Guidance document: Supplemented Foods Regulations
July 2022
Table of contents
- Executive summary
- 1.0 Introduction
- 2.0 Transition of products to the Supplemented Foods Regulations
- 2.1 Supplemented foods with Temporary Marketing Authorization Letters
- 2.2 Temporary Marketing Authorization Letter requests submitted prior to coming into force of the Supplemented Foods Regulations
- 2.3 Requirements for supplemented foods during the transition period
- 2.4 Making changes to the products during the transition period
- 2.5 Products marketed after the coming into force of the Supplemented Foods Regulations
- 3.0 Guidance for implementation of the Supplemented Foods Regulations
- 4.0 Requirements for complying with the Supplemented Foods Regulations
- 4.1 Food categories permitted under the Supplemented Foods Regulations
- 4.2 Supplemental ingredients and their conditions of use
- 4.2.1 Information included in the List of Permitted Supplemental Ingredients
- 4.2.2 Minimum amounts of supplemental ingredients
- 4.2.3 Maximum amounts of supplemental ingredients
- 4.2.4 Cautionary statements for supplemental ingredients
- 4.2.5 Overages for supplemental ingredients
- 4.2.6 Deficiencies for supplemental ingredients
- 4.2.7 Direct or indirect addition of supplemental ingredients
- 4.2.8 Supplemental ingredients made up of two parts
- 4.2.9 Quality standards for supplemental ingredients
- 4.2.10 Vitamins and mineral nutrients
- 4.2.11 Amino acids
- 4.2.12 Other supplemental ingredients
- 4.2.13 Other substances for consideration as supplemental ingredients
- 4.2.14 Ingredients that are not supplemental ingredients
- 4.3 Labelling of supplemented foods
- 4.3.1 Labelling requirements specific to supplemented foods
- 4.3.2 Front-of-package nutrition labelling
- 4.3.3 Declaring nutrients outside of the Supplemented Food Facts table
- 4.3.4 Declaring supplemental ingredients outside of the Supplemented Food Facts table
- 4.3.5 Assortments
- 4.3.6 Compendium of templates for supplemented food facts tables, supplemented food caution identifiers and lists of cautionary statements
- 4.3.7 Priority allergens, gluten sources, and sulphites
- 4.3.8 Supplemented food versus standardized food names
- 4.4 Regulatory requirements and guidance for use of nutrition and health-related statements and claims on supplemented foods
- 4.4.1 General health claims
- 4.4.2 Nutrient content claims
- 4.4.3 Types of health claims and their regulatory requirements
- 4.4.4 Health claims substantiation
- 4.4.5 Health claims general guidance
- 4.4.6 Amino acid claims for supplemented foods
- 4.4.7 Specific restrictions on the use of claims and other representations on supplemented foods
- 5.0 Compliance and enforcement of Supplemented Foods Regulations
- 6.0 Pathway to market for new products not compliant with the List of Permitted Supplemented Food Categories and/or the List of Permitted Supplemental Ingredients
- 7.0 Resources Available
- 8.0 Contact Information
- 9.0 Glossary and Abbreviations
- Appendix 1: Documents incorporated by reference into the Supplemented Foods Regulations
- Appendix 2: Is my product a supplemented food (SF)?
- Appendix 3: Ingredients not permitted in foods, including supplemented foods
- Appendix 4: Vitamins and mineral nutrients - Approach for setting maximum amounts and other conditions of use
- Appendix 5: Amino acids - Approach for setting maximum amounts and conditions of use
- Appendix 6: Taurine - Approach for setting maximum amount and other conditions of use
- Appendix 7: Applicable Health Canada guidance documents and other resources
- References Cited
List of tables
- Table 1: Examples to illustrate regulatory requirements for different types of prepackaged foods as well as natural health products
- Table 2: Description of prepackaged foods in the List of Permitted Supplemented Food Categories
- Table 3: Example of requirements in the List of Permitted Supplemental Ingredients for vitamin A (Retinol)
- Table 4: Vitamins and mineral nutrients not acceptable for addition in supplemented foods
- Table 5: Amino acids not permitted for addition to supplemented foods
- Table 6: Sources for the derivation of maximum amounts for vitamins and minerals
- Table 7: Essential and non-essential amino acids involved in protein synthesis
Table of figures
Executive summary
Supplemented foods (SFs) are prepackaged foods with added supplemental ingredients (SIs). Division 29 in Part B of the Food and Drug Regulations (FDR) has been created to set out the specific requirements for SFs. Additional amendments have been made to Division 1 of Part B and in Part D of the FDR. Documents incorporated by reference into the FDR specify additional requirements for SFs, which a product must meet as a condition of sale to be on the market. Previously, products that met established compositional and labelling conditions were allowed market access through Temporary Marketing Authorization Letters (TMALs). The new regulatory requirements in the FDR, establish a risk-based and flexible regulatory framework for SFs.
SFs that meet the requirements of the Regulations Amending the Food and Drug Regulations and the Cannabis Regulations (Supplemented Foods) (SOR/0169), hereinafter referred to as the "Supplemented Foods Regulations", the documents incorporated by reference, and all other applicable provisions of the FDR, will be eligible to gain market access without the need for Health Canada's premarket authorization.
The purpose of this document is to provide information to stakeholders to facilitate an understanding of the Supplemented Foods Regulations. The document elaborates on the categories of foods that are permitted to be SFs, the permitted SIs and their maximum amounts permitted for addition, as well as all related conditions of use. The document also provides details on foods not acceptable as SFs, ingredients not permitted for addition in SFs, and how the compositional requirements for SFs have been established.
Under the transitional provisions of the Supplemented Foods Regulations, products that are issued a TMAL or a written notice for market access will be allowed a transition period after the coming into force of the regulations. The transition allows manufacturers or distributors of SFs continued market access and provides time to make the necessary changes to comply with the requirements of the Supplemented Foods Regulations. Health Canada continued to accept requests for TMALs for new SFs received before the coming into force of the Supplemented Foods Regulations on July 21, 2022. These requests are under review and if all applicable conditions in the transitional provisions are met, Health Canada will issue a written notice to the requestor for the sale of their product(s). These products will also have the transition period to comply with the Supplemented Foods Regulations, subject to continued compliance with the transitional provisions.
Stakeholders can request a premarket assessment by Health Canada for foods or ingredients that do not meet the conditions of use set out in the documents incorporated by reference, to determine their acceptability. This document outlines when this is needed and provides an overview of the premarket assessment process.
This guidance document elaborates on the regulatory requirements for supplemented foods in the Supplemented Foods Regulations.
1.0 Introduction
The Food and Drugs Act (FDA) and Food and Drug Regulations (FDR) are intended to help protect the health and safety of people of Canada with respect to the safety of food and drug products on the Canadian market.
1.1 Policy objectives
The Regulations Amending the Food and Drug Regulations and the Cannabis Regulations (Supplemented Foods) (SOR/0169), hereinafter referred to as the "Supplemented Foods Regulations", made under the FDA, introduced into the FDR a regulatory framework for the sale of supplemented foods (SFs) in Canada. These provisions allow safe SFs to be sold in Canada and are intended to be flexible and responsive to adapt to innovation and new scientific evidence.
1.2 Scope
This guidance document is intended for stakeholders, including manufacturers and distributors of foods for sale in Canada, to facilitate the understanding of the Supplemented Foods Regulations, which came into force on July 21, 2022.
The Supplemented Foods Regulations should be read in conjunction with other provisions of the FDR applicable to pre-packaged products as well as the FDA and the documents incorporated by reference into the FDR (List of Permitted Supplemental Ingredients, List of Permitted Supplemented Food Categories, Directory of Supplemented Food Facts Table Formats, and Directory of Supplemented Food Caution Identifier Specifications), listed in Appendix 1. To account for new requirements specific to SFs in the FDR, certain consequential amendments had to be made to existing provisions in the FDR (for example, expanding labelling provisions in Part B, Division 1 applicable to the Nutrition Facts table to include the Supplemented Food Facts table). This document does not elaborate on these consequential amendments. Health Canada's webpage on Supplemented Foods provides information and resources related to the requirements for SFs.
It is the responsibility of manufacturers and distributors to comply with all applicable legislative and regulatory requirements. In case of a discrepancy between this guidance and the provisions of the FDR or documents incorporated by reference, the regulations and the documents incorporated by reference take precedence.
In this guidance document, "must" is used to express a requirement, that is, a provision of the FDR that the manufacturer or distributor is obliged to satisfy; "should" is used to express a recommendation or that which is advised but not required; and "may" is used to express an option or that which is permissible within the limits of this document.
1.3 Background
The Supplemented Foods Regulations published in the Canada Gazette, Part II on July 20, 2022 amend the FDR to allow the sale of certain foods that have added vitamins, mineral nutrients, amino acids or other substances that were otherwise not permitted under existing provisions.
Previously, a number of products in food formats containing added vitamins, mineral nutrients, amino acids, and other substances which were not in compliance with the FDR, were introduced into the marketplace as Natural Health Products (NHPs) through the Natural Health Products Regulations (NHPR). As NHPs, these products were subject to individual premarket assessment and licensing. However, Health Canada determined, based on public perception, history of use, product representation to consumers, product composition and product format, that many of those products fit the definition of a food, as per the Guidance Document: Classification of products at the food-natural health product interface: products in food formats. In October 2011, the Minister of Health announced the intent to regulate caffeinated energy drinks (CEDs), which were formerly NHPs, as foods. In April 2012, it was announced to stakeholders that other NHPs that fit the definition of a food would also be regulated as foods.
Given that these products were not compliant with the FDR as foods, Temporary Marketing Authorization Letters (TMALs) were issued in accordance with section B.01.054 of the FDR, as an interim measure to allow the sale of products that were deemed safe, while addressing data gaps needed to support the development of regulations for SFs. Products were reviewed on a case-by-case basis and were issued TMALs under certain conditions and specified product composition with respect to supplemental ingredients (SIs), and labelling requirements. As part of the TMAL conditions, manufacturers or distributors were required to provide research, annual sales data, and if applicable, annual consumption incident reports to Health Canada for their SF while it remained on the market. This data, along with scientific literature, and other information available to Health Canada, were used to inform the regulatory amendments to the FDR for SFs. Health Canada pre-published the Supplemented Foods Regulations in the Canada Gazette, Part I on June 26, 2021 for public consultation and input from interested stakeholders and Canadians until September 24, 2021. Health Canada reviewed and analyzed the comments received during the consultation period and made revisions, where supported by evidence, prior to finalizing the regulations.
2.0 Transition of products to the Supplemented Foods Regulations
Prior to the publication of the Supplemented Foods Regulations, SFs were able to gain market access if the manufacturers or distributors received a TMAL for their products. The TMALs set out strict compositional and labelling conditions for their products to meet while on the Canadian market, based on the criteria in the guidance documents Category Specific Guidance for Temporary Marketing Authorization: Supplemented Food, or the Category Specific Guidance for Temporary Marketing Authorization - Caffeinated Energy Drinks, as applicable.
With the coming into force of the Supplemented Foods Regulations, the TMALs issued for SFs are no longer valid. However, manufacturers or distributors of SFs that held a valid TMAL for their products at coming into force of the regulations are given until December 31, 2025 to come into compliance with the Supplemented Foods Regulations, subject to applicable conditions in the transitional provisions. The same transition period is provided to manufacturers or distributors of foods that submitted a request for a TMAL prior to coming into force and that receive a written notification from the Minister authorizing the sale of the food. This date aligns with Health Canada and the (CFIA's joint policy on Food Labelling Coordination. The transition period allows manufacturers or distributors of SFs continued market access and provides time to make the necessary changes to comply with the requirements of the Supplemented Foods Regulations.
The sections below provide additional information on the transitional provisions applicable to existing products with TMALs that expired on July 21, 2022, and for products for which requests for TMALs were submitted prior to the coming into force of these regulations.
2.1 Supplemented foods with Temporary Marketing Authorization Letters
SFs with valid TMALs prior to the coming into force of the Supplemented Foods Regulations are listed in Table 1 - Lists of foods that were issued TMALs as supplemented foods that expired on July 21, 2022 - of the Lists of foods that have received temporary marketing authorization letters. Products listed in Table 1 are categorized to reflect the classification criteria in the Guidance Document: Classification of products at the food-natural health product interface: products in food formats that were used during the transition of products from the NHP framework to the food framework. Manufacturers or distributors of these SFs are permitted to continue to sell their products in Canada and have until December 31, 2025, to transition their products to comply with the Supplemented Foods Regulations. The transitional provisions set out the specific conditions previously found in the TMALs that apply to manufacturers or distributors during their transition. Subject to amending clause 35 in the transitional provisions, all applicable conditions must be complied with for the duration of the transition period or until they make a change to comply with the regulations. Another reason that may disqualify a manufacturer or distributor from the transition is if the Minister of Health notifies them that their product is not safe for consumption.
Products that fall under the transitional provisions must meet all applicable provisions of the FDR as they read prior to the coming into force of the Supplemented Foods Regulations, with the exception of variations from certain requirements of the FDR authorized by the TMAL, as well as certain requirements that were amended by the Regulations Amending the Food and Drug Regulations (Nutrition Labelling, Other Labelling Provisions and Food Colours) SOR/2016-305. All exemptions from the FDR as specified in the TMAL continue to be valid throughout the transition period (see Section 2.3).
Tables 3 and 4 of the Lists of foods that have received temporary marketing authorization letters include products other than SFs that have received TMALs. These products are considered outside of the scope of the Supplemented Foods Regulations, and continue to be subject to the conditions and requirements of their TMALs.
2.2 Temporary Marketing Authorization Letter requests submitted prior to coming into force of the Supplemented Foods Regulations
Health Canada continued to accept requests for TMALs for new SFs up until July 20, 2022. Manufacturers or distributors requested TMALs for their products in accordance with the criteria in the guidance documents Category Specific Guidance for Temporary Marketing Authorization: Supplemented Food, or the Category Specific Guidance for Temporary Marketing Authorization - Caffeinated Energy Drinks, as applicable.
In accordance with the requirements of the transitional provisions, Health Canada is reviewing the requests for TMALs that were submitted prior to July 21, 2022. Manufacturers and distributors will be issued a written notice authorizing the sale of products that meet the conditions set out in the transitional provisions, including in the document Threshold Levels for Cautionary Statements and Other Conditions of Use, which is incorporated by reference into the transitional provisions. The written notice includes details related to which conditions are applicable to the products in the request. Subject to amending clause 35 (as noted in Section 2.1), and all applicable conditions in the transitional provisions, a manufacturer or distributor that receives a written notice has until December 31, 2025, to comply with the Supplemented Foods Regulations or until they make a change to comply with the new requirements.
2.3 Requirements for supplemented foods during the transition period
Manufacturers and distributors of SFs that are subject to the transitional provisions as per Sections 2.1 and 2.2 must meet all applicable compositional, labelling and claims requirements for their products. These include applicable requirements of the FDR, and those in the transitional provisions.
During the transition period, manufacturers and distributors of SFs that fall under the transitional provisions as per Sections 2.1 and 2.2:
- must meet all the conditions detailed in the transitional provisions, including all required conditions of use such as labelling requirements and any applicable cautionary statements for their products;
- for SFs that had TMALs that expired on July 21, 2022, this includes the specific conditions in the transitional provisions applicable to them, that were previously in their TMALs
- for SFs subject to a written notice, this includes all applicable conditions of the transitional provisions, including those specified in the document Threshold Levels for Cautionary Statements and Other Conditions of Use;
- must not make changes to the SIs in their products;
- are permitted to comply with some of the provisions of the FDR that were amended by the 2016 nutrition labelling regulations as they read on December 13, 2016. This is to ensure that SFs with an older version of the Nutrition Facts table and list of ingredients do not need to make label changes until they transition to the Supplemented Foods Regulations; and
- are permitted to continue to use any exemptions provided in the expired TMAL or as part of the transitional provisions.
Manufacturers or distributors can choose to fully comply with the requirements of the Supplemented Foods Regulations at any point before the end of the transition period. However, modifying a product, including its label, to come into partial compliance with the requirements would result in the loss of the transition period, making the food non-compliant.
Some of the conditions of the TMALs for SFs are no longer applicable. The transitional provisions of the Supplemented Foods Regulations specify which conditions continue to apply. The requirement to provide sales data, consumption incident reports for products with cautionary statements, as well as research data on consumption patterns of Canadians and their understanding of SF label information was intended to inform the development of the regulatory framework. With the publication of the Supplemented Foods Regulations, this data is no longer required and therefore this condition of the TMAL has not been included as part of the transitional provisions.
2.4 Making changes to the products during the transition period
Manufacturers and distributors of products that fall under the transitional provisions must meet the conditions of the transition, as described in their TMAL or written notice during the transition period. This means, any modifications to the container size or the SIs in the product formulation, i.e., addition of a new SI or removal or changing the level of an SI, would result in the loss of the transition period for that product. In such cases, the manufacturer of distributor must fully comply with all the requirements of the Supplemented Foods Regulations, which includes the List of Permitted Supplemented Food Categories and the List of Permitted Supplemental Ingredients. However, modifications related to other ingredients that were not included in the authorization for the product, and as permitted by the transitional provisions (i.e., food ingredients permitted to be added to food in accordance with the FDA and FDR as they read immediately before the coming into force of the Supplemented Foods Regulations) are permitted during the transition period. For example, substituting an approved food additive for another approved food additive in products subject to an expired TMAL or written notice would be permitted during the transition period.
Manufacturers or distributors making other modifications to their product labels such as a brand name or flavour change would likely not result in a loss of the transition period. However, they are encouraged to contact Health Canada to discuss modifications to ensure that Health Canada's database of these products, used by the CFIA, reflects the modifications. This will help ensure that products that are permitted the transition period are identifiable and not subject to enforcement by the CFIA for non-compliance with the Supplemented Foods Regulations.
2.5 Products marketed after the coming into force of the Supplemented Foods Regulations
Any new SFs, other than those described in Sections 2.1 and 2.2, coming onto the market in Canada are required to immediately comply with the Supplemented Foods Regulations. This means that manufacturers or distributors do not need TMALs for any new products that meet all regulatory requirements (i.e., belongs to a permitted food category, contains a permitted SI, meets all conditions applicable to the added SIs, and meets all applicable requirements of the Supplemented Foods Regulations and FDR). These products can go straight to market and do not require a transition period, as they must already be compliant with the Supplemented Foods Regulations.
3.0 Guidance for implementation of the Supplemented Foods Regulations
3.1 Foods regulated under the Supplemented Foods Regulations
Products with added vitamins, mineral nutrients, amino acids and other substances may be subject to different regulatory requirements (see Table 1).
The addition of vitamins, mineral nutrients and amino acids to prepackaged products is subject to specific provisions and prohibitions in the FDR. For some foods, the intent underlying the addition of a vitamin, mineral nutrient, or amino acid is fortification. With fortification, specific nutrients must or may be added to foods to achieve a nutritional purpose (for example, to prevent nutritional deficiencies in the population or to restore or improve the nutritional quality of the foods). This addition of nutrients can be mandatory, for example, the addition of vitamin D to milk to help prevent vitamin D deficiency, which can lead to rickets in children and osteomalacia (softening of the bones) in adults; or voluntary, for example, the addition of thiamin, niacin, vitamin B6, folic acid, pantothenic acid, magnesium, iron and zinc to breakfast cereals.
The Supplemented Foods Regulations also permit the addition of specific vitamins, mineral nutrients, amino acids and other substances to prepackaged products; however, the addition is not for a nutritional purpose noted in Section 3.2 a). SFs contain added SIs that can pose a risk to health if they are consumed in excess by the general population or by vulnerable populations such as children, or pregnant or breastfeeding women. The Supplemented Foods Regulations outline specific rules for SFs in terms of food categories, composition, labelling and representations.
Appendix 2 - Is my product a supplemented food? provides a simplified diagram to help stakeholders determine whether the Supplemented Foods Regulations apply to their product.
Certain prepackaged products with added vitamins, mineral nutrients, amino acids or other ingredients may share characteristics of both foods and NHPs. For these products, the appropriate regulatory framework may not be immediately apparent. NHPs are subject to the NHPR and are not considered foods. To assist manufacturers or distributors with the classification of their products, Health Canada has published the Guidance Document: Classification of products at the food-natural health product interface: products in food formats. Manufacturers or distributors should consult the classification guidance document if there is uncertainty regarding whether their product is considered a food or NHP. In addition, a request for a classification determination can be made to the Food Directorate by sending an e-mail to the Submission Management and Information Unit (SMIU) at smiu-ugdi@hc-sc.gc.ca, with the word "Classification" in the subject line.
| Conventional FoodsFootnote 1 | Fortified FoodsFootnote 2 | Supplemented Foods | Natural Health Products | |
|---|---|---|---|---|
| Description | Products in food formats that can be consumed as one desires (i.e., ad libitum) and do not contain added vitamins, mineral nutrients, or amino acids. | Products in food formats that are required or permitted to contain added vitamins, mineral nutrients or amino acids for a nutritional purpose (e.g., to restore levels of nutrients lost during processing, or to prevent/correct a deficiency in the population). | Products in food formats that are permitted to contain added vitamins, mineral nutrients, amino acids, or other ingredients for reasons other than for a nutritional purpose (i.e., fortification purposes). SFs have conditions of use (e.g., cautionary labelling). These products may not be suitable for ad libitum consumption or for vulnerable populations such as children, pregnant or breastfeeding women. | Products in dosage formats that are not typical of foods (e.g., capsules, tablets, powders, tinctures), are likely to be considered NHPs. These products are subject to consumption limits, may not be suitable for the general population and may carry cautionary labelling. |
| Examples | Cheese, vinegar, cooking oils | White flour, milk, breakfast cereals | Beverages or bars with added vitamins and mineral nutrients | Multi-vitamin tablets |
| Applicable regulatory framework | Must comply with all applicable provisions of the FDR. | Must comply with all applicable provisions of the FDR, including those in Part D. Section D.03.002 of the FDR provides a list of the foods that are permitted to contain added vitamins, mineral nutrients and amino acids. | Must comply with all applicable provisions of the FDR, including those in Division 29 of Part B. | The FDR does not apply as these products are not considered foods. Must comply with all applicable provisions of the NHPR. |
3.2 Foods excluded from the Supplemented Foods Regulations
All SFs must meet the definition of a prepackaged product in subsection B.01.001(1) of the FDR; however, not all prepackaged products are SFs. A limited number of food categories are eligible as SFs, as per the incorporated by reference document List of Permitted Supplemented Food Categories. Further information on the food categories that are permitted to be SFs is provided in Section 4.1. In addition, the following foods are not eligible as SFs because they are either subject to other provisions in the FDR, or due to their higher level of risk for consumers if they were supplemented, such as foods intended for certain vulnerable populations (e.g., young children):
- a) Foods required or permitted the addition of nutrients for fortification purposes
The addition of vitamins, mineral nutrients and amino acids is required or permitted in the FDR for certain foods for achieving the following objectives:
- to restore the nutrients lost during processing, storage or handling,
- to make a substitute food nutritionally equivalent to the food it is intended to replace,
- in response to a public health need, where a risk of deficiency has been identified, and
- to ensure the appropriate nutrient composition for foods for special dietary uses (e.g., meal replacements and nutritional supplements).
