Food and nutrition highlights 2020: Helping you maintain and improve your health
Download in PDF format
(6.24 MB, 48 pages)
Organization: Health Canada
Published: 2021-07-12
ISBN: 2563-3376
Learn about what Health Canada is doing to improve the food environment in Canada, and make it easier for Canadians to make healthier choices, as well as our other accomplishments in 2020.
On this page
- Welcome to Health Canada's annual highlights report
- Message from the Chief Medical Advisor
- Message from Chief Regulatory Officer
- Food and nutrition: 2020 in brief
- Key accomplishments
- Approved novel foods
- Approved food additives
- Approved infant formulas
- Healthy clicks: Food and nutrition at a glance
Welcome to Health Canada's annual highlights report

Assistant Deputy Minister

Associate Assistant Deputy Minister
Although the COVID-19 pandemic brought unprecedented changes to our lives and our work, we remain focused on ensuring Canadians continue to have access to safe and nutritious food. We also provided the information needed to make safe and healthy nutritional choices during these difficult times.
To support Canadians during this past year, we provided information to help them make healthy eating as easy as possible during the pandemic.
While there is no scientific evidence indicating that COVID-19 can be spread through food or food packaging, our scientists continue to monitor the situation closely and conduct research to identify any potential food safety risks. Their scientific advice informs every action we take to protect the safety of Canada's food supply.
We are also providing information on food safety during the pandemic so Canadians have the guidance they need to protect themselves and their loved ones. Our advice for safe food handling and cooking remains the same, but we have added tips for safe grocery shopping and food delivery.
This report will give you an overview of the work we undertook in 2020 to support healthy eating, food safety, and innovation, as well as how we contributed to the Government of Canada's comprehensive response to address the COVID-19 pandemic.
Message from the Chief Medical Advisor

Chief Medical Advisor
At a time when Canadians are navigating their way through a challenging time in their lives, we are providing easy-to-follow, science-based advice on how to eat a nourishing diet while guarding themselves from COVID-19. To assist Canadians in protecting themselves and their loved ones, we are offering comprehensive information on the pandemic and food safety.
If Canadians are having food delivered to their homes, we recommend using contactless payment whenever possible, and asking for contactless delivery if available (dropped in your trunk or on your doorstep, for example). Keep your distance, at least 2 metres, from the delivery person, and remember to wash your hands after receiving your order. Take care in putting your groceries away, ensuring that the items that need refrigeration find their way into the fridge, and remembering to wash your hands again after handling the food and food packaging.
Meal planning is especially important right now, to avoid shopping more than necessary. Maintaining and following a grocery list helps you be prepared at a time when we are advised to limit the number of trips to the grocery store, and number of household members doing the grocery shopping.
If you are grocery shopping in-person, use hand sanitizer, at the entrance, when it is available. Follow the 2 metres-apart rule from shoppers and staff. Wear a non-medical mask or face covering while out in public, particularly in crowded settings where it may not be possible to maintain a safe physical distance consistently from others. Avoid touching items you are not going to buy, and take care to avoid touching your eyes, nose, and mouth. As always, remember to wash your hands when you return home, and again after you've handled your grocery items.
Further, Canadians can lower the risk of infection and kill the virus that causes COVID-19 by following safe food handling and cooking practices.
As always, health and safety is our top priority. To help Canadians protect themselves, and those around them, from the pandemic, we continue to provide them with trusted, evidence-based advice.
Message from Chief Regulatory Officer

Chief Regulatory Officer
The COVID-19 pandemic dominated our work as a regulatory authority for health products and food during the past year, but Canadian laws and regulations were challenged and new pathways were used during this unprecedented time.
To help enable Canadians' continued access to specialized food products, we developed an interim order to allow designated foods to be imported into Canada in the event of a shortage caused by possible supply chain disruptions or demand surges caused by the pandemic, while protecting the health of Canadians. We took this action to ensure that vulnerable Canadians would have continued access to the specialized food products they needed, such as infant formulas for babies who needed it. We used the interim order to permit the exceptional import of two infant formulas to ensure Canadians' continued access to the product despite supply chain challenges identified by the manufacturer.
Two years ago, the Government launched a targeted regulatory review of the agri-food and aquaculture sector. In response to the findings of this review, Health Canada committed to a suite of policy and regulatory initiatives to address barriers to growth and innovation, and to bring much needed agility and coherence to our food regulations. These commitments are the foundation of our Food Regulatory Innovation Agenda, which sets out a concrete plan for how we will change the way we regulate food to meet the needs of the future. While COVID required us to adjust some of our timelines, progress on key regulatory innovation commitments was made in 2020, and we will continue to build on this momentum.
Stakeholders have told us how important it is to have an agile regulatory system that will support innovation while protecting health and safety. In order to ensure that our regulatory framework can respond to the trends, challenges, and innovative products that may be emerging during the next five to 10 years, we have launched a foresight exercise. Having a better understanding of what the future may bring will ensure the continued effectiveness of our regulations. In this exercise, we are relying on strong expertise, in policy, the development of regulations, our science, and our monitoring activities, along with the insights shared by our stakeholders, to help us anticipate the demands that we will need to address. The exercise is giving us the knowledge and insights needed to inform the next phases of modernizing our food regulations.
Food and nutrition: 2020 in brief

Director General,
Office of Nutrition Policy and Promotion

Director General,
Food Directorate
Although the importance of healthy eating and overall nutritional well-being is well recognized, the COVID-19 pandemic highlighted the difficulties Canadians face in maintaining a healthy diet.
Data have shown that, during the pandemic, Canadians have been consuming larger quantities of junk food and sweets. Trends like these are concerning because they are associated with diet-related chronic diseases, such as obesity, heart disease, hypertension, and diabetes, which are also risk factors for more severe cases of COVID-19.
The pandemic underscored the relationship between poor diets, obesity, chronic conditions and COVID-19. It has also highlighted the importance of creating environments that support healthy eating to help decrease chronic diseases rates, as identified in the Healthy Eating Strategy.
Health Canada strives to create a supportive food environment for Canadians, to make the healthier food choice easier for everyone. We do this by promoting healthy eating, assessing the health risks and benefits of food, and by providing trusted information about the safety and nutritional quality of what we eat.
Our policies, standards, regulations and guidelines are grounded in scientific evidence. We conduct research, and rely on the latest findings related to nutrition and food safety to ensure our work reflects the strongest evidence.
We remain committed to promoting healthy eating, including continuing to work to introduce new restrictions on the commercial marketing of certain foods and beverages to children, and establishing new front-of-package nutrition labelling. For a clearer picture of the extent of children's exposure to food advertising, we continued to monitor food and beverage marketing aimed at kids. We also collaborated with our international partners to test a monitoring framework, to provide further insight on how marketing affects children in this digital age.
To this end, the department continued to work throughout 2020 to promote, and implement, evidence-based nutrition policies and standards, and advance initiatives under the Healthy Eating Strategy. We also continue to promote healthy eating through our monthly food guide newsletter. To empower Canadians with reliable advice about making better eating choices in the face of difficult circumstances, we have updated our online content. Resources now include advice on meal planning, shopping for nutritious foods with longer shelf lives, and eating mindfully during the pandemic. In order to advance our work under the Healthy Eating Strategy, we also revised the sodium reduction targets for processed foods in 2020.
Healthy eating can reduce chronic disease and reduce societal costs, including health care costs. Despite progress to date, there remains much to do. In order to guide our efforts, we continue to monitor the food supply, and the impact the Healthy Eating Strategy is having on the nutritional quality of foods sold in Canada. In particular, we are studying the availability of foods that contain high amounts of saturated fat, sugars and sodium, which are nutrients of public health concern. In this report, we outline the progress we are making in improving the food environment in Canada.
