Chapter 4: Prevention - Chemoprophylaxis regimens: Canadian recommendations for the prevention and treatment of malaria
Last complete chapter revision: May 2025
On this page
- Preamble
- Key points for the health care provider
- Recommendations
- Background
- Methods
- Results
- Protection against insect bites
- Importance of completing the malaria chemoprophylaxis regimen as prescribed
- Conclusions and research needs
- Acknowledgements
- Conflicts of interest
- Appendix 1: Search strategy
- Appendix 2: AMSTAR assessment
- Appendix 3: Evidence to decision framework: Question 1
- Appendix 4: Evidence to decision framework: Question 2
- Appendix 5: Evidence to decision framework: Question 3
- Appendix 6: Assessment tables for applicability of good practice statements (GPS)
- References
Preamble
The Committee to Advise on Tropical Medicine and Travel (CATMAT) provides the Public Health Agency of Canada with ongoing and timely medical, scientific, and public health advice relating to tropical infectious disease and health risks associated with international travel. The Agency acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and medical practices, and is disseminating this document for information purposes to the medical community caring for travellers.
Persons administering or using drugs, vaccines, or other products should also be aware of the contents of the product monograph(s) or other similarly approved standards or instructions for use. Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) or other similarly approved standards or instructions for use by the licensed manufacturer(s). Manufacturers have sought approval and provided evidence as to the safety and efficacy of their products only when used in accordance with the product monographs or other similarly approved standards or instructions for use.
Key points for the health care provider
- Malaria is a life-threatening febrile illness caused by Plasmodium parasites transmitted through the bites of female Anopheles mosquitoes.
- Health care professionals are encouraged to use the below approach when counselling patients on and prescribing malaria chemoprophylaxis (MCP) for prevention of malaria (see Appendix 2 for more information):
- In most areas where malaria is present, MCP options include atovaquone-proguanil (AP), doxycycline, and mefloquine. Where the parasites are considered susceptible, chloroquine (or hydroxychloroquine) is an MCP option. Primaquine is also sometimes a suitable option in travellers where other MCP options are contraindicated or not tolerated. Note that primaquine is contraindicated in pregnancy and in people with low G6PD activity, as determined through a blood test.
- For the small subset of patients who are travelling to areas where relapsing forms of malaria are endemic, e.g., Oceania, parts of East Africa, clinicians should review the advice provided in Chapter 7 related to use of presumptive anti-relapse therapy with primaquine.
- The MCP agent tafenoquine is not licensed in Canada, so it is not covered in this chapter. It is licensed in the United States as an MCP option in addition to AP, doxycycline and mefloquine. Importantly, this drug has specific contraindications and requirements for screening related to drug metabolism/G6PD activity. These are discussed in relevant U.S. guidelinesFootnote 1 Footnote 2.
- Oral artemisinin-combination therapies are not licensed in Canada and are not recommended to be used as prophylaxis in travellers.
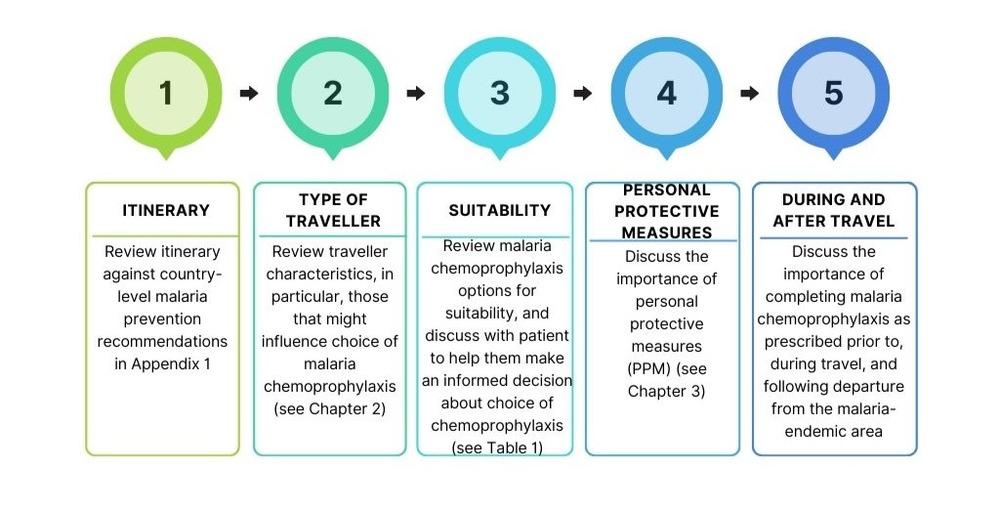
Figure 1: Text description
- Itinerary
- Review itinerary against country-level malaria prevention recommendations in Appendix 1.
- Type of traveller
- Review traveller characteristics, in particular, those that might influence choice of malaria chemoprophylaxis (see Chapter 2).
- Suitability
- Review MCP options for suitability, and discuss with patient to help them make an informed decision about choice of chemoprophylaxis (see Table 1).
- PPM
- Discuss the importance of personal protective measures (PPM) (see Chapter 3).
- During and after travel
- Discuss the importance of completing malaria chemoprophylaxis as prescribed prior to, during travel, and following departure from the malaria-endemic area.
Recommendations
1. If several MCP are considered medically suitable for the patient, CATMAT suggests atovaquone-proguanil (AP) over mefloquine or doxycycline.
(Discretionary recommendation, moderate certainty evidence)
Remarks:
- All three medications are effective for the prevention of malaria.
- Most travellers will choose AP, but some will choose mefloquine or doxycycline based on cost, convenience, past experience or other factors (see Table 1). Note that AP is contraindicated in some populations (e.g., severe renal impairment).
Rationale:
- AP, doxycycline, and mefloquine may provide a similar high level of protection against malaria (moderate certainty evidence), but
- AP likely has a lower rate of discontinuation due to adverse effects compared to mefloquine or doxycycline (moderate certainty evidence);
- AP likely has a lower rate of non-serious and concurrent neuropsychiatric adverse effects compared to mefloquine (very low to moderate certainty evidence); and,
- Serious adverse effects are similar with all options but the rate is low and therefore not critical to decision making (very low certainty evidence).
2. Where the parasites are considered susceptible to hydroxychloroquine (or chloroquine), CATMAT suggests atovaquone-proguanil (AP) over mefloquine, doxycycline or hydroxychloroquine (or chloroquine).
(Discretionary recommendation, very low certainty evidence)
Remarks:
- Most travellers will choose AP, but some will choose mefloquine, doxycycline, or hydroxychloroquine (or chloroquine) based on cost, convenience, past experience or other factors (see Table 1). Note that AP is contraindicated in some populations (e.g., severe renal impairment).
- Of note, the recommendation is based on very low certainty evidence compared to the moderate certainty evidence for recommendation 1. This specifically reflects the paucity of recent evidence for hydroxychloroquine (or chloroquine) (see rationale below).
Rationale:
- AP, doxycycline, mefloquine, and hydroxychloroquine (or chloroquine) may provide a similar high level of protection against malaria (very low certainty evidence for mefloquine compared to chloroquine);
- The committee notes that there was no recent evidence for efficacy of hydroxychloroquine against comparators and a theoretical concern that emergence of clinically relevant resistance to hydroxychloroquine in areas where malaria is currently presumed to be sensitive might be difficult to detect. This resulted in the committee placing more weight on the well-established efficacy of AP.
- AP likely has a lower rate of discontinuation due to adverse effects compared to mefloquine and doxycycline (moderate certainty evidence for AP compared to mefloquine) and may have a lower rate compared to hydroxychloroquine (or chloroquine) (very low certainty evidence for mefloquine compared to chloroquine);
- AP likely has a lower rate of non-serious and concurrent neuropsychiatric adverse effects compared to mefloquine (very low to moderate certainty evidence);
- AP may have a lower rate of non-serious adverse effects compared to hydroxychloroquine (very low to low certainty evidence for mefloquine compared to chloroquine), and
- Serious adverse effects are similar with all options (very low certainty evidence for mefloquine compared to chloroquine).
3. Where MCP is recommended and where other agents (AP, mefloquine, doxycycline, hydroxychloroquine) are contraindicated or not tolerated by the traveller, CATMAT recommends that primaquine be used for MCP.
(Strong recommendation, low certainty evidence).
Remarks:
- There are important and specific considerations related to use of primaquine for chemoprophylaxis (see Chapter 8 and Table 1). Primaquine use in any kind of preventive regimen should be avoided in those with abnormal activity detected on a qualitative G6PD screening test (see Figure 2) and is contraindicated in some populations regardless of G6PD screening results (e.g. pregnancy).
- Primaquine has causal prophylactic activity, and therefore requires only 7-days of prophylaxis following departure from the endemic area.
Rationale:
- Primaquine may provide a high level of protection against clinical malaria compared to placebo or multivitamin (low certainty evidence).
- Based on the mechanism of action, and lower efficacy in placebo-controlled studies compared to estimates from studies using other agents, it is unknown whether primaquine is as efficacious in preventing P. falciparum malaria compared to first-line MCP.
- There may be no differences in gastrointestinal adverse effects or neuropsychiatric adverse events between primaquine and placebo or multivitamin (very low to low certainty evidence).
- There may be no differences in adverse-effect associated discontinuations between primaquine and placebo or multivitamin but the rate is low and therefore not critical to decision making (very low certainty evidence).
- Clinical failure of primaquine radical cure treatment of Plasmodium vivax malaria has been documented in people who have polymorphisms in the Cytochrome P-450 2D6 gene.
4. The values and preferences of travellers towards MCP choice are varied.Clinicians should allow for discussion of this variability as part of the joint decision-making process (good practice statement).
5. Clinicians should advise travellers to adhere to personal protective measures against insect bites when they are exposed to mosquitoes in malaria-endemic areas (good practice statement).
6. Clinicians should emphasize to travellers that it is essential to complete their malaria chemoprophylaxis regimen as prescribed prior to, during and after departure from the malaria-endemic area (good practice statement).
| Consideration | Primary MCP options | Alternative options: Special circumstances | ||||
|---|---|---|---|---|---|---|
| Atovaquone-proguanil | Mefloquine | Doxycycline | Primaquine | Hydroxychloroquine (or chloroquine) | ||
Dosing (See Chapter 8 for more information on dosing) |
Adult |
250 mg atovaquone plus 100mg proguanil hydrochloride daily with fatty food |
250 mg once weekly |
100 mg daily with food and adequate amounts of fluid |
30 mg base (52.6 mg salt) daily with food |
310/300 mg base once weekly |
Pediatric |
Weight based dose daily with fatty food Atovaquone 62.5 mg Proguanil 25 mg |
5 mg/kg once weekly (max 250mg/dose) |
2 mg base/kg once daily (max 100mg daily) |
0.5 mg base/kg daily (max 30mg daily) |
5 mg base/kg once weekly |
|
When to startFootnote b (duration before arriving in malaria endemic area) |
1-2 days before |
At least 1 week before Loading dose can be given for 3 days prior if traveller is departing in < 1 week |
1-2 days before |
1-2 days before |
1 week before |
|
When to stop (duration after leaving malaria endemic area) |
7 days after |
4 weeks after |
4 weeks after |
7 days after |
4 weeks after |
|
Medication suitability |
Contraindications (See Chapter 8 and product monographs available through Health Canada's Drug Product Database for more information) |
|
|
|
|
|
Precautions (See Chapter 8 and product monographs available through Health Canada's Drug Product Database for more information) |
|
|
|
|
|
|
Patient values and preferences |
Cost |
Generally higher than alternatives |
Generally lower than alternatives |
Generally lower than AP, higher than mefloquine |
May be comparable in cost to alternatives |
Generally lower than alternatives |
Non-malaria benefits |
Not applicable |
Not applicable |
May provide protection against diseases/infectious agents susceptible to doxycycline (e.g., rickettsial infections, leptospirosis, sexually transmitted diseases) |
Not applicable |
Not applicable |
|
Trip duration/ exposure consideration |
Shorter run-in (1-2 days) period (pre-exposure) |
Longer run-in (at least 1 week) period (pre-exposure) |
Shorter run-in (1-2 days) period (pre-exposure) |
Shorter run-in (1-2 days) period (pre-exposure) |
Longer run-in (1 week) period (pre-exposure) |
|
Safety and efficacy as assessed in systematic reviewsFootnote c (See Summary of the evidence under the Results section below) |
Protection level against malaria |
Likely similar high level of protection against malaria (>90%) (moderate certainty evidence) |
High level of protection against malaria compared to placebo. |
May be similar high level of protection against malaria in chloroquine sensitive regions compared to AP, mefloquine, and doxycycline. |
||
Rate of discontinuation due to adverse effects |
Likely lower compared to mefloquine (moderate certainty evidence) |
Likely higher compared to AP (moderate certainty evidence) |
May be similar compared to mefloquine (very low certainty evidence) |
May be similar compared to placebo or multivitamin (very low certainty evidence) |
May be higher compared to AP (very low certainty evidence) |
|
Rates of serious adverse effects (SAE)Footnote d |
May be similar low rate of SAE (very low certainty evidence) |
Not assessed |
May be similar low rate of SAE (very low certainty evidence) |
|||
Rates of non-serious gastrointestinal, nervous system, and psychiatric adverse effects (See product monographs for a complete list of potential adverse effects) |
Likely lower compared to mefloquine (very low to moderate certainty evidence) |
Likely higher neuropsychiatric effects compared to AP and doxycycline (very low to moderate certainty evidence):
|
May be fewer neuropsychiatric effects but more gastrointestinal side effects than mefloquine and AP (very low certainty evidence):
|
May be no differences in gastrointestinal and neuropsychiatric adverse effects compared to placebo Not assessed compared to alternative agents |
May be fewer neuropsychiatric effects but similar rates of gastrointestinal effects compared to mefloquine (very low to low certainty evidence) |
|
Recommended use |
If several MCP are considered medically suitable for the patient, CATMAT suggests Atovaquone-proguanil (AP) over mefloquine or doxycycline (discretionary recommendation, moderate certainty evidence)Footnote e. |
Where MCP is recommended and where other agents (AP, mefloquine, doxycycline, hydroxychloroquine) are contraindicated or not tolerated by the traveller, CATMAT recommends that primaquine be used for MCP (strong recommendation, low certainty evidence).Footnote f |
Where the parasites are considered susceptible, CATMAT suggests Atovaquone-proguanil (AP) over mefloquine, doxycycline, or hydroxychloroquine (or chloroquine), (discretionary recommendation, very low certainty evidence)Footnote g |
|||
|
||||||
Background
Malaria is a life-threatening illness caused by Plasmodium parasites that are transmitted through the bites of female Anopheles mosquitoes. In addition to severe illness, malaria can result in significant out of pocket expenses if the affected person requires medical care while travelling (or living) in an endemic country.