The table to section D.03.002 of the FDR provides a list of the foods that are required or permitted to contain added vitamins, mineral nutrients and amino acids. As the provisions for fortification already exist throughout Part B and D of the FDR and addition of nutrients is based on nutritional objectives, these foods are not eligible as SFs, subject to the exceptions described below. However, the Supplemented Foods Regulations do not restrict the use of fortified foods as ingredients in the manufacture of SFs (e.g., the use of fortified juice in the manufacture of an SF is permitted).
Exceptions:
Certain foods for which the addition of nutrients for fortification purposes is required or permitted may also be eligible as SFs and would be subject to conditions of use that apply to all SFs.
Foods under items 2 and 14 of the table to section D.03.002 of the FDR are intended as SFs, they must not be represented and formulated as per their applicable fortification provisions, i.e., the products cannot be SFs and fortified foods at the same time.
For item 26 in the table to section D.03.002 of the FDR, mineral water, spring water, water in sealed containers are permitted the addition of fluoride for fortification purposes. As fluoride is not a permitted SI, these foods are permitted to be formulated and represented as per the provisions of the Supplemented Foods Regulations and other applicable fortification provisions at the same time.
In addition, Health Canada has previously issued Interim Marketing Authorizations (IMAs) for foods to address important public health needs. These include the addition of calcium, with or without vitamin D, to orange juice, and orange and tangerine juice sold as such, in fluid, concentrated, or reconstituted forms. Although the IMA periods have expired, Health Canada has adopted an Interim Policy on the Use of Expired Interim Marketing Authorizations Related to Food Fortification. If these products are intended to be SFs, they must meet the requirements of the Supplemented Foods Regulations and must not be represented and formulated as per the conditions of the expired IMA, i.e., the product cannot be an SF and fortified food at the same time.
- b) Foods for special dietary use
Foods for special dietary use (FSDU) are specially processed or formulated to meet the particular needs of a person in whom a physical or physiological condition exists as a result of a disease, disorder or injury, or for whom a particular effect, including but not limited to weight loss, is to be obtained by a controlled intake of foods. Division 24, Part B of the FDR sets out compositional, labelling and marketing requirements applicable to FSDU. Therefore, foods that are subject to Division 24 requirements are not permitted to be SFs.
Exception:
Gluten-free foods are a category of foods in Division 24, Part B of the FDR that do not have specific compositional or labelling requirements (aside from not containing any gluten protein). Gluten-free products that meet all the requirements of the Supplemented Foods Regulations can be SFs. However, a food that is both a gluten-free food and a food for special dietary use referred to in any of paragraphs of subsection B.24.003(1)(f) to (f.2) and (h) to (j) of the FDR is not permitted to be an SF. Furthermore, while section D.03.003 of the FDR allows the fortification of gluten-free foods, fortified gluten-free foods cannot be SFs.
- c) Infant foods and foods targeted to children under 4 years of age, pregnant or breastfeeding women
Infant foods are regulated under Division 25 of the FDR, which specifies compositional and labelling requirements. These foods are not eligible as SFs.
Toddlers between 1 and 3 years of age experience more rapid growth than older children, making them more susceptible to nutrient imbalances. For this reason, foods that are intended for children under four years of age are not permitted to be SFs.
Pregnant women and breastfeeding women are a particularly vulnerable life-stage group. Many SIs have never been assessed as food ingredients for their safety in pregnant or breastfeeding women and remain unapproved for such use. Therefore, foods labelled or advertised for consumption by these populations are not permitted to be SFs.
- d) Prepackaged unprocessed foods or minimally processed foods
Unprocessed foods and minimally processed foods are not permitted to be SFs. Minimally processed foods include raw fruit or vegetables that have been peeled, sliced, chopped or shredded prior to being packaged for sale. The prepackaged foods listed below are not permitted to be SFs:
- Raw single ingredient meat, meat by-product, poultry meat or poultry meat by-product;
- Raw single ingredient marine or fresh water animal product;
- Whole or cut fresh, frozen, canned or dried fruits or vegetables;
- Nuts, grains, legumes and seeds; and
- Whole eggs, including liquid, frozen or dried eggs (this also includes cooked forms), or whole egg mixes.
- e) Alcoholic beverages
Alcoholic beverages with an alcohol content of more than 0.5% are not permitted to be SFs. Manufacturers or distributors of SFs must ensure that the alcohol content of their product does not exceed 0.5% during the shelf life of the product.
- f) Specialty foods
Specialty food is defined in subsection B.01.012(1) of the FDR and means a food that has special religious significance and is used in religious ceremonies; or is an imported food that is not widely used by the population as a whole in Canada, and for which no readily available substitute is available in Canada. A food will no longer meet the definition of a specialty food if it meets the requirements of the Supplemented Foods Regulations. For additional information on specialty foods, refer to the CFIA's website on Bilingual food labelling.
- g) Novel Foods
A novel food is defined in Division 28 of Part B of the FDR as:
- a substance, including a microorganism, that does not have a history of safe use as a food;
- a food that has been manufactured, prepared, preserved or packaged by a process that
- (i) has not been previously applied to that food, and
- (ii) causes the food to undergo a major change; and
- a food that is derived from a plant, animal or microorganism that has been genetically modified such that
- (i) the plant, animal or microorganism exhibits characteristics that were not previously observed in that plant, animal or microorganism, or
- (ii) the plant, animal or microorganism no longer exhibits characteristics that were previously observed in that plant, animal or microorganism, or
- (iii) one or more characteristics of the plant, animal or microorganism no longer fall within the anticipated range for that plant, animal or microorganism
Novel foods are regulated under Division 28, Part B of the FDR and therefore must undergo a mandatory premarket assessment prior to being authorized for sale in Canada. If the premarket assessment concludes that the food is safe, novel foods are issued a letter of no objection, in which case, they may be used as ingredients in foods, including in SFs. Novel food authorizations are provided without conditions of use, thus the data must demonstrate safety for general use as food. For more information, refer to the Novel Foods website. Refer to Section 4.2.14.3 for information on novelty determination and Section 6.1 for considerations about premarket submission paths for ingredients to be added to SFs as SIs.
4.0 Requirements for complying with the Supplemented Foods Regulations
As with any food, the onus is on the food manufacturer or distributor to ensure that a food offered for sale in Canada complies with all applicable legislative and regulatory provisions, including but not limited to requirements under the FDA and the Safe Food for Canadians Act (SFCA), and the Regulations associated with these Acts. This includes, for example, ensuring compliance with food labelling requirements, provisions for the use of food additives and the prohibitions in section 4 of the FDA, which prohibits the sale of a food that contains a poisonous or harmful substance or that is adulterated. Parts 1 and Part 2 of the List of Contaminants and Other Adulterating Substances in Foods, incorporated by reference into Division 15, Part B of the FDR, set out substances and contaminants not permitted in food, or the maximum levels for those substances and contaminants. Maximum levels for certain contaminants in foods are also set out in the List of Maximum Levels for Various Chemical Contaminants in Foods, which is maintained on Health Canada's website. Appendix 3 includes a list of additional ingredients that are considered inappropriate for addition to foods, including SFs.
The addition of an SI to an SF is not considered adding a poisonous or harmful substance or adulterating the food, contrary to section 4 of the FDA, if the ingredient is added according to its specific conditions of use. However, a prepackaged product, other than an SF, is adulterated if a substance listed in column 1 of the List of Permitted Supplemental Ingredients has been added to it other than in accordance with the FDR (e.g., for an approved food additive purpose). Furthermore, an SF can only be added as an ingredient to another prepackaged product if that product is also an SF that meets the Supplemented Foods Regulations.
SFs must also comply with all the provisions of the Supplemented Foods Regulations with respect to food categories permitted as SFs (Section 4.1), SIs and their conditions of use (Section 4.2), labelling (Section 4.3), and representations (Section 4.4).
4.1 Food categories permitted under the Supplemented Foods Regulations
Only foods belonging to the food categories set out in the List of Permitted Supplemented Food Categories can be SFs.
Table 2 below provides a description of food categories outlined in the List of Permitted Supplemented Food Categories. It is the manufacturer's or distributor's responsibility to ensure that their product meets the requirements of the List and the criteria for each category outlined below. The descriptions in Table 2 provide an overview of the type of foods that belong to each permitted category and outline exclusions of foods that are not within the scope of each category. Manufacturers or distributors are encouraged to contact the Food Directorate's SMIU (smiu-ugdi@hc-sc.gc.ca) to seek clarity on whether their product is within the scope of a category in the List of Permitted Supplemented Food Categories.
A product cannot be an SF if it does not belong to a permitted food category. In order to market a product belonging to a food category that is not part of the List of Permitted Supplemented Food Categories, refer to Section 6.0 for information on the process to request an amendment to the List.
4.1.1 Description of food categories in the List of Permitted Supplemented Food Categories
A number of foods have prescribed standards of composition and identity in the FDR. Foods with prescribed standards of composition and identity must comply with every aspect of the specifications of those standards. There are no standards of identity or composition for SFs; therefore, any SF that could be mistaken for a standardized food must be labelled so as to clearly indicate how the food differs from the standard by using a modified common name. The names of food categories in the List of Permitted Supplemented Food Categories are not considered common names. For information on common names, refer to Section 4.3.8. In addition, foods captured in Section 3.2 are not permitted to be SFs unless an exception is specified.
Products belonging to the categories in Table 2 may share characteristics with both foods and natural health products. Manufacturers or distributors are encouraged to consult the Guidance Document: Classification of products at the food-natural health product interface: products in food formats to determine whether their product is a food or a natural health product or request a classification determination as per Section 3.1.
Certain food categories, as identified in the List of Permitted Supplemented Food Categories, include concentrates and mixes to be reconstituted into beverages. SFs belonging to these food categories must meet the requirements of their respective category and List of Permitted Supplemental Ingredients as consumed. As SFs are subject to specific labelling requirements, prepackaged one bite confections sold individually are excluded from permitted categories in the List since they are exempt from the labelling requirements set out in the FDR and the Safe Food for Canadians Regulations (SFCR). Additional information on one bite confections is available on the CFIAS's webpage.
| Item No. | Food Category | Description |
|---|---|---|
| (1) | Carbonated or non-carbonated water-based beveragesFootnote 1 | Ready-to-drink carbonated or non-carbonated water-based beverages, including their concentrates and mixes to be reconstituted as beverages. The beverages as consumed must be water-based, i.e., water must constitute more than 50% of the beverage. Products in dry mix formats must be packaged to allow for controlled servings. For clarity this category also encompasses the following product types:
Exclusions:
|
| (2) | Fruit drinks (from fruit concentrate), vegetable drinks (from vegetable concentrate), fruit and vegetable drinks (from fruit and vegetable concentrates), fruit nectars, fruit-based smoothies, vegetable-based smoothies, or fruit- and vegetable-based smoothiesFootnote 2 | Ready-to-drink drinks obtained from fruit and/or vegetable concentrates that are manufactured as frozen concentrates to be reconstituted. Fruit nectars, vegetable drinks, bases and mixes for vegetable drinks and a mixture of vegetable juices Ready-to-drink drinks that are manufactured by blending and/or pureeing fruits and vegetables with juice, water or other ingredients to a thick consistency Exclusions:
|
| (3) | JuicesFootnote 2 | Juices and mixed juices obtained directly from fruits or vegetables, and from concentrates prepared by the addition of water to juice from which water has been removed. |
| (4) | Single-serving prepackaged tea, coffee or herbal infusions | Single-serving prepackaged tea from Camellia sinensis or other herbal infusion products available in tea bags. Single-serving prepackaged ground coffee products to be brewed, i.e., coffee pods. Exclusions:
|
| (5) | Bars | Products pressed, extruded, baked or otherwise formed into bars, which includes:
Exclusions:
|
| (6) | Hard, soft, or semi-soft candiesFootnote 3 | Candy products in a hard, soft or semi-soft form. Includes products that are sweet treats, that may or may not have a filling, their dietetic counterparts, and products that may or may not contain cocoa. Includes gummies that are represented as foods Exclusions:
|
| (7) | Chocolate confectioneriesFootnote 3 | Chocolate confection products which are derived from one or more cocoa products. The format of products in this category can be in any form, e.g., bars, pieces, bites and squares. Exclusions:
|
| (8) | Chewing gumsFootnote 3 | Products made from natural or synthetic gum base containing flavours, sweetening agents (e.g., sucrose), aroma compounds, and food additives. Includes bubble gum and breath-freshener gum products. Exclusions:
|
| (9) | Ice pops | Refers to water-based flavoured ice products. Includes popsicles and freezies. Exclusions:
|
|
||
4.2 Supplemental ingredients and their conditions of use
The SIs permitted for addition to SFs are specified in the List of Permitted Supplemental Ingredients along with their conditions of use. An SF can contain one or more SIs permitted in the List provided that the conditions of use are met for all SIs added to the SF. A product cannot be an SF if the conditions of use for one or more SIs that it contains are not met, even if it belongs to a permitted category.
SFs are subject to the same general restrictions and prohibitions as other foods regarding the addition of ingredients to foods. The addition of SIs is subject to additional restrictions and requirements. These are referred to as conditions of use and set out in the List of Permitted Supplemental Ingredients and summarized below. Refer to Section 6.0 for information on the process to request a change to the List.
4.2.1 Information included in the List of Permitted Supplemental Ingredients
The List of Permitted Supplemental Ingredients includes the following information:
- Column 1 - Description: This column indicates the name of the SI that is permitted for addition in SFs. The SI must be declared on the label as listed in this column unless noted otherwise in column 5. The SIs are grouped into four parts in the List, specifically, Part I - Vitamins, Part II - Mineral nutrients, Part III - Amino acids, and Part IV - Other Supplemental Ingredients. Within each part in the List, the SIs are listed in alphabetical order.
- Column 2 - Permitted in: This column describes the food category, as per the List of Permitted Supplemented Food Categories, to which the SI identified in column 1 may be added. An SI is permitted for addition only to the food category that is specified in this column.
- Column 3 - Maximum amounts and units per serving of stated size: This column indicates the maximum amount that must not be exceeded for the corresponding SI listed in column 1, when added to a product belonging to the food category in column 2 of the List of Permitted Supplemental Ingredients. The maximum amounts apply to the quantity declared on the label, which must include contributions from all added and naturally occurring sources in the product. The quantity of an SI must be expressed on the label using the same unit that is specified for the maximum amount in column 3, unless noted otherwise.
- Column 4 - Cautionary statements required on the label: This column indicates the cautionary statements that are required on the label, if any, for the corresponding SI listed in column 1, and the threshold levels above which these statements are required. The threshold levels are in respect of the quantity declared on the label.
- Column 5 - Other: This column indicates any other conditions of use related to the SIs. This includes, as applicable, conditions on the forms of the ingredient permitted for addition (e.g., the use of nicotinic acid as a source of niacin is not permitted), ingredient specifications, and/or additional requirements related to labelling or representations.
| Supplemental Ingredient | Conditions of Use | |||
|---|---|---|---|---|
| Column 1 Description |
Column 2 Permitted in |
Column 3Footnote ii Maximum Amount and Units Per Serving of Stated Size |
Column 4Footnote iii, Footnote iv Cautionary Statements Required on the Label |
Column 5 Other |
Vitamin A (Retinol) |
Foods belonging to a category listed in the List of Permitted Supplemented Food Categories, other than foods belonging to category 1 that contain added caffeine and a total amount of caffeine from all sources of more than 150 ppm |
745 µg |
(a) All products containing vitamin A (retinol) require the cautionary statements:
(b) If the amount of vitamin A (retinol) declared on the label is more than 149 µg per serving, the following cautionary statement is required to replace (a)(ii):
(c) If the amount of vitamin A (retinol) declared on the label is more than 149 µg per serving, the following additional cautionary statement is required:
|
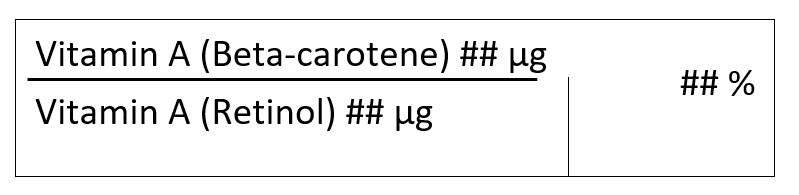
(a) When retinol, and/or its derivatives and beta-carotene are present in the supplemented food, the following requirements apply:
(b) When retinol and/or retinyl esters is the only form of vitamin A present in the supplemented food, the percent DV is determined based on the amount of vitamin A (retinol) in micrograms of RAE, calculated on the basis of the relationship set out in paragraph D.01.003 (1)(a) |
| Explanation | ||||
| Refers to the SI vitamin A (Retinol) and must be expressed in the Supplemented Food Facts table as such | Refers to the foods to which vitamin A (Retinol) can be added, which are any permitted food categories with the exception of foods belonging to category 1 that contain added caffeine and a total amount of caffeine from all sources of more than 150 ppm. As there are no other listings for the use of vitamin A (Retinol), it is not permitted to be added to any other foods as an SI. |
Refers to the maximum amount of vitamin A (Retinol) that can be declared on the label per serving when added as an SI to foods permitted in column 2. The maximum amount applies to contributions from added and naturally occurring sources. | Refers to the cautionary statements that are required at the identified threshold levels. Products with any amount of vitamin A (Retinol) added as an SI must declare on the label the cautionary statements indicated under (a). If the total amount of vitamin A (Retinol) declared on the label is more than 149 µg per serving, the modified cautionary statement indicated under (b)(i) is required instead of the cautionary statement indicated under (a)(ii). Additionally, the caution statement indicated under (c)(i) is required in addition to the caution statements indicated in (a)(i), (b)(i). See Section 4.2.4.1 for information on calculating the appropriate number of servings to include in the caution statement indicated in (c)(i). |
Refers to additional conditions of use, as applicable for the use of an SI in SFs. For vitamin A (Retinol), there are additional conditions of use when both Retinol and/or its derivatives and beta-carotene are present in the SF. See Section 4.3.1.1 for further information on declaring vitamin A (Retinol) and vitamin A (beta-carotene) on the label. |
|
||||
4.2.2 Minimum amounts of supplemental ingredients
The List of Permitted Supplemental Ingredients does not include any minimum levels of additions for SIs. As SIs are not added to SFs for a nutritional purpose, there is no required minimum amount of addition.
4.2.3 Maximum amounts of supplemental ingredients
SIs in the List of Permitted Supplemental Ingredients include a maximum amount, which is set using a risk-based approach. The levels are not related to nutritional requirements and they are not recommended levels for addition. In general, the maximum amounts apply to the quantity declared on the label, which must include contributions from all added and naturally occurring sources in the product (e.g., amounts added as an SI, other added food ingredients, and naturally occurring amounts of the SI in other ingredients). For amino acids specifically, the quantity declared on the label must be based on the total amount of free amino acids from all sources, i.e., added and naturally occurring free amino acids (e.g., in fruit juice). Therefore, for amino acids, the quantity declared on the label excludes naturally occurring protein-bound amino acids (i.e., amino acids present in the product as intact protein) and peptides (e.g., aspartame).
Details on how the maximum amounts were developed can be found in Appendix 4 (vitamins and mineral nutrients) and Appendix 5 (amino acids).
4.2.4 Cautionary statements for supplemental ingredients
The declaration of SIs above certain threshold levels may require cautionary statements to be displayed on the label of an SF in order to inform consumers of the health risks of excess consumption or consumption by vulnerable populations. Column 4 of the List of Permitted Supplemental Ingredients includes threshold levels for each SI, above which various cautionary statements may be required. Certain SIs require the applicable cautionary statements at any level. Appendix 4 and Appendix 5 include more details regarding the determination of threshold levels for cautionary statements for vitamins and mineral nutrients, and for amino acids, respectively.
A product may contain one or more added SI(s); therefore, the requirement for cautionary statement(s) must be determined by comparing the label declared amount of each SI to the applicable threshold levels for cautionary labelling. If multiple SIs require the same cautionary statement, it must only appear once on the label. This is also described in footnote iv of the List.
The requirement for cautionary statement(s) for an SI may vary depending on the permitted food category that the SI is added to, e.g., the requirements for vitamins and mineral nutrients vary when added to beverages containing added caffeine with a total amount of caffeine from all sources of more than 150 ppm compared to other food categories. These requirements are outlined in the List of Permitted Supplemental Ingredients. SFs that do not exceed the lowest threshold levels specified in column 4 for any SI are not required to carry any cautionary statements on the label.
It is the responsibility of the manufacturer or distributor to ensure that all required cautionary statements appear on the product's label. When required, cautionary statements must be grouped together, under a standardized bolded heading "Caution" on the label of an SF (see Section 4.3.1 for more details).
4.2.4.1 Cautionary statement related to the maximum number of servings
The quantity declared on the label of an SI in a single serving of an SF must not exceed the maximum amount specified for that SI in column 3 of the List of Permitted Supplemental Ingredients. However, as noted in column 4 of the List, products containing SIs above certain threshold levels require the cautionary statement "Do not [eat/drink] more than X serving(s) per day", where X is the number of servings that provides a daily amount of the SI, based on the amount declared on the label, that does not exceed the maximum amount for that SI.
- The maximum number of servings (X) indicated on the label is calculated by dividing the maximum amount of an SI by the quantity declared on the label per serving of stated size. The resulting value must be rounded down as indicating partial servings on the label would not be appropriate.