While the COVID-19 pandemic has challenged all of us, Health Canada continues to maintain its strong reputation as a global food regulator. At their core, Canada's food regulations have in place the necessary safeguards to ensure we maintain one of the safest food supplies in the world. However, over time, the structure of our regulations has become increasingly complex. Over the past two years, the Government has engaged stakeholders who have expressed the need for increased flexibility and agility in our food regulations.
In 2020, we forged ahead with our work to modernize our regulations to address the issues, irritants, and bottlenecks that affect innovation and growth in the agri-food sector. Our actions will provide the industry with more timely access to new products and technologies that will enhance the safety and quality of foods, while helping to ensure that vulnerable people have access to the food products they need. Our measures also include improving food labelling so Canadians can make healthier food choices, which is more important now than ever.
Key accomplishments
Promoting healthy eating
Healthy Eating Strategy
Healthy eating is key to the good health of Canadians, because we know that an unhealthy diet is a major risk factor for chronic diseases.
While healthy eating is one of the best ways to protect and promote good health, a wide variety of factors influence our ability to make healthy food choices. Not only are our choices shaped by the food we have available in our homes, schools, restaurants, and grocery stores, they are also influenced by social media and advertising. Moreover, the changing and conflicting messages Canadians receive about what to eat can result in a lot of confusion.
Our Healthy Eating Strategy aims to make it easier for Canadians to make healthier food choices by:
- improving the nutritional quality of foods
- improving healthy eating information
- protecting vulnerable populations
Canada's food guide is an important resource for achieving this goal.
Canada's food guide
Since 1942, Canada's food guide has been a trusted source of nutrition information. With a focus on eating habits and recommended food choices, the food guide encourages Canadians to eat a variety of nutritious foods each day, be mindful of their eating habits, cook more often, and enjoy their food.
Updated in 2019, the food guide is a mobile friendly web application, providing Canadians with easier access to information about healthy eating, wherever they are, at any time. It offers resources such as Canada's Healthy Eating Recommendations, and the food guide snapshot, an engaging visual that communicates guidance on food choices and eating habits.
It also features Canada's Dietary Guidelines for Health Professionals and Policy Makers, which provides advice on:
- nutritious foods and beverages that are the foundation for healthy eating
- foods and beverages that can have a negative impact on health when consumed on a regular basis
- the importance of food skills as a practical way to support healthy eating
- the importance of creating supportive environments for healthy eating
To help Canadians apply dietary guidance in their daily lives, the food guide includes recipes, videos, and tips on a variety of healthy eating topics.
Canada's food guide by the numbers
Number of:
- Food guide snapshots accessed*: 695,310
- Translated snapshots accessed*: 57,364
- e-newsletter subscribers: 52,300
- Website visits: 2,945,000
- Times content featured on social media: 1 million
- Food guide recipes accessed*: 800,000
* accessed = ordered + downloaded + viewed
Top three recipes:
Facilitating the use of Canada's food guide
To meet the needs of Canadians, we continue to develop new food guide tools and resources with helpful advice on adopting healthy eating practices. Canada's food guide continued to evolve with new content added throughout 2020, with advice on adjusting recipes, improving eating habits, diets and food trends, and healthy eating during pregnancy and breastfeeding. New kid friendly recipes, and recipe videos were added to the website.
Building and maintaining awareness of Canada's food guide
In collaboration with the Canadian Nutrition Society, Health Canada conducted a three-month Student Ambassador Network pilot project to encourage nutrition and dietetics students to share the food guide's healthy eating recommendations throughout their university communities. Students organized a range of activities at 13 universities, including booths, cooking classes, presentations, workshops, and social media events/posts to raise awareness of healthy eating and the new food guide. The largest event took place over social media, with more than 2,000 participants.
Reaching this demographic is important because youths and young adults represent one third of the Canadian population, and reflect Canada's diversity. Health Canada also recognizes that engaging and developing resources with and for young people can help instill long-lasting healthy eating habits. We continue to explore new, and innovative ways to involve and to reach these audiences. For example, we established two other networks of youths and post-secondary students to engage their peers about the food guide and healthy eating in 2021. These networks will also provide advice to Health Canada to inform educational activities and resources supporting use of Canada's food guide.
In addition, in order to increase awareness of healthy eating recommendations, Health Canada also collaborated with Public Health Agency of Canada to support the production of a calendar featuring recipes from the food guide. The calendar, which included recipes such as corn, bean and squash soup (Three Sisters Soup), moose stew, and pork-and-apple skillet dinner, was mailed to the communities served by the Nutrition North Canada program. In addition to recipes, the calendar highlighted the recommendations from the food guide snapshot, and featured other helpful tips.
Supporting healthy eating during the pandemic
Being asked to reduce contact with others during the COVID-19 pandemic has meant that Canadians have been cooking more at home, thinking about how to plan their meals better to avoid visiting grocery stores more than necessary, and being more aware of the ingredients they have in stock at home. At the same time, some Canadians have also been reporting that they are consuming more junk foods and sweets.
To help respond to the needs of Canadians during this challenging time, Health Canada developed webpages to support Canadians' healthy eating during the pandemic. The food guide's monthly newsletter was also adapted to provide subscribers with advice relevant to addressing the challenges many were experiencing. Newsletter topics included information on using non-perishable foods to build healthy meals, adjusting recipes when ingredients are limited, making better use of leftovers, and grocery shopping during the pandemic.
Supporting evidence-informed decision making
Assessing adherence to Canada's food guide
In collaboration with the Canadian Institutes of Health Research, Health Canada hosted a Best Brains Exchange (BBE) event in March 2020, with discussions focused on developing tools to assess how Canadians are following the recommendations outlined by the food guide. The BBE aimed to engage researchers and policy experts in an open dialogue around how the proposed tools can be improved or adapted so that they are relevant and useful to a variety of users. Health Canada is using the experiences and knowledge shared at the BBE to develop and validate these new tools in collaboration with researchers. For further information regarding the discussions, check out the event summary.
Updating the National Nutritious Food Basket
In 2020, we updated the National Nutritious Food Basket to be consistent with the 2019 food guide. The food basket includes approximately 60 nutritious foods that are commonly consumed by Canadians.
The food basket is used as a tool by provincial and territorial governments, as well as stakeholders, to monitor the cost of healthy eating within their regions. It serves as a reference to inform their health and social policies, and raises awareness about the relationship between poverty and food insecurity. Statistics Canada also uses the food basket to calculate the cost of the food included in the Market Basket Measure (MBM). The MBM of low income develops thresholds of poverty based on the cost of a basket of food, clothing, shelter, transportation, and other items for individuals and families representing a modest, basic standard of living.
Food insecurity
Our leadership in nutrition policy includes the facilitation of data collection and knowledge development on factors that promote, or are barriers to, healthy eating. There is a strong correlation between household food insecurity and negative nutritional and health outcomes, including higher rates of chronic conditions and premature deaths. To understand the impact of the COVID-19 pandemic on Canadians at risk of food insecurity, we worked with a leading Canadian food security expert and Statistics Canada to include questions on household food insecurity as part of the Canadian Perspective Survey Series. Conducted in May 2020, the survey found that almost one in seven Canadians (14.6%) reported living in a household with food insecurity in the previous 30 days. These results were more than a third higher than pre-pandemic estimates. These data help governments and other organizations across the country understand the state and nature of food insecurity, to help develop well-informed interventions, both in and outside the health sector, to support the conditions for healthy eating.
