Health Canada 2020–2021 Departmental Results Report
Table of Contents
- From the Ministers
- Results at a Glance
- Results: what we achieved
- Health Canada's Response to COVID-19
- Core Responsibility 1: Health Care Systems
- Core Responsibility 2: Health Protection and Promotion
- Internal Services
- Experimentation and GBA Plus
- Analysis of trends in spending and human resources
- Corporate Information
- Supporting information on the program inventory
- Supplementary information tables
- Federal tax expenditures
- Organizational contact information
- Appendix: definitions
- Endnotes
From the Ministers
The COVID-19 pandemic is one of the largest crises that the world has faced in our lifetime. It has tested the resilience and resolve of governments, our health care systems and of Canadians. Our public health specialists, scientists, health care workers and public servants have worked around the clock to assess and reassess the situation and implement unprecedented measures to protect the health and safety of Canadians. We are grateful for their efforts and the efforts of Canadian as we continue to work through these challenging times.
From the outset of the pandemic, Health Canada worked alongside the Public Health Agency of Canada (PHAC) and Canada's Chief Public Health Officer (CPHO) to lead a whole-of-government response to the pandemic. This report provides an overview of the Department's extensive efforts to support a comprehensive response while also continuing to deliver on its core work of protecting the health and safety of Canadians.
The Department's dedicated COVID-19 Task Force teamed with the Agency to ensure a coordinated and multi-faceted effort—one that not only brought together other federal departments and agencies, but also provinces and territories, municipalities, Indigenous communities, academic, science and technical organizations, private sector companies, and frontline workers.
Health Canada reimagined the way it delivers results to Canadians by constantly adjusting to evolving scientific evidence without compromising Canada's high standards for safety, efficacy and quality. The Department implemented innovative and agile regulatory measures to authorize clinical trials and approve vaccines, treatments and therapies, sanitizers and disinfectants, and medical devices. These expedited measures helped make health products and medical supplies for COVID-19 available to Canadians. Health Canada also supported PHAC in the roll-out of the largest vaccination campaign in history. Ensuring the availability of safe and effective COVID-19 vaccines has been an important tool to protect our families, communities and ourselves against the virus and to reduce the spread.
Furthermore, Health Canada supported provinces and territories, including health care capacity, through targeted funding and investments. We prioritized protecting communities at higher risk by establishing a $1 billion Safe Long-term Care Fund to help jurisdictions protect those in care and improve infection prevention and control in long-term care facilities across Canada. The Department also responded to drug shortages and an increased demand for various medications required to manage the pandemic by working with provinces and territories to establish a COVID-19 Critical Drug Reserve.
Innovation and experimentation resulted in new digital platforms, tools and approaches to provide direct support to Canadians in need and make critical information about the pandemic available to Canadians in a timely way. For example, recognizing the serious impact of COVID-19 on mental wellbeing, Health Canada launched Wellness Together Canada, a free, online portal that connects Canadians with mental health and substance use resources.
While Health Canada employees were responding to urgent pandemic-related priorities, they continued delivering on other key activities to support the health and safety of Canadians. We know that the pandemic has intensified the already horrific overdose crisis that is affecting many Canadians, families and communities across the country. Health Canada took urgent action with respect to the opioid crisis by assisting community-based organizations to continue their essential work to save lives, including reinforcing and adapting frontline services in the context of COVID-19. Through partnerships with community organizations, Health Canada funded the delivery of evidence-based treatments, harm reduction and prevention programs and increased awareness and prevention efforts to minimize harms on individuals, families and communities.
The Department also continued work on its core mandate to support the evolution and improvement of our health care system including supporting the implementation of medical assistance in dying (MAID) legislation, establishing a Canadian Drug Agency Transition Office and consulting on a strategy for improving access to drugs for rare diseases.
Protecting Canadians from unsafe substances and consumer products remained an important focus of Health Canada's work. The Department continued to issues recalls and increased its efforts to protect young Canadians from vaping by restricting the promotion of vaping products to youth, raising awareness of the potential harms, and taking additional compliance and enforcement actions as necessary.
We would like to once again acknowledge the fortitude and dedication of Health Canada's employees in responding to this pandemic. We take great pride in their commitment to protecting the health and safety of Canadians.
We would also like to acknowledge our country's resilience and the compassion that Canadians demonstrated by supporting one another and doing their part to protect each other. Together, we've been able to overcome great adversity and the lessons we've learned as a result will serve us well as we continue to move forward during this difficult time.

The Honourable Jean-Yves Duclos, P.C., M.P.
Minister of Health

The Honourable Carolyn Bennett, M.D., P.C., M.P.
Minister for Mental Health and Addictions and Associate Minister of Health
Results at a Glance
Resources used to achieve results for Canadians
Health Canada's total actual spending for 2020-21: $3,116,652,943
Health Canada's total actual full‑time equivalents for 2020-21: 8,627
Health Canada is the federal department responsible for helping Canadians maintain and improve their health. In keeping with the Department's commitment to making this country's population among the healthiest in the world, its main responsibilities are as a regulator, a catalyst for innovation, a funder, and an information provider.
Health Canada also administers the Canada Health Act which embodies national principles to ensure a universal and equitable publicly-funded health care system. In addition to working closely with provincial and territorial governments, the Department also works with partners in the Health Portfolio [Public Health Agency of Canada (PHAC), Canada Food Inspection Agency (CFIA), and Canadian Institutes of Health Research (CIHR)], other federal departments and agencies, non-governmental organizations, other countries, Indigenous partnersFootnote 1 and the private sector.
From coast to coast to coast, Health Canada employees—scientists and researchers, inspectors, doctors and nurses, policy analysts and administrative professionals, and many others—are working to help Canadians maintain and improve their health.
Core Responsibilities
Health Canada's Departmental Results Framework outlines two core responsibilities for the Department: Health Care Systems and Health Protection and Promotion. This reporting framework provides the structure for planned activities, which are organized according to these core responsibilities and their corresponding results.
Core Responsibility 1:
Health Care Systems
Core Responsibility 2:
Health Protection and Promotion
Under the Health Care Systems core responsibility, Health Canada provides national leadership to foster sustainable health care systems that ensure access for Canadians to appropriate and effective health care. This is mainly achieved through partnerships with provincial and territorial governments and support through targeted funding agreements to organizations and key pan-Canadian health partners that are contributing to health system improvements.
Within the Health Protection and Promotion core responsibility, Health Canada works with domestic and international partners to assess, manage and communicate the health and safety risks and benefits associated with health and consumer products, food, chemicals, pesticides, environmental factors, tobacco and vaping products, cannabis, and controlled substances. These risks are managed through rigorous regulatory frameworks and by communicating risks and benefits to Canadians so that they can make informed decisions.
Key Results
In 2020-21, Health Canada achieved the following key results that contributed to the health of Canadians and responded to the government's priorities as articulated in the 2020 Speech from the Throne.
Results: what we achieved
Health Canada's Response to COVID-19
- The magnitude of the COVID-19 pandemic required strategic and wide-ranging collaboration with all of Health Canada's partners in health – other federal government departments and agencies, provinces and territories (F/P/Ts), municipalities, Indigenous communities, academic and science and tech organizations, private sector companies, frontline workers and other domestic and international stakeholders – to enhance healthcare capacity; scale up testing and tracing; mobilize resources; approve clinical trials, vaccines, therapeutics, diagnostics, disinfectants and hand sanitizers; monitor the safety of these products after approval; and ensure that Canadians received the support and information they needed.
- Health Canada established a COVID-19 Task Force to provide leadership and direction on the Health Portfolio's response to health issues, specifically: vaccines; treatments and therapies; testing diagnostics; PPE supply and demand; modelling and coordination; and surge capacity.
- Health Canada worked collaboratively with the Health Portfolio, F/P/Ts, Indigenous partners, and the private sector to ensure that Canada's response was based on the latest science, research and the evolving situation. The Department supported health care systems and protected the health and safety of Canadians by investing in virtual care and mental health to facilitating access to health products, including vaccines. This work cut across each of the Department's Core Responsibilities.
Core Responsibility 1: Health Care Systems
- In support of its mandate, Health Canada conducted research, analysis and policy work on the following priority health care systems issues: Canada’s response to COVID-19; modelling and analytics to develop a national supply and demand picture of medical supplies, including PPE and vaccines; supporting F/P/Ts to protect populations at higher risk; health expenditures and funding; home care; access to sexual and reproductive health services; impacts of health care systems modernization on health human resources; health care systems and service delivery innovation; as well as health technology.
- The Department supported multiple pan-Canadian health organizations that directly contribute to health system improvements in areas such as digital health, health information, drugs and technologies, mental health and substance use, cancer prevention and control, patient safety and quality of care, and service delivery innovation. By leveraging technology, these investments facilitated access to virtual tools and services during the COVID-19 pandemic.
- To ensure that Canadians have access to appropriate and effective health services, the Department focused on: advancing health care policy and strategies; building surge capacity to support P/Ts in managing the pandemic; improving access to home, community and palliative care; expanding access to mental health and substance use services; expanding access to virtual and primary care; developing innovative approaches to testing and screening; establishing a Canadian Drug Agency Transition Office and launching consultations on a national strategy on drugs for rare diseases; supporting the implementation of medical assistance in dying (MAID) legislation; encouraging compliance with the Diagnostic Services Policy; providing Canadian thalidomide survivors with support; combatting cancer; improving organ, tissue and blood donation and transplantation; as well as supporting patients in official language minority communities through the Official Languages Health Program.
- The Department’s Substance Use and Addictions Program provided funding for 198 projects representing a wide range of evidence-informed and innovative problematic substance use prevention, harm reduction and treatment initiatives across Canada at the community, regional and national levels. These public education, research, and service delivery initiatives targeted a range of controlled drugs and high-risk substances, including opioids, stimulants, cannabis, alcohol, and tobacco and vaping.
Core Responsibility 2: Health Protection and Promotion
- Health Canada advanced the Regulatory Innovation Agenda arising from the Health and Biosciences Sectoral Regulatory Review Roadmap and the Agri-food and Aquaculture Regulatory Review Roadmap, consisting of initiatives designed to make the federal regulatory framework more agile and responsive.
- The Department worked to ensure that Canadians have access to safe, effective and quality health products by: facilitating access to COVID-related health products during the pandemic, including vaccines, and conducting post-market safety surveillance; stabilizing the supply of critical COVID-19 drugs and managing drug and medical device shortages; promoting timely access to other health products and modernizing processes; management of prescription drugs (including opioids); applying real-world evidence to support regulatory decision-making; strengthening regulatory oversight; modernizing compliance and enforcement; combatting antimicrobial resistance (AMR); fostering international collaboration and coordination; as well as promoting access to new and emerging technologies.
- Health Canada protected Canadians from unsafe consumer and commercial products and substances by: implementing a comprehensive approach to substance use-related harms; facilitating access to treatment services for substance use during the pandemic; regulating cannabis; managing the risks of chemicals in the home, the workplace and the environment; supporting the safety of consumer products and cosmetics; protecting Canadians from radiation; and strengthening pesticide regulation and communication.
- The Department implemented timely, comprehensive, collaborative, compassionate, and evidence-based measures that enabled the health system to address the needs of people with substance use disorder, guided by the Canadian Drugs and Substances Strategy and its 4 pillars of prevention, treatment, harm reduction and enforcement.
- Health Canada supported Canadians to make healthy choices in their day-to-day lives, by: promoting healthy eating; improving food packaging and labelling; fostering international collaboration and coordination for food safety and nutrition; reducing tobacco use and responding to the increase in youth vaping; as well as supporting Canadians in making informed decisions about cannabis use through public education, research and surveillance.
Internal Services
- Health Canada employees worked diligently to limit the spread of COVID-19 and to minimize the health, economic, and social impacts of this rapidly evolving global health crisis. The Department leveraged its scientific, regulatory, policy and administrative expertise to respond as quickly as possible.
- Through such diversity and inclusion networks as the Indigenous Employee Network, Persons with Disabilities Network, Black Employees Matter Network, and Young Professionals Network, Health Canada encouraged employees to mobilize, embrace a greater diversity of voices and make their workplaces ones where everyone’s contributions are valued.
Experimentation
Health Canada continued to increase employee capacity for experimentation and innovation via its Innovation and Experimentation Policy Framework 2020. The third year of the Department’s Solutions Fund: Powering Employee Innovation saw approval of 2 new projects that supported the Government of Canada’s (GOC) pandemic response: exploring and prototyping an AI-assisted assessment engine to enhance the accuracy and speed of assessing complex natural health products; and testing whether automating key human resource transactions can increase efficiency and prevent user error. In addition, Health Canada employees completed 8 of the 13 existing Solutions Fund projects, and experimented with other innovative projects to keep pace with emerging technologies and the evolving demands and expectations of Canadians.
Sex and Gender-Based Analysis Plus (GBA Plus/SGBA Plus)
Health Canada continued to build on the Sex and Gender Action Plan, launched in 2017, while preparing for its next phase in spring 2021. The Action Plan strengthened the Department’s foundation of integrating SGBA Plus considerations into its work and ensured its responses to the COVID-19 pandemic took into account key intersecting sex, gender and diversity issues. Each branch identified at least one signature initiative. This report details progress in the areas of: enhanced capacity building; strengthened sex and gender related evidence and expertise; as well as implementing SGBA Plus across Health Canada programs.
For more information on Health Canada’s plans, priorities and results achieved, see the “Results: what we achieved” section of this report.
Health Canada's COVID-19 response by the numbers
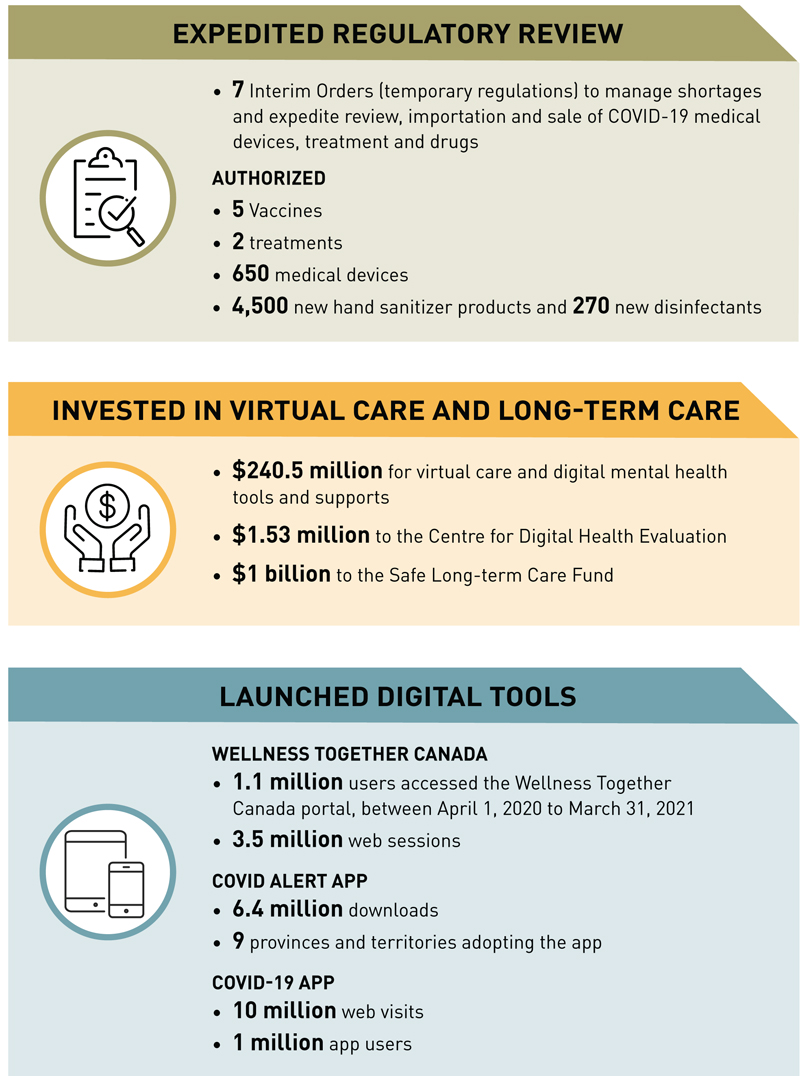
Text description
The figure illustrates some of Health Canada's COVID-19 Response by the Numbers for 2020-21. The responses are grouped in 3 categories: (1) Expedited Regulatory Review, (2) Invested in Virtual Care and Long-term Care, and (3) Launched Digital Tools. Each highlights various initiatives accomplished by the department.
Expedited Regulatory Review
- 7 Interim Orders (temporary regulations) to manage shortages and expedite review, importation and sale of COVID-19 medical devices, treatment and drugs
- Authorized
- 5 Vaccines
- 2 treatments
- 650 medical devices
- 4,500 new hand sanitizer products and 270 new disinfectants
Invested in Virtual Care and Long-term Care
- $240.5 million for virtual care and digital mental health tools and supports
- $1.53 million to the Centre for Digital Health Evaluation
- $1 billion to the Safe Long-term Care Fund
Launched Digital Tools
- Wellness Together Canada
- 1.1 million users accessed the Wellness Together Canada portal, from April 2020 to March 31, 2021
- 3.5 million web sessions
- COVID Alert App
- 6.5 million downloads
- 9 provinces and territories adopting the App
- COVID-19 App
- 10 million web visits
- 1 million App users
Health Canada's Response to COVID-19
In 2020-21, the COVID-19 pandemic dominated the lives of Canadians and created extraordinary challenges for the Canadian health system. From the outset, Health Canada, working closely with PHAC and the CPHO, assumed a leadership role not just in addressing the health impacts of the pandemic but in ensuring an integrated whole-of-government response to protecting the health and safety of Canadians.
Did you know?
The Government of Canada took strong, immediate and effective action to protect Canadians and our economy from the impacts of the global COVID-19 pandemic. The COVID-19 Emergency Response Act was enacted to stabilize the Canadian economy and protect Canadians’ health and safety. Among other components, the Act authorized the Government to make regulations that helped to prevent or alleviate shortages of drugs and medical devices (including PPE) that were needed to address the public health emergency, and to expedite the review of vaccines, therapeutics, diagnostics, disinfectants, sanitizers and clinical trials.
The magnitude of this crisis required strategic and wide-ranging collaboration with all of Health Canada’s partners in health. The Department worked with other federal government departments and agencies, provinces and territories (F/P/Ts), municipalities, Indigenous communities, academic and science and tech organizations, private sector companies, frontline workers and other stakeholders to support health care systems and at-risk populations; scale up testing; approve clinical trials, vaccines, therapeutics, diagnostics, disinfectants and hand sanitizers; monitor the safety of these products after approval; and ensure that Canadians received the support and information they needed.
To contribute to the pandemic response, working closely with PHAC and federal partners, the Department established the COVID-19 Task Force – creating new governance structures, integrating existing departmental organizations and collaborating with P/Ts and other stakeholders. The COVID-19 Task Force provided leadership and direction on the portfolio’s response on health issues, specifically: vaccines, treatments and therapies, testing diagnostics, PPE supply and demand, modelling and coordination, and surge capacity.
The resurgence of COVID-19 in ‘waves’ and the emergence of highly contagious variants exacerbated the burden on the health care system, from mental health and primary care, to long-term care and access to health care, health products and treatments. To minimize this disruption, the Department and PHAC worked with Public Safety Canada to put in place contingency plans and new tools in the event of resurgence, including the creation and renewal of health human resource rosters and expanding the Canadian Red Cross partnership and standing capacity.
The Health Portfolio also worked with partner departments and agencies to provide guidance and implementation assistance for COVID response on such key files as: protecting Canadians from fraudulent offers of COVID-19 vaccines; border management and the establishment of federal quarantine facilities; supports for Temporary Foreign Workers; building federal contact tracing capacity; increasing F/P/T testing capacity and support delivery of rapid tests to essential workplaces; as well as epidemic prevention and control training at federal correctional facilities.
Health Canada’s existing functions, roles and regulatory authorities also played a critical part in the government-wide response to the pandemic in supporting P/Ts in the delivery and administration of healthcare services. Central to the Department’s response to the urgent need for safe and effective health products, including vaccines, was the introduction of several temporary emergency measures, or Interim Orders – to ensure capacity and supply for the health care systems. An Interim Order is one of the fastest regulatory tools available to help address large-scale public health emergencies. These helped to:
- Facilitate the conduct of clinical trials and broaden access for trial participants;
- Establish temporary pathways to expedite the review of medical devices, drugs and clinical trials;
- Allow exceptional importation of drugs, medical devices or foods for a special dietary purpose;
- Provide additional tools to help prevent and alleviate shortages of drugs and medical devices that may have been caused or worsened by the pandemic.
These innovative and agile measures expedited the regulatory review and authorization of COVID-19 vaccines, enabling Health Canada to be among the first in the world to approve these without compromising safety, efficacy or quality standards, reinforcing the Department’s position as a top-tier global regulator. By March 2021, Health Canada had authorized 5 vaccines (Pfizer-BioNTech, Moderna, AstraZeneca, Serum Institute of India, and Janssen). Canada’s vaccine roll-out started in December 2020 with Pfizer and Moderna vaccines and later expanded to include AstraZeneca, albeit in smaller quantities. The Department supported PHAC in the development of a diverse portfolio of COVID-19 vaccine agreements with world-leading candidates to optimize the deployment of safe, effective, and timely vaccines to Canadians in 2020-21, and supported PHAC’s rollout of the approved vaccines across the country.
Additionally, high demand for key drugs used to treat COVID-19 symptoms and supply disruptions in manufacturing countries increased the risk of drug shortages. Health Canada established a Critical Drug Reserve with P/Ts to ensure future drug supply for medical procedures to support COVID-19 patients, such as drugs needed for ventilators and Intensive Care Units. It worked with Public Services and Procurement Canada to secure agreements with pharmaceutical companies to procure millions of rapid tests, and developed new distribution channels to ensure supply reached to those who needed them.
In summary, the Department worked collaboratively and creatively across the Health Portfolio, other federal departments and agencies, P/Ts, Indigenous partners, and the private sector, from coast to coast to coast, to ensure that Canada’s response was based on the latest science, research and the evolving situation. This work cut across each of Health Canada’s Core Responsibilities and all 5 associated Departmental Results and is described in detail below.
Core Responsibility 1: Health Care Systems
Description
Health Canada provides national leadership to support and encourage sustainable and adaptable health care systems that ensures access for Canadians to appropriate and effective health care services.
The United Nations 2030 Agenda for Sustainable Development and UN Sustainable Development Goals (SDGs)
Health Canada's results under Core Responsibility 1: Health Care Systems directly supported Canada's efforts to address the UN 2030 Agenda, particularly the Sustainable Development Goal 3 (SDG), promoting the good health and well-being of Canadians. For example:
- Promoted health care system and service delivery innovation, including expanding access to virtual health care service delivery, and strengthening Canada's health care systems with a focus on improving the capacity to protect vulnerable populations and high risk communities through various initiatives.
- Improved access to appropriate and effective health care services (including MAID, home, community and palliative care, mental health and substance use services, and cancer care); supported the health workforce; established a Canadian Drug Agency Transition Office; and launched consultations on a national strategy on drugs for rare diseases.
These results also supported the ongoing response to address the direct and indirect health impacts of COVID-19.
Results
Departmental Result 1: Canada has modern and sustainable health care systems
Canadians face an array of complex health concerns, even more so during a pandemic. For over half a century, the Canadian health care system has been strong and reliable in supporting social and economic security. Many Canadians require additional help in dealing with challenging health issues beyond COVID-19—from addressing substance use, to access to sexual and reproductive health, to navigating the complexities of end-of-life care. In 2020-21, Health Canada closely collaborated with P/Ts, providing them with the policy and financial support needed to improve the quality and sustainability of the public health care system for all Canadians.
In support of its mandate, Health Canada conducted research, analysis and policy work on the following priorities, detailed further below: Canada’s response to COVID-19; modelling and analytics to develop a national supply and demand picture of medical supplies, including PPE and vaccines; supporting F/P/Ts to protect populations at higher risk; health expenditures and funding; home care; access to sexual and reproductive health services; impacts of health care systems modernization on health human resources; health care systems and service delivery innovation; as well as health technology. Since the start of the COVID-19 pandemic in January 2020, Health Canada also began applying a COVID-19 lens to all of its research, analysis and policy activities.
What’s new?
Health Canada's Departmental Science Advisor provides arm’s length scientific advice; champions the science agenda; and, works closely with Canada’s Chief Science Advisor and other advisors. In 2020-21, they launched a new Health Canada Framework for Science and Research Excellence to advance science issues; established a Science and Research Integration Network to support and promote departmental scientists in providing advice on emerging issues; launched CanCOVID, an online platform that provides current science advice on COVID-19; chaired the Deputy Minister of Health’s Science Town halls; and provided scientific advice on departmental priorities such as sustainable use of pesticide products and the Chemical Management Plan’s environmental health approach.
The Department worked closely with PHAC, P/Ts and stakeholders to inform decision makers about COVID-19 metrics and developments, and provide expert advice on such vital topics as medical countermeasures, vaccine and PPE procurement, and outbreak management through enhanced contact tracing and rapid testing.
Canada’s response to COVID-19
Health Canada developed policy and forecasting analytical tools to guide the national response to COVID-19. The Department led F/P/T strategy and engagement; facilitated information sharing across jurisdictions and sought expert advice and guidance to support federal officials responsible for managing the pandemic; and led or supported multiple COVID-related intergovernmental and external advisory committees, including 5 interdepartmental Deputy Minister Committees (PPEs, Vaccine and Therapeutics Procurement, Vaccine Roll-Out, Testing and Tracing, and Medical Countermeasures).