X servings = maximum amount for the SI ÷ amount declared on the label per serving of stated size
For example, an SF bar containing vitamin C as an SI with the quantity of vitamin C declared on the label of 280 mg per serving would require the cautionary statement pertaining to maximum number of servings as the amount declared on the label exceeds the threshold level for this cautionary statement set out in the List of Permitted Supplemental Ingredients of 151 mg. The cautionary statement must indicate 2 (or less) servings since consuming more than two servings (756 mg ÷ 280 mg = 2.7 servings) would lead to intakes exceeding the maximum amount.
- If multiple SIs trigger a maximum number of servings cautionary statement, i.e., more than one SI in the formulation exceeds its respective threshold level for this cautionary statement, then the most conservative number of servings (X) must be applied on the label.
For example, if an SF bar contains vitamin C as an SI and the amount declared on the label is 280 mg per serving, the cautionary statement must indicate "Do not eat more than 2 servings per day". However, if the same bar also contains calcium as an SI at a label declared quantity of 200 mg per serving, then more than one serving of the bar would exceed the maximum amount for calcium (293 mg). Therefore, the cautionary statement on the label of the SF bar must indicate "Do not eat more than 1 serving per day". - The use of more conservative statements than required based on the quantity declared on the label are also acceptable on the label.
For example, if the maximum number of servings (X) for a product is determined to be 3 servings, statements indicating "Do not eat more than 1 serving per day" or "Do not eat more than 2 servings per day", would also be acceptable. - If one serving of a product is equivalent to one container, the word "container" or other appropriately descriptive term can be used in the cautionary statement instead of "serving", provided that the container is equivalent to a serving.
For example, for a beverage in a 473 mL can, since one can would be equivalent to a serving, the statement "Do not drink more than 1 can per day" would be acceptable.
4.2.4.2 Cautionary statements advising against use by certain population groups
SIs above certain threshold levels can require one or more of the following separate or combined statements that begin with "Not recommended for". Examples include:
- "Not recommended for those under 14 years old";
- "Not recommended for pregnant or breastfeeding women";
- "Not recommended for those under 14 years old or pregnant or breastfeeding women"; and
- "Not recommended for those under 14 years old, pregnant or breastfeeding women or individuals sensitive to caffeine".
When more than one of these statements are required, the statements must be grouped together to ensure the "Not recommended for" element or any population groups are not repeated, and the remaining elements are joined by means of a conjunction or punctuation.
- Example 1: When more than one cautionary statement on separate population groups, i.e., "Not recommended for those under 14 years old" and "Not recommended for pregnant or breastfeeding women" are required, the common element "Not recommended for" must be grouped together in a combined statement as "Not recommended for those under 14 years old or pregnant or breastfeeding women".
- Example 2: When more than one cautionary statement is required which include the same population groups, the combined statement must be used on the label. For example, the cautionary statements "Not recommended for those under 14 years old" and the combined cautionary statement "Not recommended for those under 14 years old or pregnant or breastfeeding women", include the population group of those under 14 years old; however, when the addition of SIs require both statements, only the combined statement must be used on the label.
An SI may not be suitable for consumption by individuals under a certain age, triggering the requirement for an age-related caution statement to appear on the label. The age-related cautionary statement, whether appearing separately or as part of a combined statement on the label, must include the most restrictive age (highest age) applicable. For example, if a product contains one SI requiring the age-related cautionary statement "Not recommended for those under 14 years old" and one SI requiring the age-related cautionary statement "Not recommended for those under 18 years old", the statement with the more restrictive age, i.e., "under 18 years old" must be used. This cautionary statement must only appear once on the label even if it is triggered by more than one SI, either on its own or as part of a combined cautionary statement. There must not be multiple ages indicated in the cautionary statements.
4.2.4.3 Cautionary statement related to use of other supplemented foods or supplements
For most SIs, the following cautionary statements are required at the same threshold levels:
- "Do not [eat/drink] on the same day as any other supplemented foods with [the same supplemental ingredients/(name specific ingredients)]"; and
- "Do not [eat/drink] on the same day as any other supplements with [the same supplemental ingredients/(name specific ingredients)]".
As a result, these are listed as the combined statement "Do not [eat/drink] on the same day as any other supplemented foods or supplements with [the same supplemental ingredients/(name specific ingredients)]", in the List of Permitted Supplemental Ingredients. For certain SIs (i.e., vitamin A (retinol)), one of the statements is required at a lower threshold; however, when both are required, only the combined statement needs to appear on the label as it would be redundant to include on the label a combined and separate statements regarding the use of supplements or supplemented foods.
4.2.5 Overages for supplemental ingredients
The maximum amounts for SIs in the List of Permitted Supplemental Ingredients do not include overages.
It is the manufacturer's responsibility to ensure that the overage added for an SI, when permitted, is in accordance with good manufacturing practice (GMP), provided that such an overage does not present a risk to health and is not misleading.
The overage for an SI should be based on the minimum amount of the ingredient that must be added to assure that the quantity declared on the label is met, within acceptable tolerances, throughout a product's shelf life. A number of variables affect the amount of overage that is needed for a specific SI, including the food matrix, method of manufacture, type of packaging, storage conditions, and shelf life. A food stability study can help the manufacturer determine the minimum amount of the SI to add during manufacturing in order to meet the quantity declared on the label at the end of the shelf life and avoid excessive overages. Manufacturers must be able to justify the overage amount, as well as ensure that the ingredient content meets the quantity declared on the label, is not likely to result in an excessive overage, and will not present a risk to the health of consumers.
The document Compliance of Supplemental Ingredients Declared on Labels of Supplemented Foods provides information on which SIs are permitted overages. To request a copy of this document, please send an e-mail to supplementedfoods-alimentssupplementes@hc-sc.gc.ca with the subject line "Requesting Compliance of supplemental ingredients declared on labels of supplemented foods".
4.2.6 Deficiencies for supplemental ingredients
Certain SIs are not permitted an overage. For these SIs, the product must not contain more than the quantity declared on the label for the duration of its shelf life. To achieve this, manufacturers should ensure that the quantity declared on the label represents the amount of the SI at the beginning of shelf life. To account for degradation over shelf life, deficiencies under the quantity declared on the label are permitted. It is the manufacturer's responsibility to ensure that the deficiencies, when permitted, are in accordance with GMP, provided that such a deficiency does not present a risk to health and is not misleading.
The document Compliance of Supplemental Ingredients Declared on Labels of Supplemented Foods mentioned in Section 4.2.5, provides information on which SIs are permitted deficiencies.
4.2.7 Direct or indirect addition of supplemental ingredients
The addition of SIs is subject to the conditions of use outlined in the List of Permitted Supplemental Ingredients regardless of whether the ingredient is added directly or indirectly. If an SF is used as an ingredient in another SF, the SIs in that first SF are considered to also be SIs in the final SF. For example, an SF juice used as an ingredient in a juice blend would be acceptable; however, the finished juice blend must be compliant with the Supplemented Foods Regulations. Adding an SF as an ingredient to a food that is not itself an SF (i.e., belongs to a permitted food category and complies with the conditions of use in respect of all SIs) is not permitted. Section B.29.004 of the FDR outlines the requirements for SFs intended solely for use as ingredients in the manufacture of other SFs that are intended for sale to consumers.
4.2.8 Supplemental ingredients made up of two parts
Ingredients added to SFs for supplemental purposes may be made up of two parts, which could both be added as SIs. When both parts of the ingredient are found in the List of Permitted Supplemental Ingredients, both are considered SIs and subject to the conditions of use as SIs. However, if both parts of the ingredient are nutrients (i.e., vitamins, mineral nutrients and/or amino acids) an exemption may apply. These are most commonly salts, such as potassium glutamate, which is a source of two nutrients, potassium and glutamic acid.
When both parts of the ingredient are nutrients found in the List of Permitted Supplemental Ingredients, and are declared in the "Supplemented with" section of the Supplemented Food Facts table (SFFt) (see Section 4.3.1), both are considered SIs and are subject to the conditions of use stated in the List (e.g., addition of potassium glutamate as a supplemental source of both potassium and glutamic acid).
If only one part of the ingredient made up of two nutrients is declared in the "Supplemented with" section of the SFFt, then only that part is considered an SI and subject to the conditions of use as an SI (e.g., addition of calcium phosphate just as a source of calcium). To facilitate the addition of the SI (e.g., calcium) the other part (e.g., phosphorus) is added as part of that ingredient but not considered as an SI. However, if the part of the ingredient that is not listed in the "Supplemented with" section of the SFFt (e.g., phosphorus) is the subject of a claim or is declared elsewhere on the label (except in the case of a core nutrient that must be listed in Section 1 of the SFFt as per section B.29.002 of the FDR (see Section 4.3.1.1 a)), it would be considered an SI. In this case, the other part (e.g., phosphorus), is considered to be added for a functional purpose, rather than as an incidental salt to deliver the SI (e.g., calcium).
4.2.9 Quality standards for supplemental ingredients
Vitamins, mineral nutrients and amino acids added to SFs, like other food ingredients, must be food-grade. Ingredients that meet internationally recognized quality standards such as those set out in the most recent versions of the Food Chemicals Codex, Codex Alimentarius International Food Standards, United States Pharmacopeia, or European Pharmacopoeia meet this requirement. While there may be specifications in the aforementioned quality standards for substances permitted as SIs other than vitamins, mineral nutrients and amino acids, they must meet relevant quality parameters set out in the List of Permitted Supplemental Ingredients. These ingredients are permitted based on what has been evaluated as part of the Food Directorate's safety assessment and considered to be acceptable for use in SFs, under certain conditions of use where/as applicable. Examples of required quality parameters could include acceptable types of extraction solvents, standardization or minimum purity of constituents, and limits on impurities.
Preparations of SIs being added in SFs must respect applicable regulatory provisions. For example, caffeine-enriched extracts added as "caffeine" must not be novel food ingredients; the use of food additives, including carrier or extract solvents, must comply with the Lists of Permitted Food Additives; and preparations must not contain any substances that violate section 4 of the FDA. Additionally, the product must not include ingredients that are not acceptable for use in foods.
4.2.10 Vitamins and mineral nutrients
The List of Permitted Supplemental Ingredients includes vitamins and mineral nutrients that are permitted to be added to SFs, as well as their maximum amounts and other conditions of use. These maximum amounts were established based on available scientific evidence to help ensure that their addition does not contribute to excessive intake, which could lead to adverse health effects. More information on how the maximum amounts for vitamins and mineral nutrients were developed is outlined in Appendix 4.
4.2.10.1 Vitamins and mineral nutrients not permitted for addition to supplemented foods
The vitamins and mineral nutrients listed in Table 4 below are currently not acceptable for addition to SFs at any level. These nutrients are associated with risks for the general population or for a specific vulnerable population for whom it is unlikely that cautionary labelling would be an effective risk mitigation tool.
| Vitamin or Mineral | Rationale | Reference |
|---|---|---|
| Folic acid | 53-73% of the Canadian population have blood values associated with intakes of folic acid above the Tolerable Upper Intake Levels (ULs)Footnote 3 set by the National Academies of Sciences, Engineering, and Medicine (NASEM), formerly known as the Institute of Medicine (IOM). Exceeding the UL has adverse effects which include masking and potentially delaying diagnosis of a vitamin B12 deficiency. | (NASEM, 1998), (MacFarlane et al., 2011) |
| Iron | Supplemental iron intake could be a hazard for individuals with undiagnosed hemochromatosis. Hereditary hemochromatosis is an inherited disorder of iron metabolism which affects 1 in every 200-250 individuals of Northern European descent. This disorder results in high rates of iron absorption, which can accumulate in the body and cause deposits in the tissues and organs. | (Dietitians of Canada, 2015) |
| Manganese | The 95th percentile of estimated intakes of manganese are at or exceeding the UL for children 4 to 8 years, males 14 to 18 years and males 19 to 30 years. Elevated blood manganese concentrations and neurotoxicity were selected as the critical adverse effects upon which the NASEM based the UL for manganese. | (NASEM, 2001), (Canadian Community Health Survey (CCHS), 2015) |
| Nicotinic acid (a form of niacin) | There are known side effects associated with excess intake of nicotinic acid, a form of niacin. The adverse effect associated with exceeding the UL set by the NASEM is flushing of the skin. However, niacinamide is another form of niacin that can be used safely in foods. | (NASEM, 1998) |
| Vitamin K | Changes to vitamin K intake can be a hazard for individuals on blood thinning medication. This risk could potentially be mitigated with a cautionary statement; however, extensive cautionary labelling such as statements related to medical conditions, medications or duration of use are not appropriate for foods. | (NASEM, 2001) |
Iodine
Iodine is currently not acceptable for addition to SFs based on 95th percentile intakes, estimated from single spot urine samples (Canadian Health Measures Survey, Cycle 2 (2009-2011)), exceeding the UL set by the NASEM for children 4 to 8 years and certain adult populations. The adverse effect associated with exceeding the UL is thyroid dysfunction.
However, more recent iodine intake estimates obtained from two day spot urine samples (Canadian Health Measures Survey, Cycle 5 (2016-2017)) have become available, which more accurately estimate iodine intakes compared to single spot urine samples.
In light of this new data, Health Canada will reassess its position on iodine as an SI. If it is found that iodine may be safely consumed as an SI added to SFs, a proposal will be made to add iodine to the List of Permitted Supplemental Ingredients as per Section 6.4.
4.2.10.2 Vitamins and mineral nutrients acceptable for addition to supplemented foods
The maximum amounts for vitamins and mineral nutrients when used as SIs were developed to protect the most vulnerable age-gender groups. The approach and methods used to determine the most vulnerable age-gender groups are detailed in Appendix 4. Two sets of maximum amounts of vitamins and mineral nutrients were developed for SFs:
- Maximum amounts for supplemented foods other than beverages containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm
The addition of vitamins and mineral nutrients as SIs to these SFs, may require cautionary statements if the quantity declared on the label exceeds the threshold levels specified in the List of Permitted Supplemental Ingredients for that SI. The cautionary statements are required to inform consumers of the potential risks of excessive intake. Section 4.2.4 includes information on the cautionary statements that may be required on an SF label for vitamins and mineral nutrients. Refer to Appendix 4 for the rationales for the requirement of cautionary statements for vitamins and mineral nutrients. - Maximum amounts for beverages containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm
Separate maximum amounts have been established for the addition of vitamins and mineral nutrients as SIs in these SFs. If the addition of a vitamin or mineral nutrient is not explicitly permitted as per column 2 of the List of Permitted Supplemental Ingredients for these SFs, then it must not be added regardless of whether the same vitamin or mineral nutrient is permitted for addition in other SF categories. For example, vitamin A (retinol) is permitted for addition in all SFs with the exception of beverages that contain added caffeine and a total amount of caffeine from all sources of more than 150 ppm.
The maximum amounts set for vitamins and mineral nutrients for these SFs are conservative, because additional variables, described in Appendix 4, were considered in their calculation. The addition of vitamins and/or mineral nutrients to these products does not trigger the requirement for cautionary statements. However, these SFs require cautionary statements triggered by the addition of other SIs (e.g., caffeine) as outlined in the List of Permitted Supplemental Ingredients.
4.2.11 Amino acids
The List of Permitted Supplemental Ingredients includes amino acids that are permitted to be added to SFs, including their maximum amounts and other conditions of use. The maximum amounts for standard amino acids involved in protein synthesis are established using a risk-based approach to ensure that the consumption of SFs does not result in excessive intakes of supplemental amino acids. Details on how the maximum amounts for amino acids were developed are outlined in Appendix 5. See Section 4.4.6 for information on amino acid claims for SFs.
4.2.11.1 Amino acids not permitted for addition to supplemented foods
Currently, only the L-isomers of amino acids are considered appropriate for addition to SFs as per the List of Permitted Supplemental Ingredients. Most D-isomers of amino acids provide little or no nutritional support to humans (Friedman & Levin, 2012), and many may have different biological or toxic effects compared to the L-isomers (Gu et al., 2020).
Amino acids listed in the table below are not currently acceptable for addition to SFs at any level.
| Amino acid | Rationale | Reference |
|---|---|---|
| Tryptophan | The addition of tryptophan or other essential amino acids to foods for reasons beyond nutritive purposes may increase the risk of imbalance for individuals with low protein intake (see Appendix 5 for details). Tryptophan has the highest excess over its requirement among all the essential amino acids in the background diet at the 5th percentile of intake for the most vulnerable subpopulation. As a result, any supplementation would increase this relative excess, potentially leading to imbalances. In addition, tryptophan functions as a precursor to the neurotransmitter serotonin, which may cause interactions with certain medications. As tryptophan is permitted for addition to certain foods as set out in subsection D.03.002 of the FDR, industry is encouraged to discuss regulatory options for their specific products with Health Canada. | (CCHS, 2015), (Kikuchi et al., 2021) |
4.2.11.2 Amino acids permitted for addition to supplemented foods
The List of Permitted Supplemental Ingredients includes maximum amounts and other conditions of use for the 20 standard amino acids involved in protein synthesis.
The addition of these L-amino acids in the free, hydrated, or anhydrous form, or as a hydrochloride or mineral salt (e.g., sodium or potassium salt) to foods belonging to the List of Permitted Supplemented Food Categories is permitted. Refer to Section 4.2.8 for more information regarding SIs made up of two parts.
Unlike vitamins and mineral nutrients, there is only one set of maximum amounts for amino acids, which applies to all categories in the List of Permitted Supplemented Food Categories.
The maximum amounts for amino acids apply to amounts declared on the label. The declared amount must include the amount of the free amino acid in an SF from amounts added as SIs, amounts contributed by food ingredients, and amounts of naturally occurring free amino acids (e.g., in fruit juice). Any naturally occurring levels of protein-bound amino acids in an SF (i.e., amino acids present in the product as intact protein) would not count towards the amino acid declaration. In addition, short-chain peptides and other amino acid metabolites or derivatives should not be considered when determining the declared value or comparing against maximum amounts for single amino acids. Short chain peptides, metabolites or derivatives of amino acids (e.g., N-acetyl-L-cysteine) may have different absorption and metabolism, and different physiological or therapeutic uses (e.g., as a natural health product) compared to single amino acids.
Based on the declared amount of an added amino acid in an SF, cautionary statements may be required to inform consumers of potential risks based on the threshold levels noted in column 4 of the List of Permitted Supplemental Ingredients. Refer to Section 4.2.4 for information on cautionary statements.
Some amino acids (L-Cysteine and L-Methionine; and L-Phenylalanine and L-Tyrosine) have a combined maximum amount, as indicated in column 3 of the List of Permitted Supplemental Ingredients. In these cases, when both amino acids are declared on the label, the sum of the declared amounts must not exceed the maximum amount identified in column 3 of the List. For example, when both L-Cysteine and L-Methionine are added as SIs, their total level declared on the label must not exceed the combined maximum amount of 413 mg.
Refer to Appendix 5 for the rationales for the requirement of cautionary statements for amino acids.
4.2.12 Other supplemental ingredients
4.2.12.1 Caffeine
Canada has a long history of permitting caffeine to be used as a food additive to characterize cola type beverages and non-alcoholic carbonated water-based flavoured and sweetened beverages other than cola type beverages. More recently, the prepackaged SFs to which caffeine was added has been allowed on a temporary basis provided this addition met established compositional and labelling requirements. The general population may also be less familiar that SFs, such as beverages or chewing gum, may contain caffeine or contain it at higher levels than previously permitted. A risk-based approach is warranted to help Canadians safely consume these products. Therefore, the oversight of these new uses of caffeine as SFs is appropriate.
Caffeine is permitted for addition as an SI to specified foods belonging to specified permitted food categories as set out in column 2 of the List of Permitted Supplemental Ingredients, and in accordance with maximum amounts and conditions of use stated in columns 3 to 5 of the List.
4.2.12.1.1 Conditions of use for caffeine as a supplemental ingredient
- a) Addition of caffeine to beverages up to 150 ppm
As per the List of Permitted Supplemental Ingredients, the addition of caffeine is permitted in non-carbonated water-based beverages belonging to item 1 of the List of Permitted Supplemented Food Categories. Non-carbonated water-based beverages belonging to item 1 of the List with added caffeine and a total caffeine amount from all sources of no more than 150 ppm must carry the statement "Contains caffeine" on the principal display panel (PDP) of the label. There are further conditions of use related to product types and labelling for SFs belonging to this category that are specified in the List of Permitted Supplemental Ingredients such as restrictions related to product composition, e.g., products, as consumed, must not contain 25% or more of juice, purée, pulp or nectar, singly or in combination.
The addition of caffeine to cola type beverages and non-alcoholic carbonated water-based flavoured and sweetened beverages other than cola type beverages continues to be subject to the food additive regulations, as set out in the List of Permitted Food Additives with Other Accepted Uses (Lists of Permitted Food Additives). Under the Supplemented Foods Regulations, the addition of caffeine or caffeine citrate to beverages other than carbonated beverages, or to carbonated beverages at levels greater than 150 ppm, will be considered an SI, not as a food additive. For the duration of the transition period for the Supplemented Foods Regulations (see Section 2.0), the maximum permitted level of use of caffeine as food additive in cola type beverages is 200 ppm; however, once the transition period ends, the addition of caffeine in these beverages as a food additive will be limited to a maximum caffeine concentration of 150 ppm. This alignment with the transition period for SFs is intended to minimize impacts to industry who may need to reformulate their cola-type beverages should they wish to use caffeine or caffeine citrate as food additive, not as an SI. The Department's intent in this regard, and the subsequent consequential modification will be published on the Government of Canada's website and aligns with the management of caffeine described in the following section b).
In the List of Permitted Supplemental Ingredients, the unit used to describe the maximum amount in beverages differs from that to be declared on the label, i.e., the maximum amount is provided in ppm; however, the quantity must be expressed in milligram (mg) on the label.
- b) Addition of caffeine to beverages where total caffeine concentration from all sources is above 150 ppm
The addition of caffeine to carbonated or non-carbonated water-based beverages for a total amount of caffeine from all sources of more than 150 ppm (and up to 400 ppm) is permitted in item 1 of the List of Permitted Supplemented Food Categories. The total amount of caffeine in beverages with added caffeine must not exceed 400 ppm, or be more than 180 mg per serving, in the beverages as consumed.