A variety of interventions exist to reduce the risk of malaria, including malaria chemoprophylaxis (MCP) (addressed in this chapter) and/or the use of personal protective measures against mosquito bites (see Chapter 3). For each individual and circumstance, the recommended approach to prevention depends on the estimated risk and impact of acquiring malaria, travellers' values and preferences, the suitability of the available drugs, including their cost, medical history, allergies etc.
The purpose of this chapter is to provide health care professionals with recommendations for the use of different MCP, and with information on the benefits, harms and uncertainties with their use, particularly for the large majority of malaria-endemic areas where the primary MCP options (in Canada) are AP, doxycycline and mefloquine.
Clinical and epidemiological features
There are five related parasites associated with human malaria. Two of these, Plasmodium falciparum (P. falciparum) and Plasmodium vivax (P. vivax), are responsible for the large majority of travel-related malaria infections. P. falciparum predominates in sub-Saharan Africa and is responsible for the bulk of travel-related malaria deaths. Among non-immune leisure travellers, it has an overall case fatality rate that can exceed 1%Footnote 3 Footnote 4, which rises to approximately 10-20% among those with severe diseaseFootnote 3 Footnote 5 Footnote 6. P. vivax is often the more common parasite in endemic countries outside of sub-Saharan Africa. Although less frequently than for P. falciparum, severe disease also occurs with P. vivaxFootnote 7 Footnote 8.
All Canadians travelling to malaria endemic areas are at risk of acquiring malaria infection. Risk varies by destination, itinerary and a variety of other factors including subpopulations of travellers. For example, travellers born in regions with endemic malaria who relocate outside the endemic area but subsequently return to visit friends and relatives (VFRs) are at relatively higher exposure/malaria riskFootnote 9 than are vacation/leisure travellersFootnote 9 Footnote 10. By contrast, if clinical disease develops, the risk of death appears to be higher in leisure travellers than VFRsFootnote 4. Other groups of concern include pregnant women who are at relatively increased risk for severe disease, and long-term travellers because they often will have higher cumulative risk for exposure and infection and might be less likely to adhere to recommendations for use of MCP compared to short-term leisure travellers.
Chemoprophylaxis drugs
For travel to the majority of the malaria endemic countries, the main MCP options are AP, doxycycline, or mefloquine. Mefloquine-resistant P. falciparum has been identified in parts of Southeast Asia and therefore mefloquine is not recommended when travelling to these regions. Hydroxychloroquine (or chloroquine) is also available as MCP for people travelling to regions where parasites are considered susceptible, mostly in certain locations in the Americas. See Appendix 1 for detailed information on MCP recommendations by destination.
Primaquine may also be a suitable MCP agent, but it is not a first-line medication. It is active against the developing liver schizont stage of all malaria parasites, and the hypnozoite stage of P. vivax and P. ovale, which distinguishes it from other MCP. However, because it does not exhibit potent activity against disease-causing blood-stage parasites at doses typically used for MCP, it might be less effective against the generally more serious P. falciparum infectionsFootnote 11 Footnote 12 Footnote 13 Footnote 14. See Chapter 8 for more information about drugs used for the prevention of malaria.
Methods
General
A scoping review was conducted to identify potential key questions to address in this chapter. After consideration of the results of the scoping review, the CATMAT committee identified 6 key questions. A comprehensive systematic or rapid process was used to search for, select, analyze and assess the evidence to inform the recommendations and statements in this chapter. The level of certainty in the evidence in the benefits and harms was assessed using GRADE for the formal recommendations and is presented as high, moderate, low or very low when used. The CATMAT committee developed the recommendations and statements after considering benefits and harms, and other factors, such as patient values and preferences, resources, acceptability, feasibility and equityFootnote 15. An Evidence to Decision framework was used to assess the evidence and make recommendationsFootnote 16.
Good practice statements were also developed in the specific situation that benefits unequivocally outweighed the harms (see Appendix 6)Footnote 17 Footnote 18.
Key questions and evidence review
The following key PICO (Population of interest, Intervention, Comparison, and Outcome) questions were identified for consideration, three of which were addressed as good practice statements. For the other questions, outcomes were selected and classified according to the GRADE approach as critical or important (see question 1 for specific outcomes)Footnote 19.
Key questions
- Should AP, mefloquine and/or doxycycline be preferred for travellers from Canada to malaria endemic areas with hydroxychloroquine (or chloroquine) resistance and for whom MCP is recommended?
- What is the efficacy of mefloquine compared to AP or doxycycline for preventing travel-associated malaria? The committee rated the outcome of clinical cases of malaria as critical for clinical decision making.
- What are the harms of mefloquine compared to AP or doxycycline when used as chemoprophylaxis by travellers? We attributed the importance of harms as follows:
- Serious adverse effects (SAE) were initially considered critical outcomes, but changed to important given the small number of events. These were as specified in reviewed evidence, and included adverse effects that are life threatening, require inpatient hospitalization or prolongation of existing hospitalization, or result in persistent or significant disability or incapacity, or is a congenital anomaly/ birth defect.
- Adverse effect (AE)-associated medication discontinuations as a critical outcome.
- Pre-specified non-serious AE as an important but not critical outcome, such as gastrointestinal disorders; nervous system disorders; psychiatric disorders.
- In areas where malaria parasites are considered susceptible, should hydroxychloroquine (chloroquine), atovaquone-proguanil (AP), mefloquine and/or doxycycline be preferred for travellers from Canada to malaria endemic areas, and for whom MCP is recommended?
- What are the benefits and harms?
- Should primaquine be recommended as an MCP alternative in travellers to malaria endemic regions?
- What are the benefits and harms?
- Should clinicians discuss patient values and preferences as part of the joint decision-making process related to choice of MCP (good practice statement)?
- Should providers advise travellers to adhere to personal protective measures against insect bites (good practice statement)?
- Should providers emphasize to travellers to complete their malaria chemoprophylaxis regimen as prescribed prior to, during and after departure from the malaria-endemic area (good practice statement)?
A systematic literature search for systematic reviews and primary studies was done up to September 2019 for questions 1-4 (see Appendix 1 for strategy). As part of this search, we identified a 2017 Cochrane systematic review and meta-analysis titled "Mefloquine for preventing malaria during travel to endemic areas"Footnote 20. The quality of this study was assessed as moderate using the AMSTAR II toolFootnote 21 (see Appendix 2) and we judged it to be of sufficient quality and relevance to use for questions 1 and 2.
For good practice statements (GPS), we applied the approach recommended by the GRADE working groupFootnote 17.
Results
Primary chemoprophylaxis regimens (AP, doxycycline and mefloquine)
Recommendation
1. If several MCP are considered medically suitable for the patient, CATMAT suggests atovaquone-proguanil (AP) over mefloquine or doxycycline.
(Discretionary recommendation, moderate certainty evidence).
Remarks:
- All three medications are effective for the prevention of malaria.
- Most travellers will choose AP, but some will choose mefloquine or doxycycline based on cost, convenience, past experience or other factors (see Table 1). Note that AP is contraindicated in some populations (e.g., severe renal impairment).
Rationale:
- AP, doxycycline, and mefloquine may provide a similar high level of protection against malaria (moderate certainty evidence), but
- AP likely has a lower rate of discontinuation due to adverse effects than mefloquine or doxycycline (moderate certainty evidence);
- AP likely has a lower rate of non-serious and concurrent neuropsychiatric adverse effects than mefloquine (very low to moderate certainty evidence); and,
- Serious adverse effects are similar with all options but the rate is low and therefore not critical to decision making (very low certainty evidence).
Literature
The 2017 Cochrane reviewFootnote 20 included 20 RCTs (11,470 participants); 35 cohort studies (198,493 participants); and four large retrospective analyses of health records (800,652 participants). CATMAT's literature search identified a single additional study of interestFootnote 22, a systematic review of deaths and parasuicides associated with mefloquine chemoprophylaxis. A second studyFootnote 23 as identified separately retrospectively evaluated possible associations between self-reported antimalarial use and mental health outcomes among U.S. military personnel. Two additional studies conducted after completion of the systematic review related to adherence were also identified and assessed for inclusionFootnote 24 Footnote 25. These studies did not meet the inclusion criteria of the Cochrane review, and therefore were not added to the meta-analyses. As the efficacy of all three MCP is well established based on historic studies, this indirect evidence was also included in the GRADE assessment for efficacy.
There has been an increasing focus, especially among military veterans, on the potential for persistent neuropsychiatric adverse effects resulting from the use of antimalarial chemoprophylaxis. In response, several political reports have been developed, including in CanadaFootnote 26, AustraliaFootnote 27 and the UKFootnote 28 Footnote 29. In updating the systematic literature search, one study was identified that considered "long-term" effects of antimalarials among U.S. service membersFootnote 30, as well as a 2020 National Academies of Sciences, Engineering and Medicine (NASEM) report on this subjectFootnote 31. We believe the NASEM study provides relevant and important information, and therefore considered it in our evidence review (see below) as well as in the development of recommendations.
Summary of the evidence
The evidence included in the Cochrane meta-analyses may not have included important subpopulations of travellers, e.g., VFR. Further, relatively few Canadian travellers were included in the trialsFootnote 32, and most of the assessed evidence for the comparison between mefloquine and AP was for relatively short duration travel. We therefore judged the evidence to suffer serious indirectness and rated down the certainty of evidence by one level for the following outcomes: Serious AE, Discontinuations due to AE, and Non-serious AE.
The certainty of evidence from cohort studies were automatically downgraded by two levels for risk of bias, as they all had methodological concerns which could result in confounding or bias. For example, several studies were conducted in long-term military personnel where drug allocation may have been impacted by previous history of neuropsychiatric conditions. Study participants and clinicians may also be more likely to attribute adverse events to mefloquine, due to its prominence in the media.
Efficacy (critical outcome): AP, doxycycline, and mefloquine likely provide a similar and high level of protection against malaria (moderate certainty evidence). Two RCTsFootnote 32 Footnote 33 (1,293 participants) compared mefloquine directly to AP among non-immune international travellers travelling to endemic areas for a period of 28 days or less. No clinical cases of malaria occurred in either treatment arm suggesting these medications had similar efficacy (moderate certainty evidence, Appendix 3). Four RCTsFootnote 33 Footnote 34 Footnote 35 Footnote 36 involving 744 participants compared mefloquine to doxycycline among non-immune short-term international travellers. Four cases of malaria occurred in individuals receiving mefloquine compared to three in those receiving doxycycline for a calculated risk ratio of 1.35, 95% CI 0.35 to 5.19 (moderate certainty evidence, Appendix 3). This is further supported by historic indirect evidence comparing to placebo or other alternatives, which shows that all three MCP regimens demonstrate >90% efficacy against P. falciparum in susceptible regionsFootnote 37 Footnote 38 Footnote 39 Footnote 40 Footnote 41.
Discontinuations due to AE (critical outcome): In three RCTs, discontinuation due to adverse effects was likely greater with mefloquine than with AP (relative risk (RR) 2.86, 95% CI 1.53 to 5.31; 1,438 participants; 4/100 more discontinuations with mefloquine; moderate certainty evidence). Evidence from nine additional cohort studies demonstrated similar results, (RR 2.73, 95% CI 1.83 to 4.08). Based on four RCTs, discontinuations due to AE may be similar for mefloquine compared to doxycycline (RR 1.08, 95% CI 0.41 to 2.87; 763 participants; 2/100 discontinuations; very low certainty evidence). Evidence from ten cohort studies similarly demonstrated no substantial difference between drugs (RR 0.92, 95% CI 0.54 to 1.55). The Cochrane review did not compare AP directly against doxycycline. However, based on an indirect comparison, we estimate 4/100 fewer AE-associated discontinuations with AP than doxycycline.
Serious AE (important outcome): AP, doxycycline, and mefloquine may result in little to no difference in serious adverse effects (very low certainty evidence). In three cohort studiesFootnote 42 Footnote 43 Footnote 44 (3,693 participants) comparing mefloquine and AP, there were 15 serious AE reported in 2,651 mefloquine users and none reported among 940 participants who took AP. Three cohort studiesFootnote 43 Footnote 45 Footnote 46 (3,722 participants) found similar rates of SAE between mefloquine (19 in 2,125 participants) and doxycycline (10 in 1,597), risk ratio 1.53, 95% CI 0.23 to 10.24.
Non-serious AE (important outcome): Mefloquine likely results in more non-serious neuropsychiatric adverse effects than AP (very low to moderate certainty evidence) as reported in a RCT and eight cohort studies (Table 2). For non-serious adverse events, mefloquine may have relatively more neuropsychiatric events and relatively fewer gastrointestinal system events than doxycycline (Table 2) (see Appendix 3; very low certainty evidence).
| Adverse effect | Mefloquine (estimated absolute risk) | AP (estimated absolute risk) |
Doxycycline (estimated absolute risk) |
Hydroxychloroquine (estimated absolute risk) |
Estimates of absolute difference if AP is used over mefloquine (per 100 people) |
|---|---|---|---|---|---|
| Serious AE | 1/100 | 0/100 | 1/100 | 0/100 | 1 fewer |
| Discontinuation due to AE | 6/100 | 2/100 | 6/100 | 4/100 | 4 fewer |
| Abnormal dreams | 14/100 | 7/100 | 1/100 | 5/100 | 7 fewer |
| Insomnia | 13/100 | 3/100 | 3/100 | 11/100 | 10 fewer |
| Anxiety | 6/100 | 1/100 | 0/100 | 1/100 | 5 fewer |
| Depressed mood | 6/100 | 1/100 | 0.5/100 | 2/100 | 5 fewer |
| Abnormal thoughts or perceptions | 1/100 | 0/100 | 0/100 | 0/100 | 1 fewer |
| Dizziness | 8/100 | 2/100 | 2/100 | 11/100 | 6 fewer |
| Headache | 7/100 | 4/100 | 6/100 | 8/100 | 3 fewer |
| Nausea | 8/100 | 3/100 | 22/100 | 8/100 | 5 fewer |
| Vomiting | 1/100 | 1/100 | 6/100 | 1/100 | No difference |
| Abdominal pain | 5/100 | 5/100 | 17/100 | 7/100 | No difference |
| Diarrhea | 8/100 | 8/100 | 29/100 | 10/100 | No difference |
| Dyspepsia | 4/100 | 8/100 | 14/100 | Not available | 4 more |
Absolute estimates were calculated using the point estimates from the systematic review by Tickell-Painter et al. (2017)Footnote 20. The Confidence Interval has not been calculated due to the very low certainty of the evidence for some comparisons. Data from cohort studies were used when data from RCTs were unavailable. |
|||||
Persistent neuropsychiatric harms: A 2020 NASEM study, cited above but not included in the meta-analyses, concluded for mefloquine: "…there is insufficient or inadequate evidence of an association between the use of mefloquine for malaria prophylaxis and persistent or latent psychiatric events, including PTSD. Current evidence suggests further study of such an association is warranted, given the evidence regarding biologic plausibility, adverse events associated with concurrent use, or data from the existing epidemiologic studies"Footnote 31. While the study authors did not apply a GRADE framework, they did undertake a systematic evidence collection and review. We suggest their conclusion be interpreted as meaning mefloquine may not be associated with an elevated likelihood of persistent or latent psychiatric events (very low certainty evidence).