Health Canada and PHAC met with P/Ts to share information, support national collaboration and improved F/P/T testing capacity, and provide advice towards ramping up testing, contact tracing, data management and infrastructure as part of a collective response to COVID-19. Building on these meetings, the Department hosted virtual Testing and Screening Knowledge Exchange Sessions focused on mutually informed decision-making.
Did you know?
Through its COVID-19 Task Force, Health Canada worked in collaboration with PHAC, CIHR, Genome Canada, the Canadian COVID Genomics Network, and P/Ts to develop and implement an integrated Variants of Concern Strategy. The $53 million initiative aims to improve and rapidly scale up the surveillance, sequencing, tracing, and research of COVID-19 variants of concern, such as B.1.1.7, B.1.351 and P.1.
In 2020-21, Health Canada drafted the Strategy and established the supporting governance structure. The initiative is a key component of the GOC’s science and evidence-based response to the pandemic. As COVID-19 virus variants continue to emerge, Canada will be prepared to detect, track, and treat these new cases.
Health Canada also developed forecasting tools to keep F/P/T partners, Parliamentarians and Canadians fully informed on public health measures and the impacts on healthcare system capacity under the pandemic. The Department tracked P/T COVID-19 statistics daily and mapped measures to support populations at higher risk as well as reported weekly on P/T public health measures as well as COVID-19 developments in the U.S., with a particular emphasis on bordering states with Canada. This information facilitated decision-making on deploying resources, procuring commodities and encouraging domestic production of PPE, among other important elements.
In addition, Health Canada coordinated with PHAC, Innovation, Science and Economic Development Canada (ISED), CIHR and the National Research Council (NRC) to conduct research on: virus origin and nature; transmission epidemiology; disease impacts; health system impacts; immunity; vaccines; treatments; testing; public health measures; and risk communications. This work supported evidence-based decision-making on testing and screening, vaccine and therapeutic investments, as well as on medical countermeasures.Footnote 2
These collaborations facilitated the flow of information and open dialogue, supported timely decision-making and strengthened trust and collaborative relationships with key federal partners, other jurisdictions and stakeholders.
Modelling and analytics to develop a national supply and demand picture of medical supplies, including PPE and vaccines
In order to better identify national needs and inform future procurement plans, Health Canada created a national model of PPE supply and demand across health care, long-term care, and 60 other economic sectors to enable strategic procurement and deployment. It also developed a National PPE and Medical Supplies Dashboard and a common set of PPE data standards to provide accurate, timely information on PPE procurement and deployment to federal and provincial decision-makers. Looking to the future, the Department worked with PHAC and other government partners to begin developing long-term strategies for PPE data sharing and PPE supply chain security.
In addition, the Department collaborated with PHAC and Statistics Canada to create a vaccines supply model that enabled PHAC’s National Operation Centre to distribute vaccines based on both the available supply and the readiness of the receiving organization to administer the doses, as well as to calculate when immunization targets set by the National Advisory Committee on Immunization (NACI)Footnote 3 could be met.
Supporting F/P/Ts to protect populations at higher risk
Health Canada researched and analyzed the impact of the pandemic on higher-risk groups, which highlighted how disproportionately these groups had been affected.
Working with PHAC and other federal departments (e.g., Employment and Social Development Canada) and P/Ts, the Department made recommendations to reduce or close policy and program gaps, such as providing paid sick leave for long-term care workers, reducing vaccine hesitancy, and increasing testing and tracing in communities most at risk. Health Canada also worked with the Canadian Food Inspection Agency (CFIA) and organizations such as the Canadian Red Cross, local jurisdictions, industry groups, and source countries to respond to outbreaks within food production and processing sectors that employ Temporary Foreign Workers, such as agri-food and agriculture. Some of these activities included:
- Strengthening information sharing among various organizations to better plan for worker arrivals;
- Preparing the Safe Volunteer Isolation Sites (launched on April 1, 2021) to support the isolation needs of individuals in congregate living situations, including Temporary Foreign Workers;
- Providing pre-arrival educational materials in the workers’ language, personalized COVID-19 testing assistance, and access to community and health supports.
Did you know?
COVID-19 took a disproportionate toll on Temporary Foreign Workers in the agri-food and agriculture sectors. Factors such as congregate living situations, barriers to taking time off work when sick, and difficulties following public health instructions due to language barriers exacerbated the impact of the pandemic on these workers. Health Canada’s COVID-19 Task Force worked with other federal departments, provinces, local jurisdictions, industry groups, source countries and other partners to reduce the risks of transmission within these sectors, as well as to promote an effective and timely response to outbreaks.
Health expenditures and funding
Health Canada researched, monitored and analyzed domestic and international health expenditures and funding, and their implications for health care delivery in Canada. This encompassed such key data sources as CIHI’s National Health Expenditure Trends; F/P/T budgets; and the Organisation for Economic Co-operation and Development trends in health spending, long-term care, and mental health, allowing the Department to forecast the drivers of future health spending growth and composition (e.g., supportive care for seniors), in Canada and internationally. This informed the GOC’s response to the pandemic and strategic decisions to ensure the future sustainability and responsiveness of the health care system.
Home care
In support of the GOC’s ongoing commitment, reconfirmed in the September 2020 Speech from the Throne, to take more action to help people stay in their homes longer, Health Canada engaged with experts and key stakeholders to advance knowledge and the adoption of proven approaches and best practices. This included funding projects related to health care policy and strategies, led by stakeholders, that: facilitated the expansion of paramedics providing preventive care and other services in homes and the community; built capacity in primary care to support Canadians with dementia at home; supported decision makers to adopt improvements in home-based palliative care; provided home care professionals with the skills to empower family caregivers; and strengthened post-acute care for seniors.
Did you know?
The monitoring, research, analysis and policy work conducted by Health Canada on issues such as timely access to care, patient safety and health care quality served to guide a number of Health Canada’s policy responses. For example, the results contributed to an analysis of the COVID-19 pandemic’s impact on the level of health services backlogs related to delay of care.
Access to sexual and reproductive health services
In 2020-21, Health Canada’s research and analysis identified barriers in access to treatments and health services. Women, youth, LGBTQ2 people, visible minority Canadians, and Indigenous populations face the highest sexual and reproductive health risks and the greatest barriers to accessing support, information, and services. This led to a Budget 2021 commitment to invest $45 million over 3 years towards funding community-based organizations that help make sexual and reproductive health care information and services more accessible to Canadians.
Impacts of health care systems modernization on health human resources
The Department conducted research and policy analysis in the area of health workforce management, including through its secretariat role for the F/P/T Committee on Health Workforce. This pan-Canadian forum for collaborative action provides policy and strategic advice to jurisdictions and to the Conference of Deputy Ministers of Health regarding health workforce challenges and emerging issues.
Health care systems and service delivery innovation
Health Canada continued to work closely with ISED to advance and communicate health system priorities within the organization’s innovation agenda. In 2020-21, this included targeted funding for COVID-related efforts, as well as support for the Strategic Innovation Fund, the Supercluster initiative, and other programs that affect the health sector. Examples of initiatives that have the potential to influence the health sector include: a blood test to detect certain types of cancer without a hospital visit to help relieve the strain on the healthcare system; modernizing home health care with artificial intelligence (AI) to meet the growing demand; disinfecting robots to keep patients and healthcare workers safe; and AI that detects where to spray for pests in fields to reduce pesticide use by up to 95%.
What’s new?
Systemic racism and racial discrimination are key determinants of health and contribute to health inequities between Canadians and Indigenous Peoples. Health Canada is committed to addressing anti-Indigenous racism in health systems in a manner informed by the lived experiences of visible minority communities and Indigenous Peoples.
In 2020-21, Health Canada worked in partnership with Indigenous Services Canada to convene key partners and stakeholders within the health sector to learn from Indigenous medical and health professionals. The departments hosted two national dialogues that brought together over 150 organizations and governments to address anti-Indigenous racism. These dialogues created opportunities for governments and organizations to build relationships for ongoing collaboration and to share action plans.
Health technology
During 2020-21, a short-term Health Canada task force conducted a focused analysis on health data and digital needs, and the implications for Canadian health systems. This work analyzed gaps, challenges and opportunities with respect to health data and digital supports within these systems. This focused initiative was supported by Statistics Canada, PHAC, and the CIHI and was strengthened through engagement with leading experts and stakeholders on the key issues. The results advanced the Department’s understanding and laid the groundwork for future efforts to respond to COVID-19, including, for example, the federal approach to supporting P/Ts with regard to the rapid deployment of needed virtual health care services.
Over the course of the fiscal year, Health Canada made important investments to support several organizations that directly contribute to health care system improvements. As part of this support, the Department provided:
- $84.4 million to Canada Health Infoway to advance digital health innovation, including the development of a pan-Canadian e-prescribing system and virtual care initiatives. As well, the funding was used to begin developing pan-Canadian standards on secure messaging and videoconferences and support P/Ts as they implement new initiatives pursuant to the bilateral agreements for the rapid roll-out of virtual care and other needed health system supports. Funding also continued to support the adoption and use of electronic health records and to better link these electronic systems to improve access for providers, institutions and patients.
- $99.6 million to the CIHI to deliver comparable and actionable data analysis and information to accelerate improvements in health care, health system performance and population health across the continuum of care. CIHI advanced the collection of pan-Canadian data in key areas, including: home care; long-term care; mental health and substance use; pharmaceuticals; acute care and hospital ICU capacity; patient-reported outcomes; organ donation and transplantation; health care workforce; virtual care; and the health of Indigenous populations. CIHI also made significant efforts to support F/P/T governments in their response to COVID-19 by pivoting a number of its core functions (health data standards, data, analysis and reporting, and capacity building) to support access to critical health data on system capacity during the pandemic.
- $26.1 million to the Canadian Agency for Drugs and Technologies in Health (CADTH) to continue strengthening the management of drugs and non-drug technologies. The funding supported health system effectiveness and sustainability by promoting the evidence-based, cost-effective and optimal use of drugs and other health technologies by health care decision-makers such as public drug plans and healthcare practitioners. In 2020-21, CADTH launched a COVID-19 evidence portal containing information to support the needs of health care decision-makers at this challenging time.
- $23.7 million to Canadian Foundation for Healthcare ImprovementFootnote 4 to identify, accelerate and scale health care innovations. Through its rapidly-developed LTC+ Program, the Foundation supported 1,000 long-term care and retirement homes and 40 shelters for people either experiencing homelessness or undergoing residential substance use treatment in preparing for and responding to the COVID-19 pandemic. The program focused on such health care improvements as more frequent communication and more consistent COVID-19 messaging with staff and families, as well as improved infection prevention and control measures.
Did you know?
Congregate living facilities participating in the Canadian Foundation for Healthcare Improvement’s LTC+ Program were coached in doing pandemic readiness assessments, provided with best practices in preparing for future waves of COVID-19 (e.g., infection prevention and control practices), and given $10,000 each in seed funding to address gaps they identified along with access to experts and coaches who could facilitate their improvement efforts.
- $7.6 million to the Canadian Patient Safety Institute to improve patient safety and quality of care. The Institute provided leadership and coordinated the work necessary to enable a culture of patient safety throughout the Canadian health care system. It launched the Canadian Quality and Patient Safety Framework, the first of its kind in Canada, which aims to align the country around 5 shared goals for safety and quality improvement, providing a roadmap for health services regardless of their jurisdiction. The Institute also updated and re-released the Safety Competencies Framework, used to embed safety practices into health care professionals’ training and educational development curriculums across the country.
- $10.9 million to the Canada Brain Research Fund Program, in matched funds to augment innovative neuroscience discovery work, administered by the Brain Canada Foundation (Brain Canada). Through the Fund, Brain Canada made strategic investments to bolster the capacity of the research community to improve care for Canadians with brain health conditions. This included securing a $3.8 million multi-year partnership with Bell Let's Talk to accelerate work on neuroscience linkages with mental health, including research on how COVID-19 affects brain function and mental health. With matching funding, Brain Canada was able to invest $27.7 million in new projects focussed on dementia, and $8.9 million on mental health. One dementia project, led by a research team at McGill University, has resulted in a retinal scan that can detect Alzheimer’s disease before symptoms progress. In 2020-21, this new technology received clearance to allow for partnerships with eye clinics to conduct scans for at-risk populations. The expectation is that this discovery will promote early detection and proactive treatment opportunities.
- $27 million to the Territorial Health Investment Fund to offset the medical transportation costs experienced by territorial governments and supported the development and implementation of innovative activities intended to transform territorial health systems. COVID-19 slowed the implementation of some Investment Fund projects, given restrictions on in-person events and pressure territories faced in responding to the emerging pandemic. Initiatives that launched in 2020-21 include:
- $7.1 million to the Northwest Territories, which piloted a new cultural competency training program for its staff. Key learnings from this phase will inform the final training model, which will be designed for broad participation across the health system, including frontline services.
- $6.4 million to the Yukon, which released the final report of its comprehensive health and social services review and continued to focus on developing collaborative care delivery models, including working with partners and stakeholders to implement the first phases of the “Aging in Place” Action Plan.
- $13.5 million to Nunavut, which increased the number of local Inuit paraprofessionals working in the territory – as of November 2020, a total of 37 mental health and addictions paraprofessionals were hired. Under the territory’s tuberculosis program, all 25 communities acquired the capacity to conduct screenings and treat active cases, while the community oral health program successfully provided services to all communities.
What’s new?
To further advance innovation in testing and public health data managementHealth Canada launched the Safe Restart Agreement contribution program. The Department made available an additional $30 million to P/Ts that submitted proposals to modernize their health data management capability in a manner that improves the sharing of relevant public health data and information across all jurisdictions.
By leveraging technology, these investments facilitated access to virtual tools and services during the COVID-19 pandemic. For example, health care providers were able to better coordinate patient care; policy makers gained access to streamlined information on the performance of the health care system and the cost-effectiveness of drugs and technologies; and promising innovations and best practices in service delivery were more readily identified and shared across jurisdictions. Progress on these initiatives was monitored through indicators on drug spending and health expenditure at the system-level.
Departmental Result 2: Canadians have access to appropriate and effective health services
Through Health Canada, the federal government is responsible for promoting and defending the core principles of the Canada Health Act – public administration, comprehensiveness, universality, portability and accessibility – and ensuring that P/T health care insurance plans provide reasonable access to health services, including insured hospital and physician services, without financial or other barriers, such as patient charges for insured services.
The GOC provided financial contributions to P/Ts to support publicly funded health care services through the Canada Health Transfer (more than $42 billion for 2020-21Footnote 5) and through targeted funding (i.e., $11 billion over 10 years starting in 2017) to support improved access to home and community care, and mental health and substance use services.
Following agreement by F/P/T Ministers on a Common Statement of Principles on Shared Health Priorities in 2017, the federal government negotiated and signed bilateral agreements with all 13 P/Ts, which are set to be renewed in 2021-22. These agreements set out details of how each jurisdiction applies federal funding, which is targeted to areas of the health system where change is most needed. In particular, it aims to help shift care from expensive hospital settings to community-based services, improve health outcomes and create more sustainable health systems.
Did you know?
Health Canada advanced the federal commitment in the Fall Economic Statement to support P/Ts in protecting individuals living in long-term care settings, by working with all parties in early 2021 to amend the agreements to target $1 billion towards safe long-term care in response to the COVID-19 pandemic. This is in addition to the Department’s continued targeted funding of $11 billion (Budget 2017) over 10 years to P/Ts to improve access to home and community care (including palliative care), as well as to mental health and addictions services.
CIHI is working with F/P/T governments, sector stakeholders, measurement experts and the public to develop and report on 12 pan-Canadian indicators to measure progress on the bilateral agreements and the Common Statement of Principles. In 2020-21, CIHI focused on results for 3 specific indicators related to home and community care and mental health and addiction services: self-harm, including suicide; caregiver distress; and new long-term care residents who potentially could have been cared for at home.
To ensure that Canadians have access to appropriate and effective health services, including enhancing health care capacity during the pandemic, the Department focused its 2020-21 efforts on the following priorities, detailed further below: advancing health care policy and strategies; building surge capacity to support P/Ts in managing the pandemic; improving access to home, community and palliative care; expanding access to mental health and substance use services; expanding access to virtual and primary care; developing innovative approaches to testing and screening; managing pharmaceuticals strategically; supporting the implementation of medical assistance in dying (MAID); encouraging compliance with the Diagnostic Services Policy; providing Canadian thalidomide survivors with support; combatting cancer; improving organ, tissue and blood donation and transplantation; as well as supporting patients in official language minority communities through the Official Languages Health Program.
Advancing health care policy and strategies
Health Canada provided $33.6 million under the Health Care Policy and Strategies Program, towards disseminating, exchanging and implementing knowledge to support innovation and implementation of best practices in areas such as: palliative and end-of-life care; home and community care; mental health; and other F/P/T emerging priorities.
What’s new?
With the support of a $4 million investment via Health Canada’s Health Care Policy and Strategies Program, the Association of Faculties of Medicine of Canada established a National Consortium on Indigenous Medical Education to provide leadership, develop educational resources, and implement Indigenous-led activities that reform physician education and advance culturally safe health care delivery. The creation of the Consortium will allow for a better understanding of the culturally-sensitive issues faced by Indigenous communities, and an improved medical education environment, with the goal of fostering access to appropriate, effective and culturally safe healthcare services for Indigenous Peoples, free from discrimination.
In 2020-21, the Program continued to support the health care priorities of Canadians by funding and advancing the following new and ongoing projects:
- The Institute for Health System Transformation and Sustainability’s “Preparing Patients and Families from Culturally Diverse Backgrounds for Enhanced Access to Palliative Care through Advance Care Planning Engagement” focused on developing culturally appropriate and linguistically acceptable tools and resources to facilitate more informed palliative and end-of-life decisions in culturally diverse communities;
- Choosing Wisely Canada (CWC) encourages clinicians and patients across Canada to engage in conversations about unnecessary tests, treatments and procedures. In 2020-21, CWC continued to work with key stakeholders and physician leaders to review and refresh its list of recommendations, including publishing new ones related to COVID-19, rural medicine, and critical care, among others;
- Heart & Stroke’s “Advancing Women’s Heart Health” supported targeted research on the prevention, diagnosis and treatment of heart disease and stroke in women, promoted collaboration between research institutions across the country, and invested in female and Indigenous scientists to build a pool of high calibre Canadian researchers focused on women’s vascular health;
- The Canadian Association of Schools of Nursing’s “Essential COVID-19 Skills for Graduating and New Nurses” created a Virtual Simulation Series to strengthen the capacity of nursing students, new frontline nurses and nurses returning to work to provide care during the COVID-19 health crisis.
Building surge capacity to support P/Ts in managing the pandemic
Health Canada worked with such partners as the Public Service Commission, Public Safety Canada, the Canadian Red Cross and PHAC to recruit and train volunteers ready to be rapidly deployed across Canada, including to remote communities. These volunteers had a broad range of skills, such as outbreak management in long-term care homes and other congregate living sites. By March 2021, approximately 24,000 from a total of some 50,000 on-call candidates were referred to P/Ts as a result of this initiative.
What’s new?
In 2020-21, Health Canada supported the Pallium Canada project Building and Bridging – Palliative Care is Everyone’s Business, which aims to strengthen home and community palliative care capacity. Funding allowed Pallium to expand its "Learning Essentials Approaches to Palliative Care" (LEAP) program, which develops and provides inter-professional palliative care education across Canada. In response to the COVID-19 pandemic, Pallium offered 10 of its LEAP training modules as well as a webinar series and other resources online, free of charge, to allow health care providers to quickly gain the knowledge they needed to care for seriously-ill patients. As of end of March 2021, over 11,000 frontline health care providers had accessed these free resources.
The Department also collaborated with Public Safety Canada and Public Services and Procurement Canada (PSPC) while preparing to deploy two federal Mobile Health Units in Ontario in response to a provincial request for assistance. When turned over to the province in April and May 2021, the units each provided 100 additional hospital beds and facilitated the transfer of non-critical care patients out of the ICU to ensure those specialized resources were available for those who needed them most.
Expanding access to home, community and palliative care
In 2020-21, federal funding for home, community and palliative care supported initiatives that continued to:
- Coordinate and integrate care;
- Enhance digital connectivity and the use of remote technology to access care from home;
- Provide caregivers with more education supports and expanded respite services;
- Improve access across the country to home care, palliative and end-of-life supports;
- Work with all partners in health towards setting new national standards for long-term care.
In 2020-21, Health Canada continued to implement the federal Action Plan on Palliative Care that was launched in 2019-20. The Department developed new initiatives to advance and improve palliative care and health system change, such as: raising awareness; supporting training and education; removing access barriers; fostering research; building capacity for standardized data collection; as well as supporting innovation, especially in response COVID-19.
Under the Action Plan, the Department funded new projects related to virtual care, such as: training volunteer navigators who help tap into local home care supports and services; developing a remote symptom monitoring system that connects patients in the home and community with palliative care experts via a daily reporting application; supporting non-specialist health care providers to effectively deliver virtual palliative care services; and improving palliative care capacity among primary care providers through the creation of virtual networks (hubs and spokes) across Canada to share information and foster mentorship from specialists built on case-based learning.
Health Canada continued to collaborate with Indigenous Services Canada on engagements with Indigenous communities to develop a distinctions-based framework on palliative care, reflecting the specific and unique priorities of First Nations, Inuit and Métis.
The COVID-19 pandemic disproportionately affected Canadians receiving long-term care (LTC) in community settings, specifically LTC facilities, assisted living facilities, and seniors’ residences. More than 2,500 care homes experienced an outbreak between March 1, 2020, and February 15, 2021, resulting in the deaths of more than 14,000 residents and close to 30 staff. This represents more than two-thirds of Canada’s overall COVID-19 deaths. About 80,000 residents and staff of LTC and retirement homes were infected, representing 10% of all COVID-19 cases in Canada. To protect seniors, the GOC committed to safe LTC, and to work with P/Ts to set new, national standards.
Did you know?
Health Canada supported PHAC in developing general guidance on indoor ventilation during the pandemic, as well as specific guidance on improving ventilation in long-term care homes and individual residences to reduce COVID-19 transmission.
In 2020-21, the GOC committed up to $1 billion for a Safe Long-term Care Fund to help P/Ts protect LTC residents and staff and support such infection prevention and control measures as: carrying out infection prevention and control readiness assessments; improving ventilation; and hiring additional staff or topping up wages.
Expanding access to mental health and substance use services
Health Canada advanced the development of national standards for access to mental health services, consulting with federal partners, Pan-Canadian Health Organizations, and other experts to gain insight on practical approaches to developing and implementing these standards, in alignment with the Common Statement of Principles priority areas: primary health service integration; children and youth; and people with complex health needs.
In the area of mental health and substance use services, federal investments in 2020-21 supported initiatives that continued to:
- Increase the availability of mental health and substance use services in the community;
- Expand access to community-based mental health and substance use services for children and youth, including school-based programs for early prevention, detection and treatment;
- Expand access to crisis intervention services and integrated multidisciplinary professional services.
In April 2020, Health Canada launched the Wellness Together Canada portal to provide Canadians with free access to live and confidential online mental health and substance use resources, available 24/7 in more than 200 languages and dialects. It also offers tailored supports for youth, adults and frontline workers. Further, there is a dedicated phone line to reach Program Navigators who can assist individuals in accessing portal resources appropriate to their needs.
Did you know?
A November 2020 survey of those who used Wellness Together Canada revealed that without the portal, 24% would not have accessed care for mental health or substance use concerns at all, 36% would have considered reaching out to a general practitioner, 6% to a walk-in clinic and 5% to a hospital emergency department. The top reasons for choosing Wellness Together Canada are that it is available online (61%), free (60%), and convenient (49%).
Wellness Together Canada also helped alleviate local pressures on service delivery by providing an alternative to in-person care. Through the portal, individuals have immediate access to a range of supports that include assessments, self-guided programming, peer-support and counselling. As of March 31, 2021, over 1.1 million individuals in all P/Ts had accessed the Wellness Together Canada portal in over 3.5 million web sessions, increasing awareness and understanding of care options that can be applied personally or are offered as a government service.
The Department also directed $14.2 million to the Mental Health Commission of Canada that helped to advance specific priorities in the area of mental health, substance use and suicide prevention. The organization, in collaboration with stakeholders, produced knowledge products, tools and training; created a Resource Hub for COVID-19 specific information and tools; and, offered free virtual crisis response training for essential workers.
Since the beginning of the COVID-19 pandemic, Health Canada implemented critical and timely measures to enable the health system to address the needs of people with substance use disorder. The Department facilitated access to medications necessary for opioid agonist treatment (e.g., suboxone and methadone), and through P/T class exemptions, enabled jurisdictions to quickly establish overdose prevention sites to address urgent public health needs (including in temporary community shelters).
The GOC also committed to providing P/Ts with $500 million towards health care to respond to the pandemic, which includes support for people experiencing challenges with substance use, mental health, or homelessness. This investment is part of the more than $19 billion invested through the Safe Restart Agreement to help P/Ts safely restart their economies and ensure Canadians have the support they need in these challenging times. P/Ts confirmed that funds would be used to bolster existing mental health and substance use programs, particularly the expansion of virtual care and the enhancement of community-based services.
In 2020-21, Health Canada invested $74.6 million to support community-based organizations responding to substance use issues and to address additional challenges posed by the COVID-19 pandemic. These investments helped provide: medications to people with substance use disorder as an alternative to the toxic illegal drug supply; opioid overdose response training; and increased access to naloxone across the country, including in rural, remote, isolated and otherwise underserved communities.