These SFs are required to carry cautionary statements as per the List of Permitted Supplemental Ingredients. Refer to Section 4.2.4 for information on cautionary statements. If the total caffeine content is above the threshold level specified in column 4 of the List, the additional cautionary statement related to maximum number of servings per day is required on the label. Section 4.2.4.1 provides information on determining the appropriate number of servings.
In addition, beverages containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm must carry the "High caffeine content" statement on the label. Labelling specifications for this statement are not prescribed, but it must adhere to general requirements (e.g., legibility requirements) for all mandatory labelling statements described in the FDA, SCFA, and the Regulations under these Acts. There are restrictions on representations for SFs carrying the "High caffeine content" statement on their label as described in Section 4.4.7.3.
- c) Addition of caffeine to foods other than beverages
As per the List of Permitted Supplemental Ingredients, the addition of caffeine to certain foods in categories other than beverages is permitted. If the total amount of caffeine is more than the threshold level of 56 mg per serving, the cautionary statements noted in column 4 of the List are required (see Section 4.2.4).
In addition, column 5 of the List specifies additional labelling requirements as noted below:
- Products that contain up to 56 mg of caffeine per serving require the statement "Contains caffeine" on the PDP.
- Products containing more than 56 mg of caffeine per serving require the statement "High caffeine content" on the label.
4.2.12.1.2 Added caffeine and ingredients containing caffeine
The listing for caffeine in the List of Permitted Supplemental Ingredients applies to products that contain added forms of caffeine as an SI. Synthetic caffeine, caffeine isolated from natural sources, as well as caffeine-containing extracts may be added forms of caffeine. With respect to caffeine-containing extracts in particular, it is determined on a case-by-case basis whether or not they would be considered added caffeine. For an ingredient to be considered a flavouring, it must be used for the purpose of providing flavour and its composition, including its caffeine content, must be consistent with that of the ingredient whose purpose is to impart a characteristic flavour. Extracts, if enriched in caffeine, or if added in amounts higher than what would be expected if they were being used as flavouring ingredients in foods, in order to increase caffeine content of the food, are likely added forms of caffeine.
Conversely, ingredients that contain caffeine as a natural constituent and are not processed to be enriched in caffeine are not likely to be added forms of caffeine. Examples of such ingredients include brewed coffee or tea, chocolate, and certain flavourings. SFs that do not contain any added forms of caffeine are not subject to the conditions of use of caffeine in the List; however, it should be noted that once the SF contains any added caffeine, the maximum amounts and other conditions of use are based on the sum of all sources of caffeine (see Section 4.2.4.1).
4.2.12.2 Taurine
Taurine is permitted as an SI in all SFs up to a maximum amount of 2000 mg, as specified in column 3 of the List of Permitted Supplemental Ingredients. SFs containing taurine above certain threshold amounts are not suitable for consumption by the general population without cautionary statements. Column 4 of the List specifies the threshold levels for taurine at which specific cautionary statements are required. More information about the maximum amount and other conditions of use, as applicable, can be found in Appendix 6.
4.2.13 Other substances for consideration as supplemental ingredients
Ingredients other than those described above may be eligible as SIs in SFs. Such ingredients may include herbal or non-herbal ingredients such as those found in natural health products. These substances, or certain levels of these substances, may not have a history of safe use as foods. However, a novel ingredient intended for use as an SI need not undergo evaluation as a novel food (see Section 6.1). Health Canada can determine conditions for their use as SIs, by taking into account the manufacture and chemistry of the ingredients, toxicological and nutritional evidence, and the appropriateness of any risk-mitigation tools such as cautionary labelling.
Refer to Section 6.0 for information on the process to request a change to the List of Permitted Supplemental Ingredients by adding a new SI.
4.2.14 Ingredients that are not supplemental ingredients
Only ingredients used according to their maximum amount and conditions of use listed in the List of Permitted Supplemental Ingredients are considered SIs. Use of an ingredient set out in column 1 of the List in a manner that is not consistent with the specified conditions of use is not considered an SI. Certain substances that are permitted as SIs can also be used for other purposes, which are subject to other provisions in the FDR. For example, some ingredients may be used as food additives, flavourings, or salt substitutes. When used only for purposes captured by other provisions, such ingredients are not considered to be SIs and do not need to comply with the conditions of use described in the List of Permitted Supplemental Ingredients. Their use will continue to be regulated under the existing provisions in the FDR, and would be acceptable for use in foods, including SFs, provided they are compliant with the applicable requirements of the regulations.
For products that were issued TMALs as SFs, certain ingredients that were assessed on a case-by-case basis for their addition to those products may not be listed in the List of Permitted Supplemental Ingredients, namely L-theanine, inositol and glucuronolactone. These ingredients are considered acceptable food ingredients (i.e., not SIs) and their addition to foods, including SFs, is subject to the existing provisions of the FDR. However, above certain levels, their safe use in foods is unknown or has not been established. Authorization for use of these ingredients could be sought through either the novel food route (Division 28, Part B of the FDR; see Section 4.2.14.3) if intended for general use in the food supply, or as SIs if the safe use of the ingredient is intended for use in food categories eligible for supplementation and/or their use requires establishing conditions of use. For information on how to add a new ingredient to the List, refer to Section 6.0 to amend the List.
4.2.14.1 Food additives
In Canada, food additives are regulated by the FDA, FDR and Marketing Authorizations (MAs) issued by the Minister of Health. Approved food additives and their permitted conditions of use are set out in the 15 Lists of Permitted Food Additives that are incorporated by reference in the corresponding 15 MAs and published by the Government of Canada on its website. The use of foods additives in SFs must be in accordance with these Lists of permitted food additives. Food additives are generally organized according to the functional classes of food additives and specify the food or foods an additive can be used in, the maximum amount in that food and any other specific conditions of use. As with conventional foods, manufacturers of SFs wishing to use an unapproved food additive, an approved food additive in a new food category or at a level not currently permitted, must file a food additive submission in accordance with section B.16.002 of the FDR to seek a modification to the relevant List(s) of Permitted Food Additives before it can used in foods. Health Canada will evaluate the submission to determine whether the requested food additive use should be permitted, prior to consulting on any proposed modification to the Lists.
4.2.14.2 Food flavouring ingredients
Flavouring ingredients used in foods must be used for the purpose of providing flavour. The composition of food flavouring ingredients must be consistent with that of the substance whose purpose is to impart a characteristic flavour. Flavouring ingredients should be food-grade quality. There are standards of identity and composition for certain flavouring preparations in Division 10 of the FDR. There are also specifications for flavouring ingredients that have been evaluated by the Joint FAO/WHO Expert Committee on Food Additives as well as specifications that are prescribed in the latest edition of the Food Chemicals Codex.
The FDR does not require premarket evaluation of most flavouring ingredients and there is no "positive" list of permitted flavours in the FDR. However, the FDA prohibits the sale of adulterated foods and Part 1 of the List of Contaminants and Other Adulterating Substances in Foods includes certain flavouring ingredients that would render a food adulterated. These substances are not permitted in food at any level. Ultimately, the seller is responsible for ensuring that flavouring ingredients in their food products do not result in a violation of section 4 of the FDA.
4.2.14.3 Novel foods
Novel foods are typically used for a conventional food purpose (e.g., for nutrition, hydration) and are subject to the requirements of Division 28, Part B of the FDR. Petitioners unsure about the novelty status of an ingredient are encouraged to seek a novelty determination from the Novel Foods Section of Health Canada's Food Directorate. A Novelty Determination Information Form can be requested by sending an e-mail to Food Directorate's SMIU (smiu-ugdi@hc-sc.gc.ca) with the subject "Novelty Determination Information Form".
More information about novelty determination and assessment can be found on Health Canada website and in the Guidelines for the Safety Assessment of Novel Foods Derived from Plants and Microorganisms, respectively. Health Canada has published a List of non-novel determinations for food and food ingredients, which stakeholders can consult regarding ingredients for which a novelty determination was requested and the outcome was that it is not novel.
4.3 Labelling of supplemented foods
All requirements pertaining to labelling and advertising in the FDA, the SFCA, and the Regulations associated with these Acts will generally apply to SFs. SFs are subject to general labelling requirements for prepackaged products such as bilingual labelling, common name, country of origin, date markings and storage instructions, name and principal place of business, list of ingredients and allergens, lot numbers, net quantity and the legibility and location of this information.
With the exception of requirements related to the Nutrition Facts table (NFt), SFs are also subject to the regulatory requirements made in the final amendments to the FDR entitled Regulations Amending the Food and Drug Regulations (Nutrition Labelling, Other Labelling Provisions and Food Colours) (SOR/2016-305) - referred to as 2016 Nutrition Labelling Regulations herein, published in the Canada Gazette, Part II on December 14, 2016. The amended provisions related to the NFt are not applicable to SFs as these foods must instead comply with the provisions related to the Supplemented Food Facts table (SFFt) in the Supplemented Foods Regulations (see Section 4.3.1). For information and guidance on recent updates to food labelling requirements, please refer to the following Health Canada webpage Regulations and compliance - nutrition labelling. The transition period for the above mentioned nutrition labelling amendments ended on December 14, 2021; however, the Canadian Food Inspection Agency (CFIA) is focusing its efforts on education and compliance promotion for the first year (until December 14, 2022). SFs that are permitted the transition period (see Section 2.0) are not required to comply with the 2016 Nutrition Labelling Regulations until the end of the transition period for SFs.
SFs are also subject to the final amendments to the FDR for front-of-package (FOP) nutrition labelling and other labelling aspects entitled Regulations Amending the Food and Drug Regulations (Nutrition Symbols, Other Labelling Provisions, Vitamin D and Hydrogenated Fats or Oils) (SOR/2022-0168).
4.3.1 Labelling requirements specific to supplemented foods
There are specific labelling requirements for SFs as part of the Supplemented Foods Regulations. The labelling of SFs provides consumers with information to distinguish these products from conventional foods, to better understand the risks associated with the consumption of certain SFs, and to make informed decisions related to their consumption. The specific labelling requirements for SFs are described below.
4.3.1.1 Supplemented Food Facts table
The Supplemented Foods Regulations requires that SFs carry a standardized SFFt. The SFFt shares a similar format to that of an NFt, maintains many of the same requirements as the NFt for core nutrients, and provides additional information specific to SIs. Specifically, the SFFt must indicate the amount and, where applicable, percent daily values (% DV) of the core nutrients. In addition, the SFFt must include the amount of each SI, expressed in the applicable unit set out in column 3 of the List of Permitted Supplemental Ingredients. For SIs that have an established DV (i.e., vitamins and mineral nutrients), the amount must also be expressed as a %DV.
A typical SFFt is shown in Figure 1, and is composed of three main sections:
- Section 1: Similar to an NFt, the SFFt must display mandatory information such as the serving size, calories, and the amounts of core nutrients (fat, carbohydrate, protein, cholesterol, sodium, potassium, calcium and iron) in metric units and % DVs. Similar to the NFt, the SFFt may also display other nutrients, either voluntarily or on a mandatory basis when they are the subject of a representation. However, if there is a supplemental source of the same nutrient, it must be declared under the subheading "Supplemented with" (Section 3 of the SFFt) instead of Section 1 of the SFFt (e.g., while calcium is a core nutrient, in the case where it is added as a SI, it must be declared under the "Supplemented with" subheading only). The declaration must be for the total amount of the nutrient in the product.
- Section 2: Similar to the NFt, the SFFt maintains the footnote (5% or less is a little, 15% or more is a lot) at the bottom of the first section of the table. This will help consumers understand if there is a little or a lot of a nutrient like sugars, sodium and potassium in the food.
- Section 3: The "Supplemented with" section must display the total amounts (naturally present + supplemental amounts + contribution from other sources) of SIs in the food. For SI that have established DVs, i.e., vitamins and mineral nutrients, the amount of the SI must also be expressed as a %DV.

Figure - Text description
Left justified at the top of the table is the heading Supplemented Food Facts and stacked below it is the heading Info-aliment supplémenté. Both are in bold and large font. The next line is Per 1 can open parenthesis 500 mL close parenthesis. The next line is pour 1 canette open parenthesis 500 mL close parenthesis. There is a thin rule below pour 1 canette open parenthesis 500 mL close parenthesis that spans the width of the table. The next line is Calories in bold followed by 160, also in bold. Right justified on the same line is the subheading percent symbol Daily Value and stacked under it is percent symbol valeur quotidienne. Both are in bold and denoted by an asterisk that refers to a footnote below. There is a thick rule under Calories that ends after the amount; it does not span the width of the table.
Left justified on the next line is Fat, forward slash, Lipides in bold followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. Indented on the next line under Fat, forward slash, Lipides is Saturated, forward slash, saturés and 0 followed by a lower case g. Indented on the next line is a plus symbol followed by Trans, forward slash, trans followed by 0 and lowercase g. Right justified and vertically centered against the saturated and trans information on the left is 0 followed by a percent symbol. There is a thin rule below the trans information that spans the width of the table.
Left justified on the next line is Carbohydrate, forward slash, Glucides in bold followed by 41 and a lowercase g. Indented on the next line under Carbohydrate, forward slash, Glucides is Fibre, forward slash, Fibres, followed by 0 and a lowercase g. Right justified on the same line is 0 followed by a percent symbol. Indented on the next line under Fibre is Sugars, forward slash, Sucres, followed by 41 and a lowercase g. Right justified on the same line is 41 followed by a percent symbol. There is a thin rule under the sugars information that spans the width of the table.
Left justified on the next line is Protein, forward slash, Protéines in bold followed by 0 and a lowercase g. There is a thin rule under the protein information that spans the width of the table.
Left justified on the next line is Cholesterol, forward slash, Cholestérol in bold followed by 0 and mg in lowercase. There is a thin rule under the cholesterol information that spans the width of the table.
Left justified on the next line is Sodium in bold followed by 150 and mg in lowercase. Right justified on the same line is 7 followed by a percent symbol. There is a thick rule under the sodium information that spans the width of the table.
Left justified on the next line is Potassium followed by 60 and mg in lowercase. Right justified on the same line is 1 followed by a percent symbol. There is a thin rule under the potassium information that spans the width of the table.
Left justified on the next line is Calcium followed by 0 and mg in lowercase. Right justified on the same line is 0 followed by a percent symbol. There is a thin rule under the calcium information that spans the width of the table.
Left justified on the next line is Iron, forward slash, Fer followed by 0 and mg in lowercase. Right justified on the same line is 0 followed by a percent symbol. There is a thick rule under the iron information that spans the width of the table.
The next two lines is the footnote to the percent Daily Value and percent valeur quotidienne. The footnote starts with an asterisk followed by the statement: 5 percent symbol or less is a little,15 percent symbol or more is a lot. On the second line is an asterisk followed by the statement: 5 percent symbol ou moins c'est peu,15 percent symbol ou plus c'est beaucoup. The terms 'a little', 'a lot', 'peu', and 'beaucoup' are in bold. There is a thick rule under the footnote information that spans the width of the table.
Left justified on the next line is Supplemented with, forward slash, Supplémenté en, in bold, followed by a dagger symbol that refers to a footnote at the bottom of the table. There is a thin rule below this information that spans the width of the table.
Left justified on the next line is Niacin, forward slash, Niacine followed by 7 and mg in lowercase. Right justified on the same line is 44 followed by a percent symbol. There is a thin rule under the niacin information that spans the width of the table.
Left justified on the next line is Pantothenate, forward slash, Pantothénate followed by 8 and mg in lowercase. Right justified on the same line is 160 followed by a percent symbol. There is a thin rule under the pantothenate information that spans the width of the table.
Left justified on the next line is Vitamin B6, forward slash, Vitamine B6 followed by 1 and mg in lowercase. Right justified on the same line is 59 followed by a percent symbol. There is a thin rule under the vitamin B6 information that spans the width of the table.
Left justified on the next line is Vitamin B12, forward slash, Vitamine B12 followed by 4 and µg in lowercase. Right justified on the same line is 167 followed by a percent symbol. There is a thin rule under the vitamin B12 information that spans the width of the table.
Left justified on the next line is Vitamin C, forward slash, Vitamine C followed by 225 and mg in lowercase. Right justified on the same line is 250 followed by a percent symbol. There is a thin rule under the vitamin C information that spans the width of the table.
Left justified on the next line is Vitamin E, forward slash, Vitamine E followed by 2 and mg in lowercase. Right justified on the same line is 13 followed by a percent symbol. There is a thin rule under the vitamin E information that spans the width of the table.
Left justified on the next line is Magnesium, forward slash, Magnésium followed by 20 and mg in lowercase. Right justified on the same line is 5 followed by a percent symbol. There is a thin rule under the magnesium information that spans the width of the table.
Left justified on the next line is Zinc followed by 5 and mg in lowercase. Right justified on the same line is 45 followed by a percent symbol. There is a thin rule under the zinc information that spans the width of the table.
The next two lines is the supplemented footnote that was referred to earlier. The footnote starts with a dagger symbol followed by the statement: Includes naturally occurring and supplemental amounts and on the second line is a dagger symbol followed by the statement: Comprend les quantités naturelles et supplémentées. This is the end of the Supplemented Food Facts table.
- a) Core and additional information declared in the Supplemented Food Facts table
As with the NFt, Section 1 of the SFFt shown in Figure 1 indicates the core information that must always be included in the SFFt and the order in which it must be presented. Exceptions exist for simplified formats, including those for SFs that are single-serving prepackaged products as well as small packages. The required information and the manner of expression are found in the table to section B.29.002 of the FDR.
Additional information may be voluntarily included, or must be included in Section 1 of the SFFt if specifically required by the regulations (e.g., for nutrients that are the subject of a claim). If such information is included in Section 1 of the SSFt, it must be declared as per the table to subsection B.29.003(1) of the FDR.
- b) Supplemental ingredient information in the Supplemented Food Facts table
Any permitted SI as per the List of Permitted Supplemental Ingredients, must be quantitatively declared in the "Supplemented with" section (Section 3) of the SFFt. An SI declared in the "Supplemented with" section must not be declared in Section 1 of the SFFt. All SIs must be shown in Section 3 of the SFFt in the same order as they appear in the List of Permitted Supplemental Ingredients.
Specific requirements for declaring SIs in the SFFt are listed below:
- When an SI is added to an SF, the total amount of the SI must be quantitatively declared in the "Supplemented with" section (Section 3 of the SFFt). For SIs with established DVs, the %DV must also be indicated next to the quantity of the SI declared on the label (see Figure 1).
- If there are no other sources contributing to the amount of the SI, the amount declared is in respect of the total added amount of the SI, excluding any overages applied. Refer to Sections 4.2.5 and 4.2.6 for information on overages and deficiencies, respectively.
- In general, if there are other sources contributing to the total amount of the SI, including amounts from naturally occurring sources and/or amounts from other sources, the amount declared must be in respect of the total from all sources, excluding any overages applied.
- When an SI added to an SF is an amino acid, the total amount of free amino acids (supplemental + naturally occurring free amino acids + contribution from other sources) must be quantitatively declared in the "Supplemented with" section (Section 3 of the SFFt). This amount does not include protein-bound amino acids.
- When an SI added to an SF is also a core nutrient (e.g., calcium, potassium), it must only be declared in the "Supplemented with" section (Section 3 of the SFFt), and the core nutrient must not be quantitatively declared or display a %DV in Section 1 of the SFFt. The declaration must be according to 1) above.
Specific requirements for declaring vitamin A in the SFFt:
Vitamin A has two main dietary forms: (i) retinol and retinyl esters, and (ii) beta-carotene, which are permitted as SIs in the List of Permitted Supplemental Ingredients. Both forms of vitamin A can be present in SFs as SIs or from non-supplemental sources (e.g., naturally occurring in other food ingredients).
- (i) When vitamin A is present as a non-supplemental source only
For SFs, when there are only non-supplemental sources of vitamin A in a product (e.g., beta-carotene from vegetable juice or retinol from milk), the total amount of vitamin A that they contribute can be voluntarily declared as "Vitamin A" in the units of retinol activity equivalents (RAE)Footnote 4 in Section 1 of the SFFt along with the %DV.
- (ii) When one or more forms of vitamin A are added as SIs
When added as SIs, retinol and beta-carotene must be declared as follows:
- a) When both forms are added as SIs, they must be declared separately in the "Supplemented with" section (Section 3 of the SFFt), in the appropriate units as per column 3 of the List of Permitted Supplemental Ingredients:
- (i.) "Vitamin A (Retinol)" in micrograms (µg), and
- (ii.) "Vitamin A (Beta-carotene)" in micrograms (µg)
- b) When only one form of vitamin A is added as an SI (e.g., retinol, but not beta-carotene), the added form must be declared in the "Supplemented with" section (Section 3 of the SFFt). If the other form of vitamin A (in this case, beta-carotene) is also present from non-supplemental sources, it must be declared separately in the "Supplemented with" section of the SFFt, as per (ii)(a)(ii) above, despite there being no supplemental source of that form in the SF.
While the absolute amounts of each form of vitamin A are declared separately in Section 3 of the SFFt, a combined %DV declaration is required (see Figure 2). The %DV declaration is based on the sum of both forms of vitamin A, in the units of RAE, since the DV for vitamin A is based on the total amount from both forms.

Figure - Text description
Left justified is a blurred example of an ingredient listed before Vitamin A in the Supplemented Food Facts table, followed a thin rule that spans the width of the table.
The next line is Vitamin A, open parenthesis, Beta-Carotene, close parenthesis, followed by a placeholder for the amount of vitamin A in the form of beta-carotene and micrograms. Below, there is a thin rule that does not span the width of the table.
The next line is Vitamin A, open parenthesis, Retinol, close parenthesis, followed by a placeholder for the amount of vitamin A in the form of retinol in micrograms. Below, there is a thin rule that spans the width of the table. There is a combined placeholder percent Daily Value for vitamin A that is right justified and vertically centred against the beta-carotene and retinol in information on the left.
The next line shows a blurred example of an ingredient listed after Vitamin A in the Supplemented Food Facts table.
- c) Rounding Rules
For nutrients that are declared in Section 1 of the SFFt (see Section 4.3.1.1 (b)), the rounding rules for conventional foods apply. Refer to the CFIA's Industry Labelling Tool on Rounding Rules for more information.
The rounding rules for SIs are set out in item 18 in the table following subsection B.29.002(10) of the FDR. Unlike the rounding rules for conventional foods, the declared value of an SI must be rounded off to the nearest whole number and expressed in the manner set out in column 3 of the List of Permitted Supplemental Ingredients.