Values and preferences of travellers: We identified a number of studies that considered, directly or indirectly, values and preferences of travellers in the context of MCP but did not meet the inclusion criteria of the Cochrane review. In summary, a variety of factors are implicated as potentially influencing decisions related to MCP, including: chemoprophylaxis side effects, duration of travel, sub-populations of traveller (e.g., VFR), age, perceived likelihood of acquiring malaria, and costFootnote 47 Footnote 48 Footnote 49 Footnote 50 Footnote 51 Footnote 52 Footnote 53 Footnote 54. This is consistent with other interventions that are targeted to travellers including vaccines. Given that divergent traveller values and preferences are expected, clinicians should allow for discussion of this as part of the joint decision-making process related to the choice of MCP (good practice statement, see Appendix 6).
Judgement
Current evidence (moderate certainty) suggests AP, mefloquine and doxycycline likely provide a similar and high level of protection against clinical malaria. However, fewer people may discontinue AP than mefloquine (4/100 fewer). As well, AP may lead to fewer (5 to 10 fewer people out of 100) non-serious neuropsychiatric side effects than mefloquine, such as abnormal dreams, insomnia, anxiety, depressed mood and dizziness (very low to moderate certainty evidence). Serious AE were infrequently reported with little difference in absolute estimates between MCP (very low certainty evidence).
The Committee believes that the majority of travellers would wish to avoid side effects and discontinuing medications, and hence would prefer to receive AP. However, the committee recognizes that there is significant uncertainty and variability related to the values and preferences of travellers, and there may be differences in costs, convenience of dosing, familiarity with a medication, ancillary therapeutic advantages, or other factors that may affect the choice of medication (see Table 1). Based on these considerations, the committee chose to make a discretionary recommendation for the use of AP where several MCP are considered medically suitable for the patient.
Other chemoprophylaxis regimens (hydroxychloroquine [chloroquine] and primaquine)
2. Where the parasites are considered susceptible to chloroquine, CATMAT suggests atovaquone-proguanil (AP) over mefloquine, doxycycline or hydroxychloroquine (chloroquine).
(Discretionary recommendation, very low certainty evidence)
Remarks:
- Most travellers will choose AP, but some will choose mefloquine, doxycycline, or hydroxychloroquine (or chloroquine) based on cost, convenience, past experience or other factors (see Table 1). Note that AP is contraindicated in some populations (e.g., severe renal impairment).
- Of note, the recommendation is based on very low certainty evidence compared to the moderate certainty evidence for recommendation 1. This specifically reflects the paucity of recent evidence for hydroxychloroquine (or chloroquine) (see rationale below).
Rationale:
- AP, doxycycline, mefloquine, and hydroxychloroquine (or chloroquine) may provide a similar high level of protection against malaria (very low certainty evidence for mefloquine compared to chloroquine).
- The committee notes that there was no recent evidence for efficacy of hydroxychloroquine against comparators and a theoretical concern that clinically relevant resistance to hydroxychloroquine in sensitive areas might be difficult to detect. This resulted in the committee placing more weight on the well-established efficacy of AP.
- AP likely has a lower rate of discontinuation due to adverse effects than mefloquine and doxycycline (moderate certainty evidence for AP compared to mefloquine) and may have a lower rate than hydroxychloroquine (or chloroquine) (very low certainty evidence for mefloquine compared to chloroquine);
- AP may have a lower rate of non-serious adverse effects than hydroxychloroquine (very low to low certainty evidence for mefloquine compared to chloroquine), and
- Serious adverse effects are similar with all options (very low certainty evidence for mefloquine compared to chloroquine).
3. Where MCP is recommended and where other agents (AP, mefloquine, doxycycline, hydroxychloroquine) are contraindicated or not tolerated by the traveller, CATMAT recommends that primaquine be used for MCP.
(Strong recommendation, low certainty evidence).
Remarks:
- There are important and specific considerations related to use of primaquine for chemoprophylaxis (see Chapter 8 and Table 1). Primaquine use in any kind of preventive regimen should be avoided in those with abnormal activity detected on a qualitative G6PD screening test (see Figure 2) and is contraindicated in some populations regardless of G6PD screening results (e.g. pregnancy).
- Primaquine has causal prophylactic activity, and therefore requires only 7-days of prophylaxis following departure from the endemic area.
Rationale:
- Primaquine may provide a high level of protection against clinical malaria compared to placebo or multivitamin (low certainty evidence).
- Based on the mechanism of action, and lower efficacy in placebo-controlled studies compared to estimates from studies using other agents, it is unknown whether primaquine is as efficacious in preventing P. falciparum malaria compared to first-line MCP.
- There may be no differences in gastrointestinal adverse effects or neuropsychiatric adverse events between primaquine and placebo or multivitamin (very low to low certainty evidence).
- There may be no differences in adverse-effect associated discontinuations between primaquine and placebo or multivitamin but the rate is low and therefore not critical to decision making (very low certainty evidence).
- Clinical failure of primaquine radical cure treatment of Plasmodium vivax malaria has been documented in people who have polymorphisms in the Cytochrome P-450 2D6 gene.
Hydroxychloroquine (chloroquine) for primary prophylaxis
Literature
The Cochrane review served as the foundation of the assessment for hydroxychloroquine (chloroquine), and no additional relevant studies were identified for inclusion from the literature reviewFootnote 20. There is a lack of recent studies on chloroquine efficacy. All studies from the Cochrane review comparing the efficacy of chloroquine with mefloquine included semi-immune populations and were conducted over 20 years ago. Further, two of the four RCTs were conducted in areas of known chloroquine resistance at the study sites, so these studies were excluded in the assessment of efficacy. As the efficacy of chloroquine in susceptible regions is well established in practice, from therapeutic efficacy studies, and based on historic challenge studies, indirect efficacy studies were also included in the GRADE assessment for this outcome.
Summary of the evidence
Efficacy (critical outcome): P. falciparum parasites in most areas of the world are not sensitive to chloroquine, and hence it only is considered a viable MCP option in a few locations (see Appendix 1). Based on two RCTs from the Cochrane review where there was no known chloroquine resistance, there may be similar protection against malaria among those who took chloroquine and those who took mefloquine. No clinical cases of malaria were reported in either study (100% efficacy, very low certainty evidence). While there is a lack of recent evidence demonstrating chloroquine efficacy due to widespread resistance of P. falciparum parasites, historic challenge studies have shown high to completely protective efficacy against P. vivax malariaFootnote 55 Footnote 56. Two therapeutic efficacy studies done in Haiti in 2011 and 2013, found a failure rate of chloroquine for treatment of uncomplicated P. falciparum of 10.3% (n=68) and 15.3% (n=39), respectively. However, it was not clear if some of these failures were misclassified, and instead represented reinfectionsFootnote 57. As of 2022, chloroquine in combination with primaquine continues to be recommended by the WHO for treatment of uncomplicated P. falciparum in some countries in the Americas, suggesting it may still be effective for prevention of malaria in these regions.
Discontinuations due to AE (critical outcome): Based on indirect evidence, AP may have fewer discontinuations than hydroxychloroquine (2/100 fewer), and hydroxychloroquine may have fewer discontinuations due to AE as compared to mefloquine and doxycycline (2/100 fewer).
Serious AE (important outcome): Hydroxychloroquine (0/100), AP (0/100), doxycycline (1/100), and mefloquine (1/100) may result in little to no difference in serious adverse effects (very low certainty evidence).
Non-serious AE (important outcome): Based on indirect evidence, AP may have fewer non-serious adverse effects than chloroquine (1 to 9/100 fewer, see Table 2). All non-serious adverse events were lower with AP, aside from the outcome of abnormal dreams, which has an estimated 2/100 more with AP. Hydroxychloroquine may have fewer neuropsychiatric AE compared to mefloquine, including abnormal dreams, anxiety, and depressed mood (1 to 5/100 compared to 6 to 14/100 for mefloquine). However, hydroxychloroquine and mefloquine may have similar rates of gastrointestinal AE (1 to 10/100 compared to 1 to 8/100 for mefloquine). Based on indirect evidence, doxycycline may have similar but different spectra of non-serious AE compared to hydroxychloroquine. Doxycycline may have fewer neuropsychiatric AE (0 to 3/100 compared to 1 to 11/100 for hydroxychloroquine). However, hydroxychloroquine may have fewer gastrointestinal AE (1 to 10/100 compared to 6 to 29/100 for doxycycline).
Judgement
Chloroquine's safety profile is well established. The efficacy and serious adverse effects may be similar for hydroxychloroquine when compared with other MCP options. However, the committee notes that there was no recent evidence for efficacy of hydroxychloroquine against comparators and a theoretical concern that emergence of clinically relevant resistance to hydroxychloroquine in areas where malaria is currently presumed to be sensitive might be difficult to detect. Further, AP may have fewer adverse effect-associated discontinuations and non-serious adverse effects than chloroquine. This resulted in the committee placing more weight on the well-established efficacy of AP and decision to make a discretionary recommendation for AP in areas where the parasites are considered susceptible to hydroxychloroquine.
Primaquine for primary prophylaxis
Literature
CATMAT's literature search identified a systematic review on primaquine for primary prophylaxis in travellers. This systematic review included seven interventional studies examining chemoprophylactic efficacy of daily-dosed primaquine in 7,060 primarily non-immune travellers to Ethiopia, Kenya, Indonesia, Papua New Guinea, or Colombia, who ranged in age from 7 to 65 years and were without clinical or microscopically confirmed malaria at the start of interventionFootnote 12. Three reviews, one pharmacokinetic study, one case report, and two case series published between 1967-2006 on primaquine benefits and harms were also identified for inclusion in the GRADE assessments and evidence summary, but were not included in the meta-analysesFootnote 58 Footnote 59 Footnote 60 Footnote 61 Footnote 62 Footnote 63 Footnote 64.
Data on the ease with which travellers could obtain G6PD screening prior to initiating primaquine primary prophylaxis in an outpatient pre-travel setting are lacking.
Summary of the evidence
Efficacy (critical outcome): Based on four RCTs which compared primaquine to placebo, evidence suggests that primaquine provides a high level of protection against clinical malaria compared to placebo or multivitamin (low certainty evidence; 87% efficacy; 34/100 fewer)Footnote 65 Footnote 66 Footnote 67 Footnote 68 Footnote 69. This is consistent with the U.S. evidence review, which suggests >85% protective efficacy of primaquine against P. falciparum and primary P. vivax infectionsFootnote 64. However, there are different baseline conditions, including malaria endemicity and dominant parasite present, across studies that might affect performance. For example, the study by Ling et al. (2002) was conducted in Papua Indonesia, where P. vivax is highly endemicFootnote 69. For most travellers, the absolute effect would be lower than 34/100, as studies assessed were conducted in highly endemic areas, such as Kenya and Papua IndonesiaFootnote 65 Footnote 68 Footnote 69. Based on Swedish surveillance data from 1997 to 2003, the crude risk for travellers was estimated to vary from 1 per 100,000 travellers to Central America and the Caribbean to 357 per 100,000 in central AfricaFootnote 70.
In several studies, the short follow-up period limited the ability to detect late relapse P. vivax cases. An observational before-and-after study among nonimmune Israeli rafters conducted in an area hyperendemic for both P. falciparum and P. vivax along the Omo River Ethiopia, demonstrated that primaquine is effective for causal prophylaxis (liver stage) of P. vivaxFootnote 71. Of the 106 travellers who received primaquine, 5.7% developed malaria (four P. falciparum, one P. vivax, and one mixed infection).
Primaquine efficacy was compared to alternative agents (mefloquine, doxycycline, and AP) among two RCTs, one of which was an open label trial, however, a meta-analysis was not completed owing to the low quality and indirectness of the evidenceFootnote 65 Footnote 68 Footnote 69. Additional studies are needed to assess direct comparisons between these agents.
Because primaquine is activated by cytochrome P-450 isoenzyme 2D6 (CYP2D6) in the liver, individuals who harbor polymorphisms resulting in low CYP2D6 enzyme activity may have sub-therapeutic levels of primaquine. Clinical failure of primaquine radical cure treatment of P. vivax infections has been documented in persons who harbor polymorphisms in cytochrome P-450 2D6 geneFootnote 72 Footnote 73. The possible contribution of these polymorphisms to documented failure of primaquine prophylaxis remains unknown, and testing for CYP2D6 polymorphisms is not easily or rapidly available.
Discontinuations due to AE (important outcome): Primaquine may have a similar, low rate of discontinuations due to adverse effects as compared to placebo or multivitamin (very low certainty evidence; 2/1,000 fewer), however, the direction of the effect differed between studies.
Non-serious AE (important outcome): Based on four RCTs which compared primaquine to placebo, evidence suggests that primaquine and placebo or multivitamin may have a similar rate of non-serious gastrointestinal (diarrhea, stomach pains, nausea, vomiting, appetite loss) (RR=0.81), and neuropsychiatric adverse effects (headache, fatigue, malaise, dizziness, insomnia) (RR=1.03) (very low to low certainty evidence).
Other undesirable effects: Primaquine is known to cause methemoglobinemia and oxidant-induced hemolytic anemia in individuals with G6PD deficienciesFootnote 59 Footnote 64. Thus, there is a need to test for G6PD deficiency before administering primaquine, which limits its implementation as radical cure treatment and chemoprophylaxis. In most cases, a qualitative test is sufficient to determine G6PD deficiency <30%. However, since G6PD deficiency is an X-linked disorder, females can be heterozygous for the allele and have variable levels of G6PD deficiencyFootnote 74. In this case, quantitative testing may be required, which may result in greater time delays and increased cost. See Figure 2 for guidance on testing for G6PD deficiency.
Uncertainties: Randomized controlled trials of primaquine as a primary prophylactic agent against P. falciparum malaria in children are limited, though available data suggest that protective efficacy is approximately 83%Footnote 65.