In addition, the Substance Use and Addictions Program (SUAP)Footnote 6 provided funding for 198 projects representing a wide range of evidence-informed and innovative problematic substance use prevention, harm reduction and treatment initiatives across Canada at the community, regional and national levels. These public education, research, and service delivery initiatives targeted a range of controlled drugs and high-risk substances, including opioids, stimulants, cannabis, alcohol, and tobacco and vaping.
From April 1, 2020 to March 31, 2021, SUAP-funded projects delivered a total of 33,300 knowledge products/learning opportunities, reaching approximately 10 million Canadians, including: adults and youth [who use drugs and/or who are at-risk, peers, Indigenous Peoples, LGBTQ2 Plus, visible minority, Fetal Alcohol Spectrum Disorder (FASD), low-income]; frontline care teams and healthcare professionals; FPT/Regional/Municipal governments; school boards/trustees; community partners (public safety, veterans); non-profit organizations; program designers; policy makers; in addition to the general public.
For specific examples of SUAP-funded projects, please see the Table on the following page.
Health Canada's targeted funding in 2020-21 through SUAP included the following:
- Investing $58 million towards 15 pilot projects to provide medications as safer alternatives to the contaminated illegal drug supply; contributing $5 million to address methamphetamine use; and, provided $20 million for naloxone kits and opioid overdose response training to communities. (For further details, see A comprehensive approach to substance use-related harms)
- Funding to the Canadian Centre on Substance Use and Addiction (CCSA) of $10 million to address problematic substance use in Canada and $2.3 million for research on the impact of cannabis legalization and regulation. CCSA works with a wide range of national and international partners to mobilize efforts, foster knowledge exchange, and develop evidence-informed actions in the problematic substance field. Funded projects included:
- 27 core initiatives targeted to educating the public about the harms of cannabis, opioids, alcohol and other high-risk substances, and to updating jurisdictional-based costs and harms of substance use in Canada;
- 149 knowledge and communication products, and more than 130 events and presentations. Social media knowledge dissemination activities by CCSA increased national and international interest in contrast to previous years;
- 24 new resources on COVID-19 and substance use, plus access to approximately
300 additional resources from more than 125 organizations, and launched a new
COVID-19 webinar series; - 29 cannabis research projects aimed at building knowledge of COVID-19 and cannabis regulation, sales, and use. Through these projects, CCSA developed 9 COVID-19 knowledge products, updated 5 cannabis research products, and delivered over
10 workshops, presentations and webinars.
- Funding to the Mental Health Commission of Canada of $3.3 million towards projects that assess the impacts of cannabis use on the mental health of Canadians. Funded projects included:
- A cannabis knowledge exchange event, in partnership with the Centre for Addiction and Mental Health, that attracted 120 policy and practice experts and led to the creation of 4 new knowledge products;
- In collaboration with CIHR and various other partners, the funding of 22 one-year Catalyst Grant research projects aimed at producing new evidence on cannabis and mental health outcomes, ranging from the risks posed by cannabis to mental health, as well as the potential therapeutic benefit of cannabis and cannabidiol in certain circumstances;
- 14 community-based research projects to investigate research gaps and priorities in cannabis and mental health, as defined by communities.
Expanding access to virtual and primary care
In spring 2020, P/Ts made tremendous strides to put in place tools and supports for virtual services. In response to the challenges of delivering health care during the pandemic, and to support P/Ts in accelerating their efforts, Health Canada worked with these jurisdictions to institute a range of digital supports that helped Canadians find the information, resources and care they needed, whenever and wherever they needed it.
The Department also formed a digital surge team to explore, assess and support the procurement of digital tools in response to COVID-19. These efforts resulted in an online self-assessment tool to help Canadians determine their symptoms and make informed decisions regarding the best actions to take. By March 31, 2021, this service had been accessed more than 10 million times. The Department also launched the Canada COVID-19 app, which had more than 1 million users, to serve as a hub for Canadians to access resources and remain informed about COVID-19.
Additionally, Health Canada invested $240.5 million to develop, maintain and expand these virtual care and mental health tools and supports. Of this funding, $150 million was provided directly to P/Ts through bilateral agreements, and $50 million allocated for Infoway, to help jurisdictions implement new technologies and approaches for virtual care. The remaining $40.5 million was invested for digital tools, which included Wellness Together Canada. As of March 31, 2021, 8 bilateral agreements were signed, with funding focused on advancing 5 shared priorities:
- Secure, end-to-end messaging and information-sharing platforms and supports;
- Secure video-conferencing technology;
- Remote patient monitoring tools;
- Patient access to COVID-19 and other laboratory results;
- The integration and alignment of new platforms, tools and approaches into existing digital health systems.
Since the outset of the pandemic, F/P/T officials worked in collaboration to share best practices and lessons learned, and to understand the impact of virtual care. In this context, the Centre for Digital Health Evaluation (CDHE), a third party organization funded by Health Canada, led work in partnership with P/Ts, CIHI, Infoway and CADTH to evaluate and understand the impact of virtual care during the pandemic. To support this work, Health Canada provided funding of $1.53 million over 3 years, starting in 2020-21, to CDHE through the Health Care Policy and Strategies Program. Together, these investments are enabling the longer-term adoption of high-quality and safe virtual health care services as a complement to in-person care.
F/P/Ts also began looking beyond the pandemic, collaborating to develop a national policy framework that identifies barriers and opportunities for longer-term adoption of virtual services as a core element of Canada’s health systems, now and into the future. In fall 2020, F/P/Ts agreed to an initial draft of this virtual care policy framework that is currently under review and validation.
Developing innovative approaches to testing and screening
Established by the Minister of Health, the COVID-19 Testing and Screening Expert Advisory Panel published 5 reports that provided the GOC with evidence-based recommendations on existing and innovative approaches to testing and screening for COVID-19, in the following areas: testing optimization; long-term care; primary and secondary schools; testing and quarantine at Canadian borders; and self-testing.
In collaboration with ISED and industry associations, Health Canada identified close-contact workplaces and secured guidance and advice on implementing workplace screening programs through Health Canada’s ad hoc Industry Advisory Roundtable on COVID-19 Testing, Screening, Tracing and Data Management. It launched rapid screening pilot projects in over 37 locations, in partnership with P/Ts and the Creative Destruction Lab Rapid Screening Consortium.
What’s New?
There is a simple but effective way to provide additional protection to Canadians in the workplace: screen regularly. In its first report, the Industry Roundtable concluded that enabling businesses to establish workplace screening programs would help protect employees and communities by catching potential infections early, particularly in contagious people who don't show symptoms.
Task shifting involves deploying a broader range of trained individuals to deliver urgent care. Several pilot projects across the country implemented this practice in 2020-21. For example, Public Health Ontario increased available resources for rapid testing by allowing paramedics, pharmacists, dentists and other health professionals to administer the tests. Other provinces, such as Alberta, Quebec and Saskatchewan, introduced similar measures.
Health Canada, with PSPC logistical support, provided rapid tests directly to private, non-profit and crown organizations as well as federal departments and agencies. By March 31, 2021, more than 385,000 tests had been shipped to 20 different organizations across Canada. The COVID-19 Task Force also contributed to the development of 2 Industry Roundtable reports on: Accelerating Rapid Screening in the Workplace and Task Shifting.
Looking to Canada’s land border with the U.S., Health Canada developed and implemented a mandatory testing initiative and a reduction in non-essential travel policy. The Department worked with F/P/T partners on effective border management, adapting as evidence on the risk of international travel evolved and applying data collected from pilot projects (i.e., a collaboration with the Government of Alberta on an International Border Testing Program; a COVID-19 Border Study led by McMaster Health Labs).
With regard to air travel, the Department developed and implemented COVID-19 testing measures, in partnership with P/Ts and airport authorities, that aligned with public health objectives while balancing the need to ensure the free flow of critical people and goods. Activities included: supporting PHAC to implement pre-departure testing and mandatory testing for international travelers; and supporting the development of testing protocols for essential workers.
Health Canada also launched COVID Alert, a nation-wide mobile exposure notification app in July 2020 to let users know when they may have been exposed to the virus. The COVID-19 Exposure Notification App Advisory Council provided advice to ensure that the app would meet the highest standards in public health outcomes, technology, and privacy. Several improvements were introduced over the year, the most significant being that the app is compatible with nearly 98% of smartphones.
By March 2021, COVID Alert had been adopted by 9 P/Ts and downloaded more than 6.4 million times. More than 23,800 individuals used the app to notify others anonymously that they may have been exposed.
Did you know?
Following a significant increase in cases in Newfoundland and Labrador in the week of February 8 to 12, the province’s Chief Medical Officer of Health (Dr. Janice Fitzgerald) tweeted out a request to all Newfoundlanders that they download the COVID Alert app. This post garnered thousands of retweets and responses and led to an impressive grassroots movement on social media. Within hours, local celebrities (including Mark Critch and Alan Doyle) as well as social media influencers, had amplified the message. In addition, several local companies asked people to send them screenshots of the downloaded app and entered them into draws for merchandise and services. This yielded a significant increase in the number of one-time-keys entered for the province and a 7-fold increase in overall national downloads compared to previous weekends.
Managing pharmaceuticals strategically
In 2020-21, Health Canada began developing a national strategy on drugs for rare diseases, in close collaboration with willing P/Ts, pharmaceutical management system stakeholders, patients and Indigenous partners. Once finalized, the strategy will aim to improve access for Canadians to effective treatments for rare diseases.
Health Canada launched a broad public and stakeholder online consultation where Canadians provided their views on the national strategy, with an emphasis on patients with rare diseases, their families and caregivers. This initiative encompassed 5 public town halls, 16 virtual stakeholder meetings and 136 questionnaires. A ‘What We Heard’ report was subsequently published in summer 2021 to summarize key themes and feedback that emerged during these sessions.
The Department established a Canadian Drug Agency Transition Office towards the goal of creating a Canadian Drug Agency. The Transition Office will advance work on pharmaceuticals management initiatives and provide dedicated leadership and resources to support F/P/T discussions.
Did you know?
There are more than 7,000 diseases that are considered rare, and although each one affects a relatively small number of Canadians, they are often life-threatening, debilitating and chronic health conditions. The high cost of many drugs for rare diseases and the limited clinical evidence (often due to small patient populations) make it difficult for patients and their families, employers and governments to decide on whether and how to pay for treatment. This can create challenges for many P/Ts looking to help families. A national strategy would help ensure more effective assessment of a drug's efficacy, better manage costs, and improve access for patients.
Supporting implementation of medical assistance in dying (MAID)
Health Canada continued work towards meeting federal commitments to support the implementation of MAID legislation, in collaboration with P/Ts.
In July 2020, using information collected under the federal MAID monitoring program, the Department released the First Annual Report on Medical Assistance in Dying (MAID) in Canada, 2019. This report provided Canadians with information about who is requesting MAID, under what circumstances, and insight into service delivery across the country.
In March 2021, the GOC announced passage of a new MAID law (Bill C-7) in response to a 2019 Québec Superior Court ruling. The new law responds to Canadians’ evolving needs, supports their autonomy and freedom of choice, and protects those who may be vulnerable. It also includes a renewed federal commitment to conduct a parliamentary review of its provisions and other considerations including a Parliamentary Review of the state of palliative care in Canada, which will take place over the course of 2021-22.
Encouraging compliance with the Diagnostic Services Policy
Over the course of 2020-21, the Department worked with British Columbia, Alberta, Quebec, Ontario, Nova Scotia and New Brunswick to encourage compliance with the Diagnostic Services Policy, which came into effect in April 2020. Due to the retroactive nature of the Canada Health Act reporting, P/Ts will report on compliance with the Policy beginning in December 2022.
Did you know?
The Diagnostic Services Policy formalizes application of the Canada Health Act to diagnostic services, such as Computerized Tomography Scan (CT Scan) and Magnetic Resonance Imaging (MRI). It is meant to end patient charges for medically necessary diagnostic services that would otherwise be covered if provided in a hospital setting. Health Canada believes Canadians should receive health care based on their level of need and not on their ability to pay – this policy goes toward protecting the principles of Medicare.
Providing Canadian thalidomide survivors with support
Health Canada continued its contribution to meeting the lifetime needs of Canadian thalidomide survivors, allowing them to age with dignity. The Canadian Thalidomide Survivors Support Program (CTSSP) applied a 3-step probability-based medical assessment process to determine eligibility. By March 2021, 198 applications had been submitted and of those, 59 were new in 2020-21. The application period remains open until June 2024.
Based on needs identified in the 2019-20 annual survey of survivors, the Department improved the Extraordinary Medical Assistance Fund. For example, it expanded the eligible expenditure categories to include ongoing costs related to medication, chiropractic care, physiotherapy, and attendant services, among others.
Based on the 2020-21 annual outreach survey, completed by 86 survivors, 82.6% reported a better ability to age with dignity, and 65% reported better access to care, treatment and/or support from ongoing payments.
Combatting cancer
In 2020-21, Health Canada provided over $12.8 million to The Terry Fox Research Institute to establish a national network of cancer centres with expertise in advancing precision medicine in cancer. The data generated will form an important resource for Canadian cancer research. Work began to designate specific centres to the Network, confirming that these organizations are ready to collaborate and execute the key safety, security, clinical, scientific, training and technical requirements expected of network members.
The Department provided $2.25 million to Ovarian Cancer Canada towards addressing gaps in knowledge about ovarian cancer. Funding was used to develop experimental models, test new treatments, and conduct various research projects, all contributing to increased knowledge of effective treatment options for ovarian cancer, including advancements in precision medicine as a management tool.
Health Canada also invested $51 million in the Canadian Partnership Against Cancer to work collaboratively with its partners – P/Ts, cancer programs and agencies, health organizations, Indigenous agencies and other key stakeholders – to leverage collective efforts to support equitable access to quality, sustainable cancer care.
Improving organ, tissue and blood donation and transplantation
The Department provided ongoing leadership and support to the Organ Donation and Transplantation Collaborative, an F/P/T initiative that manages the Organs, Tissues, and Blood Contribution Program with Canadian Blood Services. The Collaborative is working to improve Canada’s organ donation and transplantation (ODT) system, in order to give Canadians more timely and effective access to care. 2020-21 outcomes included:
- Developing best practices to remove barriers to shipping donated kidneys across Canada;
- Launching an ODT Quality Assurance project to allow for continuous improvement and better transplant outcomes;
- Developing a Communication and Knowledge Translation Strategy, including dissemination of ODT project snapshots and support for project implementation strategies;
- Supporting a call through Innovative Solutions Canada to develop machine learning technology that will improve organ donation rates and donor-recipient matches.
Health Canada provided $6.5 million to Canadian Blood Services to work with partners on activities related to blood, organ and tissue donation and transplantation. Among other activities, the funding supported:
- Ongoing research that could inform future changes to blood and plasma donation policies for men who have sex with men (MSM);
- Basic research on blood products, stem cells, and transfusion medicine;
- Development of guidelines to address the challenges of operating donation and transplantation services during the COVID-19 pandemic;
- The first community Public Education and Awareness Committee with patients and donor family members and representatives from various associations and foundations.
Supporting patients in official language minority communities through the Official Languages Health Program
In 2020-21, Health Canada invested $37.4 million under the Official Languages Health Program to community-based organizations, governments and academic institutions to improve access to health services for English-speaking communities in Quebec and French-speaking communities elsewhere in Canada. In addition, the Program supported a number of innovative projects across Canada that took into account the specific needs of patients in official language minority communities (OLMCs), including home care and mental health. These projects seek to better understand the needs of these communities and improve their access to health care services.
Key results included:
- Association des collèges et universités de la francophonie canadienne (ACUFC)-Consortium national de formation en santé (CNFS) and McGill University increased the number of bilingual health providers delivering services in OLMCs. The ACUFC-CNFS recorded 674 additional health graduates from 108 health programs and 91% of the surveyed graduates were retained to work in these communities. McGill University enrolled over 1,118 health and social services professionals in its language training program aimed at improving the English of participants so they can better serve English-speaking patients.
- The Société Santé en français supported Francophone minority communities (outside Quebec), and the Community health and social services networks supported English-speaking minority communities (in Quebec) by undertaking networking initiatives that mobilized partners in health to improve access to health services within OLMCs. A total of 39 community-based health networks and 10 satellites across Canada collaborated with various health sector stakeholders to provide support, resources and tools.
Key risk for Core Responsibility 1: Health Care Systems
Information on Key Risks is available on Health Canada’s website.
Results achieved for Core Responsibility 1: Health Care Systems
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| National health expenditure as a percentage of Gross Domestic Product (GDP) (Baseline: 10% of GDP in 2014-15) |
Between 10% and 12% | March 31, 2021 |
|
| Real per capita health expenditure (1997)DR 1 table Footnote 2 (Baseline: $4,014 per person in 2014-15) |
Between $3,864 and $4,722 | March 31, 2021 |
|
| Drug spending as a percentage of Gross Domestic ProductDR 1 table Footnote 3 (Baseline: 1.74% in 2014-15) |
Between 1% and 2% | March 31, 2021 |
|
| Percentage of family physicians using electronic medical records (Baseline: 73% in 2015) |
At least 95% | March 31, 2022 |
|
|
|||
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| Percentage of Canadians (aged 15+) with a mental disorder who have expressed that they have an unmet mental health care need (Baseline: 26% in 2012) |
At most 15% | March 31, 2021 |
|
| Percentage of Canadians (aged 18+) who have expressed that they have an unmet need for access to home care services (Baseline: 1.6% in 2015-16) |
At most 1% | March 31, 2027 |
|
| Percentage of Canada Health Act compliance issues addressed within 24 months of identification (Baseline: 53% in 2016-17) |
At least 95% | March 31, 2022 |
|
| Percentage of Canadians (aged 12+) who did not fill a prescription for medicine or skipped doses of medicine because of the cost (Baseline: 7.1% in 2014) |
At most 5% | March 31, 2022 |
|
|
|||
| 2020-21 Main Estimates |
2020-21 Planned spending |
2020-21 Total authorities available for use |
2020-21 Actual spending (authorities used) |
2020-21 Difference (Actual spending minus Planned spending) |
|---|---|---|---|---|
| 1,777,284,741 | 1,777,284,741 | 2,200,154,060 | 1,987,223,947 | 209,939,206 |
| Note: The variance of $209.9 million between actual and planned spending is mainly due to Health Canada's response to the COVID-19 pandemic for drugs, medical devices and virtual care; as well as, the safe restart agreement for the federal investments in testing, contact tracing and data management. | ||||
| 2020-21 Planned full-time equivalents |
2020-21 Actual full-time equivalents |
2020-21 Difference (Actual full-time equivalents minus Planned full-time equivalents) |
|---|---|---|
| 290 | 247 | -43 |
| Note: The variance in FTE utilization is mainly due to attrition and longer than anticipated staffing processes. | ||
Core Responsibility 2: Health Protection and Promotion
Description
Health Canada works with domestic and international partners to assess, manage and communicate the health and safety risks and benefits associated with health and consumer products, food, chemicals, pesticides, environmental factors, tobacco and vaping products, cannabis, and controlled substances.
The United Nations 2030 Agenda for Sustainable Development and UN Sustainable Development Goals (SDGs)
Health Canada's results under Core Responsibility 2: Health Protection and Promotion directly supported Canada's efforts to address the UN 2030 Agenda and the related Sustainable Development Goals (SDGs). For example, the Department:
- Worked with P/T partners to support the health sector in reducing the human health impacts of climate change (SDGs 1, 11 and 13).
- Provided information to Canadians on healthy eating, prioritizing populations at higher risk of food insecurity, and fostered international collaboration on food safety and nutrition standards support (SDG 2).
- Ensured timely access to safe, effective and quality health products through innovative and agile regulatory frameworks, including combatting antimicrobial resistance, promoting access to new and emerging technologies and managing drug and medical device shortages (SDG 3).
- Supported healthier living by preventing and minimizing substance use harms through the Canadian Drugs and Substances Strategy and Substance Use and Addictions Program; including engaging Canadians on the risks of tobacco, alcohol, cannabis use and vaping (SDG 3).
- Developed updates on the Canadian drinking water guidelines to help improve drinking water quality (SDGs 3 and 6).
- Developed and provided science-based information to Canadians on the health effects of ambient and indoor air pollution, including collaborating with PHAC on guidance for improving indoor air quality in long-term care facilities to reduce COVID-19 transmission (SDGs 3 and 11).
- Worked with Environment and Climate Change Canada and other partners to implement Canada's Chemicals Management Plan, to reduce the human health and environmental risks posed by chemicals in air, water, food, soil and in consumer and industrial products and processes (SDGs 3 and 12).
- Worked with partner departments to support the safe and sustainable use of pesticide products, contributing to Canada's efforts towards sustainable consumption and production patterns (SDG 12).
- Partnered with F/P/Ts on a collaborative approach to reduce plastic waste in the health and non-health sectors, especially generated by the increased use of PPE in the context of COVID-19 (SDG 11).
These results also supported the ongoing response to address the direct and indirect health impacts of COVID-19.
Results
Despite the impact of the pandemic in 2020-21, Health Canada continued to advance the Regulatory Innovation Agenda arising from the Health and Biosciences Sectoral Regulatory Review Roadmap and the Agri-food and Aquaculture Regulatory Review Roadmap. The Regulatory Innovation Agenda directly responds to the industry-government Health and Bio-sciences Economic Strategy Table that recommended more regulatory agility as a part of a boarder strategy to make Canada’s health and biosciences sector one of the top 3 in the world.
The Agenda cuts across multiple Departmental Results that make up Core Responsibility 2. It consists of initiatives designed to make the federal regulatory framework more agile and responsive to an innovative environment, while ensuring the system remains science and safety-based. Its continued implementation will result in health product and food regulatory frameworks that protect the health and safety of Canadians while encouraging innovation.
While the COVID-19 pandemic delayed some of Health Canada’s plans as outlined in the Roadmaps, it also reinforced the need for regulatory agility and flexible approaches to health product oversight. In its response to the pandemic, the Department launched temporary regulatory measures that helped companies in the health and biosciences sector bring urgently-needed health products, including medical supplies for COVID-19, to the market. These temporary measures have provided an opportunity to pilot many of the Health and Biosciences Roadmap’s more agile regulatory solutions, such as the use of terms and conditions. Health Canada will build on this experience to inform policy and regulatory development as it continues to implement Roadmap commitments – examples include modernizing regulations for clinical trial and licensing, as well as the Medical Device Establishment Licensing Framework and modernizing compliance and enforcement oversight for drugs.
Health Canada remained committed to its Roadmap initiatives. Key achievements in 2020-21 included:
- Supporting the implementation of new inspector authorities under the Food and Drugs Act, and a shift in the compliance and enforcement approach, as outlined in the 2018 Compliance and Enforcement Policy for Health Products (POL-0001);
- Finalizing regulations allowing the sale of human milk fortifiers that assist vulnerable infants who require these products;
- Consulting with stakeholders on a proposed policy approach on supplemented foods;
- Advancing policy work to modernize food regulations on official methods, microbiological criteria and food additives;
- Continuing to develop a regulatory framework for clinical trials of drugs, medical devices, natural health products and foods for a special dietary purpose;
- Publishing proposed amendments to Food and Drug Regulations: Export and Transhipment of Drugs in Canada Gazette, Part I. The amendments are expected to appear in Canada Gazette, Part II in Fall 2021;
- Publication of the 2021 Miscellaneous Amendments Regulations on March 31, 2021, which helped to further clarify Health Canada regulatory frameworks.
Departmental Result 3: Canadians have access to safe, effective and quality health products
In 2020-21, the Department continued to invest in meeting the needs of the health care system and in ensuring that Canadians had timely access to safe, effective and quality health products – including prescription and non-prescription drugs, biologic and radiopharmaceutical drugs, natural health products, and medical devices.
What’s new?
In 2020-21, Health Canada launched a Pediatric Drug Action Plan to address the many challenges affecting access to safe and effective health products for children and youth in Canada. Moving forward, Health Canada intends to work with its national and international partners to:
- Increase the development of essential pediatric medicines and formulations;
- Improve access to pediatric medicines and formulations;
- Provide more extensive information to Canadians.
The pandemic created an unprecedented demand on Canada's health care system and an urgent need for Health Canada to facilitate access to COVID-related health products without compromising safety, efficacy and quality standards. As part of the GOC’s broad pandemic response, the Department introduced temporary emergency measures that helped to: expedite the regulatory review of COVID-19 related health products; conduct post-market safety surveillance; stabilize the supply of critical COVID-19 drugs and manage drug and medical device shortages; as well as adapt compliance and enforcement approaches in response to pandemic restrictions.
In addition, Health Canada made progress in relation to the following priorities, detailed further below: promoting timely access to other health products and modernizing processes; management of prescription drugs (including opioids); applying real-world evidence to support regulatory decision-making; strengthening regulatory oversight; modernizing compliance and enforcement; combatting antimicrobial resistance (AMR); fostering international collaboration and coordination; as well as promoting access to new and emerging technologies.
Facilitating access to COVID-related health products, including vaccines, and conducting post-market safety surveillance
At the onset of the pandemic, there was an urgent need for safe and effective health products that would help limit the spread of the novel coronavirus. Health Canada initiated an agile regulatory response to this emergency situation, prioritizing and expediting the regulatory review of COVID-related products without compromising standards for safety, efficacy and quality.
The effort involved an unprecedented level of collaboration and engagement with international and F/P/T regulatory partners, industry and health professionals, to get COVID-19 products to market on a timely basis. The Department quickly engaged with Canadian and international product developers to track and monitor potential COVID-19 solutions, connect them with other government partners to address challenges, share information on temporary COVID-measures, and provide early regulatory advice. It worked with manufacturers of medical devices, vaccines and treatments to ensure they coordinated their data submissions with those of other major international regulators, enabling authorization of those products in line with other jurisdictions.
