reports |
|---|---|---|
| Infant (28 days to 23 months) | 2 | 6 |
| Child (2-11 years) | 9 | 19 |
| Adolescent (12-18 years) | 8 | 4 |
| Adult (19-64 years) | 87 | 96 |
| Elderly (65 years and above) | 54 | 85 |
| Unknown | 11 | 152 |
- The highest number of reports (serious and non-serious) involved influenza vaccines (240 reports) followed by herpes zoster vaccines (217 reports) and pneumococcal vaccines (31 reports) (Figure 3).
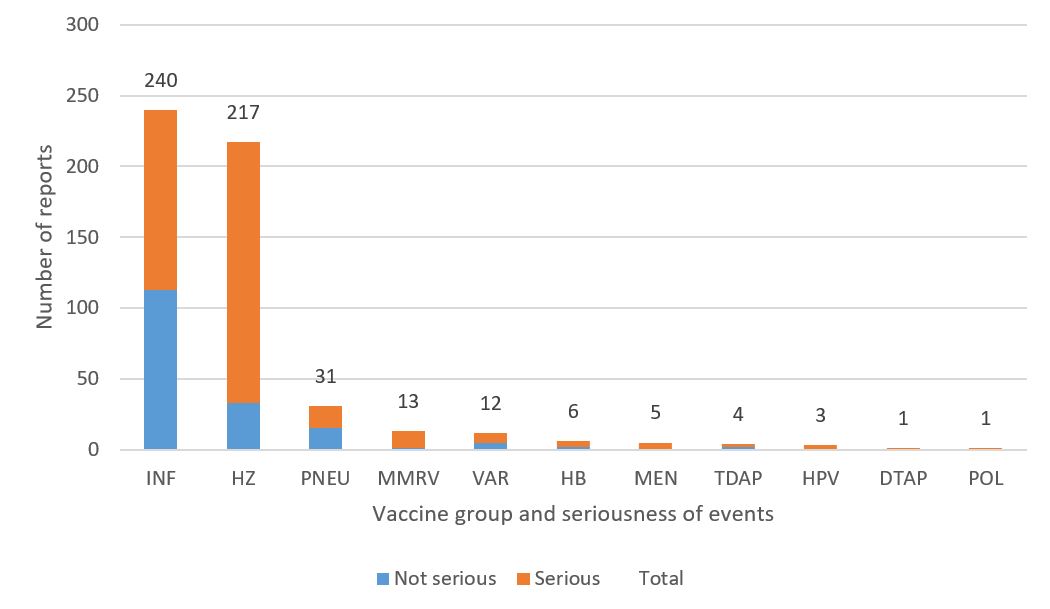
Figure 3 - Text description
| Vaccine type | Number of non-serious reports | Number of serious reports | |
|---|---|---|---|
| Influenza | INF | 113 | 127 |
| Herpes zoster | HZ | 33 | 184 |
| Pneumococcal | PNEU | 15 | 16 |
| Measles, mumps, rubella, varicella | MMRV | 1 | 12 |
| Varicella | VAR | 5 | 7 |
| Hepatitis B | HB | 2 | 4 |
| Meningococcal | MEN | 0 | 5 |
| Tetanus, diphtheria (reduced), acellular pertussis | TDAP | 2 | 2 |
| Human papillomavirus | HPV | 0 | 3 |
| Diphteria, tetanus, acellular pertussis | DTaP | 0 | 1 |
| Poliomyelitis | POL | 0 | 1 |
- Of the 533 reports, 362 (68%) were serious reports. The most frequently reported serious adverse events included herpes zoster, vaccination failure, pain, rash, swelling, erythema, dyspnea, pyrexia, pruritus, arthralgia and headache. Most of these involved patients with underlying medical conditions and/or concomitant medications, and the serious adverse events were unlikely related to the vaccination.
- There were 6 reports with an outcome of death; 2 reports involved males and 4 involved females. Five cases occurred in individuals 65 years of age and older. One case did not report age. The reported vaccines were: influenza vaccine (4), herpes-zoster vaccine (1), and pneumococcal vaccine (1). The information provided in the reports was not sufficient to assess the causal association with the vaccine.
- No new safety signals (potential safety issues) were identified during this period.
- The benefits of vaccines authorized in Canada continue to outweigh the risks.
- Health Canada, in collaboration with PHAC, will continue to closely monitor the safety of vaccines authorized in Canada.
For additional information, contact the Marketed Health Products Directorate.
Note that because of updated information received by the Canada Vigilance Program, there may be differences in the number of reports and adverse events retrieved at different dates.
Scope
This monthly publication is intended primarily for healthcare professionals and includes information on pharmaceuticals, biologics, medical devices and natural health products. It provides a summary of key health product safety information published in the previous month by Health Canada, as well as a selection of new health product safety information meant to raise awareness. New information contained in this issue is not comprehensive but rather represents a selection of clinically relevant items warranting enhanced dissemination.
Reporting Adverse Reactions
Canada Vigilance Program
Telephone: 1-866-234-2345
Fax or mail: Form available on MedEffect Canada
For more information on how to report an adverse reaction, visit the Adverse Reaction and Medical Device Problem Reporting page.
Helpful links
- MedEffectTM Canada
- Recalls and Safety Alerts Database
- New Safety and Effectiveness Reviews
- Canada Vigilance Adverse Reaction Online Database
- Drug Product Database
- Medical Devices Active Licence Listing
- Licensed Natural Health Products Database
- The Drug and Health Product Register
- Drug Shortages Canada
- Medical device shortages: List of shortages and discontinuations
- Stop Illegal Marketing of Drugs and Devices
- List of drugs for exceptional importation and sale
- Drug and vaccine authorizations for COVID-19: List of authorized drugs, vaccines and expanded indications
- Reported side effects following COVID-19 vaccination in Canada
Suggestions?
Your comments are important to us. Let us know what you think by reaching us at: infowatch-infovigilance@hc-sc.gc.ca
Health Product InfoWatch Editorial Team
Marketed Health Products Directorate
Health Canada
Address Locator 1906C
Ottawa ON K1A 0K9
Telephone: 613-954-6522
Teletypewriter: 1-800-465-7735 (Service Canada)
Copyright
© 2022 His Majesty the King in Right of Canada. This publication may be reproduced without permission provided the source is fully acknowledged. The use of this publication for advertising purposes is prohibited. Health Canada does not assume liability for the accuracy or authenticity of the information submitted in case reports.
Adverse reactions (ARs) to health products are considered to be suspicions, as a definite causal association often cannot be determined. Spontaneous reports of ARs cannot be used to estimate the incidence of ARs because ARs remain underreported and patient exposure is unknown.
Due to time constraints relating to the production of this publication, information published may not reflect the most current information.
- *
-
In the Food and Drugs Act and Regulations, a serious AR is defined as “a noxious and unintended response to a drug that occurs at any dose and that requires in-patient hospitalization or prolongation of existing hospitalization, causes congenital malformation, results in persistent or significant disability or incapacity, is life-threatening or results in death”. Other situations may also warrant a designation as serious, “such as medically important events that may not be immediately life-threatening or result in death or hospitalization but may jeopardize the patient or may require intervention to prevent one of the other outcomes listed in the definition from the Regulations”.
- †
-
Organized data collection systems include patient registries, surveys, and patient support or disease management programs.
- ‡
-
Glossary of Fields in the Canada Vigilance Adverse Reaction Online Database