Judgement
The benefits of primaquine in preventing clinical malaria largely outweigh the trivial adverse effects and the requirement for G6PD deficiency testing. The committee believes that the majority of individuals would wish to avoid clinical malaria, and accept G6PD deficiency testing, so chose to make a strong recommendation for primaquine where other agents are contraindicated or not tolerated by the traveller. The benefit of primaquine primary prophylaxis in those with G6PD deficiency is unlikely to outweigh the potential harms. Thus, primaquine use in any kind of preventive regimen should be avoided in those with abnormal activity detected on a qualitative G6PD screening test (see Figure 2). Figure 2 shows the potential resulting pathways after G6PD deficiency testing with a qualitative test. Most qualitative tests for G6PD deficiency have a threshold of 30%Footnote 74.
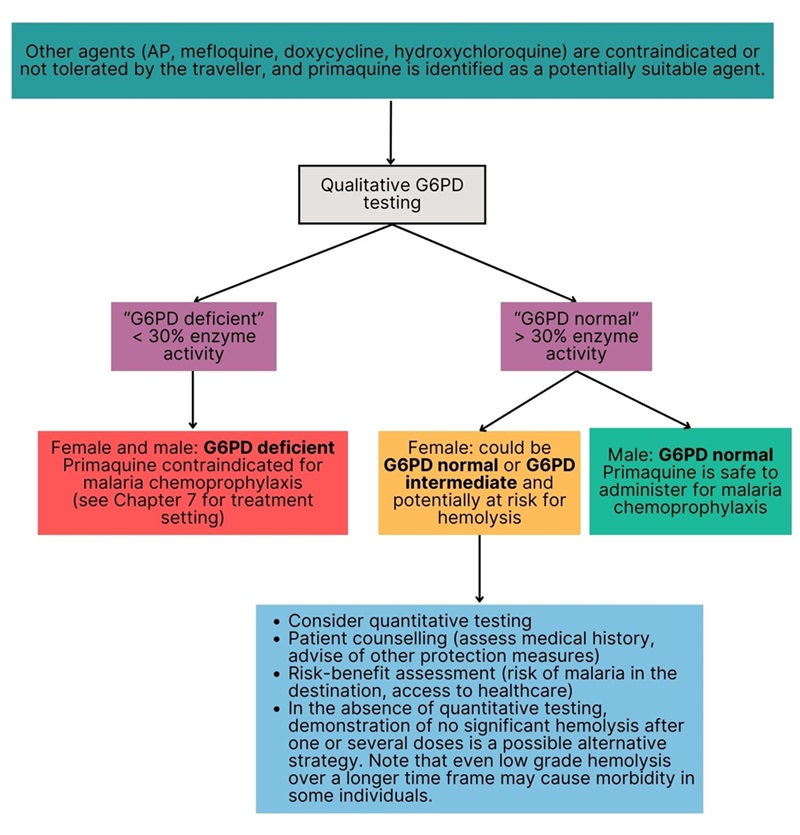
Figure 2: Text description
Where other agents (AP, mefloquine, doxycycline, hydroxychloroquine) are contraindicated or not tolerated by the traveller, and primaquine is identified as a potentially suitable agent, then a qualitative G6PD test is done.
If the result is "G6PD deficient" (<30% enzyme activity), then both females and males are considered G6PD deficient and primaquine is contraindicated for malaria chemoprophylaxis (see Chapter 7 for the treatment setting).
If the result is "G6PD normal" (>30% enzyme activity) and the patient is male, then the individual is considered G6PD normal and primaquine is safe to administer for malaria chemoprophylaxis.
If the result is "G6PD normal" (>30% enzyme activity) and the patient is female, then the individual could be G6PD normal or G6PD intermediate and potentially at risk of hemolysis. In this case, health care professionals are advised to:
- consider quantitative testing;
- patient counselling (assess medical history, advise of other protection measures);
- conduct a risk-benefit assessment (risk of malaria in the destination, access to healthcare).
- In the absence of quantitative testing, demonstration of no significant hemolysis after one or several doses is a possible alternative strategy. Note that even low grade hemolysis over a longer time frame may cause morbidity in some individuals.
Protection against insect bites
Clinicians should advise travellers to adhere to personal protective measures (PPM) against insect bites when they are exposed to mosquitoes in malaria-endemic areas (good practice statement, see Appendix 6).
Clinicians should emphasize the following implementation considerations when advising travellers:
- Use an approved and age-appropriate insect repellent.
- Protect sleeping areas against mosquito entry/activity, e.g., by staying in places that have screening in good repair, air conditioning, bed nets, no gaps in building envelope that would allow mosquito entry.
- Consider wearing clothing that has been pre-treated with permethrin.
For additional details on recommendations related to the prevention of arthropod bites, see the CATMAT statement on measures to prevent arthropod bites.
Importance of completing the malaria chemoprophylaxis regimen as prescribed
Clinicians should emphasize to travellers that it is essential to complete their malaria chemoprophylaxis regimen as prescribed prior to, during and after departure from the malaria-endemic area (good practice statement, see Appendix 6).
The efficacy reported for different antimalarial clinical trials assumes strict adherence to the dosing schedule as well as the use of PPM, such as insect avoidance and use of insect repellents. Some regimens (e.g. mefloquine) are more forgiving of missed doses while some need to be taken on a very regular schedule (e.g. doxycycline, primaquine) to ensure maximal protection. If travellers are educated on all aspects of malaria prevention and the specific issues around their prophylactic regimen, they may be more compliant with the treatment schedule.
Conclusions and research needs
To improve the certainty of evidence and better target recommendations for Canadian travellers, further clinical trials and observational studies of Canadian traveller populations are needed, including those which are representative of long-term travellers and individuals visiting friends and relatives.
Recent data are currently lacking on the efficacy of hydroxychloroquine (chloroquine) for malaria chemoprophylaxis in areas where the P. falciparum parasites remain to be susceptible. Future studies which compare the efficacy and adverse effects of primaquine to alternative agents are also warranted. CATMAT's recommendations are subject to change based on the future publication of such data.
Acknowledgements
This statement was prepared by: YG Bui, S Kadykalo, A Khatib, S Schofield, CP Yansouni, R Zimmer, and was approved by CATMAT.
CATMAT gratefully acknowledges the contribution of: C Bell, N Farmanara, M Laplante, TM Lee, M Libman, T Nguyen, T Olagunju, K Plewes, N Santesso, and the Public Health Agency of Canada Library.
CATMAT
Members: M Libman (Chair), YG Bui (Vice-Chair), K Plewes (Malaria Sub-Cttee Chair), A Acharya, I Bogoch, C Greenaway, A Khatib, P Lagacé-Wiens, J Lee, C Yansouni
Former members: A Boggild, A McCarthy
Liaison representatives: J Pernica (Association of Medical Microbiology and Infectious Disease Canada, K O'Laughlin (Centers for Disease Control and Prevention, United States), I Viel-Thériault (Canadian Paediatric Society)
Former liaison: K Angelo
Ex-officio representatives: E Ebert (National Defence and the Canadian Armed Forces), C Jensen (National Advisory Committee on Immunization [NACI] Secretariat, PHAC, D Marion (National Defence and the Canadian Armed Forces), S Schofield (National Defence and the Canadian Armed Forces), M Tunis (NACI Secretariat, PHAC) and R Zimmer (Biologic and Radiopharmaceutical Drugs Directorate, Health Canada)
Former ex-officio representatives: P McDonald, C Rossi
CATMAT Malaria Chemoprophylaxis Working Group
Members: YG Bui, A Khatib, N Santesso, S Schofield, CP Yansouni, R Zimmer
PHAC participants: S Kadykalo, M Laplante, TM Lee, T Nguyen
Former members: A Boggild, A McCarthy, P McDonald, C Rossi
Conflicts of interest
None declared.
Appendix 1: Literature search strategy
For questions 1-4, a literature search strategy was developed with the aid of a reference librarian. Six electronic databases (OVID MEDLINE, Embase, Scopus, Global Health, EBM Reviews, and Proquest Public Health) were searched using terms related to malaria prophylaxis and decision making for health care practitioners and travellers. For question 1, the systematic review time period was from the earliest available date in the database to September 3 or 4, 2019 and yielded 198 results. Three working group members reviewed the abstracts for inclusion and exclusion and 67 articles were retained. For questions 2-4, the systematic review period was also from the earliest available date in the database to September 2019, and yielded 105 results. Two working group members reviewed the abstracts for inclusion and exclusion. Papers were excluded if they were duplicates, if they were not published in English or French, or were case studies or factsheets. In cases where there was disagreement over inclusion, the paper was retained with the expectation that a full-article review would be appropriate for determining relevance.
Sample strategy
What are the harms related to malaria prophylaxis with Mefloquine or Malarone or Doxycycline in travellers?
| Search number | Search strategy | Results |
|---|---|---|
| 1 | (Doxycycline or vibramycin or azudoxt or bmy 28689 or bmy-28689 or bmy28689 or bu 3839t or bu-3839t or deoxymykoin or dossiciclina or doxiciline or doxitard or doxivetin or doxy-caps or doxy-puren or doxy-tabs or doxychel or doxycyclinum or doxysol or doxytetracycline or investin or liviatin or monodox or nordox or oracea or ronaxan or spanor or vibra-tabs or vibramycin or vibramycine or vibravenos or zenavod or adoxa or amermycin or atrax or bactidox or banndoclin or basedillin or bassado or biocolyn or biodoxi or bronmycin or calcium doxycycline or cloran or cyclidox or dentistar or deoxycycline or deoxymycin dispersal or deoxyoxytetracycline or desoxy oxytetracycline or desoxycycline or doinmycin or dosil or dotur or doxaciclin or doxacycline or doxat or doxatet or doxi-sergo or doxibiotic or doxycycline or doxilin or doximed or doximycin or doxin or doxine or doxocycline or doxsig or doxy or doxy 100 or doxy II or doxy m or doxy n tablinen or doxy p ratiopharm or doxy puren or doxy s or doxy tablinen or doxy-1 or doxybiocin or doxycen or doxycin or doxycycline or doxylag or doxylin or doxymycin or doxypuren or doxytec or doxytrim or dumoxin or duracycline or esdoxin or doxylin or etidoxina or gewacyclinor gs 3065 or ibralene or idocyclin or idocyklin or interdoxin or longamycin or lydox or magdrin or medomycin or mespafin or mildox or miraclin or nsc 56228 or paldomycin or pernox gel or radox or remycin or respidox or roximycin or serodoxy or servidoxine or servidoxyne or siadocin or siclidon or sigadoxin or supracyclin or supramycina or tenutan or tolexine or tolexine ge or torymycin or tsurupioxin or unidox or veemycin or viadoxin or vibra s or vibra tabs or vibrabiotic or vibracina or vibradox or vibramicinaor vibratab or vibraveineuse or vibravet or viradoxyl-n or wanmycin or zadorin or 564-25-0 or doryx or doxyhexal).ti. | 3559 |
| 2 | (Doxycycline or vibramycin or azudoxt or bmy 28689 or bmy-28689 or bmy28689 or bu 3839t or bu-3839t or deoxymykoin or dossiciclina or doxiciline or doxitard or doxivetin or doxy-caps or doxy-puren or doxy-tabs or doxychel or doxycyclinum or doxysol or doxytetracycline or investin or liviatin or monodox or nordox or oracea or ronaxan or spanor or vibra-tabs or vibramycin or vibramycine or vibravenos or zenavod or adoxa or amermycin or atrax or bactidox or banndoclin or basedillin or bassado or biocolyn or biodoxi or bronmycin or calcium doxycycline or cloran or cyclidox or dentistar or deoxycycline or deoxymycin dispersal or deoxyoxytetracycline or desoxy oxytetracycline or desoxycycline or doinmycin or dosil or dotur or doxaciclin or doxacycline or doxat or doxatet or doxi-sergo or doxibiotic or doxycycline or doxilin or doximed or doximycin or doxin or doxine or doxocycline or doxsig or doxy or doxy 100 or doxy II or doxy m or doxy n tablinen or doxy p ratiopharm or doxy puren or doxy s or doxy tablinen or doxy-1 or doxybiocin or doxycen or doxycin or doxycycline or doxylag or doxylin or doxymycin or doxypuren or doxytec or doxytrim or dumoxin or duracycline or esdoxin or doxylin or etidoxina or gewacyclinor gs 3065 or ibralene or idocyclin or idocyklin or interdoxin or longamycin or lydox or magdrin or medomycin or mespafin or mildox or miraclin or nsc 56228 or paldomycin or pernox gel or radox or remycin or respidox or roximycin or serodoxy or servidoxine or servidoxyne or siadocin or siclidon or sigadoxin or supracyclin or supramycina or tenutan or tolexine or tolexine ge or torymycin or tsurupioxin or unidox or veemycin or viadoxin or vibra s or vibra tabs or vibrabiotic or vibracina or vibradox or vibramicinaor vibratab or vibraveineuse or vibravet or viradoxyl-n or wanmycin or zadorin or 564-25-0 or doryx or doxyhexal).ab. /freq=3 | 2618 |
| 3 | (Malarone or atovaquone proguanil or proguanil atovaquone or proguanil hydrochloride plus atovaquone or "atovaquone and proguanil" or atovaquone mix* or atovaquone-proguanil hydrochloride mix*or proguanil hydrochloride atovanquone mix* or malanil or atovaquone plus proguanil or proguanil plus atovaquone or "atovaquone/proguanil" or "proguanil/atovaquone" or 156979-69-5).ti. | 186 |
| 4 | (Malarone or atovaquone proguanil or proguanil atovaquone or proguanil hydrochloride plus atovaquone or "atovaquone and proguanil" or atovaquone mix* or atovaquone-proguanil hydrochloride mix*or proguanil hydrochloride atovanquone mix* or malanil or atovaquone plus proguanil or proguanil plus atovaquone or "atovaquone/proguanil" or "proguanil/atovaquone" or 156979-69-5).ab. /freq=3 | 115 |
| 5 | (Mefloquine or lariam or mefloquin or mefloquina or mefloquinum or racemin mefloquina or ro 21-5998 or ro 215998 or wr 142,490 or wr 142490 or laricam or mefliam or mefloquine hydrochloride or mephaquin or mephaquine or tropicur or 53230-10-7).ti. | 1280 |
| 6 | (Mefloquine or lariam or mefloquin or mefloquina or mefloquinum or racemin mefloquina or ro 21-5998 or ro 215998 or wr 142,490 or wr 142490 or laricam or mefliam or mefloquine hydrochloride or mephaquin or mephaquine or tropicur or 53230-10-7).ab. /freq=3 | 883 |
| 7 | or/1-6 | 6222 |
| 8 | ((side* adj2 (react* or event* or effect*)) or harm* or (adverse adj2 (react* or event* or effect*))).tw. | 725593 |
| 9 | exp "Drug-Related Side Effects and Adverse Reactions"/ or exp *product surveillance, postmarketing/ or *risk assessment/ or exp *safety/ | 176211 |
| 10 | or/8-9 | 871523 |
| 11 | and/7,10 | 974 |
| 12 | doxycycline/ae or atovaquone plus proguanil/ae or mefloquine/ae | 1109 |
| 13 | or/11-12 | 1695 |
| 14 | *malaria/pc or (malaria adj2 (prophylaxis or chemoprophylaxis or prevent*)).ti. | 8538 |
| 15 | travel*.mp,jt. | 71074 |
| 16 | or/14-15 | 78705 |
| 17 | and/13,16 | 315 |
| 18 | ("20160421" or "20160422" or "20160423" or "20160424" or "20160425" or "20160426 20160427" or "20160428" or "20160429" or "20160430" or 201605* or 201606* or 201607* or 201608* or 201609* or 201610* or 201611* or 201612* or 2017* or 2018* or 2019*).ez. | 3747452 |
| 19 | and/17-18 | 19 |
Appendix 2: AMSTAR assessment
The Cochrane review was judged as being of moderate quality. Given that there was no adjustment for confounding in the analysis of non-randomized studies (NRSI) and no justification provided, we rated down one level (from high) for the overall confidence of the review and the level of confidence in the estimates. However, since most of the effect estimates based on NRSI were already at 'very low' certainty and RCTs were used wherever possible, we did not rate down further. This reflects that we have a lower confidence in the relative effect estimates for outcomes that were based on cohort studies only.