Significant collaboration with international counterparts allowed Health Canada to align strategies and guidance and share information and expertise to support the review and post-market monitoring of COVID-19 health products. These combined efforts played an important role in supporting Canada’s effective regulatory response and the timely access to urgently needed health products.
The interim orders introduced by the Department allowed drug establishment licences to be issued in relation to COVID-19 drugs in a more agile manner, taking into consideration urgent public health needs. This balanced the benefits of greater flexibility, such as the modification of certain good manufacturing practices requirements, while still protecting the health and safety of Canadians who would use these COVID-19 drugs.
The interim orders also allowed the Minister to impose or amend terms and conditions in relation to a COVID-19 drug submission, authorization, or drug establishment licence. For example restricting the use of the product to certain populations or requiring companies to provide additional information once the product was on the market.
Additionally, Health Canada supported PHAC and PSPC in negotiating 8 vaccine agreements to build a diverse portfolio of COVID-19 vaccine platforms, guided by scientific data and expert advice from the Vaccine Task Force. This required developing a rigorous decision-making framework that guided how vaccine funding would be managed and accessed. Similar support was also provided to PHAC and PSPC to procure several promising therapies for the treatment of COVID-19.
In an effort to ensure future supply, the Department also developed a strategy to fortify and accelerate Canada's domestic biomanufacturing sector in collaboration with ISED, NRC and PHAC. It also supported PHAC in facilitating Canada's participation in the COVAX Facility, a global procurement mechanism that helps secure access to COVID-19 vaccines for domestic use by member countries. COVAX also facilitates vaccine donations around the world through a process developed with the support of Canadian funding. Canada's membership in COVAX helped to further diversify Canada's vaccine portfolio.
Special Access Requests
The Special Access Program allows health care professionals to request, in emergency situations, medical devices and drugs that are not yet authorized for use in Canada. The program was heavily relied upon during the pandemic, managing over 100 significant COVID-related inquiries in 2020.
Health Canada received a total of 76 requests from practitioners related to medical devices, and authorized 43 of these. Products included hand-held scanners to facilitate screening of COVID-19 patients, and ventilators that were in short supply early in the pandemic. The Department also approved 15 special access requests for remdesivir to treat COVID-19 prior to its market authorization in July 2020. In addition, the Program facilitated the supply of a number of drugs and medical devices that were in critical need after delays were created by the pandemic (for example, in transport).
Authorizing clinical trials
Health Canada recognized early on the impact of the global disruption on healthcare systems. Nimble approaches were required to avoid contracting the virus through visits at trial sites, and to enable remote consent and visits, telemedicine, different ways of dispensing and administering trial drugs, and the decentralization of some clinical trials. The Department provided extensive guidance and flexibility regarding regulatory requirements, as well as amended hundreds of existing clinical trial applications to accommodate the impacts of the pandemic.
To support rapid investigation of promising COVID-19 therapies, Health Canada prioritized and expedited the reviews of clinical trial applications for these products, reviewing them within 15 days instead of the standard target of 30 days, without compromising on health and safety. The interim orders also helped reduce the administrative burden on sponsors conducting COVID-19 trials while maintaining patient safety, and provided greater flexibility regarding the types of health care professionals able to conduct trials.
As of March 31, 2021, Health Canada had authorized 89 clinical trials for potential COVID-19 treatments and vaccines, including 24 under the interim orders. It also authorized 11 clinical trials for COVID-19 medical devices, such as those that apply artificial intelligence for the early detection of COVID-19 in the lung, and for touch-free vital signs assessment.
Health Canada hosted 11 compliance promotion events with industry to advise them on clinical trial procedures during the pandemic, while still adhering to safety requirements. The Department conducted 14 inspections related to COVID-19 clinical trials, all remotely.
Drug and vaccine authorizations and establishment licences
In July 2020, Health Canada authorized the use of remdesivir as the first treatment for COVID-19 under the Food and Drug Regulations, prior to the introduction of an interim order regulating the importation, selling and advertising of COVID-19 drugs during the pandemic.
The interim order (introduced in September 2020) was critical in expediting the authorization of COVID-19 treatments and vaccines. This measure allowed Health Canada to review data from manufacturers as it became available instead of waiting for it to be provided all at once. This, along with additional surge capacity required for the emergency response, significantly reduced the amount of time required to get COVID-19 drugs and vaccines approved. For example, while a typical vaccine review could take an average of 397 days, the average interval in 2020-21 for COVID-19 vaccines was 82 days. This was accomplished under exceptional circumstances to meet the needs of a global public health crisis.
The order waived the fees for applications and allowed the Department to authorize drugs and vaccines with terms and conditions, such as requiring companies to provide more frequent safety updates or additional information once the product was on the market. This provided Health Canada with more agility to regulate the products across their lifecycle, from authorization and on through their sale and use.
By March 31, 2021, the Department had received 13 COVID-related submissions under the interim order – including 7 treatments and 6 vaccines – and authorized 5 of these vaccines (Pfizer-BioNTech, Moderna, AstraZeneca, Covishield, and Janssen) and 1 treatment (bamlanivimab). The others remain under review. All vaccines have been approved with terms and conditions and risk management plans to which the manufacturers must adhere, thereby assisting the Department in detecting and managing emerging safety concerns and in confirming the longer-term efficacy and safety of the vaccines.
In March 2021, Health Canada developed and published new regulations that replaced the Interim Order. This provided a sustainable pathway for the current portfolio of COVID-related drugs while retaining some of the efficiencies and flexibilities to continue expediting approval of new products without compromising evidence standards.
To detect emerging safety concerns after product approval, the Department undertook proactive post-market surveillance of COVID-19 therapeutic products, including vaccines, pharmaceutical treatments and biologic therapies. These activities included conducting environmental scans, reviewing monthly summary safety reports, requesting additional safety information from manufacturers, monitoring and assessing adverse reaction reports, and detecting emerging safety issues of products. Health Canada completed assessments of emerging safety concerns for COVID-19 products and took immediate action to address these, including: imposing new terms and conditions; updating product safety information; revising risk management plans; issuing risk communications; and updating product labelling to increase awareness among the public and health professionals.
The Department also approved 43 proposals to import critical drugs to alleviate the most critical pandemic-related drug shortages (known as ‘Tier 3’) via the interim orders.
In addition, Health Canada continued to expedite the issuing of drug establishment licences to ensure supply chains involved in the manufacturing, importation and distribution of drugs and other critical medicines could continue to supply the Canadian market. The Department issued more than 2,200 drug establishment licences, over 170 of which were directly related to COVID-19 or medically-necessary drugs.
Medical device authorizations and establishment licences
In the pandemic’s earliest days, there was no way to detect and prevent the spread of the novel coronavirus and the demand for medical devices like diagnostic tests, PPE and ventilators was significant and urgent. Through the interim orders, Health Canada was able to expedite the review of these urgently needed devices.
As of March 31, 2021, the Department had received through interim orders more than 3,600 applications for COVID-related medical devices, and authorized 650 of these. Of the total authorizations issued, 366 were for PPE, 63 for test kits, 55 for thermometers, 33 for ventilators and 133 for other devices. Over 250 different medical devices were also authorized for importation on an exceptional basis.
The Department issued medical device establishment licences within 15 days of application (vs. the pre-pandemic standard of 120 days), to ensure the supply of needed medical devices related to COVID-19 response efforts. Over 2020-21, Health Canada expedited more than 3,000 COVID-related medical device establishment licences, in addition to the pre-pandemic number of approximately 2,800 licence holders.
To detect emerging safety concerns, Health Canada undertook proactive post-market surveillance of COVID-related medical devices, including test kits and devices for respiratory protection. These activities included: conducting environmental scans; leveraging international collaborations; requiring risk management plans to monitor for the impact of the variants on the performance of the COVID testing kits; and monitoring adverse incident reports.
The Department completed assessments of emerging safety concerns for COVID-related medical devices, such as respirators and test kits, and took immediate action to address these, including revising the labelling of medical devices and issuing notices to increase awareness among health professionals. Health Canada also prioritized the entry and processing of post-market medical device incident reports involving devices authorized under the interim orders.
Throughout the pandemic, Health Canada proactively engaged with COVID-related device manufacturers and published guidance on several topics, including serology tests, ventilators, swabs, and PPE. The Department also worked with F/P/Ts and industry to address the increased plastic waste generated by the use of PPE. In collaboration with PHAC, Health Canada issued public messaging regarding reusable PPE and proper disposal of single-use PPE. Additionally, the Department collaborated with industry, NRC, and ISED to launch two challenges through the Innovative Solutions Canada (ISC) program to make PPE more compostable and recyclable.
Sanitizers, disinfectant and cleaning products
The pandemic dramatically increased the demand for sanitizers, disinfectants and cleaning products – most notably in the early days, demand was such that Canadians had difficulty finding these products on store shelves. Health Canada swiftly introduced interim measures and guidance to help mobilize a wide range of companies stepping up to meet the need for these products, including ones that did not typically make them.
From the onset of the pandemic to end of March 2021, Health Canada authorized more than 4,500 new hand sanitizer products, some 270 new disinfectants, and close to 2,050 new sites applying the interim expedited service standards for over-the-counter drugs and natural health products. The Department also developed an online list of hard-surface disinfectants effective against the virus (more than 600 products as of March 31, 2021).
What’s New?
Health Canada worked with Canadian Poison and Injury Prevention Centres through the Canadian Surveillance System for Poison Information (CSSPI) to monitor and report safety issues associated with increased exposures to cleaning products used to fight COVID-19. This involved timely sharing of safety information and prompt action to protect the health of Canadians. For example, based on Poison Centre data showing that reported incidents related to hand sanitizer exposures increased by as much as 450% in 2020 over 2019 on a month-to-month basis, the Department developed the publication Alcohol-based hand sanitizers in beverage and food containers: Packaging and labelling requirements to reduce unintentional ingestion risk.
Health Canada applied the interim orders and other regulatory flexibilities to increase Canada’s domestic and international supply of hand sanitizers and disinfectants via both importation and domestic production, ensuring that individuals, businesses and health care settings could access appropriate infection prevention and control tools. To meet the increased demand, more than 675 hand sanitizers and surface disinfectants were accepted for exceptional importation under the interim measure.
The Department faced a significant increase in requests for regulatory guidance from manufacturers, distributors and importers of sanitizers and similar-type products (i.e., UV radiation-emitting devices, self-sanitizing coatings) who sought to bring their products to market in Canada. The Department responded in a timely manner and collaborated with international counterparts to align communications and regulatory requirements to the extent possible. This surge in demand for guidance resulted in additional applications for registration of these products. Health Canada received a total of 29 applications in 2020-21 (compared to an average of 5 in previous years) and registered 7 of these.
In April 2020, Health Canada implemented temporary emergency measures to facilitate access to hand sanitizers, disinfectants, and certain workplace and household cleaning products and soaps in the event of a supply shortage. It also took immediate action in response to incidents involving methanol poisoning and suspected use of low-grade ethanol, testing 40 samples of hand sanitizers and ethanol raw material to ensure they met standards for content and purity. The Department identified unauthorized and non-compliant products being marketed and issued 129 recalls.
Stabilizing the supply of critical COVID-19 drugs and managing drug and medical device shortages
Health Canada monitored the supply and demand of certain drugs and medical devices critical to the pandemic response and introduced interim orders to help mitigate and prevent shortages, where possible, by:
- Providing the Department with 2 new tools to address shortage situations – the authority to compel information; and the ability to add terms and conditions to an authorization or drug identification number;
- Introducing new measures to help prevent bulk importation programs from causing or worsening a drug shortage in Canada. Drugs intended for the Canadian market were prohibited from being distributed or sold out-of-country if this would cause or exacerbate a shortage. The Minister was authorized to require information from a Drug Establishment Licence holder or manufacturer, related to a shortage or the risk of a shortage;
- Re-introducing regulatory flexibilities to support Canada’s supply chain during the pandemic, allowing for a rapid response to critical supply issues should food or health product shortages occur. It was made mandatory to notify the Minister of shortages of medical devices considered critical during the pandemic, and the Minister was authorized to request from the seller of a medical device, information within their control about a shortage or potential shortage.
Health Canada ensured that Canadians had access to the medicines they need by:
- Working collaboratively with P/Ts, healthcare professionals and industry to designate 37 drugs as Tier 3 shortages in Canada (i.e., critical, national shortages of medications that would have a significant impact on the healthcare system and individuals if they were not available);
- Adding 60 drugs to Heath Canada’s list of designated drugs, making them eligible to be imported and sold under the interim orders introduced by the Department;
- Leading the establishment of Canada’s COVID-19 Critical Drug Reserve in collaboration with P/Ts, to ensure that the drugs hospitals use to treat COVID-19 patients are available when they are needed. Canada began procuring an initial 3-month supply of the 12 drugs that hospitals most rely upon to treat symptoms of COVID-19. By the end of 2020-21, 4 jurisdictions (NU, NWT, BC, SK) signed the Memorandum of Understanding to participate in the reserve and other jurisdictions are moving through approvals.
In cases where importing foreign drugs was necessary, the Department issued risk communications to healthcare professionals to highlight differences between domestic and foreign products thus supporting the safe use of these products in Canada.
With respect to medical device shortages, as of March 31, 2021, 504 mandatory reports had been received. Of these, 292 were confirmed to meet the definition of a shortage and were listed on a shortages webpage. Of those posted, 144 have since been resolved by the manufacturer.
Adapting compliance and enforcement approaches
The pandemic challenged Health Canada to deliver its compliance and enforcement activities differently, while creating conditions for its programs to be more risk-based, agile and innovative. The Department introduced interim orders to expedite processes, implemented e-licensing for drug establishments, and deployed new tools for secure electronic transfer of documents to facilitate inspections. It also adapted its approach by using virtual inspection tools and desk-based reviews to monitor compliance, and allowed industry stakeholders greater flexibility in meeting regulatory requirements where the risk was deemed to be low.
While Health Canada maintained the ability to undertake onsite inspections in situations of higher risk to human health or safety, the disruption to many planned onsite inspections paved the way to transform Health Canada’s inspection delivery model by:
- Pivoting compliance and enforcement programs to hybrid approaches (virtual/onsite inspections, where necessary);
- Experimenting with virtual inspection tools, conducting online inspections for non-compliance (e.g., vaping product promotion regulations on social media), virtual compliance promotion activities, and remote sampling;
- Re-assessing risk-based decision-making models.
Due to the pandemic, many companies mobilized their services to provide safe and effective medical devices to frontline workers and Canadians. As a result, the Department enhanced its web presence (bulletins, recall postings, public advisories), and hosted virtual sessions with key stakeholders to promote compliance with regulations.
Health Canada began to assess compliance of establishment licenses for both medical devices and drugs through remote and hybrid models at the beginning of the pandemic and continued this practice throughout 2020-21. The compliance program asked more than 2,500 establishments to complete a screening questionnaire and fully assessed 1,145 of these. Remote and hybrid assessments focused on critical requirements to ensure a responsive, safe and secure supply chain for drugs and medical devices in Canada. The Department also implemented a more assertive approach, as warranted, to address non-compliant drugs and medical devices via stop sale letters and recalls (e.g., relabeling).
Health Canada continued onsite inspections at international mail centres, air cargo ports and land border crossings across the country in order to address the significant volume of shipments in Canada Border Services Agency (CBSA) and Canada Post facilities. Border admissibility determinations increased by 26% compared to 2019-20, due to an influx of COVID-related health product shipments (including PPE and test kits). The Department’s Border Centres worked closely with the CBSA to address this increased volume and focused on high risk shipments and ensuring that critical items were processed as expeditiously as possible. By meeting the increased demand, Health Canada was able to support Canadians in accessing critical COVID-19 health products throughout the pandemic.
Did you know?
In 2020-21, restricted air travel and border crossing due to COVID-19 brought additional challenges to addressing drug shortages. For example, a potential shortage of medical leeches used to treat surgical complications was averted only after close collaboration between multiple agencies. Health Canada, Global Affairs Canada, the Canadian Embassy in France and the Canadian Border Services Agency worked together to ensure leeches could continue to be imported from France and that Canadian hospitals could rely on a steady supply.
Medicinal leeches are used by surgeons after re-attachment surgery (such as finger re-attachment) and applied to skin grafts to improve blood flow. They are regulated by Health Canada as drugs under the Food and Drugs Act and Regulations.
Further to the above, Health Canada received additional funding in the Economic and Fiscal Snapshot 2020 to strengthen its approach to preventing and alleviating drug shortages. The Department created a new Drug Supply Strategies division dedicated to managing drug shortages, collaborating with supply chain partners (including P/Ts) and bolstering mitigation activities. It also increased its capacity to manage medical device shortages.
Promoting timely access to other health products and modernizing processes
In addition to responding to the pandemic in 2020-21, Health Canada continued to provide Canadians with timely access to safe health products by reviewing the safety, efficacy and quality of pharmaceutical and biologic drugs, medical devices and natural health products.
What’s new?
Nitrosamines are compounds that can form in certain drugs during manufacturing. Some nitrosamines may increase the risk of cancer if people are exposed to them over long periods of time. In 2020-21, Health Canada launched a web page to inform Canadians about nitrosamines.
The Department also held a webinar to share information and answer questions about the regulatory requirements related to nitrosamines. Over 770 stakeholders attended, including representatives from the P/Ts, industry, and international regulators.
The Department approved 89 new pharmaceutical drugs for human use, of which 24 were drugs containing new active substances not previously approved in Canada, as well as 9 new veterinary drugs for use in companion and food-producing animals. It also approved 224 new generic drugs for human use and 10 new veterinary generic drugs. As well, Health Canada approved 21 new biologic drugs for human use, of which 11 were drugs containing new active substances not previously approved in Canada. Furthermore, market authorizations were issued for 16 biosimilar drugs (ones demonstrated to be highly similar to a biologic drug previously authorized in Canada). While faced with increased workload due to COVID, the Department's approval rates for health products remain comparable to those of previous years.
Health Canada also approved 428 requests for significant changes (referred to as ‘supplements’) to pharmaceutical drugs for human use already on the market (not including generic drugs) and 215 supplements for significant changes to biologic drugs for human use already on the market. These supplements include changes such as new uses, new safety updates to the labelling material, new manufacturing methods, and new dosing recommendations for existing drugs. In addition, a total of 10,868 natural health products including hand sanitizers were approved.
The Department also approved 1,307 new Class II, III, or IV medical devices, 5,820 medical device establishment licences, as well as 1,900 requests for significant changes to medical devices already on the market, such as design changes and new manufacturing methods. A 2020 joint Audit and Evaluation report found that the Medical Devices Program made progress in balancing safety with access to medical devices. Among its efforts, the Program implemented various measures to improve industry’s understanding of the regulatory process and better aligned its requirements with those of other countries, in an effort to improve access to devices in Canada.
Health Canada assessed biocides (e.g., disinfectants and sanitizers) for their safety, efficacy and quality under the Food and Drug Regulations and the Pest Control Products Regulations, continued to leverage foreign authorizations during the COVID-19 pandemic, and advanced work towards developing a framework for biocides in 2021-22.
In order to modernize the way Health Canada provides timely access to health products, including those not readily available, the Department continued to align its drug submission review processes in 2020-21 with those of Health Technology Assessment (HTA) organizations, as part of the Regulatory Review of Drugs and Devices initiative. As of March 2021, over 50 reviews had been aligned between CADTH, l’Institut national d’excellence en santé et en services sociaux, and Health Canada. Such alignment often leads to shorter intervals between Health Canada authorizations and HTA reimbursement recommendations, ultimately contributing to faster access to drugs for Canadians.
The Department published in the Canada Gazette, Part II in October 2020, the final Regulations Amending Certain Regulations made Under the Food and Drugs Act, (Sale of a New Drug for Emergency Treatment). Introduced in support of the Special Access Program for human drugs and the Emergency Drug Release Program for veterinary drugs, these changes improved the processes used by health care providers and reduced the administrative burden for requests to access drugs that are not yet authorized for sale in Canada, for COVID-19 and other medical emergencies.
First proposed in 2019, Health Canada continued work in 2020-21 on a new regulatory framework to enable public health officials responsible for public and military health emergencies to request access to drugs that are unavailable in Canada. Since publication of the proposed Regulations Amending Certain Regulations Concerning the Sale of Drugs (Public or Canadian Armed Forces Health Emergencies) and throughout 2020, the Department reflected comments received from the Canada Gazette Part I pre-publication consultation on the regulatory proposal and continued to engage with PHAC and the Canadian Armed Forces on implementation.
Management of prescription drugs (including opioids)
In 2020-21, Health Canada pursued its efforts to mitigate the risks associated with prescription opioids by continuing to update the Canadian labelling (product monograph and prescribing information) for prescription opioid products. Updates included warnings related to: serotonin toxicity; hyperalgesia; sleep apnea; switching between methadone products; as well as hallucinations related to tramadol.
Health Canada developed and provided increased monitoring, education and communication measures for opioid prescribers, pharmacists, other healthcare professionals, and patients. In March 2021, the Department published draft guidance, Electronic media in prescription drug labelling, to clarify Health Canada’s expectations for distributing information about a prescription product using an electronic platform linked to that product’s label.
On March 31, 2021, final regulations scheduling the opioid pain reliever tramadol as a controlled substance and narcotic were published in the Canada Gazette, Part II. These regulations will provide additional safeguards around this drug to help prevent problematic use and other harms while also protecting patient access. The regulations will come into force on March 31, 2022 giving stakeholders time to comply with the new requirements.
Over the course of the fiscal year, Health Canada continued to work with all stakeholders to achieve an optimal balance between regulatory oversight and patient care and access. The Canadian Pain Task Force delivered its second report, summarizing previously-held national consultations, as well as its final report – An Action Plan for Pain in Canada – which outlines steps to ensure people with pain are recognized and supported and that pain is understood, prevented and effectively treated throughout Canada.
Applying real-world evidence (RWE) to support regulatory decision-making
RWE is evidence about the use, and potential benefits or risks, of a medical product that is gathered after a product is on the market. In 2020-21, Health Canada collaborated with international regulators to use RWE during the COVID-19 pandemic. The Department co-chaired the International Coalition of Medicines Regulatory Authorities COVID-19 RWE and Observational Studies Working Group, led the Building International Cohorts sub-working group, and participated in the Pregnancy and Vaccine Surveillance sub-working group. Efforts to date have resulted in the launch of 3 Drug Safety and Effectiveness Network (DSEN) studies, with additional studies forthcoming.
Health Canada continued to leverage RWE through the DSEN at record levels, with 14 queries submitted to the network in 2020-21, compared to 9 in 2019-20. Completed queries included 1 that contributed to the Department’s understanding of ongoing COVID-19-related research in Canada, and 3 related to pain management that supported the activities of the Canadian Pain Task Force. New evidence generated via DSEN helps Health Canada and other stakeholders to assess the risks and benefits of health products and supports decision-making on public reimbursement, as well as the safe and optimal prescribing and use of drugs within the Canadian health care system.
What’s new?
In 2020-21, the Drug Core Action Team established its Terms of Reference, and formed working groups to launch demonstration projects addressing oncology products, non-oncology products, and guidance document development. These working groups are co-chaired by representatives from Health Canada and CADTH, and will help inform the use of RWE within these 3 areas.
The Department continued to participate on the Drug Core Action Team along with CADTH, l'Institut national d'excellence en santé et en services sociaux, CIHI, and industry. This advisory body guides and supports a pan-Canadian approach to applying RWE and identifies where RWE can add value to regulatory decision-makers throughout a drug’s lifecycle.
Strengthening regulatory oversight
In 2020-21, Health Canada advanced its efforts to modernize the regulatory framework for self-care products (natural health products and non-prescription drugs). The Department developed a proposal to improve natural health product labelling, including homeopathic labelling, and sought feedback from consumer and patient safety groups, health professionals, academia, and industry associations with the goal of consulting more broadly in 2021. In addition, Health Canada launched the natural health product good manufacturing process (GMP) Inspection Pilot Program, which focuses on verifying compliance at higher risk sites.
The Department also began building the foundation to modernize and strengthen the environmental risk assessment of drugs. This included: work towards amending the Food and Drugs Act to give the Minister of Health the authority to impose measures to protect the environment from harmful risks associated with drugs and developing a regulatory regime that would require industry to provide environmental data for new drugs at different stages of their development.
If adopted, this approach will better align with the U.S. FDA and the European Medicines Agency and the environmental impact of drugs would no longer fall under the purview of the Canadian Environmental Protection Act, 1999 and the New Substances Notification Regulations (Chemicals and Polymers) and (Organisms).
Health Canada also began issuing drug establishment licences and other compliance-related certificates electronically, which supported larger greening government initiatives while aligning with pandemic public health guidance. The Department also implemented a risk-based approach to on-site GMP compliance visits, balancing regulatory requirements with emergency public health directives.
In April 2020, the Regulations Amending the Food and Drug Regulations, Completed Product Testing, were approved to support the implementation of the Canada-United States-Mexico Agreement. This resulted in removing burdensome regulatory re-testing requirements for certain low-risk products imported from the U.S. and to permit direct shipping to retailers, wholesalers or distributors.
Over the course of 2020-21, Health Canada issued more than 3,000 COVID-related medical device establishment licenses on an expedited basis, in addition to approximately 2,800 non COVID licenses. The Department will consider automating aspects of the licencing process in the future to continue improving oversight of the Canadian supply chain and ensure the quality and safety of medical devices entering the Canadian market.