In cases where naturally occurring amounts are declared in combination with the supplemental amounts in the "Supplemented with" section (Section 3 of the SFFt), the rounding rules for SIs apply.
- d) Supplemented Food Facts table formats
The regulations include requirements on i) the presentation, location and orientation of the SFFt on the label, ii) options on various formats of the SFFt (e.g., when an SF is to be prepared according to directions provided on the package or combined with other ingredients, or sold as an assortment of SFs of the same type), and iii) alternative methods of presentation and exceptions for small packages.
A hierarchy of SFFt formats and sizes are set out in the regulations, with the choice of format based on the same rule as the NFt; that is, no more than 15% of the available display surface needs to be used for the SFFt. If it is not possible to display the SFFt on 15% or less of the available display surface of the package, the regulations permit alternative methods of presentation of the SFFt, allowing it to be placed on a tag, package insert, the inner side of a label, a fold-out label or an outer sleeve, overwrap or collar. However, as a new rule specific to SFs, the SFFt would not be permitted on a package insert or on the inner side of a label if one or more cautionary statements are required on the product, as the information in the SFFt must be accessible to the consumer at the point of purchase.
To help in the label design, and to help the food and packaging industry meet format specifications of the SFFt, Health Canada has developed a Directory of Supplemented Food Facts Table Formats that is incorporated by reference into the FDR. This Directory sets out the various formats of the SFFt, including detailed requirements and graphical examples that are acceptable for SFs.
Furthermore, Health Canada created a compendium of templates which includes permitted SFFt formats (see Section 4.3.6).
- e) Small package exemption
With respect to small packages (section B.01.018 of the FDR), SFs that have an available display surface of less than 100 cm2 are not required to carry an SFFt, except when the label is required to carry a list of cautionary statements or when the SF is the subject of any representation on the label or in any or advertisement made or placed by or on the direction of the manufacturer, related to: a declaration of the SF's energy value or the amount of a nutrient or SI; any nutritional or health-related properties; or any health-related statement, name, logo, symbol, seal of approval or mark. However, these representations do not include claims related to "free of sugar" for a product that fully meets the relevant conditions set out for item 37 of the Nutrition Labelling - Table of Permitted Nutrient Content Statements and Claims that is incorporated by reference into the FDR. These representations also do not include any mandatory information, such as mandatory statements that a product would be required to carry on its label (e.g., "contains caffeine") as per a condition of use prescribed in the List of Permitted Supplemental Ingredients.
Small packages that are not required to carry an SFFt must, however, contain an indication on the outer label of how purchasers or consumers may obtain the information that would otherwise be shown in the SFFt. The indication must include a postal address, a website address or toll-free telephone number and be a minimum type size of 8 points. For those that carry a claim for "free of sugar", the claim itself must respect specified legibility requirements (section B.29.018(3)) and the label must include the energy value (Calories) and the amount (g) of sugar alcohols per serving of stated size, according to specified placement requirements.
As per section B.29.019 of the FDR, when the SFFt is required on a small package, only an abbreviated version of the SFFt is required. The SFFt must include the energy value, the amount of any nutrient or SI that is the subject of a representation related to any nutritional or health-related properties, any added sugar alcohol and any SI for which a cautionary statement is required on the label of that food. In addition, it must include the amount of any saturated fat, sugars and/or sodium that meets or exceeds the applicable threshold set out for the front-of-package (FOP) nutrition symbol.
4.3.1.2 List of Cautionary statements
Certain SIs or certain levels of SIs in SFs may pose a health risk to Canadians if consumed in excess, or if consumed by vulnerable populations such as children, or pregnant or breastfeeding women. Therefore, if the declared amount of an SI is above a certain threshold level specified in the List of Permitted Supplemental Ingredients, the label of the SF must display cautionary statements to inform consumers of the potential risk (see Section 4.2.4).
To make the cautionary information more noticeable to consumers, cautionary statements must be grouped together, under a standardized bolded heading "Caution" (also acceptable options are the heading with a colon - i.e., "Caution:" and "Caution :"), in a manner similar to how the current list of ingredients for prepackaged foods is to be declared. In the case of an assortment, the list of cautionary statements must clearly identify to which SFs in the assortment the list applies (see Section 4.3.4 for information on assortments). Figure 3 below shows an example of a list of cautionary statements on an SF label.
There are specific requirements as to how the list of cautionary statements on SFs must be displayed, as per section B.29.020 of the FDR, which include:
- the list of cautionary statements on the label of an SF must always appear adjacent to the SFFt in the same language version (e.g., if the SFFt is bilingual the list of cautionary statements must also be the bilingual version), without any intervening material between the title and the first cautionary statement;
- the list of cautionary statements must be in both English and French; and
- there are specific requirements on size, legibility and appearance of the list of cautionary statements on the label consistent with the list of ingredients and allergen labelling requirements (see section B.01.008.1 of the FDR).

Figure - Text description
There is a white box outlined by a black line. All text inside the box is horizontal in black with the first letter of each sentence capitalized. A bullet symbol separates each cautionary statement in the list.
The first line starts with the word Caution, in bold, followed by a colon. This is followed by the statement Not recommended for those under 14 years old, pregnant or breastfeeding women or individuals sensitive to caffeine. This is followed by a bullet and the statement Do not drink more than 1 serving per day. This is followed by a bullet and the statement Do not drink on the same day as any other supplemented foods or supplements with the same supplemental ingredients.
The French description starts on the next line with the word Attention, in bold, followed by a colon. This is followed by the statement Déconseillé aux individus de moins de 14 ans, aux femmes enceintes ou qui allaitent ou aux personnes sensibles à la caféine. This is followed by a bullet and the statement Ne pas boire plus de 1 portion par jour. This is followed by a bullet and the statement Ne pas boire le même jour que d'autres aliments supplémentés ou suppléments contenant les mêmes ingrédients supplémentaires.
4.3.1.3 Supplemented Food Caution Identifier
For SFs that contain one or more SIs that require cautionary statements as per the List of Permitted Supplemental Ingredients, a Supplemented Food Caution Identifier (SFCI) must be displayed on the PDP. This responds to consumer research indicating that Canadians find it useful to see cautionary labelling on the front of packages of SFs.
Health Canada has restricted the use of the SFCI to those SFs that carry cautionary labelling. As per section B.29.024 of the FDR, it is prohibited to label an SF with the SFCI unless the food is required to carry one or more cautionary statements on the label. This is to ensure that the SFCI or any graphics with a similar look are not used to promote consumption of SFs or confuse consumers as to the meaning of the SFCI. With education, this will help consumers distinguish SFs that require cautionary labelling from other SFs, and will alert them to look for additional information on the label so that they have access to information to determine if the product is appropriate and safe for them.
The SFCI must be placed in a prominent, clutter-free space on the PDP and must be displayed in black and white and include an exclamation mark on the left-hand side and the word or words "Supplemented" or "Supplemented / Supplémenté" and an attribution to Health Canada, all of which is surrounded by a solid line border (See Figure 4).
In addition, as per section B.29.025 of the FDR, the SF label must not carry a representation such as a word, phrase, illustration, sign, mark, symbol or design that resembles the SFCI. This prohibition does not apply to a high-in front-of-package (FOP) nutrition symbol when required on a label of an SF as per subsection B.29.025(2) of the FDR.

Figure - Text description
This figure shows a supplemented food caution identifier for the principal display panel of a prepackaged product. This identifier is bilingual, with the English text shown first, followed by the French text. There is a white rectangular box outlined by a thin black border. Centred vertically on the left side of the box is a large black exclamation mark. To the right of the exclamation mark is a horizontal bar. There is a small amount of white space between the exclamation mark and the bar as well as between the end of the bar and the thin black line that outlines the box. The bar is black and contains the word "Supplemented" followed by a forward slash and the word "Supplémenté", with both words in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred below the black bar are the words "Health Canada" followed by a forward slash and the words "Santé Canada", with all words in black lower case letters, except that the first letter of each word is in upper case.
The permitted SFCI format designs are set out in the FDR in a new Schedule (Schedule K.2). There are specific requirements on the appearance, placement and size of the SFCI on SF labels as prescribed in sections B.29.021 to B.29.023 of the FDR. The size of the SFCI would be proportional to the area of the principal display surface (PDS) of the package, with the SFCI size decreasing as the area of the PDS decreases.
To help label designers and the food and packaging industry meet format specifications of the SFCI, the hierarchy of formats and sizes are set out in the Directory of Supplemented Food Caution Identifier Specifications.
Furthermore, Health Canada created a compendium of templates which includes permitted SFCI formats (see section 4.3.6).
4.3.2 Front-of-package nutrition labelling
SFs sold in small packages with an available display surface of less than 100 cm2 are exempt from the requirement to carry a front-of-package (FOP) nutrition symbol except in the case where the SF is the subject of a representation, on the label or in any advertisement made or placed by or on the direction of the manufacturer, related to: a declaration of the SF's energy value or the amount of a nutrient or SI; any nutritional or health-related properties; or any health-related statement, name, logo, symbol, seal of approval or mark. In this case, however, if an SFCI is required on the label of the SF, the small package exemption for the FOP nutrition symbol still applies. For example, if an SF in a small package carries a claim, is high in sugar, and requires cautionary statements and the SFCI, the label would require the list of cautionary statements, the SFCI and the SFFt, but not the FOP nutrition symbol.
4.3.3 Declaring nutrients outside of the Supplemented Food Facts table
Quantitative declarations of energy value and the amount of nutrients per serving of stated size are permitted outside of the SFFt, on labels or in advertisements of SFs provided that the units and language requirements referred to in section B.01.301 of the FDR are respected. Refer to the CFIA's Industry Labelling Tool on Quantitative declarations outside the Nutrition Facts table for more information.
4.3.4 Declaring supplemental ingredients outside of the Supplemented Food Facts table
Quantitative declaration of SIs can be made outside of the SFFt on labels or in advertisements, subject to requirements for any representation concerning SIs in accordance with sections B.29.026 to B.29.029 and as per voluntary statements and claims on food (refer to Section 4.4.7), and provided the units and language requirements referred to in section B.01.301 of the FDR are respected.
4.3.5 Assortments
Prepackaged products containing assortments of SFs are permitted under the Supplemented Foods Regulations. Subsections B.29.005(2) and B.29.005(3) of the FDR include the requirements for a prepackaged product containing an assortment of SFs of the same type. For a prepackaged product containing an assortment of SFs, the outer package of the product must include the SFFt and, if applicable, a list of cautionary statements and SFCI. Where the information for each kind of SF contained in the product is different and a typical serving consists of only one of those foods, an aggregate format must be used to show the information in the SFFt for each SF contained in the product. In the case where the information in the SFFt for all SFs of the same type in the assortment is the same, a standard, horizontal or linear format, as applicable based on the available display surface of the product, is required for the SFFt. For assortments of SFs of the same type, where a serving consists of more than one of the SFs in the package, the information may either be set out for each SF using an aggregate format or as a composite value using a standard, horizontal or linear format, as applicable based on the available display surface of the product.
Prepackaged products containing assortments of SFs and other foods are also permitted. All other applicable labelling requirements, such as the NFt, would apply to the other foods that are not SFs in the assortment.
4.3.6 Compendium of templates for supplemented food facts tables, supplemented food caution identifiers and lists of cautionary statements
To help label designers and the food and packaging industry meet format specifications, Health Canada created a compendium of templates. The templates are the actual-size graphic illustrations for various SFFt formats and SFCI formats permitted by the FDR.
For lists of cautionary statements, there are variations that could meet the graphic specifications detailed in the FDR. Two examples have been provided within the compendium to demonstrate acceptable variations.
The Compendium of Templates was created using Adobe InDesign CC 2020. It is available in the following formats:
- INDD for files opened and edited in InDesign CC 2020 (or newer)
- IDML for files opened and edited in older versions of InDesign (CS4 and later)
To obtain an electronic copy of the document, please send an e-mail to smiu-ugdi@hc-sc.gc.ca with the subject line "Requesting labelling compendium for supplemented foods"
4.3.7 Priority allergens, gluten sources, and sulphites
As with all foods, SF products must comply with all general food labelling requirements applicable to prepackaged products. This includes the enhanced labelling requirements for priority allergens, gluten sources and added sulphites as set out in sections B.01.010.1 to B.01.010.3 of the FDR.
Manufacturers of SFs are also urged to use food allergen precautionary statements on food labels to alert the consumer to any potential inadvertent presence of priority allergens in the food (section B.01.010.4 of the FDR). Health Canada's webpage, The Use of Food Allergen Precautionary Statements on Prepackaged Foods, provides guidance on the appropriate use of precautionary statements on prepackaged foods.
4.3.8 Supplemented food versus standardized food names
As with all foods, SF products must comply with regulatory requirements regarding common names of standardized foods as per Divisions 2 to 14 and Divisions 17 to 22 of Part B of the FDR. For more information, refer to the CFIA guidance on common name on food labels. This guidance specifies that modified standardized common names must:
- Make it clear to consumers that the food so described does not meet the standard; and
- Describe to the consumer how the food differs from the standard in a clear and prominent manner.
The brand name and trademarked phrases used to market the product can be used in addition to the common name, and must be used in accordance with subsection 5(1) of the FDA and subsection 6(1) of the SFCA.
4.4 Regulatory requirements and guidance for use of nutrition and health-related statements and claims on supplemented foods
For the purpose of this document, nutrition and health-related statements and claims refer to nutrient content claims, health claims (including function claims, disease risk reduction and therapeutic claims), quantitative declarations, and any health-related statement, logo, symbol, seals of approval or mark on the label of the food, or in an advertisement for a food that is made or placed at the discretion of the manufacturer.
As with conventional foods, nutrition and health-related statements and claims may be made on the label or in advertisements of SFs on a voluntary basis. However, when they are made, they must comply with the regulatory requirements that apply to them and should comply with any applicable guidance. In order to make informed decisions about their health, consumers should always be provided with fair and balanced information about the benefits and the risks associated with the products being advertised.
In accordance with subsection 5(1) of the FDA and subsection 6(1) of the SFCA, a food must be advertised in a manner that does not create an erroneous impression regarding the product's character, value, quantity, composition, merit or safety. The general requirements for nutrition and health-related statements and claims appearing on the label or in an advertisement for prepackaged foods apply to SFs as well. In order to ensure the consumers have the appropriate information to make informed food choices and to comply with subsection 5(1) of the FDA, the food provisions of the FDR, subsection 6(1) of the SFCA, and any applicable guidance, Health Canada recommends that sellers of food through e-commerce technology make information that would be otherwise required on the label accessible to consumers at time of purchase.
Included below is general guidance on the use of nutrition and health-related statements and claims for SFs, and the regulatory requirements that apply to them.
4.4.1 General health claims
General health claims are statements intended to promote good health through healthy eating or to provide dietary guidance. These claims are broad and do not refer to a specific health effect. Examples include claims with terms such as "healthy", "balanced" or statements with a similar meaning.
Claims including the term "healthy" on food are best explained by linking the food to one of the directional statements in Canada's food guide. A statement referring to "healthy" on a food label would not be acceptable on beverages whose energy value comes primarily from sugars, as it is recommended to limit the consumption of sweet beverages, including fruit juices. Canada's food guide recommends having vegetables and fruits more often than juice. Similarly, a daily balance of nutrients is met by eating a variety of foods. No single food should be represented to be "balanced". A claim suggesting that the consumption of a product can deliver a daily balance of nutrients is unacceptable. To avoid creating false, deceptive or misleading impressions about a food and its consumption, it is recommended that the meaning of descriptive words used in relation to a food be clear. Words such as "balanced" may create an erroneous impression if not further clarified.
4.4.2 Nutrient content claims
Nutrient content claims (NCCs) are statements that characterize the energy value of the food or the amount of a nutrient contained in the food such as "free", "reduced", "source" and "excellent source". The conditions for these claims are specified in sections D.01.004 and D.02.002 and in the Nutrition Labelling - Table of Permitted Nutrient Content Statements and Claims which is incorporated by reference into the FDR. Specific requirements for NCCs concerning vitamins and minerals are set out in CFIA's guidance.
Unless permitted by regulation, no other NCCs can be made that characterize the energy value of the food or the amount of a nutrient contained in the food (see subsection B.01.502(1) of the FDR).
4.4.3 Types of health claims and their regulatory requirements
A health claim is any representation in labelling or advertising that states, suggests, or implies that a relationship exists between consumption of a food or an ingredient in the food and a person's health. Some food labels contain statements about the beneficial effects of the food, or ingredients in the food on a person's health, such as "a healthy diet low in saturated and trans fats may reduce the risk of heart disease". This type of statement is an example of a health claim.
For information about the different types of health claims, refer to Health Claims. Health claims are optional for foods. However, when they are made, they must be truthful and not misleading according to subsection 5(1) of the FDA and subsection 6(1) of the SFCA. This means that manufacturers and distributors must have scientific evidence to substantiate health claims prior to their use on food labels or in advertising.
In accordance with section 3 of the FDA, claims, express or implied, related to diseases and health conditions listed in Schedule A.1 to the FDA are prohibited on foods, including SFs, unless already permitted in the FDR (sections B.01.600 - B.01.603). These claims are subject to premarket assessment, which would involve preparing and submitting an application to Health Canada's Food Directorate in accordance with the Guidance Documents for Preparing Health Claim Submissions. If the claim is approved by Health Canada, a regulatory amendment would be made to add the claim to the table following section B.01.603 of the FDR to specifically permit the claim.
As with conventional foods, health claims that are not referring to diseases and health conditions listed in Schedule A.1 to the FDA are not subject to mandatory premarket review; however, an application may be voluntarily submitted to request a health claim assessment by Health Canada's Food Directorate (see Section 4.4.4 for more information on substantiation of health claims). For any claims that have not been reviewed and accepted by Health Canada, food manufacturers and distributors must disclose the evidence supporting these claims upon request from the CFIA.
4.4.4 Health claims substantiation
As with conventional foods, health claims used on SF labels or in advertising must be substantiated as per Health Canada standards of evidence for health claims for foods, as outlined in the Guidance Documents for Preparing Health Claim Submissions. See Section 4.4.7 for claims requirements and restrictions specific to SFs.
Health claim applications prepared in accordance with the Guidance Documents for Preparing Health Claim Submissions may be submitted to Health Canada's Food Directorate to request an opinion on the validity of a claim. Health claims that are reviewed and found acceptable are added to the list of accepted claims and may be used on all foods meeting the conditions of use stated in the applicable summary of assessment. Additional acceptable function claims are published on the CFIA's website. To make one of these accepted claims, the product must meet all the required conditions of use.
Function claims for nutrients, including SIs that are nutrients, may be made on SFs and must be made in accordance with conditions of use provided for in the FDR (sections B.01.311, B.01.312, D.01.004, D.01.006, D.02.002, and D.02.004). Additional information on acceptable nutrient function claims and conditions of use can be found on the CFIA website. For new nutrient function claims, an abbreviated process exists for documenting the supporting evidence for those that meet specified criteria (see Acceptability of New Nutrient Function Claims). Function claims for other ingredients, including non-nutrient SIs are subject to requirements and guidance as per Sections 4.4.3 and 4.4.5.
Consultation with Health Canada's Food Directorate is encouraged to ensure that applicable legislation, regulations and guidelines are followed or to assist when manufacturers or distributors are uncertain about the status of the claim they are planning to use. Questions regarding health claims can be directed to healthclaims-allegationssante@hc-sc.gc.ca. Requests for consultation before filing a health claim submission may be directed to smiu-ugdi@hc-sc.gc.ca.
4.4.5 Health claims general guidance
Developing proper claim wording that is consistent with the supporting evidence is an important part of making a health claim that is truthful and not misleading or deceptive, as per subsection 5(1) of the FDA and subsection 6(1) of the SFCA.
To help consumers to understand the claimed benefit, products with substantiated claims should display on the food label statements that:
- Link each claimed effect to specific substance(s) in the product;
- Indicate the amount of the substance or product required to achieve the effect along with the level of the substance contained in a serving of the food. Where there is a daily amount or RDI for the substance associated with the claimed effect, these amounts can be expressed as a percentage or fraction; and
- Express claimed effects in specific terms rather than general terms such that the claimed effects are both measurable and quantifiable reflecting their scientific validation. Claims that state a specific effect provide more useful information for the consumer and are less likely to be misleading or misunderstood than a claim about a general or broad effect.
4.4.6 Amino acid claims for supplemented foods
Under the Supplemented Foods Regulations, SFs are exempted from subsection B.01.305(2) of the FDR. This means that representations about amino acids used as SIs as per the List of Permitted Supplemental Ingredients are acceptable for SFs that do not meet the requirement for a "source of protein" claim. SFs carrying representations on their labels about amino acids added as SIs are not required to include a declaration regarding the amount of histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine contained in the food, unless that particular amino acid has been added as an SI. See Section 4.4.7 for restrictions that apply to nutrition and health-related statements and claims on SFs.
Despite the exemption above, SFs are subject to subsection B.01.305(1) of the FDR; SFs must meet the conditions for a "source of protein" claim set out in the Nutrition Labelling - Table of Permitted Nutrient Content Statements and Claims in order to make statements or claims about protein on their label.
4.4.7 Specific restrictions on the use of claims and other representations on supplemented foods
As noted in Section 4.4, the general requirements for claims and other representations on prepackaged foods apply to SFs. However, certain representations, express or implied, are not permitted on the label or in advertisements of certain SFs.
In the following sections, "representation" means any nutrition and health-related statements and claims, that expressly or implicitly indicates that the food has particular nutritional or health-related properties, including logos, symbols, brand names, seals of approvals and marks that are used by manufacturers or distributors on SF labels or in advertising of an SF, unless otherwise indicated.
4.4.7.1 Claims not permitted for supplemental ingredients with an associated cautionary statement
Subsection B.01.311(3) of the FDR permits the use on the label of or advertisement for a food a statement or claim to the effect that a nutrient contained in the food is generally recognized as an aid in maintaining the functions of the body necessary to the maintenance of good health and normal growth and development. It is further clarified through Guidance that the general function claims referring to a nutrient as an aid or a factor in the maintenance of good health and in normal growth and development can be made about any nutrient (e.g., "Calcium is a factor in the maintenance of good health" and "Calcium is a factor in growth and development"). Furthermore, claims about the specific role(s) of certain nutrients as listed in the Table of Acceptable Nutrient Function Claims (e.g., "Calcium helps in the formation and maintenance of bones and teeth" can be used alone or in association with the general nutrient function claims).
In order to provide consumers with adequate information to make informed choices about the food they consume, subsections B.029.026(1) and B.29.026(2) of the FDR requires that, if an SI that is a nutrient requires a cautionary statement to be shown on the label as per column 4 of the List of Permitted Supplemental Ingredients, a claim or a statement referring to this nutrient that is an SI as an aid in the maintenance of good health is prohibited unless it is accompanied by a statement or claim about the specific effect of that nutrient in maintaining the functions of the body, which is necessary to the maintenance of good health. The accompanying statement reflects and refers to the scientifically recognized specific role each nutrient has in maintaining good health as listed in the Table of Acceptable Nutrient Function Claims.
This prohibition does not apply to SIs that are nutrients if their addition to SFs does not require a cautionary statement as per column 4 of the List of Permitted Supplemental Ingredients.
For example, if the declared amount of calcium added to an SF is more than 58 mg per serving, the label of the SF must show a list of cautionary statements as per column 4 of the List of Permitted Supplemental Ingredients. In this case, the general nutrient function claim "Calcium is a factor/aid in the maintenance of good health" can be used on the food label or in an advertisement of the food, if it is accompanied by a statement about a specific role of calcium maintaining the functions of the body, for example "Calcium helps in the formation and maintenance of bones and teeth". It would also be acceptable to use the latter specific claim alone.
Conversely, if the declared amount of calcium added as an SI in an SF is lower than 58 mg, the general claim "Calcium is a factor/aid in the maintenance of good health" can be used on the SF label, with or without the specific function claim "Calcium helps in the formation and maintenance of bones and teeth".
When the two statements appear on the label, they must be of the same prominence and must appear adjacent to one another without intervening printed, written or graphic material. If the two statements are made in a radio advertisement or in the audio portion of a television advertisement for the SF, they should immediately follow one another. If made in the visual portion of a television advertisement for the SF, they should appear concurrently and for the same amount of time, adjacent to one another without any intervening printed, written or graphic material, and must be shown in letters of the same size and prominence (subsection B.029.026(3) of the FDR).
4.4.7.2 Claims not permitted on supplemented foods that are not to be consumed by a certain age group
For SFs that are required to carry a cautionary statement advising that the food is not intended to be consumed by any group of individuals under 18 years of age (e.g., those under 14 years of age), a statement or claim to the effect that a nutrient contained in the food is generally recognized as an aid in maintaining the functions of the body necessary to normal growth and development, would not be permitted on the label of or in the advertisement for the SF (section B.29.027 of the FDR). Health benefits referring to growth and development are generally more relevant to younger sub-populations and would not be appropriate when the food is not recommended for those sub-populations.
For example, if the amount of vitamin D declared on the label of an SF is more than 7 µg per serving, the cautionary statement "Not recommended for those under 14 years old" would be required as per column 4 of the List of Permitted Supplemental Ingredients. In this case, the general nutrient function claim "Vitamin D is a factor/ aid in normal growth and development" and the specific function claim about the role of vitamin D in maintaining the functions of the body as listed in the Table of Acceptable Nutrient Function Claims (e.g., Vitamin D aids in the formation of bones and teeth) would not be permitted on the label of or in the advertisement for the SF.
4.4.7.3 Claims not permitted on supplemented foods with the "High caffeine content" statement
Subsection B.29.028(1) of the FDR prohibits the use, on the label or in an advertisement of an SF that is required to carry the statement "High caffeine content" as set out in column 5 of the List of Permitted Supplemental Ingredients for caffeine, of any representation, express or implied, about any vitamins or mineral nutrients contained in the SF. Representations that refer to or imply that the SF is a source of essential nutrients such as vitamins and mineral nutrients, as well as claims (general or specific) referring to a nutrient contained in the food as an aid in maintaining the functions of the body necessary to the maintenance of good health and normal growth and development, create an impression that the product can be consumed long-term as part of a daily eating pattern. These representations are not appropriate for products with high caffeine content. As per subsection B.29.028(2) of the FDR, this prohibition does not apply to the declaration of a vitamin in the list of ingredients or in the SFFt, or to the declaration of a mineral nutrient in the list of ingredients, the FOP nutrition symbol or in the SFFt.
In the List of Permitted Supplemental Ingredients, column 2 excludes products that are subject to any of the following representations, express or implied, on the label of or in an advertisement for an SF that is required to carry the statement "High caffeine content", as set out in column 5 of the List for caffeine:
- a) Any representation related to the benefit of consuming the SF to improve physical performance (e.g., endurance, recovery, power, strength, performance, or sports) before, during or after physical activity. The consumption of SFs with high caffeine content within the context of sport performance is not recommended as their intake could lead to adverse effects, which can be more pronounced in those sensitive to caffeine or those that have limited exposure to caffeine (Rodriguez et al., 2009, Grinberg et al., 2022); and
- b) Any representation about the product suitability for hydration or for electrolyte replacement. For example, the statement "with electrolytes" would not be permitted. Representations that refer to or imply that SF beverages with high caffeine content can be used for hydration or electrolyte replacement are not consistent with the types of products in which caffeine is permitted and the conditions of use described in the List of Permitted Supplemental Ingredients (e.g., cautionary statement to limit the number of servings that can be consumed per day).
4.4.7.4 Restrictions on size of acceptable claims for supplemented foods with a supplemented food caution identifier
As per section B.29.029 of the FDR, any representation shown on the label of SFs that carry an SFCI as per section B.29.021 of the FDR, are subject to size restrictions:
- a) When the representation is displayed on a PDP that carries the SFCI:
- i. the height of the upper case letters in the representation must not exceed two times the height of the upper case letters (excluding any accents) in the SFCI, other than in the words "Health Canada" and "Santé Canada"; and
- ii. the height of the tallest ascender of the lower case letters in the representation must not exceed two times the height of the tallest ascender of the lower case letters in the SFCI, other than in the words "Health Canada" and "Santé Canada".
- b) When the representation is displayed on any continuous surface, other than on the PDP:
- i. the height of the upper case letters in the representation must not exceed two times the height of the upper case letters, excluding any accents, in the cautionary statements; and
- ii. the height of the tallest ascender of the lower case letters in the representation must not exceed two times the height of the tallest ascender of the lower case letters in cautionary statements.
The representations that are subject to the size restrictions are those made or placed at the discretion of the manufacturer and are specified in subsections B.29.029(2) and B.29.029(3) of the FDR and are as follows:
- a) any declarations of the SF energy value or the amount of a nutrient or SI contained in the SF, except when these declarations are made in the SFFt;
- b) any representation that expressly or implicitly indicates that the SF or any substance it contains has particular nutritional or health-related properties including but not limited to any statement or claim set out in column 4 of the Nutrition Labelling - Table of Permitted Nutrient Content Statements and Claims or column 1 of the table following section B.01.603 or referred to in section B.01.311, section D.01.006 or D.02.004 of the FDR; and
- c) any health-related statement, logo, symbol, seal of approval or mark, excluding the brand name or product name of the SF, and any statement required to be shown on the label of an SF in accordance with column 5 of the List of Permitted Supplemental Ingredients.
5.0 Compliance and enforcement of Supplemented Foods Regulations
The onus is on the food manufacturer or distributor to ensure that a food offered for sale in Canada complies with all rules that apply to the sale of the food, including but not limited to rules under the FDA and the SFCA, and the regulations associated with these Acts.
TMALs were issued to allow the sale of safe but non-compliant foods in order to generate in-market information to support an amendment to the FDR, for a framework for supplemented foods. The in-market information collected by TMAL holders included sales data, research data on Canadians' consumption patterns of these foods, and consumers' understanding and use of label information on the product.
For SFs that carry cautionary statements, there was an additional requirement that TMAL holders provide consumption incident reports on an annual basis for the duration of the TMA. A summary of consumption incidents reported for SFs is published on Health Canada's website.
This information, along with, scientific literature, and other information available to Health Canada, was used to inform the development of the Supplemented Foods Regulations.
Under the Supplemented Foods Regulations, there is no regulatory requirement for manufacturers or distributors of SFs to collect and provide research and in-market data related to their products. This also means that there is no longer a requirement to report consumption incidents to Health Canada. SF consumption incidents are to be managed by the CFIA in the same manner as other foods. The CFIA may take enforcement action, including recalling of non-compliant SFs, if appropriate.
In Canada, responsibility for regulatory oversight of food safety is shared between the federal Government and provincial health units or provincial departments. Food safety and labelling concerns are reported to the CFIA, except in Quebec where the Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec (MAPAQ) is the lead organization for all consumer concerns. Consumers can report consumption incidents to the CFIA using the webpage Find out where to report a food complaint or concern. In Quebec, consumers can report concerns about a food, including SFs, through MAPAQ's online form.
Sections 82 to 85 of the SFCR require manufacturers or distributors to have an effective complaint, investigation, notification and recall system in place for foods. When a food presents a risk of injury to health or is non-compliant with the SFCR, it is the license holder's responsibility to investigate, and in the case a risk of injury to health is found, immediately notify CFIA as well as take action to mitigate the risk. Additional requirements related to preparing, keeping and maintaining food complaint procedure related documents can be found on CFIA's Regulatory requirements: Preventive controls webpage.
While it is Health Canada's role to develop the Supplemented Foods Regulations, the CFIA is responsible for the enforcement of the regulations. Health Canada and the CFIA are working jointly to facilitate the transition of existing SFs into full compliance with the Supplemented Foods Regulations. All new SFs must fully comply with the regulations in place, and products considered non-compliant with the regulations will be subject to enforcement action by the CFIA. SFs that are permitted the transition period to come into compliance with the Supplemented Foods Regulations as per Section 2.0 must meet all the rules of the FDR other than the variations outlined in their expired TMAL or written notice.
6.0 Pathway to market for new products not compliant with the List of Permitted Supplemented Food Categories and/or the List of Permitted Supplemental Ingredients
Health Canada regulates SFs in a manner that allows for flexibility, while ensuring safety of new products. Only products that meet the regulatory rules including those in the List of Permitted Supplemented Food Categories and the List of Permitted Supplemental Ingredients are considered compliant with the Supplemented Foods Regulations. However, stakeholders may request a change to these Lists, which are incorporated by reference into the FDR, by submitting a premarket request to Health Canada's Food Directorate. If the outcome of the assessment supports a potential change to a List, the requested change may be implemented in the applicable List after a consultation process. Once a change is implemented in the Lists, any SF manufacturer can benefit from the new change and is not required to submit a separate premarket request. A premarket submission cannot be filed to request a change to the documents incorporated by reference into the transitional provisions in a static manner (see Appendix 1). The sections below provide an overview of the requirements and process related to premarket requests.
6.1 Triggers for premarket requests
Stakeholders who wish to request a change to the List of Permitted Supplemented Food Categories and/or the List of Permitted Supplemental Ingredients, incorporated by reference into the Supplemented Foods Regulations can file a premarket submission for any of the following changes:
- Addition of a new SI to the List of Permitted Supplemental Ingredients;
- Modification of the List of Permitted Supplemental Ingredients in respect of a SI already listed including an increase or decrease to the maximum permitted amount of addition or removal of food categories in which it can be used and change to the conditions of use; or
- Modification of the List of Permitted Supplemented Food Categories, including the addition of a new category or removal of a category.
An ingredient, such as a herbal or non-herbal ingredient, may meet the definition of a novel food (see Section 3.2 g). The Supplemented Foods Regulations introduced an amendment to the novel food definition in Division 28, Part B of the FDR that excludes SIs and SFs; thereby excluding them from the notification requirements applicable to novel foods. If, therefore, a manufacturer determines that it is more appropriate or of great interest for an ingredient or food to be used as a SI or a SF, then the manufacturer could consider filing a premarket submission to amend documents incorporated by reference into Division 29, Part B of the FDR, rather than as a novel food under Division 28.
An authorized SI or SF - their use being exactly in accordance with the conditions outlined in the List of Permitted Supplemented Food Categories and the List of Permitted Supplemental Ingredients - would no longer meet the definition of a novel food. This does not mean that the use of the same ingredient or food outside their authorized use as a SI or a SF would also be exempt from the novel food definition. For broad, unrestricted use of an ingredient or a food, a manufacturer may wish to consider filing a premarket submission as a novel food under Division 28 of the FDR.
An ingredient that is evaluated under the general novel food paradigm would be expected to be safe for use, such that it could be generally added to any food without special conditions, above and beyond those that already exist for foods, and foods containing the ingredient could be consumed without restriction by the general population.
An approved novel food, but which has not been approved as an SI, could be added to a SF, but could not be represented as a SI, such as by being included in the SFFt under the subheading "Supplemented with" (see Section 4.3.1.1). An approved novel food could subsequently at a stakeholder's request, be evaluated as a SI in order to be represented as such, in which case a premarket submission must be submitted as per the applicable data requirements.
6.2 Data requirement for premarket requests
A request for modification to the List of Permitted Supplemental Ingredients or to the List of Permitted Supplemented Food Categories, must be supported by specific information, which will depend on the type of change requested.
The following information should be provided in support of a request for a modification to the List of Permitted Supplemental Ingredients:
- Description of the proposed change;
- Description of the ingredient, including its name;
- Source of the ingredient and its method of manufacture;
- Information on the physical and chemical properties of the ingredient;
- Information on the composition and specifications (chemical and microbiological) of the ingredient or provide a detailed rationale;
- Proposed maximum amount of the ingredient per serving;
- Proposed conditions of use of the ingredient; and
- Data to establish the safety of the ingredient under its proposed conditions of use.
The following information should be provided in support of a request for a modification to the List of Permitted Supplemented Food Categories:
- Description of the proposed change;
- Description of the proposed product;
- Description of the proposed category;
- Estimated levels of consumption of foods in the proposed category; and
- Information on the SIs proposed to be permitted within the category.
For modifications to either List, stakeholders can be requested to provide any additional information that may be relevant to an assessment of the request. Health Canada intends to develop further guidance for stakeholders with detailed data requirements for requesting modifications to the Lists.
6.3 Premarket submission process
Stakeholders who wish to file a premarket submission for one of the purposes outlined in Section 6.1 can contact the SMIU (smiu-ugdi@hc-sc.gc.ca). Stakeholders are encouraged to request a pre-submission consultation with the Food Directorate to seek further guidance so that a complete submission can be filed at the outset, potentially reducing the number of requests to the applicant for clarification or additional information, or preventing the submission from being rejected for incompleteness. Pre-submission consultations on SFs may be arranged by contacting the SMIU (smiu-ugdi@hc-sc.gc.ca).
6.4 Modification of incorporated by reference Lists
Health Canada will review premarket requests to modify the List of Permitted Supplemented Food Categories and List of Permitted Supplemental Ingredients received in accordance with Sections 6.1 to 6.3. If the outcome of the premarket assessment supports the proposed change, the proposal for modifying the incorporated by reference Lists will undergo a public consultation.
Domestic and international stakeholders are notified of the proposed modifications by means of a Notice of Proposal (NOP) published on Health Canada's website. Interested stakeholders are provided a 60-75 day period from the day on which the NOP is posted to provide comments.
At the close of the comment period, all feedback received is taken into consideration. If no new scientific information is provided that would require revisions to the proposal, the Food Directorate will then proceed with formally modifying the incorporated by reference document. Notification is provided by means of a Notice of Modification (NOM) posted on Health Canada's website. The NOM will specify the changes made, which will come into effect as soon as the Lists are amended.
In addition, the Food Directorate continues to monitor new scientific data as it becomes available and will conduct or update health risk assessments for foods, including SFs, when warranted. A proposal to modify the Lists may result based on new science, an emerging health risk or the outcomes of risk assessments, should a risk concerning SFs or SIs be identified. A change proposed by Health Canada would also undergo a NOP prior to making a change through an NOM.
Further details on the incorporation by reference process and the associated timelines can be found on the Government of Canada website.
7.0 Resources Available
Additional resources that will assist SF industry stakeholders in meeting regulatory requirements for SFs are provided in Appendix 7.
8.0 Contact Information
For general inquiries related to SFs please contact the Food Directorate at supplementedfoods-alimentssupplementes@hc-sc.gc.ca. For questions related to premarket submission process please refer to Section 6.3.
9.0 Glossary and Abbreviations
This section provides a glossary of key terms and abbreviations used throughout this guidance document.
9.1 Glossary
- Conditions of use:
- Refers to the regulatory requirements for a supplemented food, related to the permitted food categories, maximum amounts of supplemental ingredients and manner of declaring them, cautionary statements, and other conditions, as prescribed in the List of Permitted Supplemental Ingredients and List of Permitted Supplemented Food Categories.
- Directory of Supplemented Food Facts Table Formats:
- Means the document entitled Directory of Supplemented Food Facts Table Formats incorporated by reference into the Food and Drug Regulations, and published by the Government of Canada on its website, as amended from time to time.
- Directory of Supplemented Food Caution Identifier Specifications:
- Means the document entitled Directory of Supplemented Food Caution Identifier Specifications incorporated by reference into the Food and Drug Regulations, and published by the Government of Canada on its website, as amended from time to time.
- List of Permitted Supplemented Food Categories:
- Means the document entitled List of Permitted Supplemented Food Categories incorporated by reference into the Food and Drug Regulations, and published by the Government of Canada on its website, as amended from time to time.
- List of Permitted Supplemental Ingredients:
- Means the document entitled List of Permitted Supplemental Ingredients incorporated by reference into the Foods and Drug Regulations, published by the Government of Canada on its website, as amended from time to time.
- List of cautionary statements:
- Means the list shown on the label of a supplemented food as per subsection B.29.020(1) of the FDR.
- Supplemented Food:
- Means a prepackaged product that belongs to a food category set out in column 1 of the List of Permitted Supplemented Food Categories and to which a supplemental ingredient has been added.
- Supplemented Food Facts Table:
- Means the supplemented food facts table required to be carried on the label of a supplemented food.
- Supplemented Food Caution Identifier:
- Means the supplemented food caution identifier required to be carried on the principal display panel of a supplemented food when one or more supplemental ingredients trigger the requirement of the supplemented food to carry cautionary statements as per the List of Permitted Supplemental Ingredients.
- Supplemental Ingredient:
- Means a vitamin, mineral nutrient, amino acid or any other substance that is listed in column 1 of the List of Permitted Supplemental Ingredients and that is added as an ingredient to a food in accordance with the applicable conditions of use set out in columns 2 to 5 of that List.
- Thresholds Levels for Cautionary Statements and Other Conditions of Use
- Means the document entitled Threshold Levels for Cautionary Statements and Other Conditions of Use, published by the Government of Canada on its website, as it reads on the day on which the Supplemented Foods Regulations come into force.
- TMAL Lists:
- Means one or more of the following lists, published by the Government of Canada on its website, as they read on the day on which the Supplemented Foods Regulations come into force: (a) the List of beverages, beverage mixes and concentrates; (b) the List of caffeinated energy drinks; and (c) the List of conventional foods.
9.2 Abbreviations
The following abbreviations are used throughout the document.
- CCHS
- Canadian Community Health Survey
- CED
- Caffeinated energy drink
- CFIA
- Canadian Food Inspection Agency
- CGI
- Canada Gazette, Part I
- CGII
- Canada Gazette, Part II
- DRI
- Dietary Reference Intake
- EFSA
- European Food Safety Authority
- FDA
- Food and Drugs Act
- FDR
- Food and Drug Regulations
- FOP
- Front-of-Package
- GMP
- Good Manufacturing Practices
- IMA
- Interim Marketing Authorization
- MAPAQ
- Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec
- NASEM
- National Academies of Science, Engineering and Medicine
- NCC
- Nutrient Content Claim
- NHP
- Natural Health Product
- NHPR
- Natural Health Products Regulations
- NOM
- Notice of Modification
- NOP
- Notice of Proposal
- PDP
- Principal Display Panel
- PDS
- Principal Display Surface
- RDI
- Reasonable Daily Intake
- SFCA
- Safe Food for Canadians Act
- SFCR
- Safe Food for Canadians Regulations
- SF
- Supplemented Food
- SFCI
- Supplemented Food Caution Identifier
- SFFt
- Supplemental Food Facts Table
- SI
- Supplemental Ingredient
- SMIU
- Submission Management and Information Unit
- TMA
- Temporary Marketing Authorization
- TMAL
- Temporary Marketing Authorization Letter
- UL
- Tolerable Upper Intake Level
Appendix 1: Documents incorporated by reference into the Supplemented Foods Regulations
The documents listed below are incorporated by reference into the FDR. Documents 1 to 4 are incorporated by reference on an ambulatory basis, meaning as amended from time to time. These technical documents are managed administratively by Health Canada's Food Directorate, to permit flexibility in the framework to respond to a premarket submission from a stakeholder, new evidence or emerging health risks. Documents 5 and 6 are incorporated by reference statically, meaning as they read on the day that the regulations came into force. These two documents are not to be modified as they are related to the transitional provisions of the regulations.
- Directory of Supplemented Food Caution Identifier Specifications
- Directory of Supplemented Food Facts Table formats
- List of Permitted Supplemented Food Categories
- List of Permitted Supplemental Ingredients
- TMAL ListsFootnote 5
- Threshold Levels for Cautionary Statements and Other Conditions of UseFootnote 5
The List of Permitted Supplemental Ingredients and the List of Permitted Supplemented Food Categories may be modified from time to time in response to a request for a change from a stakeholder, new science or an emerging health risk (see Section 6.0).

Figure - Text description
Appendix 2 is composed of a flowchart to help stakeholders identify if their products fall within the scope of the supplemented foods regulations. On the left hand side of the flow chart there are 7 questions to be answered to determine if the product in question is a supplemented food. The right hand side and the bottom of the left hand side of the flowchart contain different possible outcomes, depending on whether a yes or no answer is provided to each question.
First question:
Is the product prepackaged and intended to be sold and represented for use as a food? If unsure, refer to the Guidance Document: Classification of products at the food-natural health product interface: products in food formats, with the webpage hyperlinked in the text. If NO, Supplemented Foods Regulations only apply to prepackaged foods. If the product is a Natural Health Product, please contact the Natural and Non-prescription Health Products Directorate. If YES, proceed to the second question.
Second question:
Does the food contain added vitamins, mineral nutrients, amino acids and/or other substances? If NO, Supplemented Foods Regulations do not apply. The product must comply with all applicable rules of the FDR. If YES, proceed to the third question.
Third question:
Are the added vitamins, mineral nutrients and/or amino acids being used for a nutritional purpose (i.e., fortification purposes)? If YES, fortified foods are excluded from being supplemented foods; however, certain exceptions apply. Please refer to Section 3.2 a) for information on fortified foods, applicable exceptions, and use of fortified foods as ingredients in supplemented foods. If NO, proceed to the fourth question.
Fourth question:
Is the product an alcoholic beverage, a specialty food, a food for special dietary use, or a food targeted to infants, children under 4 years of age, pregnant or breastfeeding women? If YES, not permitted as a supplemented food. Refer to Section 3.2 for more information, including the exception applicable to gluten-free foods. If NO, proceed to fifth question.
Fifth question:
Does the food belong to a food category specified in column 1 of the List of Permitted Supplemented Food Categories? If NO, not a permitted supplemented food category. Section 4.1 includes descriptions of the permitted categories. Refer to Section 6 for information on amending the List of Permitted Supplemented Food Categories. If YES, proceed to sixth question.
Sixth question:
Does the food contain any added vitamins, minerals, amino acids and/or other substances not permitted by the FDR? If YES, non-compliant with the FDR. If the substance is intended to be added as a supplemental ingredient, refer to Section 6 for information on amending the List of Permitted Supplemental Ingredients. If NO, proceed to the seventh question.
Seventh question:
If the vitamin, mineral nutrient, amino acid or other substance is intended to be added as a supplemental ingredient, does it follow the criteria outlined in columns 2 to 5 of the List of Permitted Supplemental Ingredients? If NO, non-compliant with the Supplemented Foods Regulations. Refer to Section 6 of this document for information on amending the List of Permitted Supplemental Ingredients. If YES, the product is a supplemented food and must comply with the Supplemented Foods Regulations, along with all other applicable provisions of the FDR in order to be sold on the Canadian market.
Appendix 3: Ingredients not permitted in foods, including supplemented foods
In addition to the prohibitions and maximum levels set out in Health Canada's List of Contaminants and Other Adulterating Substances in Foods and in the List of Maximum Levels for Various Chemical Contaminants in Foods (see Section 4.0), the ingredients found in the list below are substances considered to be inappropriate for consumption as foods, including in supplemented foods. The list of ingredients below is not exhaustive and may be revised as new information becomes available.
Ingredients inappropriate for consumption as foodsFootnote 6
- Cascara sagrada (Frangula purshiana Cooper)
- Chaparral (Larrea tridentata (Sessé & Moc. ex DC.) Coville, L. divaricata Cav.)
- Ephedra (Ephedra spp.)
- Germander (Teucrium chamaedrys L.)
- Horsetail (Equisetum spp.)
- Kava-kava (Piper methysticum G.Forst.)
- Khat (Catha edulis (Vahl) Endl.)
- Senna (Senna alexandrina Mill.)
- Arnica (Arnica montana L., wolf's bane, leopard's bane)
- Comfrey (Symphytum spp.)
- Magnolia (Magnolia officinalis Rehder & E.H.Wilson)
- Pleurisy root (Asclepias tuberosa L.)
- Stephania (Stephania tetrandra S.Moore)
- Yellow jessamine (Gelsemium sempervirens (L.) J.St.-Hil.)
Appendix 4: Vitamins and mineral nutrients - Approach for setting maximum amounts and other conditions of use
Health Canada has established maximum amounts for vitamins and mineral nutrients in SFs using a risk-based approach to help ensure that their addition does not contribute to excessive intakes.
1.0 Methods for deriving maximum amounts and other conditions of use
The first step in setting the maximum amounts was to determine the total daily amount which should not be exceeded for each vitamin and mineral nutrient; hereafter, described as the "safe daily amount".
For most vitamins and mineral nutrients, the Tolerable Upper Intake Levels (ULs)Footnote 7, established by the National Academies of Science, Engineering and Medicine (NASEM), were used as the safe daily amount. Where there was no UL established by the NASEM or where other information was determined to be more appropriate, alternate scientific sources were used to establish the safe daily amount.
For the majority of nutrients, the safe daily amount, as well as background intake, were used to calculate the maximum amounts for vitamins and mineral nutrients for all SFs.
For each vitamin and mineral nutrient, calculations of the maximum amounts were based on data inputs from the most vulnerable age-gender group, i.e., the group with the smallest difference after subtracting background intakes from the safe daily amount. For most vitamins and minerals, the background intake used was the 95th percentile of usual intakeFootnote 8 obtained from the 2015 Canadian Community Health Survey (CCHS, 2015). Using the 95th percentile of usual intake protects the most vulnerable populations from risks of overconsumption of vitamins and mineral nutrients.
For a few vitamins and mineral nutrients for which food intake data was not available from the CCHS, estimated intakes were based on levels reported by the NASEM. For certain other vitamins and minerals, supplement intake data from the CCHS was considered unreliable due to extreme sampling variability. In these cases, nutrient intakes from supplements were estimated based on the composition of the top selling multi-vitamin/mineral supplements in Canada according to 2017 Nielsen data.
Refer to Table 6 in this Appendix for references used for the safe daily amount and data inputs for each vitamin and mineral nutrient specifically.
1.1 Derivation of maximum amounts for vitamins and mineral nutrients
- a) Method for calculating maximum amounts for SFs other than beverages containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm
Maximum amount formulaFootnote 9:
(safe daily amount - 95th percentile food intake) ÷ 2
For most vitamins and mineral nutrients, the maximum amounts were determined by subtracting the 95th percentile of usual food intake from the safe daily amount. The resulting values were the total daily amount of that vitamin or mineral nutrient that could be consumed through SFs without exceeding the safe daily amount. A safety factor of two (2) was applied to the resulting values to mitigate the potential risk of excess intakes if consumers do not read or follow the cautionary statements and to account for the fact that overages were not taken into consideration in calculating maximum amounts.
This formula does not account for the following factors:
- Intake from supplements;
- Consumption of multiple servings per day; and
- Consumption by individuals under 14 years of age.
Therefore, additional cautionary statements are required above certain threshold levels to mitigate potential risks of excessive intakes of vitamins and mineral nutrients added to SFs other than beverages described in (b).
- b) Method for calculating maximum amounts for beverages containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm
Maximum amount formulaFootnote 9:
(safe daily amount - 95th percentile combined food and supplement intake) ÷ 5
For most vitamins and mineral nutrients, the maximum amounts for SFs belonging to item 1 of the List of Permitted Supplemented Food Categories that contain added caffeine and a total amount of caffeine from all sources of more than 150 ppm, commonly known as caffeinated energy drinks (CEDs), were determined by subtracting the 95th percentile of combined dietary intakes from food and supplements, from the safe daily amount. The resulting values were divided by five (5) to account for the possibility that individuals might consume multiple servings (i.e., up to five servings) of SFs in one day.
No additional cautionary statements are required to mitigate the risk of excess vitamin and mineral nutrient intakes, as the formula accounts for supplement intake as well as the consumption of multiple servings.
While the formula used for SFs in this category does not account for intake by individuals under 14 years of age, the cautionary statement "Not intended for those under 14 years old" is always required for SFs in this category based on the presence of caffeine as an SI.
1.2 Rationales for the requirement of cautionary statements for vitamins and mineral nutrients
The List of Permitted Supplemental Ingredients specifies the threshold levels for each vitamin and mineral nutrient above which cautionary statements are required.
- a) Cautionary statement: "Do not [eat/drink] more than X serving(s) per day"
The TMA research suggests that a small proportion of consumers may consume up to 5 servings of SFs per day. Therefore, this cautionary statement is required if consuming 5 or less servings of an SF in a day results in total intakes exceeding the maximum amount for any vitamin or mineral nutrient.
- b) Cautionary statement: "Do not [eat/drink] on the same day as any other supplemented foods or supplements with [the same supplemental ingredients/(name specific ingredients)]"
Consuming an SF that provides vitamins and mineral nutrients at levels requiring the maximum daily servings cautionary statement, along with a supplement or another SF containing the same vitamins and mineral nutrients, could result in intakes exceeding the maximum amounts. To mitigate this risk, SFs that require the maximum daily servings cautionary statement also require the cautionary statement about combining intakes with other sources of the same vitamins and mineral nutrients (i.e., supplements and other SFs).
For most vitamins and mineral nutrients, the threshold levels for this statement are the same as those that apply to the maximum daily servings cautionary statement. However, for vitamin A and zinc, certain age-gender groups already have intakes exceeding the safe daily amount from food and supplement intake. Therefore, in order to prevent excess intakes from SFs, the cautionary statement "Do not [eat/drink] on the same day as any other supplements with [the same supplemental ingredients/(name specific ingredients)]" is required for products containing vitamin A and zinc as an SI at any level.
- c) Cautionary statement: "Not recommended for those under 14 years old"
This cautionary statement is required for SFs that contain vitamins and mineral nutrients above levels determined to be safe for individuals under 14 years of age.
For most vitamins and mineral nutrients, the threshold level for this cautionary statement is set above the amount determined by the following formula, using data from the most vulnerable age-gender group under 14 years old:
Threshold level formula:
(Safe daily amount - combined dietary intake from food and supplements) ÷ 5
These threshold levels represent the highest amount, per serving, that could be consumed by individuals under 14 years of age without additional cautionary statements, as the formula accounts for supplement intake and the consumption of up to 5 servings per day.
For a number of vitamins and mineral nutrients, the threshold level calculated using this formula resulted in an amount higher than the threshold level for other cautionary statements. In these cases, the threshold level for this statement was adjusted to match the lowest threshold level for other cautionary statements. The approach ensures that individuals under 14 years of age will not have to read and understand cautionary labelling in order to safely consume SFs.
For vitamin A and zinc, some age-gender groups under 14 years of age already have intakes exceeding the safe daily amount from food and/or supplement intake. Therefore, this cautionary statement is required on products containing these nutrients at any level.
- d) Cautionary statement: "Not recommended for pregnant or breastfeeding women"
For all vitamins and mineral nutrients with the exception of niacin, the safe daily amount used for setting the maximum amounts applied to pregnant and breastfeeding women. For niacin, the UL established by the European Food Safety Authority (EFSA) (see Table 6 below) was used as the safe daily amount, and EFSA explicitly states that the UL does not apply during pregnancy or lactation due to inadequate data. Therefore, this cautionary statement is required for products containing niacin above 30 mg per serving, based on the lowest UL established by the NASEM for pregnant and breastfeeding women. Intakes up to this level are not likely to pose a risk of adverse health effects in this population.
2.0 Additional information on the approach for maximum amounts and cautionary statements for vitamins and mineral nutrients
The maximum amounts and conditions of use for all vitamins and mineral nutrients permitted for addition to SFs are specified in the List of Permitted Supplemental Ingredients. In some cases, the maximum amounts, conditions of use, or permitted SIs, differ from those set out as part of the TMA requirements set out in the guidance documents Category Specific Guidance for Temporary Marketing Authorization: Supplemented Food and Category Specific Guidance for Temporary Marketing Authorization - Caffeinated Energy Drinks. The reasons for these changes vary and are summarized below.
2.1 Source of nutrient intake data
The maximum amounts for SFs specified in the List of Permitted Supplemental Ingredients were calculated using dietary intake data from the 2015 Canadian Community Health Survey (CCHS), which includes nutrient intake data from both food and supplements. This dataset provides a more accurate estimate of Canadians' supplement intake compared to the previous approach which based estimates on Nielsen sales data of multi-vitamin/mineral supplements in Canada.
2.2 Vitamins and mineral nutrients not permitted for addition to supplemented foods
Manganese has been added to the list of vitamins and mineral nutrients not permitted for addition to SFs (see Section 4.2.10.1). Intake data from CCHS 2015 shows that the 95th percentile of intakes of manganese are at or exceeding the UL for children 4-8 years, males 14-18 years and males 19 to 30 years of age, leaving no room for additional manganese from SFs.
2.3 Maximum amounts for supplemented foods other than beverages containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm
The List of Permitted Supplemental Ingredients specifies one set of maximum amounts for all SF categories, other than SFs belonging to item 1 of the List of Permitted Supplemented Food Categories, containing added caffeine and total caffeine concentration from all sources of more than 150 ppm caffeine, commonly known as caffeinated energy drinks (CEDs) during the TMA period. These amounts were set using the same approach used to derive the former Path 2 maximum levels established for the TMA period (see Section 1.1.a) above.
With the current approach, the threshold levels for cautionary statements are comparable to the former Path 1 maximum levels. During the TMA period, products containing vitamins or mineral nutrients at levels exceeding the Path 1 maximum levels required cautionary statements to mitigate the risk of excess vitamin and mineral nutrient intakes.
2.4 Maximum amounts for beverages containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm
The List of Permitted Supplemental Ingredients includes maximum amounts for vitamins and mineral nutrients that are permitted for addition to beverages containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm. The approach to establish the maximum amounts for SFs in this category differs from the approach used during the TMA period. The approach was revised in order to improve alignment with the approach applied for other SFs (see Section 1.1.b) above).
2.5 Approach for setting maximum amounts for B vitamins with no established Tolerable Upper Intake Level
For riboflavin, thiamine, pantothenic acid, vitamin B12 and biotin, a UL has not been established by the NASEM. During the TMA period, maximum levels permitted in the Category Specific Guidance for Temporary Marketing Authorization - Caffeinated Energy Drinks for thiamine, riboflavin and vitamin B12 were based on the threshold for maximum absorption. This is the level above which there is a significant decrease in the body's absorption for that nutrient. For pantothenic acid, levels up to 100 mg were indicated as acceptable by Health Canada in the Category Specific Guidance for Temporary Marketing Authorization - Caffeinated Energy Drinks during the TMA period. For biotin, an internal maximum level was established. While no maximum levels were set for these B vitamins for other SF categories, levels of addition higher than those found in SFs marketed at the time guidance was published were assessed on a case-by-case basis during the evaluation of a TMA submission.
Maximum amounts have been established for these B vitamins for all SF categories in the Supplemented Foods Regulations. The maximum levels set out in the Natural and Non-prescription Health Products Directorate's (NNHPD's) Multi-vitamin/mineral supplements monograph were used as the safe daily amount to derive the maximum amounts for these B vitamins (see Section 1.0 above). By setting maximum amounts for all SF categories, a consistent approach is applied and prevents SFs from containing levels that exceed those permitted in supplements.
2.6 Approach for setting maximum amounts for potassium
The NASEM has not established a UL for potassium. However, during the TMA period, maximum levels for potassium for SFs other than CEDs, were established to protect vulnerable populations with impaired potassium excretion (e.g., individuals with chronic kidney disease and individuals who use certain medications that may impair potassium excretion). The safe daily amount used to derive the maximum levels was the lowest daily amount of potassium recommended for low potassium diets in clinical settings for individuals with impaired potassium excretion.
Since the publication of the maximum levels for the TMA period, the NASEM (2019) has concluded that short-term potassium supplementation of approximately 2,500 mg/day appears to be safe for generally healthy individuals. As a result, the maximum amount for potassium has been calculated using this level as the safe daily amount (see Section 1.0 above). However, since this safe daily amount applies to short term supplementation only, a safety factor of 3 (instead of 2) is applied in the formula for determining the maximum amount for SFs other than beverages containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm.
2.7 Approach for cautionary statements related to vitamins and mineral nutrients
2.7.1 Grouping of cautionary statements
During the TMA period the following two statements were required for SFs above certain threshold levels for vitamin and mineral nutrients as per the Category Specific Guidance for Temporary Marketing Authorization: Supplemented Food:
- "If you take a daily supplement [that has the same vitamins or minerals], you may be getting too much vitamins or minerals by consuming this product; and
- "Do not consume this product with other supplemented foods [that has the same vitamins or minerals].
The intent of both of these statements is to limit consumption of the SF on days that certain other products (i.e. supplements or other SFs) are consumed which contain the same SIs. Therefore, for simplicity the statements have been combined as follows (see Section 4.2.4):
- "Do not [eat/drink] on the same day as any other supplemented foods or supplements with [the same supplemental ingredients/(name specific ingredients)]"
2.7.2 Threshold level for the cautionary statement: [Do not [eat/drink] on the same day as any other supplements with [the same supplemental ingredients/(name specific ingredients)]
During the TMA period, a statement similar in meaning ("If you take a daily supplement [that has the same vitamins or minerals], you may be getting too much vitamins or minerals by consuming this product") was required above threshold levels of vitamins and mineral nutrients that could result in intakes exceeding the safe daily amount when combined with a supplement. In the Supplemented Foods Regulations, this cautionary statement is required at levels that could result in intakes exceeding the established maximum amounts, when combined with a supplement. The revised approach preserves the safety factor for its intended purpose, i.e., to mitigate the risk of consumers not reading or following the cautionary statements on the label and to account for additional nutrient quantities added for overage purposes.
2.7.3 Wording of the cautionary statement: [Not recommended for those under 14 years old]
During the TMA period, all products containing added caffeine and a total amount of caffeine from all sources of more than 200 ppm (and below 400 ppm) were required to carry the cautionary statement "Not recommended for children…" and other SFs were required to carry the cautionary statement "Not intended for children" or "For adults only" above certain threshold levels for vitamins and mineral nutrients.
However, more recent information available to Health Canada, including the TMA research, found that some children under 14 years were still consuming SFs that carried these cautionary statements. A common source of confusion was the age at which these cautionary statements would apply. To clarify the issue, the cautionary statement has been revised to "Not recommended for those under 14 years old" for all SF categories.
2.7.4 Requirement for the cautionary statement for Niacin: [Not recommended for pregnant or breastfeeding women]
During the TMA period, the maximum amount for niacin was based on calculations that applied the UL, set by the EFSA, as the safe daily amount. Although the UL established by the EFSA was not applicable to pregnant or breastfeeding women, no additional cautionary labelling was required during the TMA period.
When establishing the maximum amount for niacin in the Supplemented Foods Regulations, Health Canada reconsidered its approach and determined that above a certain threshold level, this cautionary statement is warranted for niacin.
2.8 Research conducted during the Temporary Marketing Authorization period
The research collected during the TMA period provided information regarding consumption patterns of SFs, which helped further refine the approach for setting maximum amounts and clarify the wording of cautionary statements as described in the sections above.
2.8.1 Supplement use
The research conducted during the TMA period showed that many consumers of SFs also consume multi-vitamin/mineral supplements. One TMA research study showed that 34% of CED users reported consuming a multi-vitamin/mineral supplement daily on most days, whereas other studies showed that 23-59% of consumers of SFs other than CEDs reported taking multi-vitamin/mineral supplements.
These results support accounting for supplement intake in setting maximum amounts or the application of a cautionary statement against consumption of SFs along with supplements that contain the same SIs.
2.8.2 Youngest age of consumers
While most research did not specifically address consumption of SFs by children as young as 4 years of age, results from one study showed that children as young as 4 years of age consumed the SF in question. This data supports protecting the most vulnerable age-gender group, 4 years of age and older, in setting maximum amounts.
2.8.3 Number of servings per day
According to TMA research, a very small proportion of respondents may be consuming 5 or more CEDs and/or other SFs per day. This data supports accounting for individuals consuming up to 5 servings of SFs per day in setting the maximum amounts.
| Nutrient | Safe Daily Amount | SFs excluding those containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm | SFs containing added caffeine and a total amount of caffeine from all sources of more than 150 ppm | |
|---|---|---|---|---|
| FoodFootnote * | FoodFootnote * | SupplementsFootnote * | ||
| Beta-carotene | (UK EVM, 2003) | X | X | (Nielsen, 2017) top selling multi-vitamin/mineral supplements in Canada |
| Biotin | (NNHPD, 2018) | (NASEM, 1998) estimated food intake | (NASEM, 1998) estimated food intake | (CCHS, 2015) supplement intake |
| Choline | (NASEM, 1998) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake | |
| Niacin | (EFSA, 2006) | X | X | (CCHS, 2015) supplement intake |
| Pantothenic acid | (NNHPD, 2018) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake | |
| Riboflavin | (NNHPD, 2018) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake (for most age-gender groups) | |
| Thiamine | (NNHPD, 2018) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake (for most age-gender groups) | |
| Vitamin A | (NASEM, 2001) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake | |
| Vitamin B6 | (NASEM, 1998) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake | |
| Vitamin B12 | (NNHPD, 2018) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake (for most age-gender groups) | |
| Vitamin C | (NASEM, 2000) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake | |
| Vitamin D | (NASEM, 2011) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake | |
| Vitamin E | (EFSA, 2015) | X | X | (CCHS, 2015) supplement intake |
| Calcium | (NASEM, 2011) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake | |
| Chromium | (EFSA, 2010) (WHO, 1996) | X | X | (CCHS, 2015) supplement intake |
| Copper | (NASEM, 2001) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake (for most age-gender groups) | |
| Magnesium | (NASEM, 1997) | X | X | (CCHS, 2015) supplement intake (for most age-gender groups) |
| Molybdenum | (NASEM, 2001) | (NASEM, 2001) estimated intake | (NASEM, 2001) estimated intake | (CCHS, 2015) supplement intake |
| Phosphorus | (NASEM, 1997) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake (for most age-gender groups) | |
| Potassium | (NASEM, 2019) | X | X | (CCHS, 2015) supplement intake |
| Selenium | (NASEM, 2000) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake | |
| Zinc | (NASEM, 2001) | (CCHS, 2015) food intake | (CCHS, 2015) combined food and supplement intake | |
|
||||
Appendix 5: Amino acids - Approach for setting maximum amounts and conditions of use
Health Canada has developed maximum levels and other conditions of use for amino acids in SFs using a risk-based approach to ensure that the consumption of these products does not result in excessive intakes of supplemental amino acids, which could lead to adverse health effects.
1.0 Rationale for the approach used for setting maximum amounts for amino acids
While there were no published maximum amounts for amino acids during the TMA period, Health Canada had indicated in guidance that the use of amino acids and their levels in SFs would be assessed on a case-by-case basis taking into account the overall risk profile of each product. As part of the TMA submission review process, Health Canada had established internal maximum levels and conditions of use for amino acids, which also included cautionary statements.
In order to increase transparency and minimize regulatory burden, maximum amounts for amino acids are published in the List of Permitted Supplemental Ingredients. The approach to determining maximum amounts and other conditions of use is outlined below, and is intended to help ensure the safety of consumers, and particularly any vulnerable subpopulations. As a result, the maximum amounts outlined in the List of Permitted Supplemental Ingredients may not necessarily be the same as internal maximum levels established during the TMA period, which have been published in the incorporated by reference document Threshold Levels for Cautionary Statements and Other Conditions of Use.
2.0 Approach for setting maximum amounts for amino acids
Maximum amounts have been established for the 20 amino acids involved in protein synthesis (see Table 7). Of these 20, nine are essential amino acids that cannot be produced by the human body, and therefore must be obtained from the diet. Adequate intake of essential amino acids is important to meet human nutritional requirements for normal growth, maintenance of physiological functioning and tissue repair (NASEM, 2002).
| Non-essential amino acids | Essential amino acids |
|---|---|
|
|
Supplementation of the diet with single amino acids, rather than whole proteins, is of concern due to their much faster absorption rate and markedly increased blood concentrations (Pencharz et al., 2008, Gropper et al., 1993), in addition to their greater potential to deviate from nutritional requirements.
Animal studies, and some observations in humans (Kurpad, 2018, Harper, 1958) have shown that amino acid imbalance, caused by disproportionate amounts of essential amino acids, can cause decreases in growth rate and appetite (Harper, 1970), increases in fat accumulation in the liver (Toohey, 2014), and deficiencies of other amino acids (Salmon, 1958). Supplementation with single amino acids can also result in antagonistic effects, where amino acids compete for cellular uptake and the relative excess of one amino acid decreases the availability of its competitor (Park, 2006). Research findings emphasize the importance of total protein intake in mitigating these adverse effects of amino acid supplementation (Food and Agriculture Organization of the United Nations (FAO) Expert Consultation, 2013, Anderson & Raiten, 1992, Imamura et al., 2013, Xiao et al., 2019) and individuals with low protein intake may be more vulnerable to such adverse effects.
Since it may be difficult for some consumers to self-identify as having a low protein diet (e.g., for those who are undernourished in general, as opposed to those who have been advised to follow a low protein diet by their physician), cautionary labelling alone may not mitigate risk for those individuals.
Given the different considerations for supplemental amino acids compared to protein, as well as the approach used to determine maximum amounts as outlined below, maximum amounts for amino acids apply to the total amount of free amino acids in the product, including the supplemental amount, contributions from other food ingredients, and naturally occurring amounts of free amino acids. Maximum amounts do not include protein-bound amino acids or peptide-bound amino acids.
2.1 Maximum amounts for essential amino acids
The maximum amounts for essential amino acids are calculated such that supplementation of a single essential amino acid would not result in an amino acid score that exceeds the highest amino acid score existing in the dietary background intake of the most vulnerable age-gender group (i.e., would not significantly change the ratios of essential amino acids in the background diet). The amino acid score is the ratio of the essential amino acid content in the diet to the requirement of that amino acid for selected age groups, expressed in milligram amino acid per gram protein requirement. The following formula is used to determine the maximum amount for essential amino acids:
Maximum amount formula:
(HdSr - Hr Sd) ÷ Hr
Hd = Daily dietary intake of the essential amino acid with highest amino acid score (mg)
Hr = Requirement for the essential amino acid with highest amino acid score in the scoring pattern (mg/g)
Sd = Daily dietary intake of the supplemental amino acid (mg)
Sr = Requirement for the supplemental amino acid in the scoring pattern (mg/g)
The variables in the formula considered the following factors:
- a) Most vulnerable age-gender group
Females over 70 years of age were determined to be the most vulnerable age-gender group. This group has the highest percentage of individuals (22%) consuming amounts of protein that are insufficient to meet their Estimated Average Requirement (EAR), as per the data from 2015 CCHS. In addition, this group has the lowest overall protein intake of any age-gender group 4 years of age and older.
- b) Dietary intake
The 5th percentile of intake was used as a reference value since supplementation with amino acids may have a higher risk of adverse effects in those with the lowest protein intake. This aligns with the approach for other nutrients, which protects those in the 95th percentile, or top 5% of consumption for each nutrient (see Appendix 4). L-Tryptophan was determined to have the highest amino acid score in the background diet at the 5th percentile of intake for the most vulnerable subpopulation.
- c) Scoring pattern
The scoring pattern used in the formula above is the scoring pattern for older children, adolescents, and adults, as per the 2013 FAO report, "Dietary protein quality evaluation in human nutrition" (FAO Expert Consultation, 2013). This scoring pattern encompasses requirements for both maintenance and growth for adults, adolescents, and children as young as 3 years old. Scoring patterns for younger age groups were not considered, as SFs are not intended for children under 4 years old.
2.2 Maximum amounts for non-essential amino acids
The addition of non-essential amino acids does not change the ratios of essential amino acids relative to one another. Therefore, the maximum amounts for non-essential amino acids generally correspond to the maximum doses specified in the Natural and Non-prescription Health Products Directorate's (NNHPD's) Workout Supplements monograph. In order to ensure that these levels would be appropriate for addition to SFs, the essential amino acid scores were calculated for the background diet of the most vulnerable age-gender group, and these were compared to the amino acid scores of the same diet with the additional non-essential amino acid. Based on this analysis, for most non-essential amino acids, the levels permitted in NNHPD's Workout Supplements monograph were determined to be appropriate in SFs, with some exceptions, listed below:
- The non-essential amino acids L-cysteine and L-tyrosine are included in the scoring pattern for sulfur and aromatic amino acids, respectively. As a result, the maximum amounts for these non-essential amino acids are combined with the maximum amounts for the essential amino acids L-methionine and L-phenylalanine, respectively.
- The maximum amount for L-arginine was set at a level above which extensive cautionary statements would be required to mitigate risk to individuals with cardiovascular disease. Extensive cautionary statements are not considered an appropriate risk mitigation tool for foods.
- The maximum dose specified for L-glutamine in NNHPD's Workout Supplements monograph may have a larger effect on the limiting (lowest) amino acid score for the most vulnerable age-gender group. Therefore, the maximum amount in SFs is set at a level that would not decrease the limiting amino acid score for the most vulnerable group by more than 5%, which is an amount that has been deemed tolerable for the essential amino acids at their respective maximum amounts.
3.0 Approach for cautionary statements related to amino acids
The addition of amino acids at various threshold levels triggers the requirement for carrying cautionary statements on the label of SFs. The threshold levels above which the cautionary statements are required are identified in the List of Permitted Supplemental Ingredients. The rationales for the requirement of these statements are provided below.
- a) Requirement for cautionary statement: "Do not [eat/drink] more than X serving(s) per day"
This statement is required to ensure that consuming 5 or less servings of an SF in one day does not result in total intakes exceeding the maximum amount for any amino acid. As the TMA research indicated that a small proportion of consumers may consume up to 5 servings of SFs per day (see Section 2.8 in Appendix 4), the threshold levels were determined by dividing the maximum amount by 5.
- b) Requirement for cautionary statement: "Not recommended for those under 14 years old or pregnant or breastfeeding women"
These cautionary statements may be required on SFs as a precautionary approach to protect certain vulnerable populations from potential risks of added amino acids. The NASEM has not set a UL for amino acids for any age-gender group due to the lack of sufficient data; however, this does not mean that there are no risks associated with excessive intake. Amino acid supplementation at high levels has been shown to have adverse effects in growing animals, and therefore, these cautionary statements may help to mitigate risk to vulnerable subpopulations who experience increased rates of growth, such as children. While the available safety evidence for amino acids focuses on adults, a specific or significant risk has not been identified to increase the age limit from 14 years and older for SFs containing added amino acids up to the specified maximum amounts.
The threshold levels for these cautionary statements have been set in consideration of identified and possible uses as food flavouring ingredients for SF categories. The thresholds for L-alanine, L-arginine, and glycine have been adjusted to mitigate possible risks associated with exceeding their maximum amounts from all potential sources of dietary intake, and to provide a more consistent and predictable labelling approach for SFs.
- c) Requirement for cautionary statement: "Do not [eat/drink] on the same day as any other supplemented foods or supplements with [the same supplemental ingredients/(name specific ingredients)]"
This cautionary statement is not required for amino acids. The maximum amounts for amino acids in SFs are conservative compared to the levels at which adverse effects have been seen in humans in the general adult population. A small proportion of the Canadian population may be vulnerable to excess intakes of single amino acids (e.g., 22% of women over 70 years of age and 5-15% of many other age-gender groups do not meet their EAR for protein); however, those with low protein intake may be unlikely to consume single amino acids from multiple sources (e.g., workout supplements). Health Canada will continue to monitor intakes of amino acids from SFs, as well as new research regarding safe levels of amino acids as it becomes available, in order to determine whether additional requirements are needed to mitigate identified risks in the future.
Appendix 6: Taurine - Approach for setting maximum amount and other conditions of use
Taurine is permitted for addition in SFs with a maximum amount of 2000 mg per serving of stated size as per the List of Permitted Supplemental Ingredients.
1.0 Maximum amount for taurine during the Temporary Marketing Authorization period
The maximum level of taurine permitted during the TMA period was 3000 mg/day for CEDs, as indicated in the Category Specific Guidance for Temporary Marketing Authorization - Caffeinated Energy Drinks. This level was based on the maximum dose in the NNHPD's Taurine Monograph, and the Observed Safe Level published based on evidence of the absence of adverse effects for taurine at supplemental intakes up to 3000 mg/day (Shao & Hathcock, 2008). A maximum level for taurine was not published for SFs other than for CEDs. The addition of taurine to SFs other than CEDs was assessed on a case-by-case basis taking into account the overall risk profile of the individual product.
2.0 Approach for setting a maximum amount for taurine
The maximum level of 3000 mg/day for taurine published in the Category Specific Guidance for Temporary Marketing Authorization - Caffeinated Energy Drinks during the TMA period may not account for additional SF categories that could also contain taurine. As similar considerations would apply for taurine in other SFs (e.g., the Observed Safe Level encompasses all supplemental sources of taurine), a single maximum amount is proposed for all SF categories. To mitigate the risk of exceeding safe levels by consuming multiple SFs from different SF categories that may contain taurine, without the need for additional cautionary statements, the maximum amount for taurine has been revised to 2000 mg/day.
While studies with taurine at levels higher than 3000 mg/day tend to have relatively small sample sizes and shorter durations, Health Canada has not identified significant toxicological concerns up to 10,000 mg of taurine per day (Pearl et al., 2014, La Vieille et al., 2021). Therefore, occasional consumption of multiple servings of different SFs (e.g., up to 5 servings of SFs), each containing taurine up to the maximum amount of 2000 mg/day, would not result in adverse health effects and would not require additional cautionary statements, beyond those required for CEDs during the TMA period.
Appendix 7: Applicable Health Canada guidance documents and other resources
- Acceptable Nutrient Function Claims
- Additional Nutritional Information
- CFIA Industry Labelling Tool
- Consumption Incident Reporting for Supplemented Foods
- Contact a CFIA Office
- Daily values for vitamin and mineral nutrients (2016)
- Food Labelling and Advertising
- Front-of-package nutrition labelling
- Guidance Document: Category Specific Guidance for Temporary Marketing Authorization: Caffeinated Energy Drinks
- Guidance Document: Category Specific Guidance for Temporary Marketing Authorization: Supplemented Food
- Guidance Document: Classification of products at the food-natural health product interface: products in food formats
- Guidance Documents for Preparing Health Claim Submissions
- Guidelines for the Safety Assessment of Novel Foods Derived from Plants and Microorganisms
- Health Canada Notices of Proposal and Notices of Modification
- Health Claims
- Health Claim Assessments
- Health Claims Reviewed and Accepted
- Information on Incorporation by Reference
- Lists of Permitted Food Additives
- List of non-novel determinations for food and food ingredients
- Multi-vitamin/mineral supplements monograph
- Natural Health Product Directorate's Workout Supplements Monograph
- Novel Foods
- Novelty Determination Request for a Food or Food Ingredient
- Nutrition Information and Rounding Rules
- Nutrition Labelling Toolkit
- Report a Food Safety or Labelling Concern
- Schedule A.1 of the Food and Drug Act
- Supplemented Foods
- The Food Directorate's Premarket Submission Management Process for Food Additives, Infant Formulas and
- The Use of Food Allergen Precautionary Statements on Prepackaged Foods
- Taurine Monograph
- Workout Supplements monograph
References Cited
- Canadian Community Health Survey (CCHS). (2015). CCHS, 2015. https://www.canada.ca/en/health-canada/services/food-nutrition/food-nutrition-surveillance/health-nutrition-surveys/canadian-community-health-survey-cchs/reference-guide-understanding-using-data-2015.html?wbdisable=true
- Dietitians of Canada - Practice-based Evidence in Nutrition (PEN). (2015). Hemochromatosis/Haemochromatosis. https://www.pennutrition.com/KnowledgePathway.aspx?kpid=22567&trid=23247&trcatid=38
- European Food Safety Authority (EFSA). (2015). Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA Journal, 13(7). https://doi.org/10.2903/j.efsa.2015.4149
- European Food Safety Authority (EFSA) Panel on Food Additives and Nutrient Sources added to Food (ANS). (2010). Scientific Opinion on the safety of trivalent chromium as a nutrient added for nutritional purposes to foodstuffs for particular nutritional uses and foods intended for the general population (including food supplements). EFSA Journal, 8(12), 1882. https://doi.org/10.2903/j.efsa.2010.1882
- European Food Safety Authority (EFSA) Scientific Committee on Food. (2006). Tolerable Upper Intake Levels for Vitamins and Minerals. http://www.efsa.eu.int
- FAO Expert Consultation. (2013). Dietary protein quality evaluation in human nutrition.
- Friedman, M., & Levin, C. E. (2012). Nutritional value of d-amino acids, d-peptides, and amino acid derivatives in mice. Methods in Molecular Biology, 794, 337-353. https://doi.org/10.1007/978-1-61779-331-8_23
- Grinberg, N., Benkhedda, K., Barber, J., Krahn, A. D., & La Vieille, S. (2022). Effects of Caffeinated Energy Drinks on Cardiovascular Responses during Exercise in Healthy Adults: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Applied Physiology, Nutrition, and Metabolism. https://doi.org/10.1139/APNM-2021-0807
- Gropper, S. S., Gropper, D. M., & Acosta, P. B. (1993). Plasma Amino Acid Response to Ingestion of L-Amino Acids and Whole Protein. Journal of Pediatric Gastroenterology and Nutrition, 16(2), 143-150. https://doi.org/10.1097/00005176-199302000-00008
- Gu, S.-X., Wang, H.-F., Zhu, Y.-Y., & Chen, F.-E. (2020). Natural Occurrence, Biological Functions, and Analysis of D-Amino Acids. Pharmaceutical Fronts, 02(02), e79-e87. https://doi.org/10.1055/s-0040-1713820
- Harper, A. E. (1958). Balance and imbalance of amino acids. Annals of the New York Academy of Sciences, 69(5), 1025-1041.
- Harper, A. E. (1970). Effects of ingestion of disproportionate amounts of amino acids. Physiological Reviews, 50(3), 428-558.
- Imamura, W., Yoshimura, R., Takai, M., Yamahura, J., Kanamoto, R., & Kato, H. (2013). Adverse Effects of Excessive Leucine Intake Depend on Dietary Protein Intake: A Transcriptomic Analysis to Identify Useful Biomarkers. Journal of Nutritional Science and Vitaminology, 59(1), 45-55. https://doi.org/10.3177/jnsv.59.45
- Kikuchi, A. M., Tanabe, A., & Iwahori, Y. (2021). A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. Journal of Dietary Supplements, 18(3), 316-333. https://doi.org/10.1080/19390211.2020.1746725
- Kurpad, A. (2018). 90th Anniversary Commentary: Amino Acid Imbalances: Still in the Balance. The Journal of Nutrition, 148(10), 1647-1649.
- La Vieille, S., Gillespie, Z., Bonvalot, Y., Benkhedda, K., Grinberg, N., Rotstein, J., Barber, J., & Krahn, A. D. (2021). Caffeinated energy drinks in the canadian context: Health risk assessment with a focus on cardiovascular effects. Applied Physiology, Nutrition and Metabolism, 46(9), 1019-1028. https://doi.org/10.1139/APNM-2021-0245/SUPPL_FILE/APNM-2021-0245SUPPLA.DOCX
- Liu, N.-K., & Xu, X.-M. (2006). β-Tubulin Is a More Suitable Internal Control than β -Actin in Western Blot Analysis of Spinal Cord Tissues after Traumatic Injury. Journal of Neurotrauma, 23(12), 1794-1801. https://doi.org/10.1089/neu.2006.23.1794
- MacFarlane, A. J., Greene-Finestone, L. S., & Shi, Y. (2011). Vitamin B-12 and homocysteine status in a folate-replete population: results from the Canadian Health Measures Survey. The American Journal of Clinical Nutrition, 94(4), 1079-1087. https://doi.org/10.3945/ajcn.111.020230
- National Academies of Sciences Engineering and Medicine (NASEM). (2019). Dietary Reference Intakes for Sodium and Potassium. In Dietary Reference Intakes for Sodium and Potassium. National Academies Press. https://doi.org/10.17226/25353
- National Academies of Sciences Engineering and Medicine (NASEM) formerly the Institute of Medicine (IOM). (1998). Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press. https://doi.org/10.17226/6015
- National Academies of Sciences Engineering and Medicine (NASEM) formerly the Institute of Medicine (IOM). (2000). Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academies Press. https://doi.org/10.17226/9810
- National Academies of Sciences Engineering and Medicine (NASEM) formerly the Institute of Medicine (IOM). (2002). Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. http://www.nap.edu.
- National Academies of Sciences Engineering and Medicine (NASEM) formerly the Institute of Medicine (IOM). (2011). Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press. https://doi.org/10.17226/13050
- National Academies of Sciences Engineering and Medicine (NASEM) formerly the Institute of Medicine (IOM) Panel on Micronutrients. (2001). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press. https://doi.org/10.17226/10026
- National Academies of Sciences Engineering and Medicine (NASEM) formerly the Institute of Medicine Institute of Medicine (IOM) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. (1997). Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. https://www.ncbi.nlm.nih.gov/books/NBK109829/
- Natural and Non-prescription Health Products Directorate (NNHPD). (2018). Multi-vitamin/mineral supplements monograph. https://webprod.hc-sc.gc.ca/nhpid-bdipsn/atReq.do?atid=multi_vitmin_suppl
- Nielsen. (2017). Nielsen Market Track, National All Channels.
- Park, B. C. (2006). Amino acid imbalance-biochemical mechanism and nutritional aspects. In Asian-Australasian Journal of Animal Sciences (Vol. 19, Issue 9, pp. 1361-1368). Asian-Australasian Association of Animal Production Societies. https://doi.org/10.5713/ajas.2006.1361
- Pearl, P. L., Schreiber, J., Theodore, W. H., McCarter, R., Barrios, E. S., Yu, J., Wiggs, E., He, J., & Gibson, K. M. (2014). Taurine trial in succinic semialdehyde dehydrogenase deficiency and elevated CNS GABA. Neurology, 82(11), 940-944. https://doi.org/10.1212/WNL.0000000000000210
- Pencharz, P. B., Elango, R., & Ball, R. O. (2008). An approach to defining the upper safe limits of amino acid intake. Journal of Nutrition, 138(10), 1996S-2002S. https://doi.org/10.1093/jn/138.10.1996s
- Rodriguez, N. R., DiMarco, N. M., & Langley, S. (2009). Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. Journal of the American Dietetic Association, 109(3), 509-527. https://doi.org/10.1016/j.jada.2009.01.005
- Salmon, W. D. (1958). The Significance of Amino Acid Imbalance in Nutrition. The American Journal of Clinical Nutrition, 6(5), 487-494.
- Shao, A., & Hathcock, J. N. (2008). Risk assessment for the amino acids taurine, l-glutamine and l-arginine. Regulatory Toxicology and Pharmacology, 50(3), 376-399. https://doi.org/10.1016/j.yrtph.2008.01.004
- Toohey, J. (2014). Sulfur amino acids in diet-induced fatty liver: a new perspective based on recent findings. Molecules, 19(6), 8334-8349.
- United Kingdom Expert Group on Vitamins and Minerals Contents (UK EVM). (2003). Safe Upper Levels for Vitamins and Minerals.
- World Health Organization (WHO). (1996). Trace elements in human nutrition and health. WHO. http://www.who.int/nutrition/publications/micronutrients/9241561734/en/
- Xiao, C. W., Wood, C., & Bertinato, J. (2019). Dietary supplementation with l-lysine affects body weight and blood hematological and biochemical parameters in rats. Molecular Biology Reports, 46(1), 433-442. https://doi.org/10.1007/s11033-018-4492-1
Footnotes
- Footnote 1
-
Foods that do not contain added nutrients or other added substances, other than those used for a nutritive or technical effect (e.g., food additives) or food flavouring ingredients.
- Footnote 2
-
Foods that are required or permitted the addition of vitamins, mineral nutrients or amino acids for a nutritional purpose, i.e., to prevent nutritional deficiencies or to restore or improve their nutritional quality (see Section 3.2 a)).
- Footnote 3
-
The UL is the highest average daily nutrient intake level likely to pose no risk of adverse health effects to almost all individuals in a given life-stage and gender group.
- Footnote 4
-
As per the FDR, one microgram of retinol is equivalent to one RAE, whereas 12 micrograms of beta-carotene is equivalent to one RAE.
- Footnote 5
-
Document is static with the publication of the Supplemented Foods Regulations.
- Footnote 6
-
Gotu kola is not included in the list of ingredients inappropriate for consumption as foods. Although minimally processed gotu kola is a food, this does not mean the use of ingredients derived from gotu kola (e.g., extracts) is automatically approved for food use. Please contact Health Canada's Food Directorate for further information.
- Footnote 7
-
The UL is the highest average daily nutrient intake level likely to pose no risk of adverse health effects to almost all individuals in a given life-stage and gender group.
- Footnote 8
-
Usual dietary intake is the long-term average daily intake of a nutrient or food.
- Footnote 9
-
Inputs based on the most vulnerable age-gender group, i.e., 14 years of age and older.