Appendix 3: Evidence to decision framework for: research question 1
Question: Should atovaquone-proguanil (AP), mefloquine and/or doxycycline be preferred for travellers from Canada to malaria endemic areas with hydroxychloroquine (chloroquine) resistance, and for whom malaria chemoprophylaxis (MCP) is recommended?
Population: Travellers from Canada to malaria endemic areas with chloroquine resistance, and for whom malaria chemoprophylaxis (MCP) is recommended.
Intervention: Mefloquine
Comparison: AP or doxycycline
Main outcomes: Clinical malaria, serious adverse effects (SAE), adverse effect-associated discontinuations, pre-specified non-serious adverse effects
Setting: International travel
Perspective: Individual
Background: Malaria is a life-threatening illness caused by parasites that are transmitted through the bites of Anopheles mosquitoes. Travellers who visit areas of endemicity are at risk for malaria and, if infected, might go on to develop severe malaria, which has a case fatality rate of approximately 20%. There are a variety of interventions available to travellers to prevent malaria. These include materials and/or chemicals to prevent mosquito bites, e.g., topical skin repellents, spatial repellents and insecticide treated netting/bed nets. A cornerstone for malaria prevention among travellers is use of malaria chemoprophylaxis (MCP). MCP does not negate the need for use of other malaria prevention approaches but does offer a high level of protection against clinical disease. In Canada, there are three main MCP agents recommended for use in areas where malaria parasites are resistant to chloroquine (most endemic areas): AP, doxycycline and mefloquine. CATMAT has recommended these medications for decades without a "preference" but has not applied GRADE to inform its recommendations for MCP. In 2017, a systematic review (SR) comparing mefloquine to AP or doxycycline was published by the Cochrane collaborationFootnote 20. CATMAT assessed the evidence from this SR and applied GRADE to revisit its recommendations related to MCP choice (when it is indicated) for travel to endemic areas where chloroquine resistance is a concern.
Conflict of interests: None
Assessment
| Domain | Judgement | Research evidence and additional considerations |
|---|---|---|
Problem: Is the problem a priority? |
No Probably no Probably yes Yes Varies Don't know |
Malaria is one of the most important infectious diseases in the world. Travellers to malaria endemic areas can be exposed to malaria, and if disease develops, are at risk for severe outcomes and death. It is estimated that several hundred cases of malaria occur annually among Canadian travellers, including several severe or complicated cases (Chapter 1: Introduction to Canadian recommendations). |
Desirable effects: How substantial are the desirable anticipated effects (protection against malaria)? |
Trivial Small Moderate Large Varies Don't know |
Systematic review: A systematic literature search for systematic reviews and primary studies was done up to September 2019. As part of this search, a 2017 Cochrane systematic review and meta-analysis titled "Mefloquine for preventing malaria during travel to endemic areas" was identified and judged to be of sufficient quality and relevance to use for question 1Footnote 20. The 2017 Cochrane review included 20 RCTs (11,470 participants); 35 cohort studies (198,493 participants); and four large retrospective analyses of health records (800,652 participants). See table 5 and table 6 for a summary of the results. Additional indirect evidence:
Overall efficacy: Likely similar high levels of protection against malaria (>90%) in areas where there is no drug resistance (moderate certainty evidence). |
Undesirable effects: How substantial are the undesirable anticipated effects? |
Trivial Small to Moderate Large Varies Don't know |
See estimates of SAE and discontinuations due to AE (critical undesirable effects) in Table 5 and Table 6, and in Table 1. Serious AE: May be similar and low rates of serious AE for AP, mefloquine and doxycycline. Discontinuations due to AE:
Non-serious AE:
Persistent neuropsychiatric harms:
Additional considerations: The certainty of evidence from cohort studies were downgraded by two levels for risk of bias, as they all had methodological concerns which could result in confounding or bias. For example, several studies were conducted in long-term military personnel where drug allocation may have been impacted by previous history of neuropsychiatric conditions. Study participants and clinicians may also be more likely to attribute adverse events to mefloquine, due to its prominence in the media. |
Certainty of evidence: What is the overall certainty of the evidence of effects? |
Very low Low Moderate High No included studies |
The overall certainty of evidence for the critical outcomes (clinical malaria and discontinuations) is Moderate. Important outcomes such as adverse events and serious adverse events (given low number of events) were not considered in the overall certainty of evidence. |
Importance of outcomes to/in/for/in relation to affected population: Is there important uncertainty about or variability in how much travellers value the main outcomes? |
Important uncertainty or variability Possibly important uncertainty or variability Probably no important uncertainty or variability No important uncertainty or variability |
The guideline panel identified the following outcomes as critical or important to decision making: Desirable outcomes:
Undesirable outcomes:
Additional considerations: There is significant uncertainty related to the values and preferences of travellers as respects choice of MCP (and other travel interventions like vaccines). Clinicians should allow for discussion of this variability as part of the joint decision-making process (good practice statement, see Appendix 6). |
Balance of effects: Does the balance between desirable and undesirable effects favour the intervention or the comparison? |
Favours the comparison (AP over mefloquine) Probably favours the comparison Does not favour either the intervention (mefloquine) or the comparison (doxycycline) Probably favours the intervention Favours the intervention Varies Don't know |
Although there are trivial differences in clinical efficacy, the estimated RR for AE-associated discontinuation was higher for mefloquine compared to AP (2.86, 95% CI 1.53 to 5.31; moderate certainty evidence). Estimated rate of AE-associated discontinuation for mefloquine and doxycycline were similar (RR 1.08, 95% CI 0.41 to 2.87; very low certainty evidence). The rate of non-serious AE were higher for mefloquine than for AP. Additional considerations: CATMAT believes that there was a small-moderate advantage for AP when comparing the undesirable effects of MCP discontinuation and adverse effects. |
Resource considerations: How large are the resource requirements (costs) to the individual? |
Large costs Moderate costs Small costs Negligible costs Varies Don't know |
We did not find research evidence for the direct and indirect costs. See 'Equity' section below for relative cost comparisons. |
Equity: What would be the impact on health equity? |
Reduced Probably reduced Probably no impact Probably increased Increased Varies Don't know |
AP is generally the most expensive MCP, however the price varies between pharmacies. CATMAT acknowledges the presence of equity-related issues at the population level, however these considerations generally do not impact the decision-making for recommendation development by the Committee. CATMAT's focus on the perspective of supporting the individual traveller does not negate nor reduce the importance of population level challenges. Additional considerations: Persons receiving MCP incur personal costs. AP is generally more expensive than mefloquine or doxycycline. Hence, travellers wishing to follow the recommendation to use AP as MCP are likely to incur more expense, which might act as a barrier for some. |
Acceptability: Is the intervention acceptable to key stakeholders? |
No Probably no Probably yes Yes Varies Don't know |
All MCP are considered suitable, when medically indicated, for prevention of malaria. Additional considerations: All medications are taken orally. We are aware of a subset of clinicians that are concerned about prescribing mefloquine for its neuropsychiatric effects, and there is a tendency not to prescribe it. There are some differences in dosing and personal suitability by MCP (see Table 1 ). |
Feasibility: Is the intervention feasible to implement for the individual? |
No Probably no Probably yes Yes Varies Don't know |
There is no change in feasibility by recommending AP. Additional considerations: AP, doxycycline and mefloquine are already widely known to be suitable MCP medications. There are some concerns with adherence, however this applies to all MCP options. |
Type of recommendation
Discretionary recommendation against the intervention (mefloquine or doxycycline compared to AP)
Conclusions
Recommendation
If several MCP are considered suitable for the patient, CATMAT suggests atovaquone-proguanil (AP) over mefloquine or doxycycline.
(Discretionary recommendation, moderate certainty evidence).
Remarks:
- All three medications are effective for the prevention of malaria.
- Most travellers will choose AP, but some will choose mefloquine or doxycycline based on cost, convenience, past experience or other factors (see Table 1). Note that AP is contraindicated in some populations (e.g., severe renal impairment).
Rationale:
- AP, doxycycline, and mefloquine may provide a similar high level of protection against malaria (moderate certainty evidence), but
- AP likely has a lower rate of discontinuation due to adverse effects than mefloquine or doxycycline (moderate certainty evidence);
- AP likely has a lower rate of non-serious and concurrent neuropsychiatric adverse effects than mefloquine (very low to moderate certainty evidence); and,
- Serious adverse effects are similar with all options but the rate is low and therefore not critical to decision making (very low certainty evidence).
| Outcomes | Number of participants (studies) contributing to effect estimate |
Additional participants (studies) considered in GRADE assessment | Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
|---|---|---|---|---|---|---|
| Risk with atovaquone-proguanil | Risk difference with mefloquine | |||||
Clinical malaria |
1293 (2 RCTs) |
Not applicable |
ModerateFootnote b Footnote c Footnote d |
Not estimable |
No malaria cases occurred in either group |
|
Serious adverse effects |
3591 (3 non-randomised studies) |
976 (1 RCT) |
Very lowFootnote e Footnote f |
RR 1.40 (0.08 to 23.22) |
0 per 10,000 |
7 more per 10,000 (from 0 fewer to 124 more) |
Discontinuations due to adverse effects |
1438 (3 RCTs) |
7785 (9 non-randomised studies) |
ModerateFootnote f |
RR 2.86 (1.53 to 5.31) |
2 per 100 |
4 more per 100 (1 more to 8 more) |
Abbreviations: CI: Confidence interval |
||||||
Explanations:
|
||||||
| Outcomes | Number of participants (studies) contributing to effect estimate |
Additional participants (studies) considered in GRADE assessment | Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
|---|---|---|---|---|---|---|
| Risk with doxycycline | Risk difference with mefloquine | |||||
Clinical malaria |
744 (4 RCTs) |
Not applicable |
RR 1.35 (0.35 to 5.19) |
1 per 100 |
0 fewer per 100 (1 fewer to 3 more) |
|
Serious adverse effects |
3722 (3 non-randomised studies) |
682 (3 RCTs) |
Very lowFootnote f Footnote g Footnote h |
RR 1.53 (0.23 to 10.24) |
1 per 100 |
0 fewer per 100 (0 fewer to 6 more) |
Discontinuations due to adverse effects |
763 (4 RCTs) |
10,165 (10 non-randomised studies) |
Very lowFootnote g Footnote h Footnote i |
RR 1.08 (0.41 to 2.87) |
2 per 100 |
0 fewer per 100 (1 fewer to 4 more) |
Abbreviations: CI: Confidence interval |
||||||
Explanations:
|
||||||
Appendix 4: Evidence to decision framework for: research question 2
Question: In areas where malaria parasites are considered susceptible (see Appendix 1), should hydroxychloroquine (chloroquine), atovaquone-proguanil (AP), mefloquine and/or doxycycline be preferred for travellers from Canada to malaria endemic areas, and for whom malaria chemoprophylaxis (MCP) is recommended?
Population: Travellers from Canada to malaria endemic areas without chloroquine resistance, and for whom malaria chemoprophylaxis (MCP) is recommended.
Intervention: Hydroxychloroquine (chloroquine)
Comparison: Mefloquine, AP, or doxycycline
Main outcomes: Clinical malaria, serious adverse effects (SAE), adverse effect-associated discontinuations, pre-specified non-serious adverse effects
Setting: International travel
Perspective: Individual
Background: See the evidence to decision for Question 1 above. Hydroxychloroquine (or chloroquine) is another MCP agent that is available for use where the parasites are considered susceptible, in certain locations in the Americas.