In 2020-21, the Department supported implementation of new regulations for mandatory reporting by hospitals that came into force in December 2019. Hospitals are now required to report to all medical device incidents and serious adverse drug reactions to Health Canada within 30 days of being documented within the hospital. The Department developed a guidance document, education modules and promotional materials to support hospitals. As such, the number of reports submitted increased dramatically in fiscal year 2020-21 compared to the previous fiscal year – by 356% for incident reports related to medical devices and by 103% for adverse drug reaction reports.
Key 2020-21 accomplishments relative to Health Canada's Action Plan on Medical Devices included:
- Publishing 328 Regulatory Decision Summaries for amendments to Class III and IV medical device licences in its Drug and Health Product Register. The new information allows patients with implants to more easily monitor any relevant safety amendments or warnings.
- Meetings of the Scientific Advisory Committee on Medical Devices Used in the Cardiovascular System to review and discuss paclitaxel-coated devices and the use of medical devices in the treatment of coronary lesions and valvular heart disease.
- Meetings of the Scientific Advisory Committee on Health Products for Women with patient-focused discussions on medical devices, including surgical mesh and breast implants.
- Finalizing draft guidance on Clinical Evidence Requirements with the goal of consulting the public in the summer of 2021.
- Publishing new regulations allowing the Department to act quickly on medical devices that may pose a serious health risk (Health Canada can now request research information from manufacturers; and manufacturers must notify Health Canada of any new warnings from abroad related to their medical devices). Health Canada led well-attended webinar sessions to inform industry of the new regulations.
- Developing draft regulations governing how independent researchers and medical professionals conduct clinical trials on medical devices, including a requirement to register clinical trials online and to provide information publicly about results.
- Ongoing site visits to medical device manufacturers to verify compliance and take appropriate enforcement action where high risk is identified.
- Responding to an increased number of medical device incident reports submitted by hospitals.
Modernizing compliance and enforcement
In addition to responding to the pandemic, Health Canada committed to becoming more agile, assertive, consistent, innovative, proactive, and risk-based as part of its Compliance and Enforcement (C&E) Modernization and Transformation priority. To guide this transformation, the Department initiated work in 6 critical areas: regulatory framework development; quality management systems; training for C&E designation; IT systems and tools; data analytics; and risk management tools. In 2020-21, Health Canada:
- Began extending the use of quality management systems across more types of regulated products;
- Improved risk management and data management tools to more effectively target resources to address instances of highest risk;
- Continued to develop Occupational Health and Safety tools and C&E training programs to better equip inspectors;
- Developed Designation Directives and National Certification Standards for inspector designation under the Assisted Human Reproduction Act, the Cannabis Act, and the Controlled Drugs and Substances Act.
As with many other regulators, public health measures and restrictions introduced across the country due to the COVID-19 pandemic led to Health Canada postponing onsite inspections. While maintaining the option to undertake onsite activities in situations of higher risk to human health or safety, the Department had to adapt quickly, be flexible, and find innovative solutions to meet its mandate.
Health Canada took advantage of this opportunity to accelerate its modernization and transformation efforts, expanding its C&E oversight methods to encompass compliance verifications, document reviews, and applying technologies such as Vidcruiter, epost Connect, and video-conferencing. The Department will refine many of these measures and add them to the C&E toolkit moving forward.
Combatting antimicrobial resistance (AMR)
Recognizing that AMR is an urgent issue for the health of humans, animals, and the environment, Health Canada maintained its coordinated and collaborative One Health approach to addressing associated risks. The Department continued work to safeguard the use of available antimicrobial drugs for human use, raise awareness, promote innovation, and monitor and support the prudent use of antimicrobials in animals.
Specifically, the Department consulted on the Pathogens of Interest List, and published updates in March 2021 to ensure this list continues to capture emerging threats, such as fungal pathogens, requiring new therapeutic and/or diagnostic options in Canada.
As part of World Antimicrobial Awareness Week, Health Canada and other federal partners held a series of informative webinars featuring experts in human and animal health presenting on various aspects of antimicrobial resistance.
Did you know?
In 2020-21, Health Canada advanced its coordinated and collaborative “One Health” approach to combatting AMR across the human health, animal health, and agri-food sectors. This approach aligns with the Federal Government’s shared priorities outlined under Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action.
In the veterinary drug context, the Department published a risk-based approach to re-evaluating medically important antimicrobials being used in animals that may contribute to AMR in humans. It further outlined the first phase of this re-evaluation process, which will focus on updating labels of products with undefined or prolonged duration of use to strengthen their responsible use.
Health Canada and PHAC collaborated to publish and share the 2018 Veterinary Antimicrobial Sales data, which reflected the 2 first years of sales data submitted through the Veterinary Antimicrobial Sales Reporting system. The past year marked the third year of antimicrobial sales data collected under recent mandatory requirements for manufacturers, importers, and compounders. The data will help inform the Department’s ongoing antimicrobial surveillance, stewardship, and responsible use initiatives.
Health Canada continued to facilitate access to low-risk veterinary health products that enhance health and wellness in animals and reduce the need for routine use of antimicrobials. It also continued to collaborate with the CFIA on an interim pilot program to facilitate access to veterinary health products allowed for mixing into livestock feed, a pathway not previously accessible to livestock owners.
Fostering international collaboration and coordination
Health Canada continued to collaborate with international partners and participate on various international committees, driven in large part over 2020-21 by treatments and vaccines to combat COVID-19.
The Department co-chaired the COVID-19 working group under the International Coalition of Medicines Regulatory Authorities, supporting collaboration and alignment of scientific requirements for clinical trials and approvals of COVID-19 drugs and vaccines. It also participated in the COVID-19 Vaccine Pharmacovigilance Network of the International Coalition of Medicines Regulatory Authorities, which facilitated timely access to information about COVID-19 vaccines in other jurisdictions and supported Health Canada’s own assessments and actions.
For example, Health Canada exchanged knowledge with the European Medicines Agency (EMA) during the review of the first COVID-19 drug, Veklury (remdesivir), which was approved in Canada in July 2020. Thanks in part to this collaboration, the expedited review of this new drug submission took only 38 days (vs. a 300-day standard review time). The success of this work led to the new EMA OPEN Pilot Project that will allow enhanced review collaboration between the EMA and other international regulators (including Health Canada).
What’s new?
In 2020, Health Canada was elected to vice-chair the World Health Organization’s Paediatric Regulatory Network. This global network supports the international development, registration and pharmacovigilance of quality medical products for children and youth by facilitating communication, collaboration, training, and regulatory harmonization.
The Department played various leadership roles at the International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use, serving as the Vice Chair of the ICH Assembly and Chair of the ICH Financial Committee. It helped to develop internationally harmonized guidelines that aligned technical requirements for human drugs, including drafting and/or implementing some 39 ICH guidance documents.
Canada participated on the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products steering committee, which harmonizes technical requirements for veterinary product registration, and on Codex Alimentarius, which develops standards, guidelines and codes of practice that protect consumer health and promote fair practices in the food trade.
Health Canada continued its participation in Project Orbis. Now in its second year, the Project brings together regulators from multiple countries to review cancer drugs concurrently so that patients can receive earlier access to promising treatments. The Department collaborated with the U.S., Australia, Switzerland, Singapore, Brazil and the U.K. on the review and approval of 11 cancer drugs. Examples include Qinlock (ripretinib) for the treatment of adult patients with advanced gastrointestinal stromal tumour (GIST), and Inqovi (decitabine & cedazuridine) for the treatment of adult patients with myelodysplastic syndromes (MDS).
In 2020-21, Health Canada helped to foster international collaboration and coordination by:
- Continuing its partnership with the newly-expanded Australia-Canada-Singapore-Switzerland-U.K. (Access) consortium and published a collective statement pledging to work together to tackle COVID-19. In 2020-21, the consortium exchanged information on several COVID-19 vaccines, published joint guidance for vaccines targeting variants of concern, and approved 3 new drugs/indications, with 7 more currently under review.
- Maintaining international leadership with like-minded countries on drug shortages and working through the Pharmaceutical Inspection Cooperation Scheme and with Mutual Recognition Agreement partners to share information on drug/medicinal products GMP inspections.
- Working with the U.S. on several pilot projects, including the Medical Device Single Review program.
- Developing a Pediatric Work Plan, in collaboration with EMA, designed to promote international alignment in pediatric regulatory review.
- Maintaining its regulatory collaboration with the U.S. through the Regulatory Cooperation Council, conducting simultaneous reviews of veterinary drugs with the U.K., and reviewing products on a trilateral basis with Australia and New Zealand.
- Engaging international partners as part of the Transatlantic Task Force on Antimicrobial Resistance to increase information exchange and best practices to addressing the growing threat of AMR.
- Working with other federal partners to advance revisions to the Codex Code of Practice to Minimize and Contain Foodborne Antimicrobial Resistance.
Promoting access to new and emerging technologies
Scientific and technological advances are accelerating the pace of innovation in the healthcare system, leading to the development of innovative health products that use emerging technologies such as advanced artificial intelligence (AI) and machine learning (ML) algorithms, telerobotics, 3D printing and gene editing. Increasingly, health products are becoming personalised, developed at point of care, and manufactured, distributed, and used in significantly new and untraditional ways that simply cannot be regulated in our existing system.
In 2019, Health Canada introduced a new set of provisions to the Food and Drugs Act to regulate of advanced therapeutic products (ATP) – defined as drugs or devices that are innovative, complex, distinct, and not easily accommodated by the Department’s existing regulatory framework. This is a key component of Health Canada’s Regulatory Innovation Agenda, since it supports timely access for patients and optimizing safety and benefits while supporting innovation in the health and biosciences sector. The Department can now create tailored requirements to oversee the unique characteristics of emerging and complex technologies, while maintaining its rigorous standards for patient safety. This requires consulting those directly involved in developing and using these products, as well as with health system players such as international regulators and health technology assessors. In 2020-21, Health Canada continued to advance this work and consulted stakeholders and healthcare partners on potential advanced therapeutic product candidates and on the concept of a new ‘concierge service’ to support them in navigating the new approach.
Departmental Result 4: Canadians are protected from unsafe consumer and commercial products and substances
Helping Canadians lead healthier lives and providing protection from unsafe consumer and commercial products and substances remained an important focus of Health Canada’s work. Over the course of 2020-21, the Department’s efforts on this front concentrated on the following priorities, detailed further below: a comprehensive approach to substance use-related harms; regulating cannabis; managing the risks of chemicals in the home, the workplace and the environment; supporting the safety of consumer products and cosmetics; protecting Canadians from radiation; and strengthening pesticide regulation and communication.
A comprehensive approach to substance use-related harms
Substance use-related harms: through a COVID lens
Substance use-related harms in 2020-21 continued to cause devastating health and social effects on Canadians from every walk of life. For many people, and in many jurisdictions, the COVID-19 pandemic exacerbated these harms – evidence shows that substance-related hospitalizations and deaths increased in Canada during the pandemic compared to the same period in 2019.
In particular, the opioid overdose crisis continued to have the most acute impact on Canadian communities and families. Between January 2016 and December 2020, opioid overdoses claimed the lives of 21,174 Canadians, making it one of the most serious public health concerns in a generation.
As a result of considerable efforts among all levels of government, frontline workers, researchers, and advocates, opioid-related harms and deaths were actually trending downward at the end of 2019 and beginning of 2020. Tragically, in 2020-21, the pandemic exacerbated long-standing challenges regarding substance use and the overdose crisis with most jurisdictions reporting record high rates of overdose deaths and harms. Nationally, there were 3,351 opioid-related deaths between April and September 2020, an 82% increase compared to the same time period in 2019.
Likely contributing factors included increased feelings of isolation, stress and anxiety, and limited availability or accessibility of services for those who use drugs during the outbreak. In addition, the illegal drug supply in Canada, already contaminated with highly-potent fentanyl or fentanyl-like drugs, grew more unpredictable and toxic as supply chains were disrupted by COVID-related travel restrictions and border measures.
These same factors of isolation, stress and anxiety also played a significant role regarding ongoing harms related to problematic use of alcohol. Since the start of the COVID-19 pandemic in February 2020, Canadians reported increased consumption of alcohol to help them cope. Hospitalizations attributed to alcohol use increased by 5% in 2020 compared to 2019.
A comprehensive approach: prevention, treatment, harm reduction and enforcement
Health Canada took urgent action to address the opioid overdose crisis and other substance use-related harms using a public health-focused approach guided by the Canadian Drugs and Substances Strategy (CDSS). The Strategy encompasses 15 federal departments and agencies and outlines the GOC’s comprehensive, collaborative, compassionate, and evidence-based approach to problematic substance use, with the goal of minimizing harms on individuals, families and communities. The CDSS is based on the 4 pillars of prevention, treatment, harm reduction and enforcement, and covers a broad range of substances, including alcohol, cannabis, illegal drugs, and the problematic use of prescription drugs.
Working with its other federal partners under the CDSS, the Department continued to support efforts to: improve access to treatment and harm reduction services; strengthen enforcement to help reduce the toxic illegal drug supply; increase awareness and prevention efforts; and build the evidence base through federal investments in research and surveillance.
Health Canada also worked closely with other health partners to advance solutions to save lives and to help reverse the crisis: P/Ts, Indigenous leadership and communities, and a wide range of additional stakeholders, including people with lived and living experience, drug policy organizations and researchers, private sector organizations, and service providers.
In December 2020, for example, the Department launched the People with Living and Lived Experience Council – its mandate to provide advice and insights to inform federal responses to the opioid overdose crisis and substance use more broadly. The Ministers of Health and Labour also hosted two roundtables with union leaders and other stakeholders focused on substance use issues in relation to working-aged men employed in the trades who have been disproportionately affected by the overdose crisis.
Health Canada also invested in prevention and public health campaigns, shifting to a virtual platform to continue reaching broad and diverse audiences throughout the pandemic. This included: engaging youth and young adults on the facts of substance use; expanding public awareness around opioids and the harms of stigma; and supporting the implementation of opioid prescribing and treatment guidelines.
What’s new?
Health Canada continued to work closely with other federal departments and stakeholders to reduce substance use stigma. The Department collaborated on several initiatives in 2020-21, including: stigma reduction training for law enforcement; expanding public awareness through a multi-year campaign; and launching a virtual platform for the Know More Opioids tour, which engages with high school and post-secondary students.
In April 2020, the Department proactively exempted P/Ts from certain legislative requirements to ensure they had the tools needed to manage the compounding effects on their communities of the opioid overdose crisis and the COVID-19 pandemic. This allowed them to establish temporary overdose prevention sites within shelters or other temporary sites, as needed, to help people stay safe from overdose and respect physical distancing and self-isolation measures. This exemption also provided P/Ts with the flexibility to establish other harm reduction activities for drugs and other controlled substances. These exemptions were subsequently extended to September 2022.
In October 2020, Health Canada extended the subsection 56(1) class exemption from the Controlled Drugs and Substances Act that allowed practitioners to verbally prescribe, and authorizes pharmacists to prescribe, sell, or provide controlled substances in limited circumstances, or transfer prescriptions for controlled substances. The exemption also allowed individuals to deliver controlled substances to those in isolation.
The Department continued to build a strong evidence base through research and surveillance efforts, in order to better understand and adapt interventions to the ever-changing nature of the crisis. In 2020-21, the Health Portfolio: collected and published data on opioid-related deaths and harms; improved and used modelling that shows how the opioid overdose crisis may change subsequent to the COVID-19 pandemic; conducted rapid knowledge synthesis; and developed a range of guidance documents to support evidence-based interventions.
Health Canada engaged P/T counterparts through the Problematic Substance Use and Harms F/P/T committee, establishing working groups on: treatment and recovery; prevention; stigma; frontline community response capacity; and data and evidence. These efforts align with the committee’s work plan which includes developing practical tools and resources to help jurisdictions intervene at the community level.
The Department also worked closely with P/Ts to continue implementing the Emergency Treatment Fund, which provides $150 million to match P/T investments that, combined, will see more than $300 million directed towards improving access to treatment in the context of the opioid crisis. By the end of fiscal year, P/Ts had invested over 74% of the funding towards: reducing treatment wait times; increasing the number of treatment beds and clinics that provide rapid access to addiction medicine; improving access to culturally-appropriate care for Indigenous communities; expanding access to virtual supports; supporting providers through training opportunities; addressing methamphetamine use; as well as improving health systems and building community-level capacity.
These investments have contributed to: a significant reduction of wait times in 3 P/Ts (Nova Scotia, Newfoundland and Northwest Territories); new treatment beds in British Columbia, Manitoba and Ontario; On-the-Land Healing Camps in Nunavut; and 3 Rapid Access Addiction Medicine clinics in Saskatchewan. Funding also ensured the training of an additional 1,200 health care workers across the country, increasing the available pool of treatment providers and improving overall quality of care.
Unmanaged pain, and the trauma and complexity that often accompany it, is a primary driver of problematic substance use and a barrier to successful treatment. For that reason, Health Canada continued to support ($1 million for 2020-21) the Canadian Pain Task Force. In year two of its mandate, the Task Force conducted public online consultations and ran additional workshops, capturing the perspectives of over 2,000 people living with and affected by chronic pain across the country. These consultations, along with continued review of evidence and new economic costing analyses, formed the basis of the second report, released in October 2020. The Department adapted and accelerated the Task Force’s mandate to ensure delivery of their final report by March 31, 2021. An Action Plan for Pain in Canada makes recommendations to ensure that people with pain are recognized and supported and that pain itself is understood, prevented and effectively treated throughout Canada. The report’s public release followed in May 2021.
What’s new?
COVID-19 disrupted health care and services for people living with pain, and those who care for them. Online consultations revealed that Canadians living with chronic pain might be in need of additional supports. Accordingly, the Canadian Pain Task Force compiled and posted resources to support those living with pain through the pandemic.
In March 2021, the Minister of Health established an Expert Task Force on Substance Use to provide Health Canada with independent expert advice and recommendations on:
- The federal government's drug policy, as outlined in the CDSS, to further strengthen the public health approach to substance use;
- Potential alternatives to criminal penalties for the simple possession of controlled substances, with the goal of reducing the effects of criminal sanctions on people who use drugs, while maintaining support for community and public safety.
In 2020-21, Health Canada continued to scale up the most effective and evidence-based programs, such as increasing access to a safer supply of prescription opioids in order to better protect people with substance use disorder. Through SUAP, the Department committed almost $58 million towards 15 pilot projects designed to provide medications as safer alternatives to the contaminated illegal drug supply in Canada (these pilot projects are referred to by some stakeholders as “safer supply”).
These pilot projects will ensure communities across Canada have the tools and support they need to reduce the risk and harms for people at risk of overdose from the toxic illegal drug supply. Additionally, SUAP invested over $5 million to fund 9 projects that are exploring new approaches to addressing problematic methamphetamine use, building knowledge of effective interventions, and improving access to services.
What’s new?
In fall 2020, Health Canada conducted a Supervised Consumption Site Pandemic Survey to gather information on the impacts of the COVID-19 pandemic on the clients, staff, and operators of federally exempted supervised consumption and Urgent Public Health Need sites. The Department expanded the quarterly substance-related harms release on Public Health InfoBase beyond opioid poisoning-related deaths, hospitalizations, and EMS visits to include data on stimulants. This data helped inform understanding of the multiple-substance nature of the overdose crisis.
While both domestic and international studies support the effectiveness of using a “safer supply” in treatment programs, there is less evidence available regarding its application as a harm reduction measure. Health Canada supported a preliminary assessment of the above safer supply pilot projects to capture early findings and lessons learned, which will be completed in 2021-22. This will complement a multi-year evaluation of 5 other long-term pilot projects funded by the CIHR.
Also through SUAP, the Department provided $20 million for the distribution of naloxone kits and opioid overdose response training to support communities particularly hard hit by the opioid overdose crisis. These include individuals living in rural and remote areas, Indigenous Peoples, Northern residents, people experiencing homelessness, youth in communities at increased risk of opioid-related harms, and working-aged men.
Beyond the opioid crisis, SUAP funding also supported: an update of Canada’s Low-Risk Drinking Guidelines; the development of treatment guidelines; and projects to test and implement Managed Alcohol Programs (MAPs). MAPs offer a harm reduction approach to people who have severe alcohol use disorder and who may be experiencing homelessness at the same time. MAPs provide small amounts of alcohol to clients at regular intervals throughout the day and are often combined with or offered within housing programs.
The Department invited Canadians to provide comments on a proposal to develop new regulations under the Controlled Drugs and Substances Act for supervised consumption sites and services. It also held virtual engagement sessions with targeted stakeholders directly affected by the potential new regulations. The consultation feedback is serving to inform next steps with respect to this proposal.
In addition, Health Canada launched a portal to capture electronic submissions of loss and theft reports for controlled substances, to improve monitoring capabilities of the legal domestic drug supply chain. Following the launch, the Department followed up on 164 suspicious transactions and thefts with various establishments such as community pharmacies, hospital pharmacies, dental surgeons and veterinary clinics.
Also this fiscal year, Health Canada supported public health organizations with drug analysis services during the pandemic. The Department continued to notify Canadian law enforcement agencies and public health partners about newly identified drugs, while maintaining such harm reduction initiatives as drug checking projects with F/P/Ts and analysis of drug samples for public health purposes. In addition, it completed the first cycle of data collection for the Canadian Postsecondary Alcohol and Drugs Survey.
Regulating cannabis
Health Canada continued to support the effective implementation and objective of the Cannabis Act to protect the public health and public safety of Canadians – with a particular focus on restricting youth access to cannabis and reducing illegal activities surrounding cannabis.
The Department closely monitored usage rates of cannabis and other substances. Evidence across multiple Canadian surveys suggests that the prevalence of cannabis use among youth has remained stable. COVID-19 has influenced usage patterns among adults across various substances, including cannabis, which may confound conclusions regarding the impact of cannabis legalization and regulation. Health Canada will continue its monitoring activities to provide additional insight into the effects of the pandemic on substance use and beyond.
Over the course of 2020-21, the Department demonstrated significant progress in supporting the establishment of a tightly regulated cannabis industry capable of delivering a sufficient supply of quality-controlled products required to displace the illegal market.
The federally-regulated production of cannabis continued to expand and diversify over the past year. From April 2020 to March 2021, Health Canada granted an additional 302 licences for cultivation, processing, and/or sale of cannabis for medical purposes and 596 licences for research, analytical testing, cannabis drug and/or industrial hemp. It implemented new and updated non-binding service standards for the screening and review of applications for licences, permits and personal registrations under the Cannabis Act and its Regulations. Implementation of public reporting on the percentage of licence applications reviewed within published service standards was delayed in 2020 due to the pandemic. These results will be published in fiscal year 2021-22.
Based on results from Health Canada’s annual Canadian Cannabis Survey, 80% of people who use cannabis reported buying some of their products from legal sources. This result is reinforced by Statistics Canada’s detailed consumption survey, which found that the share of legal spending on cannabis increased from 44.5% in the first quarter of 2020 to 58.3% in the first quarter of 2021, a level of illegal market displacement consistent with the experiences of other jurisdictions with regulated access to cannabis.
What’s new?
In late 2020, Health Canada established the Science Advisory Committee on Health Products Containing Cannabis. The Committee’s mandate is to provide the Department with independent scientific and clinical advice and recommendations regarding appropriate safety, efficacy, and quality standards for health products containing cannabis, including the conditions under which certain products might be suitable for use without practitioner oversight. Their input will contribute towards the regulatory evolution of these products.
The Department also continued to administer the regulatory framework that provides reasonable access to cannabis for medical purposes. The number of active registrations for personal and designated production was 39,525 as of March 31, 2021 compared to 35,218 as of March 31, 2020, and the number of registered clients accessing cannabis for medical purposes through licence holders was 292,399 in March 2021 compared to 320,340 for the previous fiscal year.
In addition, Health Canada provided scientific advice, conducted health risk assessments and monitored, assessed, and communicated on adverse reactions, contributing to regulatory amendments, compliance and enforcement activities and public education. In 2020-21, the Department conducted 4 health risk assessments and over 400 risk-related consultations/requests, and published the Cannabis Adverse Reaction Reporting Guide to inform federal licence holders. It screened 176 unique cases of adverse reactionFootnote 7 associated with cannabis products, 33 of which required hospitalization.
To help monitor compliance with regulatory requirements, the Cannabis Regulations require federal licence holders to provide advance notice of their intent to introduce new cannabis products into the market. In 2020-21, Health Canada reviewed a total of 23,905 notices of new cannabis products and followed up on 3.5% of them due to potential non-compliance with the regulations or potential safety concerns.
The Department also conducted 208 inspections and 185 compliance verifications with an overall industry compliance rate of 93%. It undertook 134 compliance promotion sessions and tested 216 product samples (including vaping and edibles) to monitor and enforce compliance with legislative and regulatory requirements. In addition, the Department revoked 1 licence for compliance reasons, suspended another licence, and reinstated 2 licences.
From a compliance and enforcement perspective, Health Canada is responsible for regulatory oversight of the legal industry, while law enforcement agencies are responsible for enforcing the criminal prohibitions associated with cannabis (such as the illegal production, distribution or sale or cannabis). The Department continued to engage with applicants, associations, industry representatives and regulated parties to increase knowledge of, and compliance with, regulatory requirements. This occurred through: compliance promotion calls; virtual licence holder school sessions; engagement with licence holders on recalls and pesticide reporting requirements; and the delivery of a national virtual series to support law enforcement and P/T compliance regulators. The Department referred 178 cases to law enforcement agencies in fiscal year 2020-21 for review and possible enforcement action.
Did you know?