Monitoring food and beverage advertising to children
In 2020, we began implementing an evidence-based strategy to monitor food and beverage advertising across a range of media and settings. New methods and protocols were developed, where needed, to do this monitoring. This foundational work has enabled the monitoring activities currently underway, which provide Health Canada with key insights into the current state of food and beverage advertising to children.
We know that children are particularly vulnerable to advertising that influences their food preferences and choices, which can shape their eating habits for the rest of their lives. Many Canadian children are exposed to such advertising regularly via digital platforms. At the same time, the reach of online advertising can be difficult to monitor and measure due to its complex nature. To gain a better understanding of how advertising is affecting children in the digital age and support countries in policy decision making, the World Health Organization (WHO) Europe developed the CLICK Monitoring Framework (a tool to assess the extent of children's exposure to advertising). Health Canada is now collaborating with WHO Europe to test the framework's methods and tools, and will be launching a pilot project in 2021 to assess the digital marketing of foods to children in Canada.
In addition, Health Canada is monitoring the exposure of Canadian adolescents to food and beverage advertising on social media websites. The findings will generate new information regarding the levels of exposure to advertising, and insights into how adolescents engage with the food and beverage brands they see on social media.
In addition, we continue to support the child-focused component of the International Food Policy Study (IFPS), which evaluates the impact of national-level food policies by conducting surveys across Canada, the U.S., Australia, Chile, the U.K., and Mexico. This ground-breaking study will support our efforts to monitor food and beverage advertising. It will also serve to inform our other healthy eating policies, while providing evidence of the impact of healthy eating policies at the international level.
The IFPS conducts annual surveys to gather information on food and eating behaviours, including food sources and purchasing, food preparation and skills, food security, dietary patterns, and weight loss efforts. The initiative also collects data regarding the impact of dietary guidance (such as Canada's food guide), food advertising, and taxes on sugary drinks on consumption habits.
While the first two cycles of IFPS collected data from adults only, Health Canada supported the expansion of the IFPS to include children and youth between the ages of 10 and 17 years. The IFPS's inclusion of this younger age group provides Health Canada with another valuable resource to inform policy. Having data on this age group also helps us assess how factors such as food advertising, the food guide, nutrition labelling, food security, sugary drink consumption, restaurant dining, nutrition information sources, and the nutrition environment at schools are influencing the eating habits of Canadian children and youths. Data collection and analysis on the child survey took place in 2019 and 2020, and a report will be released in 2021.
Focus on…
The Canadian Nutrient File
The Canadian Nutrient File is a database that Health Canada developed and continues to update to help inform our policies, standards, regulations, risk assessments, and food-consumption surveys. The database contains information on the amount of nutrients in foods commonly consumed in Canada and reports on up to 152 nutrients in 5,690 foods. Accessible online, this valuable resource is used by hospitals, universities, food manufacturers, and the general public to positively influence healthy eating and promote better food choices.
Sodium reduction in processed foods
Our bodies need a small amount of sodium to be healthy. Too much sodium can result in high blood pressure, a risk factor for stroke and heart diseases, which are among the leading causes of death in Canada. Sodium reduction is a key component of the Healthy Eating Strategy, and Canada is committed to helping meet the World Health Organization's goal of reducing the global population's salt/sodium intake by 30 percent by 2025.
As most of the sodium we consume comes in the form of processed and commercially prepared foods, Health Canada set voluntary sodium reduction targets for these categories of food in 2012. We have made incremental progress since the targets were established. In 2004, Canadians were consuming an average of 3,400 mg of sodium per day, but their consumption has now been reduced to 2,760 mg of sodium per day on average. This represents a 19% reduction in sodium intake since 2004. We continue to work toward our commitment of reducing salt consumption to an average of 2,300 mg per day, per person, to have a positive impact on the overall health of Canadians.
After addressing the challenges faced by the food industry, we published revised voluntary reduction targets for sodium in processed foods in December 2020.
Health Canada continues to encourage and work with the food processing sector to reach these targets by 2025, and will evaluate their progress.
Promoting sodium reduction
Health Canada is working with Colleges and Institutes of Canada to help raise awareness about the importance of sodium reduction. Our collaborative work includes the development of teaching materials to educate food technology and nutrition college students, as well as professionals, on reducing the use of salt or sodium-based ingredients when preparing food. The goal of this initiative is to raise awareness on how to reduce sodium in our food supply, particularly in the food produced by small, independent restaurants and food-service establishments.
Focus on…
Release of global protocol for measuring trans fats
Thanks in part to the contributions from Health Canada's Nutrition Research Division, the World Health Organization released its Global protocol for measuring fatty acid profiles of foods with emphasis on monitoring trans fatty acids originating from partially hydrogenated oils in December 2020. The document's goal is to provide a comprehensive, global protocol for measuring the trans fat content of foods. This effort is important to the health of Canadians because the regular consumption of trans fats increases the level of bad cholesterol in the blood, and the risk of heart disease.
Nutrition Science Advisory Committee
Health Canada's newly established, Nutrition Science Advisory Committee (NSAC) held its first meeting on November 18, 2020. This group of external expert advisors was formed to provide us with scientific and technical advice on nutrition, in a timely and independent manner. The committee will provide advice in several key areas, such as emerging scientific trends that impact the nutritional health of Canadians, and the best practices for assessing and evaluating scientific evidence to inform public health nutrition policy. NSAC's advice will support Health Canada's efforts to use the best available evidence in fulfilling its mandate to support the nutritional health of Canadians.
Focus on…
Folic Acid
As part of a healthy pregnancy, folic acid is vital to the growth of the baby's spine, brain and skull. Taking a daily vitamin supplement containing folic acid, a form of folate, can reduce the risk of a baby having a neural tube defect (NTD), such as spina bifida.
The benefits of taking folic acid to reduce the risk of NTDs are highest during the very early weeks of pregnancy, at a point when most women do not yet know they are pregnant. Younger women, and those who are economically disadvantaged, tend to be more at risk for a folate deficiency, and thus of having a baby with NTD. Yet, women in other demographic groups tend to consume more than the recommended levels of folic acid. To help provide guidance, Health Canada scientists developed an optional statement that can be displayed on the packaging of pre-natal supplements, featuring clear information on the recommended folic acid dose. We also continue to monitor the population for a clearer picture of folate consumption for women of childbearing age in Canada, to reduce the risk of babies having NTD and to avoid overconsumption of the vitamin.
Monitoring the food environment
Trends in nutritional quality of the food supply
Canada's food supply is extensive and dynamic, with close to 25,000 new products introduced over the past five years. The Healthy Eating Strategy takes a comprehensive approach toward improving the food environment, with initiatives aimed at improving the quality of the food supply. To monitor and assess the impact of the Healthy Eating Strategy on nutritional quality of the Canadian food supply, we are tracking the availability of foods high in nutrients of public health concern (saturated fat, sugars and sodium) on the Canadian market. Foods containing 15% or more of the daily value are considered "high" in these nutrients.
Using 2017 nutrition data that University of Toronto collected about prepackaged foods, we established a baseline for the nutritional quality of the Canadian food supply. The baseline shows that about half the prepackaged foods sampled (just over 17,600) on Canadian store shelves in 2017 were high in at least one of the three nutrients of concern. Health Canada found that the following select food categories had a tendency to be high in nutrients of concern: bakery products, beverages, cheese, condiments and sauces, chips and snacks, frozen entrees and sides, candies, cookies, soups, deli meats, dried fruit, and plant-based meat alternatives. See the graph below.