Conflict of interest: None
Assessment
| Domain | Judgement | Research evidence and additional considerations |
|---|---|---|
Problem: Is the problem a priority? |
No Probably no Probably yes Yes Varies Don't know |
Malaria is one of the most important infectious diseases in the world. Travellers to malaria endemic areas can be exposed to malaria, and if disease develops, are at risk for severe outcomes and death. It is estimated that several hundred cases of malaria occur annually among Canadian travellers, including several severe or complicated cases (Chapter 1: Introduction to Canadian recommendations). |
Desirable effects: How substantial are the desirable anticipated effects (protection against malaria)? |
Trivial Small Moderate Large Varies Don't know |
Systematic review: The 2017 Cochrane systematic review and meta-analysis titled "Mefloquine for preventing malaria during travel to endemic areas"Footnote 20 was judged to be of sufficient quality and relevance to use for question 2. See Table 8 for a summary of the results. Additional historic and indirect evidence:
Overall efficacy: There may be similar high levels of protection against malaria (>90%) in areas where there is no drug resistance (very low certainty evidence). |
Undesirable effects: How substantial are the undesirable anticipated effects? |
Trivial Small Moderate Large Varies Don't know |
See undesirable effects in Table 8, and Table 2 for indirect comparisons to AP and doxycycline. Serious AE: May be similar and low rates of serious AE for hydroxychloroquine, AP, mefloquine, and doxycycline. Discontinuations due to AE: AP likely has relatively fewer discontinuations than mefloquine and doxycycline (4/100 fewer). AP may also have fewer discontinuations than hydroxychloroquine (2/100 fewer). Non-serious AE:
Additional considerations: The certainty of evidence from cohort studies were downgraded by two levels for risk of bias, as they all had methodological concerns which could result in confounding or bias. For example, several studies were conducted in long-term military personnel where drug allocation may have been impacted by previous history of neuropsychiatric conditions. Study participants and clinicians may also be more likely to attribute adverse events to mefloquine, due to its prominence in the media. |
Certainty of evidence: What is the overall certainty of the evidence of effects? |
Very low Low Moderate High No included studies |
The overall certainty of evidence for the critical outcomes is Very low. |
Importance of outcomes to/in/for/in relation to affected population: Is there important uncertainty about or variability in how much travellers value the main outcomes? |
Important uncertainty or variability Possibly important uncertainty or variability Probably no important uncertainty or variability No important uncertainty or variability |
The guideline panel identified the following outcomes as critical or important to decision making: Desirable outcomes:
Undesirable outcomes:
Additional considerations: There is significant uncertainty related to the values and preferences of travellers as respects choice of MCP (and other travel interventions like vaccines). Clinicians should allow for discussion of this variability as part of the joint decision-making process (good practice statement, see Appendix 6). |
Balance of effects: Does the balance between desirable and undesirable effects favour the intervention or the comparison? |
Favours the comparison Probably favours the comparison (AP) Does not favour either the intervention or the comparison Probably favours the intervention Favours the intervention Varies Don't know |
The efficacy and serious adverse effects may be similar for hydroxychloroquine when compared with other MCP options. However, the committee notes that there was no recent evidence for efficacy of hydroxychloroquine against comparators and a theoretical concern that emergence of clinically relevant resistance to hydroxychloroquine in areas where malaria is currently presumed to be sensitive might be difficult to detect. This resulted in the committee placing more weight on the well-established efficacy of AP. Further, there may be a small advantage for AP, which may have fewer adverse event-associated discontinuations and non-serious adverse effects than hydroxychloroquine. |
Resource considerations: How large are the resource requirements (costs) to the individual? |
Large costs Moderate costs Small costs Negligible costs Varies Don't know |
We did not find research evidence for the direct and indirect costs. See 'Equity' section below for relative cost comparisons. |
Equity: What would be the impact on health equity? |
Reduced Probably reduced Probably no impact Probably increased Increased Varies Don't know |
AP is generally the most expensive MCP, however the price varies between pharmacies. CATMAT acknowledges the presence of equity-related issues at the population level, however these considerations generally do not impact the decision-making for recommendation development by the Committee. CATMAT's focus on the perspective of supporting the individual traveller does not negate nor reduce the importance of population level challenges. Additional considerations: Persons receiving MCP incur personal costs. AP is generally more expensive than hydroxychloroquine, mefloquine, or doxycycline. Hence, travellers wishing to follow the recommendation to use AP as MCP are likely to incur more expense, which might act as a barrier for some. |
Acceptability: Is the intervention acceptable to key stakeholders? |
No Probably no Probably yes Yes Varies Don't know |
There is no change in acceptability by recommending AP. Additional considerations: All medications are taken orally. We are aware of a subset of clinicians that are concerned about prescribing mefloquine for its neuropsychiatric effects, and there is a tendency not to prescribe it. There are some differences in dosing and personal suitability by MCP (see Table 1). |
Feasibility: Is the intervention feasible to implement for the individual? |
No Probably no Probably yes Yes Varies Don't know |
There is no change in feasibility by recommending AP. In the past, there have been shortages of hydroxychloroquine (chloroquine) in Canada, impacting its availability for travellers. AP, doxycycline, hydroxychloroquine and mefloquine are already widely known to be suitable MCP medications. There are some concerns with adherence, however this applies to all MCP options. |
Type of recommendation
Discretionary recommendation against the intervention
Conclusions
Recommendation
Where the parasites are considered susceptible to hydroxychloroquine (chloroquine), CATMAT suggests atovaquone-proguanil (AP) over mefloquine, doxycycline, or hydroxychloroquine.
(Discretionary recommendation, very low certainty evidence).
Remarks:
- Most travellers will choose AP, but some will choose mefloquine, doxycycline, or hydroxychloroquine (chloroquine) based on cost, convenience, past experience or other factors (see Table 1). Note that AP is contraindicated in some populations (e.g., severe renal impairment).
- Of note, the recommendation is based on very low certainty evidence compared to the moderate certainty evidence for recommendation 1. This specifically reflects the paucity of recent evidence for hydroxychloroquine (or chloroquine) (see rationale below).
Rationale:
- AP, doxycycline, mefloquine, and hydroxychloroquine (or chloroquine) may provide a similar high level of protection against malaria (very low certainty evidence for mefloquine compared to chloroquine);
- The committee notes that there was not recent evidence for efficacy of hydroxychloroquine against comparators and a theoretical concern that emergence of clinically relevant resistance to hydroxychloroquine in areas where malaria is currently presumed to be sensitive might be difficult to detect. This resulted in the committee placing more weight on the well-established efficacy of AP.
- AP likely has a lower rate of discontinuation due to adverse effects than mefloquine and doxycycline (moderate certainty evidence for AP compared to mefloquine) and may have a lower rate than hydroxychloroquine (or chloroquine) (very low certainty evidence for mefloquine compared to chloroquine);
- AP may have a lower rate of non-serious adverse effects than hydroxychloroquine (very low to low certainty evidence for mefloquine compared to chloroquine), and
- Serious adverse effects are similar with all options (very low certainty evidence for mefloquine compared to chloroquine).
| Outcomes | Number of participants (studies) contributing to effect estimate | Additional participants (studies) considered in GRADE assessment | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|---|
| Risk with chloroquine | Risk difference with mefloquine | |||||
Clinical malaria |
413 (2 RCTs) |
Not applicable |
Very lowFootnote a Footnote b |
Not estimable |
No malaria cases occurred in either group |
|
Serious adverse effects |
1000 (4 RCTs) |
79257 (6 non-randomised studies) |
Very lowFootnote c Footnote d Footnote e |
RR 2.77 (0.32 to 23.85) |
0 per 1,000 |
3 more per 1,000 (0 fewer to 25 more) |
Discontinuations due to adverse effects |
815 (3 RCTs) |
55397 (6 non-randomised studies) |
Very lowFootnote c Footnote d Footnote f |
RR 1.60 (0.61 to 4.18) |
2 per 100 |
1 more per 100 (1 fewer to 5 more) |
Abnormal dreams |
359 (1 RCT) |
2845 (4 non-randomised studies) |
RR 2.70 (1.05 to 6.95) |
3 per 100 |
5 more per 100 (0 fewer to 19 more) |
|
Insomnia |
359 (1 RCT) |
56952 (5 non-randomised studies) |
Very lowFootnote d Footnote h Footnote i |
RR 1.19 (0.76 to 1.84) |
25 per 100 |
5 more per 100 (6 fewer to 21 more) |
Anxiety |
3408 (3 non-randomised studies) |
Not applicable |
Very lowFootnote d Footnote j |
RR 6.30 (4.37 to 9.09) |
3 per 100 |
16 more per 100 (10 more to 25 more) |
Depressed mood |
58855 (5 non-randomised studies) |
Not applicable |
Very lowFootnote d Footnote j Footnote k |
RR 3.14 (1.15 to 8.57) |
1 per 100 |
3 more per 100 (0 fewer to 11 more) |
Abnormal thoughts or perceptions |
4831 (4 non-randomised studies) |
Not applicable |
Very lowFootnote d Footnote i Footnote j |
RR 5.49 (2.65 to 11.35) |
0 per 100 |
2 more per 100 (1 more to 4 more) |
Dizziness |
569 (2 RCTs) |
58847 (5 non-randomised studies) |
RR 0.72 (0.35 to 1.46) |
6 per 100 |
2 fewer per 100 (4 fewer to 3 more) |
|
Nausea |
359 (1 RCT) |
58984 (6 non-randomised studies) |
RR 1.01 (0.57 to 1.79) |
13 per 100 |
0 fewer per 100 (6 fewer to 10 more) |
|
Vomiting |
359 (1 RCT) |
5577 (5 non-randomised studies) |
RR 1.12 (0.36 to 3.49) |
3 per 100 |
0 fewer per 100 (2 fewer to 8 more) |
|
Abdominal pain |
569 (2 RCTs) |
5440 (4 non-randomised studies) |
RR 0.71 (0.37 to 1.36) |
7 per 100 |
2 fewer per 100 (4 fewer to 2 more) |
|
Diarrhea |
772 (3 RCTs) |
5577 (5 non-randmosed studies) |
RR 0.83 (0.46 to 1.50) |
5 per 100 |
1 fewer per 100 (3 fewer to 3 more) |
|
Headache |
772 (3 RCTs) |
56998 (6 non-randomised studies) |
RR 0.89 (0.61 to 1.31) |
11 per 100 |
1 fewer per 100 (4 fewer to 3 more) |
|
Abbreviations: CI: Confidence interval |
||||||
Explanations:
|
||||||
Appendix 5: Evidence to decision framework for: research question 3
Question: Where other agents (AP, mefloquine, doxycycline, hydroxychloroquine) are contraindicated or not tolerated by the traveller, should primaquine be recommended as an MCP alternative in travellers to malaria endemic regions?
Population: Travellers from Canada to malaria endemic areas, for whom malaria chemoprophylaxis (MCP) is recommended, and in which other agents (AP, mefloquine, doxycycline, hydroxychloroquine) are contraindicated or not tolerated.
Intervention: Primaquine
Comparison: Placebo or multivitamin
Main outcomes: Clinical malaria, adverse effect-associated discontinuations, and pre-specified non-serious gastrointestinal and neuropsychiatric adverse effects.
Setting: International travel
Perspective: Individual
Background: In Canada, there are three main MCP agents recommended for use in areas where malaria parasites are resistant to chloroquine (most endemic areas): AP, doxycycline and mefloquine. Although not a first-line chemoprophylactic agent, primaquine can also be considered for malaria prevention. Primaquine is active against the hypnozoite stage of malaria parasites, including P. vivax and P. ovale, which distinguish it from other MCP. However, because it does not exhibit potent activity against disease causing blood-stage parasites at doses typically used for MCP, it might be less effective against the generally more serious P. falciparum infections.
Conflict of interests: None
Assessment
| Domain | Judgement | Research evidence and additional considerations |
|---|---|---|
Problem: Is the problem a priority? |
No Probably no Probably yes Yes Varies Don't know |
Malaria is one of the most important infectious diseases in the world. Travellers to malaria endemic areas can be exposed to malaria, and if disease develops, are at risk for severe outcomes and death. It is estimated that several hundred cases of malaria occur annually among Canadian travellers, including several severe or complicated cases (Chapter 1: Introduction to Canadian recommendations). |
Desirable effects: How substantial are the desirable anticipated effects (protection against malaria)? |
Trivial Small Moderate Large Varies Don't know |
Among four randomized controlled trials (RCTs), primaquine had an estimated RR of 0.13 (CI: 0.08-0.22; 87% efficacy) for clinical malaria compared to placebo or multivitamin, which resulted in an estimated 34 fewer cases of malaria per 100 (see Table 10). For most travellers, the absolute effect would be much lower, as the absolute benefit varies by intensity of transmission. Studies assessed were conducted in highly endemic areas, such as Kenya and Papua IndonesiaFootnote 65 Footnote 68 Footnote 69. Based on Swedish surveillance data from 1997 to 2003, the crude risk for travellers was estimated to vary from 1 per 100,000 travellers to Central America and the Caribbean to 357 per 100,000 in central AfricaFootnote 70. In several studies, the short follow-up period limited the ability to detect late relapse P. vivax cases. An observational before-and-after study among nonimmune Israeli rafters which was conducted in an area hyperendemic for both P. falciparum and P. vivax along the Omo River Ethiopia, demonstrated that primaquine is effective for causal prophylaxis (liver stage) of P. vivaxFootnote 71. Of the 106 travellers who received primaquine, 5.7% developed malaria (four P. falciparum infections, one P. vivax, and one mixed infection). Because primaquine is activated by cytochrome P-450 isoenzyme 2D6 (CYP2D6) in the liver, individuals who harbor polymorphisms resulting in low CYP2D6 enzyme activity may have sub-therapeutic levels of primaquine. Clinical failure of primaquine radical cure treatment of P. vivax infections has been documented in persons who harbor polymorphisms in cytochrome P-450 2D6 geneFootnote 72 Footnote 73. The possible contribution of these polymorphisms to documented failure of primaquine prophylaxis remains unknown, and testing for CYP2D6 polymorphisms is not easily or rapidly available. Additional considerations: The direction of the effect for the outcome of clinical malaria was the same across studies. However, there are different baseline conditions, including malaria endemicity and dominant parasite present, across studies that might affect performance. For example, the study by Ling and Baird was conducted in Papua Indonesia, where P. vivax is highly endemic. This is consistent with the U.S. evidence review, which suggests >85% protective efficacy against P. falciparum and primary P. vivax infectionsFootnote 64. |
Undesirable effects: How substantial are the undesirable anticipated effects? |
Trivial Small Moderate Large Varies Don't know |
See undesirable effects in Table 10. No differences were observed when comparing AE-associated discontinuations, gastrointestinal and neuropsychiatric side effects between primaquine to placebo. In all of the studies reviewed, G6PD deficiency was ruled out by blood examination before the start of chemoprophylaxis. Primaquine is known to cause methemoglobinemia and oxidant-induced hemolytic anemia in individuals with G6PD deficienciesFootnote 59 Footnote 64. G6PD deficiency is a genetic mutation which is more common in certain subgroups, such as those of Mediterranean, African, Asian, or Middle East ethnic originFootnote 75. These individuals may be disadvantaged, since G6PD deficiency is a contraindication for primaquine. Further research is needed on safety of various primaquine dosages for malaria prevention in those with G6PD deficiency. Due to concern for methemoglobinemia and hemolytic anemia, G6PD deficiency is a contraindication for primaquine, and thus there is a need to test for it before administering primaquine (see feasibility section and Figure 2). Additional considerations: Evidence from case-control studies suggests that G6PD deficiency may confer resistance against malaria, however, further research is needed to better understand this relationshipFootnote 76. |
Certainty of evidence: What is the overall certainty of the evidence of effects? |
Very low Low Moderate High No included studies |
The overall certainty of evidence for the critical outcomes is Low. Important outcomes such as non-serious adverse events and adverse-event associated discontinuations (given low number of events) were not considered in the overall certainty of evidence. |
Importance of outcomes to/in/for/in relation to affected population: Is there important uncertainty about or variability in how much travellers value the main outcomes? |
Important uncertainty or variability Possibly important uncertainty or variability Probably no important uncertainty or variability No important uncertainty or variability |
The guideline panel identified the following outcomes as critical or important to decision making: Desirable outcomes:
Undesirable outcomes:
Additional considerations: There is significant uncertainty related to the values and preferences of travellers as respects choice of MCP (and other travel interventions like vaccines). Clinicians should allow for discussion of this variability as part of the joint decision-making process (good practice statement, see Appendix 6). |
Balance of effects: Does the balance between desirable and undesirable effects favour the intervention or the comparison? |
Favours the comparison Probably favours the comparison Does not favour either the intervention or the comparison Probably favours the intervention Favours the intervention Varies Don't know |
Primaquine showed an estimated 87% reduction in the number of clinical malaria cases compared to placebo or multivitamin and had comparable rates of non-serious gastrointestinal and neuropsychiatric adverse events as placebo. Primaquine is active against the hypnozoite parasites as they are developing in the liver (causal effect). Hypnozoites can remain dormant in the liver and cause relapses by invading the bloodstream weeks or years later, so primaquine may be effective for preventing P vivax malaria. However, since primaquine does not exhibit potent activity against the disease-causing blood-stage parasites at doses typically used for MCP, it may be less effective against the generally more severe P falciparum infectionsFootnote 11 Footnote 12 Footnote 13 Footnote 14. Although uncertainty remains about how effective primaquine is in comparison to other agents (AP, doxycycline, mefloquine, hydroxychloroquine), it was agreed that the benefits of primaquine for prevention of malaria largely outweighs the negative effects of clinical malaria, trivial adverse effects, and requirement for G6PD deficiency testing. |
Resource considerations: How large are the resource requirements (costs) to the individual? |
Large costs Moderate costs Small costs Negligible costs Varies Don't know |
After screening for G6PD, primaquine may be comparable in cost to other MCP options. The time and cost for G6PD deficiency testing may be higher if a quantitative test is required (see Figure 2). However, testing only needs to be completed once in a person's lifetime, and the results can be beneficial for other medications which may also be contraindicated in those with G6PD deficiency. |
Equity: What would be the impact on health equity? |
Reduced Probably reduced Probably no impact Probably increased Increased Varies Don't know |
Equity is covered in the undesirable effects category above. CATMAT acknowledges the presence of equity-related issues at the population level, however these considerations generally do not impact the decision-making for recommendation development by the Committee. CATMAT's focus on the perspective of supporting the individual traveller does not negate nor reduce the importance of population level challenges. |
Acceptability: Is the intervention acceptable to key stakeholders? |
No Probably no Probably yes Yes Varies Don't know |
Primaquine, as an alternative MCP, has been recommended for many years. Clinicians are likely familiar with this medication, and it was agreed that they will consider it to be an acceptable intervention, which largely outweighs the risks associated with malaria. Similar to AP, primaquine has a shorter run-in (1-2 days) before arriving in the malaria endemic area, which might offer an advantage for travellers who are travelling on short notice or for shorter trips. Primaquine also requires only 7-days of prophylaxis following departure from the endemic area. |
Feasibility: Is the intervention feasible to implement for the individual? |
No Probably no Probably yes Yes Varies Don't know |
Travellers must be checked for G6PD deficiency before prescription of primaquine, so this can delay prescription if patients are waiting on bloodwork. In most cases, a qualitative test is sufficient, however G6PD deficiency is an X-linked disorder, so females can be heterozygous for the allele. In this case, quantitative testing may be required, which may result in greater time delays and increased cost. See Figure 2 for guidance on testing for G6PD deficiency. Availability of primaquine may also be limited in Canada. |
Type of recommendation
Strong recommendation for the intervention
Conclusions
Recommendation
Where MCP is recommended and where other agents (AP, mefloquine, doxycycline, hydroxychloroquine) are contraindicated or not tolerated by the traveller, CATMAT recommends that primaquine be used for MCP.