Health Canada supports federal, P/T and municipal law enforcement representatives by providing a dedicated, ‘24/7’ service to confirm, when necessary, that specific individuals are authorized to possess or produce a limited amount of cannabis for medical purposes. The Cannabis Regulations authorize Health Canada to share information that is protected under the Privacy Act in the context of an active law enforcement investigation.
To disrupt illegal cannabis sales online, Health Canada and Public Safety Canada engaged with financial institutions, companies that host payment platforms, and those that regulate the financial industry to explore ways to disrupt online illicit cannabis transactions. The two departments also developed public awareness tools on the risks of purchasing illicit cannabis and how to differentiate between legal and illegal cannabis retail websites.
As a means of continuous improvement of the regulatory framework for cannabis, the Department sought feedback from Canadians on a number of topics, including: a proposal to provide the cannabis industry with financial relief amid the COVID-19 pandemic; a proposal to regulate non-therapeutic research under the Cannabis Act; the manner that public possession limits are calculated for different types of cannabis products; packaging and labelling requirements; COVID-19 measures; micro class licensing; and draft guidance related to the personal and designated production of cannabis for medical purposes.
Managing the risks of chemicals in the home, the workplace and the environment
As part of its commitment to implementing the Chemicals Management Plan, Health Canada examined existing and emerging chemicals of concern to address questions and knowledge gaps related to the effects and exposure of chemical substances on humans.
Health Canada and Environment and Climate Change Canada continued their collaboration to assess all new substances (356 in 2020-21) before these were imported into or manufactured in Canada under the authority of the Canadian Environmental Protection Act, 1999. Where the two Departments identified risks, measures to manage them were instituted in order to protect human health and the environment.
What’s new?
Health Canada released the Report on Human Biomonitoring of Environmental Chemicals in Pooled Samples in December 2020. The report presents the first set of pooled serum data collected as part of the Canadian Health Measures Survey. This is the first nationally representative dataset in blood for these 90 specific persistent environmental chemicals. The data advances our understanding of Canadians’ exposure to chemicals, including measurements of persistent organic pollutants, and contributes to performance measurement activities for these chemicals.
Over the course of 2020-21, Health Canada continued to assess the safety of existing substances under the Chemicals Management Plan, with approximately 91% (3,974 substances) of the Program’s total planned assessments completed by the end of the fiscal year. Due to the complexity of some assessments, the Department dedicated additional resources to support this work, including the development of appropriate risk management approaches.
Through the Canadian Health Measures Survey, Health Canada analyzed nationally representative biomonitoring data to monitor the levels of environmental chemicals in the general Canadian population over 10 years (2007 to 2017). Several levels declined significantly over this period, including a 33% decrease for lead, 32% for bisphenol A (BPA), 31% for triclosan, and 75% for the plasticizer DEHP. Trends in the level of chemicals in Canadians will be used to evaluate the effectiveness of recent risk management actions intended to reduce chemical exposures.
The Department also raised public awareness about the health risks of chemicals and pollutants that may be found in and around the home via the Healthy Home Campaign. Launched in 2019, overall web visits increased by 29%, with 224,470 visits in 2020-21, partly as a result of a digital advertising campaign that delivered 7.7 million impressions and increased visits to all advertised web pages by 161%.
The Department adapted its traditional outreach materials to enable virtual delivery, including developing Healthy Home webinars and content for virtual tradeshows and presentations. It developed a set of new animated videos (including "Tips to Protect your Family from Chemicals and Pollutants"), published summaries on chemicals, and targeted communities with limited or no internet access with a direct mail campaign. Health Canada also issued updated tips and information on various drinking water pollutants – its Drinking Water webpages continued to be among the top 3 most viewed pages related to environmental health on Canada.ca.
In addition, the Department expanded its reach by investing in projects and contracts to accelerate digital transformation of outreach activities. For example, it collaborated with EcoSchools Canada to produce an environmental health learning tool that will become part of this institution’s curriculum-linked certification program, which reaches approximately 1 million students.
What’s new?
Health Canada continued to support the GOC’s comprehensive approach to reducing plastic pollution and achieve its zero plastic waste vision. In October 2020, the Department, in partnership with Environment and Climate Change Canada, released the final Science Assessment of Plastic Pollution – an assessment of the potential effects of plastics on the environment and human health.
Health Canada also contributed to the federal government’s Canada’s Plastics Science Agenda, helping to guide the science and research investments for understanding the potential impacts of plastics on human health and to strengthen the scientific basis for taking action.
Health Canada also responded to internal recommendations towards improving the Workplace Hazardous Products Program Evaluation, including strengthening compliance and enforcement activities, as well as enhancing communication and guidance materials. For example, the Department published the Safety Data Sheet (SDS) Compliance Promotion Tool to assist suppliers with preparing SDS for hazardous workplace products. The Department also undertook a survey and consulted with a wide range of stakeholders to inform its work regarding consumer product exclusions under the Hazardous Products Act.
Internationally, Health Canada collaborated with global partners in 2020-21 to advance the adoption, implementation and updating of the Globally Harmonized System of Classification and Labelling of Chemicals to facilitate international trade while promoting the safe use of chemical products. This was achieved through active participation in a United Nations sub-committee of experts on the topic. Proposed amendments to the Hazardous Products Regulations were introduced as a part of the work plan developed by the Canadian and U.S. partnership on the Regulatory Cooperation Council.
Supporting the safety of consumer products and cosmetics
In 2020-21, Health Canada notified Canadians of 171 consumer product and cosmetic recalls, 50 of which were coordinated as joint recalls with the U.S. and/or Mexico.
In order to further promote awareness of potentially unsafe consumer products, the Department participated in internationally-coordinated consumer product awareness campaigns, including the Organisation for Economic Co-operation and Development’s 2020 Global Awareness Campaign on the safety of toys sold online, which Canada co-led with Australia, as well as a joint campaign with the European Union on toy recalls.
Did you know?
lf flammable liquids are poured from a container when using a portable fireplace, firepot, or fondue pot, they can leave an invisible vapor trail that can ignite if those fumes come into contact with an open flame or very hot surface.
Known as ‘flame jetting’, this phenomenon has resulted in serious burn injuries and even deaths over the years in Canada. Flame jetting occurs instantaneously, so the user and/or bystanders are unable to react quickly enough to move away.
To raise awareness of the serious risks associated with flame jetting in firepots, Health Canada published consumer alerts and released a first-person testimonial video.
Health Canada also continued to prioritize promoting awareness of, and compliance with, regulations, while also monitoring progress towards compliance and taking enforcement action as necessary. For example, the Department informed stakeholders of its planned approach to enforce new requirements for corded window coverings, as set out in the Corded Window Coverings Regulations. It also posted Information for regulated parties on the Enforcement Approach for the General Prohibitions under the Canada Consumer Product Safety Act, to help inform industry of their responsibilities in ensuring that the consumer products they market do not represent a danger to the health or safety of Canadians.
Protecting Canadians from radiation
The Department continued efforts to raise awareness among Canadians of the risks, health impacts and mitigation strategies related to radon gas – the leading cause of lung cancer for non-smokers. In November 2020, as part of Radon Action Month, Health Canada's National Radon Program distributed over 1.6 million postcards in areas where approximately 10-15% of homes were estimated to be above the Canadian guideline levels of 200 Bq/m3. Website visits to TakeActiononRadon.ca originating from the targeted regions increased by over 4,000%. The Department’s call centre also saw a significant spike in calls compared to the same period the previous year.
Health Canada continued to conduct research and develop science-based advice for Canadians and stakeholders on the safety of radiation-emitting devices. In 2020-21, new recommended localized human exposure limits for radiofrequency electromagnetic fields (EMF) in the frequency range 6GHz to 300 GHz were published on Health Canada’s Radiation and Your Health site. Applying these limits will help ensure that Canadians are better protected from radiofrequency EMF sources in this range, including emerging wireless communication products.
Strengthening pesticide regulation and communications
Health Canada continued to promote, monitor and enforce compliance with the Pest Control Products Act and its Regulations. In order to maintain regular operations while supporting the GOC’s public health efforts, the Department modified administrative and regulatory requirements in response to the COVID-19 pandemic. It worked with pesticide registrants to focus on their highest priority applications and extended the post-market consultations to allow stakeholders affected by the pandemic to provide comments. It also offered flexibilities to registrants to facilitate the manufacturing of products that would help address shortages and meet the increased need for sanitizers and disinfectants.
What’s new?
In 2020-21, Health Canada published a Fact Sheeton PPE for anyone who works with pesticides with information on how protect themselves when applying these chemicals and tips for PPE use and care information.
Over the course of 2020-21, the Department completed foundational work on its multi-year program renewal project in order to build a stronger and sustainable pesticide regulatory system that strengthens health and environmental protection and leads to improved quality and timeliness of scientific decisions.
Health Canada consulted with internal, external and international stakeholders about an integrated lifecycle approach to pesticide regulation and summarized the feedback in a “What was heard” report. To accelerate the program’s transformation, it initiated IT and data strategies to support this integrated lifecycle approach and respond to the Minister’s mandate commitment to make science-based regulatory decisions in a timely manner.
The Department engaged with grower groups, provincial partners, Agriculture and Agri-Foods Canada, Environment and Climate Change Canada and industry to promote and discuss a new framework for data collection related to agricultural pesticides and environmental monitoring.
Work continued with Health Canada’s two-year high priority re-evaluation process that saw the Department undertake risk assessments, risk management, consultations, special reviews and final regulatory decisions for the remaining 32 priority pesticides. In 2020-21, the Department completed the review of 13 priority pesticides (re-evaluations and special reviews). Completing these large and complex re-evaluations and special reviews remains an ongoing priority for the Department, with new ones being initiated every year.
Departmental Result 5: Canadians make healthy choices
Helping Canadians make healthy choices in their day-to-day lives is an important part of Health Canada's Health Protection and Promotion core responsibility. Over the course of 2020-21, the Department focused on the following priorities, detailed further below: promoting healthy eating; improving food packaging and labelling; fostering international collaboration and cooperation for food safety and nutrition; reducing tobacco use and responding to the increase in youth vaping; as well as supporting Canadians in making informed decisions about cannabis use through public education, research and surveillance.
Promoting Healthy Eating
Health Canada continued to advance its Healthy Eating Strategy, which aims to curb the rising burden of obesity and diet-related chronic disease. The Strategy includes regulatory and non-regulatory interventions designed to make the healthy choice easier for all Canadians.
As part of the Strategy, the Department continued work towards introducing restrictions on the advertising of foods that contribute to excess consumption of sodium, sugars and saturated fats.
Health Canada continued to develop an evidence-based strategy to monitor food and beverage advertising in Canada across a range of media and settings, including the establishment of new assessment methods and protocols. Based on this foundational work, the Department is analysing marketing data and developing insights into the state of food and beverage advertising to children. This will inform further policy development as Health Canada works towards determining an appropriate approach to restricting the advertising of certain foods and beverages to children.
Health Canada supported the expansion of the International Food Policy Study, through the addition of a youth survey. The study is the first of its kind to evaluate the impact and effectiveness of national-level food policies, by examining changes to dietary behaviours. The new survey is being conducted annually in Australia, Canada, Chile, Mexico, the U.K. and the U.S. This collaborative research will support the Department’s efforts to monitor and take action on food and beverage advertising and address its impact on our youth. It will also serve to inform other federal healthy eating policies, by providing evidence of the benefits of such policies at both the national and international level.
Health Canada continued to support the availability of foods recommended by the Canada Food Guide in publicly-funded institutions. For example, the Department funded a collaboration of colleges and universities across Canada to create principles and criteria that will encourage greater access and availability to healthier food on campuses, with a particular emphasis on students who are food insecure.
To support Canadians during the pandemic, Health Canada provided information to make healthy eating as easy as possible, including recommendations for meal planning and preparation, shopping for nutritious foods with longer shelf lives, and eating mindfully during stressful times. The Department also developed and promoted new healthy eating content online, through the Canada Food Guide monthly e-newsletter and on social media, highlighting its popular recipes and videos.
Raising awareness of healthy eating behaviours and helping children, youth and young adults to understand the Canada Food Guide became particularly important during the pandemic. As such, Health Canada funded the creation of a Student Ambassador Network with Meal Exchange Canada – a group of post-secondary students from 30 academic institutions across Canada. As well, it funded the Student Commission of Canada to create two youth engagement teams, a teen cohort and a young adult cohort. Participants of these initiatives are working in their respective communities and campuses to: engage peers on the food guide; foster leadership; build capacity; and facilitate collaborations to raise awareness and increase knowledge and action on healthy eating.
To encourage further reduction in products with the highest sodium content, the Department published revised voluntary sodium reduction targets for processed foods in December 2020. Health Canada will continue to urge and work with the food-processing sector towards achieving these targets by 2025, and will monitor and evaluate progress. The Department also developed new proposed sodium reduction targets for foods sold in restaurants and foodservice establishments. However, the pandemic’s profound impact on this sector delayed plans to consult industry on these targets.
Health Canada worked with academics and institutions across Canada to help raise awareness on the importance of sodium reduction. For example, it developed teaching materials to educate food and nutrition students, as well as professionals, on reducing the use of salt or sodium-based ingredients when preparing food. The goal of this initiative is to raise awareness on how to reduce sodium in our food supply, particularly in the food produced by small, independent restaurants and food-service establishments.
In response to a March 2019 food safety program evaluation recommendation, the Department developed a Food Safety Risk Communications Action Plan that implements new approaches over the next three years (2021-22 to 2023-24) to influencing attitudes and behaviours of Canadians related to their food safety practices. The Action Plan lays out a 3-part strategy that involves: reviewing and optimizing existing food safety risk communications; increasing outreach and education efforts; and establishing an evaluation framework to assess the effectiveness of these efforts. 2020-21 saw public opinion research reports published online; development of a creative brief for a new food safety marketing campaign; and formation of an Education Working Group to coordinate communication initiatives within Health Canada.
Improving food packaging and labelling
Health Canada proposed new regulations that introduce a mandatory front-of-package nutrition symbol on prepackaged foods with levels of saturated fat, sugars and/or sodium that meet or exceed specific thresholds. This new symbol will help consumers quickly and easily identify such foods, allowing consumers to make healthier and more informed food choices for themselves and their families. Publication of the final front-of-package nutrition labelling regulations was delayed due to the COVID-19 pandemic.
The Department also worked with federal partners to publish a draft strategy for better coordination of future changes to food labelling requirements. This will provide more predictability in order to reduce the economic burden to industry associated with multiple, sequential label changes.
Fostering international collaboration and coordination for food safety and nutrition
Health Canada is an active participant in developing science-based international standards and risk management standards for food safety and nutrition and continued to co-lead Canada’s participation at the Codex Alimentarius Commission with the CFIA.
The COVID-19 pandemic severely affected meetings of the Codex Alimentarius Commission and its subsidiary bodies for 2020. To support the advancement of Codex work, the Department participated in meetings of the Executive Committee to study the impact of the pandemic on project management and to provide advice on how to proceed under adverse conditions.
What’s new?
Thanks in part to the contributions of Health Canada nutrition researchers, in December 2020 the WHO released its Global Protocol for measuring fatty acid profiles of foods, with emphasis on monitoring trans fats originating from partially hydrogenated oils. The document proposes a comprehensive, international protocol for measuring the trans fat content of foods. This effort is important to the health of Canadians and people around the world, because the regular consumption of trans fats increases the level of ‘bad’ cholesterol in the blood, a risk factor for heart disease.
Health Canada contributed to the Joint Food Agriculture Organization (FAO) and World Health Organization (WHO) initiatives as food chemical safety experts. It provided scientific advice to the 91st Meeting of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants; and responded to an urgent request from the United Nation’s World Food Programme for a health risk assessment and guidance following poisoning incidents from the consumption of food that contained high levels of natural toxins.
In 2020, Health Canada collaborated with Australia and New Zealand on a pilot project to assess the safety of a genetically-modified food (herbicide-tolerant canola) not yet authorized for use in the three countries. The Department conducted the initial assessment, which Australia and New Zealand then reviewed. All three agencies are now looking to establish a formal system to share food safety assessments. This international collaboration helped reduce duplication and provided an opportunity to share scientific expertise. It will inform future regulatory decisions, reduce regulatory assessment costs for food producers, and streamline the food approval process.
Health Canada also co-chaired the International Social Science Liaison Group, an informal forum for international government organisations involved in the social and behavioural sciences of food regulation, food safety and public health nutrition to share information and collaborate. In October 2020, the U.K., Australia and New Zealand, under the auspices of the Liaison Group, published a report on consumer knowledge, attitudes and behaviours relating to allergen declarations and precautionary allergen or advisory labelling. As a member of the Liaison Group, Health Canada reviewed drafts and provided feedback on the report.
The Department also participated in the Asia-Pacific Economic Cooperation (APEC) project-working group for Trade Facilitation through the Development of an APEC Food Safety Risk Communication Framework, and served as Secretariat to the International Food Chemical Safety Liaison Group. In addition, it attended the virtual quarterly meetings of the International Liaison Group for Nutrition Reference Values (NRVs) Methodologies, an informal forum where international organizations and government departments involved in setting nutrient reference values can share information and collaborate on methodologies and approaches. Three technical sessions covered topics related to: the integration of chronic disease outcomes into NRV frameworks; the assessment of the internal validity of individual studies when setting NRVs; and rating confidence in the body of evidence for setting NRVs.
Reducing tobacco use and responding to the increase in youth vaping
Health Canada continued to implement Canada's Tobacco Strategy, a comprehensive and integrated approach to reducing tobacco use to less than 5% by 2035. At the same time, the Department remained very concerned about the high levels at which Canadian youth use vaping products and took additional steps over the past year to protect a new generation from the risk of nicotine addiction and other vaping-related harms.
What’s new?
In 2020-21, Health Canada conducted over 260 online inspections to verify compliance with the Tobacco and Vaping Products Act and gathered intelligence for online age-gating verification that supports enforcement. Inspectors assessed vaping product promotions to ensure regulations restricting the promotion of prohibited flavours and the use of testimonials and endorsements were being met.
In 2020-21, Health Canada finalized the Vaping Products Promotion Regulations, which prohibit the promotion of vaping products to young people, including the display of vaping products at points-of-sale if they can be seen by young persons, including online. Finally, the Regulations require the display of a health warning about the hazards of vaping products in all permitted vaping product advertisements. The Department expects that these new restrictions will help protect young people from inducements to using vaping products.
The Department also pre-published the proposed Concentration of Nicotine in Vaping Products Regulations in the Canada Gazette, Part I, for a 75-day consultation period. Lowering the maximum concentration of nicotine allowed in vaping products is expected to contribute to reducing the appeal of these products to youth. A regulatory proposal to restrict flavours in vaping products was added to Health Canada’s Forward Regulatory Plan 2021-2023.
It also relaunched the youth vaping prevention campaign – “Consider the Consequences of Vaping” – to reach youth (13 to 18 years of age) and their parents. The campaign aimed to discourage youth from taking up vaping by educating them about the risks and harms, providing parents, adults and educators with resources to support conversations with youth, and increasing awareness of where to get more information. For parents, the Department launched a new Vaping Prevention Web Page to convey more information on vaping devices and liquids, mechanics, as well as highlighting the risks and harms of youth vaping.
Health Canada continued to develop a new generation of health warnings, information messages and toxicity statements for tobacco product labelling, while consulting with a variety of stakeholders and conducting public opinion research to inform the process.
Throughout the year, the Department focused on online inspections of vaping retailers, specifically on social media platforms promoting vaping products in a manner that may be appealing to youth. Health Canada also carried out remote inspections of tobacco and vaping manufacturers to assess compliance of cigarettes and little cigars with the requirements of the Tobacco Products Regulations (Plain and Standardized Appearance), as well as assessing vaping product packages/labels obtained from manufacturers under the Vaping Products Labelling and Packaging Regulations.
Supporting Canadians in making informed decisions about cannabis use through public education, research and surveillance
Through investments in public education in 2020-21, the GOC has helped Canadians make informed choices and better understand the risks associated with cannabis use. Based on the Canadian Cannabis Survey 2020, 77% of Canadians in 2020 strongly or somewhat agreed that they had access to enough trustworthy information about the health risks of cannabis to make informed decisions in comparison to 71% in 2019.
Health Canada undertook 2 cannabis public education campaigns; one targeted pregnant women, and the second targeted youth and young adults 16 to 24 years of age and focused on the risks and harms to mental health associated with cannabis use. The Department also conducted 2 surveys to monitor Canadians’ knowledge and attitudes: the Canadian Cannabis Survey and the Canadian Cannabis Vaping Survey.
Key risks for Core Responsibility 2: Health Protection and Promotion
Information on Key Risks is available on Health Canada’s website.
Results achieved for Core Responsibility 2: Health Protection and Promotion
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| Percentage of new drug decisions issued within service standardsDR 3 table Footnote 1 (Baseline: 88% in 2017-18) |
93% | March 31, 2021 |
|
| Percentage of Risk Management Plan reviews for new drug decisions completed within service standards (Baseline: 91% in 2017-18) |
90% | March 31, 2021 |
|
| Percentage of drug companies deemed to be compliant with manufacturing requirements under the Food and Drugs Act and associated regulations | Between 85% and 95% | March 31, 2021 |
|
|
|||
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| Percentage of domestic consumer product recalls communicated to Canadians in a timely manner (Baseline: 86% in 2016-17) |
At least 85% | March 31, 2021 |
|
| Percentage of actions taken in a timely manner to protect the health of Canadians from substances found to be a risk to human health (Baseline: 88% in 2018-19) |
100%DR 4 table Footnote 1 | March 31, 2021 |
|
| Percentage of actions taken in a timely manner to protect the health of Canadians from pesticides found to be a risk to human health and the environmentDR 4 table Footnote 2 (Baseline: 94% in 2018-19) |
At least 80% | March 31, 2021 |
|
|
|||
| Departmental Result Indicators | Target | Date to achieve target | Actual Results |
|---|---|---|---|
| Percentage of Canadians (aged 15+) who have used any tobacco product in the past 30 days (Baseline: 17.4% in 2015-16) |
Less than 5% | March 31, 2035 |
|
| Percentage of Canadians (aged 15-24) who have used cannabis in the last 12 months (Baseline: 25.5% in 2017 [17.5% of Canadians aged 15-17 and 28.4% of Canadians aged 18-24]) |
29% or lower for Canadians aged 15-24 (14% for Canadians aged 15-17 and 33% for Canadians aged 18-24) | March 31, 2025 |
|
| Percentage of Canadians who use dietary guidance provided by Health Canada (Baseline: 41% in 2012)DR 5 table Footnote 4 |
At least 50% | March 31, 2021 |
|
|
|||
| 2020-21 Main Estimates |
2020-21 Planned spending |
2020-21 Total authorities available for use |
2020-21 Actual spending (authorities used) |
2020-21 Difference (Actual spending minus Planned spending) |
|---|---|---|---|---|
| 635,964,234 | 635,964,234 | 985,316,315 | 660,580,250 | 24,616,016 |
| Note: The variance of $24.6 million between actual and planned spending is mainly due to Health Canada’s response to COVID-19 pandemic for the creation of a critical drug reserve in collaboration with provinces and territories to support access to drugs, which treat COVID-19 symptoms for Canadians; as well as, drugs, medical devices and support for regulatory and operational critical COVID-19 focused functions. | ||||
| 2020-21 Planned full-time equivalents |
2020-21 Actual full-time equivalents |
2020-21 Difference (Actual full-time equivalents minus Planned full-time equivalents) |
|---|---|---|
| 5,898 | 6,036 | 138 |
| Note: The variance in FTEs utilization is mainly due to the additional in-year resources received for Health Canada's response to the COVID-19 pandemic for drugs, medical devices, and support for regulatory and operational critical COVID-19 focused functions. | ||
Financial, human resources and performance information for Health Canada’s Program Inventory is available in GC InfoBase.
Internal Services
Description
Internal Services are those groups of related activities and resources that the federal government considers to be services in support of programs and/or required to meet corporate obligations of an organization. Internal Services refers to the activities and resources of the ten distinct services that support Program delivery in the organization, regardless of the Internal Services delivery model in a department.
The 10 service categories are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Management Services; Materiel Management Services; and Acquisition Management Services.
Results
Health Canada’s greatest strength is an engaged, empowered and well-equipped workforce with employees that have the competencies (including science and regulatory skill sets), tools and opportunities to succeed in the pursuit of excellence in program and service delivery.
The Clerk of the Privy Council noted in his letter to the Prime Minister in the Twenty-Seventh Annual Report on the Public Service of Canada, that during these uncertain times, certainty is found in the Public Service’s commitment to draw on all of its creativity and resourcefulness to implement GOC plans aimed at protecting and supporting Canadians.
During the pandemic, the Department leveraged its scientific, regulatory, policy and administrative expertise to respond as quickly as possible. Employees demonstrated resiliency and flexibility as they adjusted to an ever-changing environment. All of Health Canada’s Internal Services supported the Department in responding to the human resources, technological and financial needs and challenges created by the pandemic, in order to maintain operations and transition to working remotely without interruptions. In 2020-21, Health Canada set up new IT systems, created COVID-specific HR staffing processes, accelerated procurement activities, and worked with central agencies on flexible financial approaches.
Did you know?
To foster a workplace free of racism and discrimination, the Department established in 2020-21 a Leadership Council on Diversity and Inclusion, co-chaired by the Deputy Ministers and comprised of volunteers from various employment equity groups, disciplines and regions across Canada. It provides a forum for employees to discuss, validate, promote and enable departmental strategies, policies, and activities that strengthen an organizational culture of diversity and inclusion.