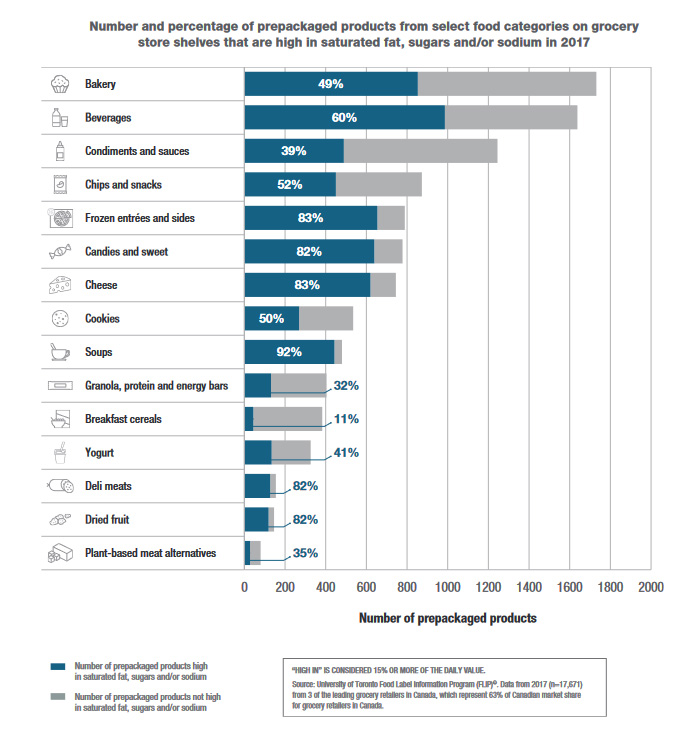
Figure 1: Number and percentage of prepackaged products from select food categories on grocery store shelves that are high in saturated fat, sugars and/or sodium in 2017: Text description
This bar graph shows the number and percentage of prepackaged products on grocery store shelves that are "high in" saturated fat, sugars and/or sodium in 2017.
There is a footnote at the bottom right of image that says: "High in" is considered 15% or more of the % Daily Value.
The graph lists each food category along the y-axis (vertically, along the left side of the graph) with a small image to the left of the text representing each food category. The number of prepackaged products is presented along the x-axis (horizontally, along the bottom of the bar chart) with a scale from 0 to 2000. There is a bar for each food category that represents the number of products in that category, and the bar is broken into the number of products that are "high in" in blue, and the number of those not "high in" in grey. The percentage of products that are "high in" is written in white font within the blue portion of the bar.
Data for each variable of a given food category that was displayed in the graph.
Bakery: The bakery bar shows a total of 1731 products. 853 products or 49% of products are high in saturated fat, sugars and/or sodium and 878 or 51% are not.
Beverage: The beverages bar shows a total of 1638 products. 986 products or 60% of products are high in saturated fat, sugars and/or sodium and 652 or 40% are not.
Condiments and sauces: The condiments and sauces bar shows a total of 1245 products. 489 products or 39% of products are high in saturated fat, sugars and/or sodium and 756 or 61% are not.
Chips and snacks: The chips and snacks bar shows a total of 873 products. 450 products or 52% of products are high in saturated fat, sugars and/or sodium and 423 or 48% are not.
Frozen entrées: The frozen entrées and sides bar shows a total of 789 products. 654 products or 83% of products are high in saturated fat, sugars and/or sodium and 135 or 17% are not.
Candies and sweets: The candies and sweets bar shows a total of 778 products. 639 products or 82% of products are high in saturated fat, sugars and/or sodium and 139 or 18% are not.
Cheese: The cheese bar shows a total of 745 products. 620 products or 83% of products are high in saturated fat, sugars and/or sodium and 125 or 17% are not.
Cookies: The cookies bar shows a total of 535 products. 269 products or 50% of products are high in saturated fat, sugars and/or sodium and 266 or 50% are not.
Soups: The soups bar shows a total of 480 products. 443 products or 92% of products are high in saturated fat, sugars and/or sodium and 37or 8% are not.
Granola, protein and energy bars: The granola, protein and energy bars bar shows a total of 404 products. 131 products or 32% of products are high in saturated fat, sugars and/or sodium and 273 or 68% are not.
Breakfast cereals: The breakfast cereals bar shows a total of 383 products. 44 products or 11% of products are high in saturated fat, sugars and/or sodium and 339 or 89% are not.
Yogurt: The yogurt bar shows a total of 326 products. 134 products or 41% of products are high in saturated fat, sugars and/or sodium and 192 or 59% are not.
Deli meats: The deli meats bar shows a total of 155 products. 127 products or 82% of products are high in saturated fat, sugars and/or sodium and 28 or 18% are not.
Dried fruit: The dried fruit bar shows a total of 146 products. 119products or 82% of products are high in saturated fat, sugars and/or sodium and 27 or 18% are not.
Plant-based meat alternatives: The plant-based meat alternatives bar shows a total of 80 products. 28 products or 35% of products are high in saturated fat, sugars and/or sodium and 52 or 65% are not.
At the bottom right, below the graph, the source is identified as the University of Toronto Food Label Information Program (FLIP)©. Data from 2017 (n=17,671) from 3 of the leading grocery retailers in Canada, which represent 63% of Canadian market share for grocery retailers in Canada.
At the bottom left below the graph there is a legend. The legend indicates that blue represents the number of prepackaged products "high in" saturated fat, sugars and/or sodium. The legend also indicates that grey represents the number of prepackaged products not "high in" saturated fat, sugars and/or sodium.
Using more recent market research data, Health Canada also looked at how the food supply changed after the launch of the revised food guide, specifically regarding plant-based foods. Canada's food guide recommends regular intake of vegetables, fruit, whole grains, and protein foods, and among protein foods, to consume plant-based more often.
Between the launch of the new food guide in January 2019, and December 2020, 120 plant-based products entered the Canadian market. Of these new products, many are dairy alternatives (30%), snack foods (26%), processed meat, fish, or egg alternatives (15%), and desserts (11%). Although plant-based, many of these products are not in line with Canada's food guide recommendations. More than half of the new plant-based, processed alternatives to meat, fish or eggs were high in sodium. In addition, the majority of the plant-based desserts were high in sugars and saturated fat (for example, containing 15% or more of the daily value for these nutrients of concern). Moreover, more than one third of dairy alternatives, snacks, and processed meat, fish, or egg alternatives were high in saturated fat.
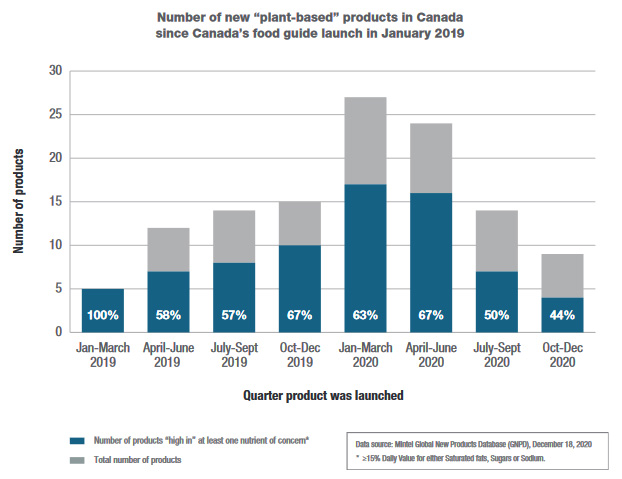
Figure 2: Number of new "plant-based" products since Canada's Food Guide launch in January 2019: Text description
| Quarter product was launched | Number of products not "high in" any nutrient of concernFootnote 1 | Number of products "high in" at least one nutrient of concernFootnote 1 | Total number of products | Percent "high in" at least one nutrient of concernFootnote 1 |
|---|---|---|---|---|
| January to March 2019 | 0 | 5 | 5 | 100% |
| April to June 2019 | 5 | 7 | 12 | 58% |
| July to September 2019 | 6 | 8 | 14 | 57% |
| October to Dec 2019 | 5 | 10 | 15 | 67% |
| January to March 2020 | 10 | 17 | 27 | 63% |
| April to June 2020 | 8 | 16 | 24 | 67% |
| July to September 2020 | 7 | 7 | 14 | 50% |
| October to December 2020 | 5 | 4 | 9 | 44% |
|
Data source: Mintel Global New Products Database (GNPD), December 18, 2020 Table Footnotes
|
||||
Focus on…
Vitamin D
Vitamin D is an essential nutrient for bone health, and many other functions in the human body. You can get vitamin D from the sun, supplements and food. In Canada, there are very few foods that contain vitamin D. The purpose of adding vitamin D to food is to help Canadians maintain the levels needed for health. Examples of foods that are sources of vitamin D include milk, fortified plant-based beverages, fatty fish, egg yolks and margarine. For Canadians over the age of 50, a daily vitamin D supplement of 400 international units is recommended.