(Strong recommendation, low certainty evidence).
Remarks:
- There are important and specific considerations related to use of primaquine for chemoprophylaxis (see Chapter 8 and Table 1). Primaquine use in any kind of preventive regimen should be avoided in those with abnormal activity detected on a qualitative G6PD screening test (see Figure 2), and is contraindicated in some populations regardless of G6PD screening results (e.g. pregnancy).
- Primaquine has causal prophylactic activity, and therefore requires only 7-days of prophylaxis following departure from the endemic area.
Rationale:
- Primaquine may provide a high level of protection against clinical malaria compared to placebo or multivitamin (low certainty evidence).
- Based on the mechanism of action, and lower efficacy in placebo-controlled studies compared to estimates from studies using other agents, it is unknown whether primaquine is as efficacious in preventing P. falciparum malaria as first-line MCP.
- There may be no differences in gastrointestinal adverse effects or neuropsychiatric adverse events between primaquine and placebo or multivitamin (very low to low certainty evidence).
- There may be no differences in adverse-effect associated discontinuations between primaquine and placebo or multivitamin but the rate is low and therefore not critical to decision making (very low certainty evidence).
- Clinical failure of primaquine radical cure treatment of Plasmodium vivax malaria has been documented in people who have polymorphisms in the Cytochrome P-450 2D6 gene.
| Outcomes | Number of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
|---|---|---|---|---|---|
| Risk with placebo or multivitamin | Risk difference with primaquine | ||||
Clinical malaria |
521 (4 RCTs) |
RR 0.13 (0.08 to 0.22) |
39 per 100 |
34 fewer per 100 (35 fewer to 30 fewer) |
|
AE-associated medication discontinuations |
488 (3 RCTs) |
Very lowFootnote a Footnote b Footnote c |
RR 0.83 (0.18 to 3.95) |
13 per 1,000 |
2 fewer per 1,000 (10 fewer to 37 more) |
Gastrointestinal adverse effects |
(4 RCTs) |
Very lowFootnote a Footnote b Footnote c |
RR 0.81 (0.45 to 1.47) |
0.03 to 1 per person week |
0.04 to 0.69 per person week |
Neuropsychiatric adverse events |
(3 RCTs) |
RR 1.03 (0.55 to 1.95) |
0.03 to 0.63 per person week |
0.03 to 0.64 per person week |
|
Abbreviations: CI: Confidence interval |
|||||
Explanations:
|
|||||
Appendix 6. Assessment tables for applicability of good practice statements (GPS)
Good practice statement: Clinicians should advise travellers to adhere to personal protective measures (PPM) against insect bites when they are exposed to mosquitoes in malaria-endemic areas.
| Criteria | Supporting information | Verification |
|---|---|---|
| Population and intervention components are clear | Not applicable | Yes |
| Message is really necessary in regard to actual health care practice | PPM against bites are an important tool to prevent exposure to vectors. Adherence to their use is often suboptimal. | Yes |
| Implementing the GPS results in a large net positive consequence | Yes, reduction in exposure to vectors will reduce exposure to the pathogens they carry. | Yes |
| Collecting and summarizing the evidence is a poor use of a guideline panel's limited time, energy or resources. The opportunity cost of collecting and summarizing the evidence is large and can be avoided. | Yes, this recommendation is about advising travellers to protect themselves. | Yes |
| There is a well-documented clear and explicit rationale connecting the indirect evidence | Yes, reducing exposure to vectors is reasonably expected to reduce likelihood of infection. | Yes |
| Clear and actionable | Not applicable | Yes |
Final judgement: development of GPS is appropriate.
Good practice statement: Clinicians should emphasize to travellers that it is essential to complete their malaria chemoprophylaxis regimen as prescribed prior to, during and after departure from the malaria-endemic area.
| Criteria | Supporting information | Verification |
|---|---|---|
| Population and intervention components are clear | Not applicable | Yes |
| Message is really necessary in regard to actual health care practice | Completion of MCP is essential to optimizing effectiveness of preventive treatment, and adherence to use of MCP including following travel is an accepted problem. | Yes |
| Implementing the GPS results in a large net positive consequence | Not applicable | Yes |
| Collecting and summarizing the evidence is a poor use of a guideline panel's limited time, energy or resources. The opportunity cost of collecting and summarizing the evidence is large and can be avoided. | Yes, following recommended practices for use of MCP including after return from a risk area is common practice. Spending resources to address this question with GRADE would be a poor use of resources. | Yes |
| There is a well-documented clear and explicit rationale connecting the indirect evidence | Yes, maximal adherence to MCP will optimize protection against malaria. | Yes |
| Clear and actionable | Not applicable | Yes |
Final judgement: development of GPS is appropriate.
Good practice statement: The values and preferences of travellers towards MCP choice are varied. Clinicians should allow for discussion of this variability as part of the joint decision-making process.
| Criteria | Supporting information | Verification |
|---|---|---|
| Population and intervention components are clear | Not applicable | Yes |
| Message is really necessary in regard to actual health care practice | Yes, individual level choices related to choice of MCP are consistently varied at the individual level. Tailoring guidance to suit the individual should increase acceptability of recommended interventions and empowers the patient. | Yes |
| Implementing the GPS results in a large net positive consequence | Not applicable | Yes |
| Collecting and summarizing the evidence is a poor use of a guideline panel's limited time, energy or resources. The opportunity cost of collecting and summarizing the evidence is large and can be avoided. | Yes, patient values and preferences are widely acknowledged to vary as respects choice of MCP, and other travel-based health interventions. Spending significant analytic effort to re-demonstrate an already well understood phenomena would be a poor use of resources. | Yes |
| There is a well-documented clear and explicit rationale connecting the indirect evidence | Yes, tailoring guidance to suit the individual may enhance adherence to MCP. | Yes |
| Clear and actionable | Not applicable | Yes |
Final judgement: development of GPS is appropriate.
References
- Footnote 1
-
Centers for Disease Control and Prevention. Choosing a Drug to Prevent Malaria [internet]. 2024 [cited 2024 Sep 19]. Available from: https://www.cdc.gov/malaria/hcp/drug-malaria/?CDC_AAref_Val=https://www.cdc.gov/malaria/travelers/drugs.html
- Footnote 2
-
Centers for Disease Control and Prevention. CDC Yellow book 2020: Health Information for International Travel. New York: Oxford University Press; 2017.
- Footnote 3
-
Kendjo E, Houzé S, Mouri O, Taieb A, Gay F, Jauréguiberry S, Tantaoui I, Ndour PA, Buffet P, Piarroux M, Thellier M. Epidemiologic trends in malaria incidence among travelers returning to metropolitan France, 1996-2016. JAMA Network Open. 2019 Apr 5;2(4):e191691-.
- Footnote 4
-
Checkley AM, Smith A, Smith V, Blaze M, Bradley D, Chiodini PL, et al. Risk factors for mortality from imported falciparum malaria in the United Kingdom over 20 years: an observational study. BMJ 2012 Mar 27;344:e2116.
- Footnote 5
-
McCarthy AE, Plourde P, Kuhn S, Bodie M. Parenteral quinine for severe malaria: five year surveillance data from the Canadian Malaria Network. 10th Conference of the International Society of Travel Medicine 2007 May 10-20.
- Footnote 6
-
Murphy GS, Oldfield EC,3rd. Falciparum malaria. Infect Dis Clin North Am 1996 Dec;10(4):747-775.
- Footnote 7
-
Baird JK. Pernicious and threatening Plasmodium vivax as reality. Am J Trop Med Hyg 2014 Jul;91(1):1-2.
- Footnote 8
-
Baird JK. Management of Plasmodium vivax risk and illness in travelers. Tropical diseases, travel medicine and vaccines. 2017 Dec; 3:1-6.
- Footnote 9
-
McCarthy AE, Morgan C, Prematunge C, Geduld J. Severe malaria in Canada, 2001-2013. Malar J 2015 Apr 11;14:151-015-0638-y.
- Footnote 10
-
Boggild AK, Geduld J, Libman M, Yansouni CP, McCarthy AE, Hajek J, et al. Malaria in travellers returning or migrating to Canada: surveillance report from CanTravNet surveillance data, 2004-2014. CMAJ Open 2016 Jul 6;4(3):E352-E358.
- Footnote 11
-
Centers for Disease Control and Prevention. Malaria Parasite Biology [internet]. 2024 [cited 2024 Sep 23]. Available from: https://www.cdc.gov/dpdx/malaria/index.html.
- Footnote 12
-
Kolifarhood G, Raeisi A, Ranjbar M, Haghdoust AA, Schapira A, Hashemi S, Masoumi-Asl H, Saadati HM, Azimi S, Khosravi N, Kondrashin A. Prophylactic efficacy of primaquine for preventing Plasmodium falciparum and Plasmodium vivax parasitaemia in travelers: a meta-analysis and systematic review. Travel Medicine and Infectious Disease. 2017 May 1;17:5-18.
- Footnote 13
-
Arnold J, Alving AS, Hockwald ES, Clayman CB, Dern RJ, Bbutler E, Flanagan CL, Jeffery GM. The antimalarial action of primaquine against the blood and tissue stages of falciparum malaria (Panama, PF-6 strain).
- Footnote 14
-
Jay S. Keystone, Robert Steffen, Phyllis E. Kozarsky, CHAPTER 126 - Health Advice for International Travel, Editor(s): Richard L. Guerrant, David H. Walker, Peter F. Weller, Tropical Infectious Diseases: Principles, Pathogens and Practice (Third Edition), W.B. Saunders, 2011, Pages 887-901, ISBN 9780702039355, https://doi.org/10.1016/B978-0-7020-3935-5.00126-9. (https://www.sciencedirect.com/science/article/pii/B9780702039355001269)
- Footnote 15
-
Lotfi T, Hajizadeh A, Moja L, Akl EA, Piggott T, Kredo T, Langendam MW, Iorio A, Klugar M, Klugarová J, Neumann I. A taxonomy and framework for identifying and developing actionable statements in guidelines suggests avoiding informal recommendations. Journal of clinical epidemiology. 2022 Jan 1;141:161-71.
- Footnote 16
-
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. bmj. 2016 Jun 28;353.
- Footnote 17
-
Committee to Advise on Tropical Medicine and Travel (CATMAT). Evidence Based Process for developing travel and tropical medicine related guidelines and recommendations. 2017.
- Footnote 18
-
Dewidar O, Lotfi T, Langendam MW, Parmelli E, Parkinson ZS, Solo K, Chu DK, Mathew JL, Akl EA, Brignardello-Petersen R, Mustafa RA. Good or best practice statements: proposal for the operationalisation and implementation of GRADE guidance. BMJ evidence-based medicine. 2023 Jun 1;28(3):189-96.
- Footnote 19
-
Brożek J, Guyatt G, Oxman A. The GRADE handbook.
- Footnote 20
-
Tickell-Painter M, Maayan N, Saunders R, Pace C, Sinclair D. Mefloquine for preventing malaria during travel to endemic areas. Cochrane Database Syst Rev 2017 Oct 30;10:CD006491.
- Footnote 21
-
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. bmj. 2017 Sep 21;358.
- Footnote 22
-
Tickell-Painter M, Saunders R, Maayan N, Lutje V, Mateo-Urdiales A, Garner P. Deaths and parasuicides associated with mefloquine chemoprophylaxis: A systematic review. Travel medicine and infectious disease. 2017 Nov 1;20:5-14.