Additionally, our collective hard work on diversity and inclusion, mental health, and harassment—as well as our experimentation with new ideas—has given momentum to renewal of the public service. Health Canada continued to support the government-wide goals of Public Service Renewal, through initiatives that foster a more inclusive, agile and equipped workforce.
Through such diversity and inclusion networks as the Indigenous Employee Network, Persons with Disabilities Network, Black Employees Matter Network, and Young Professionals Network (among others), the Department encouraged employees to mobilize, embrace a greater diversity of voices and make their workplaces ones where everyone’s contributions are valued. A highlight of this fiscal year was the formation of the Deputy Minister’s Leadership Council on Diversity and Inclusion to promote positive values and address issues of racial bias, harassment and discrimination.
Health Canada undertook the following key initiatives in 2020-21 in the area of Internal Services:
Building a healthy, diverse and inclusive workforce
Health Canada is committed to ensuring a workplace that is free of racism and discrimination, and where all employees feel safe and are treated with respect, dignity and fairness. These values are the foundation of who we are, what we do, and how we do our work. To foster a workplace free of racism and discrimination, the Department:
- Offered an in-house version of a bias-free staffing course called “The Selection of a Candidate with an Objective Eye”, when this was no longer available from the Canada School of the Public Service;
- Developed and implemented an initiative to ensure that employment equity groups have equitable access to second language training;
- Provided dedicated funding to internal Employee Equity networks chairs and executives;
- Continued to implement its Multi-Year Diversity and Employment Equity Plan, including internal anti-racism listening sessions to better understand the impacts of systemic and structural racism, discrimination and bias on employees and to identify steps towards a more diverse and inclusive workplace, free from racism and discrimination. External experts facilitated the sessions and prepared a report outlining findings and recommendations.
What’s new?
When it comes to innovations around staffing, some areas of Health Canada job posters are now national (where the work permits), and the shift to remote work has resulted in a culture change and acceptance of distributed workforces. Working remotely has resulted in a more level playing field for both potential and existing employees regardless of their location. For example, employees in the regions can now participate and contribute as if they were in the National Capital Region, with the use of MS Teams and other new ways of working.
Additionally, in order to attract and retain a diverse, inclusive, and bilingual workforce within a healthy, accessible and high performing workplace, Health Canada:
- Updated the Corporate Occupational Health and Safety Program regarding the prevention of workplace harassment and violence in response to amendments to the Canada Labour Code (Bill C-65) and developed a new directive on Work Place Harassment and Violence Prevention and Resolution;
- Implemented mental health and wellness strategies that align with the National Standard for Psychological Health and Safety in the Workplace. The focus on psychological and social support and engagement included such initiatives as developing a mental health events calendar and listening sessions to support the personal and professional mental health of employees;
- Launched the COVID-19 Mental Health Response Unit, with 3 main functions: proactive outreach to groups who need support; research into mental health best practices; and assessment, advice and coordination of holistic mental health interventions;
- Adapted orientation sessions and tools for new employees and students, as well as second language training and evaluations to a virtual environment;
- Facilitated tools for the virtual work environment, such as virtual onboarding program for new employees and adapting Official Languages training for remote learning.
Did you know?
Since the beginning of the COVID-19 pandemic, Health Canada nurses were crucial in supporting federal departments with the repatriation of Canadians as a result of COVID-19, contact tracing and surge capacity during vaccine rollout, and supporting First Nations and Inuit communities. They also continue to provide tremendous support to Global Affairs, Correctional Service Canada, Canada Border Services Agency and the Canadian Coast Guard.
Nurses at Health Canada are involved in all health services areas, including but not limited to education, developing standards and guidelines for care, contributing to health innovation and research, and participating in various program areas, including the Public Service Occupational Health Program and Employee Assistance Services.
Mobilizing to respond to COVID-19
Health Canada had to quickly develop a surge capacity to respond to the pandemic. Innovative and targeted staffing strategies, including a dedicated HR team for COVID staffing, ensured the Department was appropriately resourced in the critical pandemic response areas without overburdening other functions. The Department accelerated COVID-related HR processes to allow new hires to start as quickly as possible, without creating pay issues or compromising security clearances. The Department recruited subject matter experts from across the country, regardless of physical geographical location, in order to meet its new responsibilities and increased workload. Over 2020-21, Health Canada recruited 533 additional employees to enable the COVID-19 response, with almost 15% from outside the National Capital Region. It welcomed additional scientists, biostatisticians, medical doctors, veterinarians, economists, regulatory officers, policy analysts, administrative officers and students. All played a key role in implementing key pandemic response actions, from setting up new virtual tools to reviewing and approving treatments and vaccines.
In partnership with PHAC, Health Canada worked to provide P/Ts with surge capacity for various competencies and expertise, such as contact tracing, nursing and outbreak management. Through a bulk Interchange Agreement, Health Canada employees accepted temporary assignments to assist with outbreaks in long-term care homes and other congregate living sites, as well as case tracking and contact tracing.
Additionally, Health Canada supported PHAC in coordinating the National Emergency Strategic Stockpile Warehouse initiative to ensure appropriate and secure space for PPE and other emergency medical supplies. Partnering with the Agency, other levels of government and private sector stakeholders, the Department helped to implement travel health measures as well as safety and security assessment processes at points of entry across the country, including at four international airports (Vancouver, Calgary, Toronto and Montreal), 16 land borders and 22 Designated Quarantine Facilities.
Health Canada worked with the Office of the Chief Human Resources and employee bargaining agents to facilitate shift work that allowed for expedited reviews of promising vaccines, treatments and medical devices.
Employee Engagement
Throughout the pandemic, Health Canada employees were kept apprised of guidance and announcements from public health authorities, the Treasury Board Secretariat and the Public Service Occupational Health Program, through a variety of tools and platforms, including 6 virtual town halls, technical briefings for managers, communication kits for branches, broadcast messages, InfoCovid emails, and a COVID-19 Guide for managers and employees.
Executives and employee networks were engaged to develop and implement internal COVID-19 response programs and employee supports. Health Canada’s Labour Relations participated in significant engagement with bargaining agents, who represent the employees, in order to ensure a consistent and open flow of information related to hours of work, remote and on-site working conditions, and any senior management decisions that affected employees.
Modernizing the workplace to enable a safe and productive workforce with access to modern tools and facilities
Health Canada continued to provide employees with access to modern tools and facilities while at the same time adapting to the unique challenges posed by the COVID-19 pandemic. The abrupt need to shift to remote work required the Department to work with Shared Services Canada to secure network infrastructure that enabled all employees to work remotely, and to support modern tools while minimizing the risk of outages. The Zoom collaboration tool was initially deployed within 3 weeks of beginning remote work, followed by Microsoft 365 deployment in June. This tool equipped all employees with mobile, accessible and digital means to facilitate collaboration through chat, video and audio calling across Department desktops and mobile devices.
Approximately 14% of Health Canada employees across Canada were required to work onsite due to their critical responsibilities supporting science program operations (i.e., laboratories, offices and clinics). The Department implemented additional health and safety measures for these locations, such as distributing PPE and sanitization supplies, and applying stringent safe workplace standards.
Other initiatives implemented due to the pandemic included:
- Implementing physical distancing plans, including increased safety measures such as access to PPE and sanitization protocols in all locations nationally. Priority attention went to locations where employees were onsite in laboratories, clinics, and offices conducting critical functions;
- Liaising with building cleaning service providers and PSPC to modify cleaning and disinfection processes in buildings, with a focus on high-touch surfaces such as door handles, railings, and elevator buttons;
- Developing a strategy to prevent and reduce ergonomic hazards and to foster workplace health and well-being in a virtual environment, as well as supporting employees working remotely with accommodations where an ergonomic need was determined;
- Stabilizing infrastructure such as bandwidth and Virtual Private Network (VPN) availability for reliable remote access to the corporate network for a growing workforce;
- Enabling use of Protected B authorized public cloud;
- Equipping employees with mobile, accessible and digital tools such as M365 to facilitate employee collaboration through chat, video and audio calling across department desktops and mobile devices;
- Offering an equipment allowance to help employees set-up remote work stations;
- Maintaining HR-to-Pay timeliness at over 75% despite a high volume of critical staffing actions to support the COVID-19 response while at the same time resolving a backlog of 7,600 cases;
- Delivering 3 projects across 2 provinces and 3 cities, valued at $5.3 million as part of implementing year three of the 2018-22 National Accommodation Strategy.
Health Canada’s laboratory modernization continued to meet the Department’s needs and align with GOC enterprise direction. 2020-21 highlights included:
- Delivering 33 capital and repair projects valued at $4.3 million as part of the multi-year program across the Health Canada laboratory portfolio to replace and repair aging building systems and to improve functionality for science programs;
- Partnering with other federal science-based departments and agencies on Phase 1 of Laboratories Canada, a multi-year initiative to replace aging facilities with collaborative, flexible, green laboratories that enhance science delivery excellence for Canadians. The Department reviewed and approved the site locations for the new laboratories and began preliminary planning for cost effective and efficient configuration.
Becoming more agile in delivering results
The Centre for Ombuds, Resolution and Ethics (CORE), formerly known as Ombudsman, Integrity and Resolution Office, acted as an early warning system for the organization as a whole, identifying workplace issues and trends early on and providing recommendations on how these could be addressed. The Ombuds engaged employees and managers by taking an active role at Town Halls, executive, union and employee network meetings to raise awareness regarding the confidential support available from CORE. Tools and assistance have been provided to employees at all levels to raise, discuss and resolve workplace issues in a safe manner, through services such as coaching, facilitated discussions, training and upward feedback.
Communication Services
Health Canada continued to engage Canadians with timely and relevant information they needed to take action on their personal and collective health and safety. This was accomplished through a range of traditional, digital and innovative communication strategies that also supported the Minister in delivering on GOC priorities.
Communication channels included daily postings on Health Canada’s social media accounts, web content, digital and traditional advertising, experiential virtual events and partnerships, in addition to more traditional tools such as news releases and proactive media relations.
Health Canada provided communications in support of the GOC response to the COVID-19 pandemic, while also communicating on other important issues, such as launching virtual care tools, mental health resources, building confidence in vaccine efficacy and safety, and access to prescription medications. In 2020-21, the Department:
- Raised awareness about environmental health in a COVID context by providing advice on reducing PPE waste and on improving indoor air quality in long-term care homes and residences to reduce COVID-19 transmission;
- Worked with P/Ts to promote the COVID Alert app, designed to notify Canadians if they come into contact with an individual who has tested positive for the virus. Health Canada leveraged multiple communications channels, partnerships, influencers and stakeholders to promote COVID Alert to Canadians in jurisdictions that have embraced the app;
- Delivered public awareness campaigns to promote Wellness Together Canada through traditional and digital platforms informing Canadians of all ages that they have access to free and confidential mental health and substance use resources and support.
Health Canada delivered evidence-based and innovative public awareness campaigns and worked with P/Ts and stakeholders to inform Canadians about continuing priorities such as opioids, cannabis, vaping, tobacco, healthy eating as well as regulatory updates.
Providing timely, trusted, and evidence-based pandemic information to Canadians
As the pandemic continued to spread and evolve over 2020-21, Health Canada collaborated with all of its partners in health to deliver the most timely, trusted, accessible, and evidence-based information possible to health care providers, stakeholders and Canadians, allowing them to protect themselves, their families, communities and businesses. The Department used various communication channels to provide the latest information on vaccine authorizations, safety and efficacy to increase vaccine confidence and encourage uptake. In 2020-21, Health Canada:
- Responded to more than 7,000 media calls about COVID and organized approximately 650 news conferences/technical briefings and interviews with key spokespersons;
- Issued 136 risk communications and 249 statements;
- Expanded the Canada.ca/coronavirus web site to enable Canadians to access all federal COVID-19 related information, including vaccination, and programs from a single site;
- Responded to almost 15,000 enquiries regarding the COVID Alert app; Reached out to visible minority communities by partnering with community leaders;
- Expanded available web information on rapid test distribution and use;
- Promoted and amplified reports from the Testing and Screening Expert Advisory Panel and Industry Advisory Roundtable on COVID-19 Testing, Screening, Tracing and Data Management;
- Launched an online portal with detailed regulatory information about the vaccines and treatments authorized for COVID-19, to equip health care professionals and Canadians with the information they need about approved products;
- Collaborated with PHAC to publish weekly summaries of reports of Adverse Events Following Immunization received for all COVID-19 vaccines.
Opioids
The opioid overdose crisis remained an important communications focus, including addressing the stigma that creates barriers for those seeking treatment. Over 2020-21, the Department:
- Pursued an aggressive digital media advertising campaign to help end stigma around people who use opioids and inform Canadians about the Good Samaritan law—this resulted in approximately 98.9 million impressions;
- Expanded the reach of its “Know More tour” by transitioning to a virtual platform to deliver programming during the COVID-19 pandemic to reach 124 additional markets across Canada with 526 sessions delivered to high school classes;
- Enhanced and promoted the Interactive Map: Canada’s response to the opioid crisis on Canada.ca, which locates the full range of opioid-related prevention, harm reduction, treatment and enforcement activities and facilities across Canada.
Did you know?
Health Canada’s “Know More Tour“ has engaged nearly 155,000 youth and young adults across Canada since its launch in 2018, with 497 visits to high schools, 42 post-secondary campus visits and participation at 43 other events. The website, launched in March 2019, features resources, activities and awareness products related to opioid use, harms and stigma. In 2020-21, Health Canada created a virtual version of the tour to adapt to COVID-19 health measures which had the added benefit of expanding its reach nationally.
Cannabis
Health Canada continued to provide Canadians with the information they needed about cannabis to make informed decisions and minimize health and safety harms, by:
- Releasing the results of the latest Canadian Cannabis Survey 2020;
- Advertising about the potential mental health risks to young people who use cannabis, resulting in some 29 million impressions;
- Issuing an advisory to protect children from intentional ingestion of cannabis edibles;
- Creating engaging social media content around the risks to mental health associated with frequent cannabis use.
Vaping
The Department pursued its public education efforts to address youth vaping, including a national public education campaign – “Consider the Consequences of Vaping” – that informed youth and their parents of the associated risks and harms. The campaign included:
- Virtual educational events in schools across the country, reaching 7,990 students;
- The launch of a digital advertising campaign targeting youth and parents that generated 49.3 million impressions, 11.8 million complete video views and 156.5 thousand clicks to the website.
Tobacco
Health Canada continued to promote its “Break it Off” tobacco cessation public awareness campaign in partnership with the Canadian Cancer Society. The campaign: targeted young adults aged 20 to 24 years via social media, web resources and a mobile app; included an advertising component that featured tools and resources to quit smoking, including a cost calculator; and resulted in 21.2 million impressions, 764 thousand complete video views and 32 thousand clicks to web content.
Health Canada also launched a new digital advertising campaign (“It’s never too late”) to help adults aged 50 to 64 years old find the tools and resources to quit smoking. Consisting of web banners, mobile ads and search engine marketing, the campaign generated 9.7 million impressions, 1.5 million complete video views and 30,000 clicks to web content. In addition, the Department regularly published new web content on Canada.ca to help Canadians quit smoking.
Healthy Eating and Food Safety
Health Canada launched the “Healthy Eating at Home” campaign, targeting parents of kids aged 2 to 18 years. Advertising appeared as banner ads on websites, videos and carousel ads on YouTube, Pinterest, Facebook and Instagram social media channels. The campaign generated more than 21 million impressions, a 54% increase in web visits, and more than 2.3 million completed video views (with a completion rate of almost 41%, surpassing the GOC benchmark of 15%).
The Department also:
- Promoted Food guide tips, recipes and resources through its food guide website, monthly e-newsletter and social media posts;
- Provided relevant and timely digital information through Canada.ca and social media channels regarding healthy eating in a pandemic environment;
- Developed a COVID-19 food safety web page for consumers and issued timely social media products related to food safety.
Regulatory updates
In addition to constant communications in support of COVID-related regulations, Health Canada also:
- Launched a stakeholder and public engagement process to advance work on a National Strategy for High-Cost Drugs for Rare Diseases;
- Announced changes regarding the uses of certain neonicotinoid pesticides, namely clothianidin and thiamethoxam, to better protect aquatic insects;
- Co-led the publication of the final science assessment of plastic pollution, and supported a discussion paper on a proposed integrated management approach to plastic products, with Environment and Climate Change Canada, in October 2020.
| 2020-21 Main Estimates |
2020-21 Planned spending |
2020-21 Total authorities available for use |
2020-21 Actual spending (authorities used) |
2020-21 Difference (Actual spending minus Planned spending) |
|---|---|---|---|---|
| 314,510,185 | 314,510,185 | 549,856,462 | 468,848,746 | 154,338,561 |
| Note: The variance of $154.3 million between actual and planned spending is mainly due to Health Canada's response to the COVID-19 pandemic for the safe restart agreement for federal investments in testing, contact tracing and data management; as well as, the establishment of an internal governance structure to support operational critical COVID-19 focused functions. Also included is the operating budget carry forward of which a portion was set aside to support strategic investments in 2021-22. | ||||
| 2020-21 Planned full-time equivalents |
2020-21 Actual full-time equivalents |
2020-21 Difference (Actual full-time equivalents minus Planned full-time equivalents) |
|---|---|---|
| 1,757 | 2,344 | 587 |
| Note: The variance in FTE utilization is mainly due a technical adjustment for the provision of shared services to the Public Health Agency of Canada; as well as, additional resources received in-year to support Health Canada's response to the COVID-19 pandemic for the safe restart agreement for federal investments in testing, contact tracing, data management; and, the establishment of an internal governance structure to support operational critical COVID-19 focused functions. | ||
Experimentation and GBA Plus
Experimentation
Innovation and experimentation were more critical than ever to help drive Health Canada's response to the COVID-19 pandemic and continue to meet its mandate to protect the health and safety of Canadians.
Building on its Beyond2020 commitments, the Department continued to increase employee capacity for experimentation and innovation via its Innovation and Experimentation Policy Framework 2020. The framework outlines an action-oriented approach, across three pillars: Learn and Act, Explore and Test, and Measure and Share.
Health Canada’s Solutions Fund empowers employees to create and lead innovation and experimentation projects that will improve how it provides services to Canadians, and bring new ideas and efficiencies to how it does business. The aim of the Fund is to improve services to Canadians, improve departmental operations and functionality, and deliver greater value to taxpayers.
Two new projects exemplifying bold thinking and innovative approaches to support Health Canada’s response to the pandemic were approved for funding in 2020-21:
- Project Cognit.io is exploring and prototyping an AI-assisted assessment engine to enhance the accuracy and speed of assessing complex natural health products. This could help address the overwhelming increase in COVID-related product applications, including hand sanitizers (to be completed in 2021-22).
- Project Nitro is testing whether automating key human resource transactions can increase efficiency and prevent user error. This could help manage the significant increase in the number of such transactions since the start of the pandemic.
The Department also completed 8 of the 13 existing Solutions Fund projects, many of which supported the departmental data strategy by maximizing the use of Health Canada data. The projects included experiments that tested:
- Machine learning to improve the quality of inspection reviews;
- Use of voice technologies for recalls and safety alerts;
- Technologies such as web scraping to monitor social media for posts promoting vaping products to youth.
Health Canada employees conducted proof of concept and solutions-exploration to advance the following ongoing projects:
- PRODigy – Entering the projects second phase, Health Canada developed and began testing a new form, featuring enhanced user-experience and interface elements, for Canadians to use when reporting incidents involving consumer products and cosmetics. PRODigy will continue into 2021-22 with a live, randomized A/B test that will compare the existing and modified incident reporting forms, both on the current reporting landing page, with an aim to increase reporting rates.
- Project Kelpie – Using analytics and real-time digital data analysis, the project explored and assessed if “machines” could monitor social media for posts promoting vaping products to youth. Initial findings identified challenges with data access and privacy. Further testing is planned for 2021-22.
- FTIR spectroscopy – Health Canada completed the reference database and customized a prediction model that will significantly reduce analysis time for detecting foreign matter in cannabis, cannabis products and health products. The Department also began testing the application of FTIR spectroscopy for fungi analysis.
In 2020-21, Health Canada employees completed the solution-exploration and moved to the experimentation and pilot phase for the following 8 projects:
- Project Cyclops – In collaboration with Statistics Canada, the Department tested a mobile application that recognizes patterns to automate the information assessed during label inspections. 80% of inspectors indicated the technology would be helpful; a full pilot project is scheduled for 2021-22.
- Hummingbird Drones Cannabis Inspections – Health Canada used drones to collect satellite imagery from 11 cannabis licence holders over a 6-month period. Results showed that this technology could be used to assess inspection requirements associated with plant health and personal security. A full pilot project using both drone and satellite data will take place in 2021-22.
- Project Cipher – This new tool demonstrated that it could support risk based compliance and enforcement decision making and reduce the time required to prepare inspection reports. A full pilot project will take place in 2021-22.
- Artificial Intelligence Systematic Review – Health Canada determined that integrating systematic review principles when assessing risks to human health could improve the transparency, robustness, and scientific defensibility of risk assessments. Outcomes from the project will be shared within the Department and with other federal departments and network partners.
- Project Data On Fire – Health Canada and the Ontario Office of the Fire Marshal explored ways to increase the number of fires reported to the Department so that it could more effectively identify and communicate the risks associated with consumer products. Despite concluding that automating this process was not currently feasible in Ontario, Health Canada will continue to encourage fire professionals to report fires with the goal of removing problematic consumer products from the market.
- Individualized Accommodation Passport – In partnership with Treasury Board Secretariat and other partners, Health Canada explored ways to replace its existing paper-based Accommodations Passport with an electronic version. The Passport allows employees to share information about adaptive tools or support measures to facilitate recruitment, retention, promotion, and mobility of employees with disabilities. Work will continue in 2021-22.
- National Service Desk Online Chatbot – The Department explored the feasibility of using a chatbot to respond to IT help desk requests from employees. Although employees were willing to use the chatbot, it was not able to assist with situations that involved restricted information (such as resetting passwords). Lessons learned will be shared with GOC networks and IT communities.
- Canada Recalls and Safety Alerts Voice Project – Health Canada explored the feasibility of using voice technology to inform Canadians about product recalls and safety alerts. Lessons learned will be shared with other GOC departments and communications communities.
In other 2020-21 experimentation developments:
‘Increasing Take-Up of Cannabis Micro Applicants Using Behavioural Insights’ – Health Canada launched this project in November 2019 to examine potential barriers to legal entry for small-scale cannabis business owners that might explain the lower-than-anticipated number of applications submitted and licenses granted in the early days of the new regime. The Department held consultation sessions in early 2020, culminating in a report detailing perceived barriers to licensing for these smaller-scale (known as micro-class) applicants.
Micro-class Engagement Strategy – Based on the report from the ‘Increasing Take-Up of Cannabis Micro Applicants Using Behavioural Insights’ project, Health Canada developed strategies for reducing barriers for small-scale applicants, while deterring activity in the illicit market. Activities included improving guidance, outreach and intervention with these business owners.
Did you know?
2020-21 marked the conclusion of Phase 1 of the Innovative Solutions Canada Challenge for point-of-care diagnostics to combat antimicrobial resistance, sponsored by Health Canada. Three Canadian small businesses participated by advancing the development of innovative rapid, accurate, easy to use and low cost diagnostic tools. In the future, these devices could help healthcare professionals identify pathogens and make informed decisions when prescribing antimicrobials.
In addition, 3 projects sponsored and funded by Health Canada and the GOC’s Innovative Solutions Canada Challenge Stream successfully concluded Phase I. Recipients each received $150,000 to develop a ‘proof-of-concept’ for the following solutions to health problems:
- Exploring point of care diagnostics to combat antimicrobial resistance and help address the rise of antimicrobial resistance infections;
- Leveraging AI technologies to predict possible organ donation recipient matches and improve donation rates;
- Developing a cost-effective and reliable method to identify microbial mixtures, characterize their stability and predict possible interactions among the micro-organisms that may mask or enhance adverse effects in humans.
Funding of up to $1 million for up to 2 years could be available for eligible projects in Phase 2, which involves prototype development and early-stage testing. Note that Health Canada is also a non-funding partner in another departmental challenge to develop environmentally safer, compostable PPE.
Finally, through the Innovative Solutions Canada Testing Stream, Health Canada supported late-stage testing of pre-qualified prototypes in third party settings. In 2020-21, the Department provided expertise to support the testing of: a reusable molded elastomeric face piece developed to replace surgical masks and reduce environmental wastes; a blockchain solution to improve patient care between mental health care settings by supporting workflows, interoperability and data exchange; and an AI-enabled digital solution to support hospital emergency departments in more efficiently managing surges in patient flows and volumes.
Sex and Gender-Based Analysis Plus (SGBA Plus/GBA Plus)
Health Canada continued to build on the Sex and Gender Action Plan, launched in 2017, while preparing for its next phase in spring 2021. The Action Plan strengthened the Department’s foundation of integrating SGBA Plus considerations into its work and ensured its responses to the COVID-19 pandemic took into account key intersecting sex, gender and diversity issues.
The Action Plan: i) provides a framework that strengthens the systematic integration of sex, gender and other intersectional factors (such as age and language) in Departmental work and decision making; and ii) supports the GOC’s priorities on diversity and inclusion as well as the advancement of gender equality.
Priorities of the Action Plan are to:
- Increase departmental capacity to apply SGBA Plus;
- Strengthen the sex, gender and diversity-related evidence base and expertise;
- Increase accountability for and transparency in implementing SGBA Plus.