While Health Canada's research shows that most Canadians have adequate vitamin D levels, some population groups may be at risk of vitamin D deficiency. To learn more about these higher risk groups, Health Canada's researchers are studying more than 30,000 Canadians through the Canadian Health Measures Survey.
Ensuring the safety of Canada's food supply
Making the food supply safer
Canada's food safety system is one of the best of in the world. We consult with other governments, consumers, academia, experts and industry, to establish policies, regulations, and guidelines, as well as standards related to the safety, and nutritional quality of all food sold in Canada.
Health Canada's role is to protect Canadians from risks to health and the spread of disease. We achieve this by:
- setting food safety standards and addressing emerging risks
- conducting health-risk assessments in support of food safety investigations
- providing information to Canadians about food safety
- conducting food monitoring and surveillance
- undertaking scientific research to identify, characterize and respond to new and emerging food safety risks
The Canadian Food Inspection Agency plays the equally important role of monitoring the food industry's compliance to the regulations, and taking enforcement action when necessary. It also conducts food safety investigations and food recalls.
Canadians are important players themselves in maintaining an effective food safety system. There are safe food practices we can all follow such as:
- washing our hands with soap and water for at least 20 seconds, before and after handling food and food packaging
- using good food handling practices
- following cooking instructions
- properly storing food
- reporting a food safety or labelling concern
COVID-19 and food safety
Scientists and food safety authorities around the world are closely monitoring the spread of COVID-19. If we become aware of a potential food safety risk, we will take appropriate actions to protect the safety of Canada's food supply. While there is no scientific evidence indicating that COVID-19 can be spread through food or food packaging, we advise Canadians to follow safe food handling practices to avoid any possible exposure to the virus. Always wash your hands with soap and warm water for at least 20 seconds before and after handling food and food packaging. Also, disinfect any surfaces that have come into contact with food.
Safety assessment of technical grade ethanol in hand sanitizers
The COVID-19 pandemic caused an urgent need for hand sanitizers in Canada. Because of this demand, there were worldwide shortages of pharmaceutical and food grade ethanol. To help ensure that Canadians had access to these products, Health Canada allowed for the sale of technical grade ethanol (TGE) on a temporary basis for use in the manufacture of hand sanitizers. In support of this work, we assessed potential exposure to chemical residues on food resulting from the temporary use of technical grade ethanol in disinfectants used on surfaces in establishments where food is handled, and identified no safety concerns. For more information about hand sanitizers, check out the Drugs and Medical Devices Highlight Report.
Setting safety standards
Food contaminants are chemicals that may be present in foods at levels that could have an impact on the overall safety or quality of foods. These substances can be present in foods inadvertently (as the result of their presence in the environment, for example).
Health Canada and the Canada Food Inspection Agency conduct regular surveillance of the levels of chemical contaminants in the Canadian food supply. When a potential safety concern is identified, appropriate risk management measures are taken to protect the health of Canadians.
One risk management measure we take is developing maximum levels (MLs) for chemical contaminants, which we establish in an effort to reduce exposure to a particular contaminant in foods. We take this action as part of Health Canada's commitment to ensuring that dietary exposure to food contaminants is as low as reasonably achievable. Canadian MLs for chemical contaminants in foods are set out in the List of Contaminants and Other Adulterating Substances in Foods, as well as in our list of Maximum Levels for Various Chemical Contaminants in Foods.
Here are two examples of MLs we established in 2020:
Arsenic in rice
In June 2020, Health Canada published a notice of modification to the List of Contaminants and Other Adulterating Substances in Foods, adding MLs for inorganic arsenic in polished (white) and husked (brown) rice. Surveillance data available to Health Canada indicate that these MLs are readily achievable for rice and rice products available for sale in Canada.
Focus on…
Arsenic in rice
Because arsenic occurs naturally in the environment, it can be found at very low levels in various foods. For Canadians, rice represents a significant source of exposure to inorganic arsenic in our diets. In early 2020, Health Canada developed a targeted survey of rice-based foods, for infants and children, that are sold in Canada. We communicated that we would be using the results of the survey, conducted by the Canadian Food Inspection Agency, to determine whether additional measures to protect against arsenic exposure from rice-containing foods intended for infants and young children are needed.
Lead in infant formula
The MLs for lead in infant formula were established at a time when the sources for lead contamination in the environment were more common. Over the past 30 years, however, Canadians' exposure to lead has significantly declined due to government regulations and industry actions, including the successful phase-out of lead in gasoline, paints and the soldering used in food cans.
On July 3, 2020, in order to reflect the low concentrations of lead typically found in infant formula products today, and to align with international standards for lead in infant formula, Health Canada lowered the ML for lead in infant formula by modifying the List of Contaminants and Other Adulterating Substances in Foods. This lower ML aligns with Health Canada's approach to managing dietary exposure to lead, which aims to reduce exposure to levels that are as low as reasonably achievable, as well as our Risk Management Strategy for Lead, which recommends reducing exposure to lead from all sources.
Focus on…
Botulism Reference Service
Botulism is a rare but serious illness caused by a toxin that affects the nervous system, and can cause paralysis. The toxin is produced in foods by Clostridium botulinum, a species of bacteria found commonly in nature. There are several types of botulism, including foodborne botulism, which results from eating foods that contain the botulinum toxin. When botulism is suspected, Health Canada's Botulism Reference Service (BRS) tests clinical and food samples from hospital and provincial laboratories. In 2020, the BRS analyzed 70 samples as part of the investigation into 32 suspected botulism incidents. After analyzing the samples, the BRS confirmed two cases of infant botulism, and seven cases resulting from four outbreaks of foodborne botulism. We also released new guidance for healthcare professionals regarding the steps they should be taking with the BRS when facing a possible botulism case.
Science assessment of plastic pollution
Together with Environment and Climate Change Canada, Health Canada published a Science Assessment of Plastic Pollution in October 2020. The assessment provided a thorough scientific review of how present it is, and the potential impact of plastic pollution on the environment and human health. The report, which included a section on the occurrence of microplastic in food, will guide future research and decision making related to plastic pollution in Canada.
Although no risk to human health from exposure to microplastic in food or beverages has been identified, Health Canada recognizes this to be an emerging issue that requires further research in order to fully assess its potential risks. Health Canada routinely monitors emerging research on microplastics, and will continue to review any new information on possible sources of exposure, and the possible implications for human health. Further, to address key knowledge gaps identified in the science assessment, Health Canada is also investing in scientific research, and is collaborating with international and domestic partners to monitor scientific advancements regarding microplastics. Should Health Canada become aware of any health risk, the department would take appropriate action to address the issue.
Strong commitment to transparency
For a food to be sold in Canada, it must meet the requirements set out in the Food and Drugs Act, and the Food and Drug Regulations. However, in some cases, Health Canada may allow a non-compliant food to be sold before regulatory amendments are made. In such instances, a Temporary Marketing Authorization (TMA) may be obtained to allow a manufacturer or distributor to sell a product for the purposes of generating information in support of amending the regulations. A manufacturer or distributor may obtain a TMA to market a supplemented food, such as a caffeinated energy drink.
On September 30, 2020, we released a summary of consumption incident reports that were received for certain supplemented foods as part of the TMA agreements, reflecting the Government of Canada's commitment to greater transparency and openness. Health Canada is making more of this type of data and information available to Canadians, to raise awareness about our work.
During the same month, we also began publishing a list of food and ingredients that have been determined to be non-novel, based on the description outlined in the regulations. (Novel foods are food products that are new or changed compared to existing foods, and we review them for their safety before they can be sold in Canada.) We released the list to increase transparency of our work, and as a reference for food manufacturers, allowing them to quickly confirm whether their product would be considered novel or not.
If a food manufacturer, importer or distributor is unsure if a food or food ingredient would be classified as novel, they can ask Health Canada for guidance about novelty determination and about how we ascertain whether a food is safe for consumption.
In terms of novel foods, we also announced our plan to update our guidance regarding the regulation of novel foods. For producers who are developing new plant-based foods, particularly those that have been gene-edited, this guidance will clearly communicate the requirements for them so they understand what their responsibilities are. As part of this work, we engaged with experts from industry, academia and other sectors to provide scientific information and advice to inform the new guidance that would be consulted on in 2021. Meetings and correspondence on the development of new regulatory guidance for novel foods are listed on Health Canada's openness and transparency website.
We continue to publish stakeholder engagement activities related to the Healthy Eating Strategy, providing an online list of correspondence and meetings with stakeholders where views, opinions, and information were shared with the intent to inform the development of policies, guidance or regulations. In 2020, there were 22 meetings and eight pieces of correspondence on the strategy.
Focus on…
Award-winning research
Striving to reduce the use of animal testing in the research that informs our regulatory work, Health Canada scientists have been collaborating on finding alternative approaches to inform human health risk assessments. Recently, Health Canada scientists completed a case study, which tested a number of approaches being used for the risk assessment of a flame retardant. In recognition of their work, they received an award, in March 2020, from the Society of Toxicology for the best-published research paper demonstrating an outstanding application of advanced approaches to risk assessment. The Health Canada team used a known chemical contaminant to validate methods for predicting the threshold dose at which adverse effects appeared in animal toxicology studies. This work contributes to finding alternative approaches for hazard assessment, and aims to reduce the need for animal testing in conducting risk assessments of Canadians' exposure to environmental contaminants.
Open Data
The Canadian Total Diet Study (TDS) is a food surveillance program that monitors the concentration of chemical contaminants in foods that are typically consumed by Canadians. Since its inception in 1969, the TDS has enabled Health Canada to monitor the concentration of contaminants in food sold in Canada in order to determine the key dietary sources, assess trends, support the development of food-safety policies and regulations, and help ensure the safety of Canada's food supply. Health Canada is making TDS data more accessible by publishing newly available results on the Government of Canada's Open Data website. In 2020, we published the TDS data for radionuclides, polychlorinated biphenyls (commonly known as PCBs), and trace elements. The data is available on the Open Data Portal by typing "Total Diet Study" into the search field.
In 2020, Health Canada published the following information updates:
Ethanol in non-alcoholic fermented beverages
Fermentation is a natural process that turns sugars into ethanol (ethyl alcohol). Fermented foods, like yogurt, sauerkraut and sourdough bread, are common in our diets, and are eaten safely. We also safely consume foods such as fruit and fruit juices, which naturally contain low levels of ethanol. Fermented non-alcoholic beverages, such as kombucha, kefir and some soft drinks, like ginger beer, can also contain low levels of ethanol. Although these products are not made to be alcoholic, their ethanol content can vary depending on factors such as the fermentation process, as well as the distribution and storage conditions. The Food and Drug Regulations require beverages containing 1.1% or greater ethyl alcohol by volume (ABV) to declare the alcohol content on the label. The Public Health Agency of Canada recommends that Canadians not consume alcohol during pregnancy, or when planning a pregnancy. The Agency also advises that youths should delay drinking alcohol as long as possible, until at least reaching the legal drinking age in their province or territory. To provide consumers with advice on limiting ethanol intake from non-alcoholic fermented beverages, Health Canada published guidance on June 11, 2020.
Notice to Canadians with gluten-related disorders
To address potential food shortages in the Canadian retail sector during the pandemic, prevent food waste, and support Canada's economy without compromising food safety, temporary measures have allowed food products that were made, packaged and labelled in Canada for food-service use (but were labelled according to U.S. requirements) to be sold through retailers in Canada. As a result, some products originally labelled for the U.S. market might not identify the presence of barley (a gluten grain, which may be used in malt flavouring or extract, or in yeast extract) in the list of ingredients, because the labelling regulations for that market do not require an enhanced declaration of gluten sources. To address the issue, Health Canada published a notice to Canadians with celiac disease or other gluten-related disorders, about certain ingredients that they may wish to avoid because the ingredients could be a source of undeclared gluten in specific products during the pandemic. To inform Canadians about gluten-related disorders, and sources of gluten, we have also updated our gluten pamphlet. Moreover, information on Canada's priority allergens and gluten sources will continue to be required to appear on or with product labels.
Pre-market oversight
If a company plans to sell a novel food, a new infant formula, or a food containing a food additive which is being used in a new way, they need approval from Health Canada to sell the product in Canada. Conducting scientific, pre-market safety assessments of these products is part of Health Canada's commitment to ensuring a safe and nutritious food supply for Canadians. A product will not be approved for sale if it is found to be unsafe.
Novel Foods
Novel foods are food products that are new or changed compared to existing foods. Examples include foods that have been created using a new process, or have been genetically modified. We review novel foods for their safety before they can be sold in Canada, and we approved eight novel foods in 2020 after completing detailed assessments for each.
Food additives
A food additive is any chemical substance that is added to food during its preparation or storage. A food additive can either become a part of the food, or affect its characteristics for the purpose of achieving a particular technical effect. Examples of food additives include colouring agents, low-calorie sweeteners, and preservatives.
Health Canada's Lists of Permitted Food Additives show which additives are permitted, the foods they can be used in, and the maximum amount that can be used. In general, the 15 lists are organized according to technical function (for example, food colourings are grouped in the List of Permitted Colouring Agents). Health Canada thoroughly assesses the safety of new additives, as well as the new uses for previously permitted additives, before adding them to the list. In 2020, Health Canada approved 15 new uses for food additives after completing safety assessments, including four of which that were completely new.
Infant formulas
Health Canada recommends breastfeeding infants and young children for up to two years or more. However, Health Canada recommends commercial infant formula as a healthy and safe alternative for babies who are not being breastfed. All infant formula sold in Canada undergoes a rigorous safety assessment by Health Canada, and the department approved 12 infant formulas in 2020.
Scientific safety opinions
Health Canada conducts pre-market safety assessments of food packaging materials, incidental additives (a substance, used in processing plants, which has the potential to be left as a residue in food), and processing aids (substances used in the production of food) when representatives from the food industry ask for an evaluation. If the assessment is favourable, the department gives the food manufacturer documentation to demonstrate that the product has been found to be acceptable, from a food chemical safety standpoint. In 2020, Health Canada issued 786 of these opinions to food industry representatives.
Regulatory innovation for food
With factors such as growing populations, the increasing demand for quality food, scientific advancements, and climate change, the food system is evolving. Emerging developments have the potential to have an impact on the way food is produced, processed, distributed, packaged, consumed, and disposed.
As the food industry adapts and innovates to meet shifting demands, our traditional view of food and our approaches to regulating it must evolve as well. The Food and Drug Regulations set out the rules for food products so that they can be safely consumed by Canadians. Over time, these regulations have become more complex, making them difficult to navigate, understand and apply. Their complexity also makes it challenging to respond to advances in science, technology, or new product development. As a result, companies can face challenges in introducing new and innovative food products, or in adopting new methods designed to make products safer or better for consumers.
We are working on ways to modernize our regulations, so they can be updated more easily to address the latest scientific or technological advances while maintaining our strong safety standards. Our approach includes developing new regulations for foods containing one or more added supplemental ingredients (for example, added vitamins, minerals, amino acids, or other ingredients marketed for the purpose of providing specific physiological or health effects. This approach also involves developing regulatory oversight to improve access to safe and innovative products that will improve the health of vulnerable populations. A good example of such products are human milk fortifiers: these are foods that are added to breastmilk to increase its nutritional value. They provide necessary nutrients for certain preterm infants or other infants as medically required.
We have also been developing new guidance on the regulation of novel foods with a particular focus on foods resulting from the cross breeding of plants. This work seeks to clarify the regulatory landscape for plant breeders to allow for innovation in the sector, while continuing to ensure the safety of the Canadian food supply. During 2020, Health Canada engaged with stakeholders. These activities included a virtual panel session with plant breeders, to inform the scientific basis for the proposed guidance. The development of this guidance represents the first phase of a broader, multi-year effort to modernize guidance for all novel foods, including animals and microorganisms.
Updating regulatory requirements
Necessary for proper growth and continued wellbeing, protein is key to a healthy diet. There are different types of protein foods, and it is important to assess their quality to verify that they meet nutritional requirements. The Food and Drug Regulations currently require that only one specific method, which uses the protein efficiency ratio (PER), be used to measure the protein quality of foods. However, in recognition of concerns regarding the limitation of this particular method, we are now accepting results from a second method: the protein digestibility corrected amino acid score (PDCAAS), as of December 2020. We are in the process of updating the regulations to include both methods.
Focus on…
Allergy Prevalence in Canada
Together with the Allergy, Genes and Environment Network (AllerGen), of the Centres of Excellence (NCE), Health Canada supported the Surveying Prevalence of Food Allergy in All Canadian Environments (SPAACE) study, a research initiative led by the University of Calgary and the University of Waterloo. This large Canadian survey, launched in 2016 and released in April 2020, provides an estimate of the prevalence of common food allergies in Canada, as well as a better understanding of the attitudes and behaviours of those living with these medical conditions. It is also the first study to look at the trends over time in the overall prevalence of food allergies in Canada, but comparing the recent results to previous data collected in 2010.
Listeria policy consultation
Widely occurring in the environment, the Listeria bacteria can cause a wide range of symptoms, from mild gastrointestinal symptoms, such as nausea, to life-threatening diseases such as septicemia and encephalitis, or to miscarriage in pregnant women. Because of the prevalence of Listeria, it can contaminate food easily. To help provide advice to industry on Listeria control measures for their food products, we launched a consultation in November 2020, with the goal of updating our policy on Listeria in ready-to-eat foods. Together with the latest science and our examination of the evolving Canadian food context, the information collected as part of the consultation will inform the review of the Listeria policy.
Focus on…
Foodborne parasites
A number of parasites can be transmitted by food. The problem is an emerging issue in Canada due to factors such as the globalization of the food trade, international travel, and changes in consumer habits. Health Canada's parasitology laboratory is working on new methods for detecting and controlling parasites in food. These new methods will allow for faster and more accurate results during investigations into outbreaks of foodborne illness, or in routine food testing, thus reducing the risk of illnesses. As part of this effort to reduce the risk of foodborne parasites, our scientists made a number of significant research findings, working in collaboration with universities and provincial governments. For example, our scientists have developed a DNA test to detect a Cryptosporidium parasite in wastewater samples. Further, in collaboration with the Institut national de santé publique du Québec and the Public Health Agency of Canada, our laboratory also conducted a surveillance study on the prevalence and genotypes of Cryptosporidium parasites among the population of Québec. This data will help us identify the people who are most vulnerable to infections as we develop strategies to prevent and respond to outbreaks of foodborne parasites.
We are doing other work on detecting Toxoplasma gondii, a parasite that infects most species of warm-blooded animals, including humans. Specifically, our work is focused on detecting the parasite in "country foods", the harvested wild game in Canada's North. These studies, which also involve examining of how the parasite can be eliminated through cooking, freezing or drying wild meats, will help us develop safety guidelines for the consumption of country foods.
International collaboration
Food Standards Australia New Zealand
Health Canada is collaborating with Food Standards Australia New Zealand (FSANZ) with the goal of using each other's assessments to inform our regulatory decisions. As partners, we share similar approaches to the safety assessment of these foods, so working together reduces the duplication of work, informs future regulatory decisions, and provides an opportunity to share scientific expertise. For food producers, this collaborative initiative promises to reduce regulatory assessment costs, and streamline the food approval process. In 2020, we conducted a pilot project to assess the safety of a genetically modified (GM) food that was not yet authorized for use in Canada, Australia, or New Zealand. Health Canada undertook the assessment of the food, with FSANZ undertaking a review of the work. If both agencies are satisfied with the results, they will use the safety assessment to authorize the product in their respective countries. The aim, once the pilot project is completed in 2021, is to establish a system to share safety assessments between the agencies.
Codex Alimentarius Commission
Established in 1963, by the World Health Organization, and the Food and Agriculture Organization of the United Nations, the Codex Alimentarius Commission develops international food standards to protect consumer health and to facilitate fair practices in the food trade.
As Canada is one of Codex's 188 member countries, Health Canada works with our international partners as part of our commitment to ensure that science and evidence remain the basis of all international decisions around food safety, nutrition and food labelling.
Codex last met in-person in February 2020, before pandemic safety measures required that subsequent meetings be moved to a virtual platform.
The Commission's 43rd session, in the fall of 2020, took the form of a series of virtual meetings. Although the agenda was limited to Codex's core work, the meeting allowed participants to adopt guidance on food hygiene, and a code of practice for business operators on food allergen management. Their discussions also focused on the measures they were taking to continue their work during the pandemic.
In addition to participating at the Commission, Health Canada led work related to revising the Codex standards for chemical contaminants in foods and feeds. Health Canada also participated in many of the ongoing electronic working groups, including those focused on front-of-package nutrition labelling, allergen labelling, and guidance on control of E.coli in various foods, among other work.
Approved novel foods
- Soy Leghemoglobin (LegH) Preparation in Simulated Meat Products at Levels up to 0.8%: This preparation contains a soy leghemoglobin (LegH) protein from soybean. The preparation is added to simulated meat products to provide nutrition, and the flavour and aroma of traditional animal-derived ground beef.
- Lactospore: This is a particular strain of the microorganism Bacillus coagulans to be added to a variety of foods as a food ingredient.
- Ahiflower Oil as an Ingredient in a Broad Range of Food Categories: This oil is produced from the Buglossoides arvensis plant to be used in a broad range of food categories.
- Refined oil derived from DHA Canola NS-B50027-4: This canola was developed to produce an oil containing high levels of the omega-3 fatty acid docosahexaenoic acid (DHA). The refined oil was approved for food use.
- Herbicide Tolerant Corn - MON 87429: This corn was developed to be herbicide tolerant.
- Simplot Potato Event Z6: This potato was developed to have reduced black spot bruising, resistance to late blight, as well as reduced levels of free asparagine and reducing sugars (glucose and fructose) in tubers.
- Imidazolinone herbicide tolerant grain sorghum - ADV-IMI-R: This sorghum was developed to be herbicide tolerant.
- Enhanced Yield and Herbicide Tolerant Maize - DP-202216-6: This maize was developed to produce more grain and to be herbicide tolerant.
Approved food additives
- Glucose Oxidase from Aspergillus niger J39: The List of Permitted Food Enzymes was modified to enable the use of glucose oxidase from Aspergillus niger J39 in bread, flour, whole wheat flour, pasta, unstandardized bakery products, and in certain shredded cheeses just prior to their packaging.
- Lycopene Extract from Tomato: The List of Permitted Colouring Agents was modified to enable the use of lycopene extract from tomato in certain non-carbonated, sweetened, flavoured water-based beverages to which vitamins and minerals nutrients have been added, and in sports drinks.
- Aspergillus fijiensis as a Source Organism for Invertase: The List of Permitted Food Enzymes was modified for invertase (Aspergillus japonicus) in sucrose used in the production of fructooligosaccharides by updating this to "Aspergillus fijiensis", to reflect a re-identification of the original source organism.
- Potassium Phosphate, dibasic: Certain lists of Permitted Food Additives were modified to extend the use of potassium phosphate, dibasic, to the same foods and same levels of use as is currently permitted for sodium phosphate, dibasic.
- Phospholipase from Aspergillus oryzae AT969: The List of Permitted Food Enzymes was modified to enable the use of phospholipase from Aspergillus oryzae AT969 in bread, flour, whole wheat flour and unstandardized bakery products.
- Benzoic Acid and its Salts: The List of Permitted Preservatives was modified to enable the use of sodium benzoate as a preservative used at a maximum level of 1,000 ppm in oyster-flavoured sauce.
- Polysorbate 80: The List of Permitted Emulsifying, Gelling, Stabilizing or Thickening Agents was modified to enable the use of Polysorbate 80 as an emulsifying agent in wheat-based, ready-to-eat cereals.
- Cellulose and Microcrystalline Cellulose: The List of Permitted Anticaking Agents was modified to enable the use of cellulose and microcrystalline cellulose in cubed or diced cheeses.
- Protease from Trichoderma reesei RF8963: The List of Permitted Food Enzymes was modified to enable the use of Protease from Trichoderma reesei RF8963 in hydrolyzed animal, milk and vegetable protein.
- Steviol glycosides from Saccharomyces cerevisiae Y63348: The List of Permitted Sweeteners was modified to enable the use of steviol glycosides from Saccharomyces cerevisiae Y63348 as a sweetener in the same foods where steviol glycosides from other sources is already permitted and at the same maximum levels as are allowed for those foods.
- Spirulina extract: The List of Permitted Colouring Agents was modified to enable the use of Spirulina extract as a food colouring agent.
- Citric Acid Esters of Mono- and Diglycerides (CITREM): The List of Permitted Emulsifying, Gelling, Stabilizing or Thickening Agents was modified to enable the use of citric acid esters of mono- and diglycerides (CITREM) in whole protein-based infant formulas for special dietary purposes.
- Pectin lyase from Aspergillus niger Rung373: The List of Permitted Food Enzymes was modified to enable the use of pectin lyase from Aspergillus niger Rung373 in various standardized and unstandardized foods.
- Acid Prolyl Endopeptidase from Aspergillus niger GEP: The List of Permitted Food Enzymes was modified to enable the use of acid prolyl endopeptidase from Aspergillus niger GEP in brewers' mash, ale, beer, light beer, malt liquor, porter, stout, distiller's mash, and protein hydrolysates (hydrolyzed animal, milk and vegetable protein), cereal- and plant-derived ingredients to be further used as ingredients in the manufacture of non-alcoholic plant-based beverages.
- Cellulase from Trichoderma reesei QM9414: The List of Permitted Food Enzymes was modified to enable the use of cellulase from Trichoderma reesei QM9414 in standardized single-strength fruit juices, standardized and unstandardized fruit nectars, and unstandardized fruit and vegetable products.
Approved infant formulas
- New colorant of closures of plastic containers used for packaging of powdered infant formula: addition of a new colorant for the top closures of plastic infant formula containers used for powdered infant formula.
- Similac Advance Relaunch of S172: a relaunch of Similac S172 with a new packaging (metal cans), new fill weight (700 grams) and a new name; Similac Advance with Omega-3 and Omega-6 Infant Formula.
- Similac Pro-Advance: New infant formula with 2'-fucosyllactose (2'-FL) and short-chain fructooligosaccharides (scFOS).
- Similac Advance Step 1 and Step 2 concentrated liquid in 385 ML metal cans: minor changes to ingredient levels and labelling instructions.
- President's Choice Organics Omega + with iron infant formula: major changes to labelling instructions.
- Amendment to Arachidonic Acid (ARA) and Docosahexaenoic Acid (DHA) Fortification Levels in Non-GMO, Milk-Based Infant Formula Powder with ARA, DHA, and GOS: increases to the amounts of ARA and DHA and changes to label nutrition information.
- Major Change to Nutramigen A+ with LGG to Include a New Source of DHA (DHASCO-B) and Reduced Levels of ARA: a reformulation to include a new source of DHA (DHASCO-B) with a reduction in ARA, and increased levels of Vitamin D and inositol.
- Major Change to Nutramigen A+ 20 kcal Nursette 59 ML Plastic Bottle to include a New Source of DHA (DHASCO-B) and Reduced Levels of ARA: a reformulation to include a new source of DHA (DHASCO-B) with a reduction in arachidonic acid (ARA), and increased levels of inositol.
- Parent's Choice milk-based infant formula (658 g): New Infant Formula Parent's Choice® Milk-Based Infant Formula with ARA, DHA, GOS, and Lutein in powdered format packaged in 658 gram plastic containers.
- Parent's Choice milk-based follow up formula (658 g): New Follow-On Formula Parent's Choice® Milk-Based Infant Formula with ARA, DHA, GOS, and Lutein in powdered format packaged in 658 gram plastic containers.
- Major change to packaging for Good Start 1 Omega 3&6 with GOS and Good Start 2 Omega 3&6 with GOS Ready-to-Feed (RTF) Infant Formulas - Addition of a Resealable Cap Closure: addition of a fully resealable one-step screw cap closure to RTF Tetra packaging.
- Change to inositol level and label claim in a milk-based, non-GMO, low-lactose infant formula with ARA and DHA: an increase in the amount of inositol level in a milk-based, non-GMO, low-lactose infant formula with ARA and DHA and changes to label nutrition information.
Healthy clicks: Food and nutrition at a glance
Stay informed about our activities:
Follow us on Facebook
Follow us on Twitter
Follow us on Instagram
Follow us on YouTube
Find more information on healthy eating on our Food and Nutrition page
See the latest news from Health Canada on our website
Find other Health-related information on the Government of Canada website
Sign up to our Stakeholder Registry
Embrace healthy eating:
Consult Canada's food guide
Take inspiration from the Food guide snapshot
Check out Canada's Dietary Guidelines
Subscribe to the food guide's e-newsletter
Try one of our recipes
Seek out our food safety advice:
Protect yourself with our food safety tips
Refer to our safe cooking temperatures