- Footnote 23
-
Schneiderman AI, Cypel YS, Dursa EK, Bossarte RM. Associations between use of antimalarial medications and health among US veterans of the wars in Iraq and Afghanistan. The American journal of tropical medicine and hygiene. 2018 Sep;99(3):638.
- Footnote 24
-
Rodrigo C, Rajapakse S, Fernando SD. Compliance with Primary Malaria Chemoprophylaxis: Is Weekly Prophylaxis Better Than Daily Prophylaxis?. Patient preference and adherence. 2020 Nov 9:2215-23.
- Footnote 25
-
Ahluwalia J, Brooks SK, Weinman J, Rubin GJ. A systematic review of factors affecting adherence to malaria chemoprophylaxis amongst travellers from non-endemic countries. Malaria journal. 2020 Dec;19:1-20.
- Footnote 26
-
House of Commons Canada. EFFECTS OF MEFLOQUINE USE AMONG CANADIAN VETERANS: Report of the Standing Committee on Veterans Affairs [internet]. 2019 [cited 2024 Sep 24]. Available from: https://www.ourcommons.ca/Content/Committee/421/ACVA/Reports/RP10587302/acvarp14/acvarp14-e.pdf
- Footnote 27
-
Australia Foreign Affairs, Defence and Trade Committee Department of the Senate. Use of the Quinoline anti-malarial drugs Mefloquine and Tafenoquine in the Australian Defence Force [internet]. 2018 [cited 2024 Sep 24]. Available from: https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Foreign_Affairs_Defence_and_Trade/Mefloquine/~/media/Committees/fadt_ctte/Mefloquine/report.pdf
- Footnote 28
-
United Kingdom House of Commons Defence Committee. An acceptable risk? The use of Lariam for military personnel: Fourth Report of Session 2015-16 [internet]. 2016 [cited 2024 Sep 24]. Available from: https://publications.parliament.uk/pa/cm201516/cmselect/cmdfence/567/567.pdf
- Footnote 29
-
United Kingdom House of Commons Defence Committee. An acceptable risk? The use of Lariam for military personnel: Government Response to the Committee's Fourth Report of Session 2015-16 [internet]. 2016 [cited 2024 Sep 24]. Available from: https://publications.parliament.uk/pa/cm201617/cmselect/cmdfence/648/648.pdf
- Footnote 30
-
Eick-Cost AA, Hu Z, Rohrbeck P, Clark LL. Neuropsychiatric outcomes after mefloquine exposure among US military service members. The American Journal of Tropical Medicine and Hygiene. 2017 Jan 1;96(1):159.
- Footnote 31
-
National Academies of Sciences, Engineering, and Medicine. Assessment of long-term health effects of antimalarial drugs when used for prophylaxis.
- Footnote 32
-
Overbosch D, Schilthuis H, Bienzle U, Behrens RH, Kain KC, Clarke PD, Toovey S, Knobloch J, Nothdurft HD, Shaw D, Roskell NS. Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. Clinical Infectious Diseases. 2001 Oct 1;33(7):1015-21.
- Footnote 33
-
Schlagenhauf P, Tschopp A, Johnson R, Nothdurft H, Beck B, Schwartz E, Herold M, Krebs B, Veit O, Allwinn R, Steffen R. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. Bmj. 2003 Nov 6;327(7423):1078.
- Footnote 34
-
Arthur JD, Echeverria P, Shanks GD, Karwacki J, Bodhidatta L, Brown JE. A comparative study of gastrointestinal infections in United States soldiers receiving doxycycline or mefloquine for malaria prophylaxis. The American journal of tropical medicine and hygiene. 1990 Dec 1;43(6):608-13.
- Footnote 35
-
Ohrt C, Richie TL, Widjaja H, Shanks GD, Fitriadi J, Fryauff DJ, Handschin J, Tang D, Sandjaja B, Tjitra E, Hadiarso L. Mefloquine compared with doxycycline for the prophylaxis of malaria in Indonesian soldiers: a randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine. 1997 Jun 15;126(12):963-72.
- Footnote 36
-
Weiss WR, Oloo AJ, Johnson A, Koech D, Hoffman SL. Daily primaquine is effective for prophylaxis against falciparum malaria in Kenya: comparison with mefloquine, doxycycline, and chloroquine plus proguanil. Journal of infectious Diseases. 1995 Jun 1;171(6):1569-75.
- Footnote 37
-
Patel SN, Kain KC. Atovaquone/proguanil for the prophylaxis and treatment of malaria. Expert review of anti-infective therapy. 2005 Dec 1;3(6):849-61.
- Footnote 38
-
Boggild AK, Parise ME, Lewis LS, Kain KC. Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). American Journal of Tropical Medicine and Hygiene. 2007 Feb 1;76(2):208-23.
- Footnote 39
-
Schlagenhauf P. Mefloquine for malaria chemoprophylaxis 1992-1998: a review. Journal of travel medicine. 1999 Jun 1;6(2):122.
- Footnote 40
-
Steffen R, Fuchs E, Schildknecht J, Funk M, Schlagenhauf P, Phillips-Howard P, Nevill CG, Naef U, Sturchler D. Mefloquine compared with other malaria chemoprophylactic regimens in tourists visiting East Africa. The Lancet. 1993 May 22;341(8856):1299-303.
- Footnote 41
-
Tan KR, Magill AJ, Parise ME, Arguin PM. Doxycycline for malaria chemoprophylaxis and treatment: report from the CDC expert meeting on malaria chemoprophylaxis. The American journal of tropical medicine and hygiene. 2011 Apr 4;84(4):517.
- Footnote 42
-
Andersson H, Askling HH, Falck B, Rombo L. Well-tolerated chemoprophylaxis uniformly prevented Swedish soldiers from Plasmodium falciparum malaria in Liberia, 2004-2006. Military medicine. 2008 Dec 1;173(12):1194-8.
- Footnote 43
-
Korhonen C, Peterson K, Bruder C, Jung P. Self-reported adverse events associated with antimalarial chemoprophylaxis in peace corps volunteers. American journal of preventive medicine. 2007 Sep 1;33(3):194-9.
- Footnote 44
-
Napoletano G, Bissoli P, Bisoffi Z, Todescato A, Gottardello L, Costa S, Nguyen TM, Boscolo M, Pupo A, Valsecchi M. Malaria chemoprophylaxis-follow up of returned travellers of Veneto Region, Italy.
- Footnote 45
-
Phillips MA, Kass RB. User acceptability patterns for mefloquine and doxycycline malaria chemoprophylaxis. Journal of Travel Medicine. 1996 Mar 1;3(1):40-5.
- Footnote 46
-
Sonmez A, Harlak A, Kilic S, Polat Z, Hayat L, Keskin O, Dogru T, Yilmaz MI, Acikel CH, Kocar IH. The efficacy and tolerability of doxycycline and mefloquine in malaria prophylaxis of the ISAF troops in Afghanistan. Journal of Infection. 2005 Oct 1;51(3):253-8.
- Footnote 47
-
McCarthy AE, Coyle D. Determining utility values related to malaria and malaria chemoprophylaxis. Malar J 2010 Apr 9;9:92-2875-9-92.
- Footnote 48
-
Kain D, Findlater A, Lightfoot D, Maxim T, Kraemer MU, Brady OJ, Watts A, Khan K, Bogoch II. Factors affecting pre-travel health seeking behaviour and adherence to pre-travel health advice: a systematic review. Journal of travel medicine. 2019;26(6):taz059.
- Footnote 49
-
Frosch AE, Thielen BK, Alpern JD, Walz EJ, Volkman HR, Smith M, Wanduragala D, Holder W, Boumi AE, Stauffer WM. Antimalarial chemoprophylaxis and treatment in the USA: limited access and extreme price variability. Journal of travel medicine. 2022 May 14;29(4):taab117.
- Footnote 50
-
Landman KZ, Tan KR, Arguin PM, Centers for Disease Control and Prevention (CDC). Knowledge, attitudes, and practices regarding antimalarial chemoprophylaxis in U.S. Peace Corps Volunteers-Africa, 2013. MMWR Morb Mortal Wkly Rep 2014 Jun 13;63(23):516-517.
- Footnote 51
-
Berg J, Breederveld D, Roukens AH, Hennink Y, Schouten M, Wendt JK, et al. Knowledge, attitudes, and practices toward malaria risk and prevention among frequent business travelers of a major oil and gas company. J Travel Med 2011 Nov-Dec;18(6):395-401.
- Footnote 52
-
Voumard R, Berthod D, Rambaud-Althaus C, D'Acremont V, Genton B. Recommendations for malaria prevention in moderate to low risk areas: travellers' choice and risk perception. Malar J 2015 Apr 1;14:139-015-0654-y.
- Footnote 53
-
van Genderen PJ, van Thiel PP, Mulder PG, Overbosch D, Dutch Schiphol Airport Study Group. Trends in the knowledge, attitudes and practices of travel risk groups towards prevention of malaria: results from the Dutch Schiphol Airport Survey 2002 to 2009. Malar J 2012 May 29;11:179.
- Footnote 54
-
Widmer LL, Blank PR, Van Herck K, Hatz C, Schlagenhauf P. Cost-effectiveness analysis of malaria chemoprophylaxis for travellers to West-Africa. BMC Infect Dis 2010 Sep 22;10 :279-2334-10-279.
- Footnote 55
-
Coatney GR, Mickelsen O, Burgess RW, Young MD, Pirkle CI. Chloroquine or pyrimethamine in salt as a suppressive against sporozoite-induced vivax malaria (Chesson strain). Bulletin of the World Health Organization. 1958;19(1):53.
- Footnote 56
-
Coatney GR, Ruhe DS, Cooper WC, Josephson ES, Young MD. Studies in Human Malaria. X. The Protective and Therapeutic Action of Chloroquine (SN 7618) against St. Elizabeth Strain Vivax Malaria.
- Footnote 57
-
World Health Organization. Report on antimalarial drug efficacy, resistance and response: 10 years of surveillance (2010- 2019). Geneva; 2020. Licence: CC BY-NC-SA 3.0 IGO
- Footnote 58
-
Baird JK, Fryauff DJ, Hoffman SL. Primaquine for prevention of malaria in travelers. Clin Infect Dis 2003 Dec 15;37(12):1659-1667.
- Footnote 59
-
Fletcher KA, Evans DAP, Gilles HM, Greaves J, Bunnag D, Harinasuta T. Studies on the pharmacokinetics of primaquine. Bull World Health Organ 1981;59(3):407-412.
- Footnote 60
-
Karwacki JJ, Shanks GD, Kummalue T, Watanasook C. Primaquine induced hemolysis in a Thai soldier. Southeast Asian J Trop Med Public Health 1989 Dec;20(4):555-556.
- Footnote 61
-
Sarkar S, Prakash D, Marwaha RK, Garewal G, Kumar L, Singhi S, et al. Acute intravascular haemolysis in glucose-6-phosphate dehydrogenase deficiency. Ann Trop Paediatr 1993;13(4):391-394.
- Footnote 62
-
Salvidio E, Pannacciulli I, Tizianello A, Ajmar F. Nature of hemolytic crises and the fate of G6PD deficient, drug-damaged erythrocytes in Sardinians. N Engl J Med 1967 Jun 15;276(24):1339-1344.
- Footnote 63
-
George JN, Sears DA, McCurdy PR, Conrad ME. Primaquine sensitivity in Caucasians: hemolytic reactions induced by primaquine in G-6-PD deficient subjects. J Lab Clin Med 1967 Jul;70(1):80-93.
- Footnote 64
-
Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg 2006 Sep;75(3):402-415.
- Footnote 65
-
Weiss WR, Oloo AJ, Johnson A, Koech D, Hoffman SL. Daily primaquine is effective for prophylaxis against falciparum malaria in Kenya: comparison with mefloquine, doxycycline, and chloroquine plus proguanil. J Infect Dis 1995 Jun;171(6) :1569-1575.
- Footnote 66
-
Fryauff DJ, Baird JK, Basri H, Sumawinata I, Purnomo, Richie TL, et al. Randomised placebo-controlled trial of primaquine for prophylaxis of falciparum and vivax malaria. Lancet 1995 Nov 4;346(8984) :1190-1193.
- Footnote 67
-
Soto J, Toledo J, Rodriquez M, Sanchez J, Herrera R, Padilla J, et al. Primaquine prophylaxis against malaria in nonimmune Colombian soldiers: efficacy and toxicity. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998 Aug 1;129(3):241-244.
- Footnote 68
-
Baird JK, Lacy MD, Basri H, Barcus MJ, Maguire JD, Bangs MJ, et al. Randomized, parallel placebo-controlled trial of primaquine for malaria prophylaxis in Papua, Indonesia. Clin Infect Dis 2001 Dec 15;33(12):1990-1997.
- Footnote 69
-
Ling J, Baird JK, Fryauff DJ, Sismadi P, Bangs MJ, Lacy M, Barcus MJ, Gramzinski R, Maguire JD, Kumusumangsih M, Miller GB. Randomized, placebo-controlled trial of atovaquone/proguanil for the prevention of Plasmodium falciparum or Plasmodium vivax malaria among migrants to Papua, Indonesia. Clinical infectious diseases. 2002 Oct 1;35(7):825-33.
- Footnote 70
-
Askling HH, Nilsson J, Tegnell A, Janzon R, Ekdahl K. Malaria risk in travelers. Emerging infectious diseases. 2005 Mar;11(3):436.
- Footnote 71
-
Schwartz E, Regev-Yochay G. Primaquine as prophylaxis for malaria for nonimmune travelers: a comparison with mefloquine and doxycycline. Clinical infectious diseases. 1999 Dec 1;29(6):1502-6.
- Footnote 72
-
Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. New England Journal of Medicine. 2013 Oct 3;369(14):1381-2.
- Footnote 73
-
Choi S, Choi H, Park SY, Kwak YG, Song JE, Shin SY, Baek JH, Shin HI, Cho SH, Lee SE, Kwon JR. Association between CYP2D6 phenotype and recurrence of Plasmodium vivax infection in south Korean patients. Malaria Journal. 2022 Oct 10;21(1):289.
- Footnote 74
-
World Health Organization. Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. olvale malaria [internet]. 2016 [cited 2024 Sep 26]. Available from: https://iris.who.int/bitstream/handle/10665/250297/WHO-HTM-GMP-2016.9-eng.pdf.
- Footnote 75
-
Cappellini MD, Fiorelli GE. Glucose-6-phosphate dehydrogenase deficiency. The lancet. 2008 Jan 5;371(9606):64-74.
- Footnote 76
-
Ruwende CY, Hill A. Glucose-6-phosphate dehydrogenase deficiency and malaria. Journal of molecular medicine. 1998 Jun;76:581-8.