These priorities will remain as the Department moves into the next phase of its Action Plan, with increased considerations of diversity factors beyond sex and gender (e.g. age, race, socioeconomic status, etc.), enhanced provision of collaborative tools, resources and training, and greater accountability for the systematic integration of SGBA Plus into all areas of Health Canada’s work. In response to the COVID-19 pandemic, Health Canada incorporated SGBA Plus into its decision-making process and initiatives, and strove to develop policies and recommendations that would address inequities facing at-risk populations. For example, the Department developed and maintained an evergreen COVID-19 and intersectionality guidance document, conducted weekly environmental scans to keep abreast of the most recent trends, and researched and analysed the disproportionate impact of the pandemic on at-risk groups.
Key 2020-21 initiatives in support of the SGBA Plus Action Plan include:
Enhanced capacity building
Health Canada revised and improved its SGBA Plus tools, resources and guidance to support programs with SGBA Plus integration. The Department promoted various learning opportunities, including: training focussed on program management and integrating SGBA Plus with knowledge translation and performance measurement; an International Women's Day Event on women in the COVID-19 pandemic; customized training for Health Canada’s Scientific Advisory Committee on Health Products for Women; as well as recognition of the integration of SGBA Plus into research through the Health Canada Science Forum.
Health Canada developed a Framework for Science and Research Excellence that addresses topics such as research ethics, sex, gender, race and diversity considerations, scientific integrity and investing in our scientists and researchers.
In 2020-21, Health Canada continued to work closely with PSPC to install signage designating gender-neutral washrooms. As of March 2021, the new signage has been outfitted on over 52 single occupancy washrooms at 22 buildings nationally, thus preparing for the post-pandemic return to the worksite.
In 2020-21, Health Canada’s Employee Assistance Program worked to optimize outreach to those who tend to underuse the service, by:
- Continuing to leverage Movember-focused content in order to promote men’s mental health;
- Modifying communications to better engage visible minorities (e.g., during Black History month, the communications team crafted tweets and promoted special content that included “ask the expert” sessions and mental health awareness sessions);
- Launching a real time chat service, which enabled clients to request a referral to a counsellor via chat rather than phone. This removed an access barrier for those who might be hesitant or otherwise unable to connect. The rollout was well received and the Program is looking to continue expanding the service to additional federal departments in 2021-22;
- Delivering counselling, organizational development and critical incident support services to other federal government departments through a network of contracted mental health professionals.
The Program surveyed its network of some 850 counsellors so that clients could be better matched with and referred to a counsellor who has lived experience or other expertise relevant to a specific ethnic, cultural, or LGBTQ2 Plus identity.
Strengthened sex and gender related evidence and expertise
Did you know?
Health Canada's Chief Science Advisor provides independent scientific analysis and recommendations to ensure that the Department integrates SGBA Plus and science into its policies and decision-making, such as anti-racist science on health products.
Medical devices and drugs regulations – In collaboration with the Institute of Gender and Health at CIHR, the Department funded 2 new external SGBA Plus Health Policy-Research Partnerships that focus on how Health Canada can better integrate and apply SGBA Plus considerations to the lifecycle management of prescription drugs and medical devices. Health Canada expects the projects to conclude in 2021, and will analyze and integrate the recommendations provided as appropriate.
Adverse drug reaction submissions – The Department modified its adverse drug reaction submission forms to ensure that research queries submitted to the Drug Safety and Effectiveness Network capture sex and gender-based evidence. Ongoing discussions are underway with the CIHR Coordinating Office to ensure SGBA Plus training among Network researchers.
Health products for women – Health Canada leveraged its Scientific Advisory Committee on Health Products for Women to obtain patient-centered, scientific and medical advice on current and emerging issues regarding women’s health and the regulation of health products for women. The Department also created a full-time SGBA Plus position to provide support on internal projects related to medical devices.
The Committee advised on current and emerging issues regarding women’s health and the regulation of medical devices and drugs, such as: incorporating SGBA Plus more explicitly in the review of prescription drugs and medical devices, including vaccines against COVID-19; patient experiences and perspectives on mesh implants and breast implants; and post-market surveillance of high-risk devices such as mesh and breast implants.
Healthy eating policies – The Nutrition Science Advisory Committee was established in 2020, comprising of external experts to provide the Department with scientific and technical advice on nutrition. The Committee’s input will ensure that Health Canada is benefiting from the best available evidence in fulfilling its mandate of supporting the nutritional health of Canadians, particularly populations at increased risk, with particular attention to health inequalities. Sex and gender-related evidence will inform these considerations.
Mental Health First Aid Toolkit – Health Canada finalized sex, gender and diversity sensitive resources, developed as part of an SGBA Plus Health Policy-Research Partnership, and incorporated them into the Mental Health First Aid Toolkit for all employees. Resource tools focused on: work-life stress and mental health; work-life balance; workplace diversity, discrimination and bias; bullying and harassment; as well as stigma disclosure and access to support programs, services and resources.
Implementing SGBA Plus across Health Canada programs
Chemicals management – Health Canada began developing tools to better identify subpopulations vulnerable to chemical exposure. This will help the Department to tailor its risk-management plans – such as, considering different age groups (including infants, toddlers, children, teens, and adults) when screening for potentially toxic substances – and improve its communication products to be more explicit about which possible vulnerable subpopulations have been considered. In 2020-21, Health Canada identified two substances (cis/trans-CTAC and cis-CTAC) as harmful through potential dermal exposures of younger age groups from the use of certain sunscreens and natural health product body moisturizers. Future actions will aim to reduce the exposure of infants, toddlers, and children to these products of concern.
The Department also continued to refine its Environmental Health outreach materials to ensure inclusive outcomes for all Canadians. It used inclusive images and text, employed described video, and selected images to challenge stereotypes, such as choosing to have an image of a man doing craft exercises with children or a woman doing home renovations.
Primary health care geriatric services – The Department continued to fund the Alberta Health Services project, “Primary Health Care Integrated Geriatric Services Initiative – Phase 2”, that launched in 2019-20. This initiative seeks to better integrate health care for seniors with chronic health issues (including dementia) and their caregivers. In 2020-21, the project focused on developing community coalitions of health and social services providers to plan and implement an integrated approach to care in 5 rural communities. This work focussed on at-risk populations, based on factors such as gender, ethnicity, sexual orientation, culture and age.
Cannabis – Health Canada integrated SGBA Plus into its public opinion research on cannabis. The resulting report, “Understanding Youth and Young Adults’ Interest in, and Usage of, Flavoured Cannabis Vaping Products” included information on sex, gender, age, sexual orientation, ethnicity, and more in order to better understand the intersections between these variables and behaviours and attitudes related to the use of flavoured cannabis vaping products.
The Department also developed the Canadian Cannabis Survey 2020 and collected demographic variables including sex, gender, sexual orientation, age group, and Indigenous status to better understand cannabis use and priority populations.
Healthy Home Campaign – As part of the Healthy Home social marketing campaign, Health Canada tested short-message video communications to ensure awareness messaging would resonate with targeted subpopulations who are more challenging to reach, such as gender-diverse individuals and those with disabilities. The Department also tested its campaign communications materials with focus groups of diverse Canadians, such as seniors, newcomers, pregnant individuals, parents of children under 6, and Indigenous Peoples. Research findings will help to improve public outreach materials to better meet the needs of at-risk populations.
For more information, please refer to the supplementary information tables GBA Plus.
Analysis of trends in spending and human resources
Actual expenditures
Departmental spending trend graph
The following graph presents planned (voted and statutory spending) over time.

Text description
The figure illustrates Health Canada's spending trend from fiscal year 2018-19 to fiscal year 2023-24 where spending, in millions of dollars, is shown on the vertical axis and time period, in fiscal years, is shown on the horizontal axis.
Health Canada's actual spending for fiscal year 2018-19: $2,370 million (Voted: $2,179 million, Statutory: $191 million); 2019-20: $2,675 million (Voted: $2,489 million, Statutory: $186 million); and 2020-21: $3,117 million (Voted: $2,821 million, Statutory: $296 million).
Health Canada's planned spending for fiscal year 2021-22: $3,863 million (Voted: $3,698 million, Statutory: $165 million); 2022-23: $2,379 million (Voted: $2,230 million, Statutory: $149 million); and 2023-24: $2,372 million (Voted: $2,224 million, Statutory: $148 million).
| Core Responsibilities and Internal Services | 2020-21 Main Estimates |
2020-21 Planned spending |
2021-22 Planned spending |
2022-23 Planned spending |
2020-21 Total authorities available for use |
2020-21 Actual spending (authorities used) |
2019-20 Actual spending (authorities used) |
2018-19 Actual spending (authorities used) |
|---|---|---|---|---|---|---|---|---|
| Health Care Systems | 1,777,284,741 | 1,777,284,741 | 2,456,807,897 | 1,558,123,313 | 2,200,154,060 | 1,987,223,947 | 1,601,069,150 | 1,289,851,245 |
| Health Protection and Promotion | 635,964,234 | 635,964,234 | 1,104,086,037 | 553,041,838 | 985,316,315 | 660,580,250 | 728,899,756 | 726,841,710 |
| Subtotal | 2,413,248,975 | 2,413,248,975 | 3,560,893,934 | 2,111,165,151 | 3,185,470,375 | 2,647,804,197 | 2,329,968,906 | 2,016,692,955 |
| Internal Services | 314,510,185 | 314,510,185 | 301,904,724 | 267,393,165 | 549,856,462 | 468,848,746 | 345,420,163 | 353,056,297 |
| Total | 2,727,759,160 | 2,727,759,160 | 3,862,798,658 | 2,378,558,316 | 3,735,326,837 | 3,116,652,943 | 2,675,389,069 | 2,369,749,252 |
|
Note: At the outset of the 2020-21 fiscal year, Health Canada's planned spending was $2,727.8 million. Additional in-year funding received for Health Canada's response to the COVID-19 pandemic and the operating and capital budget carry forwards, increased Health Canada's total authorities to $3,735.3 million. The additional funding received during 2020-21 relates mainly to the following: drugs, medical devices and virtual care; the safe restart agreement for federal investments in testing, contact tracing and data management; the creation of a critical drug reserve in collaboration with P/Ts to support access to drugs, which treat COVID-19 symptoms for Canadians; as well as, the establishment of an internal governance structure to support regulatory and operational critical COVID-19 focused functions. The variance of $618.7 million between total authorities and actual spending in 2020-21 is mainly the result of the reprofile of COVID-19 funds from 2020-21 to 2021-22 in order to continue Health Canada's response to the pandemic. As well, there was a decrease in funding requirements due to lower estimated costs, for the additional safety net of key drugs for critically ill COVID-19 patients following consultations with P/Ts. The decrease of $1,484.2 million in planned spending in 2022-23 is mainly due to the expiry of budgetary authorities in 2021-22 for Health Canada's response to the COVID-19 pandemic for: long-term care and other supportive care settings; the creation of a critical drug reserve in collaboration with P/Ts to support access to drugs, which treat COVID-19 symptoms in Canadians; as well as, regulatory and operational critical COVID-19 focused functions. Also, budgetary spending authorities are expiring for the federal framework to legalize and regulate cannabis and Canada Health Infoway. The Department will have to request funding for this initiative for future years. |
||||||||
Actual human resources
| Core Responsibilities and Internal Services | 2018-19 Actual full‑time equivalents | 2019-20 Actual full‑time equivalents | 2020-21 Planned full‑time equivalents | 2020-21 Actual full‑time equivalents | 2021-22 Planned full‑time equivalents | 2022-23 Planned full‑time equivalents |
|---|---|---|---|---|---|---|
| Health Care Systems | 210 | 215 | 290 | 247 | 276 | 272 |
| Health Protection and Promotion | 5,193 | 5,785 | 5,898 | 6,036 | 5,933 | 4,962 |
| Subtotal | 5,403 | 6,000 | 6,188 | 6,283 | 6,209 | 5,234 |
| Internal Services | 2,268 | 2,164 | 1,757 | 2,344 | 1,804 | 1,636 |
| Total | 7,671 | 8,164 | 7,945 | 8,627 | 8,013 | 6,870 |
|
Note: The variance in FTE utilization in 2020-21 is mainly due to the additional in-year resources received for Health Canada's response to the COVID-19 pandemic for: the safe restart agreement for the federal investments in testing, contact tracing and data management; drugs, medical devices, and support for regulatory and operational critical COVID-19 focused functions. The decrease in FTEs in 2021-22 is mainly due to funding level decreases for the safe restart agreement for the federal investments in testing, contact tracing and data management; regulatory and operational critical COVID-19 focused functions; as well as, the expiry of budgetary authorities in 2020-21 for the Chemicals Management Plan initiative. The decrease in planned FTEs in 2022-23 is mainly due to the expiry of budgetary authorities in 2021-22 for the federal framework to legalize and regulate cannabis; funding to support regulatory and operational critical COVID‑19 focused functions; as well as, the safe restart agreement for federal investments in testing, contact tracing, data management. The Department will have to request funding for these initiatives for future years. |
||||||
Expenditures by vote
For information on Health Canada's organizational voted and statutory expenditures, consult the Public Accounts of Canada 2020-21.
Government of Canada spending and activities
Information on the alignment of Health Canada's spending with the Government of Canada's spending and activities is available in GC InfoBase.
Financial statements and financial statements highlights
Financial statements
Health Canada's financial statements (unaudited) for the year ended March 31, 2021, are available on the departmental website.
Financial statements highlights
| Financial information | 2020-21 Planned results |
2020-21 Actual results |
2019-20 Actual results |
Difference (2020-21 Actual results minus 2020-21 Planned results) |
Difference (2020-21 Actual results minus 2019-20 Actual results) |
|---|---|---|---|---|---|
| Total expenses | 3,040,946,000 | 3,450,839,000 | 2,896,523,000 | 409,893,000 | 554,316,000 |
| Total revenues | 271,342,000 | 305,849,000 | 225,366,000 | 34,507,000 | 80,483,000 |
| Net cost of operations before government funding and transfers | 2,769,604,000 | 3,144,990,000 | 2,671,157,000 | 375,386,000 | 473,833,000 |
|
The Department's total expenses in 2020-21 were $3,450.8M. There was an increase of total expenses of $409.9M when comparing actual results against planned results for 2020-21. This is primarily a result of the following:
When comparing year-over-year actual expenditures, there was an increase of $554.3M. The significant changes were:
The Department's total revenues were $305.8M in 2020-21 representing an increase of $34.5M from planned results and an increase of $80.5M over the prior year actual revenues. The year-over-year variance is primarily a result of an increase in recoveries from PHAC for communication services related to COVID-19 public announcements provided under the Shared Services Partnership agreement, annual fee increases to specific fee regimes, an increase in volume of regulatory reviews and evaluations for medical devices, drugs and vaccines available for sale and use, due to COVID-19. |
|||||
| Financial Information | 2020-21 | 2019-20 | Difference (2020-21 minus 2019-20) |
|---|---|---|---|
| Total net liabilities | 404,278,000 | 303,840,000 | 100,438,000 |
| Total net financial assets | 273,955,000 | 193,527,000 | 80,428,000 |
| Departmental net debt | 130,323,000 | 110,313,000 | 20,010,000 |
| Total non‑financial assets | 142,591,000 | 140,612,000 | 1,979,000 |
| Departmental net financial position | 12,268,000 | 30,299,000 | (18,031,000) |
|
Total net liabilities were $404.3M at the end of 2020-21, representing an increase of $100.4M from the previous year. This variance is mainly due to timing of payments on transfer payment agreements approved later in the fiscal year, decreased consumption by employees of earned vacation leave during the pandemic, and a continuing pause as instructed by the Treasury Board Secretariat, for mandatory cash out of certain leave balances while problems with the new government payroll system are addressed. The year-over-year increase in total net financial assets of $80.4M is primarily a result of an increase in amounts due from the Consolidated Revenue Fund, which is reflective of the increase in accounts payable noted above, and an increase in amounts due from PHAC for recovery of costs incurred on PHAC's behalf for Canada's response to COVID-19. |
|||
Corporate Information
Organizational profile
Appropriate Ministers: The Honourable Jean-Yves Duclos, P.C., M.P. and The Honourable Carolyn Bennett, M.D.,P.C., M.P.
Institutional Head: Dr. Stephen Lucas
Ministerial portfolio: Health
Enabling instruments: Assisted Human Reproduction Act, Canada Health Act, Canada Consumer Product Safety Act, Controlled Drugs and Substances Act, Department of Health Act, Food and Drugs Act, Cannabis Act, Hazardous Materials Information Review Act, Hazardous Products Act, Pest Control Products Act, Radiation Emitting Devices Act, Tobacco and Vaping Products Act.
List of Acts and Regulations
Year of incorporation / commencement: 1913
Raison d'être, mandate and role
“Raison d’être, mandate and role: who we are and what we do” is available on Health Canada’s website.
For more information on the department’s organizational mandate letter commitments, see the Minister’s mandate letter.
Operating context
Information on operating context is available on Health Canada's website.
Reporting Framework
Health Canada's Departmental Results Framework and Program Inventory of record for 2020-21 are shown below.

Legend:
R: Result
I: Indicator
Text description
Departmental Results Framework
Core Responsibility 1
Health Care Systems
- R1: Canada has modern and sustainable health care systems
- I1: National health expenditure as a percentage of Gross Domestic Product
- I2: Real per capita health expenditure
- I3: Drug spending as a percentage of Gross Domestic Product
- I4: Percentage of family physicians using electronic medical records
- R2: Canadians have access to appropriate and effective health services
- I5: Percentage of Canadians (aged 15+) with a mental disorder who have expressed that they have an unmet mental health care need
- I6: Percentage of Canadians (aged 15+) who have expressed that they have an unmet need for access to home care services
- I7: Percentage of Canada Health Act compliance issues addressed within 24 months of identification
- I8: Percentage of Canadians who did not fill a prescription for medicine because of the cost
- Program Inventory under core responsibility one (from one -16) as follow:
- Health Care Systems Analysis & Policy
- Access, Affordability, & Appropriate Use of Drugs & Medical Devices
- Home, Community & Palliative Care
- Mental Health
- Substance Use & Addictions
- Digital Health
- Health Information
- Canada Health Act
- Medical Assistance in Dying
- Cancer Control
- Patient Safety
- Organs, Tissue & Blood
- Promoting Minority Official Languages in the Health Care Systems
- Brain Research
- Thalidomide
- The Territorial Health Investment Fund (THIF)
Core Responsibility 2
Health Protection & Promotion
- R3: Canadians have access to safe, effective and quality health products
- I9: Percentage of new drug decisions issued within service standards
- I10: Percentage of Risk Management Plan reviews for new drug decisions completed within service standards
- I11: Percentage of drug companies deemed to be compliant with manufacturing requirements under the Food and Drugs Act and associated regulations
- R4: Canadians are protected from unsafe consumer and commercial products and substances
- I12: Percentage of consumer product recalls communicated to Canadians in a timely manner
- I13: Percentage of actions taken in a timely manner to protect the health of Canadians from substances found to be a risk to human health
- I14: Percentage of actions taken in a timely manner to protect the health of Canadians from pesticides found to be a risk to human health and the environment
- R5: Canadians make healthy choices
- I15: Percentage of Canadians (aged 15+) who have used any tobacco product in the past 30 days
- I16: Percentage of Canadians (aged 15-24) who have used cannabis in the last 12 months
- I17: Percentage of Canadians who use dietary guidance provided by Health Canada
Program Inventory under core responsibility two (from 17-33) as follow:
- Pharmaceutical Drugs
- Biologics & Radiopharmaceutical Drugs
- Medical Devices
- Natural Health Products
- Food & Nutrition
- Air Quality
- Climate Change
- Water Quality
- Health Impacts of Chemicals
- Consumer Product Safety
- Workplace Hazardous Products
- Tobacco Control
- Controlled Substances
- Cannabis
- Radiation Protection
- Pesticides
- Specialized Health Services & Internationally Protected Persons Program
Supporting information on the program inventory
Financial, human resources and performance information for Health Canada's Program Inventory is available in GC InfoBase.
Supplementary information tables
The following supplementary information tables are available on Health Canada's website:
- Reporting on Green Procurement
- Details on transfer payment programs
- Gender-based analysis plus
- Horizontal initiatives
- Response to parliamentary committees and external audits
- Up‑front multi‑year funding
Federal tax expenditures
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance Canada publishes cost estimates and projections for these measures each year in the Report on Federal Tax Expenditures. This report also provides detailed background information on tax expenditures, including descriptions, objectives, historical information and references to related federal spending programs as well as evaluations and GBA Plus of tax expenditures.
Organizational contact information
Serena Francis
Assistant Deputy Minister / Chief Financial Officer
Health Canada
Assistant Deputy Minister Office
70 Colombine Driveway, Tunney's Pasture
Ottawa, Ontario K1A 0K9
Telephone: 613-948-6358
serena.francis@hc-sc.gc.ca
Appendix: definitions
- appropriation
- Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
- budgetary expenditures
- Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
- core responsibility
- An enduring function or role performed by a department. The intentions of the department with respect to a core responsibility are reflected in one or more related departmental results that the department seeks to contribute to or influence.
- Departmental Plan
- A report on the plans and expected performance of an appropriated department over a 3‑year period. Departmental Plans are tabled in Parliament each spring.
- departmental priority
- A plan or project that a department has chosen to focus and report on during the planning period. Priorities represent the things that are most important or what must be done first to support the achievement of the desired departmental results.
- departmental result
- A Departmental Result represents the change or changes that the department seeks to influence. A Departmental Result is often outside departments' immediate control, but it should be influenced by program-level outcomes.
- departmental result indicator
- A quantitative measure of progress on a departmental result.
- departmental results framework
- A framework that connects the department's core responsibilities to its departmental results and departmental result indicators.
- Departmental Results Report
- A report on a department's actual accomplishments against the plans, priorities and expected results set out in the corresponding Departmental Plan.
- experimentation
- The conducting of activities that seek to first explore, then test and compare the effects and impacts of policies and interventions in order to inform evidence-based decision-making, and improve outcomes for Canadians, by learning what works, for whom and in what circumstances. Experimentation is related to, but distinct from innovation (the trying of new things), because it involves a rigorous comparison of results. For example, using a new website to communicate with Canadians can be an innovation; systematically testing the new website against existing outreach tools or an old website to see which one leads to more engagement, is experimentation.
- full‑time equivalent
- A measure of the extent to which an employee represents a full person‑year charge against a departmental budget. For a particular position, the full‑time equivalent figure is the ratio of number of hours the person actually works divided by the standard number of hours set out in the person's collective agreement.
- gender-based analysis plus
- An analytical process used to assess how diverse groups of women, men and gender-diverse people experience policies, programs and services based on multiple factors including race ethnicity, religion, age, and mental or physical disability.
- government-wide priorities
- For the purpose of the 2019–20 Departmental Results Report, those high-level themes outlining the government's agenda in the 2019 Speech from the Throne, namely: Fighting climate change; Strengthening the Middle Class; Walking the road of reconciliation; Keeping Canadians safe and healthy; and Positioning Canada for success in an uncertain world.
- horizontal initiative
- An initiative where two or more departments are given funding to pursue a shared outcome, often linked to a government priority.
- non‑budgetary expenditures
- Net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
- performance
- What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
- performance indicator
- A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of an organization, program, policy or initiative respecting expected results.
- performance reporting
- The process of communicating evidence‑based performance information. Performance reporting supports decision making, accountability and transparency.
- plan
- The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead up to the expected result.
- planned spending
- For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts presented in Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports.
- program
- Individual or groups of services, activities or combinations thereof that are managed together within the department and focus on a specific set of outputs, outcomes or service levels.
- program inventory
- Identifies all the department's programs and describes how resources are organized to contribute to the department's core responsibilities and results.
- result (résultat)
- A consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead they are within the area of the organization's influence.
- statutory expenditures
- Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
- target
- A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
- voted expenditures
- Expenditures that Parliament approves annually through an appropriation act. The Vote wording becomes the governing conditions under which these expenditures may be made.
Endnotes
- Footnote 1
-
The Government of Canada recognizes First Nations, the Métis Nation, and Inuit as the Indigenous Peoples of Canada, consisting of distinct, rights-bearing communities with their own histories, including with the Crown. The work of forming renewed relationships based on the recognition of rights, respect, co-operation, and partnership must reflect the unique interests, priorities and circumstances of each People. Health care policy development needs to recognize these distinctions.
- Footnote 2
-
COVID-19 medical countermeasures refer to measures to respond, detect and treat COVID-19, including but not limited to, health products (e.g., ventilators, PPE, diagnostics, etc.), vaccines, treatments, and therapeutics; community protection measures; as well as other measures used in the event of potential public health emergencies.
- Footnote 3
-
NACI is a national advisory committee of experts in the fields of pediatrics, infectious diseases, immunology, pharmacy, nursing, epidemiology, pharmacoeconomics, social science and public health. Reporting through PHAC, it provides guidance to the GOC on the use of vaccines currently or newly approved for use in Canada.
- Footnote 4
-
In 2020-21, Canadian Foundation for Healthcare Improvement amalgamated with the Canadian Patient Safety Institute, and formed Healthcare Excellence Canada.
- Footnote 5
-
The Canada Health Transfer is an ongoing annual transfer to P/Ts that grows each year in line with nominal GDP growth, or a floor of 3%.
- Footnote 6
-
SUAP is a partner in the Canadian Drugs and Substances Strategy (prevention, harm reduction and treatment pillars) and Canada's Tobacco Strategy, and supports the objectives of Health Canada's Tobacco Control, Controlled Substances and Cannabis Programs.
- Footnote 7
-
Data may be subject to change as certain non-serious adverse reactions may still be in the workflow.
Page details
- Date modified:
